Abstract
Norovirus outbreaks frequently occur in closed or semiclosed institutions. Recent studies in Catalonia and various countries indicate that, during outbreaks in these institutions, norovirus is detected in between 23% and 60% of workers, and the prevalence of infection in asymptomatic workers involved in outbreaks ranges from 17% to 40%. In this work, we carried out a prospective study to investigate the involvement of workers in closed and semiclosed institutions during outbreaks. The attack rates (ARs) and the rate ratios (RRs) were calculated according to the type of transmission and occupational category. The RRs and 95% confidence intervals (CIs) between workers and users were calculated. The mean cycle of quantification (Cq) values were compared according to the genogroup and the presence of symptoms. ARs were higher in person-to-person transmission than in common vehicle outbreaks, and 38.8% of workers were symptomatic. The RR between workers and users was 0.46 (95% CI 0.41–0.52). The ARs in workers were high, particularly in workers with closer contact with users. The mean Cq was lower in patients than in asymptomatic infected persons, although the difference was only significant for genogroup I (GI). The frequency of asymptomatic infected persons suggests that personal hygiene measures should be followed by all workers in the centers affected.
Keywords: norovirus, outbreak, acute gastroenteritis, closed institution, semiclosed institution, workers
1. Introduction
Most acute gastroenteritis (AGE) cases worldwide are caused by viruses. The causes include, in addition to norovirus, other enteric viruses such as adenovirus, astrovirus, rotavirus and sapovirus, although norovirus is the most common causal agent [1,2,3]. Noroviruses are nonenveloped RNA viruses of the Caliciviridae family. There are six genogroups (GI to GVI), although only genogroups I, II and IV are human pathogens [4,5].
The transmission mechanism is fecal–oral, and direct person-to-person contact is a very efficient mechanism. Transmission by food, water and fomites is common, and transmission by aerosols generated by vomiting has been described [6].
A systematic review estimated that human caliciviruses (including genogroup I and II norovirus and sapovirus) caused 71,000 deaths worldwide in children aged <5 years in 2011 [7].
Norovirus is highly resistant to high levels of chlorine [8], heat, cold [9], acidic pH and organic solvents [10,11], which allows its survival for long periods in the environment [12] and facilitates its transmission. The high transmissibility of norovirus is also facilitated by the short duration of immune protection [13], the low infectious dose and the frequency of asymptomatic infections and because the presence of norovirus viruses in feces may be prolonged in both symptomatic and asymptomatic infected persons.
The doses that cause infection in 50% of exposed people (ID50) described to date range from 18 to 2934 viral genomes [14,15,16,17]. About 30% of people affected by norovirus have an asymptomatic infection [18].
AGE outbreaks due to norovirus frequently occur in closed or semiclosed institutions such as long-term care facilities, daycare centers, schools, nursing homes and hotels. According to 2006–2010 data, in Catalonia (Spain), >50% of norovirus outbreaks occurred in these types of institutions [19].
The measures to control outbreaks in Catalonia include the recommendation that workers with acute gastroenteritis due to norovirus do not return to work until >48 h after the end of symptoms [20].
Recent studies in Catalonia and various countries indicate that, during outbreaks in these institutions, norovirus in feces is detected in between 23% [21] and 60% [22] of workers and the prevalence of infection in asymptomatic workers involved in outbreaks ranges from 17% [21] to 40% [22].
Although few studies have investigated the involvement of workers in AGE outbreaks due to norovirus in closed and semiclosed institutions, the attack rate among workers may vary depending on the demographic characteristics and type of occupation, the type of institution and the type of transmission causing the outbreak.
The objective of the study was to investigate the involvement of workers in outbreaks due to norovirus in closed and semiclosed institutions according to type of center, type of transmission, genogroups involved and viral load.
2. Materials and Methods
2.1. Type of Study, Study Period and Study Population
This was a prospective study of AGE outbreaks due to norovirus reported in 2017–2019 to the Notifiable Diseases System of Catalonia [23], a region in the northeast of Spain with a population of 7,496,276 in January 2017 [24].
2.2. Outbreaks Included in the Study
All laboratory-confirmed AGE outbreaks due to norovirus that occurred in closed and semiclosed institutions during the study period were included.
