Abstract
A cohort of 128 patients from 118 families diagnosed with non-syndromic or syndromic hearing loss (HL) underwent an exhaustive clinical evaluation. Molecular analysis was performed using targeted next-generation sequencing (NGS) with a custom panel that included 59 genes associated with non-syndromic HL or syndromic HL. Variants were prioritized according to the minimum allele frequency and classified according to the American College of Medical Genetics and Genomics guidelines. Variant(s) responsible for the disease were detected in a 40% of families including autosomal recessive (AR), autosomal dominant (AD) and X-linked patterns of inheritance. We identified pathogenic or likely pathogenic variants in 26 different genes, 15 with AR inheritance pattern, 9 with AD and 2 that are X-linked. Fourteen of the found variants are novel. This study highlights the clinical utility of targeted NGS for sensorineural hearing loss. The optimal panel for HL must be designed according to the spectrum of the most represented genes in a given population and the laboratory capabilities considering the pressure on healthcare.
Keywords: hearing loss, next-generation sequencing, genetics, molecular analysis, clinical evaluation
1. Introduction
Hearing loss (HL) is the most common sensory deficit in humans [1]. According to data from the World Health Organization, it is estimated that more than 5% of the world’s population suffers from this disease, that is, around 360 million people.
HL can be classified as conductive, sensorineural or mixed (a combination of both); acquired or hereditary; prelingual or postlingual; and non-syndromic (NSHL) or syndromic, as a part of a more complex phenotype, that account up to 30% of HL cases [2].
HL is one of the most common birth defects, with an incidence of 1–2 per 1000 newborns and growing as age increases, reaching more than 300 per 1000 in those over 75 years of age. This high incidence is due to both environmental and genetic factors. The genetic contribution to newborn HL has been reported to be 50–60% depending of the study and the population [3,4].
As the rate of acquired hearing loss secondary to environmental causes decreases, the significance of genetic factors that lead to deafness increases [5]. To date, over 120 genes have been associated with NSHL (Hereditary Hearing Loss Homepage: https://hereditaryhearingloss.org/), and over 400 syndromes have been associated with hearing impairment [6]. These genes encode proteins of a very diverse nature and are involved in different pathways, such as mechanotransduction, ear structures, ion homeostasis, etc.
Genetic confirmation of hearing loss is essential to the provision of genetic counseling, to ascertain the risk of recurrence and, in some cases, to determine the prognosis and select the best rehabilitation options. Furthermore, although the utility of molecular diagnosis is still limited for therapeutic approaches, a growing number of gene-based strategies to treat HL have been carried out in recent years at preclinical stages [7].
In the last decade, next generation sequencing (NGS), including custom targeted panels and whole exome sequencing, has revolutionized the genetic screening of disorders with high genetic and allelic heterogeneity, such as hearing loss, allowing hundreds of genes in several patients to be screened simultaneously in a short time and in a cost-effective manner.
In this study, we assess the efficacy of a home-designed panel for hearing loss in the Genetics Department of a tertiary university hospital.
2. Materials and Methods
2.1. Patients and Samples
A total of 128 patients from 118 families diagnosed with non-syndromic or syndromic HL were included in our study. Most patients were of Spanish origin, except for three patients that came from Eastern Europe, two patients that were from Maghreb, two patients that were of sub-Saharan origin and one patient that was from East Asia. Patients were recruited from September 2017 to December 2019. Most patients presented with non-syndromic hearing loss, but we also received for screening four patients with Usher syndrome (USH), two with Waardenburg syndrome (WS) and two patients with branchio-oto-renal syndrome (BOR). Patients were enrolled through the Department of Otolaryngology of the University Hospital La Fe, according to standard assistance procedures. Comprehensive clinical evaluations, imaging examination, pure-tone audiograms, auditory brainstem response and other relevant medical information were collected for the probands to characterize the type and severity of HL. All recruited patients presented sensorineural or mixed HL. Hearing loss severity was established as mild (between >25 and ≤40 dB), moderate (between >40 and ≤70 dB) or severe/profound (>70 dB).
Written informed consent was obtained from all participants or their legal guardians. This study was approved by the Hospital La Fe Ethics Committee in agreement with the Declaration of Helsinki (REV03/5/2014).
Genomic DNA (gDNA) from the patients and relatives was obtained and purified using the automated DNA extractor QIAsymphony (QIAGEN, Hombrechtikon, Switzerland). The concentration of the resulting DNA samples was determined with Nanodrop and Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA, USA)
2.2. Panel Design
We designed an NGS panel for the analysis of hereditary hearing loss using the SureDesign tool (Agilent Technologies, Santa Clara, CA, USA). The genes that were included in this panel were selected according to the prevalence reported in different studies [1,8,9,10] choosing those with the highest prevalence. Finally, the panel included the coding regions and flanking intronic regions (+/–25 bp) of 59 genes, 35 of them associated with non-syndromic HL, and 24 genes associated with syndromic HL (Table 1). The panel also included five deep intronic regions of the USH2A gene [11,12,13].
Table 1.
The Table Indicates the Genes Included in this Study and the Associated Phenotype.
| Gene | Phenotype | Gene | Phenotype |
|---|---|---|---|
| ACTG1 | NSHL | TRIOBP | NSHL |
| CEP250 | NSHL | CDH23 | USH/NSHL |
| CHD7 | CHARGE | CIB2 | USH/NSHL |
| CISD2 | NSHL | DFNB31 | USH/NSHL |
| CLDN14 | NSHL | MYO7A | USH/NSHL |
| COCH | NSHL | PCDH15 | USH/NSHL |
| DFNA5 | NSHL | USH1C | USH/NSHL |
| DFNB59 | NSHL | USH1G | USH/NSHL |
| ESPN | NSHL | EDN3 | WS |
| EYA4 | NSHL | EDNRB | WS |
| GJB2 | NSHL | MITF | WS |
| GJB6 | NSHL | PAX3 | WS |
| KCNQ4 | NSHL | SNAI2 | WS |
| LHFPL5 | NSHL | SOX10 | WS |
| LOXHD1 | NSHL | EYA1 | BOR |
| LRTOMT | NSHL | SIX1 | BOR |
| MYH9 | NSHL | SIX5 | BOR |
| MYH14 | NSHL | ADGRV1 | USH |
| MYO6 | NSHL | CLRN1 | USH |
| MYO15A | NSHL | USH2A | USH |
| OTOA | NSHL | KCNE1 | JLNS |
| OTOF | NSHL | KCNQ1 | JLNS |
| OTOG | NSHL | COL11A2 | Stickler/NSHL |
| OTOGL | NSHL | SEMA3E | CHARGE |
| POU3F4 | NSHL | SLC26A4 | Pendred/NSHL |
| PTPRQ | NSHL | WFS1 | WF/NSHL |
| SMPX | NSHL | chr1:215827262-215827362 | USH |
| STRC | NSHL | chr1:215967733-215967833 | USH |
| TECTA | NSHL | chr1:216039671-216039771 | USH |
| TIMM8A | NSHL | chr1:216064520-216064560 | USH |
| TMC1 | NSHL | chr1:216247426-216247526 | USH |
| TMPRSS3 | NSHL | ||
| TPRN | NSHL |
NSHL: Non-syndromic hearing loss, USH: Usher syndrome, WS: Waardenburg syndrome, BOR: BOR syndrome, JLNS: Jervell and Lange-Nielsen syndrome, Stickler: Stickler syndrome, CHARGE: Charge syndrome, Pendred: Pendred syndrome, WF: Wolfram syndrome.
We tried to include some extra probes for the regions of ESPN, OTOA and STRC genes showing high homology with their pseudogenes, in addition to the default probes generated by the SureDesign software (Agilent Technologies, Santa Clara, CA, USA). Three extra probes were designed and included for ESPN (chr1:6500314-6500500, chr1:6500686-6500868, chr1:6505724-6505995) and seven for OTOA (chr16:21742158-21742251, chr16:21752042-21752229, chr16:21756202-21756357, chr16:21763256-21763398, chr16:21763690-21763826, chr16:21768403-21768598, chr16:21771791-21772050). However, bioinformatic tools failed to design extra probes for STRC, due to the fact that STRC is 99.6% identical to its pseudogene (pSTRC).
2.3. Library Preparation and Sequencing
The library preparation was carried out according to the Bravo NGS SureSelectQXT Automated Target Enrichment protocol (Agilent Technologies, Santa Clara, CA, USA) for Illumina Multiplexed Sequencing. Sequencing analysis was performed sequentially in batches of 16 patients. The libraries were sequenced on a MiSeq instrument with a MiSeq v2 300 cycle reagent kit (Illumina, San Diego, CA, USA).
2.4. Data Analysis
The resulting sequencing data were analyzed with the Alissa software tool (Agilent Technologies, Santa Clara, CA, USA) in regard to the human assembly GRCh37/hg19. This software performs the alignment, variant calling and annotation of the variants. The annotated variants were filtered according to a minor allele frequency (MAF) value ≤ 0.02 (the frequency of the variants was explored in the Exome Aggregation Consortium (ExAC) database, genomeAD (https://gnomad.broadinstitute.org/) and 1000 genomes (https://www.internationalgenome.org/). To classify the variants, we also took into account their annotation in the dbSNP (www.ncbi.nlm.nih.gov/SNP/), their description in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), Varsome (https://Varsome.com/), HGMD (http://www.hgmd.cf.ac.uk/), LOVD (https://www.lovd.nl/) and Deafness Variation Database (http://deafnessvariationdatabase.org/) and the variant type. Novel missense variants were evaluated with the predictors included in the Varsome website and Alissa software: BayesDel_addAF, DANN, DEOGEN2, EIGEN, FATHMM-MKL, M-CAP, MVP, MutationAssessor, MutationTaster, REVEL and SIFT.
To predict the potential effect of the variants on the splicing, we used the bioinformatic tools MaxEnt and Splice AI.
Sanger sequencing (BigDye Terminator kit v1.1, Applied Biosystems, Carlsbad, CA, USA) was carried out to validate the pathogenic and likely pathogenic point variants and to perform segregation analysis when patients’ relatives were available.
To detect copy number variations (CNVs), we carried out an analysis using the DECoN v1.0.2 program [14], which is a tool that detects variants in copy number from aligned sequences based on the number of reads for each position. The CNVs obtained by this program were checked using the multiplex ligation-dependent probe amplification technique (MLPA): OTOA + STRC (P461 salsa) (MRC-Holland, Amsterdam, The Netherlands). Deletions previously described to affect the DFNB1 locus were confirmed by multiplex PCR [15]. These MLPA reagents were also performed in patients with only one pathogenic variant detected in a gene with (autosomal recessive) AR inheritance.
