Abstract
Results
SII, NLR, and PLR did not define patient groups with distinct clinicopathological characteristics. SII, NLR, and PLR cut-off values were 547, 2.13, and 88.23, as determined by ROC analysis; the corresponding areas under the curve (AUCs) were 0.625, 0.555, and 0.571, respectively. Cox regression models identified SII as independently associated with OS. Patients with low SII had prolonged OS (65 vs. 41 months, P = 0.017, HR: 3.24, 95% CI: 1.23-8.55). In the Z test, the difference in AUC between SII and NLR was statistically significant (Z = 2.721, 95% CI: 0.0194-0.119, P = 0.0065).
Conclusion
Our study suggests that the pretreatment SII value is significantly correlated with OS in breast cancer patients undergoing NAC and that the prognostic utility of SII is superior to that of NLR and PLR.
1. Introduction
Breast cancer is the most frequently diagnosed cancer in women and is the leading cause of female cancer death [1]. Although hereditary breast cancer accounts for 5% to 10% of breast cancer cases, nongenetic factors represent the principal factor for the differences in breast cancer incidence among different ethnic groups [2]. Risk factors for breast cancer include menstruation (early menarche age, late age of menopause), childbearing history (childless, late childbearing age, and fertility), use of exogenous hormones (oral contraceptive or hormone replacement therapy), and nutrition and anthropometry (weight, body fat centripetal distribution). Moreover, breastfeeding and physical activity are widely recognized as protective factors against breast cancer [3]. Survival rates for patients have improved dramatically due to advances in treatment strategies [4]. However, 20%-25% of patients are diagnosed with locally advanced breast cancer, which is associated with a higher rate of recurrence and patient mortality [5, 6].
Neoadjuvant chemotherapy (NAC), as the standard treatment for locally advanced and inoperable tumors, has been widely used in breast cancer patients. The main aim of NAC use is to improve tumor operability, reduce tumor size, and improve the likelihood of eligibility for breast-conserving management strategies [7, 8]. Molecular subtypes and biomarkers such as Ki-67 and residual cancer burden have been used to predict overall survival (OS) following NAC; however, no further markers are used to routinely aid prognostication.
In recent years, the immune system has been recognized to play an essential role in breast cancer treatment response [9, 10]. As key components of the host immune system, inflammatory biomarkers such as neutrophil (N), lymphocyte (L), and platelet (P) levels—alongside neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have been identified as prognostic factors for many other malignant tumors [11–15].
As a comprehensive hematological parameter, the systemic immune-inflammation index (SII), which is based on neutrophil (N), platelet (P), and lymphocyte (L) counts (SII = N × P/L), can better reflect the immune and inflammatory state of the body compared to the use of any one of these markers in isolation [16]. Although SII has been used to study different cancers, including liver, pancreatic, and gastric cancers [16–18], it has not been widely investigated in the context of breast cancer. This article is aimed at exploring the prognostic role of SII in breast cancer patients undergoing neoadjuvant chemotherapy.
2. Materials and Methods
2.1. Patient Selection
Between January 2014 and May 2018, a total of 249 breast cancer patients received NAC and underwent surgery at the Tumor Hospital Affiliated to Harbin Medical University, Harbin, China. The study was approved by the ethics committee of the Tumor Hospital Affiliated to Harbin Medical University, including confirmation of adherence to the Declaration of Helsinki and its later amendments. All patients gave written informed consent before study participation.
The eligibility criteria for the patients included (i) pathological confirmation of breast cancer diagnosis by core needle biopsy prior to NAC treatment, (ii) consent for NAC and surgery in our hospital, (iii) complete clinical recorded information and follow-up data, and (iv) peripheral blood samples obtained within one week prior to NAC initiation.
The exclusion criteria for the patients included (i) receipt of radiotherapy, endocrine therapy, or targeted therapy prior to NAC; (ii) diagnosis of chronic inflammatory or autoimmune disease, such as liver cirrhosis or systemic lupus erythematosus (SLE); and (iii) patients with distant metastasis.
2.2. Chemotherapy Regimens
All patients received NAC after diagnosis; the chemotherapy regimen was selected according to the results of immunohistochemistry and patients' wishes. Anthracycline- (A-) based and/or taxane- (T-) based NAC regimens were used in our study; AC regimen: anthracyclines 90 mg/m2 and cyclophosphamide (C) 600 mg/m2; AC-T regimen: anthracyclines 90 mg/m2, cyclophosphamide 600 mg/m2, and taxanes 80-100 mg/m2; AC-TH regimen: anthracyclines 90 mg/m2, cyclophosphamide 600 mg/m2, taxanes 75 mg/m2, and Herceptin (H) first dose 8 mg/kg, then 6 mg/kg; and TAC regimen: taxanes 75 mg/m2, anthracyclines 50 mg/m2, and cyclophosphamide 500 mg/m2. One cycle of the chosen regimen was repeated every 3 weeks. All patients received at least four cycles of NAC. Patients with docetaxel were given dexamethasone and diphenhydramine before chemotherapy to prevent allergy. Surgery was performed following approximately two weeks of rest period after NAC completion, according to the patient's condition.
2.3. Classification Criteria
The TNM stage system was performed according to the eighth edition of the American Joint Committee on Cancer (AJCC) [19]. Luminal A, luminal B/HER-2-positive, luminal B/HER-2-negative, triple negative, and HER-2-enriched molecular subtypes were used [20]. Age and N, L, and P count groups were divided by the median. High and low SII, NLR, and PLR groups were identified using thresholds determined by receiver operating characteristic (ROC) analysis of maximum sensitivity and specificity.
