Figure 7. Binding cycle involving OTUB1 interacting with E2 partners and K48 diUbiquitin.
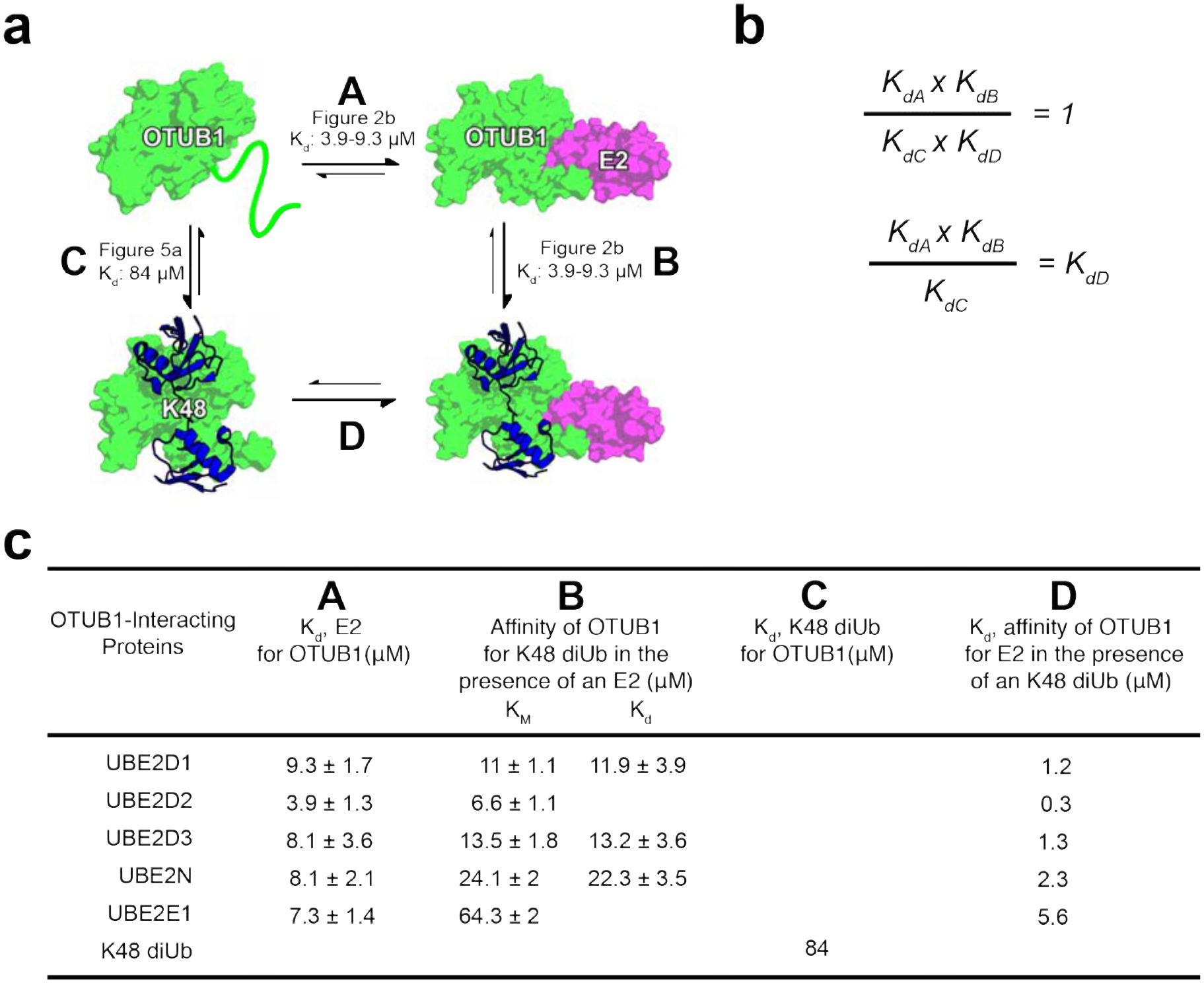
(a) Thermodynamic cycle of OTUB1 binding independently to either an E2 or K48 diUbiquitin which in turns favors binding of the other. Each side of the cycle is designated by a letter along with the binding affinities determined in this paper. (b) When comparing each side of the thermodynamic cycle with equilibrium dissociation constants, A × B must equal C × D. Therefore, the constants can be arranged so that Kd be calculated. (c) Binding affinities previously determined, A B and C, and those calculated, D. D is determined using the KM values, representing the affinity of OTUB1 for K48 diUb in the presence of an E2, since Kds were only measured for a select number of E2s.
