Abstract
Background
COVID-19 causes significant morbidity and mortality. Despite the high prevalence of delirium and delirium-related symptoms in COVID-19 patients, data and evidence-based recommendations on the pathophysiology and management of delirium are limited.
Objective
We conducted a rapid review of COVID-19-related delirium literature to provide a synthesis of literature on the prevalence, pathoetiology, and management of delirium in these patients.
Methods
Systematic searches of Medline, Embase, PsycInfo, LitCovid, WHO-COVID-19, and Web of Science electronic databases were conducted. Grey literature was also reviewed, including preprint servers, archives, and websites of relevant organizations. Search results were limited to the English language. We included literature focused on adults with COVID-19 and delirium. Papers were excluded if they did not mention signs or symptoms of delirium.
Results
229 studies described prevalence, pathoetiology, and/or management of delirium in adults with COVID-19. Delirium was rarely assessed with validated tools. Delirium affected >50% of all patients with COVID-19 admitted to the ICU. The etiology of COVID-19 delirium is likely multifactorial, with some evidence of direct brain effect. Prevention remains the cornerstone of management in these patients. To date, there is no evidence to suggest specific pharmacological strategies.
Discussion
Delirium is common in COVID-19 and may manifest from both indirect and direct effects on the central nervous system. Further research is required to investigate contributing mechanisms. As there is limited empirical literature on delirium management in COVID-19, management with non-pharmacological measures and judicious use of pharmacotherapy is suggested.
Keywords: Delirium, Confusion, Covid-19, Neuropsychiatric, Cognitive
Highlights
-
•
Delirium is common, in up to 70% of COVID-19 patients with severe respiratory failure.
-
•
Most of the literature has used terms other than delirium.
-
•
Further research is required to investigate contributing mechanisms for delirium.
-
•
Empirical literature on delirium management in COVID-19 is limited.
-
•
Non-pharmacological and judicious management with pharmacological measures is suggested.
1. Introduction
The novel coronavirus disease-19 (COVID-19), a viral illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), surged in late 2019 prompting the World Health Organization (WHO) to declare a global pandemic [1]. SARS-CoV-2 can result in severe symptoms requiring admission to the intensive care unit (ICU) and life-sustaining interventions, especially in older adults and those with co-occurring conditions [2,3].
SARS-CoV-2 typically impacts the respiratory system, causing bilateral pneumonia and respiratory distress [[4], [5], [6], [7], [8]]. However, there is emerging evidence that it has systemic effects throughout the body, including the brain [[9], [10], [11]], causing neurological symptoms and complications [12,13]. Altered consciousness is often described as a neurological symptom in up to 70% of patients with severe respiratory infection, and is also noted at presentation even in those without typical symptoms [5,13,14]. The WHO now recognizes altered consciousness and/or confusion as a core symptom of COVID-19 at presentation [15]. In addition to the commonly known risk factors for delirium, mechanisms for COVID-19 delirium have included direct and indirect brain impact [11,12,[16], [17], [18], [19], [20], [21], [22], [23]].
Delirium is associated with significant morbidity and mortality in patients with COVID-19 [24], especially in patients who are elderly. A recent review on the psychiatric and neuropsychiatric presentations of COVID-19 and other coronaviruses describes high rates of delirium [25]. This review, however, was limited to data until April 2020 and described symptom presentation rates. In addition, the evidence to date regarding the treatment of delirium in these patients is speculative and based on the general delirium management literature, case reports, and clinical experience [26,27]. Given the rapid emergence of data on COVID-19 clinical features and approaches to neuropsychiatric sequelae, including delirium, additional reviews are needed to synthesize our understanding of delirium prevalence, mechanisms, and management.
In this systematic review, we analyze the current literature on the pathoetiology, prevalence, and management of delirium in patients with COVID-19. This review aims to provide clinicians with a synthesis of the current evidence base and provide recommendations on areas for future research in COVID-19 delirium care.
2. Methods
2.1. Data sources and identification of studies
A broad systematic search of the literature was performed across six electronic databases: Medline, LitCovid, Embase, PsycInfo, WHO COVID-19 Database, and Web of Science. There were no publication type or language limits, and search results were limited to 2019–2020. The Medline search strategy used a validated COVID-19 search filter and both MeSH and keywords relevant to the delirium and its related symptoms. The Medline search was adapted to other databases (Appendix 1). Identified abstracts were imported to RefWorks and de-duplicated electronically. This list was reviewed by two authors and remaining duplicates were removed manually. The grey literature search included preprint servers and archives, as well as the websites of relevant organizations. An update of both the database and grey literature search strategies was executed before the final analysis to capture the most recent publications from August 2020. This review was registered with PROSPERO (CRD42020184857).
2.2. Study inclusion and exclusion criteria
English language studies that focused on adults with COVID-19 who exhibited symptoms suggestive of delirium were included. There were no restrictions based on study design, and both qualitative and quantitative research studies were included. Papers that did not mention symptoms or signs related to delirium were excluded. Two independent reviewers screened each article’s title and abstract. After initial screening, full texts of potentially relevant studies were reviewed to determine whether inclusion criteria were met. Disagreements on inclusion were resolved through discussion between reviewers and with the broader research team if necessary.
2.3. Data extraction and classification
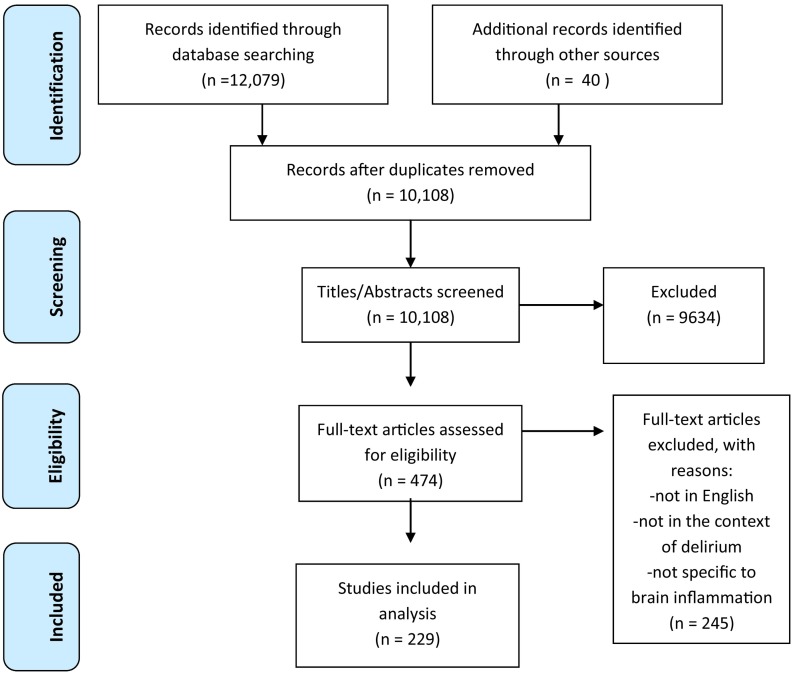
We followed the PRISMA guidelines for data analysis and article inclusion [28]. Given the heterogeneity of identified studies, a systematic review rather than a meta-analysis was undertaken. Our search criteria initially identified 12,079 articles and abstracts. Grey literature search identified an additional 40 sources. Abstracts were independently reviewed and de-duplicated by two authors (TR, SB). After removing duplicates and screening abstracts, 229 studies were included in full text review (see Fig. 1 ). Data were extracted by two researchers (PG, MR) using a data abstraction tool focused on: country of study, publication year, study setting, patient population, prevalence of delirium, described risk factors or contributors to development of delirium, described prevention or management interventions for delirium. Papers with no new data, such as editorials and commentaries were included in the review to elucidate themes and areas of future research. Please refer to Appendix 2 for a list of articles that were included in the review but not independently cited because they reiterated or reviewed concepts from prior work.
Fig. 1.
Literature search and study selection process for systematic review of the literature, published between 2019 and 2020, on individuals with COVID-19 who exhibit signs or symptoms suggestive of COVID-19. Study followed the PRISMA guidelines for conducting systematic review.
2.4. Quality assessment
Methodology, limitations, and findings of included studies were discussed. We assessed the quality of studies using the Kmet criteria for primary research papers [29]. Studies with a Kmet score of >80% were deemed “strong” and 70%-80% were deemed “good” quality [30]. Forty studies were eligible for quality ratings, excluding case reports. From the total, 19 studies, 11 studies, and 10 studies were strong, good and poor quality, respectively. Quantitative data, including prevalence rates, were listed and summarized, and qualitative descriptions were narratively synthesized.
3. Results
3.1. Prevalence and symptom presentation of delirium in adults with COVID-19
Of the papers included in the review, 77 reported on new data from case reports, case series, or observational cohorts which described the presentation and prevalence of delirium in individuals with confirmed SARS-CoV-2. As shown in Table 1 , the clinical setting varied among studies, and only nine of the 40 studies reporting prevalence used a validated tool to screen or diagnose delirium, with the majority relying on review of medical records or subjective patient self-report. The language used to indicate delirium, or its related symptoms, varied across studies (Table 1).
Table 1.
Prevalence of delirium or likely delirium in patients with COVID-19.
