Highlights
-
•
The last polio seroprevalence study in India before the tOPV-bOPV vaccine switch
-
•
Fraction IPV doses in routine immunization help to close immunity gaps
-
•
India had best immunity against polioviruses just before the tOPV-bOPV switch
-
•
Type 2 seroprevalence has been highest ever, just prior to the tOPV-bOPV switch
-
•
No cVDPV2 post tOPV- bOPV switch could be due to high population immunity
Keywords: Polio, Seroprevalence, India, 2016, tOPV, bOPV, Switch
Abstract
Introduction
This study assessed the seroprevalence against all three polioviruses among the last cohort of infants aged 6-11 months who received tOPV before the tOPV-bOPV switch and had an opportunity to receive a full dose of inactivated poliovirus vaccine introduced in the routine immunization schedule.
Methods
Serum was tested for neutralizing antibodies against polioviruses among infants residing in three different risk- category states for poliovirus transmission in India viz., Bihar historically high-risk state for polio, Madhya Pradesh a State with low routine immunization coverage and Chhattisgarh with lower acute flaccid paralysis surveillance indicators.
Results
A total of 1113 serum samples were tested across the three states. The overall seroprevalence was 98.5% (97.7-99.2), 98.9% (98.3-99.5) and 94.4% (93.0-95.8) for poliovirus types 1, 2 and 3 respectively. The median antibody titers for corresponding serotypes were 575, 362 and 181. Infants who received five doses of tOPV showed respective seroprevalence rates of 98.7%, 98.7% and 93.7% against types 1, 2 and 3 polioviruses. There was no significant difference in seroprevalence across the group of IPV recipients. The median reciprocal titers across the groups of IPV recipient was significantly higher (p = 0.006) for poliovirus-3.
Conclusion
The seroprevalence rates observed in the study are historically the highest in the series of serosurveys that India has conducted to assess the population immunity against polioviruses. Poliovirus 2 seroprevalence was very high at the time of the tOPV-bOPV switch in India effected in April 2016.
BACKGROUND
India won the war against polio with the last reported case due to wild poliovirus type 1 (WPV1) on 13 January 2011 [1], [2]. The country continued to guard against the risks of wild polioviruses importation from endemic countries and paralysis from circulating vaccine derived polio virus (cVDPV) through mass vaccination campaigns, cross-border polio vaccination, travel advisories and improvements in routine immunization coverage [3], [4]. As a part of the global polio end game strategy, India introduced inactivated poliovirus vaccine (IPV) in the routine immunization (RI) schedule and switched from trivalent OPV (tOPV) to bivalent OPV (bOPV) in April 2016 [5], [6].
The switch from tOPV to bOPV presented increased risk of emergence of cVDPV type-2 (cVDPV2) due to circulating vaccine virus from previous vaccination with tOPV but no immunity against type-2 serotype from bOPV in the cohorts born after the switch. The needed type 2 immunity could be obtained by introducing IPV in the routine immunization (RI) schedule, as a risk mitigation strategy [7]. India launched IPV in the routine schedule as one full dose of intramuscular IPV in 2015 starting with high risk states and subsequently included all states and union territories in a phased manner. Subsequently the country moved to nationwide implementation of two fractional (0.1 ml intradermal) doses of IPV replacing one full dose.
India periodically assessed the population immunity in the high-risk areas since 2007. The previous serosurvey in 2014 showed neutralizing antibody seroprevalence of 98%, 98% and 91% for polioviruses type-1, type-2 and type-3 respectively, among infants 6-11 months, residing in the states at high risk of poliovirus transmission [8]. This serosurvey in 2016 was conducted to assess immunity prior to the tOPV-bOPV switch. Earlier the Global Polio Eradication Initiative (GPEI) had set high population immunity against type 2 at the time of the tOPV-bOPV switch, as a prerequisite to ward off the risk of emergence of VDPV2 [9].
We conducted the study with the primary objective to assess sero-prevalence against polioviruses among infants living in the high-risk categories for polio transmission in India; historically high-risk state for polio, areas with low RI coverage and areas with sub-optimal acute flaccid paralysis (AFP) surveillance for polio at the time of the vaccine switch.
MATERIALS AND METHODS
Study Design and Methods
The study is a cross-sectional study of prevalence of serum neutralizing antibodies against all three poliovirus types among infants 6-11 months of age and staying in three different risk category areas for polio transmission. Subjects with reciprocal antibody titers ≥ 1:8 were considered seropositive for each poliovirus type.
Risk state categories of target populations
-
1)
Historical high-risk State for polio-Bihar: In Bihar, central region and the Kosi riverine areas had posed insurmountable challenges to the polio eradication program before they could be overcome. These areas were the last vestiges of polio transmission in India [10], [11].
