Highlights
-
•
Self-management programmes are a popular way to engage patients in their own care.
-
•
Inconclusive results stem from inconsistency in study conditions, design, and reporting.
-
•
Behaviour change theories can identify appropriate outcome measures for evaluation.
-
•
Programmes should be developed with theory and patient-centricity in mind.
Keywords: Chronic disease, Self-management, Patient education, COPD, Complex interventions, Behaviour change, Health knowledge, Attitudes, Practice, Patient participation, Health behaviour, Health promotion, Health literacy, Risk reduction behaviour, Disease management, Effectiveness
Abstract
Objective
The study aims to evaluate the ability of self-management programmes to change the healthcare-seeking behaviours of people with Chronic Obstructive Pulmonary Disease (COPD), and any associations between programme design and outcomes.
Methods
A systematic search of the literature returned randomised controlled trials of SMPs for COPD. Change in healthcare utilisation was the primary outcome measure. Programme design was analysed using the Theoretical Domains Framework (TDF).
Results
A total of 26 papers described 19 SMPs. The most common utilisation outcome was hospitalisation (n = 22). Of these, 5 showed a significant decrease. Two theoretical domains were evidenced in all programmes: skills and behavioural regulation. All programmes evidenced at least 5 domains. However, there was no clear association between TDF domains and utilisation. Overall, study quality was moderate to poor.
Conclusion
This review highlights the need for more alignment in the goals, design, and evaluation of SMPs. Specifically, the TDF could be used to guide programme design and evaluation in future.
Practice implications
Practices have a reasonable expectation that interventions they adopt will provide patient benefit and value for money. Better design and reporting of SMP trials would address their ability to do so.
1. Introduction
1.1. Rationale
As chronic illnesses represent a growing share of the global disease burden, patients are increasingly expected to become active participants in their healthcare, rather than passive recipients [1]. Health systems have been optimistic about the ability of patients, via self-management, to improve clinical outcomes while also reducing healthcare service use and its attendant costs [2].
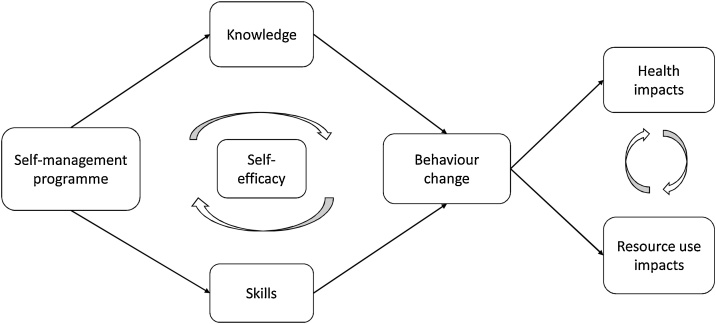
Self-management can refer to a wide range of activities, including exercise, symptom monitoring, and asking follow-up questions in healthcare appointments [3]. Patients often develop these skills on an ad-hoc basis, but increasingly health services have begun to offer ‘self-management programmes’ (SMPs) to teach and support patients to self-manage more effectively. The chain of events that leads to improvements from an SMP is long and complex, but can be clarified via a programme-theory logic model. Logic models serve to ground evaluation of complex interventions in theory, and surface implicit assumptions of the programme design [4]. In Fig. 1, we propose a mechanism by which SMPs may lead to observed changes in health outcomes, healthcare utilisation, and other stated aims:
Fig. 1.
COPD Self-management programmes promote behaviour change that can lead to improvements in health status and resource use.
Fig. 1, which was developed by the research team in consultation with respiratory experts and patients, is an extension of the model set out by Bourbeau, et al. in 2004 [5]. In this model SMPs provide disease-management knowledge and skills. Each participant’s ability to take on new knowledge and skills is mediated by their self-efficacy, a trait that in turn may be increased through gains in knowledge and skills. Participants are expected to utilise this new information to change their behaviour, to both improve their health and optimise interactions with the healthcare system. For instance, a participant with COPD may learn how their lungs work and why they experience breathlessness, use new airway clearance skills to manage their symptoms on a daily basis, and in turn have less frequent exacerbations because of their proactive management, leading to higher quality of life, better health status, and fewer visits with healthcare professionals.
However, reality is clearly much more complicated. Previous reviews have been cautiously optimistic about SMPs’ ability to improve quality of life and reduce emergency visits, but results have been mixed [6]. As always, correlation does not equal causation, and Fig. 1 shows how challenging it is to attribute a change in those downstream effects to the SMP instead of other factors. Disentangling the ambiguous impacts of interventions like SMPs requires theory-informed evaluations, as well as empirically-informed revision of the theories that guide implementation [7]. This review set out to evaluate SMPs in light of behaviour change theory, with respect to the outcomes that most indicate changes in patient behaviour – patterns of healthcare utilisation.