| Authors | Country | Sample Size |
Population setting | Delirium Assessment | Prevalence of delirium (or specified delirium syndrome term) |
||
|---|---|---|---|---|---|---|---|
| COVID + | COVID − | COVID + | COVID − | ||||
| ICU | |||||||
| Khan [32] | USA | 144 | n/a | ICU, two hospitals | CAM-ICU | 106/144 (73.6%) | n/a |
| Scullen [174] | USA | 76 | n/a | ICU | Medical record | 26/76 (34.2%) Altered mental status | n/a |
| Helms [31] | France | 58 | n/a | ICU with ARDS | CAM-ICU with RASS; n = 40) | 26/40 (65%) confusion” “positive findings” CAM | n/a |
| 14/39 (36%) Dysexecutive syndrome | |||||||
| Helms [33] | France | 140 | ICU with ARDS | CAM-ICU, if RASS appropriate, neurological exam | 118/140 (84.3%) delirium +/- abnormal neuro exam | n/a | |
| 97/122 (79.5%) CAM-ICU positive | |||||||
| 84/97 (86.6%) hyperactive delirium | |||||||
| 13/97 (14.4%) hypoactive delirium | |||||||
| Jackel [175] | Germany | 44 | ICU with ARDS | NuDesc, RASS | 20/44 (45.4%) with COVID | n/a | |
| Fan [176] | China | 86 | ICU | Medical record, DSM Criteria | 11/86 (12.8%) | n/a | |
| 11/80 (13.8%) ischemic stroke | |||||||
| 0/6 (0%) no ischemic stroke | |||||||
| Sultan [177] | USA | 10 | n/a | ECMO | Medical record | 10% (1/10) “confused” | n/a |
| Hospital | |||||||
| Liguori [178] | Italy | 103 | n/a | COVID Hospital | Anamnestic interview | 23/103 (22.3%) Confusion | n/a |
| Chen [5] | China | 274 | n/a | COVID hospital | Medical record | 26/274 (9.4%) Disorders of consciousness | n/a |
| 25/113 died; 1/161 recovered | |||||||
| Chen [135] | China | 99 | n/a | COVID hospital | Medical record | 9/99 (9%) confusion | n/a |
| Mao [13] | China | 214 | n/a | COVID hospital | Medical record and interview | 16/214 (7.5%) “Impaired consciousness” | n/a |
| 3/126 (2.4%) non-severe COVID | |||||||
| 13/88 (14.8%) Severe COVID | |||||||
| Cao [35] | China | 80 | n/a | Hospital | Medical record | 5/80 (6.2%) Confusion | n/a |
| 0/53 (0%) Non-severe COVID | |||||||
| 5/27 (18.5%) Severe COVID | |||||||
| Vena [55] | Italy | 317 | n/a | Hospital | Medical record | 29/317 (9.1%) “mental confusion” | n/a |
| Garcez [39] | Brazil | 707 | n/a | Hospital | CHART-DEL method | 234/707 (33%) | n/a |
| 86/707 (12%) at admission, | |||||||
| 22/30 (73%) with dementia | |||||||
| Romero-Sanchez [34] | Spain | 841 | n/a | Hospital; ICU (n = 77) | Medical record | 165/841 (19.6%) Any disorder of consciousness | n/a |
| 69/841 (8.2%) Acute confusional syndrome | |||||||
| 85/841 (10.1%) Bradypsychia, disorientation | |||||||
| 117/841 (13.9%) Any depressed consciousness | |||||||
| 7.2% non-severe | |||||||
| 38.9% Severe | |||||||
| Bianchetti [43] | Italy | 627 | n/a | Medical wards | Medical record and assessment | 55/82 (67.1%) Symptom at admission | n/a |
| 41/55 (74.5%) Hypoactive | |||||||
| 17/55 (31%) Hyperactive | |||||||
| Luigetti [179] | Italy | 213 | 218 | Non-intensive COVID unit | Medical record, interview if possible | 75/213 (35.2%) Encephalopathy- fever/hypoxia | 46/218 (21.1%) Encephalopathy-fever/hypoxia |
| 11/213 (5.1%) Not due to fever or hypoxia | 8/218 (3.6%) Not due to fever or hypoxia | ||||||
| Iltaf [180] | Pakistan | 350 | n/a | Outpatient + inpatient tertiary hospital | Medical record | 7/350 (2%) Altered level of consciousness | n/a |
| Mcloughlin [45] | UK | 71 | n/a | Hospital, point prevalence with 4 week follow up | DSM-IV + 4AT | 31/71 (42%) | n/a |
| 37% hypoactive | |||||||
| 53% hyperactive | |||||||
| Xiong [181] | China | 917 | n/a | Hospital | Medical record | 7/917 (0.8%) impaired consciousness/delirium | n/a |
| Frontera [182] | USA | 4645 | n/a | Hospital | Medical record | Rates of “encephalopathy” | n/a |
| 237/3079 (8%) Normonatremia | |||||||
| 82/1032 (7%) Mild hyponatremia | |||||||
| 40/305 (9%) Mod hyponatremia | |||||||
| 16/36 (44%) Severe hyponatremia | |||||||
| Karadas [183] | Turkey | 239 | n/a | Hospital | Neuro exam + interview | 23/239 (9.6%) impaired consciousness-confusion | n/a |
| Pinna [51] | USA | 50 | n/a | Hospital | Medical record | 30/50 (60%) Altered mental status | n/a |
| Agarwal [52] | USA | 404 | n/a | Outpatient clinic and hospital | Medical record | 86/404 (21.3%) Altered mental status | n/a |
| Varatharaj [49] | UK | 153 | n/a | Case registry | Medical record | 39/125 (31%) altered mental status | n/a |
| Geriatrics | |||||||
| DeSmet [184] | Belgium | 81 | n/a | Geriatrics Unit | Medical record | 34/81 (42%) | n/a |
| Zerah [40] | France | 821 | n/a | Geriatric ward | CAM | 330 (40%) | n/a |
| Knopp [38] | UK | 217 | n/a | Hospital, >70 yo | Medical record | 64/217 (29%) | n/a |
| Marengoni [36] | Italy | 91 | n/a | Geriatric ward, >70 yo | DSM-5 criteria | 25/91 (27.5%) 1/91 (1.1%) at admission | n/a |
| 24/91 (26.4%) during hospital stay | |||||||
| Poloni [37] | Italy | 59 | n/a | Dementia facility, >65 yo | DSM-5, CAM, Medical record | 21/57 (36.8%) delirium as sole onset manifestation | n/a |
| 11/21 (52.4%) hypoactive delirium | |||||||
| Palliative | |||||||
| Lovell [42] | UK | 101 | n/a | Palliative care consult service | Medical record | 24/101 (23.8%) delirium at time of referral | n/a |
| 43/101 (42.6%) agitation | |||||||
| Heath [44] | UK | 31 | n/a | Palliative care consultation service | Medical record | 24/31 (77%) agitation/delirium | n/a |
| Neurology | |||||||
| Benussi [24] | Italy | 56 | 117 | Neuro-COVID Unit with neurological disease | CAM | 15/56 (26.8%) | 9/117 (7.7%) |
| 9/43 (20.9%) In cerebrovascular disease | 4/68 (5.9%) In cerebrovascular disease | ||||||
| Pilotto [41] | Italy | 147 | 358 | ED with neurological symptoms | Medical record | 38/147 (25.9%) Altered mental state/agitation | 36/358 (10.1%) Altered mental state/agitation |
| Radmard [48] | USA | 33 | n/a | Neurology consultation | Medical record | 12/33 (36.4%) Depressed, fluctuation, impaired consciousness | n/a |
| Kremer [46] | France | 64 | n/a | Hospital with neurological manifestation and MRI | Medical record | 25/64 (39%) Disturbance of consciousness | n/a |
| 34/64 (53%) Confusion 20/64 (31%) Agitation | |||||||
| Paterson [50] | UK | 43 | n/a | Referred for neurological case review | Medical record | 10/43 (23.2%) | n/a |
| Studart-Neto [47] | Brazil | 89 | n/a | Neurological consultation service | Medical record + exam | 35/89 (39.3%) referrals for altered level of consciousness, with 28/35 (80%) diagnosed with encephalopathy | n/a |
| 12/89 (13.5%) referrals for agitation, with 7/12 (58.3%) diagnosed with encephalopathy | |||||||
| Giorgianni [53] | Italy | 26 | n/a | Hospital with neurological symptoms and neuroimaging | Not specified | 4/26 (15.4%) confusional state | n/a |
| Emergency Department | |||||||
| Chachkhiani [54] | US | 250 | n/a | ED | Medical record | 19/250 (7.6%) altered mental status at presentation | n/a |
| 73/250 (29.2%) altered mental status during hospitalization | |||||||
Rates of delirium differed substantially depending on the study population and setting. Prevalence of delirium was highest in those admitted to the ICU, ranging from 65% to 79.5% [[31], [32], [33]]. In studies which stratified groups by COVID-19 severity, higher rates were reported in those with severe respiratory disease (disorder of consciousness: 7.2% vs. 38.9%; OR 8.18, CI 5.5–12.2; acute confusional syndrome: 3.9% vs. 14.9%; OR 4.31 CI 2.5–7.4; p < .001 [34]; confusion: 0% vs 18.5%; p < 0.01 [35]; impaired consciousness: 2.4% vs 14.8%; p < 0.001 [13]). Similarly, studies of older adults found that significant proportions experienced delirium while hospitalized with COVID-19, often associated with age and frailty ranging from 29% to 40% [[36], [37], [38], [39], [40]]. Nine studies reported delirium motor subtype, agitation or specifically hyper- or hypo-active delirium, with mixed results [31,37,[41], [42], [43], [44], [45], [46], [47]]. Patients admitted to acute medical wards demonstrating hypoactive delirium ranged from 31% to 74.5% [37,43,45,46]. Other studies found substantial proportions of patients with agitation in the palliative setting (69% [31]; 77% [44]) and ICU (42.6% [42]; 86.6% [33]). In contrast, Khan et al. (2020) found that, on first assessment, most ICU patients (86.8%) had hypoactive delirium [32]. Although few studies included a comparison group, rates of delirium were substantially higher in those with COVID-19 than those without [24,41]. Numerous studies reported on patients presenting at the emergency department (ED) or admitted to hospital with neurological symptoms. Individuals presenting with neurological symptoms and COVID-19 were more likely to have delirium/altered mental status than those who presenting with neurological symptoms who did not have COVID-19 (26.8% vs 7.7%, p = .003 (full sample), 20.9% vs 5.9%, p = .05 (subset of those with cerebrovascular disease [24]; 25.9% vs. 10.1%, no significance value reported)[41]. Changes in mental status and level of consciousness, consistent with delirium, were considered common neurological manifestations [[46], [47], [48], [49], [50], [51], [52], [53]]. There was limited exploration of the association between delirium and clinical outcomes; however, delirium was associated with worse outcomes including greater ICU and hospital length of stay [32,54], increased mortality [36,37,[39], [40], [41],54,55], poorer physical function [45], and dysexecutive symptoms at discharge [31].
We found 37 case reports and case series describing presentations indicative of delirium in individuals with COVID-19. Similar to the cohort studies, some authors used the word “delirium”, while others used terms including confusion, altered mental status, acute onset of psychotic symptoms, disorientation, decreased level of consciousness, cognitive dysfunction, and encephalopathy. The primary focus of these papers was the dissemination of “atypical” SARS-CoV-2 presentations. In numerous cases, patients presented to medical attention with symptoms of delirium, but without any or with only mild respiratory or systemic symptoms [[56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68]]. In one case, the patient was initially admitted to a psychiatric ward and later transferred to a general hospital after having a seizure [69]. Outcomes ranged from full recovery to death.
4. Proposed pathoetiology of delirium in adults with COVID-19
Eighty-nine papers described possible pathoetiology of delirium in individuals with COVID-19. While pathoetiology was sometimes discussed in the context of delirium directly, it was more often indirectly mentioned through discussion of the broader neurological manifestations of COVID-19. A substantial proportion of papers in this section consisted of reviews, letters to the editor, and commentaries that discussed possible mechanisms of pathoetiology extrapolated from studies of related viruses and animals. Review of published case reports and case studies also elucidated several proposed mechanisms.
Many papers hypothesized how complications of COVID-19 may exacerbate known risk factors and causes of delirium, while others proposed potential mechanisms by which SARS-CoV-2 may directly impact the central nervous system (CNS). It is important to recognize that social distancing and policies limiting non-essential patient contact can further precipitate delirium by limiting the support patients receive from familiar caregivers. This can be disorienting and isolating, especially in those already vulnerable to delirium [23,70]. Personal protective equipment (PPE), such as gowns, masks, and face shields, can also be disorienting to older patients who may have pre-existing sensory or cognitive impairments [23].
4.1. Complications of severe COVID-19 as a possible etiology of delirium
The complications of COVID-19 infection, such as pneumonia and ARDS, as well as the interventions used to manage them, are known risk factors for delirium. ARDS, which results in hypoxia, respiratory acidosis, and respiratory failure, and, for some, disseminated intravascular coagulation (DIC) and systemic organ dysfunction, can contribute to delirium [[71], [72], [73], [74], [75], [76], [77]]. Additionally, ARDS often necessitates prolonged admission to the ICU for ventilator support and sedation, interventions which can in and of themselves be deliriogenic [[78], [79], [80]]. COVID is also believed to be associated with a hypercoagulable state, which may predispose patients to further CNS insult through ischemic brain injury [[81], [82], [83], [84], [85]].
The role of systemic inflammation in precipitating delirium, independent of the virus infecting the CNS, has also been discussed [71,[86], [87], [88]]. The systemic inflammation seen in severe cases of COVID-19 can lead to the release of cytokines such as TNF-α, IL-1, and IL-6 [72,[89], [90], [91]] and can increase the permeability of the blood-brain barrier (BBB) allowing inflammatory cells to enter the brain, where they too can release cytokines causing neuronal damage [22,71,[92], [93], [94], [95], [96]]. One manifestation of this theorized mechanism, illustrated in a case report of a patient presenting with altered mental status, is acute necrotizing encephalopathy [92]. This condition is thought to involve an intracranial cytokine storm mediated by BBB permeabilization rather than direct viral neuronal damage [17,92,97,98]. These hypotheses are supported by a recent study involving CSF analysis in COVID-19 patients with encephalitis, where signs of CSF inflammation were identified in the absence of viral RNA, suggestive of an autoimmune or inflammatory process [46].
4.2. Direct entry of SARS-CoV-2 in the CNS as a possible etiology of delirium
Now that anosmia and hypogeusia are well-recognized presenting symptoms of COVID-19, numerous papers have considered whether SARS-CoV-2 can directly enter the CNS [21,[99], [100], [101]]. There is a well-established precedent of neurotropism among related human coronaviruses such as SARS-CoV, which binds to the same ACE2 receptor as SARS-CoV-2 to gain entry into host cells, including neurons and glial cells, via its spike (S) protein [18,[102], [103], [104], [105], [106], [107], [108], [109], [110]]. SARS-CoV-2 has been shown to be capable of infecting neurons of human ACE2 transgenic mice, as well as dopaminergic neurons derived from human pluripotent stem cells [83,111,112].
In addition, there exists some histological, laboratory, and imaging evidence to support the direct involvement of SARS-CoV-2 in the CNS. A case report of a patient with COVID-19, which presented postmortem tissue analysis, demonstrated viral structures in frontal lobe tissue [113]. Furthermore, although the majority of studies published have not identified detectable levels of SARS-CoV-2 in cerebrospinal fluid (CSF) [[114], [115], [116], [117]], there have been isolated reports of SARS-CoV-2 detected in CSF [[118], [119], [120], [121]]. Additionally, a recent study of patients who died of COVID-19 identified structural brain abnormalities on postmortem brain imaging, with 21% of subjects having asymmetric olfactory bulbs [122]. Taken together, these findings suggest the potential for direct CNS infection by SARS-CoV-2.