A closed institution was defined as one in which users remained the whole day, including the night, although they might leave for short periods for exceptional reasons. Within this category, we included nursing homes, long-term care facilities and summer camps, among others. A semiclosed institution was defined as one carrying out social or educational activities (nonoccupational) in which users remained for >8 h per day, and in which most users consumed at least one meal. Daycare centers, preschool centers, schools and hotels were included in this category.
AGE was defined as sudden-onset diarrhea accompanied by nausea, vomiting, abdominal pain or fever. A norovirus outbreak was defined as AGE in ≥2 people with a common vehicle or person-to-person transmission with norovirus in stool samples identified by real-time reverse transcription polymerase chain reaction (RTqPCR) [25,26].
2.3. Data Collection
Data on the outbreaks included were collected by technicians of the Epidemiological Surveillance Services of the Public Health Agency of Catalonia and the Public Health Agency of Barcelona.
In all reported outbreaks, the type of institution, the number of users, the number of workers and the type of transmission (person-to-person or common vehicle) were recorded.
All persons exposed were questioned about sociodemographic variables (sex and date of birth) and their relationship with the institution (worker or user). Information on the type of occupation and the presence of clinical symptomatology was also collected in workers.
Samples of feces were collected from workers and users to identify norovirus genogroups I, II and IV by RTqPCR. Samples were analyzed in the Microbiology Laboratory of Vall d’Hebron University Hospital. The specific primers described by Kageyama et al. were used to detect norovirus GI and GII [25]. A modification of the primer described by Farkas et al. [26] and Kageyama et al. [25] was used to detect norovirus GIV.
2.4. Data Analysis and Management
The proportions of the study variables and their 95% confidence intervals (CIs) were calculated. The global attack rates, the attack rates and the rate ratios (RRs) and their 95% CIs were calculated for the mode of transmission (person-to-person or common vehicle), type of institution and type of work activity.
To estimate the risk of workers becoming ill with respect to users, the RRs and their 95% CIs were calculated.
To estimate the viral load, the mean cycle of quantification (Cq) values obtained by RTqPCR [27] were calculated. The means of Cq were compared using the Student’s t-test. Statistical significance was established as p < 0.05. Data were collected and handled using Microsoft Access 12.0 database manager, and the PASW Statistics 18.0.2 statistical package was used for the statistical analysis.
3. Results
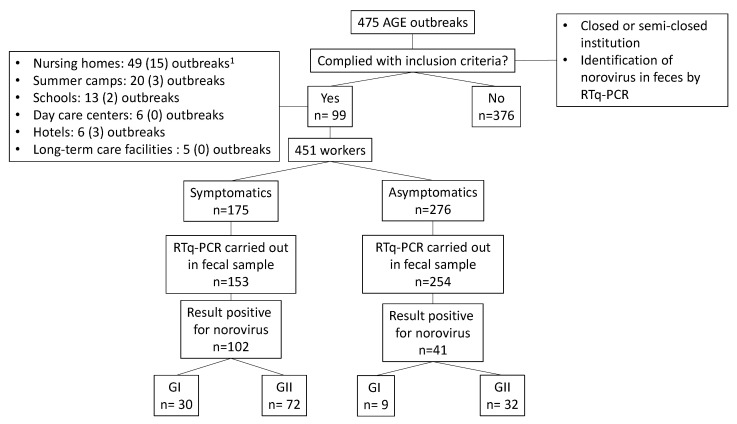
During the study period, 99 AGE outbreaks due to norovirus were detected in closed or semiclosed institutions (26 in 2017, 33 in 2018 and 40 in 2019): 49 in nursing homes (49.5%), 20 in summer camps (22.2%), 13 in schools (13.1%), 6 in daycare centers (6.1%), 6 in hotels (6.1%) and 5 in long-term care facilities (5.1%) (Figure 1).
Figure 1.
Study flowchart. 1 In brackets, the number of outbreaks in which the first case was a worker.
In 74 outbreaks (74.8%), transmission was person-to-person; in the remaining 25 (25.2%), it was due to a common vehicle.
The epidemiological survey was answered by 451 workers and 1015 users from the affected centers. Of the workers, 175 (38.8%) were symptomatic and 276 (61.2%) had no symptoms.
The attack rate was 32.03% in males and 41.49% in females. No significant differences in attack rates were found according to sex or age group (Table 1).
Table 1.