1.2. Theoretical model of behaviour change
The Theoretical Domains Framework (TDF) was developed to translate theories of behaviour change into an actionable framework for implementation research. The TDF is a synthesis of 33 theories of behaviour change, and is rooted in the Behaviour Change Wheel (BCW) that connects psychological and environmental factors to interventions [8]. The BCW is founded on a ‘behaviour system’ such that capability, opportunity, and motivation interact to produce behaviour (COM-B). The COM-B system connects to interventions and policy levers around the BCW that give a theoretical basis for behaviour change interventions [9]. This model posits that people’s behaviour is a product of
-
•
their capabilities (what they know and are able to do),
-
•
the opportunities they have to engage in the behaviour (including social and physical resources),
-
•
and their motivation to engage in the behaviour.
The TDF expands on this framework to incorporate findings from other theoretical and empirical research, to yield 14 domains of behaviour change, which are comprised of 93 behaviour change techniques [[8], [9], [10]]. The definitions of the 14 domains are reproduced with results below in Table 3. The TDF has been validated as a tool to evaluate behaviour change by implementation research experts, and has been used in a variety of healthcare contexts [8,10].
Table 3.
Theoretical Domains in COPD Self-Management Programmes.
| Theoretical Domain | Definition from Cane, et al. 20121 | Programmes Exhibiting Domain |
|---|---|---|
| Skills | Ability or proficiency acquired through practice | 19 |
| Behavioural Regulation | Activities or supports aimed at managing or changing objectively observed actions (e.g. self-monitoring, action planning) | 19 |
| Knowledge | Awareness of the existence of something (including knowledge of condition and procedural knowledge) | 18 |
| Goals | End states or outcomes individual wants to achieve (e.g. target setting, action planning, priorities) | 16 |
| Reinforcement | Increasing probability of desired behaviour by introducing dependency between stimulus and response (e.g. incentives, rewards, punishments) | 14 |
| Intentions | Resolve to act in a certain way, or perform a certain behaviour | 14 |
| Environmental Context and Influences | Any circumstance of the environment that encourages or discourages the development of skills, independence, or other adaptive behaviours (e.g. resources, organisational culture, environmental stressors) | 14 |
| Memory, Attention, and Decision Processes | Ability to retain information, focus selectively, and choose between options | 13 |
| Social Influences | Interpersonal processes that can cause individuals to change thoughts, feelings, and behaviours (e.g. social pressures and norms, power, social supports) | 9 |
| Emotion | A pattern of experiential, behavioural, and physiological reactions to significant matters or events (e.g. fear, anxiety, depression, stress) | 9 |
| Beliefs about Capabilities | Acceptance of true abilities, talents, or facilities (e.g. self-efficacy, perceived behavioural control, self-esteem, empowerment) | 8 |
| Beliefs about Consequences | Acceptance of true outcomes of a behaviour in a given situation (anticipated outcomes, anticipated regret, consequences of actions) | 6 |
| Social/Professional Role and Identity | Coherent set of displayed personal qualities in social or work setting | 2 |
| Optimism | Confidence that desired goals will be attained | 1 |
Evidence-based principles of behaviour change can be used to strengthen the design of interventions [11], but theory has not historically been used to develop healthcare interventions [12,13]. This is a documented problem [[14], [15], [16]], and recent efforts have been made to correct it [17,18]. However, even in cases when the TDF was not used to develop interventions, it can be used to analyse their effects and redress implementation problems [8].
1.3. Chronic obstructive pulmonary disease (COPD)
COPD is characterised by persistent airflow limitation associated with enhanced chronic inflammatory response [19]. It is more precisely a syndrome than a single condition, in that both symptoms and underlying pathology can vary quite widely. COPD is primarily comprised of chronic bronchitis (narrowing of the airways) and emphysema (breakdown of air sacs), both of which make respiration more difficult [20]. COPD is caused most frequently, but not exclusively, by smoking and environmental exposure to tobacco smoke [21]. Conversely, not all smokers develop COPD. Since airway obstruction can have multiple causes, experts suspect a genetic component to the disease [21].
Treatment of COPD takes place primarily through inhalers; either beta-agonists or anti-muscarinic agents. Steroid inhalers may also be used for patients with frequent exacerbations, and antibiotics are common in treating exacerbations. Non-pharmaceutical interventions like pulmonary rehabilitation and smoking cessation programmes often complement the medical regimen [22].
1.4. Objectives
The aim of this study was to evaluate the ability of self-management programmes to change the healthcare-seeking behaviours of people with Chronic Obstructive Pulmonary Disease (COPD). We hypothesised that observable changes to healthcare utilisation would be positively associated with a robust programme design.