Two pathways which would allow for SARS-CoV-2 to directly access the CNS are frequently proposed; namely, the trans-synaptic and hematogenous routes. Mouse models of SARS-CoV have demonstrated that the virus can enter the brain through the olfactory bulb, with significant effects on the thalamus and brainstem [[123], [124], [125], [126]]. These neurons may provide a refuge for the virus from CD8 T-cells because they cannot present antigens required for T-cell activation [127]. A transcribrial route to the olfactory bulb is also suspected because of anosmia noted clinically [21,[99], [100], [101]]. Moreover, a recent autopsy report showed damage to neurons and glial cells that became progressively worse from the olfactory nerve to the brainstem with detection of SARS-CoV-2 virions along this pathway [128]. Other trans-synaptic routes for CNS infection have been hypothesized but have not been as thoroughly investigated, such as those originating in the gastrointestinal (GI) tract or the lungs [103,[129], [130], [131], [132], [133], [134]].
Beyond the trans-synaptic routes, hematogenous spread has also surfaced as a plausible route for viral CNS infection. Endothelial cells, including those that comprise the BBB, express ACE2 and are susceptible to infection, indicating that SARS-CoV-2 may be capable of infiltrating the CNS in this way [96,113,[135], [136], [137], [138], [139], [140], [141]]. Additionally, there is precedent for endothelial damage from the systemic release of cytokines and activation of the complement cascade, which holds relevance for the integrity of the BBB during severe infection with COVID-19 [20,142].
If the virus were to invade the CNS, delirium could manifest either directly due to neuronal damage within the brain or indirectly due to hypoxia caused by disruptions in the function of the medullary respiratory centers [23,98,123,132,[143], [144], [145]]. Ultimately, further investigation is required before any conclusions can be drawn regarding deliriogenic pathoetiology and whether direct neuroinvasion by SARS-CoV-2 plays a role.
5. Prevention and management of delirium in adults with COVID-19
At the time of this review, no randomized clinical trials have evaluated treatment of delirium in patients with COVID-19. Nevertheless, several articles have described the treatment of delirium in patients infected with SARS-CoV-2 [23,26,27,[146], [147], [148], [149], [150]].
Despite the limited literature on delirium management in COVID-19 patients, several hospitals have developed protocols for the treatment of delirium in these patients based on experience with other coronaviruses and expert consensus [26,27,71,95,146]. The focus of these recommendations is variable, with some concentrating on the management of delirium in the intensive care unit [23] and others discussing the treatment of specific symptoms related to delirium in COVID-19 patients [150,151]. Most have suggested that treatment decisions be based on symptom presentation, underlying medical comorbidities, and consideration for medication interactions and side effect profiles [26,152].
Expert consensus articles suggest that in conjunction with daily screening, implementing non-pharmacological preventative interventions be implemented whenever possible in COVID-19 patients [23,146,147,153,154]. Numerous papers have commented on COVID-19 specific considerations that may limit the implementation of preventative measures to reduce delirium rates [23,155]. These include increased social isolation for patients (e.g. lack of family involvement and reduced staff care time with patients due to infection risk), stressful work environments, and increased healthcare demands on staff, which may limit the accuracy and speed of identifying emerging delirium [156,157].
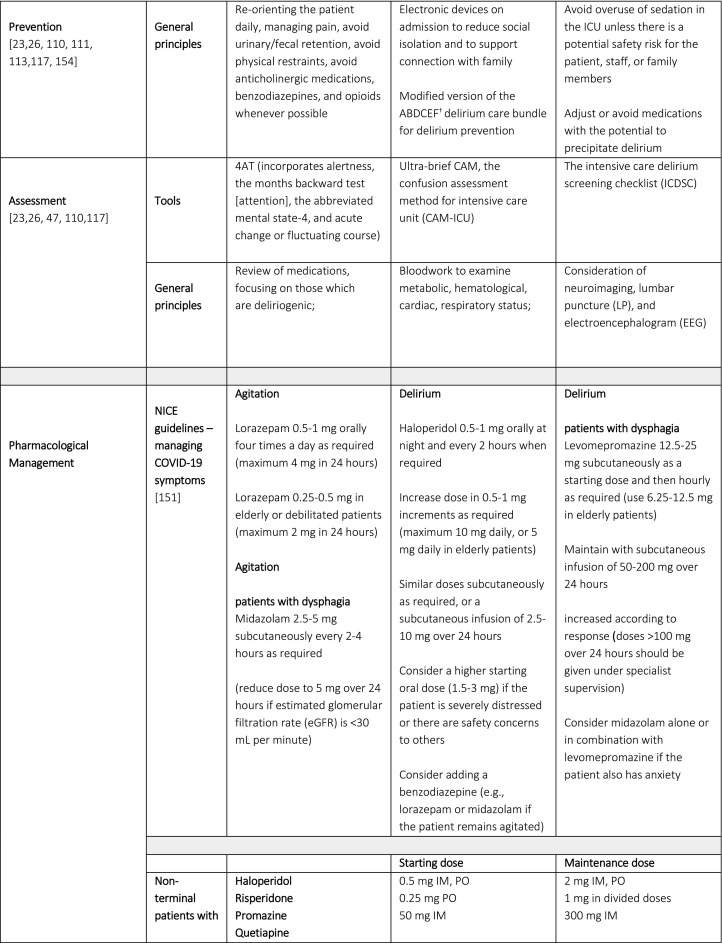
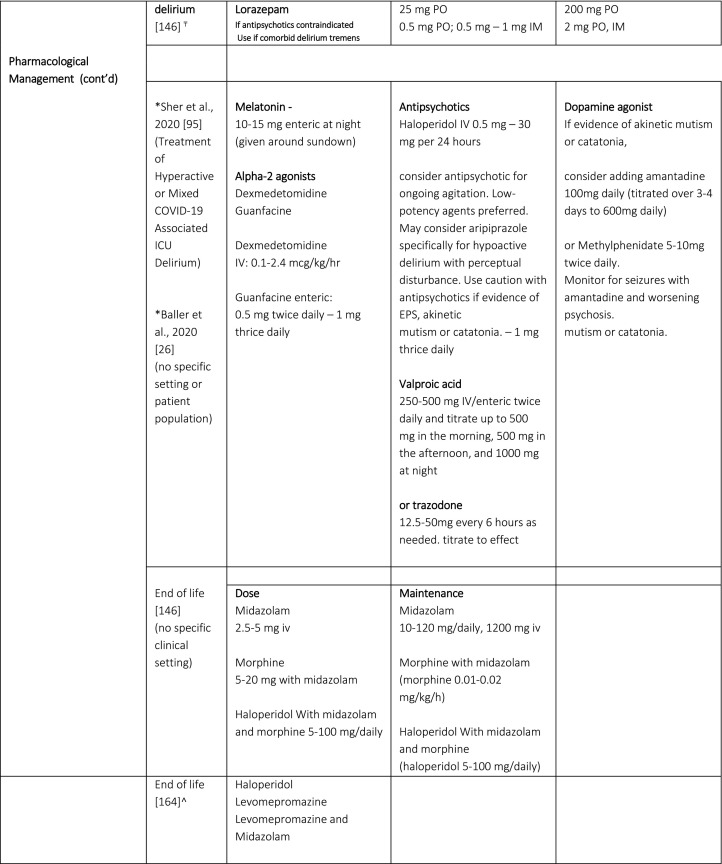
Once delirium presents, investigation to determine the underlying and contributory etiologies, and a combination of non-pharmacological and pharmacological interventions, are recommended [26,71,146,147]. Table 2 summarizes proposed prevention, assessment and pharmacological management strategies for COVID-19 delirious patients. It is important to note that the utility of the electroencephalogram (EEG) remains unclear. In a study of patients with COVID-19 and completed CT or MRI brain, altered mental status was the most common indication for brain imaging but few of these patients demonstrated acute pathology [158]. As highlighted by the case reports noted in this review, LP and EEG are being used to assess for and rule out different etiologies potentially contributing to delirium in COVID-19, although the test for detecting the SARS-CoV-2 virus in the CSF is not yet available in the US and EEG findings tend to be non-specific. Authors advocate a judicious symptom-directed approach, using these tests when there is evidence of seizure or focal neurological deficit.
Table 2.
Proposed Prevention, Assessment and Pharmacological Management Strategies in Patients with Delirium with COVID-19.
†ABCDEF; Assess, Prevent, and Manage Pain; Both Spontaneous Awakening Trials (SAT) and Spontaneous Breathing Trials (SBT); Choice of analgesia and sedation; Delirium: Assess, Prevent, and Manage; Early mobility and Exercise; and Family engagement and empowerment)
₸these recommendations are based on the NICE guidelines and the Scottish Intercollegiate Guidelines Network on Delirium
*Baller et al, provides a clear algorithm; though does not provide specific dosages for the recommended medications. Medication doses from Sher et al., 2020.
^prospective, longitudinal, descriptive study of end-of-life standardized care plan - Royal Surrey County Hospital NHS Foundation Trust, Guildford, UK)
A recent rapid review of the pharmacological treatment of hyperactive delirium in people with COVID-19 underscored the lack of data on the management of delirium in these patients [159]. To date, the literature on the psychopharmacological management of patients with delirium has focused on prevention and a symptom-based treatment approach, such as insomnia and symptoms of agitation and psychosis [26]. In the absence of randomized clinical trials, guidance on delirium psychopharmacology, including dosing, is driven by expert consensus and generalization of delirium management principles from before COVID-19.
Despite the lack of research and evidence in this area, the National Institute for Health and Care Excellence (NICE) has issued guidelines for managing COVID-19 symptoms in the community [151]. These guidelines highlight, and distinguish between delirium, anxiety, and agitation in COVID-19 patients. A distinction between patients with and without dysphagia was also made (Table 2). Anxiety treatment is not discussed here as it is out of scope for this review.
Two articles propose an algorithm for the pharmacological treatment of delirium in patients with COVID-19 [26,95]. Sher et al. (2020) recommends considering dexmedetomidine for acute agitation at nighttime and to consider guanfacine to wean other sedation [95]. Valproic acid is suggested for the management of hyperactive and/or mixed (i.e., fluctuating between agitation and hypoactivity) delirium. Valproic acid has been described as having unique qualities including being potentially anti-inflammatory, neuroprotective and antioxidant; it may potentially decrease the transcription of interleukin-6 (implicated in cytokine storm) [95] and may assist in reducing the need for sedative agents in delirious and/or agitated patients in the ICU [95,160,161]. Several authors propose using melatonin as a first-line agent in the management of delirium in COVID-19 patients given its safety compared to other agents (e.g. antipsychotics) [26,95,150]. Melatonin has also been recommended to optimize sleep in the context of COVID-19 delirium [147]. It has been found to have anti-inflammatory, antioxidative, and immune-enhancing features [95,162,163], which may reduce or interrupt the development of a cytokine storm for some patients [150]. It has also been proposed that melatonin can be advantageous for critically ill patients, reducing vascular permeability, agitation, and improving quality of sleep [162]. However, no studies have examined the efficacy of melatonin in delirium treatment or prevention in COVID-19 patient populations.
Antipsychotics are considered for the management of delirium, ongoing agitation, and end-of-life care in patients with COVID-19 [26,151,164]. However, only one study has explored this, using intramuscular aripiprazole for the management of hyperactive delirium in a series of patients with COVID-19 [165]. Aripiprazole appears to effectively reduce symptoms of delirium and agitation, and it was well tolerated. Otherwise, no other antipsychotic has been studied in patients with COVID-19.
In general, antipsychotics should not be used unless there is a safety risk to the patient or others [23,95,146,147,159]. However, some anecdotal reports suggest using antipsychotic medications earlier in the treatment of COVID-19 patients with hyperactive delirium [95]. When antipsychotics are required, consideration for dosing should be made based on the patient population being treated. Sanders et al. (2020) propose using higher doses of antipsychotics when managing risky behaviors, which put the patient and others at risk of harm secondary to spreading infection, after accounting for the risks and benefits of such interventions [27]. In contrast, for the elderly and for people with neurological conditions, other guidelines suggest conservative dosing when treating delirium in patients with COVID-19 [27,146,151]. Experts cautioned the use of antipsychotics in the elderly and those with neurological conditions such as Parkinson’s disease [146]. Specific antipsychotic use varies among studies that described the pharmacological management of these patients. Quetiapine has been suggested as an option for the management of delirium in the elderly and in patients with neurological conditions based on its tolerability and wide therapeutic range [146]. Olanzapine appears to be a preferred agent in the management of agitation in delirious patients with COVID-19 due to its sedative capacity and lower drug-drug-interaction potential with antiviral medications, as noted by one author [166]. In a recent review, Ostuzzi et al. suggests not using haloperidol, quetiapine, or lorazepam for the management of delirium in patients with COVID-19 due to the potential for drug-drug interaction with COVID-19 drugs [159]. At least two authors suggest close monitoring for respiratory depression in delirious patients treated with antipsychotics, based on a theoretical potential for antipsychotic related respiratory depression [159,166]. It is important to also monitor for QTc prolongation in these patients if antipsychotics are prescribed [159]. Whether antipsychotics have other benefits beyond assisting in the management of behaviors and/or neuropsychiatric symptoms associated with delirium is yet to be determined. However, haloperidol appears to offer some possible immunological benefits. It has been found to be an effective antagonist of sigma-1 receptors, which, in theory, might potently protect against oxidative stress-related cell death [95]. No papers recommend using medications to prevent delirium [27].
