Abstract
Background:
Chemotherapy-induced mucositis (CIM) complicates cancer therapy and limits maximum tolerated doses and efficacy. Rodent models do not reproducibly mimic clinical CIM, so alternative models are needed.
Methods:
CIM severity was assessed after weaned pigs were treated with doxorubicin (5 and 3.75 mg/kg) using clinical observations, laboratory parameters and gastrointestinal structure and functions. Bovine colostrum was provided as an experimental intervention to the pigs treated receiving the 3.75 mg/kg dose.
Results:
Doxorubin at 3.75 mg/kg decreased food intake and weight gain (p < 0.05) and caused diarrhea and vomiting that coincided with damage to the small intestine mucosa based on histological scoring (p < 0.05). It resulted in higher serum TNF-α concentrations, increased chloride secretion and reduced brush border membrane disaccharidase activities and carrier-mediated glucose uptake (all p < 0.05). The gastrointestinal damage and dysfunction resemble the clinical and laboratory features of CIM in humans; these can be partially prevented by providing cow colostrum.
Conclusion:
The weaned pig is a relevant large animal for studying CIM and evaluating existing and experimental interventions for mucositis.
Keywords: Chemotherapy-induced mucositis, Doxorubicin, Weaned-pig model, Bovine colostrum
Introduction
Many chemotherapeutic agents used for cancer therapy induce painful inflammation and gastrointestinal (GI) tract dysfunction that can extend from the mouth to the anus [1]. Indeed, chemotherapy-induced mucositis (CIM) is among the most common toxicities of cancer therapy. Clinical manifestations of CIM include painful ulcers in the oral cavity, nausea, vomiting, abdominal pain, diarrhea, inflammation and ulceration throughout the GI tract. Patients with CIM eat less and many stop eating, have problems tolerating oral medications and are at a higher risk of systemic infections and other complications due to direct chemotherapeutic damage to and anorexia-induced atrophy of the GI tract. The adverse GI responses to chemotherapy impose an economic burden [2], delay the therapy in some cases and are the limiting factor in deciding the maximum tolerated dosage for many chemotherapeutic agents, thereby limiting the efficacy of treatment. A recent Cochrane review [3] of 131 randomized trials of agents to prevent CIM found that 10 agents provided some protection (Aloe vera, amifostine, cryotherapy, granulocyte-colony stimulating factor, intravenous glutamine, honey, keratinocyte growth factor, laser, polymixin/tobramycin/amphotericin antibiotic pastille/paste and sucralfate), but none has achieved universal acceptance and the protective effects reported were modest, which leaves room for the development of new, more effective strategies. It may be that combinations of agents with different mechanisms of action could provide additional protection, but studies to determine this require careful preclinical and clinical trials to document their efficacy, which highlights the need for animal models that accurately reflect the human disease.
The internal GI damage and dysfunction caused by chemotherapeutic agents and the efficacy and safety of interventions for CIM are difficult to assess in humans. Of necessity, observations of patients are limited to reports of symptoms, evaluation of the oral cavity, assessments of general health and indirect measurements of GI function (e.g. sugar permeability, breath hydrogen, plasma citrulline, cytokines, blood chemistry and cultures). By definition, supportive care dictates that clinicians minimize CIM-associated symptoms and complications. Combined with other ethical limitations associated with having patients as research subjects, this makes it difficult to assess the severity of CIM in clinical trials on CIM interventions.
To date, the majority of preclinical CIM research has used rodent models, with the rat as the dominant model [4]. Although laboratory rodent models have provided valuable insights into the pathology of CIM and interventions, there are limitations that can lead to questions about their clinical relevance [5]. There is a need for translational nonrodent animal models that are compatible with clinical settings and chemotherapy regimens and amenable for evaluating existing and experimental interventions for mucositis and that can be adopted by investigators. The pig is increasingly recognized as a translational, large-animal model for the human GI tract [6] and other aspects of physiology, metabolism and disease [7]. Juvenile pigs have been used to evaluate a surgical procedure used for patients with rectal cancer [8]. A previous report describes CIM in a porcine model using 5-fluorouracil (5FU) that is accompanied by GI inflammation, diarrhea and other indications of GI epithelial disruption and dysfunction [9]. However, this model imposed malnutrition, and the 14-day induction period was longer than the more rapid onset of mucositis symptoms typically reported for humans receiving chemotherapy. A recent neonatal pig model of CIM for infants not yet weaned [10] is based on myeloablative chemotherapy by administering a combination of busulfan and cyclophosphamide. Though relevant to fields such as hematopoietic cell transplantation, this has little correlative value as a preclinical model for CIM associated with nonmyeloablative chemotherapy such as that used in common regimens for the primary treatment of pediatric cancers.
Our objective was to develop a translational large-animal model to address a critical need for such a model, considered relevant for patients undergoing nonmyeloablative cancer chemotherapy. We describe model that uses newly weaned pigs with CIM induced by doxorubicin, which is commonly used for oncology patients with hematologic and solid tumors. The decision to use pigs was based on the recognition of GI characteristics that are similar to humans [6, 7], the ability to apply clinical procedures for the care of the animals and the sampling of blood and tissues as well as the availability of pigs with a uniform and well-characterized genetic background. The severity of CIM induced by doxorubicin was evaluated by a combination of clinical features and direct measurements of the structural and functional characteristics of the GI tract. One component of the study was to evaluate the efficacy of bovine colostrum as an intervention for reducing the severity of CIM.
Materials and Methods
Pigs and Their Care
All aspects of the research were approved by the University of Memphis Institutional Animal Care and Use Committee. A total of 21 weaned specific pathogen-free farm pigs of a consistent genetic lineage were obtained at approximately 28 days of age from a commercial source. The pigs were housed individually in PolyDome Litter Saver Pig Nurseries divided into two sections of 61 × 76 cm and located in a room maintained at 22°C (+1°C). Each pig was provided with environmental enrichment and fed the Prime Quality Pig Starter diet (150 g, 3× daily; Cargill Animal Nutrition, Overland Park, Kans., USA). At each feeding, the pigs were allowed access to the food for 15 min, after which the remaining food was weighed and the intensity of feeding was recorded. Water was available ad libitum. The pigs were weighed daily and had frequent human interactions to facilitate chemotherapy administration and handling.
After the pigs had adapted and were eating and gaining weight (3–4 days after arrival), a catheter (3.5-Fr single-lumen Umbili-Cath™; Utah Medical Products, Inc., Midvale, Utah, USA) was surgically placed in the right jugular vein and exteriorized on the dorsal surface at the base of the neck for the collection of blood and the administration of doxorubicin (Pfizer Labs, New York, N.Y., USA). Prior to surgery, the pigs were sedated (Telazol, 3–4 mg/kg; Fort Dodge Animal Health, Fort Dodge, Iowa, USA), anesthesia was maintained using isoflurane (2% with oxygen) and a sterile surgical field was prepared. After the surgery, the pigs received analgesia (Carprofen®, 2–4 mg/kg i.m.; Fort Dodge Animal Health) and a prophylactic antibiotic (Baytril®, 10 mg/kg p.o.; Bayer Animal Health). The catheters were flushed 2–3 times daily with heparinized saline (100 units/ml).
Administration of Doxorubicin
The pigs were allowed to recover for a minimum of 2 days after surgery or until presurgery feeding intensity was regained before the administration of doxorubicin. The first group of treated pigs (n = 4) received doxorubicin at 5 mg/kg (equivalent to a human dose of 100 mg/m 2) administered via the catheter over a 30-min period. All 4 animals rapidly developed severe diarrhea, vomiting and rapid weight loss, such that euthanasia was necessary within 4 days. Further studies used pigs treated with doxorubicin (n = 7) at a dose of 3.75 mg/kg (human dose equivalent: 75 mg/m 2). Control pigs (n = 5) were provided an equivalent volume of saline. The feeding regimen was maintained after the administration of doxorubicin or saline.
The Pig as a Model for Evaluation of Interventions to Reduce Mucositis
An additional 5 pigs were used to determine the suitability of the weaned pig for investigating the efficacy and safety of interventions for CIM. The pigs were dosed with doxorubicin at 3.75 mg/kg to induce mucositis and were provided bovine colostrum at 5 ml/kg body weight 3× daily, i.e. a total of 15 ml/kg each day, beginning the day before and continuing after the doxorubicin had been administered. The colostrum was obtained fresh and unprocessed from local dairies and was from the first two milkings after parturition. Colostrum from 4 cows was pooled and frozen in 500-ml volumes until fed. The colostrum was provided between meals using a syringe and was readily consumed by the pigs prior to the administration of doxorubicin. Colostrum was selected as a trial intervention because of the recognized benefits provided to the newborn intestine and the potential to alleviate the adverse impact of doxorubicin on GI structure and functions.
Clinical Observations
Each pig was observed a minimum of 3 times each day after the administration of doxorubicin for general health status, behavior and interactions with the care staff, and vomiting frequency and stool consistency [assessed on a Likert scale from 0 (normal) to 3 (severe diarrhea)] were recorded. Food consumption (intensity of feeding and quantity eaten in grams) and body weight were measured daily for each pig.
Blood samples were collected in EDTA tubes immediately prior to euthanasia. The plasma was frozen (–70°C) until being used to measure cytokine and endotoxin concentrations.
Necropsy and Tissue Collection
The pigs stopped eating and drinking within 3–4 days, so the latest point at which necropsy was performed was 5 days after doxorubicin administration. Pigs received palliative care (100 ml of lactated Ringers via the catheter twice daily) after cessation of eating or when diarrhea was severe. The animals were euthanized (Euthasol, 1 ml/4.5 kg; Virbac Animal Health, Fort Worth, Tex., USA) 12–24 h after the start of palliative care.
The mouth was examined for lesions, and tissues with lesions were removed and placed in 10% neutral buffered formalin. The entire GI tract was exposed and the location of externally visible lesions was recorded. A segment of esophagus (1–2 cm) immediately distal to the pharynx was removed and placed in neutral buffered formalin. The stomach was opened, the interior was observed and a full-thickness patch (1–2 cm 2) from the greater curvature was fixed in formalin. The small intestine was removed, the associated mesenteries were cut and the length from the pyloric sphincter to the ileocolonic junction was measured in a relaxed state. Intact segments of 15–20 cm were removed from the proximal, mid and distal thirds of the small intestine (fig. 1). From each of these regions, a segment (1–2 cm) was fixed in formalin, another segment (5–7 cm) was flash-frozen in liquid nitrogen and stored at −70°C, and the remainder of the segment was placed in chilled (2–4°C) mammalian Ringers and transferred to the laboratory for in vitro studies. Segments of 1–2 cm were removed from the proximal, mid and distal colon and fixed in formalin.
Fig. 1.