The objectives of the study were to:
-
1
Describe the types of SMPs that have been developed for COPD,
-
2
Identify commonalities and differences by classifying programmes using the TDF, and
-
3
Evaluate the effect of SMPs on COPD patients’ healthcare utilisation, and any associations between programme design and outcomes.
2. Methods
2.1. Study identification and selection
The study methods have been outlined in a previously published protocol [23]. The study was also registered prospectively on PROSPERO (CRD42018104753).
A systematic search of the literature was performed in MEDLINE, EMBASE, HMIC, and PsycINFO on 22 April 2020. Studies were eligible if they were published between 1998–2020, in English, and employed a randomised controlled trial (RCT) design. A first-stage scoping review was conducted to select a long-term condition for the focus of the review. A plurality of studies that otherwise met these eligibility criteria reported outcomes for patients with COPD, which became the subject of this review in the second stage. The reference lists of relevant articles and related systematic reviews were also screened to ensure all eligible studies were captured.
The full search string and inclusion and exclusion criteria can be found in Appendix D and Appendix E, respectively. In summary, articles were included if adult patients with COPD were randomised to receive either a self-management intervention (treatment) or usual care or other intervention (control). Interventions, such as pulmonary rehabilitation, that encourage behaviour change but not through a self-management pathway were excluded a priori. Studies that evaluated a self-management programme as one of several simultaneous interventions (e.g. care coordination) were also excluded. Included studies were required to report at least one measure of healthcare utilisation, and measures of health-related quality of life and mortality were also recorded if available.
Initial screening of studies, conducted by two independent investigators, was based on titles and abstracts. Full-paper screening was conducted by KRS, and a 10 percent sample was reviewed by LA. Inclusion and exclusion decisions were made by following a formalised full-text selection process (Appendix E). Any disagreements were resolved by a third investigator (EKM).
2.2. Data collection
Data extraction was conducted in full by KRS, with a 10 percent sample independently extracted by LA. A modified version of the Cochrane EPOC Good Practice Data Collection form for Intervention Review (Randomised and non-randomised trials) was used to identify relevant datapoints [24]. The TIDieR Checklist was used to guide the extraction of programme description data [25]. Data was managed in Mendeley, and data extraction and decisions were recorded in Excel.
The data collected for each study included: name of first author, year of publication, intervention components and characteristics, study duration, participant and setting characteristics, and outcomes. Intervention components were recorded in detail, and included: educational content, any validated intervention or tools used, other materials, number and duration of interactions during the study period, and healthcare professionals involved in the intervention. Unique components of the intervention were also noted.
All healthcare utilisation outcomes that were reported in the study were extracted. Because of the diversity in measurement and reporting, outcomes were recorded in terms of the direction of the effect (positive, negative, or no significant difference) rather than quantitatively. Four measures of health-related quality of life, two patient-reported outcome measures, and mortality were extracted in the same way.
2.3. Risk of bias and quality assessment
Risk of bias was assessed for each study using Cochrane’s Risk of Bias criteria [24]. For each potential source of bias (selection, performance, detection, reporting, and attrition), the risk was assessed to be high, low, or unclear. In addition, the appropriateness of the statistics used to evaluate outcomes were judged against CONSORT guidelines for statistical reporting of clinical trials [26,27]. The associated risk of reporting bias from the analytic plan was also judged to be high, low, or unclear.
By convention, RCTs are assumed to have a ‘good’ quality of evidence, which may be downgraded based on various facets of study design, to ‘moderate,’ ‘poor,’ or ‘very poor’ based on characteristics of the study. Quality of evidence was defined as:
-
•
Good quality of evidence = low risk of bias for six or more of the potential sources of bias
-
•
Moderate quality evidence = low risk of bias from four or five sources
-
•
Poor quality evidence = low risk of bias from two or three sources
-
•
Very poor quality of evidence = low risk of bias on one or no domains of potential bias
Two independent reviewers scored the selected studies based on these criteria, and provided justifications (KRS and LA). Consensus was achieved on all quality designations, but authors prospectively agreed that disagreements would be resolved through discussion with a third author (EKM). Risk of bias analysis was conducted for each study individually, and then summarised in a risk of bias graph. Individual judgments can be found in Appendix A in the supplementary material.
2.4. Framework analysis
The programme components of each intervention were analysed using the Theoretical Domains Framework (TDF). First, information on programme design was extracted from each study using a modified TIDieR checklist. In cases where programme descriptions in the included studies were not sufficient, related articles were sought out, and if there were none, the authors of the original studies were contacted to provide more information.
