Abstract
Carbapenem-resistant Acinetobacter baumannii (CRAB) is a critical health concern for the treatment of infectious diseases. The aim of this study was to investigate the molecular epidemiology of CRAB emphasizing the presence of oxacillinase (OXA)-type β-lactamase-encoding genes, one of the most important carbapenem resistance mechanisms. In this study, a total of 183 non-repetitive CRAB isolates collected from 11 tertiary care hospitals across Thailand were investigated. As a result, the blaoxa-51-like gene, an intrinsic enzyme marker, was detected in all clinical isolates. The blaoxa-23-like gene was presented in the majority of isolates (68.31%). In contrast, the prevalence rates of blaoxa-40/24-like and blaoxa-58-like gene occurrences in CRAB isolates were only 4.92% and 1.09%, respectively. All isolates were resistant to carbapenems, with 100% resistance to imipenem, followed by meropenem (98.91%) and doripenem (94.54%). Most isolates showed high resistance rates to ciprofloxacin (97.81%), ceftazidime (96.72%), gentamicin (91.26%), and amikacin (80.87%). Interestingly, colistin was found to be a potential drug of choice due to the high susceptibility of the tested isolates to this antimicrobial (87.98%). Most CRAB isolates in Thailand were of ST2 lineage, but some belonged to ST25, ST98, ST129, ST164, ST215, ST338, and ST745. Further studies to monitor the spread of carbapenem-resistant OXA-type β-lactamase genes from A. baumannii in hospital settings are warranted.
Keywords: resistance mechanism, carbapenem, carbapenemase, oxacillinase, multidrug resistance
1. Introduction
Acinetobacter baumannii is a Gram-negative coccobacillus that has the ability to easily acquire antibiotic resistance and to persist in hospital environments [1]. This organism is considered an opportunistic pathogen responsible for nosocomial infections, especially in intensive care units [2]. A. baumannii commonly causes bacteremia, nosocomial-acquired pneumonia or ventilator-associated pneumonia, catheter-related infections, meningitis, peritonitis, skin and wound infections, urinary tract infections, and endocarditis [3]. The ability to survive in dry or moist conditions at various pH levels and temperatures renders it able to grow in the hospital environment [1].
A. baumannii is one of the ESKAPE pathogens, along with Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter spp., which are responsible for the majority of nosocomial infections and are capable of “escaping” the bactericidal activity of antimicrobial agents [4,5]. A. baumannii can develop resistance to many classes of commonly used antimicrobial agents [6,7]. The emergence of carbapenem-resistant A. baumannii (CRAB), multidrug-resistant (MDR) A. baumannii, or even extensively drug-resistant (XDR) A. baumannii has been progressively increasing globally over the last decade [8,9].
Carbapenems have long been considered as a last resort to treat infections caused by MDR Gram-negative bacteria, but recently, carbapenem resistance has been increasingly common in A. baumannii [10]. Several resistance mechanisms of A. baumannii against carbapenems have been reported, including antimicrobial-inactivating enzymes, efflux pump, loss of the CarO outer membrane porin, and decreased target access [3,11,12]. One of the most important carbapenem resistance mechanisms is the production of class D β-lactamases (oxacillinase; OXA). This group of enzymes can hydrolyze oxacillin and the third-generation cephalosporins, but possesses weak activity against carbapenems [13]. OXA-producing A. baumannii was first reported in 1993 from the blood culture of a patient at the Edinburgh Royal Infirmary in 1985 [14]. At present, several subtypes of OXA-type enzyme have been reported, such as OXA-23, OXA-40/24, OXA-48, OXA-51, OXA-58, OXA-143, and OXA143 [15,16]. These enzyme-coding genes can be detected on either chromosomes or plasmids. The presence of OXA-23 has been widely reported in clinical isolates in many countries [17,18,19]. OXA-23-like β-lactamase enzymes have been found in outbreak isolates collected in the UK, East Asia, and South America, while OXA-40/24-like β-lactamases were found in the United States and Europe [20,21,22]. Therefore, the aim of this study was to investigate the molecular epidemiology of CRAB, focusing on the spread of isolates with Class D β-lactamases. Multi-locus sequence typing (MLST) was also applied to investigate the sequence typing of clinical isolates from a multicenter collection in Thailand.