-
2)
Low routine immunization coverage-Madhya Pradesh: Madhya Pradesh was identified as the State with the lowest fully immunized rates among states with large population as per the survey data of the National Family Health Survey (NFHS4, 2015-16) [12].
-
3)
Sub-optimal AFP surveillance: Chhattisgarh was selected for the third risk category with the lowest AFP surveillance index- a product of non-polio AFP rate and stool adequacy rate [13].
Sample size and study area selection
Keeping in view the primary objective of the study and compensating for the rejection of blood samples (inadequate amount and hemolysis), 360 subjects were proposed to be enrolled in each state with 95% confidence level and 3% precision (total 1080 subjects in three states).
Five districts were identified from each of the three States. In Bihar, the 5 districts were selected based on number of years of poliovirus transmission and the number of poliovirus type-1 cases during 2005-2011. Five districts with lowest RI coverage from Madhya Pradesh and five districts with least performing AFP surveillance were included from Chhattisgarh. For operational feasibility, one block (sub-administrative unit) per district was randomly chosen in each district. Thus, a total of 15 blocks were selected across the three study states. In each block, microplans of the polio vaccination campaign teams were used to randomly select sample collection areas. Twelve areas were randomly selected through probability proportional to size (PPS) method. Thus, a total of 60 polio team areas were randomly chosen in each state. From each polio team area, six infants were selected in the study, making a total of 360 children in each state.
Screening for age eligible infants and subject enrolment
The field implementation of the study was conducted during July 2016. A few days prior to blood sample collection, the study teams visited households in the study areas to screen infants in the age eligible group of 6-11 months. Screening started from a random first household in the area and then moving consecutively as per the polio microplan. The number of children listed during the screening was about two times the required enrolment to account for loss of participants due to absence, non-participation, sickness etc. on the day of enrolment. On the day of actual enrolment, a medical officer visited the study area and selected infants for enrolment. Any infant 6-11-month-old, residing for > 1 month since birth in the study area and whose parents provided written informed consent were included in the study, while infants with contraindication for venipuncture and sick child requiring hospitalization or undergoing treatment for a major illness were excluded during the selection process. Six infants (two each from age groups of 6-7, 8-9, and 10-11 months) from each study area were thus selected during the household visits and transported to the nearby health facility set up for blood collection and other study procedures. The 6-11 months of age in the study is expected to have received all the routine doses and the maternal antibodies among them would have subsided to undetectable levels. We distributed sampling equally in the three age sub- groups of 6-7, 8-9 and 10-11 months because every added age of 2 months could make a difference in terms of exposure to SIA doses and the resulting seroprevalence.
Study process and laboratory testing
Government health facilities were used as study sites. At each study site, a study physician conducted a physical examination, obtained written consent from participants’ parent and enrolled the eligible participants. Also, the physician administered a short questionnaire to collect demographic information and vaccination status. An experienced phlebotomist collected 1.0 ml blood by venipuncture.
In addition, as part of the study protocol, test for hemoglobin was conducted on infants and willing mothers through hemocue method and results shared immediately. Hemoglobin less than 11 gm/dL in infants and < 12 gm/dL among mothers was considered as anemia.
The blood samples for poliovirus antibodies were centrifuged every day, sera separated in cryovials and stored below −20 °C until shipped in dry ice to The Enterovirus Research Centre,
Mumbai on completion of enrolments. The samples were tested to determine neutralizing antibodies against all three poliovirus types using Sabin OPV strains in a modified micro- neutralization assay following a standard protocol [14]. Serial two-fold dilutions of test serum sample (1:8 to 1:1024) were reacted with 100 CCID50 of each of the three poliovirus types. HEp-2(C) cells were used to detect virus infectivity. All samples for polio antibodies were tested in triplicate beginning at a 1:8 dilution. Internal reference serum and virus back titration was included in each test run. Antibody titers were determined by Karber method. Positive controls were included in every run, to determine the antibody titers.
Data management and analysis of results
Samples were made anonymous for data analysis by removing participant identifiers. The laboratory test results were merged with the questionnaire database using a common identifier. Children with a reciprocal antibody titer ≥1:8 were considered seropositive against the poliovirus type. State level estimates and corresponding 95% confidence intervals for seroprevalence to each type of poliovirus was calculated using the standard binomial proportion methods.
Ethical approvals for the study
Institutional Ethics Committee (IEC) of Enterovirus Research Center (ERC), Mumbai and the Ethics Review Committee (ERC) at WHO, Geneva provided the approval for the study.
