Abstract
Preeclampsia (PE) is a severe pregnancy complication, which may be considered as a systemic response in the second half of pregnancy to physiological failures in the first trimester, and can lead to very serious consequences for the health of the mother and fetus. Since PE is often associated with proteinuria, urine proteomic assays may represent a powerful tool for timely diagnostics and appropriate management. High resolution mass spectrometry was applied for peptidome analysis of 127 urine samples of pregnant women with various hypertensive complications: normotensive controls (n = 17), chronic hypertension (n = 16), gestational hypertension (n = 15), mild PE (n = 25), severe PE (n = 25), and 29 patients with complicated diagnoses. Analysis revealed 3869 peptides, which mostly belong to 116 groups with overlapping sequences. A panel of 22 marker peptide groups reliably differentiating PE was created by multivariate statistics, and included 15 collagen groups (from COL1A1, COL3A1, COL2A1, COL4A4, COL5A1, and COL8A1), and single loci from alpha-1-antitrypsin, fibrinogen, membrane-associated progesterone receptor component 1, insulin, EMI domain-containing protein 1, lysine-specific demethylase 6B, and alpha-2-HS-glycoprotein each. ROC analysis of the created model resulted in 88% sensitivity, 96.8% specificity, and receiver operating characteristic curve (AUC) = 0.947. Obtained results confirm the high diagnostic potential of urinary peptidome profiling for pregnancy hypertensive disorders diagnostics.
Keywords: urine peptidomics, preeclampsia, hypertension, proteomics, mass-spectrometry
1. Introduction
Preeclampsia (PE) is the most severe hypertensive pathology complicating 2–8% of pregnancies and is associated with increased risk of miscarriage, premature birth, disability in the newborns, and development of severe cardiovascular pathologies in women after pregnancy, as well as neonatal and maternal deaths [1,2,3,4]. Unlike other hypertensive pregnancy complications (chronic or gestational hypertension), PE occurs after the 20th week of pregnancy and is often associated with proteinuria and other signs of multiple organ dysfunction [5,6,7,8,9,10]. Timely and appropriate treatment is of particular significance to avoid the fateful outcomes. The identification of women at high risk before pregnancy or at least until the 13th week of gestation is extremely important for choosing an adequate management as abnormal placentation leads to more severe forms of the pathology and its early onset [11]. However, late onset PE (≥34 weeks’ gestation) may occur due to other intrinsic pathologies that may be triggered by pregnancy and are also related with abnormal uteroplacental and vascular remodeling, as well as with redistribution of blood flow, which is laid in the first trimester of pregnancy [12,13,14,15].
Several physiological pathways related to genetic, epigenetic, and environmental factors may be involved in PE [16]. Main risk factors include primipregnancy, long interval between pregnancies, advanced maternal age, pre-pregnancy obesity, diabetes, hypertension, antiphospholipid syndrome, ethnic background, and a previous and/or family history of PE [11,17,18,19]. The etiology of PE essentially relates to hypoxia resulting from abnormal trophoblast invasion and vascular and uterine remodeling. Predisposing genetic factors relate to polymorphisms in a number of proteins/genes essential for regulation of blood coagulation, vascular endothelial function, blood pressure, inflammation, and immunity, which are also essential in other systemic pathologies not associated with pregnancy, such as hypertension, vascular disease, thrombophilia, and systemic inflammation [19,20,21,22,23,24]. The dysregulation in expression of SERPIN proteins A3, A5, A8, B2, E1, E2, and G1 shown earlier may reflect some compensatory mechanisms in PE-placentas [16]. Thus, the maternal PE syndrome that appears in the second half of pregnancy can be considered as a systemic response to systemic failures in the first trimester, which can occur under various scenarios, depending on different combinations of numerous genetic and non-genetic factors, and may not depend only on a few of them.
Since the real causes of PE are laid long before its manifestation, there is an opportunity for timely diagnostics and appropriate management to improve maternal and perinatal outcomes. However, reliable screening tests for clinical application have not yet been developed. Among protein markers, abundant expression of metalloproteinases MMP-2 and MMP-9 (gelatinases A and B) in extravillous trophoblasts is highly related to extracellular matrix (ECM) degradation [25,26,27]. Levels of MMP-2 and its inhibitor TIMP-1 were shown to be significantly increased in PE [28,29]. A decreased circulation level of placental growth factor (PLGF) may predict the development of PE in the following 2 weeks [30,31]. Soluble fms-like tyrosine kinase-1 (sFlt-1) can block vascular endothelial growth factor (VEGF) and PLGF via their binding. Its increased level (or increased sFlt-1/PLGF ratio) may precede PE from the second trimester onwards [31,32,33]. The increased degree of circulating anti-angiogenic soluble endoglin (sEng) strongly correlates with PE severity [34,35]. Decreased levels of placental protein-13 and pregnancy-associated protein A in the first trimester as well as increased levels of pro-inflammatory cytokines and hypoxia induced factor 1α may also suggest PE; however, these features are less specific [36,37,38,39,40].
Urine seems to be the most convenient subject for research due to its relative stability and non-invasive collection. Since PE is often associated with renal pathologies and proteinuria, urine proteomic analysis may provide valuable information, for example it may distinguish PE from other hypertensive pregnancy complications, and may estimate the degree of disease severity, which is essential for further management. Mass spectrometry (MS)-based approaches proved to be the most effective in previous urine peptidome/proteome studies and provided most of the current information [41,42,43,44,45,46,47]. Currently, the proposed urine PE markers include reduced levels of PlGF, prostaglandin-H2 D-isomerase, and perlecan [41,48], as well as increased levels of specific fragments of α-1-antitrypsin (SERPINA1), albumin, fibrinogen alpha chain, collagen alpha chain, uromodulin [41,42,43,44,45], and of some unidentified proteins [46,47]. Using high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS), we found 35 specific urine peptides originating from SERPINA1, uromodulin, and collagen alpha-1 chains (I and III), which reliably distinguished a particular PE group (10—mild PE; 10—severe PE) from normal controls [45]. However, the results of various studies are not consistent enough, and none of the particular markers described to date show sufficient sensitivity. We believe that creation of a differentiating peptide marker panel can essentially improve PE diagnostics, and remains of particular importance as a basis for the further development of accessible clinical methods.
Here, based on the analysis of 127 urine samples of pregnant women (including normotesive controls, chronic hypertension (CH), gestational hypertension (GH), moderate PE (mPE), and severe PE (sPE)), and using peptide grouping, we propose a variant of such peptide panel with a high differentiating capacity, which consists of 22 peptide groups and also includes markers described earlier.
2. Materials and Methods
2.1. Patients
Patients were diagnosed in V.I. Kulakov’s National Medical Research Center of Obstetrics, Gynecology and Perinatology in accordance with the ACOG Practice Bulletin (2002). PE was diagnosed at blood pressure (BP) > 140/90 mmHg, proteinuria > 0.3 g/L daily, edema, manifestations of multisystem organ insufficiency. Severe PE was diagnosed with at least one of the following symptoms: BP ≥ 160/100 mmHg (twice within 6 h); proteinuria ≥ 5 g/L daily or > 0.3 g/L in separate urine samples; oliguria ≤ 500 mL/day; epigastric pain and/or pain in right hypochondrium; pulmonary edema or pulmonary failure; more than twice increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST); neurological complications; thrombocytopenia (less than 100 × 109/L); intrauterine growth restriction (IUGR) (fetal weight below 10 percentiles).
Normotensive pregnant women with urine protein content < 0.1 mg/mL were enrolled into the control group. The exclusion criteria included: multiple pregnancy, pregnancy after assisted reproductive technology (ART), diabetes, transplanted organs, autoimmune diseases, oncological diseases, decompensated kidney disease, chromosomal abnormalities in the fetus, congenital malformations of the fetus, and antenatal fetal death. CH and GH were diagnosed in patients who did not meet the PE criteria: CH in patients, who had hypertension before pregnancy; GH in patients with hypertension experienced during pregnancy.
In total, the study included 127 urine samples from 126 pregnant women: 17 from normotensive pregnant controls, 16 from patients with chronic arterial hypertension (CH; two of the samples were obtained from one patient), 15 with gestational arterial hypertension (GH), 25 with mild PE (mPE), 25 with severe PE (sPE), and 29 samples were from patients with complicated diagnoses that were considered to be unrelated to any group. The latter included 14 samples from patients with PE superimposed on CH (PE-CH); 4 samples from patients diagnosed as CH with suspected PE; 1 sample from the patient with GH who later developed mPE and sPE; 1 sample with mPE superimposed on a non-hypertensive pathology; and 9 samples with suspected but not diagnosed PE. Clinical and demographical data are shown in Table 1.