Finally, although benzodiazepines have been found to worsen delirium, experts highlighted the use of benzodiazepines in COVID-19 patients with suspected alcohol-withdrawal delirium [146].
6. Discussion
Based on the current literature on patients with COVID-19, delirium is common in patients affected by SARS-CoV-2 across the severity spectrum. It can be a core symptom at presentation, even in the absence of respiratory symptoms. Similar to pre-COVID-19 pandemic, delirium may be under-recognized and under-diagnosed which limits clinicians’ ability to manage its underlying causes and symptoms, as well as appreciate its short- and long-term impact on patients [45,167,168]. The current literature investigating rates and course of delirium in COVID-19 is limited by the lack of systematic screening and diagnosis, as well as the challenges in controlling for other factors when comparing to groups without COVID-19. In this review, validated delirium screening tools were seldomly used and many papers referred to terms such as encephalopathy, confusion, or altered mental status rather than delirium [167]. Rates of delirium were often determined by positive scores on screening tests, rather than clinical assessment, which may reflect the challenges of conducting research and in-person assessments during COVID-19. There is a need for greater focus on delirium in individuals with COVID-19 given its association with poor outcomes, including prolonged cognitive difficulties, increased length of stay, and increased mortality [14].
Individuals who are older, have dementia, and more severe illness, appear to be more susceptible to developing delirium, similarly to delirium from other causes. Identification of well-recognized risk factors for delirium in COVID-19 patients remains central to preventing and managing delirium [78,79,169]. The policies designed to prevent the spread of COVID-19 present logistical challenges in the prevention of delirium through disorienting PPE or lack of in-person support from caregivers [23,70].
There currently remains insufficient evidence to definitively elucidate the pathophysiological role of SARS-CoV-2 in the development of delirium; however, the literature suggests that development of delirium in individuals with COVID-19 is likely multifactorial, and may include systemic inflammation due to COVID-19-related pneumonia or ARDS [22,92]. It should be noted, however, that the use of the term “cytokine storm” in the context of COVID-19 has been controversial [170]. Indeed, the levels of IL-6 in patients with severe COVID-19, are 10–40 times lower than the median values recorded by trials conducted by the National Heart, Lung and Blood Institute’s ARDS Network [170,171]. This may point to an entity involving lower levels of cytokine release, distinct from what is traditionally thought of as cytokine storm associated with ARDS.
The neurological symptoms associated with COVID-19 may suggest a role for SARS-CoV-2 directly injuring the CNS [21,99]. Trans-synaptic transport of the virus from peripheral nerves [123] and/or hematogenous spread either through direct infection of the BBB endothelial cells or permeabilization of the BBB have been suggested. However, it remains a subject of debate. Furthermore, direct neuronal damage, particularly within the respiratory structures of the medulla oblongata has also been proposed [145]. There is no evidence to suggest that the routes of entry or mechanisms of viral damage discussed in this review are mutually exclusive, indeed these processes may all be occurring simultaneously. The myriad of plausible deliriogenic mechanisms in patients with COVID-19, both indirectly and directly mediated by SARS-CoV-2, suggests the need for a multifactorial hybrid model to explain the clinical picture of delirium reported during the pandemic [71]. A nuanced approach addressing the many variables precipitating delirium is necessary to prevent and manage COVID-19 delirium.
The management of delirium in patients with COVID-19 is in its infancy with no randomized clinical trials published to date. Reviewed literature on the management of delirium in COVID-19 patients was difficult to analyze due to heterogeneity of the article type (e.g., case report(s), anecdotal/experiential) and variable treatment settings (e.g. ICU vs. medical ward). Despite the limited literature, expert consensus articles suggest that the primary management of delirium in COVID-19 patients is the implementation of preventative measures whenever possible [23,146,147].
Once delirium presents, experts recommend investigating for underlying and contributory etiologies, while concomitantly implementing non-pharmacological and pharmacological interventions [71]. These strategies align with the broader delirium literature [168,172]. Notwithstanding the many similarities in the management of delirium in patients with COVID-19 to patients without this condition, there seem to be some peculiarities in the pharmacological and non-pharmacological management of COVID-19 delirious patients. Recommendations on pharmacological symptom management from the literature reviewed were sometimes conflicting. For example, some authors noted that higher dosages of medications may be required [27], while others suggested their use at lower dosages for the management of agitation, as these patients appear to commonly present with catatonic features [26,71]. Alpha-2 agonists may be particularly useful in these patients because they do not cause respiratory depression. Valproate was considered a good option for those who did not respond to antipsychotics and for those who are at increased risk of cardiac morbidity and QTc prolongation because of specific medications used in the treatment of SARS-CoV-2 infected patients, with some early promising anecdotal data in the treatment of hyperactive delirium in patients with COVID-19 [95]. Although there is no rigorous evidence to date to suggest that these pharmacological agents are safe and effective in these patients, these agents have been found to be safe in the broader delirium literature [173]. Nevertheless, higher quality research and evidence are needed before any evidence-based recommendation can be made for the pharmacological management of delirium in patients with COVID-19.
Based on this review, there is insufficient evidence to suggest one medication over another for the treatment of delirium in COVID-19 patients. Clinical judgement, medical comorbidities, and clinical presentation are imperative in choosing a psychopharmacological intervention in the management of delirium in this population.
Non-pharmacological interventions remain the cornerstone of delirium management in any setting. However, patients with COVID-19 are isolated, and interventions such as re-orientation, provision of sensory aids, and early mobilization can be extremely challenging with staff minimizing contact, visitor limitations, and use of PPE which can impair communication. It is important to consider how best to adapt recommended these non-pharmacological delirium interventions to those with COVID-19. For example, using technology to promote contact with family and staff, and taking into account PPE when developing strategies to orient and communicate with patients.
7. Strengths and limitations
This rapid review used a systematic literature review approach to identify and evaluate the most current literature on the pathoetiology, prevalence, and management of delirium in people with COVID-19. An experienced librarian collaborated with clinicians to develop the broad search strategy, including grey literature, multiple databases, and preprints. The evidence for studies examining delirium management in the context of COVID-19 was appraised. Limitations of this review include the focus on peer-reviewed publications and literature published in English, which could limit generalizability.
8. Conclusion
This review suggests that delirium in COVID-19 infected patients is highly prevalent and likely a result of multiple factors including purported direct effects of SARS-CoV-2 on the CNS. Regular assessment with validated delirium screening tools and implementation of prevention interventions are widely recommended. Ensuring that delirium is documented as such, rather than using terms such as acute confusional state and disorder of consciousness, will help strengthen the literature in this area. Given the rapidly evolving pandemic and the lack of studies regarding management of delirium in general, it is important to acknowledge that there is a role for consensus and expert opinion as well as extrapolation from delirium management approaches in other medically ill populations for the management of these patients. Further research is needed to elucidate specific patient risk factors for and to determine the effectiveness of prevention and management approaches to COVID-19 delirium.
Statement of ethics
Ethical approval was not required as it is a systematic review with anonymous data.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2020.110350.
Appendix 1
Search strategy
Database: Ovid MEDLINE: Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® <1946-Present>
1. exp Neurotoxicity Syndromes/
2. exp Neuropsychology/
3. exp Neuropsychiatry/
4. exp Central Nervous System/
5. exp Confusion/
6. exp Affective Symptoms/
7. exp Euphoria/
8. exp Psychomotor Agitation/
9. exp Attention/
10. exp Cognition/
11. exp Brain Diseases/
12. exp Encephalitis/
13. exp Meningitis/
14. neuro$.mp.
15. (central nervous adj3 system$).mp.
16. (systema adj3 nervosum adj3 centrale$).mp.
17. cns.mp.
18. cerebrospinal$.mp.
19. cerebro spinal$.mp.
20. brain$.mp.
21. confus$.mp.
22. deliriu$.mp.
23. conscious$.mp.
24. unconscious$.mp.
25. letharg$.mp.
26. psychomotor$.mp.
27. psycho motor$.mp.
28. cogniti$.mp.
29. euphori$.mp.
30. agitat$.mp.
31. (emotion$ adj3 (symptom$ or disturb$)).mp.
32. (disorganiz$ adj3 (think$ or thought$)).mp.
33. attent$.mp.
34. inattent$.mp.
35. (sleep$ adj3 disturb$).mp.
36. (affective adj3 (symptom$ or disturb$)).mp.
37. (emotion$ adj3 (symptom$ or disturb$)).mp.
38. encephalit$.mp.
39. encephalopath$.mp.
40. meningiti$.mp.
41. icu syndrome$.mp.
42. pachymening$.mp.
43. icu psychosis$.mp.
44. (intensive care adj3 psychosis).mp.
45. (intensive care adj3 syndrome$).mp.
46. reversible dementia$.mp.
47. transien$ organic brain syndrome$.mp.
48. organic mental syndrome$.mp.
49. organic mental disorder$.mp.
50. reversible cognitive dysfunct$.mp.
51. subacute befudd$.mp.
52. pseudosenilit$.mp.
53. pseudo senilit$.mp.
54. exogenous psychosis$.mp.
55. acute brain syndrome$.mp.
56. acute organic psychosis$.mp.
57. toxic psychosis$.mp.
58. nCov.mp.
59. CoV2.mp.
60. CoV 2.mp.
61. COVID19$.mp.
62. COVID2019$.mp.
63. COVID 19$.mp.
64. COVID 2019$.mp.
65. (Novel adj3 Coronavirus$).mp.
66. (Novel adj3 Corona virus$).mp.
67. (novel adj3 coronaravirus$).mp.
68. (novel adj3 coronara virus$).mp.
69. SARS COV 2.mp.
70. SARS COV2.mp.
71. Severe Acute Respiratory Syndrome Corona$ 2.mp.
72. (coronavirus$ adj3 disease$ adj3 "2019").mp.
73. (coronavirus$ adj3 disease$ adj3 "19").mp.
74. (corona virus$ adj3 disease$ 2019).mp.
75. (corona virus$ adj3 disease$ 19).mp.
76. SARS Corona$ 2.mp.
77. SARS CoV2.mp.
78. Severe Acute Respiratory Syndrome Corona virus$ 2.mp.
79. Severe Acute Respiratory Syndrome CoV 2.mp.
80. Severe Acute Respiratory Syndrome CoV2.mp.
81. HCoV 19.mp.
82. novel cov.mp.
83. exp Seafood/
84. exp Meat/
85. exp Animals, Wild/
86. seafood$.mp.
87. sea food$.mp.
88. fish?.mp.
89. meat?.mp.
90. wildlife$.mp.
91. wild life$.mp.
92. (Animal$ adj3 feral).mp.
93. (Animal$ adj3 nondomest$).mp.
94. (Animal$ adj3 non domest$).mp.
95. (Animal$ adj3 wild).mp.
96. (Animal$ adj3 stray$).mp.
97. exp Food Contamination/
98. exp Food Handling/
99. exp Food Supply/
100. food?.mp.
101. market$.mp.
102. exp Pneumonia/
103. exp Respiratory Tract Diseases/
104. exp coronavirus/
105. exp Coronavirus Infections/
106. (coronavirus$ or coronoravirus$ or coronaravirus$ or corona virus$ or coronora virus$ or coronara virus$ Coronavirinae$).mp.
107. pneumonia$.mp.
108. outbreak$.mp.
109. (respirator$ adj3 (illness$ or disease$ or symptom$ or infect$)).mp.