3. Results
We aimed to obtain a median read depth greater than 100×. Coverages obtained were around 150×–200×, and 98% of analyzable target regions were covered by at least 20 reads. However, some regions of 3 genes with homologous pseudogenes (ESPN, OTOA and especially STRC) were not well covered. These regions are detailed in Table S1.
We detected the variant(s) responsible for the disease in 47 out of 118 families (40%), 27 with an AR inheritance pattern, 18 with AD and 2 with an X-linked pattern (Table 2). Detailed clinical data from the diagnosed patients are shown in Table 2.
Table 2.
Disease Causing Variants Detected and Clinical Data of the Diagnosed Patients.
| (A) Patients Diagnosed with Autosomal Recessive Deafness | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Patient | Sex | Age | Diagnosis | Gene | Allele 1 | Allele 2 | Phenotype | ||||
| 1 | 33311 | M | 1 | NSHL |
GJB2 NM_004004.5 |
c.35del/p.(Gly12Valfs *2) [16] |
c.35del/p.(Gly12Valfs *2) [16] |
SNHL, bilateral, symmetrical, prelingual, severe, stable | ||||
| 2 | 35961 | F | 2 | NSHL |
GJB2 NM_004004.5 |
c.35del/p.(Gly12Valfs *2) [16] |
c.35del/p.(Gly12Valfs *2) [16] |
SNHL, bilateral, symmetrical, prelingual, moderate, stable | ||||
| 3 | 39026 | F | 0 | NSHL |
GJB2 NM_004004.5 |
c.35del/p.(Gly12Valfs *2) [16] |
c.35del/p.(Gly12Valfs *2) [16] |
SNHL, bilateral, symmetrical, prelingual, severe-profound, stable | ||||
| 4 | 39611 | F | 5 | NSHL |
GJB2 NM_004004.5 |
c.596C > T/p.(Ser199Phe) [17] |
c.35del/p.(Gly12Valfs *2) [16] |
SNHL, bilateral, symmetrical, postlingual, severe, stable | ||||
| 5 | 40372 | M | 5 | NSHL |
GJB2 NM_004004.5 |
c.617A > G/p.(Asn206Ser) [18] |
c.269dup/p.(Val91Serfs *11) [19] |
SNHL, bilateral, symmetrical, postlingual, mild–moderate, stable | ||||
| 6 | 42105 | M | 0 | NSHL |
GJB2 NM_004004.5 |
c.101T > C/p.(Met34Thr) [20] |
c.427C > T/p.(Arg143Trp) [21] |
SNHL, bilateral, symmetrical, prelingual, moderate, stable | ||||
| 7 | 28981 | M | 0 | NSHL |
GJB2 NM_004004.5 |
c.35del/p.(Gly12Valfs *2) [16] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | |||||
|
GJB6 NM_001110219.2 |
del(GJB6-D13S1830) [22] |
|||||||||||
| 8 | 34307 | M | 0 | NSHL |
GJB2 NM_004004.5 |
c.269dup/p.(Val91Serfs *11) [19] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | |||||
|
GJB6 NM_001110219.2 |
del(GJB6-D13S1830) [22] |
|||||||||||
| 9 | 37468 | M | 1 | NSHL |
GJB2 NM_004004.5 |
c.617A > G/p.(Asn206Ser) [18] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | |||||
|
GJB6 NM_001110219.2 |
del(GJB6-D13S1830) [22] |
|||||||||||
| 10 | 37439 | F | 5 | NSHL |
STRC NM_153700.2 |
Whole gene deletion (15q15) [23] |
Whole gene deletion (15q15) [23] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | ||||
|
GJB2 NM_004004.5 |
c.101T > C/p.(Met34Thr) [20] |
|||||||||||
| 11 | 33416 | F | 7 | NSHL |
STRC NM_153700.2 |
Whole gene deletion (15q15) [23] |
Whole gene deletion(15q15) [23] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | ||||
| 12 | 37112 | M | 4 | NSHL |
STRC NM_153700.2 |
Whole gene deletion (15q15) [23] |
Whole gene deletion (15q15) [23] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | ||||
| 13 | 31410 | M | 5 | NSHL |
OTOF NM_194248.2 |
c.4275G > A/p.(Trp1425 *) [24] |
c.2485C > T/p.(Gln829 *) [25] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | ||||
| 14 | 40184 | F | 0 | NSHL |
OTOF NM_194248.2 |
c.2485C > T/p.(Gln829 *) [25] |
c.2485C > T/p.(Gln829 *) [25] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | ||||
| 14 | 41793 | F | 0 | NSHL |
OTOF NM_194248.2 |
c.2485C > T/p.(Gln829 *) [25] |
c.2485C > T/p.(Gln829 *) [25] |
SNHL, bilateral, symmetrical, prelingual, profound, stable | ||||
| 15 | 34197 | F | 54 | NSHL |
LOXHD1 NM_144612.6 |
c.3419dup/p.(Leu1140Phefs *5) | c.3419dup/p.(Leu1140Phefs *5) | SNHL, bilateral, symmetrical, postlingual, moderate–severe, stable | ||||
| 16 | 34865 | M | 7 | NSHL |
LOXHD1 NM_144612.6 |
c.4480C > T/p.(Arg1494 *) [26] |
c.4480C > T/p.(Arg1494 *) [26] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | ||||
| 17 | 29440 | M | 33 | NSHL |
OTOA NM_144672.3 |
c.877C > T/p.(Gln293 *) | Whole gene deletion (16q12.2 region) [27] |
SNHL, bilateral, postlingual, moderate, stable | ||||
| 18 | 37140 | M | 4 | NSHL |
OTOA NM_144672.3 |
Whole gene deletion (16q12.2 region) [27] |
Whole gene deletion (16q12.2 region) [27] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | ||||
| 19 | 29865 | F | 46 | NSHL |
TMPRSS3 NM_024022.2 |
c.1276G > A/p.(Ala426Thr) [28] |
c.1159G > A/p.(Ala387Thr) [29] |
SNHL, bilateral, symmetrical, postlingual, mild–moderate, progressive | ||||
| 19 | 38198 | F | 40 | NSHL |
TMPRSS3 NM_024022.2 |
c.1276G > A/p.(Ala426Thr) [28] |
c.235T > C/p.(Cys79Arg) | SNHL, bilateral, symmetrical, postlingual, profound, progressive | ||||
| 20 | 42108 | F | 1 | NSHL |
MYO15A NM_016239.3 |
c.8968-1G > T [30] |
c.8968-1G > T [30] |
SNHL, bilateral, symmetrical, prelingual, severe, stable | ||||
| 21 | 37513 | M | 4 | NSHL/EVA |
SLC26A4 NM_000441.1 |
c.1540C > A/p.(Gln514Lys) [31] |
c.1540C > A/p.(Gln514Lys) [31] |
SNHL, bilateral, asymmetrical, postlingual, Right: profound Left: moderate, stable, EVA | ||||
| 22 | 36777 | F | 1 | NSHL |
OTOG NM_001277269.1 |
c.2140dup/p.(Ser714Lysfs *22) | c.2140dup/p.(Ser714Lysfs *22) | SNHL, bilateral, symmetrical, prelingual, moderate, stable | ||||
| 23 | 39949 | F | 18 | NSHL |
TECTA NM_005422.2 |
c.4055G > A/p.(Cys1352Tyr) [32] |
c.4055G > A/p.(Cys1352Tyr) [32] |
SNHL, bilateral, moderate | ||||
|
MYO7A NM_000260.3 |
c.5648G > A/p.(Arg1883Gln) [33] |
|||||||||||
| 24 | 40453 | F | 40 | NSHL |
MYO7A NM_000260.3 |
c.1232T > C/p.(Val411Ala) [34] |
c.6025del/p.(Ala2009Profs *32) [35] |
SNHL, bilateral, symmetrical, postlingual, mild, stable | ||||
| 25 | 27862 | M | 30 | USH |
ADGRV1 NM_032119.3 |
c.12528-1G > T [36] |
c.17933A > G/p.(His5978Arg) [37] |
SNHL, congenital, moderate, retinitis pigmentosa |
||||
| 26 | 30816 | F | 1 | USH |
CDH23 NM_022124.5 |
c.310G > T/p.(Glu104*) | c.2289 + 1G > A [38] |
SNHL, bilateral, symmetrical, prelingual, profound, stable, bilateral, vestibular areflexia, retinitis pigmentosa |
||||
| 27 | 27734 | F | 49 | USH | USH2ANM_206933.2 | c.9799T > C/p.(Cys3267Arg) [39] |
c.9676C > T/p.(Arg3226 *) [40] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable, retinitis pigmentosa |
||||
| (B) Patients Diagnosed with Autosomal Dominant Deafness | ||||||||||||
| Family | Patient | Sex | Age | Diagnosis | Gene | Allele 1 | Allele 2 | Phenotype | ||||
| 28 | 32954 | M | 46 | NSHL |
MYO6 NM_004999.3 |
c.2545C > T/p.(Arg849 *) [41] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
| 28 | 32955 | F | 15 | NSHL |
MYO6 NM_004999.3 |
c.2545C > T/p.(Arg849 *) [41] |
SNHL, bilateral, symmetrical, moderate, stable | |||||
| 29 | 35197 | F | 37 | NSHL |
MYO6 NM_004999.3 |
c.1666C > T/p.(Arg556 *) | SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
| 30 | 40488 | F | 30 | NSHL |
MYO6 NM_004999.3 |
c.1224-9del | SNHL, bilateral, asymmetrical, postlingual, severe–profound, progressive | |||||
| 31 | 31110 | M | 42 | NSHL |
MYO6 NM_004999.3 |
c.1674 + 1G > A | SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
| 31 | 36163 | M | 32 | NSHL |
MYO6 NM_004999.3 |
c.1674 + 1G > A | SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
|
ESPN NM_031475.2 |
c.2467C > T/p.(Gln823 *) | |||||||||||
| 32 | 29272 | M | 46 | NSHL |
MYO6 NM_004999.3 |
c.2751dup/p.(Gln918Thrfs *24)[42] | SNHL, postlingual | |||||
|
ESPN NM_031475.2 |
