Abstract
OBJECTIVES
Percentage-predicted forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLCO), and their predicted postoperative (ppo) values are established prognostic factors for postoperative pulmonary complications after thoracotomy. However, their predictive value for minimally invasive pulmonary resections remains controversial. This study assessed the incidence of pulmonary complications after robotic lobectomy for primary lung cancer and analysed the predictive significance of FEV1 and DLCO.
METHODS
This was a retrospective analysis of patients who underwent robotic lobectomy from 4 institutions. Descriptive and comparative analyses were performed for patients who experienced pulmonary complications versus patients who did not, in relation to FEV1 and DLCO values. To identify thresholds for increased complications, patients were categorized into groups of 10% incremental increases in FEV1 and DLCO, and their ppo values.
RESULTS
From November 2002 to April 2018, 1088 patients underwent robotic lobectomy. Overall, 169 postoperative pulmonary complications occurred in 141 patients. Male gender and Eastern Cooperative Oncology Group grade ≥1 were associated with increased pulmonary complications on univariable analysis. Patients who experienced pulmonary complications had increased mortality (2.1% vs 0.2%, P = 0.017) and longer hospitalizations (9 vs 4 days, P < 0.001). Pulmonary complications were associated when FEV1 ≤60% and DLCO ≤50%, and when ppo FEV1 or DLCO was ≤50%; ppo FEV1 ≤50% (P < 0.001) and ppo DLCO ≤50% (P = 0.031) remained statistically significant on multivariable analysis.
CONCLUSIONS
Both FEV1 and DLCO were shown to be significant predictors of pulmonary complications. Furthermore, thresholds of percentage-predicted and ppo FEV1 and DLCO values were identified, below which pulmonary complications occurred significantly more frequently, suggesting their predictive values are particularly useful in patients with poorer pulmonary function.
Keywords: Robotics, Lobectomy, Forced expiratory volume in 1 s, Diffusing capacity for carbon monoxide, Pulmonary complications
INTRODUCTION
Pulmonary complications are a major source of morbidity and mortality after thoracic surgery. The percentage-predicted forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLCO), as well as their predicted postoperative (ppo) values, have been established as important prognostic factors for postoperative pulmonary complications after thoracotomy [1, 2]. However, with the increased utility of video-assisted thoracoscopic surgery (VATS), some institutional studies and databases have reported a reduced predictive value of pulmonary function tests for patients who undergo minimally invasive pulmonary resections [3, 4]. These findings have not been consistent in other reports, and there remains a lack of consensus among surgeons about the acceptable threshold of pulmonary function results for VATS lobectomy candidates [5, 6].
The use of robotic-assisted thoracoscopic surgery in the treatment of primary lung cancer has grown exponentially in recent years [7]. There is a paucity of clinical data on the prognostic value of pulmonary function tests on postoperative pulmonary complications for patients who undergo robotic pulmonary resections. The aim of the present study was to assess the incidence of pulmonary complications after robotic lobectomy for patients with primary lung cancer and the predictive value of FEV1 and DLCO for these adverse events. Secondary aims of the study were to assess the impact of pulmonary complications on mortality and length of hospitalization.
MATERIALS AND METHODS
This was a retrospective analysis of consecutively operated patients who underwent robotic lobectomy from 4 participating institutions. The study was approved by institutional review boards, and a data transfer agreement was made to Memorial Sloan Kettering Cancer Center, New York, USA. The primary end point was postoperative pulmonary complications, which was defined a priori as pneumonia, acute respiratory distress syndrome, atelectasis, bronchopleural fistula, pneumothorax, chylothorax, empyema, pleural effusion, tracheostomy and prolonged air leak. Pulmonary complications were recorded if they were grade III or higher according to the Clavien–Dindo Classification, whereby grade III required surgical, endoscopic or radiological intervention, grade IV was life-threatening and required intensive care unit management and grade V resulted in death [8]. In addition, grade II air leaks (beyond 5 days but not requiring radiological or surgical intervention) were also included, in accordance with previously published reports [2, 4]. Pulmonary complications were included for analysis if the adverse event occurred within 30 days since the date of operation, or the same admission, whichever one was longer. Predicted postoperative lung function was calculated using the functional segment technique.