Programme descriptions were carefully compared to the description of each TDF domain for evidence that they sought to address that domain. Domains that were addressed by programme content were coded with a ‘1’, whereas others received a ‘0.’ For example, the TDF defines Goals as “End states or outcomes individual wants to achieve (e.g. target setting, action planning, priorities).” If a programme encouraged participants to set priorities or goals for their health, or provided an action plan, they were scored with a ‘1’ on this domain. SMPs received a ‘1’ on the Reinforcement domain if they provided incentives or rewards to participants, consistent with the TDF definition of that domain as “Increasing probability of desired behaviour by introducing dependency between stimulus and response (e.g. incentives, rewards, punishments).”
Each included intervention received a score based on the sum of the TDF domains addressed by the programme. The frequency with which different domains of the TDF were used in programme design were also analysed by summing the number of interventions with a ‘1’ for each domain.
Interventions that resulted in multiple included studies were only included once in this analysis. Where this was the case, the results were pooled and the first author of each relevant paper was noted.
3. Results
3.1. Characteristics of included studies
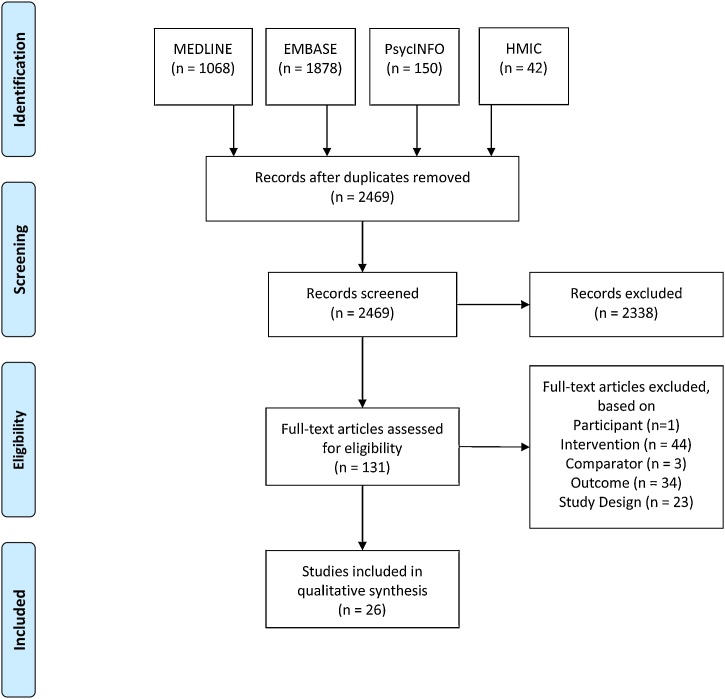
The systematic search yielded a large volume (n = 2,469) of potentially eligible studies. After screening of titles and abstracts, 131 articles remained for full-text review. Ultimately, 26 studies were included in the qualitative synthesis. We considered 16 studies for meta-analysis, but due to data heterogeneity this was not possible. Fig. 2 shows a PRISMA flow diagram of study inclusion [28].
Fig. 2.
PRISMA Flow Diagram.
Studies were screened for inclusion in duplicate. Cohen’s kappa of inter-coder agreement was 0.78 [29]. A 10 percent sample of full texts were reviewed by two authors, with a Cohen’s kappa of 1.0.
Table 1 describes the characteristics of the included articles.
Table 1.
Characteristics of Included Studies.