Screening and enrolments
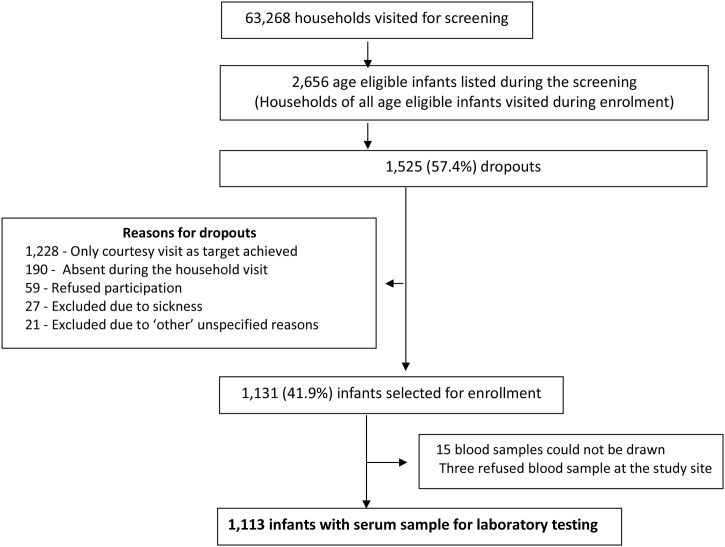
During the screening phase, the field staff visited 63,268 households and identified 2,656 age eligible subjects. On the enrolment day, field staff visited the households of all age eligible infants. Of these, 1,131 (42.6%) infants were enrolled in the study for blood sample collection. Blood sample could not be drawn in 15 subjects while three parents refused blood sample after enrolment, giving a total of 1113 samples for laboratory testing. Of these 366 were from Bihar, 370 from Chhattisgarh and 377 from Madhya Pradesh and uniformly distributed in the age sub-groups of 6-7 months (N = 376), 8-9 months (N = 369) and 10-11 months (N = 368). Remaining 1525 (57.4%) age eligible subjects were not enrolled in the study due to various reasons listed in Fig. 1.
Fig. 1.
Subject enrolment overview
RESULTS
Demographic characteristics of study participants by state
Both genders were equally distributed across the three states. The religion of the families significantly differed across the states. Literacy of both parents was significantly better in Chhattisgarh (Table 1).
Table 1.
Epidemiological characteristics of study participants by state
| State → Characteristics↓ | Bihar | Chhattisgarh | Madhya Pradesh | P value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Gender | Female | 169 | 46.2% | 177 | 47.8% | 190 | 50.4% | P = 0.50 |
| Male | 197 | 53.8% | 193 | 52.2% | 187 | 49.6% | ||
| Religion | Hindu | 303 | 82.8% | 339 | 91.6% | 333 | 88.3% | P = 0.00 |
| Muslim | 63 | 17.2% | 19 | 5.1% | 44 | 11.7% | ||
| Others | 0 | 0.0% | 12 | 3.2% | 0 | 0.0% | ||
| Mother's education | ≥12 years of education | 51 | 13.9% | 72 | 19.5% | 78 | 20.7% | P = 0.00 |
| 5-10 years of education | 133 | 36.3% | 221 | 59.7% | 147 | 39.0% | ||
| Illiterate | 182 | 49.7% | 76 | 20.5% | 152 | 40.3% | ||
| Father's education | ≥12 years of education | 74 | 20.2% | 92 | 24.9% | 95 | 25.2% | P = 0.00 |
| 5-10 years of education | 181 | 49.5% | 235 | 63.5% | 169 | 44.8% | ||
| Illiterate | 110 | 30.1% | 43 | 11.6% | 113 | 30.0% | ||
OPV doses received by subjects
Infants from all three states had opportunity to received two tOPV doses from two national polio campaigns (January and February 2016), apart from the tOPVs from the routine schedule. While children from Madhya Pradesh had no additional opportunity, infants from Bihar had additional opportunity to receive two bOPV (November 2015 and June 2016) and a tOPV (April 2016) from subnational polio campaigns. Children from Raipur district of Chhattisgarh too had an additional opportunity for tOPV (April 2016) from subnational polio campaigns. The median OPV doses (Routine plus polio campaigns) received by the study infants was 8 in Bihar and 6 in Chhattisgarh and Madhya Pradesh. The median tOPV doses received through routine immunization was 3 in Bihar and 4 in Chhattisgarh and Madhya Pradesh. The median number of OPV received by the infants was six for all three age sub-groups.
State wise seroprevalence and median titers
State wise seroprevalence and median titers against each poliovirus type is depicted in Table 2. Overall, seroprevalence rates (95% confidence intervals) across three states for types 1, 2 and 3 polioviruses were 98.5% (97.7-99.2), 98.9% (98.3-99.5) and 94.4% (93.0-95.8) respectively. A total of 93.4% participants were seropositive against all three poliovirus types. Chhattisgarh had better seroprevalence compared to Madhya Pradesh against type 1 (p = 0.026) and type 3 (p = 0.003).
Table 2.