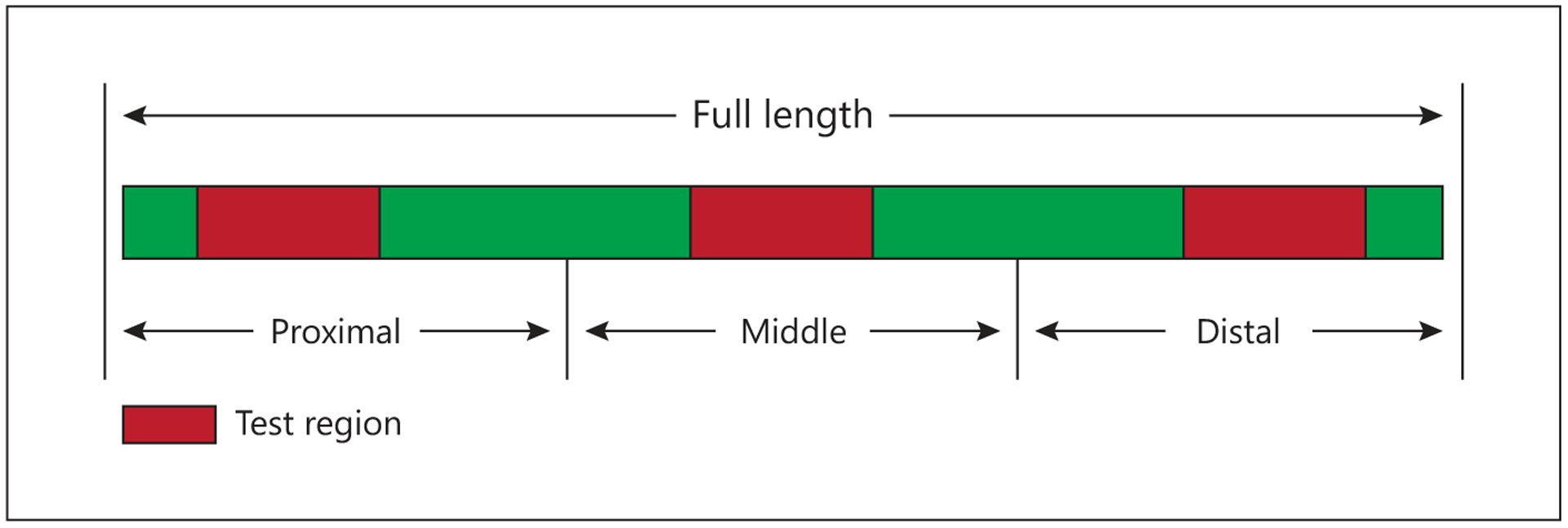
Locations of sections (red) removed from the small intestine for histology and measurements of functions.
Histologic Evaluation
The full-thickness, formalin-fixed tissues of the small intestine and colon were embedded in paraffin, sectioned (5 μm) and stained with hematoxylin and eosin for histopathology by a veterinary pathologist who was blinded to the treatment groups. The tissues were scored using a system that was originally developed, validated and previously published for GVHD (graft-versus-host disease) [11], using a Likert scale from 0 (normal tissue) to 5 (extreme damage). The tissues of the small intestine were evaluated for villus atrophy, inflammation and crypt necrosis and regeneration. Colon tissues were scored based on crypt loss, inflammation, crypt regeneration and crypt abscess/apoptosis following patterns previously published for GVHD [11].
Epithelial Functions
A portion of the proximal small intestine was used for Ussing chamber measurements of electrogenic chloride secretion. Briefly, duplicate tissues were stripped of the seromuscular layers and mounted in Ussing chambers; the apical and basolateral sides were then preexposed to indomethacin to reduce inflammatory responses, followed by amiloride to inhibit the epithelial sodium channels, and tetraethylammonium to inhibit potassium channels. After a stable baseline was achieved, forskolin was added to the apical side to stimulate chloride secretion via intracellular cyclic adenosine monophosphate signaling and activation of the cystic fibrosis transmembrane regulator (CFTR). Resulting changes in the short-circuit current (Isc) were recorded and considered as representative of CFTR-mediated Cl secretion. An additional segment was exposed to 25 mM adenosine as a secondary inducer of CFTR-mediated chloride secretion [12]. Additional segments from the proximal, mid and distal small intestine were used to measure in vitro accumulation of D-glucose [13]. Specifically, 1-cm sleeves of everted intestine from each region were exposed to mammalian Ringers with either the saturating concentration of 50 mM D-glucose or tracer (0.0002 mM). Glucose accumulation by the tissues was quantified using tracer concentrations of [14C] D-glucose. Glucose associated with the adherent tissue fluid and absorbed passively, independent of transporters, was corrected for by adding tracer levels of the [3H] L-glucose. Calculated rates of carrier-mediated glucose uptake were normalized to tissue mass and length.
The activities of the brush border membrane (BBM) disaccharidases, lactase and maltase, were determined using the flash-frozen segments of the proximal, mid and distal small intestine. The BBM was isolated using a CaCl2 precipitation approach [14], and the associated activities of lactase (EC 3.2.1.23) and maltase (3.2.1.20) were determined using lactose and maltose as substrates and by measuring the release of glucose. Protein content was measured by the Coomassie Blue method (Bio-Rad Laboratories, Hercules, Calif., USA) with bovine serum albumin as the standard. Enzyme activities [micromoles of substrate hydrolyzed per minute (IU)] were normalized to protein content.
Blood Biomarkers
Plasma samples from blood collected at necropsy were used to measure concentrations of inflammatory cytokines (IL-6, TNF-α) and anti-inflammatory cytokines (IL-10) using enzyme-linked immunosorbent assays (ELISA; R&D Systems) and following the manufacturer’s instructions. Endotoxin (e.g. lipopolysaccharide) concentrations were measured as an indicator of intestinal epithelial integrity and using a commercial kit (QCL-1000; Lonza, Walkersville, Md., USA)
Statistical Analysis
Values are presented as means and standard errors. Significance between groups was assessed with the 2-tailed Student test, with p < 0.05 considered significant.
Results
Food Consumption, Body Weight Gain and General Health after Administration of Doxorubicin
Control pigs continued to eat aggressively throughout the experiment, whereas pigs that received doxorubicin at 3.75 and 5 mg/kg had decreased their food intake within 24 h, with the decrease becoming more pronounced each day (fig. 2). Within 24 h, the treated pigs lost interest in enrichment, interacted less with caretakers and became lethargic, whereas the control pigs remained active and interactive. Vomiting was first seen 24 h after the dosage and became severe on day 3, by which time food consumption was <20% of the preadministration intake. Vomiting declined with cessation of feeding and thus was not seen after day 4. Similarly, diarrhea developed soon after the administration of doxorubicin, and particularly after day 2, with profuse watery diarrhea occurring thereafter in all treated animals until necropsy on day 5. Vomiting was not seen in any of the control pigs and stool consistency remained firm. Corresponding with these observations, the control pigs gained body weight, whereas the treated pigs lost weight (fig. 3), exhibited signs of wasting by day 4 (prominence of bony processes associated with the pelvis, scapula and spine and reduced abdominal adipose tissue and muscle tone). At necropsy, they had lost 5–20% of their body mass at the start of chemotherapy. The decline in food consumption, the weight loss and the clinical symptoms were more pronounced in the pigs treated with the higher (5 mg/kg) dose of doxorubicin. Providing colostrum to pigs treated with 3.75 mg/kg initially reduced food consumption. The colostrum did not reduce the magnitude of weight loss.
Fig. 2.
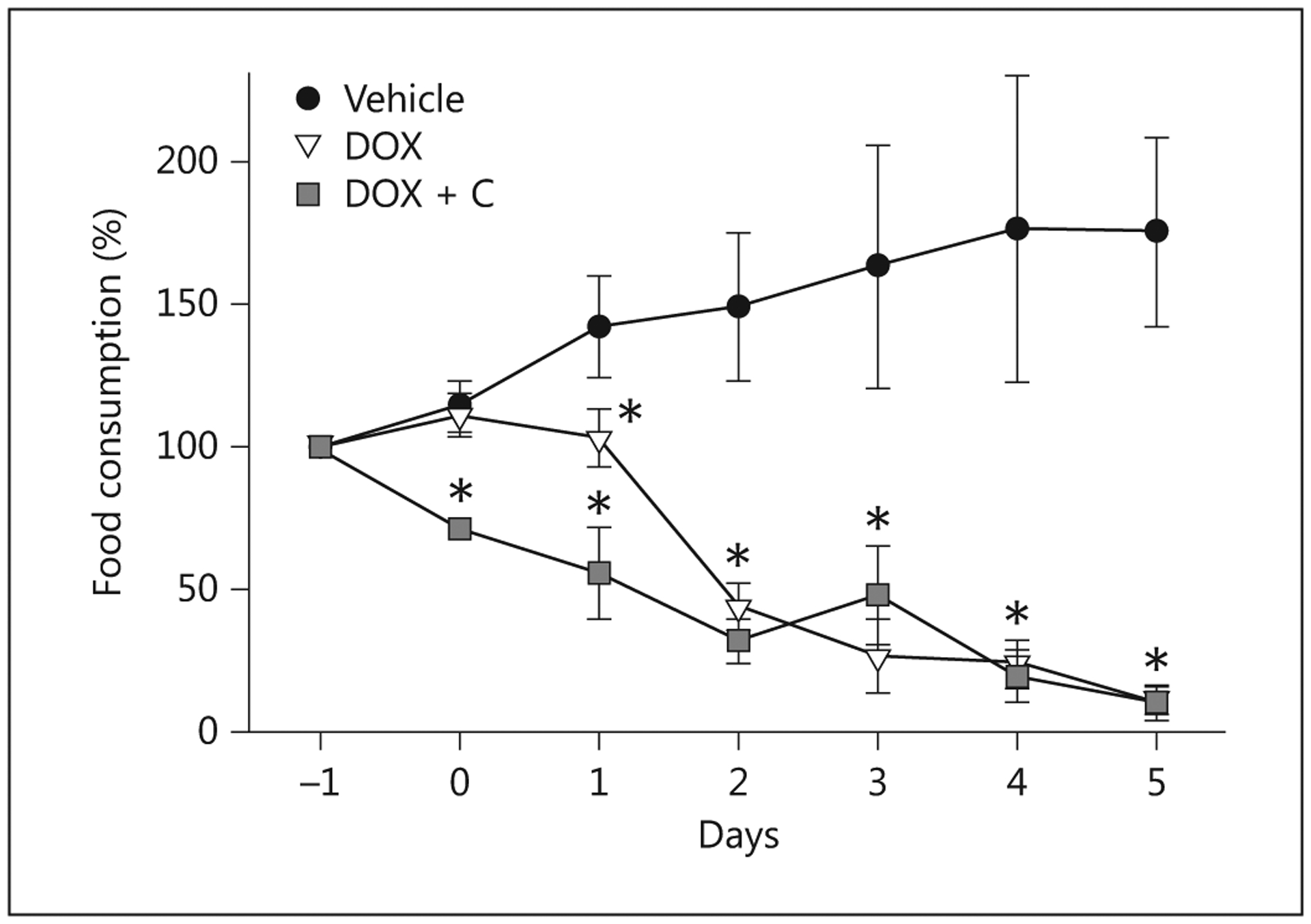
Food consumption expressed as a percentage of the amount consumed at baseline, i.e. the day before the administration of 3.75 mg/kg doxorubicin (DOX), doxorubicin and colostrum (DOX + C) or saline (Vehicle). *p < 0.05, significantly different compared to controls.