Patient selection criteria
Patients older than 18 years with biopsy-proven or pathologically confirmed primary lung cancer who underwent robotic video-assisted thoracic surgery (RVATS) lobectomy were included for analysis. Patients with benign pathology, small cell lung cancer or who underwent wedge resections were excluded from the centralized database. Patients who had neither FEV1 nor DLCO values were also excluded from analysis.
Statistics
Descriptive and comparative analyses were performed for patients who experienced pulmonary complications versus patients who did not. To identify a potential threshold for increased complications, patients were categorized into groups of 10% incremental increases in FEV1 and DLCO values, as well as their ppo values, to analyse the likelihood of experiencing a pulmonary complication at different points. For univariable analysis, χ2 and Fisher’s exact tests were used to determine significant differences in categorical variables, and Mann–Whitney U-tests were used to compare the distributions of continuous variables. To determine the factors predictive of pulmonary complications, a multivariable analysis was performed by including all variables with a P-value ≤0.2 identified on univariable analysis. A significant difference was predetermined to be a P-value ≤0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25 (IBM Corp., Armonk, NY).
RESULTS
From November 2002 to April 2018, 1088 consecutive patients who underwent robotic lobectomies from participating institutions met the selection criteria to be included in a centralized database. The median follow-up period was 6.8 months. The median age of the entire cohort was 69 years (interquartile range 63–74), with 43.5% male. Performance status was reported as ECOG (Eastern Cooperative Oncology Group) 0 in 73.2%, ECOG 1 in 24.8%, ECOG 2 in 1.8% and ECOG 3 in 0.2%. Histopathology included adenocarcinoma (74.1%), squamous cell carcinoma (12.2%), carcinoid tumours (6.3%), large cell carcinoma (1.3%) and ‘other’ types of non-small-cell lung cancer (6.1%). Surgical resections included right upper lobe (35.6%), right middle lobe (7.4%), right lower lobe (18.6%), left upper lobe (25.2%) and left lower lobe (13.3%). The perioperative mortality rate for the entire study cohort was 0.5%. The median length of hospitalization was 4 days (interquartile range 3–6).
Overall, 169 postoperative pulmonary complications occurred in 141 patients (13.0%), including 104 grade II air leaks, 53 grade III events, 4 grade IV events and 4 grade V events. Forty-four patients (4.0%) experienced at least one major pulmonary complication graded III or above. Types of pulmonary complications included prolonged air leak (10.4%), pneumonia (0.9%), acute respiratory distress syndrome (0.6%), atelectasis (0.7%), bronchopleural fistula (0.5%), pneumothorax (0.6%), chylothorax (0.4%), empyema (0.3%), pleural effusion (0.4%) and respiratory failure requiring tracheostomy (0.4%). On univariable analysis, patients who had a postoperative pulmonary complication were more likely to be male (54.6% vs 41.8%, P = 0.005) and had lower FEV1 (median 90% vs 95%, P = 0.005) and DLCO (71% vs 81%, P = 0.002) when compared to patients who did not have a pulmonary complication. Patients who experienced a pulmonary complication had a higher risk of mortality (2.1% vs 0.2%, P = 0.017) and prolonged hospitalization (9 vs 4 days, P < 0.001) compared to those who did not. A summary of these findings is presented in Table 1.