2. Results
2.1. Antimicrobial Susceptibility Pattern
A total of 183 CRAB isolates were collected from 11 tertiary care hospitals across Thailand during 2016–2017. All isolates were obtained from clinical samples from non-duplicated patients (Table 1). The Acinetobacter isolates were phenotypically identified by microbiological and biochemical methods. The molecular detection of the blaoxa-51-like gene revealed a 353 bp band in all clinical isolates, which preliminarily confirmed the identification of the clinical isolates as being A. baumannii. The class D blaoxa-51-like gene is chromosomally encoded and appears to be intrinsic to A. baumannii [23].
Table 1.
Specimen types from which carbapenem-resistant Acinetobacter baumannii isolates were recovered.
| Isolation Site | No. of Isolates (%) |
|---|---|
| Sputum | 147 (80.33) |
| Pus | 17 (9.29) |
| Blood | 8 (4.37) |
| Urine | 9 (4.92) |
| Tissue | 2 (1.09) |
| Total | 183 (100) |
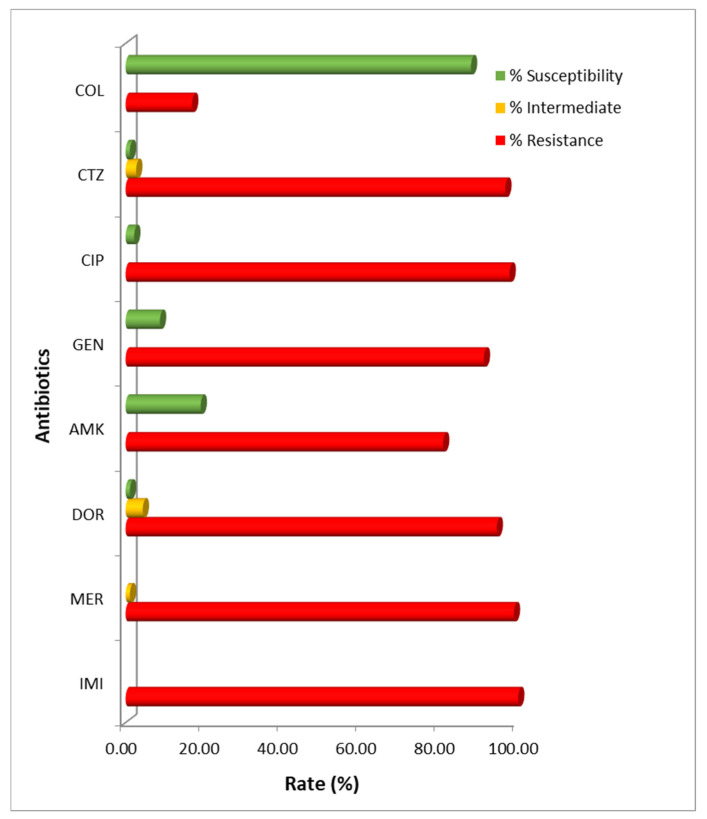
All clinical isolates were subjected to antimicrobial susceptibility testing utilizing the drugs currently used for A. baumannii treatment. The results demonstrated that all isolates were resistant to carbapenems, with 100% resistance to imipenem, followed by meropenem (98.91%) and doripenem (94.54%). High susceptibility was also observed with colistin (87.98%). Nevertheless, most of them showed high resistance rates to ciprofloxacin (97.81%), ceftazidime (96.72%), gentamicin (91.26%), and amikacin (80.87%) (Figure 1). The MIC50/MIC90 values were calculated for each antimicrobial agent, as follows: imipenem (32/64), meropenem (16/64), doripenem (16/64), ciprofloxacin (64/256), ceftazidime (512/>512), gentamicin (>512/>512), amikacin (>2048/>2048), and colistin (0.25/4), in micrograms per milliliter (Table 2). The MIC/MBC values of the control strain, A. baumannii ATCC 19606, were also examined for each antimicrobial agent: imipenem (≤1/≤1), meropenem (≤1/≤1), doripenem (≤1/≤1), ciprofloxacin (≤1/≤1), ceftazidime (16/16), gentamicin (16/16), amikacin (32/32), and colistin (2/2), in micrograms per milliliter.
Figure 1.
Antimicrobial susceptibility pattern of carbapenem-resistant A. baumannii isolates in Thailand. IMI: imipenem; MER: meropenem; DOR: doripenem; AMK: amikacin; GEN: gentamicin; CIP: ciprofloxacin; CTZ: ceftazidime; COL: colistin.
Table 2.