2.4. Follow-Up
All patients received a 3-monthly follow-up for two years after surgery, a 6-monthly follow-up for the next three years, and then an annual follow-up. Laboratory tests, physical examination (breast and lymph node palpation), breast ultrasonography, liver ultrasound, mammography, and other suitable examinations are used to assess the physical condition of patients at follow-up.
2.5. Statistical Analysis
Statistical analyses were conducted by SPSS software (version 17.0) and MedCalc software (version 19.0.7). ROC analysis was used to determine the optimal cut-off value for patient dichotomization thresholds. Categorical variables are described using frequencies and percentages (%); differences were assessed by a chi-squared test or Fisher's exact test. OS time was determined using the Kaplan-Meier product limit estimator method; survival associations were determined by Kaplan-Meier plots and log-rank test. Cox proportional hazards regression models were used to examine the independence of prognostic factors. Z tests were used to compare the difference in predictive ability between different groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Clinicopathologic Characteristics of all Breast Cancer Patients Divided by SII, NLR, and PLR
Two hundred and forty-nine breast cancer patients were classified by SII, NLR, and PLR. All cases were female; the median patient age was 51 years. BMI was calculated and classified according to WHO criteria: under normal weight (BMI < 18.5; 8 patients, 3.2%), normal weight (BMI ≥ 18.5; 154 patients, 61.8%), overweight (BMI ≥ 25; 71 patients, 28.5%), and obese (BMI ≥ 30; 16 patients, 6.4%). 50 patients (20.0%) achieved complete pathological response (pCR).
The clinicopathological characteristics of patients in different SII, NLR, and PLR groups are shown in Tables 1–3. ROC analysis was used to determine the optimal cut-off value for patient grouping by SII (547 × 109/L), NLR (2.13 × 109/L), and PLR (88.23 × 109/L). Hence, patients were dichotomized by these markers: low SII (<547 × 109/L, 183 patients, 73.5%) and high SII (≥547 × 109/L; 66 patients, 26.5%), low NLR (<2.13 × 109/L; 177 patients, 71.1%) and high NLR (>2.13 × 109/L; 72 patients, 28%), and low PLR (<88.23 × 109/L; 49 patients, 19.7%) and high PLR (>88.23 × 109/L; 200 patients, 80.3%).
Table 1.
Association between clinicopathological factors and different SII groups.
| Parameters | n = 249 (%) | SII ≤ 547 | SII > 547 | X 2 | P value |
|---|---|---|---|---|---|
| Cases | n = 183 (%) | n = 66 (%) | |||
| Age (years) | 2.493 | 0.114 | |||
| ≤51 | 134 (53.8%) | 93 (50.8%) | 41 (62.1%) | ||
| >51 | 115 (46.2%) | 90 (49.2%) | 25 (37.9%) | ||
| Tumor position | 0.059 | 0.807 | |||
| Left | 139 (55.8%) | 103 (56.3%) | 36 (54.5%) | ||
| Right | 110 (44.2%) | 80 (43.7%) | 30 (45.5%) | ||
| BMI | 0.865 | 0.864 | |||
| <18.5 | 8 (3.2%) | 7 (3.8%) | 1 (1.5%) | ||
| ≥18.5 | 154 (61.8%) | 113 (61.7%) | 41 (62.1%) | ||
| ≥25 | 71 (28.5%) | 52 (28.4%) | 19 (28.8%) | ||
| ≥30 | 16 (6.4%) | 11 (6.0%) | 5 (7.6%) | ||
| Parturition | 2.166 | 0.537 | |||
| 0 | 33 (13.3%) | 26 (14.2%) | 7 (10.6%) | ||
| 1 | 150 (60.2%) | 110 (60.1%) | 40 (60.6%) | ||
| 2 | 54 (21.7%) | 36 (19.7%) | 18 (27.3%) | ||
| >2 | 12 (4.8%) | 11 (6.0%) | 1 (1.5%%) | ||
| cT stage | 4.379 | 0.201 | |||
| T1 | 22 (8.8%) | 17 (9.3%) | 5 (7.6%) | ||
| T2 | 187 (75.1%) | 141 (77.0%) | 46 (69.7%) | ||
| T3 | 35 (14.1%) | 23 (12.6%) | 12 (18.2%) | ||
| T4 | 5 (2.0%) | 2 (1.1%) | 3 (4.5%) | ||
| ER | 1.612 | 0.204 | |||
| Negative | 86 (34.5%) | 59 (32.2%) | 27 (40.9%) | ||
| Positive | 163 (65.5%) | 124 (67.8%) | 39 (59.1%) | ||
| PR | 0.613 | 0.434 | |||
| Negative | 118 (47.4%) | 84 (45.9%) | 34 (51.5%) | ||
| Positive | 131 (52.6%) | 99 (54.1%) | 32 (48.5%) | ||
| HER-2 | 4.019 | 0.045 | |||
| Negative | 161 (64.7%) | 125 (68.3%) | 36 (54.5%) | ||
| Positive | 88 (35.3%) | 58 (31.7%) | 30 (45.5%) | ||
| Ki-67 | 0.699 | 0.403 | |||
| ≤14% | 73 (29.3%) | 51 (27.9%) | 22 (33.3%) | ||
| >14% | 176 (70.7%) | 132 (72.1%) | 44 (66.7%) | ||
| Subtype | 5.369 | 0.252 | |||
| Luminal A | 25 (10.0%) | 20 (10.9%) | 5 (7.6%) | ||
| Luminal B/HER-2- | 100 (40.2%) | 77 (42.1%) | 23 (34.8%) | ||
| Luminal B/HER-2+ | 42 (16.9%) | 30 (16.4%) | 12 (18.2%) | ||
| TNBC | 36 (14.5%) | 28 (15.3%) | 8 (12.1%) | ||
| HER-2 enriched | 46 (18.5%) | 28 (15.3%) | 18 (27.3%) | ||
| P53 | 0.327 | 0.567 | |||
| Negative | 117 (47.0%) | 84 (45.9%) | 33 (50.0%) | ||
| Positive | 132 (53.0%) | 99 (54.1%) | 33 (50.0%) | ||
| pCR | 1.804 | 0.179 | |||
| No | 199 (79.9%) | 150 (82.0%) | 49 (74.2%) | ||
| Yes | 50 (20.1%) | 33 (18.0%) | 17 (25.8%) |
Abbreviation: BMI: body mass index; cT stage: clinical T stage; ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor-2; TNBC: triple negative breast cancer; pCR: pathologic complete response.