Attack rates in workers by age and sex.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Age (Years) | Symptomatic | Exposed | Attack Rate | Symptomatic | Exposed | Attack Rate | p-Value |
| 17–25 | 10 | 17 | 58.82% | 29 | 45 | 64.44% | 0.69 |
| 26–35 | 11 | 38 | 28.95% | 28 | 58 | 48.28% | 0.06 |
| 36–45 | 10 | 27 | 37.04% | 20 | 55 | 36.36% | 0.95 |
| 46–55 | 5 | 24 | 20.83% | 31 | 83 | 37.35% | 0.13 |
| 56–65 | 4 | 14 | 28.57% | 14 | 37 | 37.84% | 0.54 |
| NA | 1 | 8 | 12.50% | 12 | 45 | 26.67% | 0.35 |
| Total | 41 | 128 | 32.03% | 134 | 323 | 41.49% | 0.06 |
NA: not available.
The attack rate in workers was 43.9% in person-to-person outbreaks and 32.6% in outbreaks with a common vehicle (Table 2). The risk of workers being symptomatic was higher in person-to-person outbreaks than in those with a common vehicle (RR 1.35; 95% CI 1.05–1.74). Analysis by type of institution showed the RR of attack rates was only significant for schools (RR 1.93; 95% CI 1.07–3.49).
Table 2.
Attack rates and rate ratios (RRs) in workers according to type of institution and type of transmission.
| Type of Institution (Total Affected) 1 | Symptomatic | Exposed | Attack Rate | RR (95% CI) |
|---|---|---|---|---|
| Summer camp | ||||
| Person-to-person (74) | 1 | 11 | 9.09% | 0.17 (0.02 to 1.30) |
| Common vehicle (105) | 12 | 32 | 37.5% | 1 |
| Mixed transmission (4) | 0 | 8 | 0% | NC |
| Total (183) | 13 | 51 | 25.49% | |
| School | ||||
| Person-to-person (144) | 15 | 56 | 26.79% | 1.93 (1.07 to 3.49) |
| Common vehicle (24) | 11 | 69 | 15.94% | 1 |
| Total (168) | 26 | 125 | 20.80% | |
| Daycare center | ||||
| Person-to-person (69) | 6 | 14 | 42.86% | NC |
| Common vehicle (0) | 0 | 0 | 0% | |
| Total (69) | 6 | 14 | 42.86% | |
| Hotel | ||||
| Person-to-person (14) | 9 | 40 | 22.5% | 0.93 (0.44 to 1.95) |
| Common vehicle (48) | 5 | 21 | 23.81% | 1 |
| Total (62) | 14 | 61 | 22.95% | |
| Nursing home | ||||
| Person-to-person (400) | 77 | 133 | 57.89% | 1.03 (0.78 to 1.38) |
| Common vehicle (93) | 28 | 50 | 56.00% | 1 |
| Total (493) | 105 | 183 | 57.37% | |
| Long-term care facility | ||||
| Person-to-person (55) | 11 | 17 | 64.70% | NC |
| Common vehicle (0) | 0 | 0 | 0% | |
| Total (55) | 11 | 17 | 64.70% | |
| Total | ||||
| Person-to-person (756) | 119 | 271 | 43.91% | 1.35 (1.05 to 1.74) |
| Common vehicle (270) | 56 | 172 | 32.56% | 1 |
| Mixed transmission (4) | 0 | 8 | 0% | NC |
| Total (1030) | 175 | 451 | 38.80% |
1 The total number of affected persons, including users and workers, is shown in parentheses. NC: Not calculable.
A total of 1015 users responded to the epidemiological survey, of whom 854 were users who were symptomatic (attack rate 84.1%). The RR of attack rates between workers and users was 0.46 (95% CI 0.41–0.52). The lower risk of workers compared with users was also observed separately for each type of institution (Table 3).
Table 3.
Attack rates and rate ratios (RRs) in workers and users according to type of institution.