Table 1.
Clinical and demographic data of patients.
| Parameter | Control (n = 17) |
CAH (n = 15) |
GAH (n = 15) |
Mild PE (n = 25) | Severe PE (n = 25) |
|---|---|---|---|---|---|
| Age (years) | 30.5 ± 4.1 | 31.8 ± 5 | 32.6 ± 4.2 | 28.5 ± 4.2 | 32.2 ± 4.5 |
| Height (cm) | 168.6 ± 4.8 | 167.4 ± 5 | 166.8 ± 7.1 | 164.6 ± 5.2 | 163.9 ± 5.8 |
| Weight (kg) | 70.1 ± 8.3 | 83 ± 15.5 | 76.5 ± 10.3 | 76.1 ± 14.9 | 74.2 ± 14.2 |
| BMI (kg/m2) | 24.7 ± 3.1 | 28.6 ± 3.4 | 27.2 ± 3.1 | 28.1 ± 5 | 27.6 ± 5.1 |
| Kidney disease | 2 (11.1%) | 2 (10.5%) | 4 (25%) | 2 (7.7%) | 4 (15.4%) |
| Previous PE | 1 (5.6%) | 6 (31.6%) | 1 (6.2%) | 0 (0%) | 6 (23.1%) |
| Primiparous | 5 (27.8%) | 7 (36.8%) | 5 (31.2%) | 16 (61.5%) | 11 (42.3%) |
| Start of hypertension (days) | - | 28 ± 9.1 | 29.2 ± 5.4 | 34.3 ± 5.1 | 28.2 ± 8.2 |
| Start of proteinuria (days) | - | 36.5 ± 0.7 | 33.5 ± 0.2 | 36.7 ± 2.1 | 32 ± 4.6 |
| sFlt-1/PLGF | 6.7 ± 1.8 | 33.4 ± 31.1 | 67.8 ± 78.1 | 229.8 ± 209.6 | 346.3 ± 281.9 |
| Delivery (weeks) | 39 ± 1.1 | 37.8 ± 1.9 | 37.6 ± 2.2 | 37.9 ± 1.6 | 33.6 ± 4.4 |
| Maximal SP | 117.2 ± 5.5 | 143.1 ± 14.4 | 146.7 ± 10.9 | 151.5 ± 12.6 | 157.7 ± 11.3 |
| Maximal DP | 77.8 ± 3.9 | 90.9 ± 7.3 | 94.7 ± 8.9 | 96.0 ± 6.9 | 100.8 ± 7.5 |
| Maximal Pu (g/l) | 0 ± 0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 1.2 ± 1.0 | 2.3 ± 1.6 |
| LDH | 393.3 ± 0 | 327.6 ± 14.4 | 366.2 ± 33.8 | 334.5 ± 91.0 | 445.8 ± 117.1 |
| ALT | 13.8 ± 2.2 | 15 ± 6.6 | 17.9 ± 7 | 24.6 ± 28.7 | 36.1 ± 20.1 |
| AST | 17.3 ± 3.4 | 18.9 ± 4.6 | 16.8 ± 4.8 | 25.0 ± 12.2 | 33.9 ± 15.7 |
| ALP | 129.7 ± 3.4 | 201 ± 156.3 | 164.3 ± 68.3 | 168.5 ± 47 | 183.2 ± 89.2 |
| Platelet count | 217.3 ± 56.5 | 244.1 ± 73.4 | 251.5 ± 55.2 | 218.8 ± 67.8 | 199.5 ± 77.2 |
| UAPI | 0.9 ± 0 | 0.9 ± 0.3 | 1 ± 0.3 | 1.4 ± 0.6 | 2.0 ± 1.0 |
| UMAPI | 0.9 ± 0 | 1.1 ± 0.4 | 1 ± 0.3 | 1.3 ± 0.5 | 1.6 ± 1.3 |
| MCAPI | 1.6 ± 0 | 2.8 ± 0.7 | 1.8 ± 0.3 | 2.6 ± 1.2 | 2.8 ± 1.1 |
| IUGR | 0 (0%) | 0 (0%) | 2 (12.5%) | 6 (23.1%) | 17 (65.4%) |
| Child weight (g) | 3361.8 ± 505.7 | 3035.3 ± 518.6 | 2961 ± 562.5 | 2768.3 ± 540.5 | 1783.3 ± 834.4 |
| Child height (cm) | 51.4 ± 2.4 | 50.5 ± 2.6 | 49.9 ± 3.6 | 48.8 ± 2.6 | 40.9 ± 8 |
| Apgar 1 min | 8.1 ± 0.3 | 7.9 ± 0.3 | 7.8 ± 0.5 | 7.7 ± 0.5 | 6.6 ± 1.7 |
| Apgar 5 min | 9.1 ± 0.4 | 8.7 ± 0.6 | 8.6 ± 0.6 | 8.7 ± 0.5 | 7.7 ± 1.3 |
UAPI—uterine artery pulsatility index, UMAPI—fetal umbilical artery pulsatility index, MCAPI—fetal middle cerebral artery pulsatility index, Pu—proteinuria, LDH—lactate dehydrogenase, IUGR—intrauterine growth restriction.
In the intergroup analysis incidence of hypertension in family history in PE, CH and PE-CH did not differ statistically between the groups—46.4%, 50%, and 50%, correspondingly—but was significantly more common as compared to the control group: 20% (p < 0.01). Thus, hypertension in relatives is a risk factor for hypertensive disorders in pregnancy. In the PE group, women were significantly more likely to have had adverse pregnancy outcomes: antenatal fetal death (7.1%), early neonatal death (3.6%), and history of preeclampsia (17.9%) compared to women with PE superimposed on CH (0%, 0%, and 12.5%, respectively), and to the control group where these complications were not observed (p < 0.05).
2.2. Urine Sample Collection and Peptide Isolation
Urine collection was performed before treatment after a written informed consent of participants in accordance with the protocol approved by the Ethical Committee (Record No12 from 17 November 2016) of V.I. Kulakov’s National Medical Research Center of Obstetrics, Gynecology and Perinatology.
Urine samples were centrifuged (2000 g, 10 min, 4 °C) within 20 min after collection, and the supernatant was stored at −80 °C. The peptide fraction was obtained as described earlier [45,49]. Particularly, 1.5 mL of urine was diluted with 3 volumes of denaturing buffer (4M urea, 20 mM ammonium hydroxide, 0.2% sodium dodecyl sulfate), transferred to Vivaspin-4 10 kDa MWCO (Sartorious) filters and centrifuged at 4000 g for 20 min at room temperature. The filtrate (2.5 mL) was further subjected to gel-filtration on a PD-10 Column (GE Healthcare; Sephadex™ G-25 Medium, equilibration and elution with 0.01% ammonium hydroxide). An amount of 2 mL of the eluate was lyophilized and dissolved in 100 µl of deionized water prior to analysis.
2.3. HPLC-MS/MS Analysis
HPLC-MS/MS analysis was performed on a nano-HPLC Agilent 1100 system (Agilent Technologies, Santa Clara, CA, USA) using a homemade capillary column (id 75 μm × length 12 cm, Reprosil-Pur Basic C18, 3 μm, 100 A; Dr. Maisch HPLC GmbH, Ammerbuch-Entringen, Germany) in combination with a 7-Tesla LTQ-FT Ultra mass spectrometer (Thermo Electron, Bremen, Germany) equipped with an in-house nanospray ion source. Gradient chromatography was implemented by changing the relative concentration of solvent B (100% acetonitrile/0.1% formic acid) in flow of solvent A (0.1% formic acid). Main elution time was 15–45 min: linear gradient from 3% to 50% of solvent B, elution of most hydrophobic peptides was 45–50 min: linear gradient from 50% to 90% of solvent B. Each sample was analyzed in 4 runs.