| First Author | Title | Year | Country | Intervention Group: Sample Size (Retention rate %) | Control Group: Sample Size (Retention rate %) | Comparator | Stated Rationale/ Hypothesis | Primary Outcome | TDF Domains |
|---|---|---|---|---|---|---|---|---|---|
| Blackstock [30] | Comparable improvements achieved in chronic obstructive pulmonary disease through pulmonary rehabilitation with and without a structured educational intervention: a randomized controlled trial | 2014 | Australia | 141 (80.1) | 126 (67.5) | Exercise training | Improve health outcomes | Disease-specific HRQOL1 and functional exercise capacity | 9 |
| Bourbeau2 [31] | Reduction of Hospital Utilization in Patients With Chronic Obstructive Pulmonary Disease | 2003 | Canada | 95 (90.5) | 95 (83.2) | Usual care | Reduce the number of hospital admissions | Percent of exacerbations resulting in hospitalisation | 7 |
| Bucknall [32] | Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial | 2012 | UK | 232 (100) | 232 (100) | Usual care | Reduce readmissions | Time to first hospitalisation w/COPD exacerbation and/or mortality | 6 |
| Casas [33] | Integrated care prevents hospitalisations for exacerbations in COPD patients | 2006 | Spain and Belgium | 65 (73.8) | 90 (80.0) | Usual care | Prevent hospitalisations for exacerbations | Re-hospitalisation for COPD exacerbation | 10 |
| Dewan3 [34] | Economic evaluation of a disease management program for chronic obstructive pulmonary disease | 2011 | US | 372 (100) | 371 (100) | Usual care | Improve overall health status and reduce costs | Direct healthcare and programme costs | 6 |
| Dritsaki4 [35] | An economic evaluation of a self-management programme of activity, coping and education for patients with chronic obstructive pulmonary disease | 2016 | UK | 89 (96.6) | 95 (100) | Usual care | Cost-effectiveness of SMP on HRQoL | Incremental cost-effectiveness | 11 |
| Fan [36] | A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: A randomized, controlled trial | 2012 | US | 209 (92.3) | 217 (90.8) | Usual care | Reduce risk of COPD-related hospitalisation | Time to first hospitalisation w/COPD exacerbation | 10 |
| Farmer [37] | Self-Management Support Using a Digital Health System Compared With Usual Care for Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial | 2017 | UK | 110 (84.5) | 56 (85.7) | Usual care | Improve quality of life and clinical outcomes | Quality of life – SGRQ8 | 6 |
| Gadoury 1 [38] | Self-management reduces both short- and long-term hospitalisation in COPD | 2005 | Canada | 96 (94.8) | 95 (88.4) | Usual care | Reduce the number of hospital admissions | All-cause hospitalisation | 7 |
| Gallefoss5 [39] | Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease | 2000 | Norway | 31 (83.9) | 31 (87.1) | Usual care | Reduce GP visits and absenteeism from work | Self-reported GP6 visits, absenteeism from work, hospital days | 11 |
| Gallefoss5 [40] | Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD--a 1-year follow-up randomized, controlled trial | 2002 | Norway | 31 (83.9) | 31 (87.1) | Usual care | Reduce GP visits and absenteeism from work | Direct and indirect costs of care | 11 |
| Gallefoss5 [41] | The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial | 2004 | Norway | 31 (83.9) | 31 (87.1) | Usual care | Reduce GP visits and absenteeism from work | Number of GP visits, proportions in need of GP visit, use of rescue meds, and patient satisfaction | 11 |
| Johnson-Warrington3 [42] | Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial | 2016 | UK | 39 (89.7) | 39 (92.3) | Usual care | Reduce readmissions | Respiratory-related hospital readmissions | 11 |
| Khdour7 [43] | Clinical pharmacy-led disease and medicine management programme for patients with COPD | 2009 | UK | 86 (82.6) | 87 (82.8) | Usual care | ‘impact on clinical and humanistic outcomes’ | Hospital admission rate for acute exacerbation | 9 |
| Khdour7 [44] | Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD | 2011 | UK | 86 (74.4) | 87 (72.4) | Usual care | Improve health status and reduce healthcare utilisation | HRQOL; cost-utility | 9 |
| Koff [45] | Proactive integrated care improves quality of life in patients with COPD | 2009 | US | 20 (95.0) | 20 (95.0) | Usual care | Increase quality of life and decrease healthcare costs | Quality of life – SGRQ8 | 8 |
| Martin [46] | Care plans for acutely deteriorating COPD: a randomized controlled trial | 2004 | NZ | 44 (100) | 49 (100) | Usual care | Reduce healthcare utilisation – avoid unnecessary GP visits and hospitalisations | Primary care utilisation | 6 |
| McGeoch [47] | Self-management plans in the primary care of patients with chronic obstructive pulmonary disease | 2006 | NZ | 86 (97.7) | 73 (95.9) | Usual care | Increase self-management knowledge, improve health and quality of life | Quality of life – SGRQ | 9 |
| Mitchell3 [48] | A self-management programme for COPD: a randomised controlled trial | 2014 | UK | 89 (100) | 95 (100) | Usual care | Reduce symptom burden | Dyspnoea | 11 |
| Rea [49] | A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease | 2004 | NZ | 83 (85.5) | 52 (88.5) | Usual care | Reduce hospitalisations and length of stay, improve quality of life | Mean hospital bed days | 9 |
| Rice2 [50] | Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial | 2010 | US | 372 (90.3) | 371 (87.1) | Usual care | Improve health status and reduce costs using a less-resource intensive programme | COPD-related hospitalisation and ED9 visits per patient | 6 |
| Rose [51] | Program of Integrated Care for Patients with Chronic Obstructive Pulmonary Disease and Multiple Comorbidities (PIC COPD+): a randomised controlled trial | 2018 | Canada | 237 (87.3) | 238 (80.3) | Usual care | Improve early exacerbation recognition and self-management, and integrate hospital and community care | ED visits per patient | 12 |
| Sanchez-Nieto [52] | Efficacy of a self-management plan in exacerbations for patients with advanced COPD | 2016 | Spain | 51 (92.2) | 45 (84.4) | Usual care | Reduce the use of healthcare resources, especially on hospitalisations for exacerbation | Exacerbations resulting in ED visit or hospitalisation | 5 |
| Trappenburg [53] | Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial | 2011 | Netherlands | 111 (82.0) | 122 (83.6) | Usual care | Prompt intervention leading to ‘faster recovery in symptoms and health status’ | Time to recovery of health status after exacerbation | 8 |
| Wakabayashi [54] | Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire | 2011 | Japan | 52 (80.8) | 50 (86.0) | Usual care | Improve health outcomes by meeting patients’ information needs | Lung Information Needs Questionnaire (LINQ) score | 9 |
| Wang [55] | The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease | 2020 | China | 77 (93.5) | 77 (92.2) | Usual care | Evaluate impact on hospitalisations, exercise capacity, and quality of life | COPD-related hospitalisation and ED9 visits per patient | 11 |
HRQOL is health-related quality of life.