The antimicrobial susceptibility results for all 183 carbapenem-resistant A. baumannii (CRAB) isolates.
| Antibiotics | MIC Range a | MIC50 a | MIC90 a | Percentage (n) | ||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||||
| Carbapenems | ||||||
| Imipenem | 8–1028 | 32 | 64 | 0.00 (0) | 0.00 (0) | 100.00 (183) |
| Meropenem | 4–256 | 16 | 64 | 0.00 (0) | 1.09 (2) | 98.91 (181) |
| Doripenem | 0.125–256 | 16 | 64 | 1.09 (2) | 4.37 (8) | 94.54 (173) |
| Ciprofloxacin | 0.125–>512 | 64 | 256 | 2.19 (4) | 0.00 (0) | 97.81 (179) |
| Ceftazidime | 4–>512 | 512 | >512 | 1.09 (2) | 2.69 (5) | 96.17 (176) |
| Gentamicin | 0.5–>512 | >512 | >512 | 8.74 (16) | 0.00 (0) | 91.26 (167) |
| Amikacin | 1–>2048 | >2048 | >2048 | 19.13 (35) | 0.00 (0) | 80.87 (148) |
| Colistin | 0.0625–512 | 0.25 | 4 | 87.98 (152) | 0.00 (0) | 16.94 (31) |
a Values are presented in µg/mL.
2.2. Distribution of OXA-Type Carbapenemases
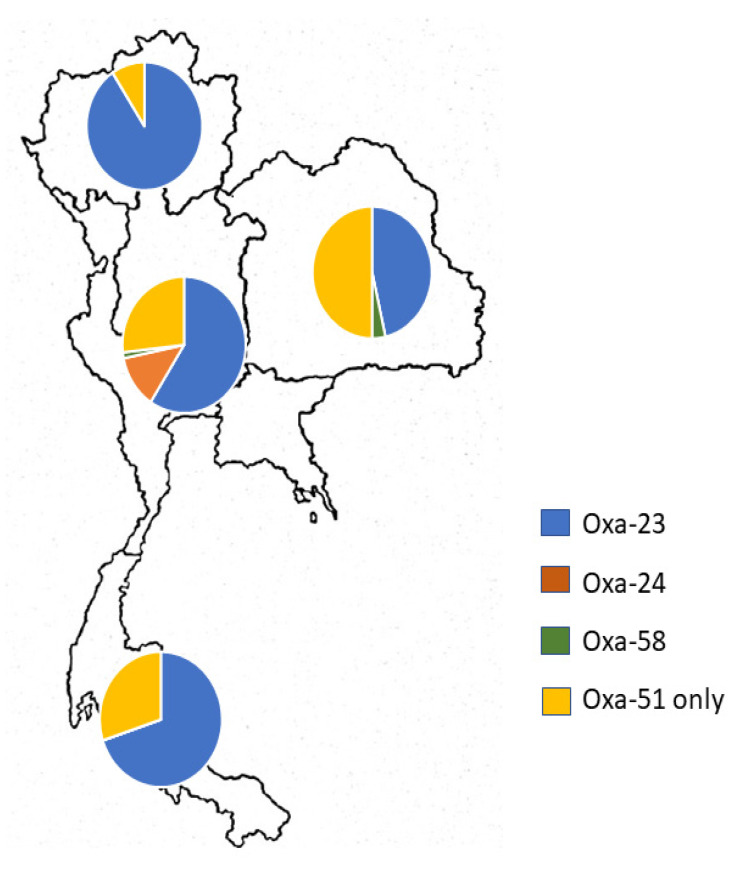
All 183 non-repetitive CRAB isolates were further investigated for the presence of OXA-type carbapenemase-encoding genes. The PCR results showed that 68.31% of the clinical isolates (125/183) were positive for the blaoxa-23-like gene. Geographically, blaoxa-23-like-carrying CRAB isolates were predominantly distributed in the northern region (90.74%), followed by the southern (70%), central and metropolitan (59.15%), and eastern (46.43%) regions of Thailand (Figure 2).
Figure 2.
Geographical distribution of oxacillinase (OXA)-type carbapenemase-encoding genes present in carbapenem-resistant A. baumannii isolates from across Thailand.