2.4. Urinary Proteome Data Base Development
To facilitate the search procedure, a small data base was created for identification and semiquantitative analysis of the massive HPLC-MS/MS data (see below) using available computing power. The detailed description of the urinary proteome data base development is out of the scope of this manuscript. Briefly, to develop the data base, proteome analysis was performed for three pooled urine samples and their 10 fractions prepared by Isoelectic Focusing Electrophoresis (IEF) using the PROTEAN system (Bio-Rad). Pooled samples were prepared from 60 individual samples (10-Control, 10-GH, 10-CH, 10-(PE-CH), 10-mPE, 10-sPE) which were used for proteome and peptidome analysis. All samples were measured on a Q-Exactive HF operating in data dependent (DDA) mode. In total, 8423 tryptic peptides from 1029 protein groups were identified and archived into the library. In an attempt to increase the coverage of the library, additional 95 individual samples (from 127 patients-2.1. Patients) were selected for additional proteomic analysis and measured in data independent (DIA) mode. Overall, this resulted in a spectral library containing 11,131 peptide ions and 1472 proteins, 32% and 43% more than the initial DDA library, respectively. Finally, the database was expanded with preliminary peptide search data and peptides described previously in PE [42,43,44,45,46,47].
2.5. Data Analysis
Urinary peptides were identified using PEAKS Studio 8.5 and MaxQuant (version 1.6.7.0) search programs across the developed urinary proteome data base (see above) with the following parameters: non-specific enzyme; mass accuracy for the precursor ion-20ppm; mass accuracy for MS/MS fragments—0.50 Da; possible variable modification–oxidation of methionine, lysine, and proline residues: up to 5 variable modifications per peptide, minimal peptide length was set to 5 amino acid residues, maximal peptide weight—10 kDa, false discovery rate (FDR) ≤ 0.01, minimal score for unmodified peptides—30, for modified—40. For semiquantitative label-free analysis, alignment of chromatograms was used; the particular peptide peak intensity values were normalized to the total intensity of all peaks in a particular sample. Significant differences in the representation of peptides in different patient groups were estimated using the Mann–Whitney U-test. The Venn diagram was build using the http://bioinformatics.psb.ugent.be/webtools/Venn/ resource, and heat-maps were created with the http://heatmapper.ca/ resource [50].
Multivariate data analysis of the semiquantitative proteomic data was performed using partial least squares analysis (PLS) with the ropls package [51] to create a classification model: normotensive pregnancy (control), hypertension without PE (CH and GH) and PE (mild and severe). The Y variables for PLS model training were set from 1 to 3 for samples of different groups (specified in Figures). The quality of statistical models was estimated by R2 (fraction of data that the model can explain using the latent variables) and Q2 (fraction of data predicted by the model according to the cross validation) parameters. The model performance was assessed by calculating the area under receiver operating characteristic curve (AUC).
3. Results
3.1. Urine Peptidome Analysis
Analysis of MS data obtained for 127 samples allowed the identification of a total of 3869 peptides with FDR < 0.1% from 43 proteins when using the PEAKS program for identification (Table S1). However, a much smaller number of peptides was found to be substantially represented in at least one of the diagnostic groups: calculation of the median intensity values revealed only 340 (including PTM variations) substantially represented peptides, whose medians exceeded the ”zero” level (Table 2 and Table S2).
Table 2.
Number and origin of substantially represented peptides in urine of pregnant women (CH—chronic hypertension; GH—gestational hypertension; Mpe—mild preeclampsia; sPE—severe preeclamsia).
| Originating Protein | Number of Peptides | Number of Samples a | Diagnostic Groups b,c |
|---|---|---|---|
| COL1A1 | 196 | 127 | All groups |
| COL3A1 | 80 | 127 | All groups |
| COL1A2 | 10 | 110 | All groups |
| UMOD | 10 | 102 | All groups |
| FGA | 9 | 121 | All groups |
| COL18A1 | 8 | 122 | Contr, CH, GH, mPE, sPE |
| KISS1 | 4 | 58 | CH, GH, mPE |
| COL4A4 | 3 | 74 | Contr, CH, GH, mPE |
| FGB | 2 | 90 | Contr, CH, GH, mPE |
| COL2A1 | 2 | 74 | Contr, CH, GH, mPE |
| EMID1 | 2 | 74 | Contr, CH, GH, mPE |
| COL5A1 | 2 | 48 | CH, GH, mPE |
| COL8A1 | 2 | 43 | CH, GH |
| COL15A1 | 1 | 74 | Contr, CH, GH, mPE |
| COL17A1 | 1 | 74 | Contr, CH, GH, mPE |
| FXYD2 | 1 | 59 | Contr, mPE |
| PGRMC1 | 1 | 49 | CH |
| VGF | 1 | 49 | CH,GH |
| INS | 1 | 47 | CH,GH |
| KDM6B | 1 | 42 | sPE |
| PIGR | 1 | 31 | GH |
a Values for the most represented peptides are given. b Only diagnostic groups for which the median intensity values were above zero for at least one peptide from the corresponding protein are indicated. c Gray background indicates consistent data for PEAKS and MaxQuant search programs.
Data in Table 1 indicates that the vast majority of substantially represented peptides are derived from collagen alpha-1(I) (COL1A1) and alpha-1(III) (COL3A1) chains. Single peptides from membrane-associated progesterone receptor (PGRMC1), neurosecretory protein VGF, insulin (INS), and lysine-specific demethylase 6B (KDM6B) are of particular interest as they have a predominant distribution in the hypertensive or PE groups. It is important to note that MaxQuant results are mostly consistent with those of PEAKS (Table 2, Table S3 and S4); however, PEAKS de novo sequencing is a definite advantage that essentially expands its identification capabilities. So, further analysis was mainly performed on the PEAKS data.
Unexpectedly, none of the SERPINA1 peptides demonstrated a substantial presence in any of the groups, although to date, their presence in urine of pregnant women has been considered as the main PE marker [42,45]. Nevertheless, the total number of identified SERPINA1 peptides (506 of 3869) is in third place after COL1A1 and COL3A1 ones (1493 and 917, correspondingly, Table S1). This suggests that SERPINA1 peptides are actually substantially represented, but their excision sites are highly variable. At the same time, the mostly represented SERPINA1 peptides (up to 37 samples, Table S1) were found to correspond to the C-terminus and have overlapping sequences. Therefore, it was suggested that combining peptides with overlapping sequences could take SERPINA1 peptides into consideration, as well as essentially expand the number of differentiating peptides. For this, we further considered peptide groups and compared the summed intensities of overlapping peptides.
3.2. Peptide Groups
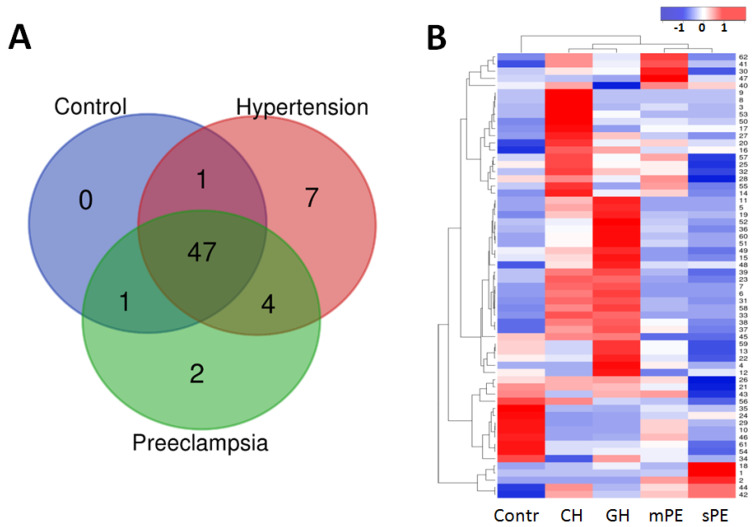
The initial 3869 peptides (PEAKS) were subdivided into 116 groups (loci) in accordance with the overlapping sequences (Table S5), and 62 of these groups were found to be substantially represented in at least one patient group (Figure 1, Table 3). Interestingly, these substantial groups mostly originate from the proteins mentioned in Table 2, and only one COL5A1 and one SERPINA1 (C-terminal) loci were added. The number of samples, in which a particular peptide group was identified, increased in comparison with corresponding individual peptides (Table 3). It seems very important that the peptide group distribution mainly coincides with the distribution of individual essentially represented peptides, and thus suggests that consideration of peptides with overlapping sequences as one group is quite reasonable, especially since it allows information from a significantly higher number of peptides to be taken into account.
Figure 1.