| Type of Institution | Symptomatic | Exposed | Attack Rate | RR (95% CI) |
|---|---|---|---|---|
| Summer camp | 0.30 (0. 19 to 0. 48) 1 |
|||
| Workers | 13 | 51 | 25.49 | |
| Users | 170 | 201 | 84.58 | |
| Total | 183 | 252 | 72.62 | |
| Schools | 0.25 (0.17 to 0.35) 1 |
|||
| Workers | 26 | 125 | 20.80 | |
| Users | 141 | 167 | 84.43 | |
| Total | 167 | 292 | 57.19 | |
| Daycare center | 0.53 (0.28 to 0.97) 1 |
|||
| Workers | 6 | 14 | 42.86 | |
| Users | 62 | 76 | 81.58 | |
| Total | 68 | 90 | 75.56 | |
| Hotel | 0.26 (0.16 to 0.42) 1 |
|||
| Workers | 14 | 61 | 22.95 | |
| Users | 48 | 55 | 87.27 | |
| Total | 62 | 116 | 34.83 | |
| Nursing home | 0.69 (0.61 to 0.79) 1 |
|||
| Workers | 105 | 183 | 57.38 | |
| Users | 388 | 468 | 82.91 | |
| Total | 493 | 651 | 75. 73 | |
| Long-term care facility | 0.69 (0.48 to 0.99) 1 |
|||
| Workers | 11 | 17 | 64.71 | |
| Users | 45 | 48 | 93.75 | |
| Total | 56 | 65 | 86.15 | |
| Total | 0.46 (0.41 to 0.52) 1 |
|||
| Workers | 175 | 451 | 38.80 | |
| Users | 854 | 1015 | 84.14 | |
| Total | 1029 | 1466 | 70.19 |
The attack rates differed according to the type of institution; the highest rates were for long-term care facilities (86.15%) and nursing homes (75.73%), and the lowest rates were for hotels (34.83%). Globally, in all types of institutions, workers had significantly lower attack rates than users.
With respect to the type of occupation, caregivers in nursing homes and healthcare workers had an increased risk of becoming ill, while being a kitchen worker was a protective factor against infection (Table 4).
Table 4.
Attack rates and rate ratios (RRs) in workers according to type of occupation.
| Type of Occupation | Attack Rate | RR 1 (95% CI) |
|---|---|---|
| Cook | 9.8% | 0.26 (0.12.0.56) |
| Kitchen assistant | 15.0% | 0.36 (0.23.0.59) |
| Waiter | 37.5% | 1.13 (0.45. 2.79) |
| Dining monitor | 27.8% | 0.79 (0.54. 1.14) |
| Caregiver or healthcare worker | 71.6% | 3.18 (2.32. 4.35) |
| Global attack rate | 38.8% |
1 Workers in each type of occupation compared with all other workers.
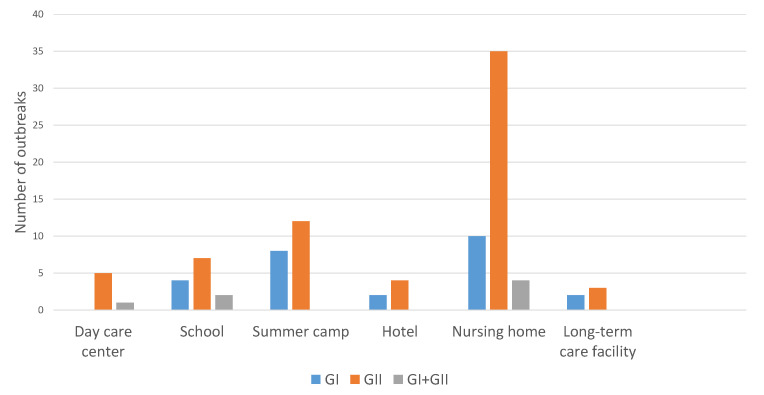
The most common genogroup was norovirus GII with 66 outbreaks (66.7%); 26 were due to GI (26.3%), and the etiology was mixed in 7 (7.1%). No outbreak due to GIV was detected. GII was more frequently involved in all types of institutions than GI (Figure 2).
Figure 2.
Outbreaks according to type of center and genogroup.
Norovirus was detected by RTqPCR in 143 workers (102 symptomatic and 41 asymptomatic) and 687 users (603 symptomatic and 84 asymptomatic). Norovirus GI was detected in 30 symptomatic workers, 9 asymptomatic workers, 144 symptomatic users and 19 asymptomatic users. Norovirus GI was detected in 72 symptomatic workers, 32 asymptomatic workers, 459 symptomatic users and 65 asymptomatic users.
The mean viral load, measured indirectly by Cq, was 27.69 for GI and 31.61 for GII, although the values are not comparable as the measurements were made using different tests.
Mean Cq was lower in symptomatic persons than in asymptomatic infected persons, with a higher viral load in symptomatic persons, for genogroups GI and GII. For GI, a mean Cq of 36.97 (SD 5.13) was observed in asymptomatic infected persons and a mean Cq of 30.01 (SD 5.51) was observed in symptomatic persons (p = 0.002). Although the Cq was also higher for GII in asymptomatic compared with symptomatic persons (29.19; SD 5.26 vs. 27.01; SD 5.84), the differences were not statistically significant (p = 0.07) (Table 5).