Fig. 3.
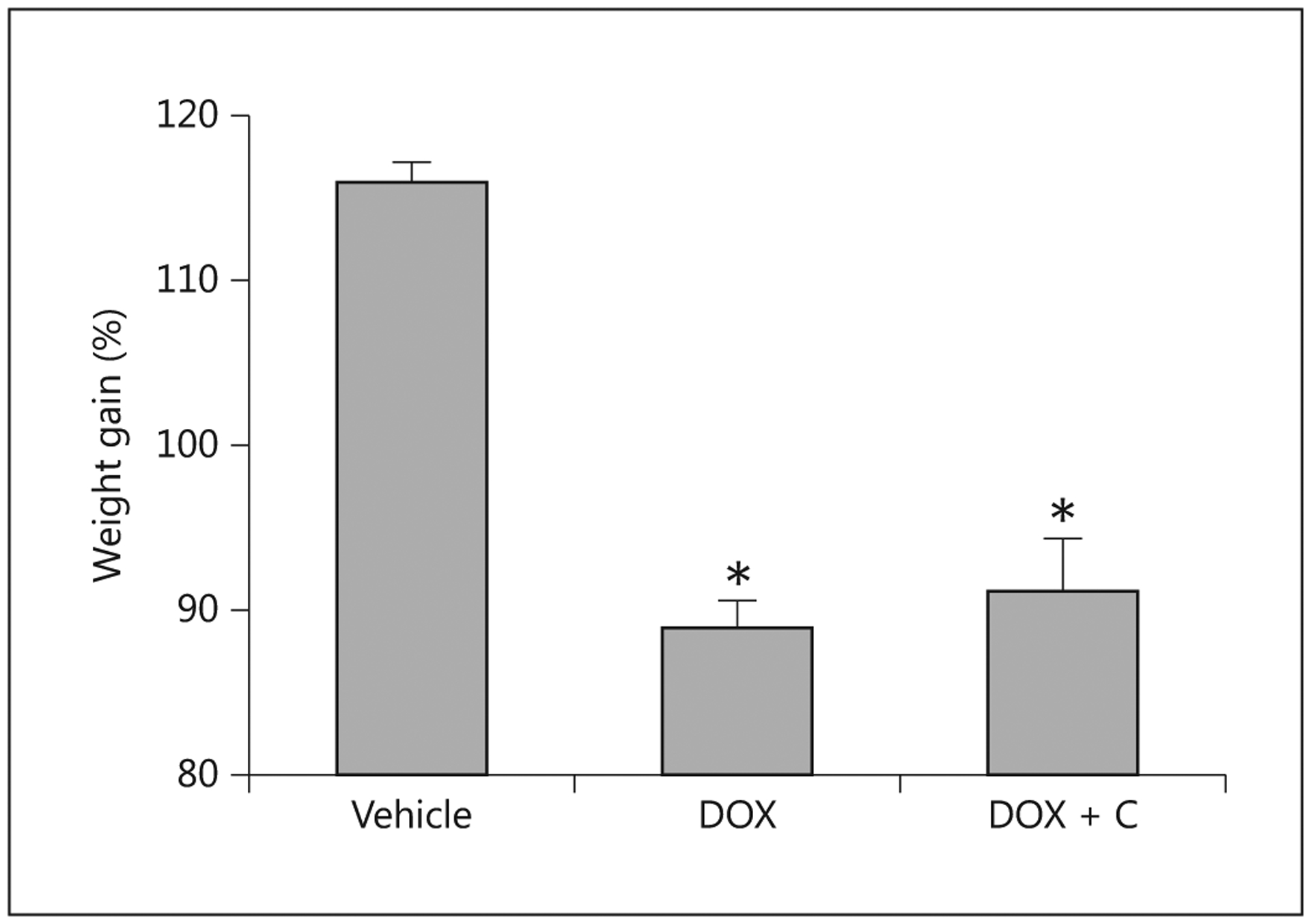
Change in body weight relative to the day of the administration of 3.75 mg/kg doxorubicin (DOX; n = 7), doxorubicin and colostrum (DOX + C; n = 5) or saline (Vehicle; n = 5). * Indicates that a significant difference was detected relative to controls.
Histopathology
The jejunum and proximal colon of the pigs receiving 3.75 mg/kg of doxorubicin had a disrupted morphology and structural integrity that was evident externally (fig. 4, 5). Microscopic examination of the control pigs did not reveal any evidence of structural or cellular alterations or any significant histological changes. Evaluation of the tissues based on a validated scoring system for GVHD confirmed that doxorubicin induced damage to the mucosa in the mid small intestine and the colon (fig. 6). Control pigs provided with saline vehicle had minimal or no signs of damage in the small intestine and colon. Interestingly, colostrum consumption reduced the damage in the small intestine of pigs that received 3.75 mg/kg of doxorubicin but did not protect the colon. The reasons for the regional differences in colostrum responses are unknown and are an area of ongoing investigation.
Fig. 4.
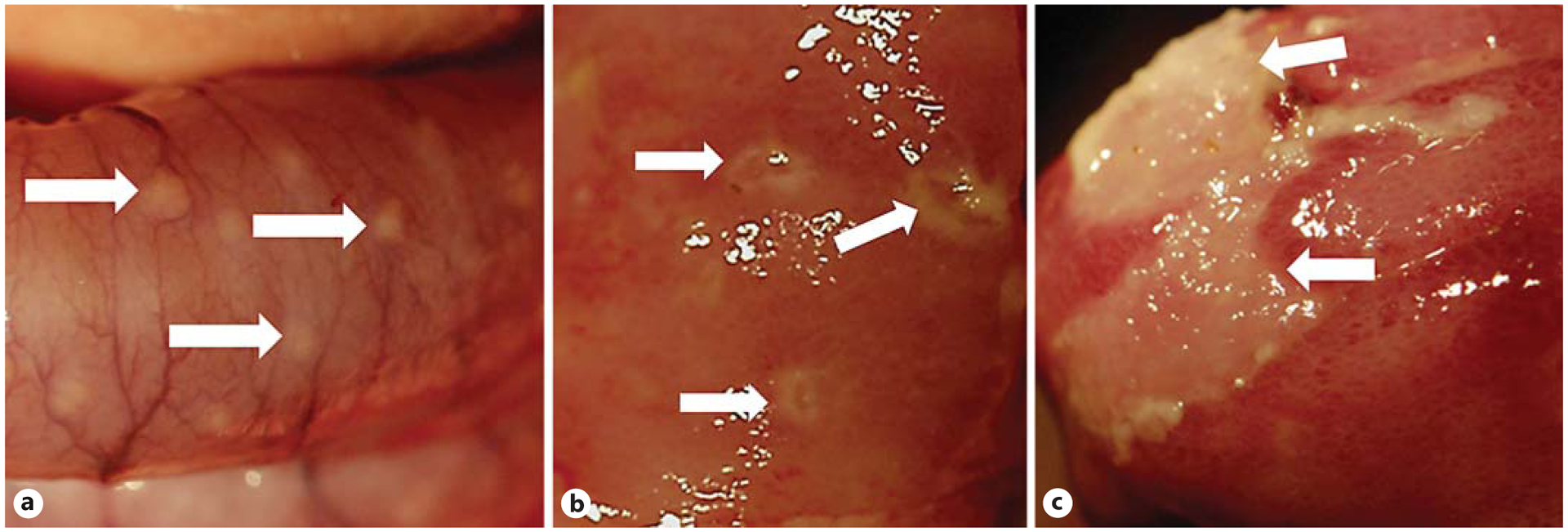
a An external photograph of the colon of a pig treated with 3.75 mg/kg doxorubicin shows some of the pustules (arrows). Internal photographs of the small intestine of doxorubicin-treated pigs show focal spots of inflammation (b) and a larger patch of inflammation (c) with tissue damage.
Fig. 5.
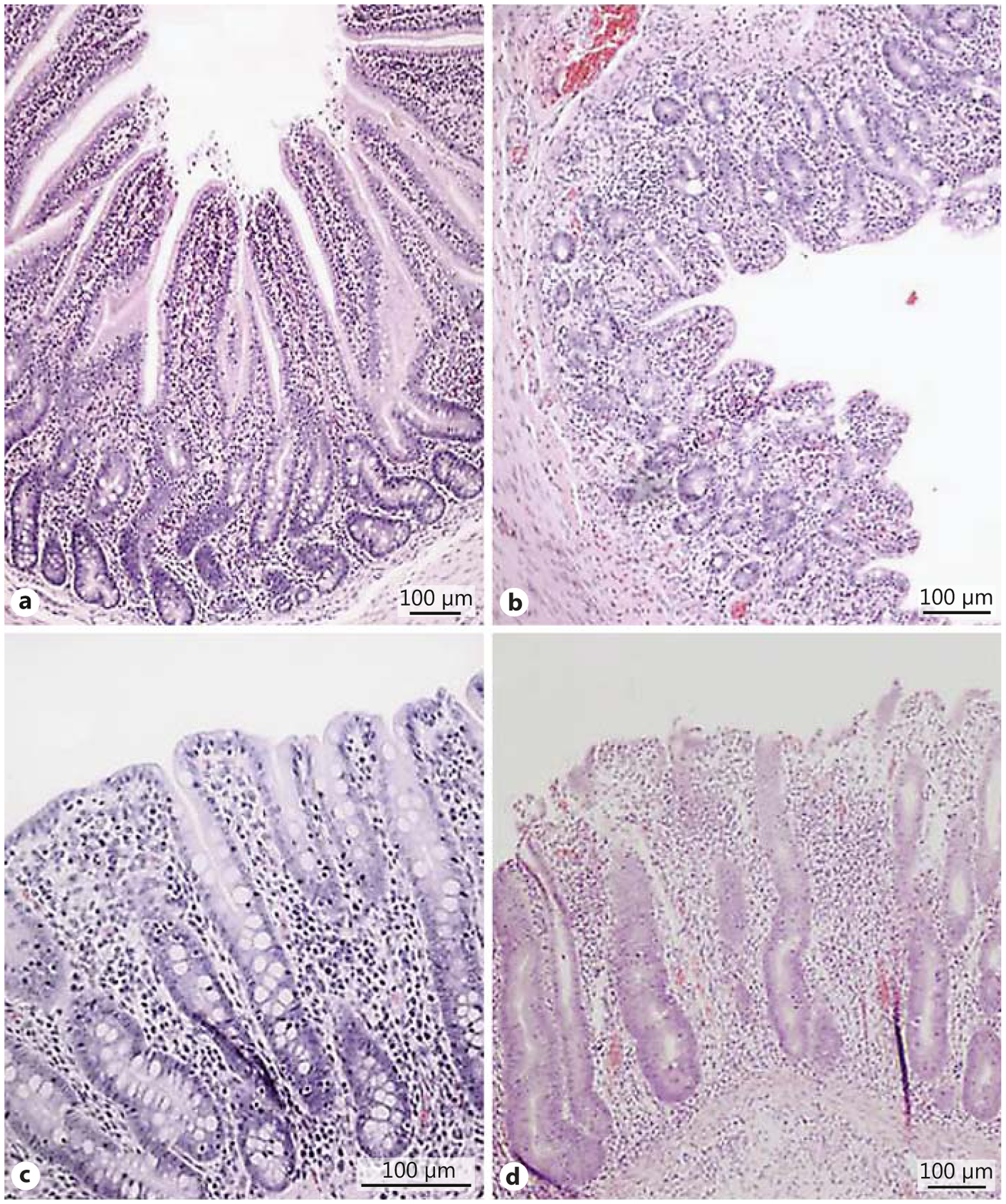
Photomicrographs of tissues from the jejunum (a) and proximal colon (c) of a control pig, and the jejunum (b) and proximal colon (d) of a pig treated with 3.75 mg/kg doxorubicin. Note the reduced villus height and the disrupted morphology and structural integrity caused by the doxorubicin. HE.