The distribution of 62 substantially represented peptide loci in control, hypertension (CH and GH), and preeclampsia (mPE and sPE) patient groups. (A) Venn diagram demonstrating peptide group intersections. (B) Hierarchical clustering of the peptide loci’s median intensity values and pregnancy associated hypertensive disorders groups. The Kendal’s Tau distance measurement method and average linkage clustering were used. The higher values are shown in red, the lower—in blue.
Table 3.
Protein affiliation of the 62 substantial peptide groups in urine of pregnant women.
| Originating Protein |
Number of Samples * | Intersection in Venn Diagram Number of Peptide Groups |
|||||
|---|---|---|---|---|---|---|---|
| Core | Control/ CH+GH |
Control/ PE |
CH+GH/ PE |
CH+GH | PE | ||
| Intersection | 47 | 1 | 1 | 4 | 7 | 2 | |
| UMOD | 118 (102) | 1 | - | - | - | - | - |
| KISS1 | 85 (58) | 1 | - | - | - | - | - |
| EMID1 | 103 (74) | 1 | - | - | - | - | - |
| FGA | 122 (121) | 1 | - | - | - | - | - |
| FGB | 95 (90) | 1 | - | - | - | - | - |
| COL2A1 | 106 (74) | 1 | - | - | - | - | - |
| COL8A1 | 93 (43) | 1 | - | - | - | - | - |
| COL15A1 | 92 (74) | 1 | - | - | - | - | - |
| COL17A1 | 93 (74) | 1 | - | - | - | - | - |
| COL3A1 | 127 (127) | 14 | 1 | - | - | - | - |
| COL1A1 | 127 (127) | 16 | - | - | 1 | - | - |
| COL18A1 | 125 (122) | 2 | - | - | 1 | - | - |
| COL1A2 | 114 (110) | 5 | - | - | - | 1 | - |
| COL4A4 | 106 (74) | 1 | - | - | - | 1 | - |
| FXYD2 | 59 (59) | - | - | 1 | - | - | - |
| PIGR | 57 (31) | - | - | - | 1 | - | - |
| COL5A1 | 53 (48) | - | - | - | 1 | 1 | - |
| COL5A2 | 48 (-) | - | - | - | - | 1 | - |
| INS | 50 (47) | - | - | - | - | 1 | - |
| VGF | 52 (49) | - | - | - | - | 1 | - |
| PGRMC1 | 51 (49) | - | - | - | - | 1 | - |
| KDM6B | 62 (42) | - | - | - | - | - | 1 |
| SERPINA1 | 47 (-) | - | - | - | - | - | 1 |
* Given values correspond to the most represented peptide groups; values in brackets indicate the numbers corresponding to the most represented peptides originating from each protein (data from Table 2).
The data in Table 3 indicate that the bulk of the peptide loci is represented in all patient groups and belongs to the core, which mostly includes collagen (COL1A1, COL3A1, and others), KISS1, EMID1, fibrinogen, and uromodulin peptides. The heat-map in Figure 1B, however, suggests the essential differences in the percentage of these core loci in samples with different diagnoses. Single peptide groups from PGRMC1, VGF, insulin, and COL5A2 are substantially represented in CH and GH samples. The group of KDM6B peptides is substantially present in PE samples, like SERPINA1 peptides. It is noteworthy that consideration of overlapping peptides together as a single group essentially increased the number of samples with accounted KDM6B peptides (from 42 to 62), and reinforced the indication that these peptides could be potential PE markers.
Pairwise data comparison for all sample groups using the Mann–Whitney U-test revealed a significantly different distribution for 52 of the 62 peptide loci (Table S6). No significant difference was shown for 4 loci from COL3A1, 3 of COL1A2, as well as single EMID1, FXYD2, and uromodulin groups. Among the significantly differentiating peptide groups, 17 seem to be especially characteristic for the control, hypertensive, or PE samples, according to their p-values (Table S6, Figure 2).
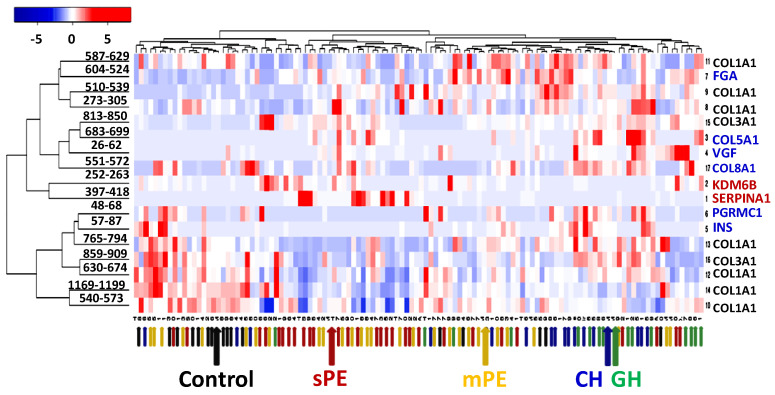
Figure 2.
Hierarchical clustering of individual sample data and 17 characteristic peptide groups using Kendal’s Tau distance measurement method and average linkage clustering. The small colored arrows indicate the clinical diagnosis in each particular sample; the large arrows show the median data of the corresponding patient groups: black—control, blue—CH, green—GH, yellow—mPE, and red—sPE.
The clustering in Figure 2 shows a good trend in the distribution of samples in accordance with the clinical diagnoses. This suggests that these urinary peptide groups can be considered as an appropriate basis for further development of a PE diagnostic panel.
3.3. Predictive Performance and Model for PE Differentiation
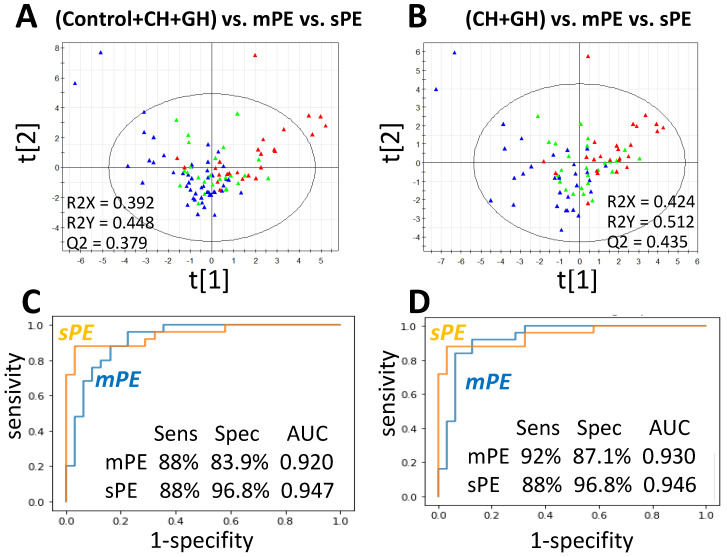
The PLS method was further applied to build a statistical model differentiating PE from hypertensive and control groups. In the beginning, the set of 570 individual peptides represented in at least 15 samples (Table S7) was analyzed to estimate whether some of potentially differentiating peptides could be lost upon selection of substantially represented peptides (and their groups). The clustering of three sample groups (control versus CH+GH versus PE) on these 570 peptides revealed 103 VIP-peptides, which give the largest contribution to the projection on hidden structures (Table S8), of which only the peptide 323–339 from alpha-2-HS-glycoprotein (AHSG) did not fall into consideration in the field of view above. Therefore, its peptide group was also taken into account and further PLS analysis was performed for 63 peptide loci (Table S5). Multiple clustering with different combinations of samples revealed the best parameters for combinations ”Control+CH+GH versus mPE versus sPE” and ”CH+GH versus mPE versus sPE” (Figure 3A,B). The comparison of VIP-peptide groups obtained upon multiple clustering showed that 22 of them were most often selected as VIPs upon clustering of different combinations of samples (Table 4). They included 15 different collagen loci as well as single groups from SERPINA1, KDM6B, INS, PGRMC1, EMID1, FGA, and AHSG; 13 of them were also part of the set of 17 selected in the previous section (Figure 2). Clustering by these 22 peptide loci showed the best parameters for differentiating mPE and sPE from ”Control+CH+GH”, while the best parameters for PE differentiation from hypertensive samples only (CH+GH) was obtained with only 20 of these groups (excluding loci ”540–573” and ”798–812” of COL1A1, Table 4).
Figure 3.