Seroprevalence and median reciprocal titers against poliovirus types in the study states
| Poliovirus Type | Seroprevalence & Median Titers | Bihar N = 366 | Chhattisgarh N = 370 | Madhya Pradesh N = 377 | Total N = 1113 |
|---|---|---|---|---|---|
| Type 1 | % Seroprevalence (95% CI) | 99.2% (98.1 - 100) | 99.2% (98.7 -100) | 97.1% (94.4 - 99) | 98.5% (97.7 - 99.2) |
| Median Titers (95% CI) | 574.7 (456.1 - 574.7) | 574.7 (515.4 - 724.1) | 574.7 (456.1 - 574.7) | 574.7 (574.7 - 574.7) | |
| Type 2 | % Seroprevalence (95% CI) | 98.9% (96.9 - 99.6) | 99.2% (97.8 - 100) | 98.7% (96.4 - 100) | 98.9% (98.3 - 99.5) |
| Median Titers (95% CI) | 362.0 (324.7 - 362.0) | 456.1 (362.0 - 456.1) | 362.0 (362.0 - 456.1) | 362.0 (362.0 - 362.0) | |
| Type 3 | % Seroprevalence (95% CI) | 93.7% (90.5 - 96.2) | 97.6% (95.2 -99.1) | 92.0% (86.7 - 94.4) | 94.4% (93.0 - 95.8) |
| Median Titers (95% CI) | 228.1 (181.1 - 287.4) | 181.0 (181.0 - 228.1) | 181.0 (143.7 - 181.0) | 181.0 (181.0 - 228.1) |
The overall median antibody titers were 574.7 (574.7-574.7), 362.0 (362.0-362.0) and 181.0 (181.0-228.1) for polioviruses 1, 2 and 3 respectively. The median titers were similar across the state for poliovirus-1, while significantly more in Chhattisgarh (p = 0.008) for poliovirus-2 and in Bihar (p = 0.046) for poliovirus-3.
Age-wise seroprevalence
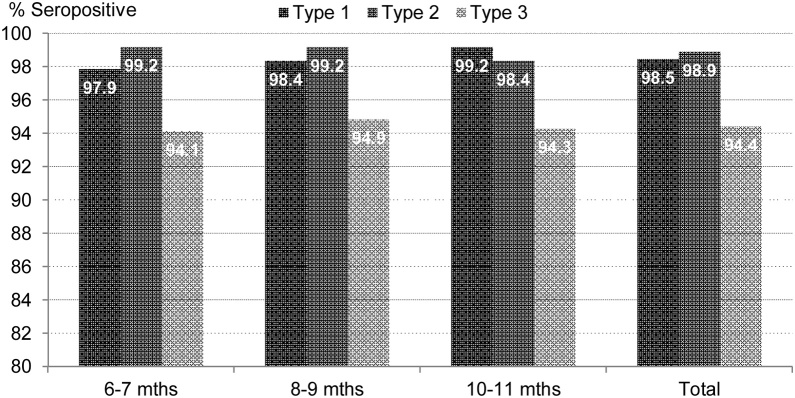
As shown in the Fig. 2, the seroprevalence against poliovirus-1 among 6-7, 8-9 and 10-11 months old was 97.9% (96.3-99.2), 98.4% (96.7-99.5) and 99.2% (98.1-100.0) respectively. For
Fig. 2.
Age wise seroprevalence among study infants
poliovirus-2, the corresponding figures are 99.2% (98.1-100.0), 99.2% (98.1-100.0) and 98.4%
(97.0-99.5) and for poliovirus-3, 94.1% (91.8-96.3), 94.9% (92.4-96.7) and 94.3% (91.8-96.5).
There is no significant difference within the age groups for any poliovirus type (p value >0.05).
Seroprevalence by some epidemiological variables
There was no significant difference in seroprevalence between gender, religions or child’s anemic conditions. However, the infants belonging to illiterate mothers have lower seroprevalence for all three poliovirus types compared to infants who have educated mothers; significantly so for types 1 and 3. Similarly Infants belonging to illiterate father have lower seroprevalence to all poliovirus types but significantly so for type 3 (Table 3).
Table 3.
Seroprevalence by few epidemiological variables.