c.2230G > A/p.(Asp744Asn) [43] |
|||||||||||
| 32 | 41950 | F | 61 | NSHL |
MYO6 NM_004999.3 |
c.2751dup/p.(Gln918Thrfs *24) [42] |
SNHL, postlingual | |||||
|
ESPN NM_031475.2 |
c.2230G > A/p.(Asp744Asn) [43] |
|||||||||||
| 33 | 41268 | M | 18 | NSHL |
MYO6 NM_004999.3 |
c.494T > G/p.(Leu165Arg) | SNHL, bilateral, symmetrical, postlingual, profound, progressive, tinnitus | |||||
|
MYO7A NM_000260.3 |
c.1997G > A/p.(Arg666Gln) [44] |
c.3527G > A/p.(Ser1176Asn) [8] |
||||||||||
| 34 | 33945 | M | 3 | NSHL |
TECTA NM_005422.2 |
c.5668C > T/p.(Arg1890Cys) [45] |
SNHL, bilateral, asymmetrical prelingual, stable | |||||
| 35 | 35453 | M | 2 | NSHL |
TECTA NM_005422.2 |
c.5383 + 5_5383 + 8del [46] |
SNHL, bilateral, symmetrical, prelingual, moderate, stable | |||||
| 36 | 38971 | F | 0 | NSHL |
TECTA NM_005422.2 |
c.5509T > G/p.(Cys1837Gly) [47] |
SNHL, bilateral, symmetrical, prelingual, moderate, stable | |||||
| 36 | 39927 | F | 0 | NSHL |
TECTA NM_005422.2 |
c.5509T > G/p.(Cys1837Gly)[47] | SNHL, unilateral, asymmetrical, prelingual, moderate–severe, progressive | |||||
| 37 | 4293 | M | 6 | NSHL |
COL11A2 NM_080680.2 |
c.1748G > A/p.(Gly583Asp) | SNHL, bilateral, symmetrical, postlingual, stable | |||||
| 37 | 31449 | M | 35 | NSHL |
COL11A2 NM_080680.2 |
c.1748G > A/p.(Gly583Asp) | SNHL, bilateral, asymmetrical, postlingual, Right: mild–moderate; Left: moderate–severe, stable | |||||
| 38 | 35238 | M | 6 | NSHL/Stickler |
COL11A2 NM_080680.2 |
c.4392 + 1G > A [48] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable, flattened facial profile, sunken nasal root, short nose with anteverted nostrils, osteorticular problems | |||||
| 38 | 42783 | F | 37 | NSHL/Stickler |
COL11A2 NM_080680.2 |
c.4392 + 1G > A [48] |
SNHL, flattened facial profile, osteorticular problems and maxillofacial alterations |
|||||
| 39 | 40431 | M | 5 | NSHL |
WFS1 NM_006005.3 |
c.1463_1474dup/p.(Val491_Pro492insLeuIleThrVal) | SNHL, bilateral, asymmetrical, postlingual, Right: profound Left: severe, progressive | |||||
| 40 | 42125 | F | 5 | NSHL |
WFS1 NM_006005.3 |
c.2108G > A/p.(Arg703His) [49] |
SNHL, bilateral, symmetrical, postlingual, severe–profound, progressive | |||||
| 41 | 36655 | M | 7 | NSHL |
KCNQ4 NM_004700.3 |
c.857A > G/p.(Tyr286Cys) [50] |
SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
| 41 | 44138 | M | 46 | NSHL |
KCNQ4 NM_004700.3 |
c.857A > G/p.(Tyr286Cys) [50] |
||||||
| 42 | 39490 | F | 45 | NSHL |
ACTG1 NM_001199954.1 |
c.895C > G/p.(Leu299Val) [29] |
SNHL, bilateral, asymmetrical, postlingual, right: moderate left: severe, progressive | |||||
| 43 | 40519 | M | 40 | NSHL |
EYA4 NM_004100.4 |
c.988C > T/p.(Gln330 *) [51] |
SNHL, bilateral, asymmetrical, postlingual, right: profound left: severe, progressive, tinnitus, decrease in size of both cochlear nerves |
|||||
| 44 | 12227 | M | 34 | WS |
MITF NM_198159.2 |
c.943C > T/p.(Arg315 *) [52] |
HL, prelingual, White forelock, Heterochromia iridis | |||||
|
GJB6 NM_001110219.2 |
del(GJB6-D13S1830) [22] |
|||||||||||
| 45 | 37350 | M | 2 | BOR |
EYA1 NM_000503.5 |
c.1540_1542del/p.(Leu514del) [53] |
Mixed HL, bilateral, symmetrical, prelingual, severe, stable, 2nd branchial arch fistula, facial dysmorphia |
|||||
| (C) Patients Diagnosed with X-Linked Deafness | ||||||||||||
| Family | Patient | Sex | Age | Diagnosis | Gene | Allele 1 | Allele 2 | Phenotype | ||||
| 46 | 34796 | M | 1 | NSHL |
POU3F4 (XLR) NM_000307.4 |
c.977T > C/p.(Phe326Ser) | Mixed HL, bilateral symmetrical, prelingual, moderate, stable, bilateral corkscrew cochlea, incomplete splitting of turns, absence of meatus and stapes fixation |
|||||
| 47 | 14285 | M | 3 | NSHL |
SMPX (XLD) NM_014332.2 |
c.20del/p.(Pro7Glnfs *74) | SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
| 47 | 41863 | F | 30 | NSHL |
SMPX (XLD) NM_014332.2 |
c.20del/p.(Pro7Glnfs *74) | SNHL, bilateral, symmetrical, postlingual, moderate, stable | |||||
The table indicates the patient and family code, sex, age (indicated in years), diagnosis, mutated gene, variants and phenotype. The variants described in the table are pathogenic or probably pathogenic, and novel variants are marked in bold. M: male, F: female, NSHL: non-syndromic hearing loss, NHL: sensorineural hearing loss, HL: hearing loss, EVA: enlarged vestibular aqueduct, USH: Usher syndrome, WS: Waardenburg syndrome, BOR: branchio–oto–renal, XLR: recessive X-linked, XLD: dominant X-linked.
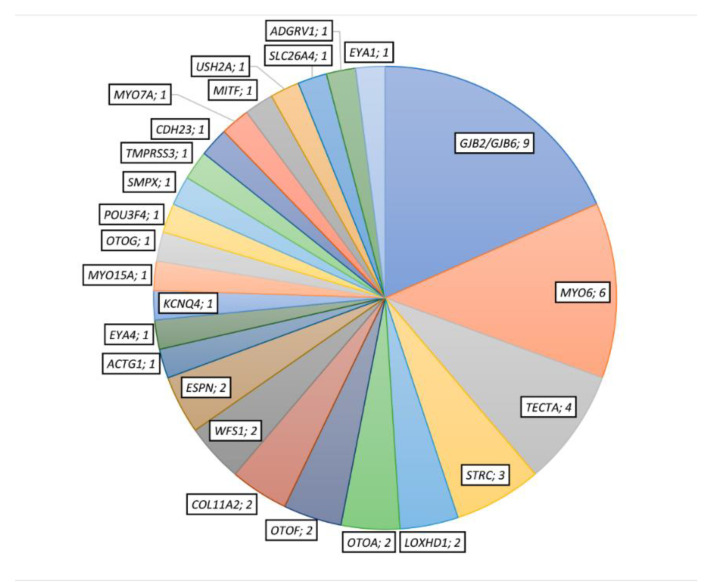
We identified candidate variants in 26 different genes, 15 with AR inheritance pattern, 9 with AD and 2 with an X-linked pattern (Figure 1). Among the 54 different candidate variants detected, 24 were missense, 7 frameshift, 11 nonsense, 2 inframe ins/del, 3 CNVs and 7 affected to the splice-site. Fourteen out of 54 variants were novel (Table 2 and Table 3).
Figure 1.
Number of diagnosed patients with putative disease-responsible variants in each represented gene.
Table 3.
Classification of Novel Variants Identified in this Study.
| Variant | Frequency | Pathogenicity Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Nucleotide | Protein | Classification | GnomAD Exomes | GnomAD Genomes | Deafness Variation Database | Missense Pathogenicity Scores | Conservation Score (GERP) | MaxEnt |
|
LOXHD1 NM_144612.6 |
c.3419dup | p.(Leu1140Phefs *5) | Pathogenic | 0.0000267 | NF | NF | NA | 5.05 | - |
|
OTOA NM_144672.3 |
c.877C > T | p.(Gln293 *) | Pathogenic | NF | NF | NF | NA | 5.41 | - |
|
TMPRSS3 NM_024022.2 |
c.235T > C | p.(Cys79Arg) | Likely Pathogenic | NF | NF | NF | 11/13 | 5.23 | - |
|
OTOG NM_001277269.1 |
c.2140dup | p.(Ser714Lysfs *22) | Pathogenic | NF | NF | NF | NA | 4.9 | - |
|
CDH23 NM_022124.5 |
c.310G > T | p.(Glu104 *) | Pathogenic | NF | NF | NF | NA | 5.43 | - |
|
MYO6 NM_004999.3 |
c.1666C > T | p.(Arg556 *) | Pathogenic | 0.0000119 | NF | Unknown significance–Impact High | NA | 5.77 | - |
|
MYO6 NM_004999.3 |
c.1224-9del | - | VUS | NF | NF | NF | NA | 5.23 | AS broken (from 7.08 to −4.37) |
|
MYO6 NM_004999.3 |
c.1674 + 1G > A | - | Pathogenic | 0.00000736 | NF | Unknown significance-Impact High | NA | 5.77 | DS broken (from 7.94 to −0.24) |
|
ESPN NM_031475.2 |
c.2467C > T | p.(Gln823 *) | Pathogenic | NF | NF | NF | NA | 4.28 | - |
|
MYO6 NM_004999.3 |
c.494T > G | p.(Leu165Arg) | VUS | NF | NF | NF | 13/13 | 5.45 | - |
|
COL11A2 NM_080680.2 |
c.1748G > A | p.(Gly583Asp) | Likely Pathogenic | NF | NF | NF | 11/11 | 3.89 | - |
|
WFS1 NM_006005.3 |
c.1463_1474dup | p.(Val491_Pro492insLeuIleThrVal) | VUS | NF | NF | NF | NA | 4.25 | - |
|
POU3F4 NM_000307.4 |
c.977T > C | p.(Phe326Ser) | Likely Pathogenic | NF | NF | NF | 10/10 | 5.07 | - |
|
SMPX NM_014332.2 |
c.20del | p.(Pro7Glnfs *74) | Pathogenic | NF | NF | NF | NA | 5.78 | - |
NF: not found; NA: not available; AS: acceptor splice-site; DS: donor splice-site. “Classification”: Variants are classified according to the guidelines of the ACMG [54].“Pathogenicity Scores” refer to the number of in silico tools that classify the variant as pathogenic/likely pathogenic versus the total of predictors used. The scores were obtained from https://Varsome.com/ (accessed November 2020) and included the followings predictors: BayesDel_addAF, DANN, DEOGEN2, EIGEN,FATHMM-MKL, LIST-S2, M-CAP, MVP, MutationAssessor, MutationTaster, PrimateAI, REVEL and SIFT. Not all predictors were available for all analyzed variants. GERP is a conservation score. The values range from −12.3 to 6.17, with 6.17 being the most conserved.