In contrast, the prevalence rates of the blaoxa-40/24-like and blaoxa-58-like genes were only 4.92% and 1.09% in CRAB isolates, respectively. It was noteworthy that all blaoxa-40/24-like-carrying CRAB isolates were collected from the central region. There were only two blaoxa-58-like-carrying CRAB isolates from the central and southern regions of Thailand. Interestingly, concomitant existence of carbapenem-hydrolyzing class D β-lactamases (excluding the intrinsic OXA-51-like gene) was found in 10 isolates. Co-existence of the blaoxa-23-like and blaoxa-40/24-like genes was detected in nine isolates, while only one isolate carried the blaoxa-23-like and blaoxa-58-like genes. It was remarkable that these isolates simultaneously carried triple OXA carbapenemase genes, including the blaoxa-51-like gene. A. baumannii ATCC 19606, a standard control strain, displayed only the intrinsic blaoxa-51-like gene.
2.3. Molecular Typing by Sequence Type (ST) Analysis
The sequence types (STs) of the CRAB isolates were determined according to the Institute Pasteur MLST scheme. The results demonstrated that most CRAB isolates in Thailand were of ST2 lineage. There were also some isolates belonging to ST25, ST98, ST129, ST164, ST215, ST338, and ST745. In addition, ST2 was the most dominant type for blaoxa-23-like-carrying CRAB isolates. Besides ST2, ST338, and ST745 were also represented among the blaoxa-40/24-like-carrying CRAB isolates, while an isolate with the OXA-58-like gene belonged to ST164.
3. Discussion
The increasing incidence of CRAB infections is becoming a pivotal concern for public health since the carbapenem drug group is considered the last resort for the treatment of severe infections caused by A. baumannii [10]. Notably, the World Health Organization (WHO) lists CRAB in the critical priority category according to the urgency of their need for new antimicrobial drugs [24]. Moreover, high incidence rates of CRAB infections have been reported in Southeast Asia. A. baumannii is naturally resistant to many antimicrobial drugs and is well-recognized in the acquisition of antibiotic-resistant genes. The increasing resistance rate of this particular microorganism is considered a public health threat due to the limited treatment options. Effective treatment of CRAB infection is now based on colistin, tigecycline, or sulbactam in combination with ampicillin [25,26]. This study revealed that most CRAB isolates from Thailand were still susceptible to colistin, although high resistance rates were observed for ciprofloxacin, ceftazidime, gentamicin, and amikacin. The MIC50/MIC90 values of these drugs were exceedingly high (>4-fold MIC breakpoints), except for those of colistin, indicating more complicated treatment by the currently used drugs. This was possibly due to the multiple resistance mechanisms commonly found in A. baumannii.
In general, several mechanisms are involved in carbapenem resistance, such as the production of carbapenem-hydrolyzing β-lactamases or carbapenemases, reduced permeability, and efflux pump. Among these, carbapenemase production is the most effective mechanism [27]. Therefore, CRAB isolates from a multicenter collection in Thailand were investigated for the prevalence of class D β-lactamases in this study. Here, we report that the blaoxa-23-like gene was predominantly present in Thailand (68.31%). These results are in agreement with those of previous studies which described the spread of OXA-23-producing Acinetobacter isolates in various locations worldwide. In China, about 80.6% of CRAB isolates carried the blaoxa-23 gene [28]. In addition, the predominant OXA group associated with carbapenem resistance across India was blaoxa-23-like (97%) [29]. In the Middle Eastern region, including Egypt, Qatar, and the United Arab Emirates, the blaoxa-23-like gene of class D β-lactamases was mainly identified among isolated A. baumannii strains [30,31]. High prevalence of the blaoxa-23-like gene (82.6%) was also reported among all A. baumannii isolates, and this gene was detected in 79.1% of CRAB isolates, from hospitals in some regions of Thailand during 2013–2015 [32]. Our study has shown that the blaoxa-23-like gene is still the most widely spread drug-resistance gene in CRAB, while the presence of other OXA-type β-lactamase genes is significantly low. The opposite trend has been observed for the occurrence of the blaoxa-40/24-like and blaoxa-58-like genes in Thai CRAB isolates, with rates of only 4.92% and 1.09%, respectively.