| Poliovirus types → | Type 1 |
Type 2 |
Type 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | p Value | N | % | p Value | N | % | p Value | ||
| Gender | Female | 527 | 98.3% | 0.69 | 531 | 99.1% | 0.65 | 511 | 95.3% | 0.20 |
| Male | 569 | 98.6% | 570 | 98.8% | 540 | 93.6% | ||||
| Religion | Hindu | 959 | 98.4% | 0.70 | 965 | 99.0% | 0.79 | 919 | 94.3% | 0.63 |
| Muslim | 125 | 99.2% | 124 | 98.4% | 120 | 95.2% | ||||
| Others | 12 | 100.0% | 12 | 100.0% | 12 | 100.0% | ||||
| Mother's education | >= 12 years education | 201 | 100.0% | 0.02 | 200 | 99.5% | 0.20 | 197 | 98.0% | 0.00 |
| 5-10 years education | 496 | 99.0% | 498 | 99.4% | 479 | 95.6% | ||||
| Illiterate | 398 | 97.1% | 402 | 98.0% | 374 | 91.2% | ||||
| Father's education | >= 12 years education | 258 | 98.9% | 0.16 | 259 | 99.2% | 0.87 | 254 | 97.3% | 0.03 |
| 5-10 years education | 579 | 99.0% | 579 | 99.0% | 553 | 94.5% | ||||
| Illiterate | 258 | 97.0% | 262 | 98.5% | 243 | 91.4% | ||||
| Hemoglobin | Anemic | 777 | 98.6% | 0.605 | 777 | 98.60% | 0.121 | 777 | 94.0% | 0.322 |
| Normal | 336 | 98.2% | 336 | 99.70% | 336 | 95.5% | ||||
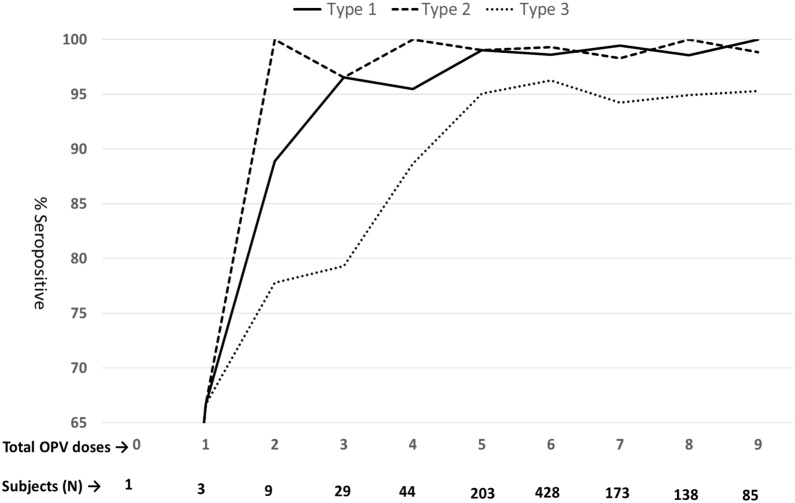
Seroprevalence with number of OPV doses
As can be observed from the Fig. 3, after 3 doses of OPV (tOPV and or bOPV), the seroprevalence reached 96.2% (86.2-100),100% (100-100) and 76.9% (59.3-92) for poliovirus types 1, 2 and 3 respectively. After 5 OPV doses the corresponding
Fig. 3.
Seroprevalence with number of OPV doses taken
seroprevalence rates were 98.7% (96.6-100), 98.7% (96.9-100) and 93.7% (89.5-97.1).
Seroprevalence with additional IPV dose received
A total of 476 (42.8%) infants received additional intramuscular dose/s of inactivated poliovirus vaccine (IPV) in the routine immunization schedule (471 infants-one dose and 5 infants- 3 doses; apparently addition IPV doses from private sector). Those infants who did not get any IPV dose had seroprevalence of 97.8%, 98.4 and 93.4% for poliovirus type 1,2 and 3 respectively. The corresponding figures for those infants who received one dose of IPV was 99.4%, 99.6% and 95.8%. All infants who received 3 additional IPV doses were found to be seropositive. There was no significant difference in seroprevalence across the group of IPV recipients. However, Median reciprocal titers across the groups of IPV recipient was
significantly higher (p = 0.006) for poliovirus-3 but insignificant for poliovirus types 1 &2 (Table 4).
Table 4.
Seroprevalence with additional doses of IPV
| Additional IPV doses | Type 1 | Type 2 | Type 3 | |
|---|---|---|---|---|
| 0 | 97.8% (96.5 - 98.9) |
98.4% (97.5 - 99.4) |
93.4% (91.7 - 95.3) |
|
| 1 |
% Seropositive |
99.4% (98.5 - 100) |
99.6% (98.9 - 100.0) |
95.8% (93.6 - 97.5) |
| 3 | (95% CI) | 100% (100.0 - 100.0) |
100% (100.0 - 100.0) |
100% (100.0 - 100.0) |
| P value across the IPV group | 0.10 | 0.18 | 0.21 | |
| 0 | 574.7 (456.1 - 574.7) |
362.0 (362.0 - 456.1) |
181.0 (181.0 - 181.0) |
|
| 1 |
Median titers |
574.7 (474.7 - 724.0) |
362.0 (362.0 - 362.0) |
228.1 (205.1 - 287.4) |
| 3 | (95% CI) | 1149.4 (287.4 - 1448.2) |
724.1 (287.4 - 912.3) |
456.1 (114.0 - 1448.2) |
| P value across the IPV group | 0.653 | 0.328 | 0.006 |
DISCUSSION
In 1988, India committed itself to the global aim of polio eradication [15]. Booth based polio immunization campaigns covering children up to 3 years of age were started in Delhi in 1994 and extended to the whole country in 1995. From 1996-97, children up to 5 years of age were immunized in the polio vaccination campaigns. House to house component was added in 2000-01 as a part of intensification of polio campaigns. Though the program initially relied exclusively on the use of tOPVs, monovalent OPVs (mOPV1 and mOPV3) and later bivalent OPV (against poliovirus types 1and 3) were introduced in the program [16]. Though most part of the country was covered with two national rounds of polio campaigns with tOPV (National Immunization Days-NID), there were additional rounds of sub-national immunization days (SNID) in the high-risk areas of transmission for poliovirus. bOPV was used in the these SNIDs from 2010 onwards.