Fig. 6.
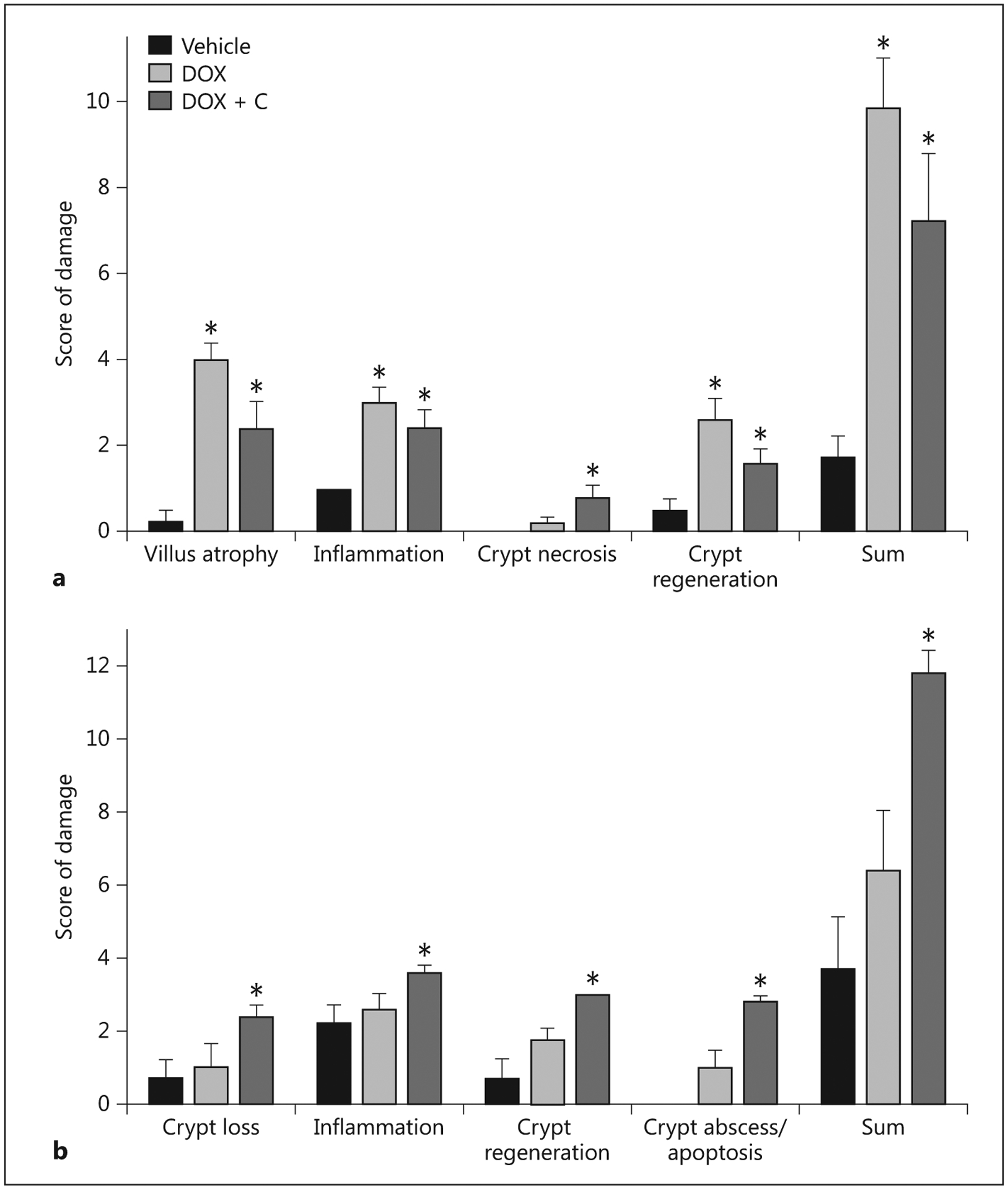
Histopathology scores for the small intestine (a) and colon (b) of pigs 5 days after administering saline (Vehicle; n = 5), 3.75 mg/kg doxorubicin (DOX; n = 7) or doxorubicin with colostrum provided as an intervention (DOX + C; n = 5).
Chloride Secretion
Addition of forskolin stimulated increases in Isc among the controls and the treated pigs. However, pigs receiving doxorubicin at 5 mg/kg had significantly higher chloride secretion relative to the other groups (fig. 7). The exposure of tissues from the proximal intestine to adenosine monophosphate also elicited an increase in Isc, with the magnitude of response 3.4-fold and 2.7-fold higher in tissue from pigs that received 5 and 3.75 mg/kg doxorubicin, respectively, compared to tissue from the controls (p < 0.05). The response to adenosine monophosphate was not evaluated for the pigs that received colostrum.
Fig. 7.
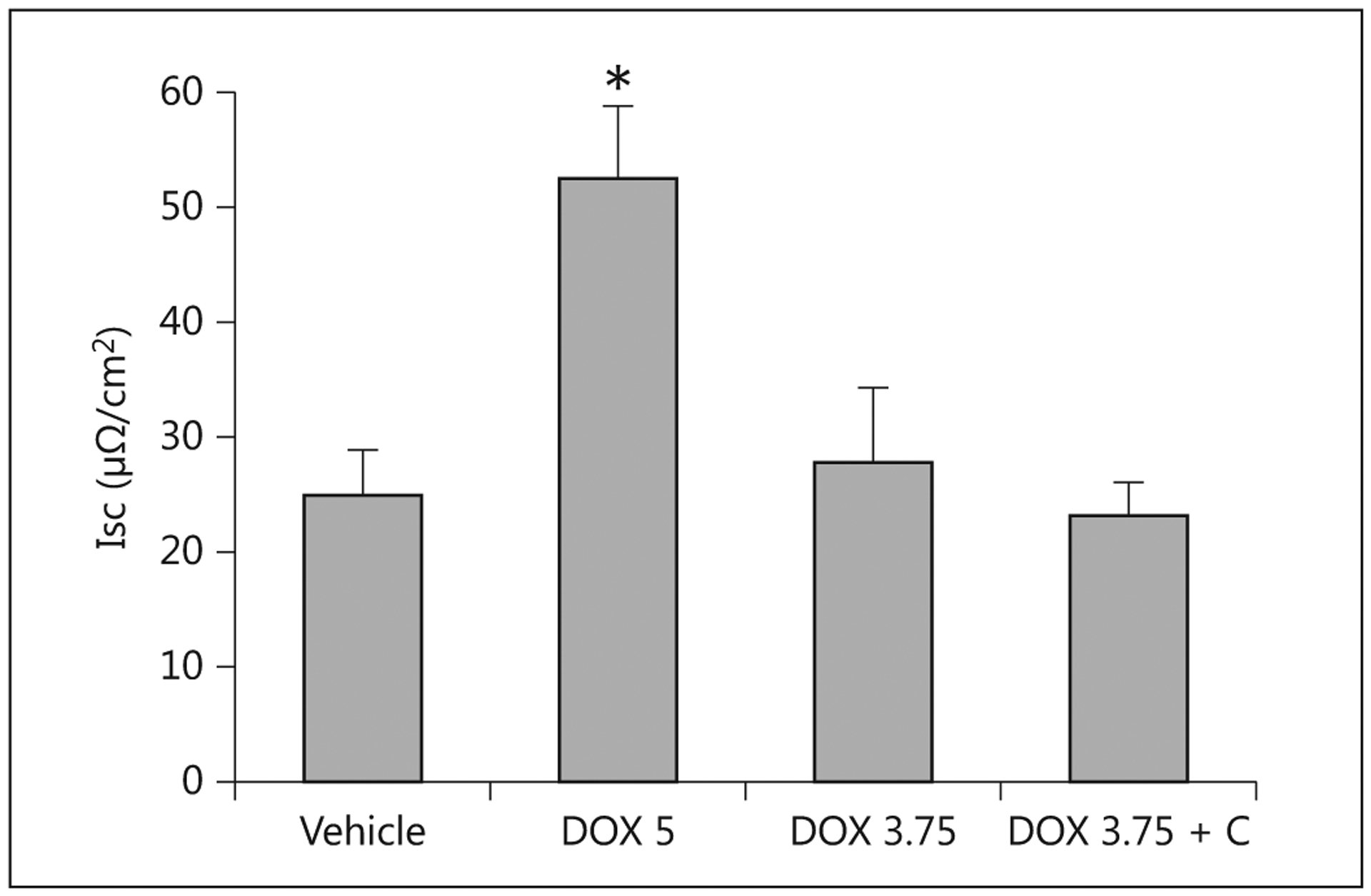
Isc for proximal small intestine tissues from pigs receiving saline (Vehicle), 5 mg/kg doxorubicin (DOX 5; n = 4), 3.75 mg/kg doxorubicin (DOX 3.75; n = 7) or 3.75 mg/kg doxorubicin with colostrum provided as an intervention (DOX 3.75 + C; n = 5). * Indicates that a significant difference was detected relative to controls.