Table 5.
Difference in viral load between symptomatic and infected asymptomatic persons according to genogroup.
| Genogroup | Symptomatic | N | Mean Cq | SD | p-Value |
|---|---|---|---|---|---|
| GI | Yes | 30 | 30.01 | 5.51 | 0.002 |
| No | 9 | 36.97 | 5.13 | ||
| GII | Yes | 72 | 27.01 | 5.84 | 0.07 |
| No | 32 | 29.19 | 5.26 |
4. Discussion
The attack rate in workers in institutions where outbreaks included in the study occurred was 38.8%, higher than the 10.45% found by Wu et al. in workers involved in norovirus outbreaks in Shanghai between 2015 and 2017 [21] and the 30% described by Sabria et al. in food handlers and healthcare workers in outbreaks in Catalonia between 2010 and 2012 [22]. The higher attack found in our study may be because the studies mentioned were not limited to closed or semiclosed institutions, in which transmission occurs more easily than in other types of institutions.
We found that 16.14% of asymptomatic workers in AGE outbreaks due to norovirus in closed and semiclosed institutions were infected.
Asymptomatic norovirus infection is common, even among people without known exposure. Qi et al., in a meta-analysis of published studies on asymptomatic norovirus infection, found a prevalence of infection of 7% worldwide in the general population [28]. Yu et al. found that 3.3% of food handlers unrelated to outbreaks were asymptomatically infected [29], and Okabayashi et al. found a rate of asymptomatic infections of up to 12% in workers in institutions [30].
Wang et al. found norovirus infections in 4.04% of the inhabitants of municipalities related to oyster cultivation, with no differences between workers in oyster farms and the rest of the population [31].
Other studies of workers involved in AGE outbreaks due to norovirus have found very similar rates to those described in our study. Wu et al. found an infection rate of 17% in asymptomatic workers in institutions where outbreaks occurred [21], a rate very similar to ours. Qi et al. found the prevalence of asymptomatic infected people to be 18% in workers related to outbreaks [28]. Our results, in common with other reports [32], found no significant differences in attack rates between male and female workers.
The main transmission route of norovirus is direct person-to-person contact, and its dissemination is facilitated by the conditions in which the outbreak occurs.
Increased personal contact between individuals, such as in nursing homes, schools and daycare centers, is likely to facilitate greater transmission [33,34]. Godoy et al. found an increased risk in workers in an AGE outbreak due to norovirus in a nursing home when workers had more direct contact with residents [35].
Our results showed the greatest risk of transmission was direct person-to-person transmission rather than transmission by a common vehicle (RR 1.35 95%; CI 1.05 to 1.74) and that the greatest risk was in caregivers in nursing homes and healthcare workers, whose occupational activity involves closer and longer-lasting contact with users. In contrast, kitchen workers, who have less direct contact with users, had a lower risk of being symptomatic.
GII was the most frequently identified genogroup, both in symptomatic and asymptomatic infected persons. The predominance of the GII genogroup, both in isolated cases of AGE and in outbreaks or asymptomatic infections, has been described by various authors. Yu et al. identified GII in 65% of food handlers in elementary schools in the Incheon region (Korea) [29]. Park et al. identified GII in 75% of positive samples from workers in nursing homes with norovirus outbreaks [36].
Likewise, 90.5% of asymptomatic children studied by Qi et al. in a nursery in Changzhou, China, were infected by genogroup GII [37], and in the United States between 2009 and 2015, 81% of norovirus outbreaks were caused by GII [38].
The viral load was higher in symptomatic persons than in asymptomatic infected persons in all cases. While differences in the mean Cq of symptomatic persons vs. asymptomatic infected persons were statistically significant for GI (p = 0.002), no significant differences were found for GII (p = 0.073).
Teunis et al. found no differences in the viral load between symptomatic persons and asymptomatic infected persons in workers and users of nursing homes and hospitals involved in GII outbreaks [39]. Kabue et al. found that, in children in rural South Africa, the viral load of GII was higher in symptomatic than in asymptomatic persons, but there were no significant differences for GI [40].