Despite the increasing prevalence of A. baumannii in hospital settings, little is known about which genomic components contribute to the clinical presentation of this pathogenic bacterium. Multi-locus sequence typing is highly discriminative and has been a powerful tool for epidemiological studies globally. MLST has been applied to characterize and monitor clinically important bacterial pathogens, including A. baumannii [33]. Since data on the sequence types (STs) of CRAB in Thailand are limited, CRAB isolates were examined to determine the most dominant type in this investigation. Here, we report that the most prevalent sequence type among CRAB in Thailand was ST2. There were some isolates belonging to ST25, ST98, ST129, ST164, ST215, ST338, and ST745. The sequence type distributions of A. baumannii in Asia have been published in many reports. In India, the predominant ST was ST848 (20%), followed by ST451 (12%), ST195 (7%), and other less common STs [29]. A similar ST distribution to the Indian clinical isolates was reported in extensively drug-resistant A. baumannii isolates in Iran [34]. However, none of this study’s isolates had STs similar to those of Indian and Iranian isolates. In Asia, ST2 has been described as the most prevalent sequence type among CRAB in China and Lebanon [28,35]. ST2 belongs to CC2 in the Pasteur scheme and corresponds to CC92 in the Oxford scheme and international clone II, as previously identified [36]. CC92 was reported to be the majority clone of CRAB isolates in India [37]. Similar to the results of a study in Malaysia, most of the isolates were grouped under the CC92 clonal complex [38]. It was noteworthy that the predominant ST in Malaysia was ST195, but it was ST2 in Thailand. Even though the sample numbers of previous studies were quite limited, they still reflected the distribution of CC92 in the Asian region.
4. Materials and Methods
4.1. Bacterial Collection and Identification
A total of 183 CRAB isolates were collected from 11 tertiary care hospitals across Thailand during 2016–2017. All A. baumannii isolates were obtained from clinical samples (sputum, urine, pus, tissue, or blood) from non-duplicated patients. Geographically, all isolates were collected from three hospitals in the central region, three hospitals in the northern region, three hospitals in the eastern region, and two hospitals from the southern region. A. baumannii ATCC 19606 was obtained from the American Type Culture Collection (ATCC), VA, USA. The Acinetobacter isolates were identified using conventional microbiological and biochemical methods. The samples were kept in glycerol stock and stored at −80 °C before use. The study was approved by the Ethical Review Committee, Faculty of Dentistry and Faculty of Pharmacy, Mahidol University (MU-DT/PY-IRB 2016/008.0404).
4.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing for CRAB identification was carried out using disc diffusion and broth dilution methods with carbapenems (imipenem, meropenem, and doripenem) and other antimicrobial agents generally used for A. baumannii infections, namely, ceftazidime, ciprofloxacin, gentamicin, amikacin, fosfomycin, piperacillin/tazobactam, and colistin. A. baumannii isolates were grown in Mueller-Hinton broth (MH broth), and then diluted with MH broth to 0.5 McFarland before adding them into 96-well plates containing antibiotics in triplicate. Finally, the plate was kept at 37 °C for 18 h. The results were evaluated by the MIC values from the minimum concentration of drugs that gave no visible growth. The Clinical and Laboratory Standards Institute (CLSI) breakpoints were applied for susceptibility determination [39]. In this study, CRAB was defined as an isolate resistant to at least one agent among the carbapenems. MDR-A. baumannii was defined as an isolate resistant to ≥1 agent in ≥3 classes of antimicrobial categories among aminoglycosides, antipseudomonal carbapenems, antipseudomonal fluoroquinolones, antipseudomonal penicillin combined with β-lactamase inhibitors, extended-spectrum cephalosporins, folate pathway inhibitors, penicillin plus β-lactamase inhibitors, polymyxins, and tetracyclines [40].
4.3. Genotypic Determination of Carbapenemases
Genomic DNA from Acinetobacter species was prepared using the boiling method [41]. Quantification of the extracted DNA was determined by spectroscopy at 260 nm. The polymerase chain reaction (PCR) method was performed for amplification of blaoxa-23-like, blaoxa-24/40-like, blaoxa-51-like, and blaoxa-58-like A. baumannii isolates. The blaoxa-51-like gene, an intrinsic enzyme marker, was utilized as a reliable marker for the identification of A. baumannii according to the study by Turton and colleagues [23]. The specific primers and product sizes are shown in Table 3 [42]. The amplification process for the intrinsic OXA-23-like gene was initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 25 s, 55 °C for 40 s, and 72 °C for 50 s, and a final elongation at 72 °C for 6 min. The amplification process for the intrinsic OXA-24/40-like and OXA-51-like genes was initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 25 s, 58 °C for 40 s, and 72 °C for 50 s, and a final elongation at 72 °C for 6 min. The amplification process for the intrinsic OXA-58-like gene was initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 25 s, 64 °C for 40 s, and 72 °C for 50 s, and a final elongation at 72 °C for 6 min.
Table 3.