Bourbeau (2003) and Gadoury (2005) report on the same study.
Dewan (2011) and Rice (2010) report on the same study.
Dritsaki (2016) and Mitchell (2014) report on the same study. Johnson-Warrington (2016) is a separate trial by the same team using the same intervention.
Gallefoss (2000), (2002), and (2004) report on the same study.
GP refers to general practice or primary care visits in an outpatient setting.
Khdour (2009) and (2011) report on the same study.
SGRQ refers to the St George’s Respiratory Questionnaire, a measure of quality of life for people with chronic respiratory diseases.
ED visits refers to attendance at an Emergency Department or Accident and Emergency.
3.2. Utilisation outcomes
The included studies reported a heterogeneous set of outcome measures. Fifteen of the studies listed a measure of utilisation as their primary outcome, compared to nine with another outcome (e.g. quality of life) as the primary endpoint. As shown in Table 1 above, hospitalisation and/or emergency visit was the most common category of primary outcome, with twelve studies reporting. Other measures of utilisation reported as a primary outcome were GP visits (3 studies), cost of care (2 studies), and cost-effectiveness (1 study). Studies with non-utilisation measures as primary outcomes focused on quality of life (4 studies), symptomology and disease severity (2 studies), and hospital length of stay (1 study).
Because of differences in measure specification, statistical tests used and methods of reporting, outcomes could not readily be standardised for comparison. Instead, effects were recorded for this review as an improvement, decline, or insignificant result. Table 2 summarises these effects using a vote-counting method. The full dataset for this analysis is reproduced in Appendix B.
Table 2.
Effect of self-management programmes on utilisation outcomes.
| Outcomes | Number of Studies | Studies showing decrease | Studies showing increase | Studies with no significant result |
|---|---|---|---|---|
| Total Resource Use | 5 | 1 | 0 | 4 |
| Hospitalisations | ||||
| All-cause | 17 | 4 | 0 | 13 |
| Respiratory-related | 10 | 2 | 0 | 8 |
| Readmissions | 3 | 1 | 0 | 2 |
| Emergency visits | ||||
| All-cause | 11 | 4 | 0 | 7 |
| Respiratory-related | 5 | 2 | 0 | 3 |
| General practitioner visits | 15 | 7 | 0 | 8 |
| Other physician visits | 9 | 1 | 0 | 8 |
| Antibiotic use | 8 | 1 | 1 | 6 |
| Steroid use | 8 | 1 | 2 | 5 |
Note: Most studies reported on multiple outcomes, therefore the ‘Number of Studies’ column does not sum to 24. Statistical significance is as reported in original studies. Results for similar metrics have been combined (e.g. COPD-related hospitalisation and pulmonary hospitalisation have been grouped together as ‘respiratory-related hospitalisations’ in the table). All-cause hospitalisation and emergency visits are metrics in their own right, not a summation of related metrics. Results for general practice visits include both planned and unplanned visits. Additional File 2 shows all outcomes.
Hospitalisation was the most commonly reported outcome, with 17 studies reporting all-cause hospitalisation rates, 10 reporting admissions due to respiratory causes, and three reporting readmissions. Emergency visits were second-most common, with 11 studies reporting all-cause emergency attendances and five reporting respiratory-related emergency visits. Across all utilisation outcomes, most results were not statistically significant. While 7 studies each showed a statistically significant decrease in hospitalisations and GP visits respectively, this represents fewer than half of the studies reporting any given outcome.