Table 2.
Association between clinicopathological factors and different NLR groups.
| Parameters | n = 249 (%) | NLR ≤ 2.13 | NLR > 2.13 | X 2 | P value |
|---|---|---|---|---|---|
| Cases | n = 177 (%) | n = 72 (%) | |||
| Age (years) | 1.422 | 0.233 | |||
| ≤51 | 134 (53.8%) | 91 (51.4%) | 43 (59.7%) | ||
| >51 | 115 (46.2%) | 86 (48.6%) | 29 (40.3%) | ||
| Tumor position | 0.624 | 0.429 | |||
| Left | 139 (55.8%) | 96 (54.2%) | 43 (59.7%) | ||
| Right | 110 (44.2%) | 81 (45.8%) | 29 (40.3%) | ||
| BMI | 2.702 | 0.437 | |||
| <18.5 | 8 (3.2%) | 7 (4.0%) | 1 (1.4%) | ||
| ≥18.5 | 154 (61.8%) | 109 (61.6%) | 45 (62.5%) | ||
| ≥25 | 71 (28.5%) | 52 (29.4%) | 19 (26.4%) | ||
| ≥30 | 16 (6.4%) | 9 (5.1%) | 7 (9.7%) | ||
| Parturition | 3.684 | 0.294 | |||
| 0 | 33 (13.3%) | 25 (14.1%) | 8 (11.1%) | ||
| 1 | 150 (60.2%) | 106 (59.9%) | 44 (61.1%) | ||
| 2 | 54 (21.7%) | 35 (19.8%) | 19 (26.4%) | ||
| >2 | 12 (4.8%) | 11 (6.2%) | 1 (1.4%) | ||
| cT stage | 5.760 | 0.109 | |||
| T1 | 22 (8.8%) | 18 (10.2%) | 4 (5.6%) | ||
| T2 | 187 (75.1%) | 136 (76.8%) | 51 (70.8%) | ||
| T3 | 35 (14.1%) | 21 (11.9%) | 14 (19.4%) | ||
| T4 | 5 (2.0%) | 2 (1.1%) | 3 (4.2%) | ||
| ER | 0.848 | 0.357 | |||
| Negative | 86 (34.5%) | 58 (32.8%) | 28 (38.9%) | ||
| Positive | 163 (65.5%) | 119 (67.2%) | 44 (61.1%) | ||
| PR | 0.650 | 0.420 | |||
| Negative | 118 (47.4%) | 81 (45.8%) | 37 (51.4%) | ||
| Positive | 131 (52.6%) | 96 (54.2%) | 35 (48.6%) | ||
| HER-2 | 4.879 | 0.027 | |||
| Negative | 161 (64.7%) | 122 (68.9%) | 39 (54.2%) | ||
| Positive | 88 (35.3%) | 55 (31.1%) | 33 (45.8%) | ||
| Ki-67 | 0.788 | 0.375 | |||
| ≤14% | 73 (29.3%) | 49 (27.7%) | 24 (33.3%) | ||
| >14% | 176 (70.7%) | 128 (72.3%) | 48 (66.7%) | ||
| Subtype | 5.261 | 0.262 | |||
| Luminal A | 25 (10.0%) | 20 (11.3%) | 5 (6.9%) | ||
| Luminal B/HER-2- | 100 (40.2%) | 75 (42.4%) | 25 (34.7%) | ||
| Luminal B/HER-2+ | 42 (16.9%) | 27 (15.3%) | 15 (20.8%) | ||
| TNBC | 36 (14.5%) | 27 (15.3%) | 9 (12.5%) | ||
| HER-2 enriched | 46 (18.5%) | 28 (15.8%) | 18 (25.0%) | ||
| P53 | 0.369 | 0.544 | |||
| Negative | 117 (47.0%) | 81 (45.8%) | 36 (50.0%) | ||
| Positive | 132 (53.0%) | 96 (54.2%) | 36 (50.0%) | ||
| pCR | 0.787 | 0.375 | |||
| No | 199 (79.9%) | 144 (81.4%) | 55 (76.4%) | ||
| Yes | 50 (20.1%) | 33 (18.6%) | 17 (23.6%) |
Abbreviation: BMI: body mass index; cT stage: clinical T stage; ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor-2; TNBC: triple negative breast cancer; pCR: pathologic complete response.