3.1. Autosomal Recessive HL
Twenty-nine cases belonging to 27 families carried biallelic pathogenic or likely pathogenic variants associated with an autosomal recessive pattern of inheritance (Table 2A).
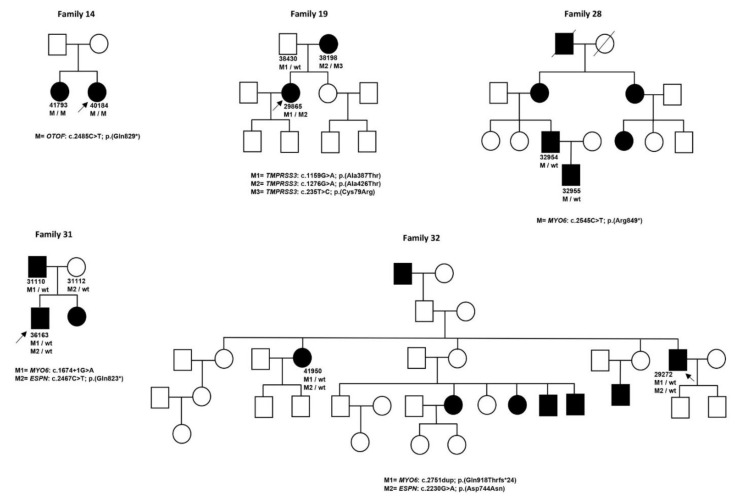
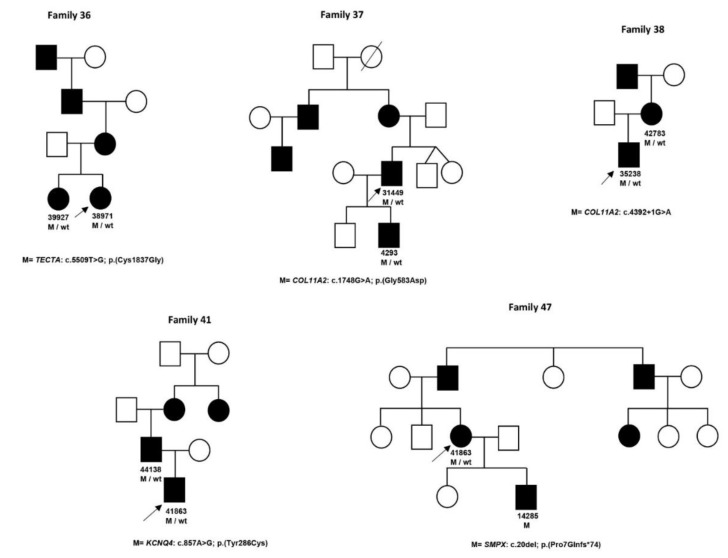
Twenty-six cases presented with NSHL. These were linked to GJB2/GJB6 (DFNB1) (nine cases), STRC (three cases), OTOF (three cases belonging to two families), LOXHD1 (two cases), OTOA (two cases), TMPRSS3 (two cases belonging to one family) and one case in the MYO15A, SLC26A4, OTOG, TECTA and MYO7A genes (Figure 1). Family trees for families with more than one affected patient are displayed in Figure 2.
Figure 2.
Pedigrees of the families and segregation analysis of the detected pathogenic or likely pathogenic variants. Arrows indicate the proband case, M indicates the pathogenic or likely pathogenic variant and wt indicates wild type sequence.
The remaining three solved cases suffered from Usher syndrome due to putative pathogenic variants in ADGRV1, CDH23 and USH2A, one family for each gene.
The most prevalent variants found were c.35del (GJB2) and del (GJB6-D13S1830), both affecting DFNB1 locus, followed by the complete deletion of the STRC gene. In all cases, the deletion of STRC was associated with mild to moderate postlingual hearing loss.
Five of the detected pathogenic variants were novel. Four of them produced a premature stop codon: three frameshift variants (c.3419dup/p.(Leu1140Phefs *5) in LOXHD1, c.877C > T/p.(Gln293 *) in OTOA and c.2140dup/p.(Ser714Lysfs *22) in OTOG) and one nonsense variant (c.310G > T/p.(Glu104 *) in CDH23). The only novel missense variant detected was c.235T > C/p.(Cys79Arg) in TMPRSS3.
3.2. Autosomal Dominant HL
We identified variants responsible for the disease associated with an autosomal dominant pattern of inheritance in 25 patients belonging to 18 families (Table 2B).
Twenty-four of these patients had been referred as non-syndromic HL. Nine patients belonging to six families presented variants in MYO6, four patients from three families in TECTA, four patients from two families in COL11A2, two patients from two families in WFS1 and two patients from the same family in KCNQ4; pathogenic variants in ACTG1 and EYA4 were detected in one patient each (Table 2B and Figure 2).
One of the families linked to COL11A2 (family 38) was found to present the pathogenic variant c.4392 + 1G > A, previously described by Brunner et al. (1994) [48] as associated with Stickler syndrome without eye affectation. This family was clinically re-evaluated and re-classified as Stickler syndrome.
Additionally, we also detected pathogenic variants in two families with syndromic hearing loss. We found the variants responsible for the disease in one patient diagnosed with Waardenburg syndrome, presenting the variant responsible for the disease in MITF, and one patient diagnosed with BOR syndrome was found to present with the pathogenic variant in EYA1.
No prevalent pathogenic variants associated with an autosomal dominant (AD) pattern of inheritance was detected. Seven of the AD pathogenic variants identified in the present study were novel. One novel stop codon (c.1666C > T/p.(Arg556 *)) was detected in MYO6. Two splicing variants, none previously described, were detected; one of them was located at a canonical site (c.1674 + 1G > A in MYO6), and the other was located at c.1224-9del in MYO6. Furthermore, an in-frame novel duplication was found in WFS1, c.1463_1474dup/p.(Val491_Pro492insLeuIleThrVal) and two missenses variants in COL11A2 (c.1748G > A/p.(Gly583Asp)) and MYO6 (c.494T > G/p.(Leu165Arg)) (Table 3).
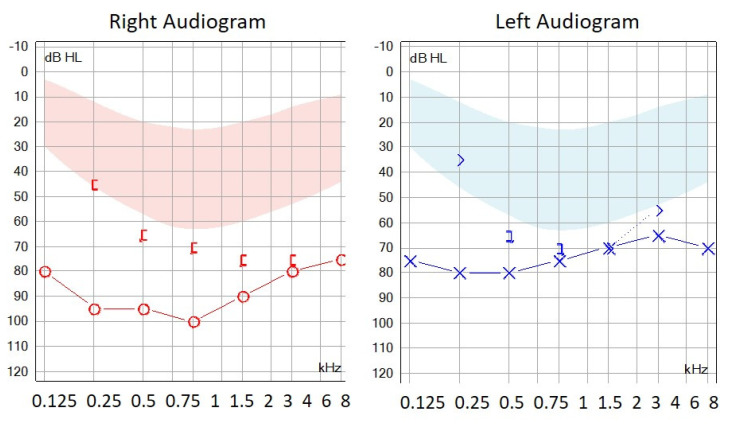
The audiogram of patient 40431, harboring the c.1463_1474dup/p.(Val491_Pro492insLeuIleThrVal) variant, showed a characteristic profile with severe threshold increases for low-frequency tones (Figure 3).
Figure 3.
Audiogram performed in patient 40431 harboring the c.1463_1474dup/p. (Val491_Pro492insLeuIleThrVal) variant in the WFS1 gene.
3.3. X-Linked HL
Variants responsible for the disease associated with an X-linked pattern of inheritance were found in three cases belonging to two families (Table 2C). One case presented a novel missense variant in POU3F4 (recessive X-linked) and the other two cases were a boy and his mother, both carrying a novel frameshift variant in SMPX (dominant X-linked) (Figure 2 and Table 3).
3.4. Partially Diagnosed Patients
In 11 patients we detected one or several pathogenic variants in the heterozygous state in genes with an AR inheritance pattern. In seven cases we identified a pathogenic variant in only one gene: USH2A (2), GJB2 (2), STRC (1), OTOF (1) and CDH23 (1). In four patients we detected pathogenic variants in several different genes (Table 4)
Table 4.
Patients with only One Heterozygous Pathogenic or Likely Pathogenic Variant in Genes Associated with an Autosomal Recessive Inheritance Pattern.
| Patient | Diagnosis | Gene | Allele 1 |
|---|---|---|---|
| 40056 | NSHL |
USH2A NM_206933.2 |
c.4325T > C/p.(Phe1442Ser) [55] |
| 31443 | USH |
USH2A NM_206933.2 |
c.2431_2432del/p.(Lys811Aspfs*11) [35] |
| 28523 | NSHL |
USH2A NM_206933.2 |
c.2135del/p.(Ser712*) [56] |
|
MYO7A NM_000260.3 |
c.5581C > T/p.(Arg1861*) [57] |
||
| 37248 | NSHL |
USH2A NM_206933.2 |
c.9244A > G/p.(Ile3082Val) [58] |
|
GJB2 NM_004004.5 |
c.109G > A/p.(Val37Ile) [59] |
||
| 37986 | NSHL |
GJB2 NM_004004.5 |
c.269T > C/(p.Leu90Pro) [19] |
| 39353 | NSHL |
GJB2 NM_004004.5 |
c.445G > A/p.(Ala149Thr) [60] |
| 12228 | NSHL |
STRC NM_153700.2 |
Complex rearrangement |
| 28358 | NSHL |
OTOF NM_194248.2 |
c.2485C > T/p.(Gln829*) [25] |
| 35862 | NSHL |
OTOF NM_194248.2 |
c.2485C > T/p.(Gln829*) [25] |
|
CDH23 NM_022124.5 |
c.4762C > T/p.(Arg1588Trp) [61] |
||
| 33335 | USH |
CDH23 NM_022124.5 |
c.2289 + 1G > A [38] |
| 34978 | NSHL |
TMC1 NM_138691.2 |
c.1763 + 3A > G [62] |
|
TMPRSS3 NM_024022.2 |
c.280G > A/p.(Gly94Arg) [29] |
NSHL: non-syndromic hearing loss, USH: Usher syndrome. Novel variants are marked in bold.