Table 1:
A summary of baseline characteristics and clinical outcomes for patients who underwent robotic lobectomy for primary lung cancer
| All patients (n = 1088) | Pulmonary complication n = 141 (13.0%) | No complication n = 947 (87.0%) | P-value | |
|---|---|---|---|---|
| Age (years), n = 1088 (100%) | 69 (63–74)a | 70 (64.5–75)a | 69 (63–74)a | 0.366 |
| Male gender, n = 1088 (100%) | 473 (43.5) | 77 (54.6) | 396 (41.8) | 0.005 |
| ECOG >1, n = 935 (85.9%) | 235 (25.1) | 40 (30.8) | 195 (24.2) | 0.127 |
| FEV1 predicted, n = 1088 (100%) | 94 (82–106)a | 90 (76–104)a | 95 (83–106)a | 0.005b |
| DLCO predicted, n = 880 (80.9%) | 81 (67–95)a | 71 (60–91)a | 81 (69–95)a | 0.002b |
| Histopathology, n = 1088 (100%) | 0.062 | |||
| Adenocarcinoma | 815 (74.9) | 111 (78.7) | 704 (74.3) | |
| Squamous | 125 (11.5) | 19 (13.5) | 106 (11.2) | |
| Large cell | 16 (1.5) | 3 (2.1) | 13 (1.4) | |
| Carcinoid | 62 (5.7) | 1 (0.7) | 61 (6.4) | |
| Other | 70 (6.4) | 7 (5.0) | 63 (6.7) | |
| Clinical stage, n = 1087 (99.9%) | 0.554 | |||
| I | 799 (73.5) | 698 (73.8) | 101 (71.6) | |
| II | 178 (16.4) | 150 (15.9) | 28 (19.9) | |
| III | 98 (9.0) | 88 (9.3) | 10 (7.1) | |
| IV | 12 (1.1) | 10 (1.1) | 2 (1.4) | |
| Lobe resected, n = 1088 (100%) | 0.479 | |||
| Right upper lobe | 387 (35.6) | 53 (37.6) | 334 (35.3) | |
| Right middle lobe | 80 (7.4) | 10 (7.1) | 70 (7.4) | |
| Right lower lobe | 202 (18.6) | 32 (22.7) | 170 (18.0) | |
| Left upper lobe | 274 (25.2) | 32 (22.7) | 242 (25.6) | |
| Left lower lobe | 145 (13.3) | 14 (9.9) | 131 (13.8) | |
| Mortality, n = 1088 (100%) | 5 (0.5) | 3 (2.1) | 2 (0.2) | 0.017 |
| Hospital LOS, n = 1087 (99.9%) | 4 (3–6)a | 9 (6–13)a | 4 (3–5)a | <0.001 |
Continuous variables are reported as median and interquartile range.
Significant on multivariable analysis.
DLCO: diffusing capacity for carbon monoxide; ECOG: Eastern Cooperative Oncology Group; FEV1: percentage predicted forced expiratory volume in 1 s; LOS: length of stay.
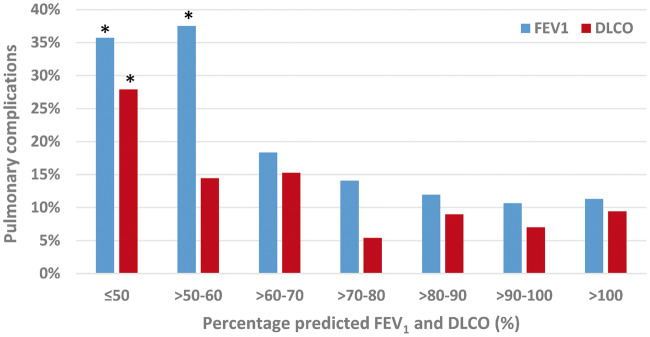
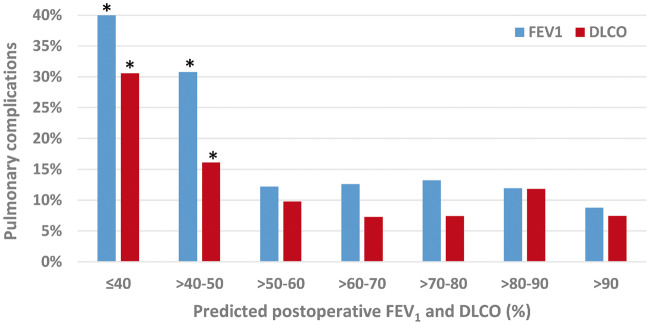
To further stratify the predictive value of pulmonary function tests, patients were divided into grouped intervals based on their percentage-predicted and ppo FEV1 and DLCO, as shown in Figs 1 and 2, respectively. The incidences of postoperative pulmonary complications were presented within each 10% incremental increase of pulmonary function values. Statistical analysis of these incremental groups found significantly more pulmonary complications in patients when FEV1 ≤60% and DLCO ≤50%, and when ppo FEV1 or DLCO was ≤50%. Multivariable analysis found ppo FEV1 ≤50% (P < 0.001) and ppo DLCO ≤50% (P = 0.031) to remain statistically significant, as shown in Table 2.