The interpretation of the significance of these differences is difficult, given the discrepancies in the results obtained in different studies, but in all cases, viral loads were detected in asymptomatic infected persons, indicating the potential of these people to act as sources of contagion during outbreaks.
The study has some limitations. Firstly, 12.6% of symptomatic workers and 8% of asymptomatic workers were not analyzed using RTqPCR, which could bias the results. However, the differences between these percentages were not significant (p = 0.11), and therefore the comparisons between the two categories of infected persons are valid.
Secondly, the fact that in some studies significant differences in relation to an increased viral load in symptomatic versus asymptomatic persons were observed for genogroup GII compared with genogroup GI may be explained by the sample size or confounding factors, and this could be the subject of further studies.
Thirdly, our study was carried out using the surveys answered, and it may be that people who became ill were more willing to collaborate in responding to the survey than those who did not present symptoms, which could have resulted in an overestimate of the attack rates.
Fourthly, some data were not available, such as the theoretical total capacity of the affected centers, the density of occupancy at the time of the outbreak or the ratio between workers and users, so the possible influence of these factors on the attack rates observed could not be analyzed.
The main strength of our study was that it was carried out in the context of epidemiological surveillance, and therefore the study coverage was universal in the target population.
5. Conclusions
The attack rate in workers in closed and semiclosed institutions was high and was related to the type of activity, being higher in workers with closer contact with users.
The frequency of asymptomatic infected persons suggests that in an AGE outbreak due to norovirus, personal hygiene measures should be followed by all workers in the institution where the outbreak occurred.
Although the genogroup I viral load in symptomatic persons was significantly higher than that in asymptomatic infected persons, the viral loads of asymptomatic infected persons were high for both genogroups GI and GII, indicating the potential of these asymptomatic people as a source of infection.
Author Contributions
Conceptualization, formal analysis and writing—original draft preparation, I.P. and À.D.; writing—review and editing and data curation, I.B., M.A., C.I. and C.R.; methodology, S.G. and M.J.; investigation and resources, T.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Instituto de Salud Carlos III through the project PI16/02005 (Co-funded by the European Regional Development Fund “Investing in your future”) and the Catalan Agency for the Management of Grants for University (AGAUR Grant Number 2017/SGR 1342).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mead P.S., Slutsker L., Dietz V., McCaig L.F., Bresee J.S., Shapiro C., Griffin P.M., Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereciartu A., Bok K., Gómez J. Identification of viral agents causing gastroenteritis among children in Buenos Aires, Argentina. J. Clin. Virol. 2002;25:197–203. doi: 10.1016/S1386-6532(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 3.Marie-Cardine A., Gourlain K., Mouterde O., Castignolles N., Hellot M.F., Mallet E., Buffet-Janvresse C. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin. Infect. Dis. 2002;34:1170–1178. doi: 10.1086/339807. [DOI] [PubMed] [Google Scholar]
- 4.Atmar R.L., Ramani S., Estes M.K. Human noroviruses: Recent advances in a 50-year history. Curr. Opin. Infect. Dis. 2018;31:422–432. doi: 10.1097/QCO.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 5.International Committee on Taxonomy of Viruses ICTV 9th Report. [(accessed on 24 November 2020)];2011 Available online: https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/253/caliciviridae.