4. Discussion
The genetic diagnosis of hereditary hearing loss is highly difficult due to its enormous underlying genetic heterogeneity (more than 120 genes described up to date), which is a reflection of the high complexity of the ear structure and organization.
In the last 10 years (from 2006 to 2016), the genetic analysis of patients with hearing loss in our tertiary hospital was restricted to detect the most frequent pathogenic variants responsible for hereditary sensorineural hearing loss in Spain, specifically the complete coding sequence of the GJB2 gene, the deletions D13S1830 and delD13S1854 in the GJB6 gene and the OTOF p.Q829X variants. The implementation of our custom NGS panel containing 59 HL genes improved the management of our patients, as it has allowed us to detect putative pathogenic variants in 26 different genes. Furthermore, we have been able to genetically diagnose syndromic cases suffering from Deafness Infertility syndrome, Usher syndrome, Stickler syndrome, Waardenburg syndrome and BOR syndrome.
However, pathogenic variants in a few genes still explain a great number of hearing loss cases. The main example is GJB2, encoding connexin 26. Pathogenic variants in this gene are the most common cause of hereditary hearing loss in many populations [63]. In the present work, biallelic variants in GJB2, together with GJB6 (DFNB1 locus), were responsible for the disease in nine families with AR inheritance, followed by pathogenic variants in STRC (three AR families). Regarding AD inheritance families, heterozygous pathogenic variants in MYO6 were found in six families, followed by pathogenic variants in TECTA (four AD families). An additional patient presented a homozygous AR pathogenic variant in TECTA. All inheritance patterns have been described for HL: recessive, dominant, X-linked and mitochondrial. In some genes (like MYO6, TECTA or ESPN), a group of variants follow a dominant inheritance pattern, whereas others follow a recessive inheritance pattern, complicating the interpretation of genetic analysis [64]. Another feature that complicates the genetic studies of HL is the existence of some pseudogenes with high homology to some prevalent genes (like STRC, OTOA or ESPN). In the panel design, we tried to include some extra probes for the regions of these genes showing high homology with their pseudogenes, in addition to the default probes generated by SureDesign. However, low coverage was still obtained, and those point variants suspected to be pathogenic had to be confirmed by Sanger sequencing using primers specifically designed to hybridize only with the gene, not the pseudogene [65,66].
When a CNV affecting STRC or OTOA was suspected after DECoN v1.0.2 analysis, its presence was confirmed by MLPA using SALSA P461 (MRC Holland).
Several pathogenic variants identified in this study are reported in a large number of studies, suggesting a high prevalence. The pathogenic variant in OTOF c.2485C > T/p.(Gln829 *) is the third most frequent in the Spanish population that causes prelingual hearing loss [67], and STRC deletions are the second most frequent cause of mild-to-moderate hearing loss after the DFNB1 locus [68]. The variant c.1540C > A/p.(Gln514Lys) is the most frequent variant in SLC26A4 in the Spanish population, described in more than 36 Spanish families to date [69]. Furthermore, the pathogenic change c.9799T > C/p.(Cys3267Arg) in USH2A is one of the most frequent variants in the Spanish population, specifically the third most common cause of Usher syndrome [70,71]. Finally, the pathogenic variant c.5668C T/p.(Arg1890Cys) that affects the TECTA gene has been described in some families from Spain, America and The Netherlands. In the most unrelated families, patients present the same haplotype, which suggests that the variant is derived from a common ancestor (founder effect) [46].
Nowadays, all known HL genes can be simultaneously analyzed thanks to the technological development of NGS. Even so, the rate of genetic diagnosis using NGS in patients with hearing loss varies around 40–60% [8,29,64,72,73,74,75,76] depending on many factors: the degree of HL (profound, severe, moderate), age of HL onset, the existence of family history, the ethnic origin or the number of genes contained in the NGS panel. The highest rates have usually been obtained for patients with a positive family history or when the HL was congenital and symmetric [8]. In the present work, the global diagnostic yield was 40%. This is a satisfactory yield, since our custom NGS panel included a limited number of genes (59), and the exclusion criteria for the genetic testing was very lax. Thus, the analyzed patient sample was very heterogeneous, including all types of sensorineural/mixed hearing loss (congenital, prelingual and postlingual; mild, moderate, severe and profound; and stable and progressive) with ages ranging from 0 to 61 years.
4.1. Novel VUS/Likely Pathogenic Variants
The development of NGS has revolutionized the field of genetic diagnosis, especially in extremely genetically heterogeneous diseases, such as hereditary HL. However, an elevated number of genetic variants of uncertain clinical significance (VUS) has been detected using this technology [77]. Variants predicted to generate direct stop codons or changes in the reading frame of the proteins and variants located at canonical splice sites (+/–1 and +/–2 positions of introns) are usually classified as pathological for proteins for which loss of function is reported as cause of the disease. However, the interpretation of missense, isocoding and intronic variants located out of canonical splice sites is more complex, and many times these variants remain classified as VUS. In these cases, bioinformatics predictions, segregation analyses or functional studies are required to infer the pathological character of these variants. In our study, a lot of a priori VUS variants were detected, and only seven of them were classified as likely pathogenic based upon bioinformatics predictions and/or segregation analyses: four missense, one intronic variant and one in-frame duplication.
Missense variants: The c.235T > C/p.(Cys79Arg) change in TMPRSS3 was not found in gnomAD exomes/genomes, and 11 computational programs predicted it as pathogenic in Varsome. Furthermore, it was found in trans with other previously described pathogenic variants in the TMPRSS3 (see Table 2 and Figure 2). The MYO6 (c.494T > G/p.(Leu165Arg)) variant was found in patient 41268. He was referred to as AD non-syndromic hearing loss, being her mother, her sister and her sister´s son were also affected. Although this variant was classified as VUS following the ACMG guidelines, we should not rule it out since it was not found in healthy control databases, had a high conservation score and showed a pathogenic computational verdict based on 13 pathogenic predictions. The COL11A2 (c.1748G > A/p.(Gly583Asp)) change was found in a patient and his affected father (family 37). This variant was not present in healthy population databases, and it showed pathogenic predictions in the Alissa Interpret program based on MutationTaster, MutationAssessor, LRT, PolyPhen2 and PROVEAN. Finally, the c.977T > C/p.(Phe326Ser) (POU3F4) variant was found in a boy with mixed hearing loss and cochlear malformations (bilateral corkscrew cochlea, incomplete splitting of turns, absence of meatus and stapes fixation); clinical characteristics of hearing loss are linked to this gene. Furthermore, this change was absent in healthy controls databases, and it showed a pathogenic computational verdict based on 10 pathogenic predictions in Varsome.
Intronic variant: The c.1224-9del variant in MYO6 was found in a patient with an AD pattern of inheritance in her family, given that her mother was also affected. Unfortunately, the patient´s mother refused to collaborate in the genetic study. This variant was not found in healthy control population databases, and the MaxEnt bioinformatic tool predicted the loss of the wild-type acceptor site. This variant was classified as VUS following the AMCG, but we consider that MYO6 c.1224-9del could be a good candidate, and functional studies at the RNA level would be necessary to definitively confirm or discard the pathologic effect of this novel variant.
In frame duplication: The WFS1 in-frame duplication (c.1463_1474dup/p.(Val491_Pro492insLeuIleThrVal)) was detected in a patient presenting HL also in a cousin and her son, but they did not collaborate in the study. This change was classified as VUS according to the ACMG. However, we consider that it is necessary to take this variant into account since it is not described in the population databases, has an acceptable value of conservation and, following the criteria of the ACMG, if it had been possible to show that the variant segregates correctly within the family, the WFS1 c.1463_1474dup/p.(Val491_Pro492insLeuIleThrVal) variant would be directly classified as likely pathogenic. Additionally, the clinical phenotype of this patient is similar to other patients with pathogenic variants in WFS1, showing a characteristic audiogram with low frequencies more affected (Figure 3).
4.2. Patients with Pathogenic Variants in Two Different Genes
NGS panels allow the simultaneous analysis of a great number of genes, and, sometimes, pathogenic variants in different genes are found in the same patient.
In the present work, the 37439 patient presented the AR c.101T > C/p.(Met34Thr) variant in GJB2 in addition to the homozygous STRC whole gene deletion. Patient 39949 presented the AR c.5648G > A/p.(Arg1883Gln) variant in MYO7A in addition to the homozygous c.4055G > A/p.(Cys1352Tyr) AR variant in TECTA. These findings have important implications for reproductive genetic counseling.
The 36163 patient was found to carry two different heterozygous novel pathogenic variants in two different genes: c.1674 + 1G > A in MYO6 and c.2467C > T/p.(Gln823 *) in ESPN. Segregation analysis in this family showed that the affected father also carried the variant in MYO6, whereas the healthy mother carried the variant in ESPN. From these results it can be deduced that the variant in MYO6 is responsible for AD hearing loss, whereas the ESPN variant presents an AR inheritance pattern (Figure 2).
The 29272 and the 41950 patients from the same family carried two different previously described AD pathogenic variants in two different genes: c.2751dup/p.(Gln918Thrfs *24) in MYO6 and c.2230G > A/p.(Asp744Asn) in ESPN. These two patients belong to a large family with more affected members, but these were geographically dispersed, and it was not possible to segregate these two variants with all family members in order to definitely elucidate the genetic basis and the inheritance pattern of HL in this case (Figure 2).
Finally, the likely pathogenic novel MYO6 (c.494T > G/p.(Leu165Arg)) variant was found in patient 41268, referred to as AD non-syndromic hearing loss. Furthermore, two previously described AR pathogenic variants in MYO7A (c.1997G > A/p.(Arg666Gln) and c.3527G > A/p.(Ser1176Asn)) were found in this patient. Segregation analysis would be necessary to definitely elucidate the genetic basis and the inheritance pattern of HL in this family and to offer accurate genetic reproductive genetic counseling.
4.3. Syndromic Cases
Most patients included in this study suffered from non-syndromic hearing loss, but eight cases were referred as syndromic: four patients with Usher syndrome (USH), two Waardenburg syndrome (WS) patients and two branchio-oto-renal syndrome (BOR) patients. We could find the variants responsible for the disease in five of them (Table 2A,B).