In the globally synchronized tOPV-bOPV switch, India switched to bOPV on 25 April 2016. Before this IPV was launched in the country in November 2015 in the EPI schedule. Bihar and Madhya Pradesh introduced a single dose of IPV in the routine immunization schedule on 30 November 2015 while Chhattisgarh did so on 4 January 2016. Starting with one full intramuscular dose at week 14, the whole country introduced two fractional (Intradermal) doses of IPV at weeks 6 and 14 around the OPV switch time. OPV switch was a well-planned process preceded by trainings across the country and appropriate communication strategies. Independent observers monitored the switch process [17].
The present study was an opportunity to assess the population immunity against polio in the last cohort of infants (6-11 months) who received tOPV before the tOPV-bOPV switch was implemented on 25 April 2016. This was the first polio seroprevalence study in India after the introduction of a full dose IPV at OPV3 / Penta3 contact (week 14) in the routine schedule in November 2015. The purpose of IPV was to maintain poliovirus-2 population immunity and minimize the risk of emergence of cVDPV2 following the tOPV-bOPV switch [18]. The overriding objective of the study, therefore, was to assess immunity against poliovirus type-2. However, due to the risk of importation of Wild poliovirus-1 from endemic countries, it was critical to maintain immunity against polioviruses types 1 and 3 as well.
The infants in the present study had a maximum opportunity to receive 4 tOPVs from RI and 2 tOPVs from the two national polio campaigns. Bihar had three additional bOPV sub-national campaigns. The median tOPV dose received by study infants through RI was 4. Infants in the high-risk states of India increasingly received more tOPVs through RI schedule until just before the tOPV-bOPV switch. The median RI tOPV doses received during 2010 was 3 in Bihar and 1 in Uttar Pradesh [19]. The median dose improved further in 2014 to 3 tOPVs among children in Bihar, MP and Mumbai [8]. The improved RI coverage in the study reiterates India’s focus on strengthening RI as a part of the polio endgame strategy and polio legacy transitioning.
Previous serosurveys in India showed poliovirus-2 antibody prevalence rates of 70% (2007) and 36.7% (2008-09) before rising again steadily to 98 % in 2014 [8], [20], [21]. The increasing seroprevalence to type 2 could be ascribed to the improving coverage with tOPV as discussed in the preceding paragraph. During 2008-09, the priority to eradicate poliovirus-1 and use of mOPV1 in western Uttar Pradesh led to low seroprevalence against poliovirus-2 during the period. This resulted in outbreaks of cVDPV2 in western Uttar Pradesh in 2009 [22]. The seroprevalence against poliovirus-2 was observed to be highest ever (98.9%) in the present study, compared to all previous serosurveys conducted in India. The neutralizing antibody titer against type 2 is also high.
Like poliovirus-2, the seroprevalence against poliovirus-1 and poliovirus-3 were also highest ever in 2016 in India (98.5% for type 1 and 94.4% for type 3 in 2016 compared to 98.3% and 91.1% in 2014) [8], [20], [21]. All three states separately have >97% seroprevalence against poliovirus-1 and >92% against poliovirus-3. These two serotypes also have high median antibody titers. Chhattisgarh participating as poor polio surveillance indicator state, for the first time in any polio seroprevalence study in India, has also shown high seroprevalence against all three poliovirus serotypes. The study also demonstrates that infants as young as 6-7 months are well protected against all three poliovirus serotypes similar to older infants.
The present study demonstrates that 5 doses of OPV in the pre-OPV switch period (tOPV in RI schedule and national polio campaigns and bOPVs in the sub-national campaigns) provides
>95% population immunity against types 1 & 3. Five doses of bOPV (3 routine bOPV plus 2 in polio campaigns) in the post-OPV switch period could provide still better protection against types 1 & 3; by superiority of bOPV over tOPV [23], [24]. However, the median titers against poliovirus type-3 differed significantly across the groups of IPV recipients in the study. Indian infants presently receive two intradermal fractional doses of IPV (fIPV) in the RI schedule at weeks 6 and 14. The study infants in our study would have been born between 11 September 2015 and 11 January 2016; just around the time they could benefit from the IPV launched in the country. Despite initial operational challenges in rolling out IPV across the country, about 43% of these initial cohort received the single dose of IPV at week 14 in the routine immunization. However, unless the coverage with IPV is escalated rapidly, low type-2 immunity would continue to carry the risk of circulation of type two Sabin virus strain. A high coverage with fIPVs in the RI schedule is however, expected to provide a good sero-protection against type 2 as well [25], [26].