Table 3.
Association between clinicopathological factors and different PLR groups.
| Parameters | n = 249 (%) | PLR ≤ 88.23 | PLR > 88.23 | X 2 | P value |
|---|---|---|---|---|---|
| Cases | 49 (%) | 200 (%) | |||
| Age (years) | 5.552 | 0.018 | |||
| ≤51 | 134 (53.8%) | 19 (38.8%) | 115 (57.5%) | ||
| >51 | 115 (46.2%) | 30 (61.2%) | 85 (42.5%) | ||
| Tumor position | 0.279 | 0.597 | |||
| Left | 139 (55.8%) | 29 (59.2%) | 110 (55.0%) | ||
| Right | 110 (44.2%) | 20 (40.8%) | 90 (45.0%) | ||
| BMI | 7.153 | 0.052 | |||
| <18.5 | 8 (3.2%) | 4 (8.2%) | 4 (2.0%) | ||
| ≥18.5 | 154 (61.8%) | 24 (49.0%) | 130 (65.0%) | ||
| ≥25 | 71 (28.5%) | 17 (34.7%) | 54 (27.0%) | ||
| ≥30 | 16 (6.4%) | 4 (8.2%) | 12 (6.0%) | ||
| Parturition | 2.166 | 0.537 | |||
| 0 | 33 (13.3%) | 6 (12.2%) | 27 (13.5%) | ||
| 1 | 150 (60.2%) | 27 (55.1%) | 123 (61.5%) | ||
| 2 | 54 (21.7%) | 12 (24.5%) | 42 (21.0%) | ||
| >2 | 12 (4.8%) | 4 (8.2%) | 8 (4.0%) | ||
| cT stage | 5.607 | 0.111 | |||
| T1 | 22 (8.8%) | 7 (14.3%) | 15 (7.5%) | ||
| T2 | 187 (75.1%) | 39 (79.6%) | 148 (74.0%) | ||
| T3 | 35 (14.1%) | 3 (6.1%) | 32 (16.0%) | ||
| T4 | 5 (2.0%) | 0 (0.0%) | 5 (2.5%) | ||
| ER | 0.416 | 0.519 | |||
| Negative | 86 (34.5%) | 15 (30.6%) | 71 (35.5%) | ||
| Positive | 163 (65.5%) | 34 (69.4%) | 129 (64.5%) | ||
| PR | 0.323 | 0.570 | |||
| Negative | 118 (47.4%) | 25 (51.0%) | 93 (46.5%) | ||
| Positive | 131 (52.6%) | 24 (49.0%) | 107 (53.5%) | ||
| HER-2 | 0.193 | 0.660 | |||
| Negative | 161 (64.7%) | 33 (67.3%) | 128 (64.0%) | ||
| Positive | 88 (35.3%) | 16 (32.7%) | 72 (36.0%) | ||
| Ki-67 | 0.229 | 0.633 | |||
| ≤14% | 73 (29.3%) | 13 (26.5%) | 60 (30.0%) | ||
| >14% | 176 (70.7%) | 36 (73.5%) | 140 (70.0%) | ||
| Subtype | 2.081 | 0.721 | |||
| Luminal A | 25 (10.0%) | 7 (14.3%) | 18 (9.0%) | ||
| Luminal B/HER-2- | 100 (40.2%) | 18 (36.7%) | 82 (41.0%) | ||
| Luminal B/HER-2+ | 42 (16.9%) | 9 (18.4%) | 33 (16.5%) | ||
| TNBC | 36 (14.5%) | 8 (16.3%) | 28 (14.0%) | ||
| HER-2 enriched | 46 (18.5%) | 7 (14.3%) | 39 (19.5%) | ||
| P53 | 0.933 | 0.334 | |||
| Negative | 117 (47.0%) | 20 (40.8%) | 97 (48.5%) | ||
| Positive | 132 (53.0%) | 29 (59.2%) | 103 (51.5%) | ||
| pCR | 2.334 | 0.127 | |||
| No | 199 (79.9%) | 43 (87.8%) | 156 (78.0%) | ||
| Yes | 50 (20.1%) | 6 (12.2%) | 44 (22.0%) |
Abbreviation: BMI: body mass index; cT stage: clinical T stage; ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor-2; TNBC: triple negative breast cancer; pCR: pathologic complete response.
Patient groups defined by SII, NLR, and PLR demonstrated similar clinicopathological characteristics (Tables 1–3). Low SII value was significantly associated with the HER-2 subgroup (X2 = 4.019, P = 0.045) and low NLR (X2 = 4.879, P = 0.027). PLR status was significantly associated with patient age (X2 = 5.552, P = 0.018).
3.2. Cox Regression Survival Analyses
Follow-up time ranged from 4 to 72 months; 5 patients were followed up less than 12 months. The median follow-up time was 34 and 28 months in the low and high SII groups, respectively. The median follow-up time of low NLR, high NLR, low PLR, and high PLR groups was 34, 28, 35, and 32 months, respectively. Median OS time was only reached in the high SII and high NLR groups (39 months and 48 months, respectively). However, the mean OS time in patients with low SII, low NLR, and low PLR is significantly longer than that in those patients with high SII, high NLR, and high PLR, as determined by the log-rank test (65 vs. 41 months, P ≤ 0.001; 64 vs. 50 months, P ≤ 0.001; and 61 vs. 59 months, P = 0.007, respectively) (Figures 1–3).
Figure 1.