The patient 35238 and his mother (42783) were referred as NSHL, but they were found to carry a pathogenic variant in COL11A2: c.4392 + 1G > A. This variant had been previously reported by Brunner et al. (1994) [48] associated with Stickler syndrome without eye affectation. These patients were clinically re-evaluated, and both presented with osteoarticular problems and flattened facial profiles (Table 2B). Thus, this family was re-classified as Stickler syndrome.
Three unrelated cases with bilateral, symmetrical, postlingual, moderate and stable HL (33416, 37112 and 37439) presented biallelic contiguous-gene deletions at chromosome 15q15.3 that included both CATSPER2 and STRC. This deletion causes deafness–infertility syndrome (DIS) in males due to CATSPER haploinsufficiency results in sperm abnormalities [78]. The patient 37112 was a male of 4 years old. Thus, the patient´s parents were informed that their son will be infertile in adulthood.
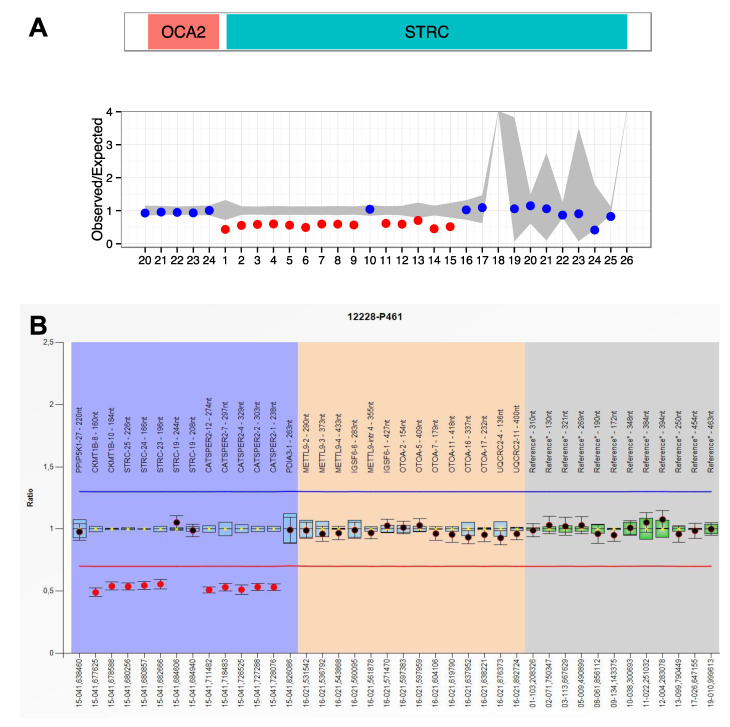
In another case (12228), a complex rearrangement involving STRC and CATSPER2 was detected (Table 3 and Figure 4). DECoN analysis using NGS data showed a partial deletion involving exons 1–15 of STRC. However, based on coverage data from NGS, we could not differentiate between STRC and pSTRC. Thus, an MLPA analysis was performed using P-461 SALSA (MRC Holland). This SALSA includes specific probes only for exons 19, 24–25 of STRC and also some specific probes for some exons of CATSPER2. MLPA results showed a partial deletion affecting exons 23, 24 and 25 of STRC (chr15:41680256-41682666), whereas STRC exon 19 showed a normal dosage (chr15:41684606-41684940). However, chromosome coordinates chr15:41711482-41728076 corresponding to CATSPER2 showed again a ratio of 0.5. Segregation analysis would be helpful in this case to find out if this complex rearrangement is carried in the same chromosome or if there are two different deletions affecting the 15q15.3 locus, located in different alleles.
Figure 4.
Complex rearrangement identified in patient 12228 in the STRC gene. (A) Result obtained from Decon software. The x-axis represents the exon number. Blue points reflect a normal value. Red points reflect a possible deletion for the exon. (B) MLPA representation of patient 12228 with the P461 salsa using the Coffalyzer.Net program (MRC Holland). Normal range: 0.7–1.3 (indicated with red and blue line, respectively).
5. Conclusions
A large number of genes has been associated with HL, but still many cases remain unexplained. Novel HL genes are expected to be discovered and also genetic variants affecting regulatory regions of the genome, which are currently not screened in diagnosis genetic testing. Furthermore, the possibility of multigenic inheritance patterns must be explored in the near future [79].
Nowadays, a huge number of DNA variants are being detected in countless genetic diagnostic laboratories around the world, and a non-negligible number of them are possibly being misinterpreted. It is necessary to share this information with the scientific community and to establish close collaborations to interpret the functional implications of DNA variability. Working altogether, we will be able to decipher the secrets that we still ignore about the human genome.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/12/1467/s1, Table S1: Regions of the panel design with a poor coverage.
Author Contributions
Conceptualization, M.A.-C., J.M.M. and E.A.; Formal analysis, A.B.-S., P.G.-D., R.V.-A., S.J.-R. and C.d.P.-V.; Funding acquisition, J.M.M.; Investigation, G.G.-G., A.B.-S., M.A.-C., R.V.-A., S.J.-R. and T.J.; Methodology, G.G.-G., T.J. and E.A.; Project administration, L.C.-G., M.A.-C., J.M.M. and E.A.; Validation, A.B.-S., R.V.-A. and S.J.-R.; Writing—original draft, G.G.-G. and E.A.; Writing—review and editing, C.d.P.-V., L.C.-G., T.J., M.A.-C. and J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants from the Institute of Health Carlos III (ISCIII), including the Center for Biomedical Research Network on Rare Diseases (CIBERER), FIS (PI19/00303). GGG is a recipient of a senior postdoctoral contract from CIBERER.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shearer A.E., Smith R.J.H. Massively Parallel Sequencing for Genetic Diagnosis of Hearing Loss: The New Standard of Care. Otolaryngol. Head Neck Surg. 2015;153:175–182. doi: 10.1177/0194599815591156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoefsloot L.H., Feenstra I., Kunst H.P.M., Kremer H. Genotype phenotype correlations for hearing impairment: Approaches to management. Clin. Genet. 2014;85:514–523. doi: 10.1111/cge.12339. [DOI] [PubMed] [Google Scholar]
- 3.Morton C.C., Nance W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 4.Smith R.J.H., Bale J.F., White K.R. Sensorineural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 5.Kochhar A., Hildebrand M.S., Smith R.J.H. Clinical aspects of hereditary hearing loss. Genet. Med. 2007;9:393–408. doi: 10.1097/GIM.0b013e3180980bd0. [DOI] [PubMed] [Google Scholar]
- 6.Alford R.L., Arnos K.S., Fox M., Lin J.W., Palmer C.G., Pandya A., Rehm H.L., Robin N.H., Scott D.A., Yoshinaga-Itano C., et al. American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet. Med. Off. J. Am. Coll. Med. Genet. 2014;16:347–355. doi: 10.1038/gim.2014.2. [DOI] [PubMed] [Google Scholar]
- 7.Delmaghani S., El-Amraoui A. Inner Ear Gene Therapies Take Off: Current Promises and Future Challenges. J. Clin. Med. 2020;9:2309. doi: 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloan-Heggen C.M., Bierer A.O., Shearer A.E., Kolbe D.L., Nishimura C.J., Frees K.L., Ephraim S.S., Shibata S.B., Booth K.T., Campbell C.A., et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum. Genet. 2016;135:441–450. doi: 10.1007/s00439-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan D., Xiang G., Chai X., Qing J., Shang H., Zou B., Mittal R., Shen J., Smith R.J.H., Fan Y.-S., et al. Screening of deafness-causing DNA variants that are common in patients of European ancestry using a microarray-based approach. PLoS ONE. 2017;12:e0169219. doi: 10.1371/journal.pone.0169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domínguez-Ruíz M. Estudio Molecular de Genes Implicados en Hipoacusia No Sindrómica Autosómica Recesiva Mediante Secuenciación Sanger y de Nueva Generación. UAM; Madrid, Spain: 2015. [Google Scholar]
- 11.Baux D., Vaché C., Blanchet C., Willems M., Baudoin C., Moclyn M., Faugère V., Touraine R., Isidor B., Dupin-Deguine D., et al. Combined genetic approaches yield a 48% diagnostic rate in a large cohort of French hearing-impaired patients. Sci. Rep. 2017;7:16783. doi: 10.1038/s41598-017-16846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liquori A., Vaché C., Baux D., Blanchet C., Hamel C., Malcolm S., Koenig M., Claustres M., Roux A.-F. Whole USH2A Gene Sequencing Identifies Several New Deep Intronic Mutations. Hum. Mutat. 2016;37:184–193. doi: 10.1002/humu.22926. [DOI] [PubMed] [Google Scholar]
- 13.Vaché C., Besnard T., Le Berre P., García-García G., Baux D., Larrieu L., Abadie C., Blanchet C., Bolz H.J., Millan J., et al. Usher syndrome type 2 caused by activation of an USH2A pseudoexon: Implications for diagnosis and therapy. Hum. Mutat. 2012;33:104–108. doi: 10.1002/humu.21634. [DOI] [PubMed] [Google Scholar]
- 14.Fowler A., Mahamdallie S., Ruark E., Seal S., Ramsay E., Clarke M., Uddin I., Wylie H., Strydom A., Lunter G., et al. Accurate clinical detection of exon copy number variants in a targeted NGS panel using DECoN. Wellcome Open Res. 2016;1:20. doi: 10.12688/wellcomeopenres.10069.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Castillo F.J., Rodríguez-Ballesteros M., Alvarez A., Hutchin T., Leonardi E., de Oliveira C.A., Azaiez H., Brownstein Z., Avenarius M.R., Marlin S., et al. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 2005;42:588–594. doi: 10.1136/jmg.2004.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelante L., Gasparini P., Estivill X., Melchionda S., D’Agruma L., Govea N., Milá M., Monica M.D., Lutfi J., Shohat M., et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997;6:1605–1609. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- 17.Green G.E., Scott D.A., McDonald J.M., Woodworth G.G., Sheffield V.C., Smith R.J. Carrier rates in the midwestern United States for GJB2 mutations causing inherited deafness. JAMA. 1999;281:2211–2216. doi: 10.1001/jama.281.23.2211. [DOI] [PubMed] [Google Scholar]
- 18.Marlin S., Garabédian E.N., Roger G., Moatti L., Matha N., Lewin P., Petit C., Denoyelle F. Connexin 26 gene mutations in congenitally deaf children: Pitfalls for genetic counseling. Arch. Otolaryngol. Head Neck Surg. 2001;127:927–933. doi: 10.1001/archotol.127.8.927. [DOI] [PubMed] [Google Scholar]
- 19.Denoyelle F., Marlin S., Weil D., Moatti L., Chauvin P., Garabédian E.N., Petit C. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: Implications for genetic counselling. Lancet. 1999;353:1298–1303. doi: 10.1016/S0140-6736(98)11071-1. [DOI] [PubMed] [Google Scholar]