- 6.Godoy P., Alsedà M., Bartolomé R., Clavería D., Módol I., Bach P., Mirada G., Domínguez À. Norovirus gastroenteritis outbreak transmitted by food and vomit in a high school. Epidemiol. Infect. 2016;144:1951–1958. doi: 10.1017/S0950268815003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keswick B.H., Satterwhite T.K., Johnson P.C., DuPont H.L., Secor S.L., Bitsura J.A., Gary G.W., Hoff J.C. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 1985;50:261–264. doi: 10.1128/AEM.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butot S., Putallaz T., Sánchez G. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int. J. Food Microbiol. 2008;126:30–35. doi: 10.1016/j.ijfoodmicro.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Cook N., Knight A., Richards G.P. Persistence and elimination of human norovirus in food and on food contact surfaces: A critical review. J. Food Prot. 2016;79:1273–1294. doi: 10.4315/0362-028X.JFP-15-570. [DOI] [PubMed] [Google Scholar]
- 11.Wobus E., Green K.Y. Caliciviridae: The viruses and their replication. In: Howley P.M., Knipe D.M., Whelan S., editors. Fields Virology, Emerging Viruses. 7th ed. Volume 1. Wolters Kluwer; Philadelphia, PA, USA: 2020. pp. 129–169. [Google Scholar]
- 12.Wu H.M., Fornek M., Schwab K.J., Chapin A.R., Gibson K., Schwab E., Spencer C., Henning K. A norovirus outbreak at a long-term-care facility: The role of environmental surface contamination. Infect. Control Hosp. Epidemiol. 2005;26:802–810. doi: 10.1086/502497. [DOI] [PubMed] [Google Scholar]
- 13.Ramani S., Estes M.K., Atmar R.L. Correlates of protection against norovirus infection and disease—Where are we now, where do we go? PLoS Pathog. 2016;12:e1005334. doi: 10.1371/journal.ppat.1005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teunis P.F., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S., Le Pendu J., Calderon R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 15.Kirby A.E., Teunis P.F., Moe C.L. Two human challenge studies confirm high infectivity of Norwalk virus. J. Infect. Dis. 2015;211:166–167. doi: 10.1093/infdis/jiu385. [DOI] [PubMed] [Google Scholar]
- 16.Guix S., Fuentes C., Pintó R.M., Blanco A., Sabrià A., Anfruns-Estrada E., Garrido V.R., Alonso M., Bartolomé R., Cornejo T., et al. Infectivity of norovirus GI and GII from bottled mineral water during a waterborne outbreak, Spain. Emerg. Infect. Dis. 2020;26:134–137. doi: 10.3201/eid2601.190778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atmar R.L., Opekun A.R., Gilger M.A., Estes M.K., Crawford S.E., Neill F.H., Ramani S., Hill H., Ferreira J., Graham D.Y. Determination of the 50% human infectious dose for Norwalk virus. J. Infect. Dis. 2014;209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura F., Matsuyama R., Nishiura H. Estimating the asymptomatic ratio of norovirus infection during foodborne outbreaks with laboratory testing in Japan. J. Epidemiol. 2018;28:382–387. doi: 10.2188/jea.JE20170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez A., Torner N., Broner S., Bartolomé R., Guix S., De Simón M., Godoy P., Moreno A., Company M., Balanyà P.J., et al. Norovirus: A growing cause of gastroenteritis in Catalonia (Spain)? J. Food Prot. 2013;76:1810–1816. doi: 10.4315/0362-028X.JFP-12-544. [DOI] [PubMed] [Google Scholar]
- 20.Barberà E., Bartolomé R., Bosch A., Cardeñosa N., Cabedo L., Domínguez A., Ferrer M.D., Godoy P., Martínez A., Masó M., et al. Guia per a la Prevenció i Control de les Toxiinfeccions Alimentàries. 2nd ed. Generalitat de Catalunya; Barcelona, Spain: 2006. [(accessed on 26 November 2020)]. pp. 175–176. Available online: https://scientiasalut.gencat.cat/bitstream/handle/11351/1930/guia_prevencio_control_toxiinfeccions_alimentaries_2006.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 21.Wu Q.-S., Xuan Z.-L., Liu J.-Y., Zhao X.-T., Chen Y.-F., Wang C.-X., Shen X.-T., Wang Y.-X., Wang L., Hu Y. Norovirus shedding among symptomatic and asymptomatic employees in outbreak settings in Shanghai, China. BMC Infect. Dis. 2019;19:592. doi: 10.1186/s12879-019-4205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabrià A., Pintó R.M., Bosch A., Bartolomé R., Cornejo T., Torner N., Martínez A., De Simón M., Domínguez À., Guix S. Norovirus shedding among food and healthcare workers exposed to the virus in outbreak settings. J. Clin. Virol. 2016;82:119–125. doi: 10.1016/j.jcv.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Generalitat de Catalunya Decret 203/2015 de 15 de setembre, pel qual es crea la Xarxa de Vigilància Epidemiològica i es regulen els sistemes de notificació de malalties de declaració obligatòria i brots epidèmics. Diari Oficial de la Generalitat de Catalunya, Núm 6958, 1–19. [(accessed on 26 November 2020)]; Available online: https://portaldogc.gencat.cat/utilsEADOP/PDF/6958/1444533.pdf.
- 24.Institut Estadistic de Catalunya Population on 1 January. By Sex. [(accessed on 18 October 2020)]; Available online: https://www.idescat.cat/indicadors/?id=anuals&n=10328&col=1&lang=en.