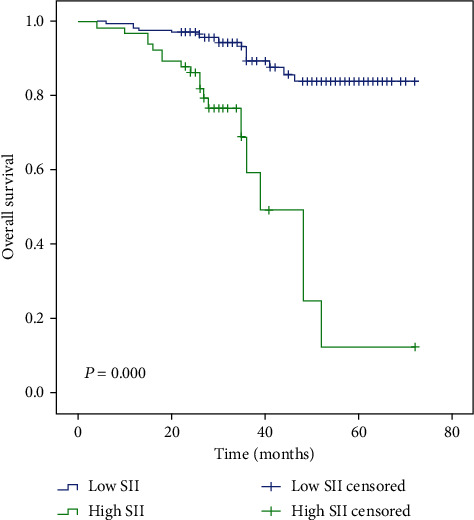
Kaplan-Meier analysis of OS in patients of high and low SII with breast cancer.
Figure 2.
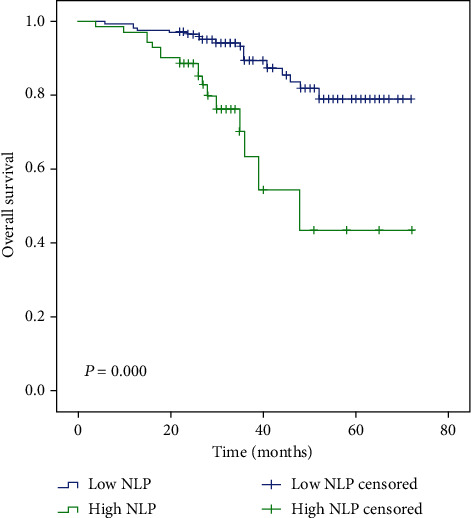
Kaplan-Meier analysis of OS in patients of high and low NLR with breast cancer.
Figure 3.
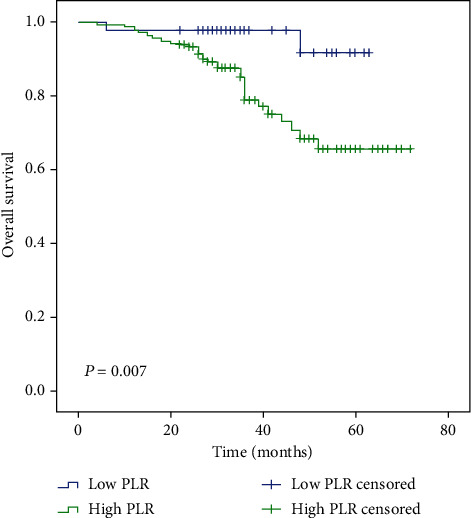
Kaplan-Meier analysis of OS in patients of high and low PLR with breast cancer.
In univariate analysis, clinical T stage, ER status, PR status, molecular subtype, P, SII, NLR, and PLR were significantly associated with differential OS. However, multivariable analysis identified only SII as independently associated with OS, with the low SII group demonstrating prolonged OS time (P = 0.017, HR:3.24, 95% CI: 1.23-8.55) (Table 4).
Table 4.
Univariate and multivariate Cox regression survival analyses of the SII for the prediction of the OS in patients with breast cancer.
| Parameters | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (≤51/>51years old) | 1.145 (0.590-2.224) | 0.688 | ||
| Position (left/right) | 1.692 (0.866-3.306) | 0.124 | ||
| BMI (U+N/OW+OB) | 0.910 (0.457-1.813) | 0.789 | ||
| Parturition (0/≥1) | 1.052 (0.408-2.716) | 0.916 | ||
| cT stage (T1+T2/T3+T4) | 2.115 (1.014-4.413) | 0.046 | 1.214 (0.558-2.641) | 0.624 |
| ER (negative/positive) | 0.330 (0.168-0.650) | 0.001 | 0.526 (0.112-2.472) | 0.416 |
| PR (negative/positive) | 0.305 (0.143-0.652) | 0.002 | 0.428 (0.140-1.314) | 0.138 |
| HER-2 (negative/positive) | 1.537 (0.786-3.007) | 0.209 | ||
| Ki-67 (≤14%/>14%) | 2.284 (0.886-5.891) | 0.087 | ||
| Subtype (A/B-/B+/TNBC/HER-2) | 1.527 (1.186-1.966) | 0.001 | 0.902 (0.494-1.648) | 0.738 |
| P53 (negative/positive) | 1.166 (0.593-2.291) | 0.656 | ||
| pCR (no/yes) | 0.843 (0.350-2.033) | 0.704 | ||
| N (≤3.65/>3.65) | 1.544 (0.795-3.039) | 0.197 | ||
| P (≤252/>252) | 2.298 (1.142-4.626) | 0.020 | 1.288 (0.576-2.878) | 0.538 |
| L (≤2.19/>2.19) | 0.733 (0.376-1.427) | 0.361 | ||
| SII (≤547/>547) | 6.302 (3.149-12.62) | 0.000 | 3.244 (1.230-8.554) | 0.017 |
| NLR (≤2.13/>2.13) | 4.032 (2.033-7.996) | 0.000 | 1.769 (0.672-4.659) | 0.248 |
| PLR (≤88.23/>88.23) | 5.621 (1.345-23.49) | 0.018 | 3.539 (0.782-16.02) | 0.101 |
Abbreviation: BMI: body mass index; U+N: under normal weight (BMI < 18.5) and normal weight (BMI ≥ 18.5); OW+OB: overweight (BMI ≥ 25) and obesity (BMI ≥ 30); cT stage: clinical T stage; ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor-2; A: luminal A; B-: luminal B/HER-2-; B+: luminal B/HER-2+; TNBC: triple negative breast cancer; HER-2: HER-2 enriched; pCR: pathologic complete response; N: neutrophil; P: platelet; L: lymphocyte; SII: systemic immune-inflammation index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
3.3. Comparison of Prognostic Ability of SII, NLR, and PLR
In order to determine the value of different hematological parameters, AUC values were compared by ROC analysis. SII had a significantly higher AUC value compared to NLR and PLR (AUC = 0.625, P = 0.018) (Table 5 and Figure 4). In the Z test, the difference of AUC between SII and NLR is also statistically significant (Z = 2.721, 95% CI: 0.0194-0.119, P = 0.0065). By contrast, comparisons of SII versus PLR and NLR vs. PLR did reveal statistically significant differences (Z = 1.039, 95% CI: -0.0478-0.156, P = 0.2986; Z = 0.255, 95% CI: -0.103-0.134, P = 0.7989, respectively) (Table 6).