110. exp china/
111. Wuhan$.mp.
112. China$.mp.
113. Chinese$.mp.
114. Hubei?.mp.
115. or/1-56 [delirium/brain inflammation set]
116. or/58-82 [COVID-19 keywords]
117. (or/83-100) and (or/101-109) and (or/110-114) [ first outbreak in China set]
118. 115 and (116 or 117)
119. limit 118 to yr="2019 –Current”
Appendix 2
A. Abdullahi, S.A. Candan, M.A. Abba, A.H. Bello, M.A. Alshehri, E.A. Victor, N.A. Umar, B. Kundakci, Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis, Frontiers in Neurology. 11 (2020). doi:10.3389/fneur.2020.00687.
A. Orsini, M. Corsi, A. Santangelo, A. Riva, D. Peroni, T. Foiadelli, S. Savasta, P. Striano, Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients, Neurological Sciences. 41 (2020) 2353–2366. doi:10.1007/s10072-020-04544-w.
A.M. Franceschi, O. Ahmed, L. Giliberto, M. Castillo, Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a Manifestation of COVID-19 Infection, American Journal of Neuroradiology. 41 (2020) 1173–1176. doi:10.3174/ajnr.a6595.
D Garg, A.K. Srivastava, R.K. Dhamija, Beyond Fever, Cough and Dyspnea: The Neurology of COVID-19, Journal of the Association of Physicians of India. 68(9) (2020) 62-66.
D. Azim, S. Nasim, S. Kumar, A. Hussain, S. Patel, Neurological Consequences of 2019-nCoV Infection: A Comprehensive Literature Review, Cureus. (2020). doi:10.7759/cureus.8790.
D. Dinakaran, N. Manjunatha, C.N. Kumar, B.M. Suresh, Neuropsychiatric aspects of COVID-19 pandemic: A selective review, Asian Journal of Psychiatry. 53 (2020) 102188. doi:10.1016/j.ajp.2020.102188.
D. Nuzzo, P. Picone, Potential neurological effects of severe COVID-19 infection, Neuroscience Research. 158 (2020) 1–5. doi:10.1016/j.neures.2020.06.009.
E. Taherifard, E. Taherifard, Neurological complications of COVID-19: a systematic review, Neurol. Res. 42 (2020) 905–912.
G. Nepal, J.H. Rehrig, G.S. Shrestha, Y.K. Shing, J.K. Yadav, R. Ojha, G. Pokhrel, Z.L. Tu, D.Y. Huang, Neurological manifestations of COVID-19: a systematic review, Critical Care. 24 (2020). doi:10.1186/s13054-020-03121-z.
G. Tsivgoulis, L. Palaiodimou, A.H. Katsanos, V. Casio, M. Kohrman, C. Molina, C. Cordonnier, U. Fischer, P. Kelly, V.K. Sharma, A.C. Chan, R. Zane, A. Saraj, P. Schellinger, K.I. Voumvourakis, N. Grigoriadis, A.V. Alexandrov, S. Tsiodras, Neurological manifestations and implications of COVID-19 pandemic, Therapeutic Advances in Neurological Disorders. 13 (2020) 1-14. doi:10.1177%.
H. Vespignani, D. Colas, B.S. Lavin, C. Soufflet, L. Maillard, V. Pourcher, O. Paccoud, S. Medjebar, P. Frouin, Report on Electroencephalographic Findings in Critically Ill Patients with COVID -19, Annals of Neurology. 88 (2020) 626–630. doi:10.1002/ana.25814.
H. Wang, Delirium: A suggestive sign of COVID-19 in dementia, EClinicalMedicine. 26 (2020) 100524. doi:10.1016/j.eclinm.2020.100524.
I. Cani, V. Barone, R. D’Angelo, L. Pisani, V. Allegri, L. Spinardi, P. Malpassi, L. Fasano, R. Rinaldi, S. Fanti, P. Cortelli, M. Guarino, Frontal encephalopathy related to hyperinflammation in COVID-19, Journal of Neurology. (2020). doi:10.1007/s00415-020-10057-5.
K. Hassanzadeh, H.P. Pena, J. Dragotto, L. Buccarello, F. Iorio, S. Pieraccini, G. Sancini, M. Feligioni, Considerations around the SARS-CoV-2 Spike Protein with Particular Attention to COVID-19 Brain Infection and Neurological Symptoms, ACS Chemical Neuroscience. 11 (2020) 2361–2369. doi:10.1021/acschemneuro.0c00373.
K. Verstrepen, L. Baisier, H. De Cauwer, Neurological manifestations of COVID-19, SARS and MERS, Acta Neurologica Belgica. 120 (2020) 1051–1060. doi:10.1007/s13760-020-01412-4.
L. Al-Harthi, E. Campbell, J.A. Schneider, D.A. Bennett, What HIV in the Brain Can Teach Us About SARS-CoV-2 Neurological Complications?, AIDS Research and Human Retroviruses. (2020). doi:10.1089/aid.2020.0161.
L.J.W. Canham, L.E. Staniaszek, A.M. Mortimer, L.F. Nouri, N.M. Kane, Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: A case series, Clinical Neurophysiology Practice. 5 (2020) 199–205. doi:10.1016/j.cnp.2020.06.001.
M. Adamczyk-Sowa, N. Niedziela, K. Kubicka-Bączyk, K. Wierzbicki, J. Jaroszewicz, P. Sowa, Neurological symptoms as a clinical manifestation of COVID-19: implications for internists, Polish Archives of Internal Medicine. (2020). doi:10.20452/pamw.15575.
M. Pennisi, G. Lanza, L. Falzone, F. Fisicaro, R. Ferri, R. Bella, SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms, International Journal of Molecular Sciences. 21 (2020) 5475. doi:10.3390/ijms21155475.
M. Sharifian-Dorche, P. Huot, M. Osherov, D. Wen, A. Saveriano, P.S. Giacomini, J.P. Antel, A. Mowla, Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic, Journal of the Neurological Sciences. 417 (2020) 117085. doi:10.1016/j.jns.2020.117085.
M.E.V. Collantes, A.I. Espiritu, M.C.C. Sy, V.M.M. Anlacan, R.D.G. Jamora, Neurological Manifestations in COVID-19 Infection: A Systematic Review and Meta-Analysis, Can. J. Neurol. Sci. (2020) 1–11.
N. Deigendesch, L. Sironi, M. Kutza, S. Wischnewski, V. Fuchs, J. Hench, A. Frank, R. Nienhold, K.D. Mertz, G. Cathomas, M.S. Matter, M. Siegemund, M. Tolnay, L. Schirmer, A.-K. Pröbstel, A. Tzankov, S. Frank, Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology, Acta Neuropathologica. 140 (2020) 583–586. doi:10.1007/s00401-020-02213-y.
O. Sinanovic, Medical Faculty, University of Tuzla, Tuzla, B.A. Herzegovina, M. Muftic, S. Sinanovic, Sarajevo Medical School, University Sarajevo, School of Science and Technology, Sarajevo, B.A. Herzegovina, Sarajevo Medical School, University Sarajevo, School of Science and Technology, Sarajevo, B.A. Herzegovina, Faculty of Health Sciences University of Sarajevo, Sarajevo, B.A. Herzegovina, Medical Faculty, University of Tuzla, Tuzla, B.A. Herzegovina, COVID-19 PANDEMIA: NEUROPSYCHIATRIC COMORBIDITY AND CONSEQUENCES, Psychiatria Danubina. 32 (2020) 236–244. doi:10.24869/psyd.2020.236.
R. Bridwell, B. Long, M. Gottlieb, Neurologic complications of COVID-19, The American Journal of Emergency Medicine. 38 (2020) 1549.e3–1549.e7. doi:10.1016/j.ajem.2020.05.024.
R.K. Garg, V.K. Paliwal, A. Gupta, Encephalopathy in patients with COVID-19: A review, Journal of Medical Virology. (2020). doi:10.1002/jmv.26207.
R.T. Pinzon, V.O. Wijaya, R.B. Buana, A. Al Jody, P.N. Nunsio, Neurologic Characteristics in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis, Frontiers in Neurology. 11 (2020). doi:10.3389/fneur.2020.00565.
S. Alomari, Z. Abou-Mrad, A. Bydon, COVID-19 and the central nervous system, Clinical Neurology and Neurosurgery. 198 (2020). doi:10.1016/j.clineuro.2020.106116.
S. Kihira, B.N. Delman, P. Belani, L. Stein, A. Aggarwal, B. Rigney, J. Schefflein, A.H. Doshi, P.S. Pawha, Imaging Features of Acute Encephalopathy in Patients with COVID-19: A Case Series, American Journal of Neuroradiology. 41 (2020) 1804–1808. doi:10.3174/ajnr.a6715.
S. Mariotto, A. Savoldi, K. Donadello, S. Zanzoni, S. Bozzetti, S. Carta, C. Zivelonghi, D. Alberti, F. Piraino, P. Minuz, D. Girelli, E. Crisafulli, S. Romano, D. Marcon, G. Marchi, L. Gottin, E. Polati, P. Zanatta, S. Monaco, E. Tacconelli, S. Ferrari, Nervous system: subclinical target of SARS-CoV-2 infection, Journal of Neurology, Neurosurgery & Psychiatry. 91 (2020) 1010–1012. doi:10.1136/jnnp-2020-323881.
S. Sultana, V. Ananthapur, COVID-19 and its impact on neurological manifestations and mental health: the present scenario, Neurological Sciences. 41 (2020) 3015–3020. doi:10.1007/s10072-020-04695-w.
S.S. Al Mazrouei, G.A. Saeed, A.A. Al Helali, M. Ahmed, COVID-19-associated encephalopathy: Neurological manifestation of COVID-19, Radiol Case Rep. 15 (2020) 1646–1649.
X. Chen, S. Laurent, O.A. Onur, N.N. Kleineberg, G.R. Fink, F. Schweitzer, C. Warnke, A systematic review of neurological symptoms and complications of COVID-19, J. Neurol. (2020). doi:10.1007/s00415-020-10067-3.
Y. Yachou, A. El Idrissi, V. Belapasov, S.A. Benali, Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients, Neurological Sciences. 41 (2020) 2657–2669. doi:10.1007/s10072-020-04575-3.