PLS model differentiating mPE and sPE urine samples from control, CH, and GH samples. (A,B) PLS score plot of semiquantitative urinary peptidomic data: blue—”Control+CH+GH” (A) or ”CH+GH” (B); green—mPE; red—sPE. (C,D) ROC analysis for mPE or sPE versus ”Control+CH+GH” (C) or ”CH+GH” (D) according to the results of clustering on 22 and 20 VIP-peptide groups (for C and D, correspondingly).
Table 4.
Urine VIP-peptide groups mostly differentiating mPE and sPE from control, CH, and GH samples.
| Peptide Group | Start-End Position |
Originating Protein | Number of Samples | Other Studies |
|---|---|---|---|---|
| GAAGEPGKAGERGVPGPPGAVGPAGKDGEAGAQGPPGPAGPAG | 587–629 | COL1A1 | 114 | |
| EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ | 57–87 | INS | 50 | |
| LMIEQNTKSPLFMGKVVNPTQK | 397–418 | SERPINA1 | 47 | [42,45] |
| ERGSPGPAGPKGSPGEAGRPGEAGLPGAKG | 510–539 | COL1A1 | 98 | [44] |
| LLGPKGPPGPPGPPGVT | 683–699 | COL5A1 | 39 | |
| GRDGEPGTPGNPGPPGPPGPPGPPG | 150–174 | COL2A1 | 106 | |
| GPAGPPGPPGPPGTSGHPGSPGSPGYQGPPGEPGQAGPSGPPG | 174–216 | COL3A1 | 113 | |
| GQPGPPGPPGPPG | 1021–1033 | COL4A4 | 49 | |
| AGPPGRDGIPGQPGLPGPPGPPGPPGPPGLGGN | 126–158 | COL1A1 | 116 | |
| GPQGQPGLPGPPGPPGPPGPPA | 551–572 | COL8A1 | 93 | |
| MGVVSLGSPSGEVSHPRKT | 321–339 | AHSG | 26 | |
| GDQPAASGDSDDDEPPPLPRL | 48–68 | PGRMC1 | 51 | |
| ADEAGSEADHEGTHSTKRGHAKSRPV | 604–624 | FGA | 122 | [44] |
| ERGEQGPAGSPGFQGLPGPAGPPGEAGKPGEQGVPGDLGAPGPSG | 630–674 | COL1A1 | 127 | |
| VKGERGSPGGPGAAGFPGARGLPGPPGSNGNPGPPGPSGSPGKDGPPGPAG | 859–909 | COL3A1 | 125 | |
| GERGPPGPPGRDGEDGPTGPPGPPGPPGPPGLGGNFA | 42–78 | COL1A2 | 100 | |
| LTGPIGPPGPAGAPGDKGESGPSGPAGPTG | 765–794 | COL1A1 | 110 | [44] |
| PPPPPPPPPPPP | 252–263 | KDM6B | 62 | |
| LDGAKGDAGPAGPKGEPGSPGENGAPGQMGPRG | 273–305 | COL1A1 | 114 | |
| PGERGPPGPPGPPGPPGPPAP | 241–260 | EMID1 | 103 | |
| LTGSPGSPGPDGKTGPPGPAGQDGRPGPPGPPGA | 540–573 | COL1A1 | 127 | |
| APGDRGEPGPPGPAG | 798–812 | COL1A1 | 126 |
The results of ROC-analysis (Figure 3C,D) suggest that the obtained VIP-peptide groups can be considered as a differentiating panel for PE diagnosis. It is noteworthy that the relative content of common peptide loci may have an essential PE differentiating capacity, in addition to the presence of particular marker peptides.
3.4. PE Markers
SERPINA1-derived peptides are currently one of the main commonly accepted urine PE markers [42,45,47,52,53]. It is noteworthy that among the 3869 peptides identified in this study, 506 derived from SERPINA1. In accordance with their sequences, they can be grouped into 11 loci (Table S5) and cover 64.8% of the full sequence. Although the C-terminal locus (397–418) was shown to be the only one substantially represented in PE-samples, C-terminal peptides were also found in 2 CH samples and 16 samples with complicated diagnoses. From the 50 PE-samples, these C-terminal peptides were found in 28 (14 mPE and 14 sPE), and only 2 PE samples contained other SERPINA1 peptides while missing the C-terminal locus. At the same time, peptides from other SERPINA1 groups though each was met in only a small number of samples, were found in PE samples only, thus suggesting that identification of all SERPINA1 peptides may still remain reasonable for PE diagnosis. However, 20 of the PE samples (40%) did not contain any SERPINA1 peptides and required other diagnostic marker(s).
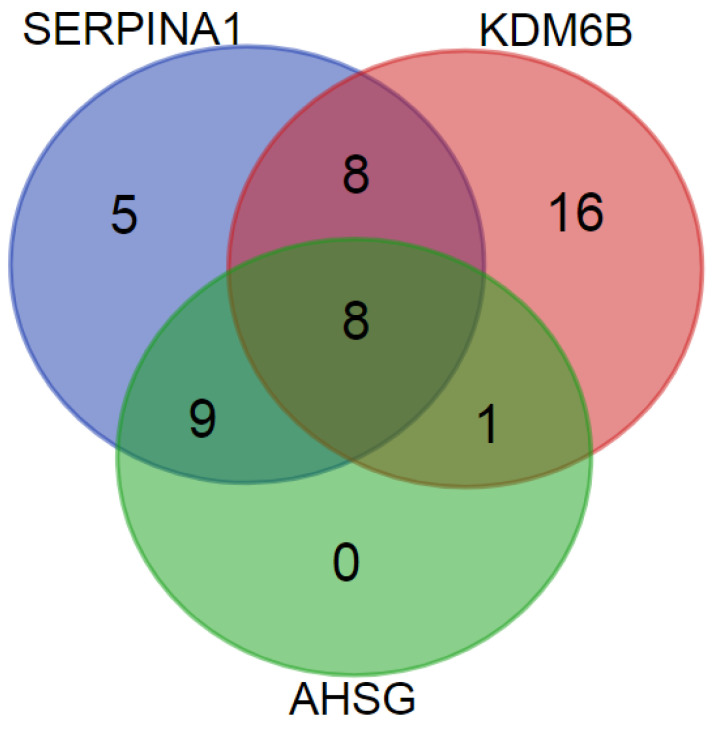
The KDM6B-derived poly-proline peptides 252–257/263 may be another PE-marker as they show predominant significantly different distribution in PE patient groups (in 16 mPE and 17 sPE samples). The data in Figure 4 implicate that together SERPINA1 and KDM6B peptides identify 47 of the 50 PE samples. However, KDM6B peptides demonstrated lower specificity in comparison with SERPINA1 and were also found in 25% normotensive and CH samples and in 46.7% GH samples. Nevertheless, the poly-proline peptide group is the essential integral component of the described above differentiating panel.
Figure 4.
Distribution of SERPINA1, KDM6B, and AHSG peptides among 50 PE urine samples.
The AHSG peptide group 321–339 is one another possible PE marker. Although it is much less represented, only in 8 mPE and 10 sPE samples, it seems to be rather specific. However, these peptides were found only in samples, which also contained SERPINA1 or poly-proline KDM6B peptides, and did not increase the total number of identified PE samples (Figure 4). Nevertheless, this peptide group also deserves particular attention due to its selection into PLS-VIPs (Table 4).