Carrier-Mediated Glucose Uptake
Accumulation of d-glucose by intact tissues at the saturating concentration of 50 mM and at a tracer concentration (0.006 mM) was reduced in all three regions of the pigs treated with doxorubicin at 3.75 mg/kg (fig. 8). At the 50-mM concentration, the provision of colostrum did not result in a marked increase in uptake; at the tracer concentration, it resulted in uptakes that were comparable to those measured in the controls.
Fig. 8.
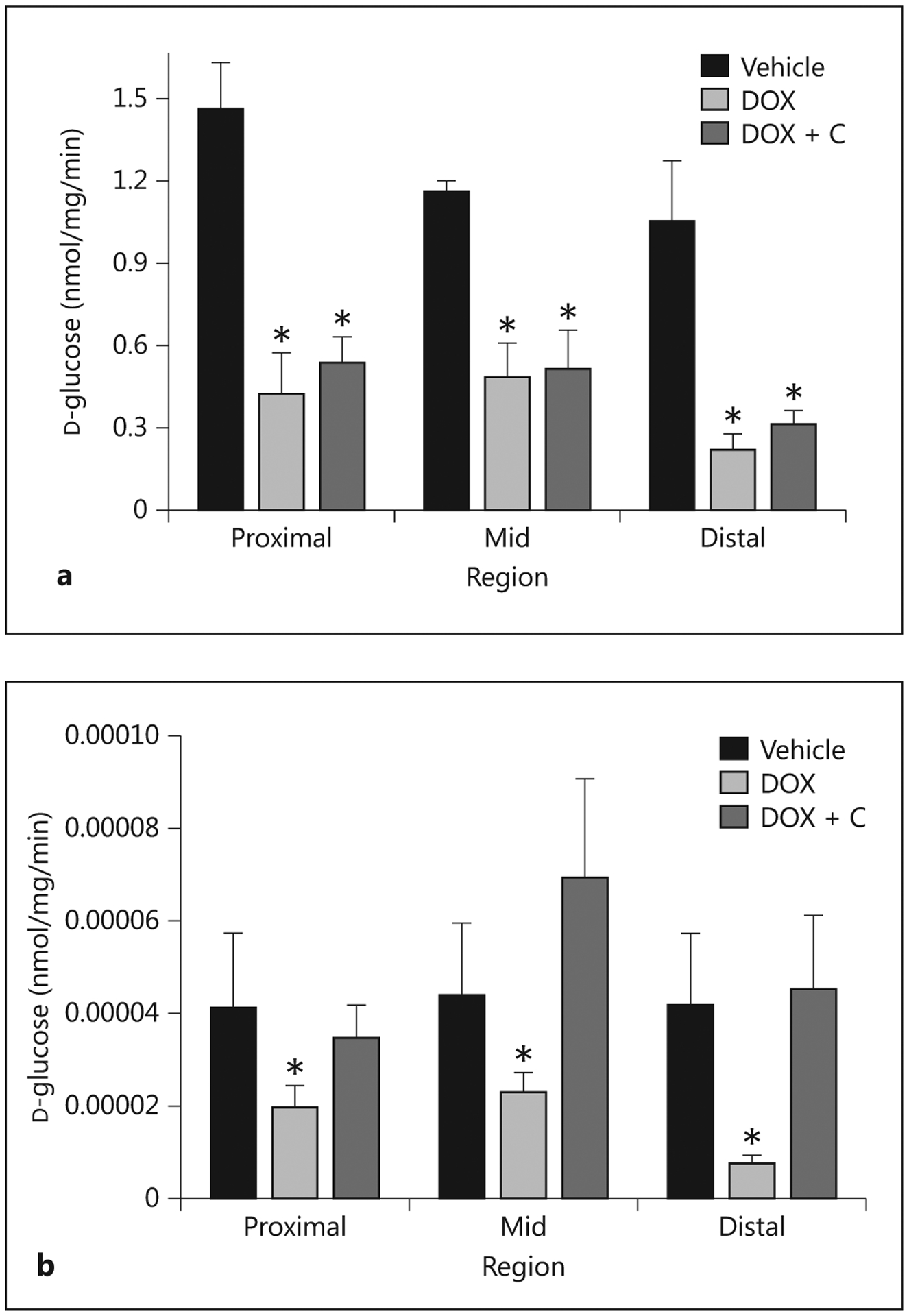
Rates of d-glucose accumulation by proximal, mid and distal small intestine exposed to 50-mM (a) and tracer (0.006 mM; b) concentrations of D-glucose. Tissues were harvested 5 days after administering saline (Vehicle; n = 5), 3.75 mg/kg doxorubicin (DOX; n = 7) or doxorubicin with colostrum provided as an intervention (DOX + C; n = 5). * Indicates that a significant difference was detected relative to controls.
The impact of doxorubicin was also evident from estimates of the glucose-absorptive capacities of the entire small intestine, calculated as the product of glucose uptake per unit of intestine × estimated total intestinal mass (data not presented). Specifically, the combination of lower small intestine mass and reduced rates of uptake per unit of intestine caused by doxorubicin resulted in total glucose uptake capacities being only 25% of those estimated for the control pigs (p < 0.05). The provision of colostrum to the treated pigs improved glucose uptake capacities, with the benefit more apparent at tracer concentration.
BBM Disaccharidases
Administration of doxorubicin reduced lactase activity in the proximal and mid regions compared to those regions in the control pigs, and more so at 5 mg/kg. The impact on lactase activity was not appreciable in the distal region due to activity already being low. Provision of colostrum partially restored lactase activity in the proximal and distal regions, and actually increased activity in the distal segment compared to the vehicle-control pigs. Maltase activity was higher than lactase activity, which is as expected in weaned pigs. Similar to lactase activity, doxorubicin reduced maltase activity in the proximal and mid region, more so at the 5 mg/kg dose than at the 3.75 mg/kg dose, but with the impact also obvious in the distal small intestine. Colostrum increased maltase activity in the mid and distal regions of the treated pigs, but not in the proximal region (fig. 9).
Fig. 9.
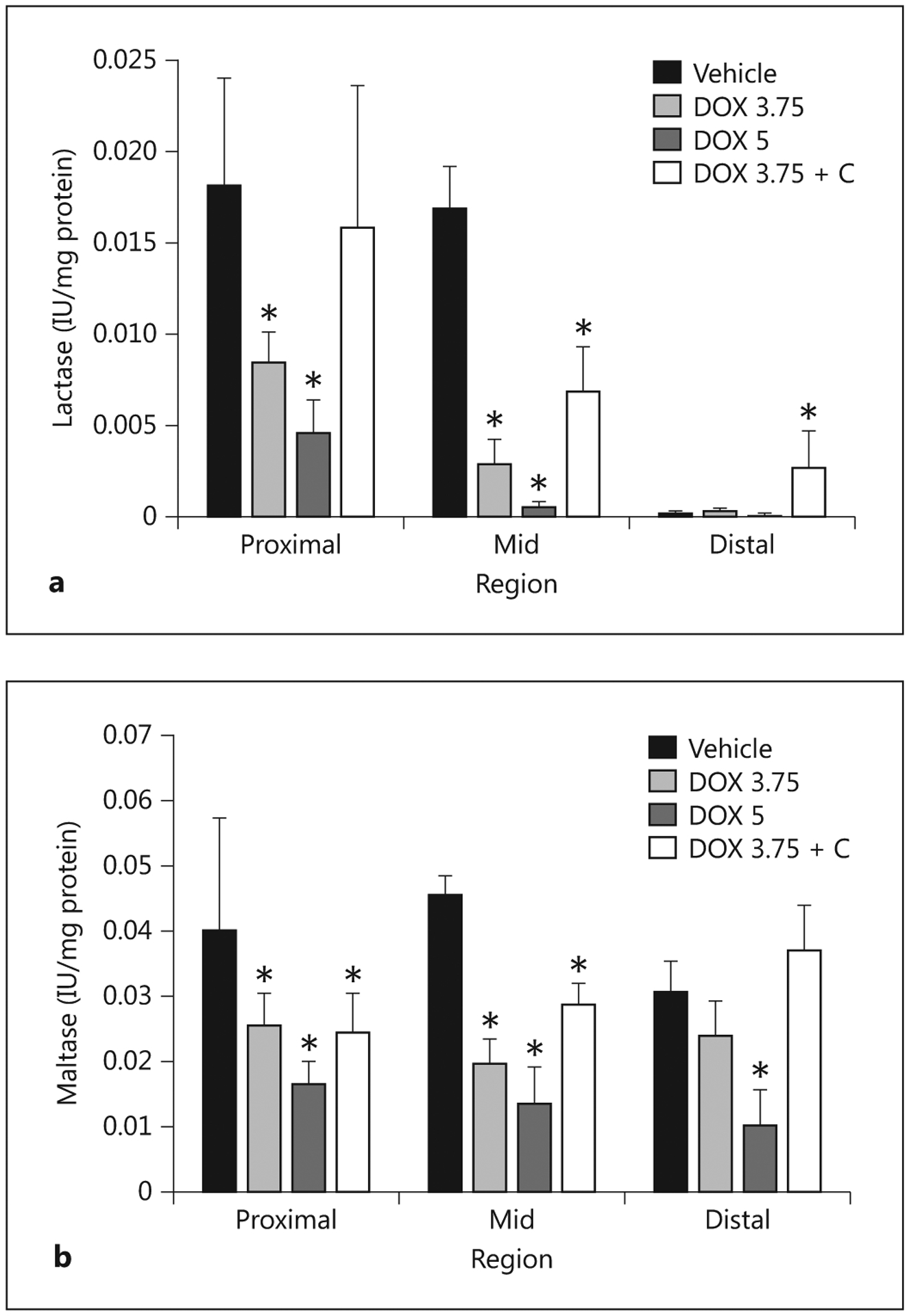
Activities of lactase (a) and maltase (b) associated with the BBM fraction isolated from the proximal, mid and distal small intestines harvested from pigs 5 days after administering saline (Vehicle; n = 5), doxorubicin at 5 mg/kg (DOX 5; n = 4), at 3.75 mg/kg (DOX 3.75; n = 7) or at 3.75 mg/kg with colostrum provided as an intervention (DOX 3.75 + C; n = 5). * Indicates that a significant difference was detected relative to controls.
The diminished BBM carbohydrase activities after the administration of doxorubicin combined with the atrophy of the intestine diminished the total intestinal capacity to hydrolyze disaccharides. Conversely, the recovery of BBM carbohydrase activities and intestinal mass associated with the provision of colostrum improved the capacity of the small intestine to digest lactose and maltose.