- 25.Kageyama T., Kojima S., Shinohara M., Uchida K., Fukushi S., Hoshino F.B., Takeda N., Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas T., Singh A., Le Guyader F.S., La Rosa G., Saif L., McNeal M. Multiplex real-time RT-PCR for the simultaneous detection and quantification of GI, GII and GIV noroviruses. J. Virol. Methods. 2015;223:109–114. doi: 10.1016/j.jviromet.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Phillips G., Lopman B., Tam C.C., Iturriza-Gomara M., Brown D., Gray J. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect. Dis. 2009;9:63. doi: 10.1186/1471-2334-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi R., Huang Y.T., Liu J.W., Sun Y., Sun X.F., Han H.J., Qin X.R., Zhao M., Wang L.J., Li W., et al. Global prevalence of asymptomatic norovirus infection: A meta-analysis. EClinicalMedicine. 2018;2:50–58. doi: 10.1016/j.eclinm.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J.H., Kim N.Y., Lee E.J., Jeon I.S. Norovirus infections in asymptomatic food handlers in elementary schools without norovirus outbreaks in some regions of Incheon, Korea. J. Korean Med. Sci. 2011;26:734–739. doi: 10.3346/jkms.2011.26.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okabayashi T., Yokota S., Ohkoshi Y., Ohuchi H., Yoshida Y., Kikuchi M., Yano K., Fujii N. Occurrence of norovirus infections unrelated to norovirus outbreaks in an asymptomatic food handler population. J. Clin. Microbiol. 2008;46:1985–1988. doi: 10.1128/JCM.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A., Huang Q., Qin L., Zhong X., Li H., Chen R., Wan Z., Lin H., Liang J., Li J., et al. Epidemiological characteristics of asymptomatic Norovirus infection in a population from oyster (Ostrea rivularis Gould) farms in southern China. Epidemiol. Infect. 2018;146:1955–1964. doi: 10.1017/S0950268818002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo L., Gu Y., Wang X., Zhang Y., Zhan L., Liu J., Yan H., Liu Y., Zhen S., Chen X., et al. Epidemiological and clinical differences between sexes and pathogens in a three-year surveillance of acute infectious gastroenteritis in Shanghai. Sci. Rep. 2019;9:9993. doi: 10.1038/s41598-019-46480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo H.L., Ajami N., Atmar R.L., DuPont H.L. Noroviruses: The principal cause of foodborne disease worldwide. Discov. Med. 2010;10:61–70. [PMC free article] [PubMed] [Google Scholar]
- 34.Wikswo M.E., Hall A.J. Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2012;61:1–12. [PubMed] [Google Scholar]
- 35.Godoy P., Artigues A., Bartolomé R., Domínguez A., Plasència A. Norovirus gastroenteritis outbreak by person-to-person transmission in a nursing home. Med. Clin. 2006;127:538–541. doi: 10.1016/S0025-7753(06)72322-3. [DOI] [PubMed] [Google Scholar]
- 36.Park G.W., Williamson K.J., DeBess E., Cieslak P.R., Gregoricus N., De Nardo E., Fricker C., Costantini V., Vinjé J. High hand contamination rates during norovirus outbreaks in long-term care facilities. Infect. Control Hosp. Epidemiol. 2018;39:219–221. doi: 10.1017/ice.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi R., Ye C., Chen C., Yao P., Hu F., Lin Q. Norovirus prevention and the prevalence of asymptomatic norovirus infection in kindergartens and primary schools in Changzhou, China: Status of the knowledge, attitudes, behaviors, and requirements. Am. J. Infect. Control. 2015;43:833–838. doi: 10.1016/j.ajic.2015.04.182. [DOI] [PubMed] [Google Scholar]
- 38.Marsh Z., Shah M.P., Wikswo M.E., Barclay L., Kisselburgh H., Kambhampati A., Cannon J.L., Parashar U.D., Vinjé J., Hall A.J. Epidemiology of foodborne norovirus outbreaks—United States, 2009–2015. Food Saf. 2018;6:58–66. doi: 10.14252/foodsafetyfscj.2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teunis P.F.M., Sukhrie F.H.A., Vennema H., Bogerman J., Beersma M.F.C., Koopmans M.P.G. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol. Infect. 2015;143:1710–1717. doi: 10.1017/S095026881400274X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabue J.P., Meader E., Hunter P.R., Potgieter N. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J. Clin. Virol. 2016;84:12–18. doi: 10.1016/j.jcv.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]