Due to high type 2 seroprevalence in the study, the independent effect of IPV in the RI
schedule on type 2 immunity couldn’t be analyzed.
CONCLUSION
The findings from the present study confirm that India has not only sustained high seroprevalence achieved during the years prior to 2016, but also improved the immunity against all three poliovirus serotypes in the subsequent years in its high-risk populations. Type 2 seroprevalence has been highest ever, just prior to the tOPV-bOPV switch. There is no evidence of any cVDPVs in India after the tOPV-bOPV switch. High seroprevalence accompanied by high mean titers as observed in the present study has probably helped India to sail through the risk of emergence of cVDPV2 in the immediate period following the tOPV- bOPV switch. As the study suggests, bOPV doses to infants through strengthened routine immunization and a couple of national campaigns could sustain high levels of sero-protection against serotypes 1 and 3. While it is expected that good coverage with two fractional doses of IPV (fIPV) in the RI schedule could provide a good immunity base against type-2 serotype, further studies focusing on type-2 population immunity can assess the impact of these fIPV doses in the cohort of children born after the tOPV-bOPV switch.
FUNDING SOURCE
This work was supported by the World Health Organization through a grant by Rotatory International (Grant number PP16BMF0005).
ETHICAL APPROVAL
Institutional Ethics Committee (IEC) of Enterovirus Research Center (ERC), Mumbai and the Ethics Review Committee (ERC) at WHO, Geneva provided approvals for the study.
DECLARATION OF INTERESTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Financial support
This work was supported by the World Health Organization through a grant by Rotatory International (Grant number PP16BMF0005).
Potential conflicts of interest
No author reported any conflict of interest.
ACKNOWLEDGEMENT
We thank the Government of India, Ministry of Health and Family Welfare and the state governments of Bihar, Chhattisgarh and Madhya Pradesh for providing administrative support and facilitating the field operations. We acknowledge the dedicated involvement of district health authorities and Medical Officers, ANMs, AWWs, ASHAs, Health Supervisors and other health functionaries in the study.
We deeply appreciate and thank the involvement of Surveillance Medical Officers of the WHO- National Polio Surveillance Project (WHO India-NPSP) and the field volunteers who participated in the study and very diligently coordinated the field implementation across multiple districts. We thank all the supervisory officers and the technical team of the WHO India-NPSP for their technical oversight and coordination.
We are grateful to the laboratory staff at the Enterovirus Research Center, Mumbai for testing the study samples.
References
- 1.Denyer S. India advances in battle to eradicate polio. The Washington Post, 12 January 2012. Available at: https://www.washingtonpost.com/world/asia_pacific/india-advances-in-battle-to-eradicate- polio/2012/01/11/gIQAZbulsP_story.html?noredirect=on&utm_term=.1e03e51ef552. Accessed 5 August 2020.
- 2.World Health Organization . 2012. Media Centre. News Release. Available at: http://www.who.int/mediacentre/news/releases/2012/polio_20120113/en/. Accessed 5 August 2020. [Google Scholar]
- 3.Press Trust Of India . 2017. To Keep India Polio-Free, How Government Is Strengthening Immunization Programme. January 30. Available at: https://everylifecounts.ndtv.com/to-keep-india-polio-free-how-government-is- strengthening-immunization-programme-9600. Accessed 5 August 2020. [Google Scholar]
- 4.The Global Polio Eradication Initiative . 2014. Polio vaccination requirements at borders help to stop polio in its tracks. 1 November. Available at: http://polioeradication.org/news-post/polio-vaccination-requirements-at-borders-help-to- stop-polio-in-its-tracks/. Accessed 28 June 2018. [Google Scholar]
- 5.The Global Polio Eradication Initiative. Polio Eradication and Endgame Strategic Plan 2013–2018. Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed on 6 August 2020.
- 6.India moves closer to polio end-game . 2013. WHO South-East Asia. Immunization. Available at: http://www.searo.who.int/entity/immunization/topics/polio/polio_india_nid_2013/en/. Accessed 29 July 2014. [Google Scholar]
- 7.World Health Organization. IPV introduction and RI strengthening. Available at: http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/inactivated_polio_vaccine/en/. Accessed 15 November 2018.