Figure 1:
Incidence of pulmonary complications according to percentage predicted FEV1 and DLCO in patients who underwent robotic-assisted lobectomy. Asterisk denotes values statistically significant in predicting pulmonary complications on univariable analysis. DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s.
Figure 2:
Incidence of pulmonary complications according to predicted postoperative FEV1 and DLCO in patients who underwent robotic-assisted lobectomy. Asterisk denotes values statistically significant in predicting pulmonary complications on univariable analysis. DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s.
Table 2:
Multivariable analysis of potential factors associated with postoperative pulmonary complications following robotic lobectomy
| Covariate | Odds ratio (95% CI) | P-value |
|---|---|---|
| ppo FEV1 <50% | 4.121 (1.860–9.132) | <0.001 |
| ppo DLCO <50% | 1.834 (1.058–3.181) | 0.031 |
| Male gender | 1.540 (0.942–2.518) | 0.085 |
| ECOG >1 | 1.063 (0.585–1.934) | 0.840 |
| Histopathology | 0.356 | |
| Adenocarcinoma | Reference | |
| Squamous cell carcinoma | 0.624 (0.289–1.343) | 0.228 |
| Large cell carcinoma | 1.582 (0.418–5.983) | 0.500 |
| Carcinoid | 0.231 (0.031–1.737) | 0.155 |
| Other | 0.709 (0.284–1.773) | 0.463 |
CI: confidence interval; DLCO: diffusing capacity for carbon monoxide; ECOG: Eastern Cooperative Oncology Group; FEV1: forced expiratory volume in 1 s; ppo: predicted postoperative.
DISCUSSION
Pulmonary function has long been recognized as an important predictor of postoperative pulmonary complications from historical studies [9, 10]. Prospective studies and outcomes from the Society of Thoracic Surgeons General Thoracic Surgery Database have identified percentage-predicted FEV1 and DLCO to be inversely proportional to the incidence of postoperative pulmonary complications for patients with non-small-cell lung cancer who underwent anatomical lung resections [1, 2]. These studies and others have established the inclusion of FEV1 and DLCO as part of the routine preoperative assessment in international guidelines [11, 12].
More recently, with the introduction and popularization of VATS, some surgeons have proposed that patients with poor pulmonary function are able to tolerate major lung resections with acceptable morbidity and mortality due to reduced pain and preserved chest wall mechanics associated with thoracoscopic surgery [4, 13]. Berry et al. [4] presented a retrospective, single-institutional study that demonstrated pulmonary function tests to be predictive of pulmonary complications after thoracotomy, but not VATS, especially for patients with poor pulmonary function. These findings were reproduced, although to a lesser degree, from the Society of Thoracic Surgeons General Thoracic Surgery Database presented by Ceppa et al. [3]. The paradigm shift in the accepted threshold for VATS lobectomy among thoracic surgeons was demonstrated in a cross-sectional survey at the 20th Anniversary of VATS Lobectomy Conference, in which 76% of surgeons considered <30% predicted FEV1 to be a contraindication for lobectomy and 64% considered <30% predicted DLCO a contraindication [6]. Despite these findings, a comprehensive report by Zhang et al. maintained that ppo FEV1 and ppo DLCO were predictive of pulmonary complications, irrespective of the open thoracotomy or VATS approach [5].
The present study represents the largest report to examine the predictive value of FEV1 and DLCO for patients who underwent robotic lobectomy. Other strengths of our study included a standardized, predefined list of pulmonary complications with a grading system consistent with previous reports [2, 4, 8]. Key findings from our study included a relatively low incidence of all pulmonary complications, at 13.0%, with 4.0% of patients experiencing at least one ≥ grade III major complication and 0.5% mortality rate. Both FEV1 and DLCO values were shown to be significant predictors of pulmonary complications on univariable and multivariable analyses. Furthermore, thresholds of percentage predicted and ppo FEV1 and DLCO values were identified, below which pulmonary complications occurred significantly more frequently. These findings suggest that pulmonary function tests remain useful assessment tools for patients who undergo robotic lobectomy, but their predictive value for pulmonary complications are particularly useful in patients with poorer pulmonary function.