Specific primers used for PCR amplification of OXA carbapenemase genes.
| Gene | Primer Sequences (5′ to 3′) | Product Size (bp) | |||
|---|---|---|---|---|---|
| Forward | Tm (°C) | Reverse | Tm (°C) | ||
| Class D | |||||
| oxacillinases | |||||
| blaoxa-23-like | GAT CGG ATT GGA | 60 | ATT TCT GAC CGC | 56 | 501 bp |
| GAA CCA GA | ATT TCC AT | ||||
| blaoxa-24/40-like | TAA TGC TTT GAT | 60 | AGT TGA GCG | 58 | 246 bp |
| CCC TTA AA | CAT CTT GG | ||||
| blaoxa-51-like | TAA TGC TTT GAT | 58 | TGG ATT GCA CTT | 58 | 353 bp |
| CGG CCT TG | CAT CTT GG | ||||
| blaoxa-58-like | AAG TAT TGG GGC | 60 | CCC CTC TGC GCT | 64 | 599 bp |
| TTG TGC TG | CTA CAT AC | ||||
4.4. Multi-Locus Sequence Typing Analysis
MLST was performed on CRAB isolates carrying either the blaoxa-23-like, blaoxa-24/40-like, or blaoxa-58-like gene. According to the Institute Pasteur protocol, the primers specific for seven housekeeping genes (recA, gltA, fusA, cpn60, pyrG, rplB, and rpoB) were used for PCR amplification, and PCR products were purified with a commercial kit according to the manufacturer’s instructions (Favorgen, Ping-Tung, Taiwan) [33]. Nucleotide sequencing was carried out by Bio Basic Asia Pacific Pte Ltd., Singapore. The nucleotide sequences were analyzed by comparing them to those in the Institute Pasteur database (https://pubmlst.org/abaumannii/).
5. Conclusions
Carbapenem resistance in A. baumannii occurs mainly as a result of the acquisition of OXA-type β-lactamase-encoding genes. The effect of the OXA β-lactamases in conferring carbapenem resistance has demonstrated a significant clinical impact on the ability to treat nosocomial infections. In this study, we report the recent situation covering CRAB isolates from multiple centers across Thailand. To date, the blaoxa-23-like gene, the first OXA-type β-lactamase gene to be identified from CRAB, still remains the most prevalent globally. The prevalence of the blaoxa-23-like gene was found to be relatively high in Thailand. The co-occurrence of two distinct carbapenemase-encoding genes in a single isolate was also detected in this study. In addition, the high occurrence rate of ST2 clinical isolates suggests that ST2, belonging to CC2 in the Pasteur scheme and CC92 in the Oxford scheme, could be an emerging lineage spreading carbapenem resistance in Thailand. Further studies are necessary to monitor the spread of carbapenem-resistant OXA-type β-lactamase genes from A. baumannii in hospital settings since they are becoming a significant cause of carbapenem resistance.
Acknowledgments
The authors wish to thank all staff in the Department of Microbiology for their technical support and suggestions on this work. The authors also thank all staff at the hospital sites. Their support and collaboration are gratefully acknowledged.
Author Contributions
Conceptualization, M.T.C.; methodology, T.S. and J.H.; validation, K.T. and P.K.; formal analysis, P.K.; investigation, T.S.-a.-n. and J.H.; resources, J.H. and P.M.; data curation, P.K. and M.T.C.; writing—original draft preparation, K.T. and M.T.C.; writing—review and editing, K.T. and M.T.C.; supervision, P.M., P.K., K.T. and M.T.C.; project administration, M.T.C.; funding acquisition, M.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Thailand Research Fund (Grant number BRG6080004) and Mahidol University (Basic Research Fund: fiscal year 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tosi M., Roa E., De Biasi S., Munari E., Venturelli S., Coloretti I., Biagioni E., Cossarizza A., Girardis M. Multidrug resistant bacteria in critically ill patients: A step further antibiotic therapy. J. Emerg. Crit. Care Med. 2018;2:103. doi: 10.21037/jeccm.2018.11.08. [DOI] [Google Scholar]