- 20.Kelsell D.P., Dunlop J., Stevens H.P., Lench N.J., Liang J.N., Parry G., Mueller R.F., Leigh I.M. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 21.Brobby G.W., Müller-Myhsok B., Horstmann R.D. Connexin 26 R143W mutation associated with recessive nonsyndromic sensorineural deafness in Africa. N. Engl. J. Med. 1998;338:548–550. doi: 10.1056/NEJM199802193380813. [DOI] [PubMed] [Google Scholar]
- 22.del Castillo I., Villamar M., Moreno-Pelayo M.A., del Castillo F.J., Alvarez A., Tellería D., Menéndez I., Moreno F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N. Engl. J. Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Malekpour M., Al-Madani N., Kahrizi K., Zanganeh M., Lohr N.J., Mohseni M., Mojahedi F., Daneshi A., Najmabadi H., et al. Sensorineural deafness and male infertility: A contiguous gene deletion syndrome. J. Med. Genet. 2007;44:233–240. doi: 10.1136/jmg.2006.045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Ballesteros M., del Castillo F.J., Martín Y., Moreno-Pelayo M.A., Morera C., Prieto F., Marco J., Morant A., Gallo-Terán J., Morales-Angulo C., et al. Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF) Hum. Mutat. 2003;22:451–456. doi: 10.1002/humu.10274. [DOI] [PubMed] [Google Scholar]
- 25.Migliosi V., Modamio-Høybjør S., Moreno-Pelayo M.A., Rodríguez-Ballesteros M., Villamar M., Tellería D., Menéndez I., Moreno F., Del Castillo I. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non-syndromic hearing loss. J. Med. Genet. 2002;39:502–506. doi: 10.1136/jmg.39.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eppsteiner R.W., Shearer A.E., Hildebrand M.S., Deluca A.P., Ji H., Dunn C.C., Black-Ziegelbein E.A., Casavant T.L., Braun T.A., Scheetz T.E., et al. Prediction of cochlear implant performance by genetic mutation: The spiral ganglion hypothesis. Hear. Res. 2012;292:51–58. doi: 10.1016/j.heares.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahin H., Walsh T., Rayyan A.A., Lee M.K., Higgins J., Dickel D., Lewis K., Thompson J., Baker C., Nord A.S., et al. Five novel loci for inherited hearing loss mapped by SNP-based homozygosity profiles in Palestinian families. Eur. J. Hum. Genet. EJHG. 2010;18:407–413. doi: 10.1038/ejhg.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wattenhofer M., Di Iorio M.V., Rabionet R., Dougherty L., Pampanos A., Schwede T., Montserrat-Sentis B., Arbones M.L., Iliades T., Pasquadibisceglie A., et al. Mutations in the TMPRSS3 gene are a rare cause of childhood nonsyndromic deafness in Caucasian patients. J. Mol. Med. Berl. Ger. 2002;80:124–131. doi: 10.1007/s00109-001-0310-6. [DOI] [PubMed] [Google Scholar]
- 29.Miyagawa M., Nishio S., Ikeda T., Fukushima K., Usami S. Massively parallel DNA sequencing successfully identifies new causative mutations in deafness genes in patients with cochlear implantation and EAS. PLoS ONE. 2013;8:e75793. doi: 10.1371/journal.pone.0075793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalay E., Uzumcu A., Krieger E., Caylan R., Uyguner O., Ulubil-Emiroglu M., Erdol H., Kayserili H., Hafiz G., Başerer N., et al. MYO15A (DFNB3) mutations in Turkish hearing loss families and functional modeling of a novel motor domain mutation. Am. J. Med. Genet. Part A. 2007;143:2382–2389. doi: 10.1002/ajmg.a.31937. [DOI] [PubMed] [Google Scholar]
- 31.Pera A., Villamar M., Viñuela A., Gandía M., Medà C., Moreno F., Hernández-Chico C. A mutational analysis of the SLC26A4 gene in Spanish hearing-impaired families provides new insights into the genetic causes of Pendred syndrome and DFNB4 hearing loss. Eur. J. Hum. Genet. EJHG. 2008;16:888–896. doi: 10.1038/ejhg.2008.30. [DOI] [PubMed] [Google Scholar]
- 32.Hutchin T., Coy N.N., Conlon H., Telford E., Bromelow K., Blaydon D., Taylor G., Coghill E., Brown S., Trembath R., et al. Assessment of the genetic causes of recessive childhood non-syndromic deafness in the UK—Implications for genetic testing. Clin. Genet. 2005;68:506–512. doi: 10.1111/j.1399-0004.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang X.M., Yan D., Du L.L., Hejtmancik J.F., Jacobson S.G., Nance W.E., Li A.R., Angeli S., Kaiser M., Newton V., et al. Characterization of Usher syndrome type I gene mutations in an Usher syndrome patient population. Hum. Genet. 2005;116:292–299. doi: 10.1007/s00439-004-1227-2. [DOI] [PubMed] [Google Scholar]
- 34.Kothiyal P., Cox S., Ebert J., Husami A., Kenna M.A., Greinwald J.H., Aronow B.J., Rehm H.L. High-throughput detection of mutations responsible for childhood hearing loss using resequencing microarrays. BMC Biotechnol. 2010;10:10. doi: 10.1186/1472-6750-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nájera C., Beneyto M., Blanca J., Aller E., Fontcuberta A., Millán J.M., Ayuso C. Mutations in myosin VIIA (MYO7A) and usherin (USH2A) in Spanish patients with Usher syndrome types I and II, respectively. Hum. Mutat. 2002;20:76–77. doi: 10.1002/humu.9042. [DOI] [PubMed] [Google Scholar]
- 36.García-García G., Besnard T., Baux D., Vaché C., Aller E., Malcolm S., Claustres M., Millan J.M., Roux A.-F. The contribution of GPR98 and DFNB31 genes to a Spanish Usher syndrome type 2 cohort. Mol. Vis. 2013;19:367–373. [PMC free article] [PubMed] [Google Scholar]
- 37.Besnard T., Vaché C., Baux D., Larrieu L., Abadie C., Blanchet C., Odent S., Blanchet P., Calvas P., Hamel C., et al. Non-USH2A mutations in USH2 patients. Hum. Mutat. 2012;33:504–510. doi: 10.1002/humu.22004. [DOI] [PubMed] [Google Scholar]
- 38.Astuto L.M., Bork J.M., Weston M.D., Askew J.W., Fields R.R., Orten D.J., Ohliger S.J., Riazuddin S., Morell R.J., Khan S., et al. CDH23 mutation and phenotype heterogeneity: A profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am. J. Hum. Genet. 2002;71:262–275. doi: 10.1086/341558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aller E., Jaijo T., Beneyto M., Nájera C., Oltra S., Ayuso C., Baiget M., Carballo M., Antiñolo G., Valverde D., et al. Identification of 14 novel mutations in the long isoform of USH2A in Spanish patients with Usher syndrome type II. J. Med. Genet. 2006;43:e55. doi: 10.1136/jmg.2006.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhaus C., Eisenberger T., Decker C., Nagl S., Blank C., Pfister M., Kennerknecht I., Müller-Hofstede C., Charbel Issa P., Heller R., et al. Next-generation sequencing reveals the mutational landscape of clinically diagnosed Usher syndrome: Copy number variations, phenocopies, a predominant target for translational read-through, and PEX26 mutated in Heimler syndrome. Mol. Genet. Genomic Med. 2017;5:531–552. doi: 10.1002/mgg3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanggaard K.M., Kjaer K.W., Eiberg H., Nürnberg G., Nürnberg P., Hoffman K., Jensen H., Sørum C., Rendtorff N.D., Tranebjaerg L. A novel nonsense mutation in MYO6 is associated with progressive nonsyndromic hearing loss in a Danish DFNA22 family. Am. J. Med. Genet. Part A. 2008;146:1017–1025. doi: 10.1002/ajmg.a.32174. [DOI] [PubMed] [Google Scholar]
- 42.Kwon T.-J., Oh S.-K., Park H.-J., Sato O., Venselaar H., Choi S.Y., Kim S., Lee K.-Y., Bok J., Lee S.-H., et al. The effect of novel mutations on the structure and enzymatic activity of unconventional myosins associated with autosomal dominant non-syndromic hearing loss. Open Biol. 2014;4 doi: 10.1098/rsob.140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donaudy F., Zheng L., Ficarella R., Ballana E., Carella M., Melchionda S., Estivill X., Bartles J.R., Gasparini P. Espin gene (ESPN) mutations associated with autosomal dominant hearing loss cause defects in microvillar elongation or organisation. J. Med. Genet. 2006;43:157–161. doi: 10.1136/jmg.2005.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet C., Riahi Z., Chantot-Bastaraud S., Smagghe L., Letexier M., Marcaillou C., Lefèvre G.M., Hardelin J.-P., El-Amraoui A., Singh-Estivalet A., et al. An innovative strategy for the molecular diagnosis of Usher syndrome identifies causal biallelic mutations in 93% of European patients. Eur. J. Hum. Genet. EJHG. 2016;24:1730–1738. doi: 10.1038/ejhg.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plantinga R.F., de Brouwer A.P.M., Huygen P.L.M., Kunst H.P.M., Kremer H., Cremers C.W.R.J. A novel TECTA mutation in a Dutch DFNA8/12 family confirms genotype-phenotype correlation. J. Assoc. Res. Otolaryngol. JARO. 2006;7:173–181. doi: 10.1007/s10162-006-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hildebrand M.S., Morín M., Meyer N.C., Mayo F., Modamio-Hoybjor S., Mencía A., Olavarrieta L., Morales-Angulo C., Nishimura C.J., Workman H., et al. DFNA8/12 caused by TECTA mutations is the most identified subtype of nonsyndromic autosomal dominant hearing loss. Hum. Mutat. 2011;32:825–834. doi: 10.1002/humu.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno-Pelayo M.A., del Castillo I., Villamar M., Romero L., Hernández-Calvín F.J., Herraiz C., Barberá R., Navas C., Moreno F. A cysteine substitution in the zona pellucida domain of alpha-tectorin results in autosomal dominant, postlingual, progressive, mid frequency hearing loss in a Spanish family. J. Med. Genet. 2001;38:E13. doi: 10.1136/jmg.38.5.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunner H.G., van Beersum S.E., Warman M.L., Olsen B.R., Ropers H.H., Mariman E.C. A Stickler syndrome gene is linked to chromosome 6 near the COL11A2 gene. Hum. Mol. Genet. 1994;3:1561–1564. doi: 10.1093/hmg/3.9.1561. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y., Cheng J., Lu Y., Li J., Lu Y., Jin Z., Dai P., Wang R., Yuan H. Identification of two novel missense WFS1 mutations, H696Y and R703H, in patients with non-syndromic low-frequency sensorineural hearing loss. J. Genet. Genom. Yi Chuan Xue Bao. 2011;38:71–76. doi: 10.1016/j.jcg.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Escribano L.B. Epidemiología Genética en Pacientes Españoles con Hipoacusia de Herencia Autosómica Dominante, Sindrómica y No Sindrómica, Utilizando Herramientas de Nueva Generación: Array-CGH y Secuenciación Masiva. UAM; Madrid, Spain: 2016. [Google Scholar]