Appendix A. Supplementary data
Supplementary material
References
- 1.W.H. Organization . 2020. General’s Opening Remarks at the Media Briefing on COVID-19. [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L. Others, Covid-19 in critically ill patients in the Seattle region—case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y., Chen S., Yang Z., Guan W., Liu D., Lin Z., Zhang Y., Xu Z., Liu X., Li Y. SARS-CoV-2 viral load in clinical samples of critically ill patients. Am. J. Respir. Crit. Care Med. 2020;201(11):1435–1438. doi: 10.1164/rccm.202003-0572LE. https://www.atsjournals.org/doi/pdf/10.1164/rccm.202003-0572LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. HCA lung biological network, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Geng X., Tan Y., Li Q., Xu C., Xu J., Hao L., Zeng Z., Luo X., Liu F., Wang H. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hanlon S., Inouye S.K. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. 2020;49:497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization and International Severe Acute Respiratory and Emerging Infection Consortium. COVId-19 Core Case Report Form. https://media.tghn.org/medialibrary/2020/05/ISARIC_WHO_nCoV_CORE_CRF_23APR20.pdf Available from:
- 16.Das G., Mukherjee N., Ghosh S. Neurological insights of COVID-19 pandemic. ACS Chem. Neurosci. 2020;11:1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- 17.Conde Cardona G., Quintana Pájaro L.D., Quintero Marzola I.D., Ramos Villegas Y., Moscote Salazar L.R. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J. Neurol. Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 19.Kabbani N., Olds J.L. Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol. Pharmacol. 2020;97:351–353. doi: 10.1124/molpharm.120.000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steardo L., Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020;229 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Liu T., Yang N., Han D., Mi X., Li Y., Liu K., Vuylsteke A., Xiang H., Guo X. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020 doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benussi A., Pilotto A., Premi E., Libri I., Giunta M., Agosti C., Alberici A., Baldelli E., Benini M., Bonacina S., Brambilla L., Caratozzolo S., Cortinovis M., Costa A., Piccinelli S.C., Cottini E., Cristillo V., Delrio I., Filosto M., Gamba M., Gazzina S., Gilberti N., Gipponi S., Imarisio A., Invernizzi P., Leggio U., Leonardi M., Liberini P., Locatelli M., Masciocchi S., Poli L., Rao R., Risi B., Rozzini L., Scalvini A., di Cola F. Schiano, Spezi R., Vergani V., Volonghi I., Zoppi N., Borroni B., Magoni M., Pezzini A., Padovani A. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020 doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 25.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/s2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baller E.B., Hogan C.S., Fusunyan M.A., Ivkovic A., Luccarelli J.W., Madva E., Nisavic M., Praschan N., Quijije N.V., Beach S.R., Smith F.A. Neurocovid: pharmacological recommendations for delirium associated with COVID-19. Psychosomatics. 2020 doi: 10.1016/j.psym.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders B.J., Bakar M., Mehta S., Reid M.C., Siegler E.L., Abrams R.C., Adelman R.D., Lachs M.S. Hyperactive delirium requires more aggressive management in patients with COVID-19: temporarily rethinking “Low and Slow”. J. Pain Symptom Manag. 2020;60:e31–e32. doi: 10.1016/j.jpainsymman.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Kmet L.M., Lee R.C. Alberta heritage foundation for medical research, standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Herit. Found. Med. Res. 2004:1–20. [Google Scholar]
- 30.Lee L., Packer T.L., Tang S.H., Girdler S. Self-management education programs for age-related macular degeneration: a systematic review. Aust. J. Ageing. 2008;27:170–176. doi: 10.1111/j.1741-6612.2008.00298.x. [DOI] [PubMed] [Google Scholar]
- 31.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan S.H., Lindroth H., Perkins A.J., Jamil Y., Wang S., Roberts S., Farber M.O., Rahman O., Gao S., Marcantonio E.R., Boustani M., Machado R., Khan B.A. Delirium incidence, duration and severity in critically Ill patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.31.20118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms J., Kremer S., Merdji H., Schenck M., Severac F., Clere-Jehl R., Studer A., Radosavljevic M., Kummerlen C., Monnier A., Boulay C., Fafi-Kremer S., Castelain V., Ohana M., Anheim M., Schneider F., Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Z., Li T., Liang L., Wang H., Wei F., Meng S., Cai M., Zhang Y., Xu H., Zhang J., Jin R. Clinical characteristics of coronavirus disease 2019 patients in Beijing, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marengoni A., Zucchelli A., Grande G., Fratiglioni L., Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020;49:923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poloni T.E., Carlos A.F., Cairati M., Cutaia C., Medici V., Marelli E., Ferrari D., Galli A., Bognetti P., Davin A., Cirrincione A., Ceretti A., Cereda C., Ceroni M., Tronconi L., Vitali S., Guaita A. Prevalence and prognostic value of Delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. EClinicalMedicine. 2020;26:100490. doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knopp P., Miles A., Webb T.E., Mcloughlin B.C., Mannan I., Raja N., Wan B., Davis D. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. medRxiv. 2020 doi: 10.1101/2020.06.07.20120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcez F.B., Aliberti M.J.R., Poco P.C.E., Hiratsuka M., Takahashi S. de F., Coelho V.A., Salotto D.B., Moreira M.L.V., Jacob-Filho W., Avelino-Silva T.J. Delirium and adverse outcomes in hospitalized patients with COVID-19. J. Am. Geriatr. Soc. 2020;68(11):2440–2446. doi: 10.1111/jgs.16803. https://onlinelibrary.wiley.com/doi/abs/10.1111/jgs.16803?casa_token=sWSzBiI5htoAAAAA:JQjYTiVo5c34ysHPF-91U1JFsEfSAKG9EHnIzAN9Uc_LBXXeOgFoIuMU-6jJkX-qBf3-rbYn093TJA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerah L., Baudouin É., Pépin M., Mary M., Krypciak S., Bianco C., Roux S., Gross A., Toméo C., Lemarié N., Dureau A., Bastiani S., Ketz F., Boully C., de Villelongue C., Romdhani M., Desoutter M.-A., Duron E., David J.-P., Thomas C., Paillaud E., de Malglaive P., Bouvard E., Lacrampe M., Mercadier E., Monti A., Hanon O., Fossey-Diaz V., Bourdonnec L., Riou B., Vallet H., Boddaert J., APHP/Universities/Inserm COVID-19 research collaboration Clinical characteristics and outcomes of 821 older patients with SARS-Cov-2 infection admitted to acute care geriatric wards. J. Gerontol. A Biol. Sci. Med. Sci. 2020 doi: 10.1093/gerona/glaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilotto A., Benussi A., Libri I., Masciocchi S., Poli L., Premi E., Alberici A., Baldelli E., Bonacina S., Brambilla L. COVID-19 impact on consecutive neurological patients admitted to the emergency department. Journal of Neurology, Neurosurgery, and Psychiatry. 2020 doi: 10.1136/jnnp-2020-323929. https://www.medrxiv.org/content/10.1101/2020.05.23.20110650v1.abstract [DOI] [PubMed] [Google Scholar]
- 42.Lovell N., Maddocks M., Etkind S.N., Taylor K., Carey I., Vora V., Marsh L., Higginson I.J., Prentice W., Edmonds P., Sleeman K.E. Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J. Pain Symptom Manag. 2020;60:e77–e81. doi: 10.1016/j.jpainsymman.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G., Bianchetti L., Trabucchi M. Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 2020;24:560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heath L., Yates S., Carey M., Miller M. Palliative care during COVID-19: data and visits from loved ones. Am. J. Hosp. Palliat. Care. 2020;37:988–991. doi: 10.1177/1049909120943577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcloughlin B.C., Miles A., Webb T.E., Knopp P., Eyres C., Fabbri A., Humphries F., Davis D. Functional and cognitive outcomes after COVID-19 delirium. Eur. Geriatr. Med. 2020;11:857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kremer S., Lersy F., Anheim M., Merdji H., Schenck M., Oesterlé H., Bolognini F., Messie J., Khalil A., Gaudemer A., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Jager L., Nesser P., Mba Y.T., Hmeydia G., Benzakoun J., Oppenheim C., Ferré J.-C., Maamar A., Carsin-Nicol B., Comby P.-O., Ricolfi F., Thouant P., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Constans J.-M., Zorn P.-E., Mathieu M., Baloglu S., Ardellier F.-D., Willaume T., Brisset J.-C., Caillard S., Collange O., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., Meyer N., Helms J., Cotton F. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 47.Studart-Neto A., Guedes B.F., Tuma R. de L.E., Filho A.E. Camelo, Kubota G.T., Iepsen B.D., Moreira G.P., Rodrigues J.C., Ferrari M.M.H., Carra R.B., Spera R.R., Oku M.H.M., Terrim S., Lopes C.C.B., Neto C.E.B. Passos, Fiorentino M.D., Souza J.C.C.D.E., Baima J.P.S., Silva T.F.F.D.A., Moreno C.A.M., Silva A.M.S., Heise C.O., MendonÇa R.H., Fortini I., Smid J., Adoni T., GonÇalves M.R.R., Pereira S.L.A., Pinto L.F., Gomes H.R., Zanoteli E., Brucki S.M.D., Conforto A.B., Castro L.H.M., Nitrini R. Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq. Neuropsiquiatr. 2020;78:494–500. doi: 10.1590/0004-282x20200089. [DOI] [PubMed] [Google Scholar]
- 48.Radmard S., Epstein S.E., Roeder H.J., Michalak A.J., Shapiro S.D., Boehme A., Wilson T.J., Duran J.C., Bain J.M., Willey J.Z. Inpatient neurology consultations during the onset of the SARS-CoV-2 New York City pandemic: a single center case series. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00805. https://www.ncbi.nlm.nih.gov/pmc/articles/pmc7365853/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., Coles J.P., Manji H., Al-Shahi Salman R., Menon D.K., Nicholson T.R., Benjamin L.A., Carson A., Smith C., Turner M.R., Solomon T., Kneen R., Pett S.L., Galea I., Thomas R.H., Michael B.D., CoroNerve Study Group Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinna P., Grewal P., Hall J.P., Tavarez T., Dafer R.M., Garg R., Osteraas N.D., Pellack D.R., Asthana A., Fegan K., Patel V., Conners J.J., John S., Silva I.D. Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J. Neurol. Sci. 2020;415:116969. doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal P., Ray S., Madan A., Tyson B. Neurological manifestations in 404 COVID-19 patients in Washington State. J. Neurol. 2020 doi: 10.1007/s00415-020-10087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giorgianni A., Vinacci G., Agosti E., Mercuri A., Baruzzi F. Neuroradiological features in COVID-19 patients: first evidence in a complex scenario. J. Neuroradiol. 2020;47:474–476. doi: 10.1016/j.neurad.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chachkhiani D., Soliman M.Y., Barua D., Isakadze M., Villemarette-Pittman N.R., Devier D.J., Lovera J.F. Neurological complications in a predominantly African American sample of COVID-19 predict worse outcomes during hospitalization. Clin. Neurol. Neurosurg. 2020;197:106173. doi: 10.1016/j.clineuro.2020.106173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vena A., Giacobbe D.R., Di Biagio A., Mikulska M., Taramasso L., De Maria A., Ball L., Brunetti I., Loconte M., Patroniti N.A., Robba C., Delfino E., Dentone C., Magnasco L., Nicolini L., Toscanini F., Bavastro M., Cerchiaro M., Barisione E., Giacomini M., Mora S., Baldi F., Balletto E., Berruti M., Briano F., Sepulcri C., Dettori S., Labate L., Mirabella M., Portunato F., Pincino R., Russo C., Tutino S., Pelosi P., Bassetti M. GECOVID study group, Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin. Microbiol. Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butt I., Sawlani V., Geberhiwot T. Prolonged confusional state as first manifestation of COVID-19. Ann. Clin. Transl. Neurol. 2020;368:m810. doi: 10.1002/acn3.51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward C.F., Figiel G.S., McDonald W.M. Altered mental status as a novel initial clinical presentation for COVID-19 infection in the elderly. Am. J. Geriatr. Psychiatry. 2020;28:808–811. doi: 10.1016/j.jagp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zayet S., Ben Abdallah Y., Royer P.-Y., Toko-Tchiundzie L., Gendrin V., Klopfenstein T. Encephalopathy in patients with COVID-19:“Causality or coincidence?”. J. Med. Virol. 2020 doi: 10.1002/jmv.26027. https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.26027?casa_token=1IcDhK79CNEAAAAA:gvfb63DOnkpwwGYhstQPvw1M3NveJLB-DgbfHAX7U5Ed6chYAifZFZ9rthJaenLpkK4A_izgWg0zhQ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alkeridy W.A., Almaglouth I., Alrashed R., Alayed K., Binkhamis K., Alsharidi A., Liu-Ambrose T. A unique presentation of delirium in a patient with otherwise asymptomatic COVID-19. J. Am. Geriatr. Soc. 2020;68(7):1382–1384. doi: 10.1111/jgs.16536. https://onlinelibrary.wiley.com/doi/abs/10.1111/jgs.16536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tay H.S., Harwood R. Atypical presentation of COVID-19 in a frail older person. Age Ageing. 2020;49:523–524. doi: 10.1093/ageing/afaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin R., Feng W., Wang T., Chen G., Wu T., Chen D., Lv T., Xiang D. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi M., Sahashi Y., Baba Y., Okura H., Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J. Neurol. Sci. 2020;415:116941. doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hosseini A.A., Shetty A.K., Sprigg N., Auer D.P., Constantinescu C.S. Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palomar-Ciria N., Blanco Del Valle P., Hernández-Las Heras M.Á., Martínez-Gallardo R. Schizophrenia and COVID-19 delirium. Psychiatry Res. 2020;290:113137. doi: 10.1016/j.psychres.2020.113137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Djellaoui A., Seddik L., Cleret De Langavant L., Cattan S., Bachoud-Lévi A.C., Hosseini H. Posterior reversible encephalopathy syndrome associated with SARS-CoV-2 infection. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-323923. [DOI] [PubMed] [Google Scholar]