Blood Biomarkers
At necropsy, the pigs dosed with 3.75 mg/kg doxorubicin had higher plasma concentrations of TNF-α than the control pigs (fig. 10), but levels of IL-6 and the anti-inflammatory cytokine IL-10 did not differ between the groups. Provision of colostrum reduced the level of TNF-α, but did not affect the level of IL-6 and IL-10. Endotoxin levels for the vehicle-control pigs were significantly lower than those for treated pigs (fig. 11). Provision of colostrum did not reduce circulating levels of endotoxin.
Fig. 10.
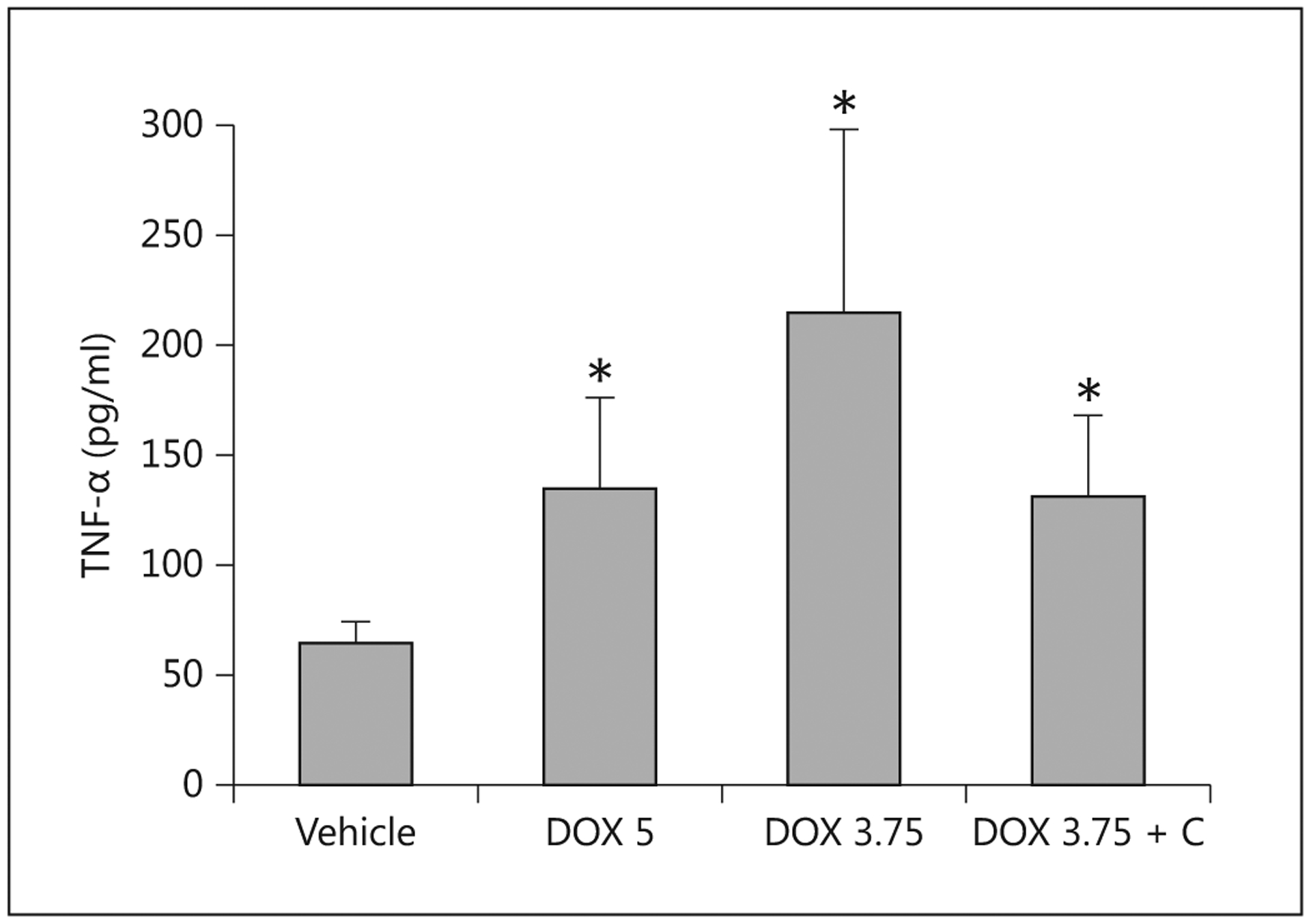
Concentrations of TNF-α measured in serum 5 days after the administration of saline (Vehicle; n = 5), doxorubicin at 5 mg/kg (DOX 5; n = 4), at 3.75 mg/kg (DOX 3.75; n = 7) or at 3.75 mg/kg with colostrum as an intervention (DOX 3.75 + C; n = 5) to pigs. * Indicates that a significant difference was detected relative to controls.
Fig. 11.
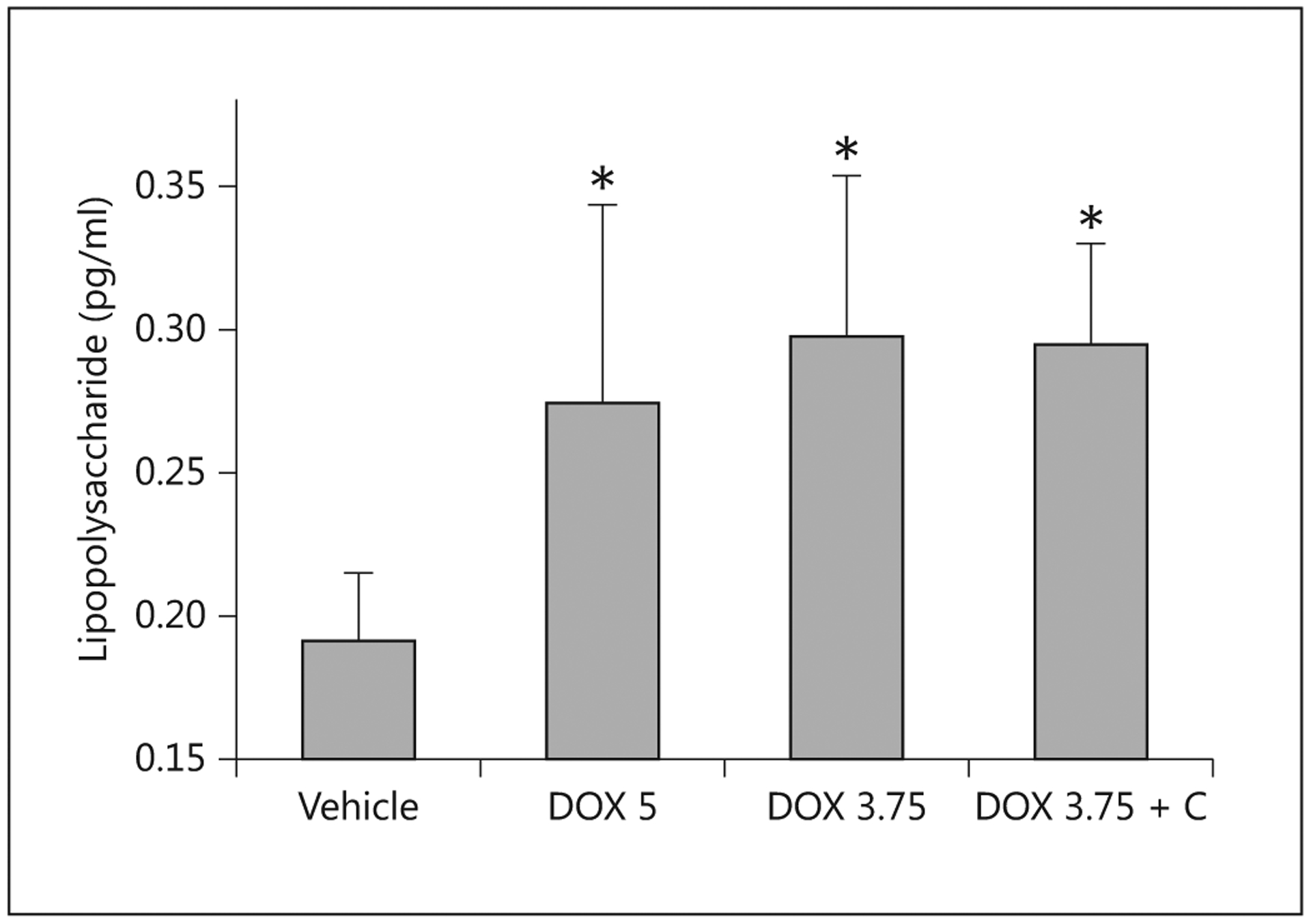
Concentrations of lipopolysaccharide measured in serum 5 days after the administration of saline (Vehicle; n = 5), doxorubicin at 5 mg/kg (DOX 5; n = 4), at 3.75 mg/kg (DOX 3.75; n = 7) or at 3.75 mg/kg with colostrum as an intervention (DOX 3.75 + C; n = 5) to pigs.* Indicates that a significant difference was detected relative to controls.
Discussion
Administration of doxorubicin to recently weaned pigs at a dose mimicking those commonly encountered in pediatric cancer chemotherapy elicits GI disturbances and dysfunctions that are similar to those of pediatric oncology patients suffering from CIM. The dose equivalent of 75 mg/m2 (3.75 mg/kg) is similar to the doxorubicin doses used for non-Hodgkin lymphomas and higher than those used for leukemias and most solid tumors, so any agent that protects against mucositis caused at this dose should effectively prevent CIM at lower doses [15]. The extreme toxicity evoked by administering 5 mg/kg (equivalent to a human dose of 100 mg/m 2, higher than the dose in any regimen used) is also consistent with the adverse reactions that limit high-dose clinical regimens. The alterations in the structure and the compromised functions of the intestine are similar to reports on laboratory rodents treated with chemotherapeutic agents known to cause mucositis [5, 16] including doxorubicin [17]. The following sections compare and contrast our findings for the weaned-pig model of doxorubicin-induced CIM with reports on rodents, two other pig models of CIM and pediatric oncology patients.
Clinical Symptoms
Among human subjects, nausea, vomiting and diarrhea are listed as the first, third and eighth most distressing consequences of chemotherapy, respectively [18]. Although the oral pain caused by mucositis is ranked much lower, the associated disturbances of the GI epithelium that occur with oral mucositis contribute to nausea, vomiting, diarrhea and anorexia.
Although nausea is difficult to quantify directly in animal models, the rapid reduction in food consumption that preceded the onset of vomiting and diarrhea after administering the doxorubicin suggests that the pigs experienced a rapid onset of nausea, causing their disinterest in eating and reduced desire to interact with the caretakers. This is similar to the acute phase of food disinterest and lethargy suffered by some pediatric patients receiving chemotherapy [19]. The later onset of diarrhea and vomiting observed in the weaned pigs is similar to the delayed GI symptoms that occur in humans on chemotherapeutic regimens. Rodents develop diarrhea within hours of administration of mucositis-inducing chemotherapeutic agents. Rodents differ from both pigs and humans by being unable to vomit.