Limitations
Limitations of our study included its retrospective nature, and potential patient selection and other biases associated with this study design. There were a number of potentially important baseline patient characteristics that were not included in the centralized database, including cardiac comorbidities. In addition, there were heterogenous processes between institutions in patient selection, operative technique and postoperative management, such as pain control and physiotherapy, which may have potentially affected the primary end point of pulmonary complications. Robotic technology had evolved from the earlier Si models to the current Xi model (da Vinci, Intuitive Surgical, Sunnyvale, California), and surgeons varied in their ‘total portal’ versus ‘robotic-assisted’ approach, sometimes within their individual experience, and also between institutions at different timeframes. Unfortunately, there was insufficient granularity within our database to analyse whether these different surgical approaches affected any clinical outcomes, but this heterogeneity probably reflected the real-world experience. Participating institutions were also tertiary centres with established robotic lobectomy programmes, which may not be representative of clinical outcomes in other institutions. Finally, only 80.9% of patients provided DLCO values, which was a reflection of the differences in preoperative assessment between participating institutions.
CONCLUSION
In conclusion, our multi-institutional study on patients who underwent robotic lobectomy found FEV1 and DLCO to be significant predictors of postoperative pulmonary complications, and their predictive value may be particularly useful in patients who have poorer pulmonary function. Although the overall incidence of pulmonary complications in our series was relatively low, we believe that routine assessment of pulmonary function tests should be considered mandatory, and patients with poor function below our identified thresholds should undergo additional postoperative measures such as closer monitoring and aggressive physiotherapy to minimize pulmonary complications.
Acknowledgements
The authors would like to thank Joe Dycoco for his assistance with data collection.
Conflict of interest: Dr Franca Melfi is a proctor for Intuitive Surgical. Dr Giulia Veronesi is a proctor for ABI Medica, speaker honoraria and consultant for Medtronic and Johnson & Johnson. Dr Brian Louie's disclosed research grant is not directly related to this manuscript.
REFERENCES
- 1. Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815–18. [DOI] [PubMed] [Google Scholar]
- 2. Ferguson MK, Gaissert HA, Grab JD, Sheng S. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg 2009;138:1297–302. [DOI] [PubMed] [Google Scholar]
- 3. Ceppa DP, Kosinski AS, Berry MF, Tong BC, Harpole DH, Mitchell JD et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berry MF, Villamizar-Ortiz NR, Tong BC, Burfeind WR Jr, Harpole DH, D’Amico TA et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang R, Lee SM, Wigfield C, Vigneswaran WT, Ferguson MK. Lung function predicts pulmonary complications regardless of the surgical approach. Ann Thorac Surg 2015;99:1761–7. [DOI] [PubMed] [Google Scholar]
- 6. Yan TD, Cao C, D'Amico TA, Demmy TL, He J, Hansen H et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633–9. [DOI] [PubMed] [Google Scholar]
- 7. Rajaram R, Mohanty S, Bentrem DJ, Pavey ES, Odell DD, Bharat A et al. Nationwide assessment of robotic lobectomy for non-small cell lung cancer. Ann Thorac Surg 2017;103:1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kearney DJ, Lee TH, Reilly JJ, DeCamp MM, Sugarbaker DJ. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest 1994;105:753–9. [DOI] [PubMed] [Google Scholar]
- 10. Ferguson MK, Little L, Rizzo L, Popovich KJ, Glonek GF, Leff A et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894–900. [PubMed] [Google Scholar]
- 11. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S–90S. [DOI] [PubMed] [Google Scholar]
- 12. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181–4. [DOI] [PubMed] [Google Scholar]
- 13. Burt BM, Kosinski AS, Shrager JB, Onaitis MW, Weigel T. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19–28; dicussion 28–9.e11. [DOI] [PubMed] [Google Scholar]