3.5. Samples from Patients with Complicated Diagnoses
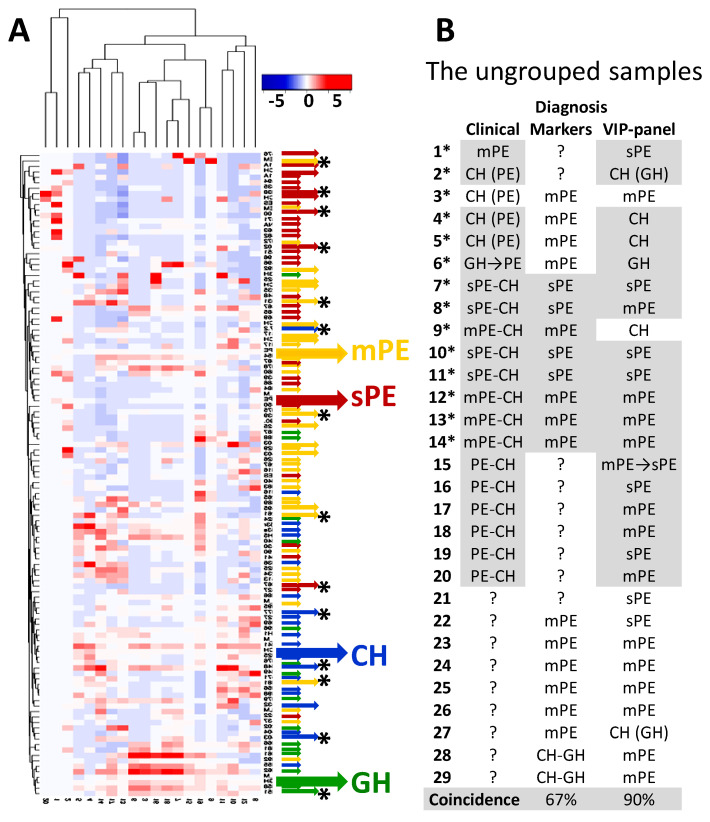
MS data for 29 ungrouped samples were analyzed both for the presence of PE markers (peptides from SERPINA1, KDM6B, and AHSG) and for the characteristic clustering of VIP-peptide loci identified above. When diagnosed by markers, based on the data obtained for each patient group, the presence of SERPINA1 or AHSG peptides most likely indicated PE; sPE was highly probable if normalized intensity values for SERPINA1 C-terminal peptide locus exceeded ”3”. The KDM6B poly-proline peptide group could suggest PE if its normalized intensity values were higher than ”0.1”. For diagnosis with VIP-peptide groups, the data of ungrouped samples were co-clustered with the data of the grouped samples, which were used above for the identification of VIP-peptides. The particular co-clustering suggested the most likely diagnosis (Figure 5).
Figure 5.
Co-clustering of ungrouped and grouped samples by 20 VIP-peptide groups. (A) Hierarchical co-clustering using Kendal’s Tau distance measurement method and average linkage clustering. The small colored arrows indicate the established clinical diagnoses in grouped samples; the middle arrows without asterisks—the probable diagnoses of ungrouped samples (with asterisks —established clinical diagnoses for ungrouped samples); the large arrows show the mean data of corresponding patient groups: blue—CH, green—GH, yellow—mPE, and red—sPE. (B) The comparison of clinical diagnoses with diagnoses proposed by using markers and VIP-peptide clustering. Grey background shows results consistent with clinical diagnoses (ignoring PE subdivision).
According to the results in Figure 5, VIP-peptide co-clustering is essentially more sensitive and specific than diagnosis by markers, since it gives 90% coincidence with clinical diagnoses and is able to differentiate any sample, whereas the markers give only a 67% coincidence and differentiate only 20 of the 29 samples.
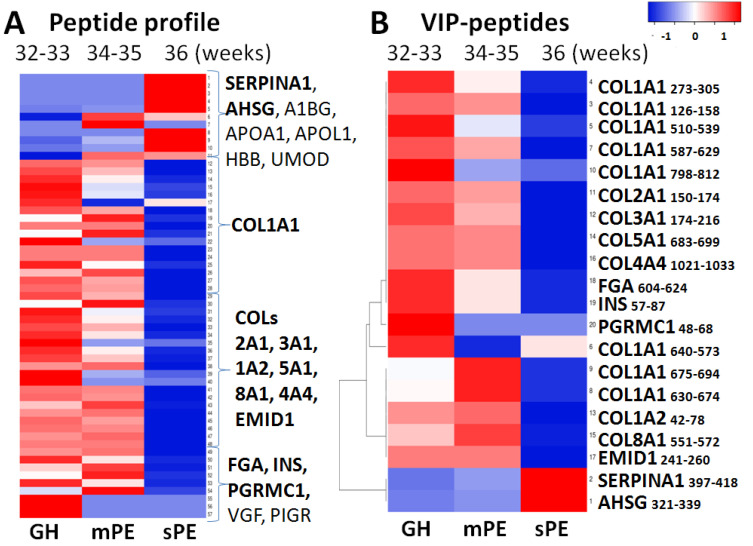
The sample from the patient, who was clinically diagnosed as GH and further developed mPE and sPE (number 6*, green arrow with asterisk in Figure 5), is of particular interest, since the two later samples were also analyzed (and enrolled into mPE and sPE diagnostic groups). The illustrated in Figure 6 dynamic changes of the peptide profile and VIP-peptide groups for this patient suggest that in addition to a significant increase in PE markers, the significant decrease in the content of collagen, INS, PGRMC1, and other peptides has a quite pronounced tendency and may reflect PE severity as well as appearance in the profile of a large number of peptides originating from a variety of plasma proteins (such as APOA1, A1BG, HBB, and others).
Figure 6.
Dynamic urine peptidome changes upon GH progression into PE, on the example of three samples obtained from the same patient at different gestation ages. (A) Profiling of the most represented peptide groups (without clustering). (B) Hierarchical clustering of VIP-peptide groups found in the compared samples using Kendal’s Tau distance measurement method and average linkage clustering.
Importantly, SERPINA1 peptides were found in all three samples of this (initially GH) patient, although their content was very significantly different (Table S9). The fact that VIP-peptide clustering still differentiated the first sample as GH in spite of the presence of SERPINA1 peptides (what coincided to the clinical diagnosis) once again implicates the high diagnostic capacity of the obtained VIP-panel. At the same time, in the example of this patient, it can be assumed that the appearance of SERPINA1 peptides in urine may indicate the increased risk of GH progression to PE.
In general, the results obtained with ungrouped samples from patients with complicated diagnosis suggest the essential diagnostic potential of the obtained VIP-peptide panel.
4. Discussion
Creation of a urine peptide panel for PE differentiation remains an urgent research task. In an attempt to create such a panel, a special strategy was applied in this study. Urine peptides with overlapping sequences were grouped and considered together as one locus and were found to have the same distribution as their most represented peptides. The combination of peptides seemed appropriate given that no specific cleavage sites and amino-acid modifications were detected for any diagnosis, at least in the analyzed 127 samples. It was assumed that peptide grouping and comparison of their summed normalized intensities should smooth out the insignificant differences of sequences and focus the attention on the presence of particular peptide locus and its relative content in samples with specific diagnoses. Additionally, this strategy might essentially decrease the discrepancies when measuring different series of samples and even reduce dissimilarities in the results of different studies. The obtained results suggested that the application of this strategy proved to be quite appropriate and led to the creation of a variant of a peptide panel, which differentiates PE with high sensitivity and specificity.
Among members of the obtained panel there are several coincidences with the results of other studies (Table 4). In particular, two COL1A1 groups, ”510–539” and ”765–794”, and the FGA ”604–624” locus contain peptides earlier described as PE markers by Carty D.M. et al. [44]. Among other substantially represented groups obtained here, COL1A1 ”1007-1041”; COL3A1 ”446-477”, COL1A2 ”451–472”, and ”917–952”; and the uromodulin locus also have coincidences with the results of [44]. However, here, they were not selected into VIP-groups, and moreover, the distribution of uromodulin peptides was not found to be significantly different (remarkably, all of the revealed uromodulin peptides belonged to the same locus ”589–607”). It is noteworthy that all of these peptide groups (including 3 VIPs) were identified in most of the analyzed samples and, hence, their presence by itself is not a marker. Nevertheless, their relative content (and in particular the content of VIP-groups) proved to be extremely important for PE differentiation.
As for direct markers of PE, the obtained results support the findings of I.A. Buhimschi et al. concerning SERPINA1 peptides [42]. However, despite the wide variety of identified SERPINA1 peptides, only the C-terminal group was shown to have a diagnostic significance since it had the highest representation. Again, consideration of the C-terminal peptide locus ”397–418” also proved to be advantageous compared to its most represented peptide (398–418) since the group was detected in 47 different samples, while the peptide itself in only 37 of them. However, SERPINA1 peptides were found in only 60% of PE samples and the rest required other markers.