- 51.Shinagawa J., Moteki H., Nishio S.-Y., Ohyama K., Otsuki K., Iwasaki S., Masuda S., Oshikawa C., Ohta Y., Arai Y., et al. Prevalence and clinical features of hearing loss caused by EYA4 variants. Sci. Rep. 2020;10:3662. doi: 10.1038/s41598-020-60259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nobukuni Y., Watanabe A., Takeda K., Skarka H., Tachibana M. Analyses of loss-of-function mutations of the MITF gene suggest that haploinsufficiency is a cause of Waardenburg syndrome type 2A. Am. J. Hum. Genet. 1996;59:76–83. [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C.-C., Tsai C.-Y., Lin Y.-H., Chen P.-Y., Lin P.-H., Cheng Y.-F., Wu C.-M., Lin Y.-H., Lee C.-Y., Erdenechuluun J., et al. Genetic Epidemiology and Clinical Features of Hereditary Hearing Impairment in the Taiwanese Population. Genes. 2019;10:772. doi: 10.3390/genes10100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Méndez-Vidal C., González-Del Pozo M., Vela-Boza A., Santoyo-López J., López-Domingo F.J., Vázquez-Marouschek C., Dopazo J., Borrego S., Antiñolo G. Whole-exome sequencing identifies novel compound heterozygous mutations in USH2A in Spanish patients with autosomal recessive retinitis pigmentosa. Mol. Vis. 2013;19:2187–2195. [PMC free article] [PubMed] [Google Scholar]
- 56.Bernal S., Medà C., Solans T., Ayuso C., Garcia-Sandoval B., Valverde D., Del Rio E., Baiget M. Clinical and genetic studies in Spanish patients with Usher syndrome type II: Description of new mutations and evidence for a lack of genotype—Phenotype correlation. Clin. Genet. 2005;68:204–214. doi: 10.1111/j.1399-0004.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 57.Adato A., Weil D., Kalinski H., Pel-Or Y., Ayadi H., Petit C., Korostishevsky M., Bonne-Tamir B. Mutation profile of all 49 exons of the human myosin VIIA gene, and haplotype analysis, in Usher 1B families from diverse origins. Am. J. Hum. Genet. 1997;61:813–821. doi: 10.1086/514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y., Guan L., Shen T., Zhang J., Xiao X., Jiang H., Li S., Yang J., Jia X., Yin Y., et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum. Genet. 2014;133:1255–1271. doi: 10.1007/s00439-014-1460-2. [DOI] [PubMed] [Google Scholar]
- 59.Abe S., Usami S., Shinkawa H., Kelley P.M., Kimberling W.J. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J. Med. Genet. 2000;37:41–43. doi: 10.1136/jmg.37.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabionet R., Zelante L., López-Bigas N., D’Agruma L., Melchionda S., Restagno G., Arbonés M.L., Gasparini P., Estivill X. Molecular basis of childhood deafness resulting from mutations in the GJB2 (connexin 26) gene. Hum. Genet. 2000;106:40–44. doi: 10.1007/s004390051007. [DOI] [PubMed] [Google Scholar]
- 61.Wagatsuma M., Kitoh R., Suzuki H., Fukuoka H., Takumi Y., Usami S. Distribution and frequencies of CDH23 mutations in Japanese patients with non-syndromic hearing loss. Clin. Genet. 2007;72:339–344. doi: 10.1111/j.1399-0004.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 62.de Heer A.-M.R., Collin R.W.J., Huygen P.L.M., Schraders M., Oostrik J., Rouwette M., Kunst H.P.M., Kremer H., Cremers C.W.R.J. Progressive sensorineural hearing loss and normal vestibular function in a Dutch DFNB7/11 family with a novel mutation in TMC1. Audiol. Neurootol. 2011;16:93–105. doi: 10.1159/000313282. [DOI] [PubMed] [Google Scholar]
- 63.Duman D., Tekin M. Autosomal recessive nonsyndromic deafness genes: A review. Front. Biosci. 2012;17:2213–2236. doi: 10.2741/4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shearer A.E., Hildebrand M.S., Smith R.J. Hereditary Hearing Loss and Deafness Overview. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; Seattle, WA, USA: 1993. [Google Scholar]
- 65.Mandelker D., Amr S.S., Pugh T., Gowrisankar S., Shakhbatyan R., Duffy E., Bowser M., Harrison B., Lafferty K., Mahanta L., et al. Comprehensive diagnostic testing for stereocilin: An approach for analyzing medically important genes with high homology. J. Mol. Diagn. 2014;16:639–647. doi: 10.1016/j.jmoldx.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Zwaenepoel I., Mustapha M., Leibovici M., Verpy E., Goodyear R., Liu X.Z., Nouaille S., Nance W.E., Kanaan M., Avraham K.B., et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl. Acad. Sci. USA. 2002;99:6240–6245. doi: 10.1073/pnas.082515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carvalho G.M.D., Ramos P.Z., Castilho A.M., Guimarães A.C., Sartorato E.L. Molecular study of patients with auditory neuropathy. Mol. Med. Rep. 2016;14:481–490. doi: 10.3892/mmr.2016.5226. [DOI] [PubMed] [Google Scholar]
- 68.Yokota Y., Moteki H., Nishio S., Yamaguchi T., Wakui K., Kobayashi Y., Ohyama K., Miyazaki H., Matsuoka R., Abe S., et al. Frequency and clinical features of hearing loss caused by STRC deletions. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-40586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yazdanpanahi N., Tabatabaiefar M.A., Bagheri N., Azadegan Dehkordi F., Farrokhi E., Hashemzadeh Chaleshtori M. The role and spectrum of SLC26A4 mutations in Iranian patients with autosomal recessive hereditary deafness. Int. J. Audiol. 2015;54:124–130. doi: 10.3109/14992027.2014.944276. [DOI] [PubMed] [Google Scholar]
- 70.Jaijo T., Aller E., García-García G., Aparisi M.J., Bernal S., Avila-Fernández A., Barragán I., Baiget M., Ayuso C., Antiñolo G., et al. Microarray-based mutation analysis of 183 Spanish families with Usher syndrome. Invest. Ophthalmol. Vis. Sci. 2010;51:1311–1317. doi: 10.1167/iovs.09-4085. [DOI] [PubMed] [Google Scholar]
- 71.Blanco-Kelly F., Jaijo T., Aller E., Avila-Fernandez A., López-Molina M.I., Giménez A., García-Sandoval B., Millán J.M., Ayuso C. Clinical aspects of Usher syndrome and the USH2A gene in a cohort of 433 patients. JAMA Ophthalmol. 2015;133:157–164. doi: 10.1001/jamaophthalmol.2014.4498. [DOI] [PubMed] [Google Scholar]
- 72.Butz M., McDonald A., Lundquist P.A., Meyer M., Harrington S., Kester S., Stein M.I., Mistry N.A., Zimmerman Zuckerman E., Niu Z., et al. Development and Validation of a Next-Generation Sequencing Panel for Syndromic and Nonsyndromic Hearing Loss. J. Appl. Lab. Med. 2020;5:467–479. doi: 10.1093/jalm/jfaa021. [DOI] [PubMed] [Google Scholar]
- 73.Cabanillas R., Diñeiro M., Cifuentes G.A., Castillo D., Pruneda P.C., Álvarez R., Sánchez-Durán N., Capín R., Plasencia A., Viejo-Díaz M., et al. Comprehensive genomic diagnosis of non-syndromic and syndromic hereditary hearing loss in Spanish patients. BMC Med. Genom. 2018;11:58. doi: 10.1186/s12920-018-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diaz-Horta O., Duman D., Foster J., Sırmacı A., Gonzalez M., Mahdieh N., Fotouhi N., Bonyadi M., Cengiz F.B., Menendez I., et al. Whole-exome sequencing efficiently detects rare mutations in autosomal recessive nonsyndromic hearing loss. PLoS ONE. 2012;7:e50628. doi: 10.1371/journal.pone.0050628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tekin D., Yan D., Bademci G., Feng Y., Guo S., Foster J., Blanton S., Tekin M., Liu X. A next-generation sequencing gene panel (MiamiOtoGenes) for comprehensive analysis of deafness genes. Hear. Res. 2016;333:179–184. doi: 10.1016/j.heares.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou S., Mei X., Yang W., Zhu R., Yang T., Hu H. Whole-exome sequencing identifies rare pathogenic and candidate variants in sporadic Chinese Han deaf patients. Clin. Genet. 2020;97:352–356. doi: 10.1111/cge.13638. [DOI] [PubMed] [Google Scholar]
- 77.Vears D.F., Sénécal K., Borry P. Reporting practices for variants of uncertain significance from next generation sequencing technologies. Eur. J. Med. Genet. 2017;60:553–558. doi: 10.1016/j.ejmg.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 78.Hoppman N., Aypar U., Brodersen P., Brown N., Wilson J., Babovic-Vuksanovic D. Genetic testing for hearing loss in the United States should include deletion/duplication analysis for the deafness/infertility locus at 15q15.3. Mol. Cytogenet. 2013;6:19. doi: 10.1186/1755-8166-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kremer H. Hereditary hearing loss; about the known and the unknown. Hear. Res. 2019;376:58–68. doi: 10.1016/j.heares.2019.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.