Table 5.
Receiver operating characteristics analyses of SII, NLR, and PLR in patients with breast cancer.
| Parameters | Cut-off value | AUC (95% CI) | Sensitivity | Specificity | P value |
|---|---|---|---|---|---|
| SII | 547 | 0.625 (0.515-0.735) | 0.543 | 0.780 | 0.018 |
| NLR | 2.13 | 0.555 (0.435-0.676) | 0.486 | 0.748 | 0.293 |
| PLR | 88.23 | 0.571 (0.467-0.675) | 0.943 | 0.220 | 0.179 |
Figure 4.
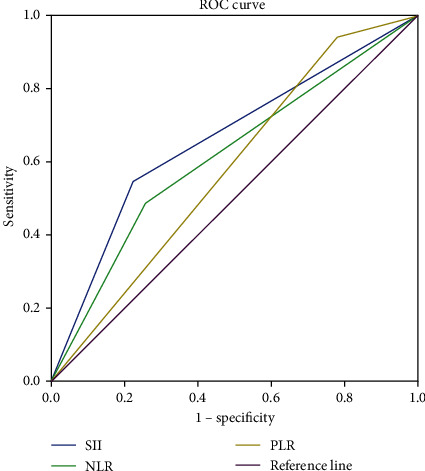
Comparison of prognostic ability of SII, NLR, and PLR.
Table 6.
The difference between SII, NLR, and PLR.
| Parameters | Difference of AUC | SE | 95% CI | Z statistic | P value |
|---|---|---|---|---|---|
| SII vs. NLR | 0.0694 | 0.0255 | 0.0194-0.119 | 2.721 | 0.0065 |
| SII vs. PLR | 0.0540 | 0.0520 | -0.0478-0.156 | 1.039 | 0.2986 |
| NLR vs. PLR | 0.0154 | 0.0605 | -0.103-0.134 | 0.255 | 0.7989 |
4. Discussion
We investigated the clinical significance of SII in breast cancer patients receiving NAC and compared the prognostic capacity of SII, PLR, and NLR. We demonstrate that high SII is significantly associated with shorter OS and that SII outperforms NLR as a prognostic marker in breast cancer patients receiving NAC.
Peripheral venous blood markers are known to reflect the condition of the whole immune system. The link between systemic inflammation and malignant tumors has been demonstrated in numerous studies [21–25]. Moreover, levels of neutrophils, platelets, and lymphocytes are prognostic in multiple cancer types. Neutrophils play a role in the proliferation and metastasis of tumors by releasing inflammatory mediators such as neutrophil elastase, interleukin-8, matrix metalloproteinase-9, and vascular endothelial growth factor [26–28]. Platelets can promote tumor angiogenesis and metastasis and protect tumor cells from antitumor immune response [29–31]. By contrast, lymphocytes represent a key component of the antitumor immune response, preventing tumor growth and metastasis, prolonging patient survival [32–34].
Few studies have investigated the impact of SII in breast cancer patients receiving NAC. In a study by Liu and colleagues, SII was identified as an independent prognostic factor in TNBC patients [35]. In two other studies investigating HER-2-positive breast cancer, SII was also related to OS time [36, 37]. By contrast, Chen and colleagues comprehensively evaluated the prognostic effects of SII on a nonsubtype-specific manner [38]; this study demonstrated differential 3-, 5-, and 10-year DFS and OS according to SII status [38]. In our study, high SII is related to the shorter mean OS time compared to low SII (65 vs. 41 months, P ≤ 0.001). Multivariate Cox regression analysis identified that SII is the only factor independently associated with survival (P = 0.017, HR: 3.244, 95% CI: 1.230-8.554).
A number of studies have investigated NLR and PLR; however, these have failed to produce consistent results. A meta-analysis demonstrated that high NLR was significantly associated with poor pathological response [39] but failed to demonstrate a significant association with DFS and OS in breast cancer patients. By contrast, a separate meta-analysis identified poor OS and high recurrence risk for breast cancer patients with high NLR and PLR [40]. Similarly, Bun et al. identified NLR as a significant and independent factor associated with OS time [41]. However, a separate study by Malek et al. showed that NLR was not associated with pCR [42]. In the current study, the low NLR and low PLR groups demonstrated better mean OS time (64 vs. 50 months, P ≤ 0.001; 61 vs. 59 months, P = 0.007, respectively). However, we did not identify a significant independent association between NLR or PLR and OS time upon multivariable analysis (P > 0.05).