Primary outcomes (as designated by each included study) were no more likely to achieve a statistically significant result. Eleven studies reported a statistically significant primary outcome, and 15 did not. This appears unrelated to the size of the study; studies that achieved statistical significance on their primary outcome included 40–743 participants, compared with 62–743 participants for studies that did not achieve statistical significance on their primary outcome.
3.3. Risk of bias and quality assessment
3.3.1. Bias in individual studies
The quality of the evidence in the included studies was mixed, but generally poor. Based on the criteria specified above, one study had a ‘good’ quality of evidence, nine were ‘moderate’, 10 were ‘poor’, and six were ‘very poor’.
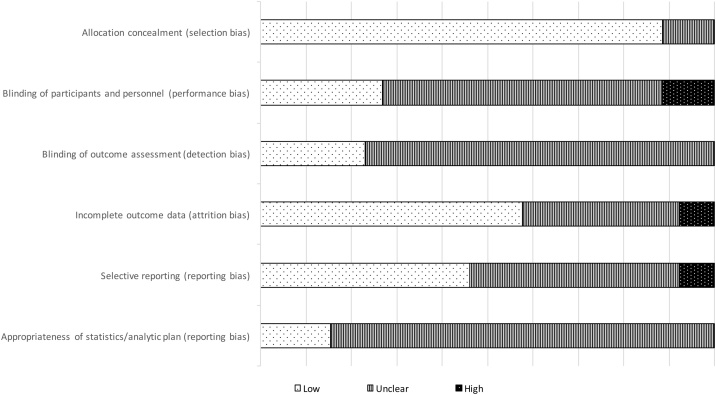
Fig. 3 shows the seven sources of Risk of Bias (RoB) evaluated for the included studies (full RoB analyses with justifications can be found in Appendix A). Generally, a higher RoB corresponds to a lower quality of evidence, and thus reduced confidence that the observed result reflected the true effect size. KRS and LA conducted this analysis independently, achieving a Cohen’s kappa of 0.926. Consensus on all designations was achieved through subsequent discussion.
Fig. 3.
Risk of Bias in Individual Studies.
3.3.1.1. Selection Bias
Nearly all included studies used random sequence generation, lowering the risk of selection bias. Of the three with unclear risk of selection bias, one used a minimisation procedure for a small sample rather than a purely random sequence, and two did not describe their procedure. Similarly, 13 did not describe their allocation concealment procedure, and three only partially concealed allocation, raising selection bias to ‘unclear’. Three studies were determined to have a high RoB for selection because they were unable to conceal allocation for operational reasons.
3.3.1.2. Performance and detection Bias
Double-blinding of participants and personnel was not possible in any of the studies because of the nature of SMP interventions as behavioural trials. This limitation to the study design can be mitigated but not eliminated. For instance, the six studies with low RoB from performance bias blinded the researchers collecting and analysing the data. Thirteen studies did not state one way or the other whether participants or personnel were blinded. One study blinded participants but not personnel. Two studies were deemed to have a high RoB for detection bias, because the researcher conducting the outcomes assessment was not blinded (for operational reasons). The studies that were judged to have a low RoB on this measure employed an independent assessor that was blinded to the allocation. Seven studies did not report whether they blinded the outcome assessment.
3.3.1.3. Attrition Bias
Studies deemed to have a low risk of attrition bias (n = 12) performed an intention-to-treat analysis to account for missing outcome data. This is considered superior to other methods, such as per-protocol analysis, which risks overestimating the treatment effect if attrition is due to some facet of the intervention [56]. Nine studies did not describe how they dealt with missing data.
3.3.1.4. Reporting Bias
Most studies did not publish an a priori protocol (n = 22), which under Cochrane guidelines means there is an unclear risk of reporting bias. Of those, three retrospectively registered on controlled-trials.com. Three studies were prospectively registered on either clinicaltrials.gov or controlled-trials.com, and thus judged to have low risk of reporting bias.
In this study, we also took into consideration the appropriateness of the analytic plan and reporting of outcomes, judged against CONSORT reporting guidelines [26,27]. Only 10 of the 26 studies analysed their outcomes in a way that introduced a low risk of bias. The others, with varying degrees of severity, chose statistics that were inappropriate to answer the research question, or reported results in ways that could be misleading. For instance, four studies reported the outcome of interest as a magnitude only, with no measure of variation or precision of the estimate. On the other hand, some studies employed analytic techniques to account for confounding variables, such as regressing the outcome on individual- or practice-level factors or showing the difference in differences pre- and post-intervention. These studies (n = 10) were deemed to have low risk of bias in this domain.
3.3.2. Bias across studies
Risk of bias exists not only within individual studies, but also across a group of studies as a whole. For instance, a lack of publications showing negative results is indicative of publication bias. That only two papers showed statistically significant increase in utilisation (antibiotic and steroid usage), suggests there is a potential for this type of bias.