The weight loss and wasting observed in the pigs corresponds well with the loss of body weight among patients with CIM. Hence, the weaned-pig model provides opportunities to evaluate nutritional support regimens to reduce weight loss or accelerate weight recovery. As the objective of our study was to determine the extent of the GI damage and dysfunction associated with doxorubicin-induced CIM, and not actually the recovery from CIM, parenteral nutrition was not provided to the pigs. The placement of central lines in weaned pigs, which we have demonstrated here as being feasible, will allow future studies to improve nutritional support to reduce weight loss and accelerate recovery.
Blood Work
Increases in proinflammatory cytokines have been explored as an indicator of mucositis in response to different chemotherapeutic agents [20]. The majority of studies have examined responses at the tissue level. Our aim was to determine if changes in proinflammatory or anti-inflammatory cytokines that have been implicated in CIM could be detected in blood samples. The higher concentration of TNF-α measured in the treated pigs 5 days after administering 3.75 mg/kg doxorubicin is consistent with reports of increased levels after treating patients [21] and mice [20] with doxorubicin. The lower TNF-α concentration measured in the pigs treated at 5 mg/kg may have been caused by the shorter period between administration and necropsy (3–4 vs. 5 days, respectively). The lack of difference between control and treated pigs in the level of IL-6 may reflect the use of doxorubicin, which may not elicit changes, or else the changes may have occurred prior to necropsy. Alternatively, changes that do occur at the tissue level may not produce enough IL-6 to be detectable systemically.
The higher circulating concentrations of endotoxin in treated pigs are indicative of increased mucosal permeability, sometimes referred to as ‘mucosal barrier injury’ [22]. The disruption of the GI epithelium allows normally excluded solutes and bacteria to gain access to the systemic circulation. The higher endotoxin levels measured in the pigs treated with doxorubicin is indicative of mucosal barrier injury. Evaluation of mucosal barrier injury of patients subjected to myeloablative regimens and using the indigestible sugars lactulose and rhamnose as permeability markers has revealed that damage can be prolonged [23].
Tissue Responses
The histopathology findings correspond with findings and localization of damage throughout the GI tract caused by the administration of doxorubicin in rodent models [16]. Based on human studies, the mucositis was expected to develop within 5–10 days of doxorubicin administration; the observations in weaned pigs are consistent with this timing.
At the functional level, administration of chemotherapy induces rapid changes in patterns of gene expression by GI tissues that can be detected as early as 1 h and may persist for days. The affected genes are associated with multiple cellular functions and pathways, and are predicted to contribute to mucositis [24]. The wide diversity of affected cell functions explains why chemotherapy-associated diarrhea is considered to have multiple causes and involve multiple mechanisms [25]. The combination of decreased digestion and absorption of solutes, increased electrolyte and fluid secretion and lower tight-junction integrity permeability that we measured are consistent with events associated with other pathologic conditions causing malabsorptive diarrhea [26].
The increased chloride secretion in the proximal small intestine after administration of 5 mg/kg of doxorubicin would contribute to increased electrolyte and fluid losses and is consistent with the rapid onset of severe diarrhea suffered by this group of pigs, and leading to early severe wasting and the need for euthanasia. The increased chloride secretion is at least partly explained by increased recruitment of CFTR to the apical member in response to doxorubicin [27]. This pathway of CFTR activation may have been exacerbated at 5 mg/kg by increased localized production of the inflammatory cytokines IL-1β and TNF-α [28], even though systemic concentrations were not elevated at 3.75 mg/kg. Measurements of chloride secretion in distal regions of the GI tract would be required to determine the magnitude and clinical relevance of increased secretion of electrolytes and fluids.
The decrease in the BBM activities of lactase and maltase and rate of glucose uptake are consistent with the disruption of tissue architecture, and are comparable to that reported for juvenile pigs treated with 5FU [9] and neonatal pigs subjected to myeloablative therapy [10]. Sucrase activity also decreases when rats are treated with doxorubicin [16, 17] and other chemotherapeutic agents, including 5FU [29], methotrexate, etoposide, irinotecan or cyclophosphamide [16]. The decline in the BBM enzymes has been attributed to the increase in inflammatory cytokines that reduce the expression of sucrase-isomaltase [30] and P-glycoprotein, an inducible plasma membrane drug efflux molecule which, when induced, enhances efflux of doxorubicin from within cells [31]. The lower sucrase activity associated with chemotherapy is the basis for the sucrose breath test developed for evaluating mucosal damage in pediatric patients undergoing chemotherapy [32]. The sucrose breath test has been validated for the pig [33], providing another clinically relevant diagnostic tool for assessing the severity of mucositis.
The decline in glucose uptake after the administration of doxorubicin was expected and is in agreement with the doxorubicin-induced decline in absorption of the matrix metalloproteinase inhibitor COL-3 [34]. The more pronounced decline in glucose uptake when measured at tracer concentration suggests doxorubicin induces a decline in transporter abundance per unit (length or mass) of intestine. However, administration of chemotherapeutic agents does not cause a universal decline in nutrient absorption. For example, glucose accumulation by BBM vesicles prepared from the intestines of rats is actually higher after treatment with 5FU, but is lower when rats are treated with methotrexate [35]; this parallels the decreased glucose uptake by pigs treated with doxorubicin. The contrasting response of glucose uptake to different chemotherapeutic agents, despite the common presence of mucosal damage and lower activities of BBM enzymes, is not fully understood. Contrasting findings have also been reported for amino acid absorption. Expression of several different amino acid and nutrient transporters decreases after rats are treated with 5FU [36], whereas children undergoing chemotherapy and with verified mucositis do not exhibit a decline in leucine systemic availability, an indicator of intestinal absorption [37]. These differences may be explained by the use of different chemotherapeutic agents and approaches for estimating amino acid absorption. Interestingly, absorption of peptides via the proton-coupled transporter PEPT1 is enhanced by 5FU [36].
Interventions for CIM
Although the nausea, vomiting and some other GI issues (e.g. diarrhea and constipation) associated with chemotherapy can be controlled, there is a need to identify interventions that will reduce the severity of mucositis and thereby permit higher and more effective chemotherapeutic dosage regimens. Although several interventions can reduce the incidence and severity of CIM (e.g. keratinocyte growth factor 1, palifermin) [38], once mucositis occurs, there are no specific therapies to shorten its duration. Supportive care measures can decrease the morbidity associated with mucositis, including the prevention of superinfection, the reduction of inflammation and nutritional support to reduce the wasting caused by GI dysfunction and a reduced caloric intake [39, 40]. Although glucagon-like peptide 2 and other growth factors have been investigated and have shown promise for oral mucositis, these are still experimental [41].
Colostrum as an Intervention for CIM
A critical shortcoming for guiding clinical decisions about interventions for mucositis has been the lack of relevant preclinical evidence about the efficacy and safety of treatments [42]. Our preliminary evaluation further validates the pig as a relevant model to assess existing and experimental interventions for mucositis. The reduced severity of mucositis in the small intestine among the pigs receiving colostrum is promising, though the concomitant lack of protection provided to the colon requires further investigation.
Our interest in colostrum as a test intervention was based on the protection provided to preterm pigs that were at risk of necrotizing enterocolitis [43] as well as against the GI damage caused by nonsteroidal anti-inflammatory drugs [44] and on the potential use of the peptide growth factors in colostrum for the treatment of various GI disorders such as inflammatory bowel disease [45]. Previous studies have shown how the components of colostrum and milk, alone or in combination with other bioactive molecules, reduce the severity of mucositis [46–48]. The unprocessed bovine colostrum provided to the pigs is a complex solution of relatively undefined composition. As a result, the identity of the bioactive fractions that provide protection against mucosal injury or enhance mucosal repair remains unknown.
Future Efforts
There is a need to better understand how chemotherapy-induced changes in the GI microbiome contribute to mucositis [49–51]. Of particular interest is the definition of the mechanisms and pathways leading to the mucosal inflammation associated with mucosal injury such as that seen in CIM. Although receptor-mediated responses to bacterial antigens have been implicated in mucositis [52], sterile inflammation occurs with doxorubicin [53]. The activation of NF-ĸB by chemotherapy and subsequent proinflammatory cytokine production has been implicated in mucositis [20, 53], as have chemotherapy-induced upregulation of the cyclooxygenase pathway and the production of proinflammatory prostaglandins [54]. However, therapeutic agents that target these pathways have not yet been translated into clinical care, partly because of the lack of a relevant animal model for preclinical trials. Biomarkers for the changes in the microbiome and the associated host responses to chemotherapy may address the need for additional noninvasive indicators of the severity of mucositis [31, 55].
Our study exposed weaned pigs to a single dose of doxorubicin. Additional studies are needed to examine the impact of multiple doses and interactions with other drugs as well as to understand potentially confounding variables like age and sex. Other toxicities associated with chemotherapy, such as the cardiotoxicity of doxorubicin [56], can also be explored using the pig model.