We demonstrate that, compared to NLR and PLR, SII has statistically significantly higher AUC and specificity by ROC analysis (AUC = 0.625, 95% CI: 0.515-0.735, specificity = 0.780, P = 0.018). Pairwise comparison of SII, NLR, and PLR using Z tests identified a significantly higher AUC for SII than NLR (P = 0.0065). Together, these results suggest that SII is of greater prognostic utility than NLR.
As hematological parameters, SII, NLR, and PLR are easy to obtain and are easy to measure repeatedly; at least some of these measurements have been demonstrated—by ourselves and others—as prognostic indicators with potential clinical utility. We demonstrate that, compared with NLR and PLR, SII appears to be of the greatest independent value.
We acknowledge several limitations of our study; firstly, it is a single-center retrospective study with no control group. Secondly, our cohort has a relatively short follow-up; longer follow-up time is required to determine the impact of SII, PLR, and NLR on long-term clinical outcomes. Independent validation in a large breast cancer patient cohort is needed.
5. Conclusion
Our study comprehensively analyzes the prognostic value of SII in breast cancer patients undergoing NAC and compared the relative prognostic performance of SII, NLR, and PLR. Our findings suggest that the pretreatment SII is significantly associated with OS in breast cancer independent of other factors and that SII is superior to NLR and PLR. SII is therefore worthy of further investigation as a prognostic hematologic parameter for breast cancer patients.
Data Availability
The measurement and enumeration data used to support the findings of this study are restricted by the ethics committee of Tumor Hospital Affiliated to Harbin Medical University in order to protect patient privacy.
Ethical Approval
This study was approved by the ethics committee of Tumor Hospital Affiliated to Harbin Medical University. It confirmed the standards of the Declaration of Helsinki and its later amendments.
Consent
All enrolled patients provided written informed consent before the study.
Disclosure
None of the executives of the related businesses has had any involvement in the manuscript writing, editing, approval, or decision to publish.
Conflicts of Interest
All the authors report no conflicts of interest in this work.
Authors' Contributions
Cong Jiang and Yuanxi Huang conceived and designed the experiments. Yubo Lu and Shiyuan Zhang analyzed the studies and extracted data. All authors participated in the writing, reading, and revising of the manuscript and approved the final version of the manuscript.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler R. G., Hoover R. N., Pike M. C., et al. Migration patterns and breast cancer risk in Asian-American women. Journal of the National Cancer Institute. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 3.Brinton L. A., Gaudet M. M., Gierach G. L. Breast cancer. In: Thun M. J., Linet M. S., Cerhan J. R., Haiman C. A., Schottenfeld D., editors. Cancer Epidemiology and Prevention. 4th. New York, NY, USA: Oxford University Press; 2018. pp. 861–888. [Google Scholar]
- 4.Patel D. A., Xi J., Luo J., et al. Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Research and Treatment. 2019;174(2):443–452. doi: 10.1007/s10549-018-05106-7. [DOI] [PubMed] [Google Scholar]
- 5.Wang M., Hou L., Chen M., et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Scientific Reports. 2017;7(1, article 44673) doi: 10.1038/srep44673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinacki M., Badzio A., Wełnicka-Jaśkiewicz M., et al. Pattern of care in locally advanced breast cancer: focus on local therapy. Breast. 2011;20(2):145–150. doi: 10.1016/j.breast.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Xie H., Liu J., Yu S., et al. Patterns of use of docetaxel-containing adjuvant chemotherapy among Chinese patients with operable breast cancer: a multicenter observational study. Advances in Therapy. 2019;36(1):131–146. doi: 10.1007/s12325-018-0841-7. [DOI] [PubMed] [Google Scholar]
- 8.Franceschini G., Di AL N. M., Sanchez M. A., Masett R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Annali Italiani di Chirurgia. 2018;89:p. 290. [PubMed] [Google Scholar]
- 9.Andre F., Dieci M. V., Dubsky P., et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clinical Cancer Research. 2013;19(1):28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- 10.Bianchini G., Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncology. 2014;15(2):e58–e68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 11.Elyasinia F., Keramati M. R., Ahmadi F., et al. Neutrophil-lymphocyte ratio in different stages of breast cancer. Acta Medica Iranica. 2017;55(4):228–232. [PubMed] [Google Scholar]
- 12.Ma J. Y., Hu G., Liu Q. Prognostic significance of the lymphocyte-to-monocyte ratio in bladder cancer undergoing radical cystectomy: a meta-analysis of 5638 individuals. Disease Markers. 2019;2019:8. doi: 10.1155/2019/7593560.7593560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Hao Y., Cong X., et al. Peripheral venous blood platelet-to-lymphocyte ratio (PLR) for predicting the survival of patients with gastric cancer treated with SOX or XELOX regimen neoadjuvant chemotherapy. Technology in Cancer Research & Treatment. 2019;18:p. 153303381982948. doi: 10.1177/1533033819829485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S., Xu H., Wang W., et al. The systemic inflammation response index predicts survival and recurrence in patients with resectable pancreatic ductal adenocarcinoma. Cancer Management and Research. 2019;Volume 11:3327–3337. doi: 10.2147/CMAR.S197911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandaliya H., Jones M., Oldmeadow C., Nordman I. I. C. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI) Translational Lung Cancer Research. 2019;8(6):886–894. doi: 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz M. H., Sideras K., Aziz N. A., et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels. Annals of Surgery. 2019;270(1):139–146. doi: 10.1097/SLA.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 17.Wang B., Huang Y., Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma. Medicine (Baltimore) 2020;99(1, article e18571) doi: 10.1097/MD.0000000000018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. Journal of Gastrointestinal Oncology. 2019;10(5):965–978. doi: 10.21037/jgo.2019.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Research and Treatment. 2018;168(1):269–275. doi: 10.1007/s10549-017-4577-x. [DOI] [PubMed] [Google Scholar]