- 66.Soysal P., Kara O. Delirium as the first clinical presentation of the coronavirus disease 2019 in an older adult. Psychogeriatrics. 2020;20:763–765. doi: 10.1111/psyg.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sirous R., Taghvaei R., Hellinger J.C., Krauthamer A.V., Mirfendereski S. COVID-19-associated encephalopathy with fulminant cerebral vasoconstriction: CT and MRI findings. Radiol Case Rep. 2020;15:2208–2212. doi: 10.1016/j.radcr.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zambreanu L., Lightbody S., Bhandari M., Hoskote C., Kandil H., Houlihan C.F., Lunn M.P. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry. 2020;91:1229–1230. doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- 69.Bernard-Valnet R., Pizzarotti B., Anichini A., Demars Y., Russo E., Schmidhauser M., Cerruti-Sola J., Rossetti A.O., Du Pasquier R. Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1101/2020.04.17.20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cipriani G., Danti S., Nuti A., Carlesi C., Lucetti C., Di Fiorino M. A complication of coronavirus disease 2019: delirium. Acta Neurol. Belg. 2020;120:927–932. doi: 10.1007/s13760-020-01401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., Fricchione G.L., Smith F.A. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Payus A.O., Lin C.L.S., Noh M.M., Jeffree M.S., Ali R.A. SARS-CoV-2 infection of the nervous system: a review of the literature on neurological involvement in novel coronavirus disease (COVID-19) Bosnian J. Basic Med. Sci. 2020 doi: 10.17305/bjbms.2020.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicholson P., Alshafai L., Krings T. Neuroimaging findings in patients with COVID-19. AJNR Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abboud H., Abboud F.Z., Kharbouch H., Arkha Y., El Abbadi N., El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed M.U., Hanif M., Ali M.J., Haider M.A., Kherani D., Memon G.M., Karim A.H., Sattar A. Neurological manifestations of COVID-19 (SARS-CoV-2): a review. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol. Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Northwell COVID-19 Research Consortium, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A., COVID-19 Lombardy ICU Network, Nailescu A., Corona A., Zangrillo A., Protti A., Albertin A., Molinari A. Forastieri, Lombardo A., Pezzi A., Benini A., Scandroglio A.M., Malara A., Castelli A., Coluccello A., Micucci A., Pesenti A., Sala A., Alborghetti A., Antonini B., Capra C., Troiano C., Roscitano C., Radrizzani D., Chiumello D., Coppini D., Guzzon D., Costantini E., Malpetti E., Zoia E., Catena E., Agosteo E., Barbara E., Beretta E., Boselli E., Storti E., Harizay F., Mura F. Della, Lorini F.L., Sigurtà F. Donato, Marino F., Mojoli F., Rasulo F., Grasselli G., Casella G., De Filippi G., Castelli G., Aldegheri G., Gallioli G., Lotti G., Albano G., Landoni G., Marino G., Vitale G., Perego G. Battista, Evasi G., Citerio G., Foti G., Natalini G., Merli G., Sforzini I., Bianciardi L., Carnevale L., Grazioli L., Cabrini L., Guatteri L., Salvi L., Poli M. Dei, Galletti M., Gemma M., Ranucci M., Riccio M., Borelli M., Zambon M., Subert M., Cecconi M., Mazzoni M.G., Raimondi M., Panigada M., Belliato M., Bronzini N., Latronico N., Petrucci N., Belgiorno N., Tagliabue P., Cortellazzi P., Gnesin P., Grosso P., Gritti P., Perazzo P., Severgnini P., Ruggeri P., Sebastiano P., Covello R.D., Fernandez-Olmos R., Fumagalli R., Keim R., Rona R., Valsecchi R., Cattaneo S., Colombo S., Cirri S., Bonazzi S., Greco S., Muttini S., Langer T., Alaimo V., Viola U. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehta S., Cook D., Devlin J.W., Skrobik Y., Meade M., Fergusson D., Herridge M., Steinberg M., Granton J., Ferguson N., Tanios M., Dodek P., Fowler R., Burns K., Jacka M., Olafson K., Mallick R., Reynolds S., Keenan S., Burry L., SLEAP Investigators, Canadian Critical Care Trials Group Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit. Care Med. 2015;43:557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 81.Wenting A., Gruters A., van Os Y., Verstraeten S., Valentijn S., Ponds R., de Vugt M. COVID-19 neurological manifestations and underlying mechanisms: a scoping review. Front. Psychiatry. 2020;11:860. doi: 10.3389/fpsyt.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020;76:3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183 doi: 10.1016/j.cell.2020.08.028. 16–27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is associated with an unusual pattern of brain microbleeds in critically Ill patients. J. Neuroimaging. 2020;30:593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doo F.X., Kassim G., Lefton D.R., Patterson S., Pham H., Belani P. Rare presentations of COVID-19: PRES-like leukoencephalopathy and carotid thrombosis. Clin. Imaging. 2020;69:94–101. doi: 10.1016/j.clinimag.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Najjar S., Najjar A., Chong D.J., Pramanik B.K., Kirsch C., Kuzniecky R.I., Pacia S.V., Azhar S. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J. Neuroinflammation. 2020;17:231. doi: 10.1186/s12974-020-01896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kempuraj D., Selvakumar G.P., Ahmed M.E., Raikwar S.P., Thangavel R., Khan A., Zaheer S.A., Iyer S.S., Burton C., James D., Zaheer A. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26:402–414. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 88.Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., Gautier-Vargas G., Keller N., Kremer S., Fafi-Kremer S., Moulin B., Benotmane I., Caillard S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2020 doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L., Shen Y., Li M., Chuang H., Ye Y., Zhao H., Wang H. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J. Neurol. 2020 doi: 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balcioglu Y.H., Yesilkaya U.H., Gokcay H., Kirlioglu S.S. May the central nervous system be fogged by the cytokine storm in COVID-19?: an appraisal. J. NeuroImmune Pharmacol. 2020 doi: 10.1007/s11481-020-09932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lahiri D., Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12 doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santis G.D., De Santis G. SARS-CoV-2: A new virus but a familiar inflammation brain pattern. Brain Behav. Immun. 2020;87:95–96. doi: 10.1016/j.bbi.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020;38:1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sher Y., Rabkin B., Maldonado J.R., Mohabir P. COVID-19–associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report. Psychosomatics. 2020 doi: 10.1016/j.psym.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R., Hernandez T., Stock A., Zhao Z., Al Rasheed M., Chen J., Li L., Wang D., Corben A., Haines K., Westra W., Umphlett M., Gordon R.E., Reidy J., Petersen B., Salem F., Fiel M., El Jamal S.M., Tsankova N.M., Houldsworth J., Mussa Z., Liu W.-C., Veremis B., Sordillo E., Gitman M., Nowak M., Brody R., Harpaz N., Merad M., Gnjatic S., Donnelly R., Seigler P., Keys C., Cameron J., Moultrie I., Washington K.-L., Treatman J., Sebra R., Jhang J., Firpo A., Lednicky J., Paniz-Mondolfi A., Cordon-Cardo C., Fowkes M. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 doi: 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- 97.Serrano-Castro P.J., Estivill-Torrús G., Cabezudo-García P., Reyes-Bueno J.A., Ciano Petersen N., Aguilar-Castillo M.J., Suárez-Pérez J., Jiménez-Hernández M.D., Moya-Molina M.Á., Oliver-Martos B., Arrabal-Gómez C., de Fonseca F.R. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurología (English Edition). 2020;35:245–251. doi: 10.1016/j.nrleng.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Román G.C., Spencer P.S., Reis J., Buguet A., El Alaoui Faris M., Katrak S.M., Láinez M., Medina M.T., Meshram C., Mizusawa H., Öztürk S., Wasay M. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020;414:116884. doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pallanti S. Importance of SARs-Cov-2 anosmia: from phenomenology to neurobiology. Compr. Psychiatry. 2020;100:152184. doi: 10.1016/j.comppsych.2020.152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mankad K., Perry M.D., Mirsky D.M., Rossi A. COVID-19: a primer for neuroradiologists. Neuroradiology. 2020;62:647–648. doi: 10.1007/s00234-020-02437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aaroe A.E., Majd N., Weathers S.-P., de Groot J.F. Potential neurologic and oncologic implications of the novel coronavirus. Neuro-Oncology. 2020;22:1050–1051. doi: 10.1093/neuonc/noaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu K., Pan M., Xiao Z., Xu X. Neurological manifestations of the coronavirus (SARS-CoV-2) pandemic 2019–2020. J. Neurol. Neurosurg. Psychiatry. 2020;91:669–670. doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- 103.Calcagno N., Colombo E., Maranzano A., Pasquini J., Sarmiento I.J.K., Trogu F., Silani V. Rising evidence for neurological involvement in COVID-19 pandemic. Neurol. Sci. 2020;41:1339–1341. doi: 10.1007/s10072-020-04447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sellner J., Taba P., Öztürk S., Helbok R. The need for neurologists in the care of COVID-19 patients. Eur. J. Neurol. 2020 doi: 10.1111/ene.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin H., Hong C., Chen S., Zhou Y., Wang Y., Mao L., Li Y., He Q., Li M., Su Y., Wang D., Wang L., Hu B. Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc. Neurol. 2020;5:146–151. doi: 10.1136/svn-2020-000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwong K.C.N.K., Ng Koy Chong, Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang H.-Y., Li X.-L., Yan Z.-R., Sun X.-P., Han J., Zhang B.-W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420917830. 175628642091783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acharya A., Kevadiya B.D., Gendelman H.E., Byrareddy S.N. SARS-CoV-2 infection leads to neurological dysfunction. J. NeuroImmune Pharmacol. 2020;15:167–173. doi: 10.1007/s11481-020-09924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sepehrinezhad A., Shahbazi A., Negah S.S. COVID-19 virus may have neuroinvasive potential and cause neurological complications: a perspective review. J. Neurovirol. 2020;26:324–329. doi: 10.1007/s13365-020-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song E., Zhang C., Israelow B., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Wilen C.B., Horvath T.L., Louvi A., Farhadian S.F., Bilguvar K., Iwasaki A. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. Cold Spring Harbor. Lab. 2020 doi: 10.1101/2020.06.25.169946. 2020.06.25.169946. [DOI] [Google Scholar]
- 112.Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., Tang X., Zhu J., Zhao Z., Jaffré F., Zhang T., Kim T.W., Harschnitz O., Redmond D., Houghton S., Liu C., Naji A., Ciceri G., Guttikonda S., Bram Y., Nguyen D.-H.T., Cioffi M., Chandar V., Hoagland D.A., Huang Y., Xiang J., Wang H., Lyden D., Borczuk A., Chen H.J., Studer L., Pan F.C., Ho D.D., ten Oever B.R., Evans T., Schwartz R.E., Chen S. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27 doi: 10.1016/j.stem.2020.06.015. 125–136.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al Saiegh F., Ghosh R., Leibold A., Avery M.B., Schmidt R.F., Theofanis T., Mouchtouris N., Philipp L., Peiper S.C., Wang Z.-X., Rincon F., Tjoumakaris S.I., Jabbour P., Rosenwasser R.H., Gooch M.R. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry. 2020;91:846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 115.Espíndola O. de M., Espíndola O. de Melo, Siqueira M., Soares C.N., de Lima M.A.S.D., Celestino Ana Claudia, Araujo A.Q.C., Brandão C.O., Silva M.T.T. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int. J. Infect. Dis. 2020;96:567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Espinosa P.S., Rizvi Z., Sharma P., Hindi F., Filatov A. 2020. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy, MRI Brain and Cerebrospinal Fluid Findings: Case 2, Cureus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guilmot A., Slootjes S.M., Sellimi A., Bronchain M., Hanseeuw B., Belkhir L., Yombi J.C., De Greef J., Pothen L., Yildiz H., Duprez T., Fillée C., Anantharajah A., Capes A., Hantson P., Jacquerye P., Raymackers J.-M., London F., El Sankari S., Ivanoiu A., Maggi P., van Pesch V. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2020 doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang Y.H., Hanna Huang Y., Jiang D., Huang J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87 doi: 10.1016/j.bbi.2020.05.012. 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saleki K., Banazadeh M., Saghazadeh A., Rezaei N. The involvement of the central nervous system in patients with COVID-19. Rev. Neurosci. 2020;31:453–456. doi: 10.1515/revneuro-2020-0026. [DOI] [PubMed] [Google Scholar]