- 3.Moubareck C.A., Halat D.H. Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. 2020;9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 5.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B., Cha C.J., Jeong B.C., Lee S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment option. Front. Cell Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifert H., Stefanik D., Olesky M., Higgins P.G. In vitro activity of the novel fluorocycline TP-6076 against carbapenem-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2020;55:105829. doi: 10.1016/j.ijantimicag.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 8.El-Badawy M.F., Abdelwahab S.F., Alghamdi S.A., Shohayeb M.M. Characterization of phenotypic and genotypic traits of carbapenem-resistant Acinetobacter baumannii clinical isolates recovered from a tertiary care hospital in Taif, Saudi Arabia. Infect. Drug Resist. 2019;12:3113–3124. doi: 10.2147/IDR.S206691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paiboonvong T., Rodjun V., Houngsaitong J., Chomnawang M., Montakantikul P., Chulavatnatol S. Comparative in vitro activity of sitafloxacin against multidrug-resistant and carbapenem-resistant Acinetobacter baumannii clinical isolates in Thailand. Pharm. Sci. Asia. 2020;47:37–42. doi: 10.29090/psa.2020.01.019.0012. [DOI] [Google Scholar]
- 10.Elshamy A.A., Aboshanab K.M. A review on bacterial resistance to carbapenems: Epidemiology, detection and treatment options. Future Sci. OA. 2020;6:FSO438. doi: 10.2144/fsoa-2019-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon N.C., Wareham D.W. Multidrug-resistant Acinetobacter baumannii: Mechanisms of virulence and resistance. Int. J. Antimicrob. Agents. 2010;35:219–226. doi: 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.J., Kim S.I., Kim Y.R., Hong K.W., Wie S.H., Park Y.J. Carbapenem-resistant Acinetobacter baumannii: Diversity of resistant mechanisms and risk factors for infection. Epidemiol. Infect. 2012;140:137–145. doi: 10.1017/S0950268811000744. [DOI] [PubMed] [Google Scholar]
- 13.Lin M.F., Lan C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases. 2014;2:787–814. doi: 10.12998/wjcc.v2.i12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codjoe F.S., Donkor E.S. Carbapenem resistance: A review. Med. Sci. 2017;6:1. doi: 10.3390/medsci6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paton R., Miles R.S., Hood J., Amyes S.G., Miles R.S. ARI 1: Beta-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents. 1993;2:81–87. doi: 10.1016/0924-8579(93)90045-7. [DOI] [PubMed] [Google Scholar]
- 16.Evans B.A., Amyes G.B. OXA β-lactamases. Clin. Microbiol. Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Bayssari C., Dabboussi F., Hamze M. Detection of expanded-spectrum β-lactamases in Gram-negative bacteria in the 21st century. Expert Rev. Anti-Infect. Ther. 2015;13:1139–1158. doi: 10.1586/14787210.2015.1066247. [DOI] [PubMed] [Google Scholar]
- 18.D’Onofrio V., Conzemius R., Varda-Brkić D., Bogdan M., Grisold A., Gyssens I.C., Bedenić B., Barišić I. Epidemiology of colistin-resistant, carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Croatia. Infect. Genet. Evol. 2020;81:104263. doi: 10.1016/j.meegid.2020.104263. [DOI] [PubMed] [Google Scholar]
- 19.AlAmri A.M., AlQurayan A.M., Sebastian T., AlNimr A.M. Molecular surveillance of multidrug-resistant Acinetobacter baumannii. Curr. Microbiol. 2020;77:335–342. doi: 10.1007/s00284-019-01836-z. [DOI] [PubMed] [Google Scholar]
- 20.Kuo S.C., Huang W.C., Huang T.W., Wang H.Y., Lai J.F., Chen T.L., Lauderdale T.L. Molecular epidemiology of emerging blaOXA-23-Like- and blaOXA-24-Like-carrying Acinetobacter baumannii in Taiwan. Antimicrob. Agents Chemother. 2018;62:e01215-17. doi: 10.1128/AAC.01215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel L., Nordmann P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006;50:1442–1448. doi: 10.1128/AAC.50.4.1442-1448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suárez C.J., Lolans K., Villegas M.V., Quinn J.P. Mechanisms of resistance to beta-lactams in some common Gram-negative bacteria causing nosocomial infections. Expert. Rev. Anti Infect. Ther. 2005;3:915–922. doi: 10.1586/14787210.3.6.915. [DOI] [PubMed] [Google Scholar]
- 23.Turton J.F., Woodford N., Glover J., Yarde S., Kaufmann M.E., Pitt T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006;44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. [(accessed on 1 October 2020)];2017 Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- 25.Oliveira M.S., Prado G.V., Costa S.F., Grinbaum R.S., Levin A.S. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J. Antimicrob. Chemother. 2008;61:1369–1375. doi: 10.1093/jac/dkn128. [DOI] [PubMed] [Google Scholar]