- 8.Ahmad M., Bahl S., Kunwar A. Cross-sectional Serologic Assessment of Immunity to Poliovirus in Differential Risk Areas of India: India Seroprevalence Survey – 2014. Indian Pediatrics. 2016;53 Supplement 1. August 15. [PubMed] [Google Scholar]
- 9.The Global Polio Eradication Initiative. OPV Cessation. Plan for tOPV Campaigns Immediately Prior to tOPV to bOPV Switch. Draft version 14 October 2014. Available at: http://www.who.int/immunization/sage/meetings/2014/october/5_Plan_for_tOPV_Campai gns_v21.pdf. Accessed 30 June 2018.
- 10.The 20th Meeting of the India Expert Advisory Group for Polio Eradication: Conclusions and Recommendations. Delhi, India, 24-25 June 2009.
- 11.Global Polio Eradication Initiative . World Health Organization; Geneva: 2010. Global Polio Eradication Initiative: Annual report 2009. Available at: http://www.who.int/iris/handle/10665/70865. Accessed on 13 November 2018. [Google Scholar]
- 12.National Family Health Survey, India. Key findings from NFHS-4. Available at: http://rchiips.org/NFHS/factsheet_NFHS-4.shtml. Accessed 8 August 2020.
- 13.World Health Organization India- National Polio Surveillance Project. AFP Surveillance Data, 2014-2015.
- 14.World Health O. 1993. Standard procedure for determining immunity to poliovirus using the microneutralization test. WHO/EPI/GEN 93.9. [Google Scholar]
- 15.New initiatives help India achieve improved coverage and quality of immunization. Press Information Bureau, Government of India, Ministry of Health and Family Welfare. Available from: http://pib.nic.in/newsite/erelease.aspx?relid=73623. Accessed 12 September 2012.
- 16.Introductory note on Pulse Polio Programme-2012-2013 with Proposed Newer Initiatives- an appraisal. Ministry of Health and Family Welfare, Government of Delhi. Available online: http://delhi.gov.in/wps/wcm/connect/doit_health/Health/Home/Family+Welfare/Pulse+Poli o+Immunization+Program. Accessed September 12, 2019.
- 17.World Health Organization . 2016. Regional Review on Preparedness for the Switch from tOPV to bOPV in the South-East Asia Region. 24–25 February. New Delhi, India. Available at: http://www.searo.who.int/immunization/documents/regional_review_preparedness_topv_ bopv.pdf. Accessed 12 September 2019. [Google Scholar]
- 18.World Health Organization The introduction of IPV, the OPV switch, and risk mitigation. Information Note, Version: 11 April. 2016. http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/inactivated Available at.
- 19.Sunil B., Concepción F.E., Roland W.S. Cross- sectional Serologic Assessment of Immunity to Poliovirus Infection in High-Risk Areas of Northern India. The Journal of Infectious Diseases, Volume 210, Issue suppl_1, 1 November. 2014:S243–S251. doi: 10.1093/infdis/jit492. [DOI] [PubMed] [Google Scholar]
- 20.Jagadish M.D., Sunil B., Bidyut K.S. Assessing Population Immunity in a Persistently High-Risk Area for Wild Poliovirus Transmission in India: A Serological Study in Moradabad, Western Uttar Pradesh. The Journal of Infectious Diseases, Volume 210, Issue suppl_1, 1 November. 2014:S225–S233. doi: 10.1093/infdis/jiu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunil B., Howard E.G., Hamid J. An Acute Flaccid Paralysis Surveillance–Based Serosurvey of Poliovirus Antibodies in Western Uttar Pradesh, India. The Journal of Infectious Diseases, Volume 210, Issue suppl_1, 1 November. 2014:S234–S242. doi: 10.1093/infdis/jiu379. ttps://doi.org/10.1093/infdis/jiu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . 2011. Update on Vaccine-Derived Polioviruses Worldwide, July 2009--March 2011. Morbidity and Mortality Weekly Report (MMWR). July 1. / 60(25);846-850. July 1, 2011 / 60(25);846-850. [PubMed] [Google Scholar]
- 23.Sutter R.W., John T.J., Jain H. Trial of bivalent type 1 and 3 oral poliovirus vaccine. Lancet. 2010;376:1682–1688. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- 24.Tara D.M., Bruce A., Michael M. Key issues in the persistence of poliomyelitis in Nigeria: a case-control study. Lancet Glob Health. 2014;2:e90–97. doi: 10.1016/S2214-109X(13)70168-2. [DOI] [PubMed] [Google Scholar]
- 25.Abhijeet A., Zaman K., Concepción F.E. Early priming with inactivated poliovirus vaccine (IPV) andintradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine. 2015;33:6816–6822. doi: 10.1016/j.vaccine.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resik S., Tejeda A., Sutter R.W. Priming after a Fractional Dose of Inactivated Poliovirus Vaccine. New England Journal of Medicine. 2013;368:416–424. doi: 10.1056/NEJMoa1202541. [DOI] [PubMed] [Google Scholar]