Poly-proline (from 6 to 12 poly-P) peptides, SERPINA1 peptides, were found to be significantly distributed in PE patient groups and essentially enhanced PE differentiation; together with SERPINA1 peptides, poly-P covered 94% of PE samples. Unlike SERPINA1 peptides, poly-P peptides are not absolutely specific markers; nevertheless, their relative content is definitely an essential indicator, first identified in this study. The possible relation of KDM6B to PE is actually unclear, especially taking into account that this protein is associated with chromatin and localized in the nucleus. However, it was shown to be involved in the inflammatory response by participating in macrophage differentiation via the regulation of gene expression [54]. Since PE is usually associated with a systemic inflammation, it may be assumed that KDM6B peptides in urine may originate from the degraded macrophages. On the other hand, the appearance of KDM6B peptides may reflect the epigenetic regulation of compensatory mechanisms, which, in particular, may lead to changes in the expression level of different SERPINs [16]. In addition, poly-P peptides may actually have different origin. In particular, many cytosolic, membrane, and cytoskeletal proteins such as large proline-rich protein BAG6, WASP homolog-associated protein with actin, protein diaphanous homologm1, MAP3K4 kinase, Ras-associated and pleckstrin homology domains-containing protein 1, and junction-mediating and -regulatory protein contain poly-P motives and may be the source of poly-P peptides in cell degradation. Still, the mechanism underlying the increase in urine poly-P in PE remains questionable.
Among the PE markers identified in this study, AHSG seems to be the most doubtful, since it was found only in 26 of 127 samples. Additionally, there are several other peptide groups with similar representativeness that did not fall into the differentiating panel: apolipoprotein A1 ”254–267” (28 mostly PE samples), complement C4-A ”1423–1440” (27 samples except for controls), clusterin ”390–423” (39 samples except for controls) and some other (Table S5). In general, it is important to note that sPE is often associated with a large variety of urine peptides originating from different plasma proteins, and these peptides may look highly PE-specific. Whether it is appropriate to consider all of such peptides as possible markers remains a question.
In sum, it is worth noting that the obtained differentiating panel for the most part consists of peptides that are represented in most samples of all diagnostic groups. The relative content of these peptides implicates the important criterion for diagnosis that is similar to the presence of specific markers. The panel still requires further validation and may be essentially reorganized. However, the differentiating role of the substantially represented peptides and their groups is expected to be the most promising.
5. Conclusions
High-resolution mass spectrometry applied for analysis of the peptidome of 127 patients including normotensive controls (n = 17), chronic hypertension (n = 16), gestational hypertension (n = 15), mild PE (n = 25), severe PE (n = 25), and 29 patients with complicated diagnosis reveal sample diversity and new features for PE diagnostics. A panel consisting of 22 peptide loci from collagens (COL1A1, COL3A1, COL2A1, COL5A1, COL8A1, and COL4A4), fibrinogen alpha-chain, insulin, membrane-associated progesterone receptor component 1, EMI domain-containing protein 1, alpha-1-antitrypsin, lysine-specific demethylase 6B, and alpha-2-HS-glycoprotein was developed. Reliable differentiation of preeclampsia from chronic or gestation hypertension and from normotensive cases was demonstrated with 88% sensitivity and 96.8% specificity (AUC = 0.947). Overall, the obtained results confirm the high diagnostic potential of urinary peptidome profiling and can serve as the basis for further creation of new reliable methods for clinical diagnostics of preeclampsia.
Acknowledgments
Authors wish to thank anonymous reviewers for their essential remarks, the Shared Research Facilities of N.N. Semenov Federal Research Center for Chemical Physics RAS and the Core Facility “New Materials and Technologies” of the Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences for providing equipment for sample analysis, and the Laboratory for the collection and storage of biological material (Biobank) at V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology for providing urine samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/12/1039/s1, Table S1: PEAKS data, the peptides identified in 127 analyzed samples, Table S2: PEAKS data for substantialy represented peptides, Table S3: MaxQuant data for peptides identified in 127 analyzed samples, Table S4: MaxQuant data for substantialy represented peptides, Table S5: PEAKS data for 116 peptide groups, Table S6: Mann-Whitney U-test for 62 substantially represented peptide groups using PEAKS data, Table S7: 570 peptides represented in >15 samples, Table S8: Results of PLS clustering on 570 peptides, Table S9: Dynamic changes of peptide profile in 3 samples from the same patient.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided.Conceptualization, G.T.S. and E.N.N.; methodology, A.S.K., N.V.Z., V.A.S., A.E.B., M.I.I., N.L.S., W.S. and P.P.; software, V.A.S., M.I.I., W.S. and P.P.; investigation, A.S.K., N.V.Z., V.A.S., A.E.B., M.I.I., N.L.S., K.T.M. and I.P.; resources, K.T.M., I.A.P., Z.S.K., R.G.S. and V.E.F.; writing—original draft preparation, A.S.K., N.V.Z., V.A.S. and N.L.S.; writing—review and editing, A.E.B., M.I.I., I.A.P. and E.N.N.; supervision, A.E.B., Z.S.K., R.G.S. and V.E.F.; project administration, A.S.K. and N.L.S.; funding acquisition, I.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement # 075-00337-20-02, project # 0714-2020-0006).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Say L., Chou D., Gemmill A., Tuncalp O., Moller A.B., Daniels J., Gulmezoglu A.M., Temmerman M., Alkema L. Global causes of maternal death: A WHO systematic analysis. Lancet. Glob. Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Fortunato S.J., Menon R. Distinct molecular events suggest different pathways for preterm labor an premature rupture of membranes. Am. J. Obstet. Gynecol. 2001;184:1399–1405. doi: 10.1067/mob.2001.115122. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L., Casas J.P., Hingorani A.D., Williams D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systrmatic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backes C.H., Markham K., Moorehead P., Cordero L., Nankervis C.A., Giannone P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan L.J., Morton J.S., Davidge S.T. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21:4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 6.Buhimschi I.A., Nayeri U.A., Zhao G., Shook L.L., Pensalfini A., Funai E.F., Bernstein I.M., Glabe C.G., Buhimschi C.S. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci. Transl. Med. 2014;6:245ra92. doi: 10.1126/scitranslmed.3008808. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy F.P., Adetoba A., Gill C., Bramham K., Bertolaccini M., Burton G.J., Girardi G., Seed P.T., Poston L., Chappell L.C. Urinary congophilia in women with hypertensive disorders of pregnancy and preexisting proteinuria or hypertension. Am. J. Obstet. Gynecol. 2016;215:464–e1. doi: 10.1016/j.ajog.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajappa C., Rangappa S.S., Suryanarayana R., Balakrishna S. Urinary congophilia in preeclampsia: Experience from a rural tertiary-care hospital in India. Pregnancy Hypertens. 2018;13:83–86. doi: 10.1016/j.preghy.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Roberts J.M., Hubel C.A. Oxidative stress in preeclampsia. Am. J. Obstet. Gynecol. 2004;190:1177–1178. doi: 10.1016/j.ajog.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Borzychowski A.M., Sargent I.L., Redman C.W. Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.English F.A., Kenny L.C., McCarthy F.P. Risk factors and effective management of preeclampsia. Integr. Blood Press. Control. 2015;8:7–12. doi: 10.2147/IBPC.S50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redman C.W., Sargent I.L., Staff A.C. IFPA Senior Award Lecture: Making sense of pre-eclampsia—two placental causes of preeclampsia? Placenta. 2014;35:S20–S25. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Valdes G., Kaufmann P., Corthorn J., Erices R., Brosnihan K.B., Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod. Biol Endocrinol. 2009;7:79. doi: 10.1186/1477-7827-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanbe A.F., Khalil R.A. Circulating and vascular bioactive factors during hypertension in pregnancy. Curr. Bioact. Compd. 2010;6:60–75. doi: 10.2174/157340710790711737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Khalil R.A. Matrix matelloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017;148:87–165. doi: 10.1016/bs.pmbts.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chelbi S.T., Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol. Cell. Endocrinol. 2008;282:120–129. doi: 10.1016/j.mce.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Duckitt K., Harrington D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg T.J., Garbers S., Lipkind H., Chiasson M.A. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am. J. Public. Health. 2005;95:1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima T., Jorde L.B., Ishigami T., Umemura S., Emi M., Lalouel J.M., Inoue I. Nucleotide diversity and haplotype structure of the human angiotensinogen gene in two populations. Am. J. Hum. Genet. 2002;70:108–123. doi: 10.1086/338454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward K., Taylor R.N. Chesley’s Hypertensive Disorders in Pregnancy. 4th ed. Academic Press; Cambridge, MA, USA: 2015. Genetic factors in the etiology of preeclampsia/eclampsia; pp. 57–80. Chapter 4. [DOI] [Google Scholar]