- 122.Coolen T., Lolli V., Sadeghi N., Rovai A., Trotta N., Taccone F.S., Creteur J., Henrard S., Goffard J.-C., Dewitte O., Naeije G., Goldman S., De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95:e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 123.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao H.C.Y., Yashavantha Rao H.C., Jayabaskaran C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J. Med. Virol. 2020;92:786–790. doi: 10.1002/jmv.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iroegbu J.D., Ifenatuoha C.W., Ijomone O.M. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol. Sci. 2020;41:1329–1337. doi: 10.1007/s10072-020-04469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roe K. Explanation for COVID-19 infection neurological damage and reactivations. Transbound. Emerg. Dis. 2020;67:1414–1415. doi: 10.1111/tbed.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bulfamante G., Chiumello D., Canevini M.P., Priori A., Mazzanti M., Centanni S., Felisati G. First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86 doi: 10.23736/s0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- 129.Esposito G., Pesce M., Seguella L., Sanseverino W., Lu J., Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav. Immun. 2020;87:93–94. doi: 10.1016/j.bbi.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pereira A. Long-term neurological threats of COVID-19: a call to update the thinking about the outcomes of the coronavirus pandemic. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bostancıklıoğlu M. Temporal correlation between neurological and gastrointestinal symptoms of SARS-CoV-2. Inflamm. Bowel Dis. 2020 doi: 10.1093/ibd/izaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Toljan K. Letter to the editor regarding the viewpoint “Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism”. ACS Chem. Neurosci. 2020;11:1192–1194. doi: 10.1021/acschemneuro.0c00174. [DOI] [PubMed] [Google Scholar]
- 134.Saavedra J.M. COVID-19, angiotensin receptor blockers, and the brain. Cell. Mol. Neurobiol. 2020;40:667–674. doi: 10.1007/s10571-020-00861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen S., Lu H., Liu Z., Yuan W. Comment on “Central nervous system involvement by severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2)”. J. Med. Virol. 2020 doi: 10.1002/jmv.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chigr F., Merzouki M., Najimi M. Comment on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J. Med. Virol. 2020;92:703–704. doi: 10.1002/jmv.25960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baig A.M. Updates on what ACS reported: emerging evidences of COVID-19 with nervous system involvement. ACS Chem. Neurosci. 2020;11:1204–1205. doi: 10.1021/acschemneuro.0c00181. [DOI] [PubMed] [Google Scholar]
- 138.Holmes E.A., O’Connor R.C., Hugh Perry V., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Silver R.C., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M., Yardley L., Cowan K., Cope C., Hotopf M., Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/s2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paybast S., Emami A., Koosha M., Baghalha F. Novel coronavirus Disease (COVID-19) and central nervous system complications: what neurologist need to know. Acta Neurol. Taiwanica. 2020;29(1):24–31. [PubMed] [Google Scholar]
- 140.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Sousa A.K., de Sousa A.K., de Aguiar Magalhães D., dos Santos Ferreira J., dos Reis Barbosa A.L. SARS-CoV-2-mediated encephalitis: role of AT2R receptors in the blood-brain barrier. Med. Hypotheses. 2020;144:110213. doi: 10.1016/j.mehy.2020.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020 doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Werner C., Scullen T., Mathkour M., Zeoli T., Beighley A., Kilgore M.D., Carr C., Zweifler R.M., Aysenne A., Maulucci C.M., Dumont A.S., Bui C.J., Keen J.R. Neurological impact of coronavirus disease of 2019: practical considerations for the neuroscience community. World Neurosurg. 2020;139:344–354. doi: 10.1016/j.wneu.2020.04.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Z., Huang Y., Guo X. The brain, another potential target organ, needs early protection from SARS-CoV-2 neuroinvasion. Sci. China Life Sci. 2020;63:771–773. doi: 10.1007/s11427-020-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tassorelli C., Mojoli F., Baldanti F., Bruno R., Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur. J. Neurol. 2020 doi: 10.1111/ene.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.di Giacomo E., di Giacomo E., Bellelli G., Peschi G., Scarpetta S., Colmegna F., Girolamo G., Clerici M. Management of older people during the COVID-19 outbreak: recommendations from an Italian experience. Int. J. Geriatr. Psychiatr. 2020;35:803–805. doi: 10.1002/gps.5318. [DOI] [PubMed] [Google Scholar]
- 147.LaHue S.C., James T.C., Newman J.C., Esmaili A.M., Ormseth C.H., Wesley Ely E. Collaborative delirium prevention in the age of COVID -19. J. Am. Geriatr. Soc. 2020;68:947–949. doi: 10.1111/jgs.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.More advice on the use of ibuprofen for COVID-19Drug Ther. Bull. 2020;58 doi: 10.1136/dtb.2020.000032. 101. [DOI] [PubMed] [Google Scholar]
- 149.Simpson R., Robinson L. Rehabilitation following critical illness in people with COVID-19 infection. Am. J. Phys. Med. Rehabil. 2020;1 doi: 10.1097/phm.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zambrelli E., Canevini M., Gambini O., D’Agostino A. Delirium and sleep disturbances in COVID-19: a possible role for melatonin in hospitalized patients? Sleep Med. 2020;70:111. doi: 10.1016/j.sleep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.N.I.F.H.A.C.E. (nice) in C.W.N.E.A.N Improvement, National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement, Managing COVID-19 symptoms (including at the end of life) in the community: summary of NICE guidelines. BMJ. 2020 doi: 10.1136/bmj.m1461. m1461. [DOI] [Google Scholar]
- 152.Bilbul M., Paparone P., Kim A.M., Mutalik S., Ernst C.L. Psychopharmacology of COVID-19. Psychosomatics. 2020 doi: 10.1016/j.psym.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Emmerton D., Abdelhafiz A. Delirium in older people with COVID-19: clinical scenario and literature review. SN Compr. Clin. Med. 2020;2:1790–1797. doi: 10.1007/s42399-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gómez-Arnau J., Hernández-Huerta D. Agitation management during the COVID-19 pandemic. Prim. Care Companion CNS Dis. 2020;22 doi: 10.4088/pcc.20com02734. [DOI] [PubMed] [Google Scholar]
- 155.Wong A.H., Roppolo L.P., Chang B.P., Yonkers K.A., Wilson M.P., Powsner S., Rozel J.S. Management of agitation during the COVID-19 pandemic. West. J. Emerg. Med. 2020;21:795–800. doi: 10.5811/westjem.2020.5.47789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Castro R.E.V., de Castro R.E.V., Garcez F.B., Avelino-Silva T.J. Patient care during the COVID-19 pandemic: do not leave delirium behind. Braz. J. Psychiatry. 2020 doi: 10.1590/1516-4446-2020-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee S. COVID-19 pandemic and care of older adults at risk for delirium and cognitive vulnerability. West. J. Emerg. Med. 2020;21 doi: 10.5811/westjem.2020.5.47800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the united states. AJNR Am. J. Neuroradiol. 2020;41:1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ostuzzi G., Papola D., Gastaldon C., Schoretsanitis G., Bertolini F., Amaddeo F., Cuomo A., Emsley R., Fagiolini A., Imperadore G., Kishimoto T., Michencigh G., Nosé M., Purgato M., Dursun S., Stubbs B., Taylor D., Thornicroft G., Ward P.B., Hiemke C., Correll C.U., Barbui C. Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations. BMC Med. 2020;18 doi: 10.1186/s12916-020-01685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crowley K.E., Urben L., Hacobian G., Geiger K.L. Valproic acid for the management of agitation and delirium in the intensive care setting: a retrospective analysis. Clin. Ther. 2020;42:e65–e73. doi: 10.1016/j.clinthera.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 161.Gagnon D.J., Fontaine G.V., Smith K.E., Riker R.R., Miller R.R., Lerwick P.A., Lucas F.L., Dziodzio J.T., Sihler K.C., Fraser G.L. Valproate for agitation in critically ill patients: a retrospective study. J. Crit. Care. 2017;37:119–125. doi: 10.1016/j.jcrc.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 162.Juybari K.B., Pourhanifeh M.H., Hosseinzadeh A., Hemati K., Mehrzadi S. Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res. 2020;287:198108. doi: 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chitimus D.M., Popescu M.R., Voiculescu S.E., Panaitescu A.M., Pavel B., Zagrean L., Zagrean A.-M. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules. 2020;10:1211. doi: 10.3390/biom10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alderman B., Webber K., Davies A. An audit of end-of-life symptom control in patients with corona virus disease 2019 (COVID-19) dying in a hospital in the United Kingdom. Palliat. Med. 2020;34:1249–1255. doi: 10.1177/0269216320947312. [DOI] [PubMed] [Google Scholar]
- 165.Martinotti G., Barlati S., Prestia D., Palumbo C., Giordani M., Cuomo A., Miuli A., Paladini C., Amore M., Bondi E., Vita A., Fagiolini A., Di Giannantonio M. Psychomotor agitation and hyperactive delirium in COVID-19 patients treated with aripiprazole 9.75 mg/1.3 ml immediate release. Psychopharmacology. 2020;237:3497–3501. doi: 10.1007/s00213-020-05644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anmella G., Arbelo N., Fico G., Murru A., Llach C.D., Madero S., Gomes-da-Costa S., Imaz M.L., López-Pelayo H., Vieta E., Pintor L. COVID-19 inpatients with psychiatric disorders: real-world clinical recommendations from an expert team in consultation-liaison psychiatry. J. Affect. Disord. 2020;274:1062–1067. doi: 10.1016/j.jad.2020.05.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Slooter A.J.C., Otte W.M., Devlin J.W., Arora R.C., Bleck T.P., Claassen J., Duprey M.S., Ely E.W., Kaplan P.W., Latronico N., Morandi A., Neufeld K.J., Sharshar T., MacLullich A.M.J., Stevens R.D. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46:1020–1022. doi: 10.1007/s00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marcantonio E.R. Delirium in hospitalized older adults. N. Engl. J. Med. 2018;378:96–97. doi: 10.1056/NEJMc1714932. [DOI] [PubMed] [Google Scholar]
- 169.Kuo C.-L., Pilling L.C., Atkins J.L., Masoli J.A.H., Delgado J., Kuchel G.A., Melzer D. APOE e4 genotype predicts severe Covid-19 in the UK Biobank community cohort. medRxiv. 2020 doi: 10.1101/2020.05.07.20094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sinha P., Matthay M.A., Calfee C.S. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 171.Sinha P., for the NHLBI ARDS Network, Delucchi K.L., Thompson B. Taylor, McAuley D.F., Matthay M.A., Calfee C.S. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thom R.P., Levy-Carrick N.C., Bui M., Silbersweig D. Delirium. Am. J. Psychiatry. 2019;176:785–793. doi: 10.1176/appi.ajp.2018.18070893. [DOI] [PubMed] [Google Scholar]
- 173.Markowitz J.D., Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont) 2008;5 29. [PMC free article] [PubMed] [Google Scholar]
- 174.Scullen T., Keen J., Mathkour M., Dumont A.S., Kahn L. Coronavirus 2019 (COVID-19)–associated encephalopathies and cerebrovascular disease: the new orleans experience. World Neurosurg. 2020;141:e437–e446. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jäckel M., Bemtgen X., Wengenmayer T., Bode C., Biever P.M., Staudacher D.L. Is delirium a specific complication of viral acute respiratory distress syndrome? Crit. Care. 2020;24 doi: 10.1186/s13054-020-03136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan S., Xiao M., Han F., Xia P., Bai X., Chen H., Zhang H., Ding X., Zhao H., Zhao J., Sun X., Jiang W., Wang C., Cao W., Guo F., Tian R., Gao P., Wu W., Ma J., Wu D., Liu Z., Zhou X., Wang J., Guan T., Qin Y., Li T., Xu Y., Zhang D., Chen Y., Xie J., Li Y., Yan X., Zhu Y., Peng B., Cui L., Zhang S., Guan H. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front. Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sultan I., Habertheuer A., Usman A.A., Kilic A., Gnall E., Friscia M.E., Zubkus D., Hirose H., Sanchez P., Okusanya O., Szeto W.Y., Gutsche J. The role of extracorporeal life support for patients with COVID-19: preliminary results from a statewide experience. J. Card. Surg. 2020;35:1410–1413. doi: 10.1111/jocs.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M., Ora J., Mina G.G., Puxeddu E., Balbi O., Pezzuto G., Magrini A., Rogliani P., Andreoni M., Mercuri N.B. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav. Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luigetti M., Iorio R., Bentivoglio A.R., Tricoli L., Riso V., Marotta J., Piano C., Primiano G., Zileri Del Verme L., Lo Monaco M.R., Calabresi P., GEMELLI AGAINST COVID-19 group Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur. J. Neurol. 2020 doi: 10.1111/ene.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iltaf S., Fatima M., Salman S., Salam J.-U., Abbas S. Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus. 2020 doi: 10.7759/cureus.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., Li N., Liu J., Yang D., Gao H., Zhang Y., Lin M., Shen S., Zhang H., Chen L., Wang G., Luo F., Li W., Chen S., He L., Sander J.W., Zhou D. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479–e1487. doi: 10.1212/wnl.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 182.Frontera J.A., Valdes E., Huang J., Lewis A., Lord A.S., Zhou T., Ethan Kahn D., Melmed K., Czeisler B.M., Yaghi S., Scher E., Wisniewski T., Balcer L., Hammer E. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit. Care Med. 2020;48:e1211–e1217. doi: 10.1097/ccm.0000000000004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karadaş Ö., Öztürk B., Sonkaya A.R. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol. Sci. 2020 doi: 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S., Lemahieu W., Symons R., Ho E., Frans J., Smismans A., Laurent M.R. Frailty and mortality in hospitalized older adults With COVID-19: retrospective observational study. J. Am. Med. Dir. Assoc. 2020;21 doi: 10.1016/j.jamda.2020.06.008. 928–932.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material