References
- 1.Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ, Yazbeck R, Elad S, Bowen JM; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO): Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 2013; 21: 313–326. [DOI] [PubMed] [Google Scholar]
- 2.Carlotto A, Hogsett VL, Maiorini EM, Razulis JG, Sonis ST: The economic burden of toxicities associated with cancer treatment: review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics 2013; 31: 753–766. [DOI] [PubMed] [Google Scholar]
- 3.Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, McCabe MG, Meyer S, Khalid T: Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011;CD000978. [DOI] [PubMed] [Google Scholar]
- 4.Bateman E, Bowen J, Stringer A, Mayo B, Plews E, Wignall A, Greenberg N, Schiffrin E, Keefe D: Investigation of effect of nutritional drink on chemotherapy-induced mucosal injury and tumor growth in an established animal model. Nutrients 2013; 5: 3948–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen JM, Gibson RJ, Keefe DM: Animal models of mucositis: implications for therapy. J Support Oncol 2011; 9: 161–168. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Widmer G, Tzipori S: A pig model of the human gastrointestinal tract. Gut Microbes 2013; 4: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuzmuk KN, Schook LB: Pigs as a model for biomedical sciences; in Rothschild MF, Ruvinsky A (eds): The Genetics of the Pig, ed 2 Wallingford, CAB International, 2011. [Google Scholar]
- 8.Chun J, Lee D, Stewart D, Talcott M, Fleshman J: Comparison of the compression anastomosis ring (EndoCAR) with a circular stapled anastomosis in a porcine model. Surg Innov 2011; 18: 235–240. [DOI] [PubMed] [Google Scholar]
- 9.Manzano M, Bueno P, Rueda R, Ramirez-Tortosa CL, Prieto PA, Lopez-Pedrosa JM: Intestinal toxicity induced by 5-fluorouracil in pigs: a new preclinical model. Chemotherapy 2007; 53: 344–355. [DOI] [PubMed] [Google Scholar]
- 10.Pontoppidan PL, Shen RL, Petersen BL, Thymann T, Heilmann C, Müller K, Sangild PT: Intestinal response to myeloablative chemotherapy in piglets. Exp Biol Med (Maywood) 2014; 239: 94–104. [DOI] [PubMed] [Google Scholar]
- 11.van der Merwe M, Abdelsamed HA, Seth A, Ong T, Vogel P, Pillai AB: Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplantation. J Immunol 2013; 191: 5764–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leipziger J: Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 2003; 284:F419–F432. [DOI] [PubMed] [Google Scholar]
- 13.Thymann T, Møller HK, Stoll B, Støy AC, Buddington RK, Bering SB, Jensen BB, Olutoye OO, Siggers RH, Mølbak L, Sangild PT, Burrin DG: Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol 2009; 297:G1115–G1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz J, Preiser H, Maestracci D, Ghosh BK, Cerda JJ, Crane RK: Purification of the human intestinal brush border membrane. Biochim Biophys Acta 1973; 323: 98–112. [DOI] [PubMed] [Google Scholar]
- 15.Laver JH, Kraveka JM, Hutchison RE, Chang M, Kepner J, Schwenn M, Tarbell N, Desai S, Weitzman S, Weinstein HJ, Murphy SB: Advanced-stage large-cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate-dose methotrexate and high-dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. J Clin Oncol 2005; 23: 541–547. [DOI] [PubMed] [Google Scholar]
- 16.Howarth GS, Tooley KL, Davidson GP, Butler RN: A non-invasive method for detection of intestinal mucositis induced by different classes of chemotherapy drugs in the rat. Cancer Biol Ther 2006; 5: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Brook CL, Whittaker AL, Lawrence A, Yazbeck R, Howarth GS: Effects of Streptococcus thermophilus TH-4 in a rat model of doxorubicin-induced mucositis. Scand J Gastroenterol 2013; 48: 959–968. [DOI] [PubMed] [Google Scholar]
- 18.de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, Verweij J: Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 1997; 76: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holdsworth MT, Raisch DW, Frost J: Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer 2006; 106: 931–940. [DOI] [PubMed] [Google Scholar]
- 20.Logan RM, Stringer AM, Bowen JM, Yeoh AS, Gibson RJ, Sonis ST, Keefe DM: The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 2007; 33: 448–460. [DOI] [PubMed] [Google Scholar]
- 21.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK, Butterfield DA: 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med 2011; 50: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 22.Blijlevens NM, Donnelly JP, De Pauw BE: Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transpl 2000; 25: 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blijlevens NM: Implications of treatment-induced mucosal barrier injury. Curr Opin Oncol 2005; 17: 605–610. [PubMed] [Google Scholar]
- 24.Bowen JM, Gibson RJ, Tsykin A, Stringer AM, Logan RM, Keefe DM: Gene expression analysis of multiple gastrointestinal regions reveals activation of common cell regulatory pathways following cytotoxic chemotherapy. Int J Cancer 2007; 121: 1847–1856. [DOI] [PubMed] [Google Scholar]
- 25.Gibson RJ, Stringer AM: Chemotherapy-induced diarrhoea. Curr Opin Support Palliat Care 2009; 3: 31–35. [DOI] [PubMed] [Google Scholar]
- 26.Sandle GI: Infective and inflammatory diarrhoea: mechanisms and opportunities for novel therapies. Curr Opin Pharmacol 2011; 11: 634–639. [DOI] [PubMed] [Google Scholar]
- 27.Maitra R, Shaw CM, Stanton BA, Hamilton JW: Increased functional cell surface expression of CFTR and DeltaF508-CFTR by the anthracycline doxorubicin. Am J Physiol Cell Physiol 2001; 280:C1031–C1037. [DOI] [PubMed] [Google Scholar]
- 28.Baniak N, Luan X, Grunow A, Machen TE, Ianowski JP: The cytokines interleukin-1β and tumor necrosis factor-α stimulate CFTR-mediated fluid secretion by swine airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 2012; 303:L327–L333. [DOI] [PubMed] [Google Scholar]
- 29.Yazbeck R, Howarth GS, Borges L, Geier MS, Smith CL, Davidson GP, Butler RN: Non-invasive detection of a palifermin-mediated adaptive response following chemotherapy-induced damage to the distal small intestine of rats. Cancer Biol Ther 2011; 12: 399–406. [DOI] [PubMed] [Google Scholar]
- 30.Ziambaras T, Rubin DC, Perlmutter DH: Regulation of sucrase-isomaltase gene expression in human intestinal epithelial cells by inflammatory cytokines. J Biol Chem 1996; 271: 1237–1242. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann G, Vassileva V, Piquette-Miller M: Impact of endotoxin-induced changes in P-glycoprotein expression on disposition of doxorubicin in mice. Drug Metab Dispos 2005; 33: 820–828. [DOI] [PubMed] [Google Scholar]
- 32.Tooley KL, Saxon BR, Webster J, Zacharakis B, McNeil Y, Davidson GP, Butler RN: A novel non-invasive biomarker for assessment of small intestinal mucositis in children with cancer undergoing chemotherapy. Cancer Biol Ther 2006; 5: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 33.Tooley KL, Howarth GS, Lymn KA, Butler RN: Optimization of the non-invasive 13C-sucrose breath test in a rat model of methotrexate-induced mucositis. Cancer Chemother Pharmacol 2010; 65: 913–921. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Zhou S, Huynh H, Duan W, Chan E: Alteration of the pharmacokinetics of COL-3, a matrix metalloproteinase inhibitor, due to acute gastrointestinal toxicity of doxorubicin. Pharm Res 2005; 22: 1954–1963. [DOI] [PubMed] [Google Scholar]
- 35.Tomimatsu T, Horie T: Enhanced glucose absorption in the rat small intestine following repeated doses of 5-fluorouracil. Chem Biol Interact 2005; 155: 129–139. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Miyamoto KI, Morita K, Haga H, Segawa H, Shiraga T, Fujioka A, Kouda T, Taketani Y, Hisano S, Fukui Y, Kitagawa K, Takeda E: Regulation of the PepT1 peptide transporter in the rat small intestine in response to 5-fluorouracil-induced injury. Gastroenterology 1998; 114: 714–723. [DOI] [PubMed] [Google Scholar]
- 37.de Koning BA, van der Schoor SR, Wattimena DL, de Laat PC, Pieters R, van Goudoever JB: Chemotherapy does not influence intestinal amino acid uptake in children. Pediatr Res 2007; 62: 195–199. [DOI] [PubMed] [Google Scholar]
- 38.Finch PW, Mark Cross LJ, McAuley DF, Farrell CL: Palifermin for the protection and regeneration of epithelial tissues following injury: new findings in basic research and preclinical models. J Cell Mol Med 2013; 17: 1065–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yazbeck R, Howarth GS: Complementary medicines: emerging therapies for intestinal mucositis. Cancer Biol Ther 2009; 8: 1629–1631. [DOI] [PubMed] [Google Scholar]
- 40.Keefe DM, Rassias G, O’Neil L, Gibson RJ: Severe mucositis: how can nutrition help? Curr Opin Clin Nutr Metab Care 2007; 10: 627–631. [DOI] [PubMed] [Google Scholar]
- 41.Li E, Trovato JA: New developments in management of oral mucositis in patients with head and neck cancer or receiving targeted anticancer therapies. Am J Health Syst Pharm 2012; 69: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 42.Feller L, Essop R, Wood NH, Khammissa RA, Chikte UM, Meyerov R, Lemmer J: Chemotherapy- and radiotherapy-induced oral mucositis: pathobiology, epidemiology and management. SADJ 2010; 65: 372–374. [PubMed] [Google Scholar]
- 43.Støy AC, Heegaard PM, Thymann T, Bjerre M, Skovgaard K, Boye M, Stoll B, Schmidt M, Jensen BB, Sangild PT: Bovine colostrum improves intestinal function following formula-induced gut inflammation in preterm pigs. Clin Nutr 2014; 33: 322–329. [DOI] [PubMed] [Google Scholar]
- 44.Playford RJ, MacDonald CE, Calnan DP, Floyd DN, Podas T, Johnson W, Wicks AC, Bashir O, Marchbank T: Co-administration of the health food supplement, bovine colostrum, reduces the acute non-steroidal anti-inflammatory drug-induced increase in intestinal permeability. Clin Sci (Lond) 2001; 100: 627–633. [PubMed] [Google Scholar]
- 45.Playford RJ, Macdonald CE, Johnson WS: Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am J Clin Nutr 2000; 72: 5–14. [DOI] [PubMed] [Google Scholar]
- 46.Howarth GS, Francis GL, Cool JC, Xu X, Byard RW, Read LC: Milk growth factors enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J Nutr 1996; 126: 2519–2530. [DOI] [PubMed] [Google Scholar]
- 47.Boukhettala N, Ibrahim A, Aziz M, Vuichoud J, Saudan KY, Blum S, Déchelotte P, Breuillé D, Coëffier M: A diet containing whey protein, free glutamine, and transforming growth factor-beta ameliorates nutritional outcome and intestinal mucositis during repeated chemotherapeutic challenges in rats. J Nutr 2010; 140: 799–805. [DOI] [PubMed] [Google Scholar]
- 48.Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN: Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr 2003; 77: 1296–1303. [DOI] [PubMed] [Google Scholar]
- 49.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ: The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 2010; 6:e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorpe DW, Stringer AM, Gibson RJ: Chemotherapy-induced mucositis: the role of the gastrointestinal microbiome and Toll-like receptors. Exp Biol Med (Maywood) 2013; 238: 1–6. [DOI] [PubMed] [Google Scholar]
- 51.Kvakkestad KM, Gammelsrud KW, Brandtzaeg P, Høiby EA: Unchanged antibiotic susceptibility in Escherichia coli and Pseudomonas aeruginosa after long-term in vitro exposure to antineoplastic drugs. Chemotherapy 2012; 58: 118–122. [DOI] [PubMed] [Google Scholar]
- 52.Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, Cauwels A, Takahashi N, Magez S, Bachert C, Vandenabeele P: TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ 2011; 18: 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowen JM, Keefe DM: New pathways for alimentary mucositis. J Oncol 2008; 2008: 907892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lalla RV, Pilbeam CC, Walsh SJ, Sonis ST, Keefe DM, Peterson DE: Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer 2010; 18: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Dasooqi N, Bowen JM, Gibson RJ, Logan RM, Stringer AM, Keefe DM: Selection of housekeeping genes for gene expression studies in a rat model of irinotecan-induced mucositis. Chemotherapy 2011; 57: 43–53. [DOI] [PubMed] [Google Scholar]
- 56.Dong Q, Chen L, Lu Q, Sharma S, Li L, Morimoto S, Wang G: Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol 2014;171: 4440–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]