- 21.Van Dijk M., Oudejans C.B. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J. Pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Zhang Y., Dai L., Song Y., Wang Y., Zhou B., Zhou R. Association between polymorphishs in CXCR2 gene and preeclampsia. Mol. Genet. Genomic. Med. 2019;2019:e578. doi: 10.1002/mgg3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papazoglou D., Galazios G., Koukourakis M.I., Kontomanolis E.N., Maltezos E. Association of −634G/C and 936C/T polymorphisms of the vascular endothelial growth factor with spontaneous preterm delivery. Acta Obstet. Gynecol. Scand. 2004;83:461–465. doi: 10.1111/j.0001-6349.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 24.Alrahmani L., Willrich M.A.V. The complement alternative pathway and preeclampsia. Curr. Hypertens. Rep. 2018;20:40. doi: 10.1007/s11906-018-0836-4. [DOI] [PubMed] [Google Scholar]
- 25.Shimonovitz S., Hurwitz A., Dushnik M., Anteby E., Geva-Eldar T., Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: A possible mechanism for control of trophoblast invasion. Am. J. Obstet. Gynecol. 1994;171:832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 26.Isaka K., Usuda S., Ito H., Sagawa Y., Nakamura H., Nishi H., Suzuki Y., Li Y.F., Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24:53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 27.Su M.T., Tsai P.Y., Tsai H.L., Chen Y.C., Kuo P.L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors. 2017;43:210–219. doi: 10.1002/biof.1325. [DOI] [PubMed] [Google Scholar]
- 28.Montagnana M., Lippi G., Albiero A., Scevarolli S., Salvagno G.L., Franchi M., Guidi G.C. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J. Clin. Lab. Anal. 2009;23:88–92. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eleuterio N.M., Palei A.C., Rangel Machado J.S., Tanus-Santos J.E., Cavalli R.C., Sandrim V.C. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens. 2015;5:205–208. doi: 10.1016/j.preghy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Chappell L.C., Duckworth S., Seed P.T., Griffin M., Myers J., Mackillop L., Simpson N., Waugh J., Anumba D., Kenny L.C., et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation. 2013;128:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 31.March M.I., Geahchan C., Wenger J., Raghuraman N., Berg A., Haddow H., McKeon B.A., Narcisse R., David J.L., Scott J., et al. Circulating Angiogenic Factors and the Risk of Adverse Outcomes among Haitian Women with Preeclampsia. PLoS ONE. 2015;10:e0126815. doi: 10.1371/journal.pone.0126815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bian Z., Shixia C., Duan T. First-Trimester Maternal Serum Levels of sFLT1, PGF and ADMA Predict Preeclampsia. PLoS ONE. 2015;10:e0124684. doi: 10.1371/journal.pone.0124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y.M., Bdolah Y., Lim K.H., Yuan H.T., Libermann T.A., et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 35.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., Sibai B.M., Epstein F.H., Romero R., Thandhani R., et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 36.Ali S.M., Khalil R.A. Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert Opin. Ther. Targets. 2015;19:1495–1515. doi: 10.1517/14728222.2015.1067684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah D.A., Khalil R.A. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem. Pharmacol. 2015;95:211–226. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chafetz I., Kuhnreich I., Sammar M., Tol Y., Gibor Y., Meiri H., Cuckle H., Wolf M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2007;197:e1–e35. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Spencer C.A., Allen V.M., Flowerdew G., Dooley K., Dodds L. Low levels of maternal serum PAPP-A in early pregnancy and the risk of adverse outcomes. Prenat. Diagn. 2008;28:1029–1036. doi: 10.1002/pd.2116. [DOI] [PubMed] [Google Scholar]
- 40.Karumanchi S.A. Biomarkers of Kidney Disease. 2nd ed. Academic Press; Cambridge, MA, USA: 2017. Biomarkers in preeclampsia; pp. 555–594. Chapter 14. [DOI] [Google Scholar]
- 41.Guo H.-X., Zhu Y.-B., Wu C.-P., Zhong M., Hu S.-W. Potential urine biomarkers for gestational hypertension and preeclampsia. Mol. Med. Rep. 2019;19:2463–2470. doi: 10.3892/mmr.2019.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buhimschi I.A., Zhao G., Funai E.F., Harris N., Sasson I.E., Bernstein I.M., Saade G.R., Buhimschi C.S. Proteomic profiling of urine identifies specific fragments of SERPINA1 and albumin as biomarkers of preeclampsia. Am. J. Obstet. Gynecol. 2008;199:551–e1. doi: 10.1016/j.ajog.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G., Zhang Y., Jin X., Zhang L., Zhou Y., Niu J., Chen J., Gu Y. Urinary proteomics analysis for renal injury in hypertensive disorders of pregnancy with iTRAQ labeling and LC-MS/MS. Proteom. Clin. Appl. 2011;5:300–310. doi: 10.1002/prca.201000100. [DOI] [PubMed] [Google Scholar]
- 44.Carty D.M., Siwy J., Brennand J.E., Zurbig P., Mullen W., Franke J., McCulloch J.W., Noth R.A., Chappell L.C., Mischak H., et al. Urinary proteomics for prediction of preeclampsia. Hypertension. 2011;57:561–569. doi: 10.1161/HYPERTENSIONAHA.110.164285. [DOI] [PubMed] [Google Scholar]
- 45.Kononikhin A.S., Starodubtseva N.L., Bugrova A.E., Shirokova V.A., Chagovets V.V., Indeykina M.I., Popov I.A., Kostyukevich Y.I., Vavina O.V., Muminova K.T., et al. An untargeted approach for the analysis of the urine peptidome of women with preeclampsia. J. Proteom. 2016;149:38–43. doi: 10.1016/j.jprot.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Lee S.M., Park J.S., Norwitz E.R., Kim S.M., Kim B.J., Park C.-W., Jun J.K., Syn H.C. Characterization of discriminatory urinary proteomic biomarkers for severe preeclampsia using SELDI-TOF mass spectrometry. J. Perinat. Med. 2011;39:391–396. doi: 10.1515/jpm.2011.028. [DOI] [PubMed] [Google Scholar]
- 47.Kolialexi A., Mavreli D., Tounta G., Mavrou A., Papantoniou N. Urine proteomic studies in preeclampsia. Proteom. Clin. Appl. 2015;9:501–506. doi: 10.1002/prca.201400092. [DOI] [PubMed] [Google Scholar]
- 48.Levine R.J., Thadhani R., Qian C., Lam C., Lim K.-H., Yu K.F., Blink A.L., Sachs B.P., Epstein F.H., Sibai B.M., et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 49.Kononikhin A.S., Sergeeva V.A., Bugrova A.E., Indeykina M.I., Starodubtseva N.L., Chagovets V.V., Popov I.A., Frankevich V.E., Pedrioli P., Sukhikh G.T., et al. Methodology for Urine Peptidome Analysis Based on Nano-HPLC Coupled to Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Methods Mol. Biol. 2018;1719:311–318. doi: 10.1007/978-1-4939-7537-2_20. [DOI] [PubMed] [Google Scholar]
- 50.Babicki S., Arndt D., Marcu A., Liang Y., Grant J.R., Maciejewsky A., Wishart D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thevenot E.A., Roux A., Xu Y., Ezan E., Junot C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Prot. Res. 2015;14:3322–3335. doi: 10.1021/acs.jproteome.5b00354. [DOI] [PubMed] [Google Scholar]
- 52.Sergeeva V.A., Zakharova N.V., Bugrova A.E., Starodubtseva N.L., Indeykina M.I., Kononikhin A.S., Frankevich V.E., Nikolaev E.N. The high-resolution mass spectrometry study of the protein composition of amyloid-like urine aggregates associated with preeclampsia. Eur. J. Mass. Spectrom. 2020;26:158–161. doi: 10.1177/1469066719860076. [DOI] [PubMed] [Google Scholar]
- 53.Starodubtseva N., Nizyaeva N., Baev O., Bugrova A., Gapaeva M., Muminova K., Kononikhin A., Frankevich V., Nikolaev E., Sukhikh G. SERPINA1 peptides in urine as a potential marker of preeclampsia severity. Int. J. Mol. Sci. 2020;21:914. doi: 10.3390/ijms21030914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Santa F., Totaro M.G., Prosperini E., Notarbartolo S., Testa G., Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.