- 20.Sandberg L. J., Clemens M. W., Symmans W. F., et al. Molecular profiling using breast cancer subtype to plan for breast reconstruction. Plast Reconstr Surg. 2017;139(3):586e–596e. doi: 10.1097/PRS.0000000000003050. [DOI] [PubMed] [Google Scholar]
- 21.Shinko D., Diakos C. I., Clarke S. J., Charles K. A. Cancer-related systemic inflammation: the challenges and therapeutic opportunities for personalized medicine. Clinical Pharmacology and Therapeutics. 2017;102(4):599–610. doi: 10.1002/cpt.789. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Cordoba S., Meneghini E., Sant M., et al. Decoding immune heterogeneity of triple negative breast cancer and its association with systemic inflammation. Cancers. 2019;11(7):p. 911. doi: 10.3390/cancers11070911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stovgaard E. S., Nielsen D., Hogdall E., Balslev E. Triple negative breast cancer - prognostic role of immune-related factors: a systematic review. Acta Oncologica. 2018;57(1):74–82. doi: 10.1080/0284186X.2017.1400180. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L.-Y., Yang D.-D., Ma X.-K., et al. The prognostic value of aspartate aminotransferase to lymphocyte ratio and systemic immune-inflammation index for overall survival of hepatocellular carcinoma patients treated with palliative treatments. Journal of Cancer. 2019;10(10):2299–2311. doi: 10.7150/jca.30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanghera C., Teh J. J., Pinato D. J. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver International. 2019;39(11):2008–2023. doi: 10.1111/liv.14220. [DOI] [PubMed] [Google Scholar]
- 26.Houghton A. M. G., Rzymkiewicz D. M., Ji H., et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine. 2010;16(2):219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walz W., Cayabyab F. S. Neutrophil infiltration and matrix metalloproteinase-9 in lacunar infarction. Neurochemical Research. 2017;42(9):2560–2565. doi: 10.1007/s11064-017-2265-1. [DOI] [PubMed] [Google Scholar]
- 28.Tan K. W., Chong S. Z., Wong F. H. S., et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122(22):3666–3677. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- 29.Franco A. T., Corken A., Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L., Luan Y., Miao X., et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF- integrin cooperative signalling. British Journal of Cancer. 2017;117(5):695–703. doi: 10.1038/bjc.2017.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter D. G., Kopetz S., Hawk E., et al. Platelet "first responders" in wound response, cancer, and metastasis. Cancer Metastasis Reviews. 2017;36(2):199–213. doi: 10.1007/s10555-017-9682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohme M., Riethdorf S., Pantel K. Circulating and disseminated tumour cells -- mechanisms of immune surveillance and escape. Nature Reviews Clinical Oncology. 2017;14(3):155–167. doi: 10.1038/nrclinonc.2016.144. [DOI] [PubMed] [Google Scholar]
- 33.Ali H. R., Provenzano E., Dawson S. J., et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Annals of Oncology. 2014;25(8):1536–1543. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 34.Velaei K., Samadi N., Barazvan B., Rad J. S. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast. 2016;30:92–100. doi: 10.1016/j.breast.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Shi Z., Bai Y., Liu L., Cheng K. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Management and Research. 2019;Volume 11:4471–4480. doi: 10.2147/CMAR.S197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang L., Fang J., Ding J. High systemic immune-inflammation index predicts poor survival in patients with human epidermal growth factor receptor-2 positive breast cancer receiving adjuvant trastuzumab. Cancer Management and Research. 2020;Volume 12:475–484. doi: 10.2147/CMAR.S231444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y., Li W., Li A. J., Su H., Yue J., Yu J. <p>Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients</p>. Cancer Management and Research. 2019;Volume 11:3153–3162. doi: 10.2147/CMAR.S190335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L., Kong X., Wang Z., Wang X., Fang Y., Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. Journal of Cellular and Molecular Medicine. 2020;24(5):2993–3021. doi: 10.1111/jcmm.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue L. B., Liu Y. H., Zhang B., et al. Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: meta-analysis. Medicine. 2019;98(1, article e13842) doi: 10.1097/MD.0000000000013842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo W., Lu X., Liu Q., et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Medicine. 2019;8(9):4135–4148. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bun A., Fujimoto Y., Higuchi T., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in luminal breast cancers with low levels of tumour-infiltrating lymphocytes. Anticancer Research. 2020;40(5):2871–2880. doi: 10.21873/anticanres.14263. [DOI] [PubMed] [Google Scholar]
- 42.Eryilmaz M. K., Mutlu H., Salim D. K., Musri F. Y., Tural D., Coskun H. S. The neutrophil to lymphocyte ratio has a high negative predictive value for pathologic complete response in locally advanced breast cancer patients receiving neoadjuvant chemotherapy. Asian Pacific Journal of Cancer Prevention. 2014;15(18):7737–7740. doi: 10.7314/apjcp.2014.15.18.7737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The measurement and enumeration data used to support the findings of this study are restricted by the ethics committee of Tumor Hospital Affiliated to Harbin Medical University in order to protect patient privacy.