- 26.Garnacho-Montero J., Amaya-Villar R., Ferrándiz-Millón C., Díaz-Martín A., López-Sánchez J.M., Gutiérrez-Pizarraya A. Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii bacteremia. Expert Rev. Anti-Infect. Ther. 2015;13:769–777. doi: 10.1586/14787210.2015.1032254. [DOI] [PubMed] [Google Scholar]
- 27.Kamolvit W., Sidjabat H.E., Paterson D.L. Molecular epidemiology and mechanisms of carbapenem resistance of Acinetobacter spp. in Asia and Oceania. Microb. Drug Resist. 2015;21:424–434. doi: 10.1089/mdr.2014.0234. [DOI] [PubMed] [Google Scholar]
- 28.Yaowen C., Guangxin L., Ying X., Yanhong W., Min S., Chi Z., Wei Z., Jinwei H., Jingni Y., Xu J., et al. Characterization of carbapenem-resistant Acinetobacter baumannii isolates in a Chinese teaching hospital. Front. Microbiol. 2015;6:910. doi: 10.3389/fmicb.2015.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayakumar S., Mathur P., Kapil A., Das B.K., Ray P., Gautam V., Sistla S., Parija S.C., Walia K., Ohri V.C., et al. Molecular characterization & epidemiology of carbapenem-resistant Acinetobacter baumannii collected across India. Indian J. Med. Res. 2019;149:240–246. doi: 10.4103/ijmr.IJMR_2085_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019;18:1. doi: 10.1186/s12941-018-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolain J.M., Loucif L., Al-Maslamani M., Elmagboul E., Al-Ansari N., Taj-Aldeen S., Shaukat A., Ahmedullah H., Hamed M. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase in Qatar. New Microbes New Infect. 2016;11:47–51. doi: 10.1016/j.nmni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leungtongkam U., Thummeepak R., Wongprachan S., Thongsuk P., Kitti T., Ketwong K., Runcharoen C., Chantratita N., Sitthisak S. Dissemination of bla(OXA-23), bla(OXA-24), bla(OXA-58), and bla(NDM-1) genes of Acinetobacter baumannii isolates from four tertiary hospitals in Thailand. Microb. Drug Resist. 2018;24:55–62. doi: 10.1089/mdr.2016.0248. [DOI] [PubMed] [Google Scholar]
- 33.Bartual S.G., Seifert H., Hippler C., Luzon M.A.D., Wisplinghoff H., Rodríguez-Valera F. Development of a Multilocus Sequence Typing Scheme for Characterization of Clinical Isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saffari F., Monsen T., Karmostaji A., Azimabad F.B., Widerström M. Significant spread of extensively drug-resistant Acinetobacter baumannii genotypes of clonal complex 92 among intensive care unit patients in a university hospital in southern Iran. J. Med. Microbiol. 2017;66:1656–1662. doi: 10.1099/jmm.0.000619. [DOI] [PubMed] [Google Scholar]
- 35.Nawfal Dagher T., Al-Bayssari C., Chabou S., Antar N., Diene S.M., Azar E., Rolain J.M. Investigation of multidrug-resistant ST2 Acinetobacter baumannii isolated from Saint George hospital in Lebanon. BMC Microbiol. 2019;19:29. doi: 10.1186/s12866-019-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkshoorn L., Aucken H., Gerner-Smidt P., Janssen P., Kaufmann M.E., Garaizar J., Ursing J., Pitt T.L. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 1996;34:1519–1525. doi: 10.1128/JCM.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S., Patil P.P., Singhal L., Ray P., Patil P.B. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates reveals the emergence of blaOXA-23 and blaNDM-1 encoding international clones in India. Infect. Genet. Evol. 2019;75:103986. doi: 10.1016/j.meegid.2019.103986. [DOI] [PubMed] [Google Scholar]
- 38.Rao M., Rashid F., Shukor S., Hashim R., Ahmad N. Detection of antimicrobial resistance genes associated with carbapenem resistance from the whole-genome sequence of Acinetobacter baumannii isolates from Malaysia. Can. J. Infect. Dis. Med. 2020;2020:5021064. doi: 10.1155/2020/5021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. pp. 46–49. CLSI Document M100. [Google Scholar]
- 40.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 41.Falah F., Shokoohizadeh L., Adabi M. Molecular identification and genotyping of Acinetobacter baumannii isolated from burn patients by PCR and ERIC-PCR. Scars Burn Health. 2019;5:2059513119831369. doi: 10.1177/2059513119831369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]