Another source of bias across studies is the lack of consensus around what self-management programmes should provide and achieve, and which endpoints are most appropriate to evaluate that. Variation in the structure of the programmes themselves likely lead to differential outcomes, and the degree of fidelity to the programmes as set out in their protocols will also influence results.
3.4. Theoretical domains framework analysis
We analysed SMP design in terms of the theoretical domains evidenced in either their study methods or other supplemental material (Appendix C contains programme summaries). If the SMP description provided evidence of a domain it was coded as ‘1’, and otherwise it was coded as ‘0’. We then summed across all programmes for each domain, and across domains for each programme. Of the 14 theoretical domains laid out in the framework, two were evidenced in all programmes: skills and behavioural regulation. All programmes gave evidence of at least 5 domains.
In Table 3, we provide the definitions of each domain as outlined by the TDF developers and the number of programmes that show evidence of each domain in their programme design.
Programmes also differed in the number of domains of the TDF they utilised within their SMPs. The Rose [51] programme was most robust, with 12 domains, and the relatively parsimonious Sanchez-Nieto [52] study called on five of the 14 domains. On average, programmes employed 8.53 domains, with a standard deviation of 2.06. A full matrix of TDF domains mapped onto SMPs follows in Table 4.
Table 4.
Theoretical Domains in SMPs.
| Blackstock | Bourbeau/ Gadoury |
Bucknall | Casas | Dewan/Rice | Dritsaki/ Johnson-Warrington/ Mitchell |
Fan | Farmer | Gallefoss 2000, 2002, and 2004 | Khdour 2009 and 2010 | Koff | Martin | McGeoch | Rea | Rose | Sanchez-Nieto | Trappenburg | Wakabayashi | Wang | Total studies | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knowledge | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 |
| Skills | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 19 |
| Social/Professional Role and Identity | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Beliefs about Capabilities | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 8 |
| Optimism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Beliefs about Consequences | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Reinforcement | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 |
| Intentions | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 14 |
| Goals | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Memory, Attention, and Decision Processes | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Environmental Context and Influences | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 14 |
| Social Influences | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 9 |
| Emotion | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 9 |
| Behavioural Regulation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 19 |
| Total elements | 9 | 7 | 6 | 10 | 6 | 11 | 10 | 6 | 11 | 9 | 8 | 6 | 9 | 9 | 12 | 5 | 8 | 9 | 11 |
3.5. Synthesis
The a priori hypothesis of this review was that a more robust programme design, as evidenced by the number of TDF domains used in the intervention, would be associated with greater effect sizes in terms of reductions in healthcare utilisation. This review found no clear association between the presence of a TDF domain and an effect on utilisation. Heterogeneity in the study design, outcomes chosen, and method of reporting meant that such an association was difficult to establish. Overall, the magnitude of effect on utilisation measures was small, most studies were not powered to detect these outcomes, and the quality of evidence across the studies was moderate to poor.
4. Discussion and conclusion
4.1. Discussion
Chronic diseases require patients to engage in SM to successfully monitor and mitigate their symptoms, and to interact effectively with healthcare providers. SM education and skills training should result in better clinical outcomes, slower rates of deterioration, reduced need for healthcare services, and reduced costs of care. This review set out to understand the extent to which SM initiatives change the healthcare-seeking behaviours of people with COPD. Findings suggest studies have not yet been able to establish a link between these two. Reason for this are manifold, and include but are not limited to: 1) inconsistencies in the study designs, analysis methods, and reporting; 2) an unclear programme logic; and 3) and lack of consensus on anticipated outcomes of SMPs, all of which make a comparisons between studies challenging.
Any behaviour change is the result of an interplay between features of the intervention and characteristics of the individual receiving it. For instance, disease severity would be expected to influence both the need to engage in self-management behaviours, and the ability to do so. Cultural norms, social supports, and other environmental factors may also influence a person’s willingness and ability to participate.
Further, certain facets of a multi-component intervention may be more fundamental than others. Specific to COPD, smoking cessation has been well established as a primary treatment to lead to improved lung function [57]. However, a subgroup analysis of participants who smoked and those who were not often conducted. It is therefore unclear which patients undertook this specific behaviour change, if those who did participate stopped smoking, or if accounting for this would have modified the results.
Behavioural interventions in real-world settings have multiple layers of complexity. Social and cultural contexts, fidelity to the intervention, and patient-level factors such as self-control and motivation can contribute to creating systematically biased results [58,59]. Concurrent policy interventions like pay-for-performance may influence results by changing the environment in which behavioural RCTs take place, incentivising specific processes and outcomes. For instance, evidence suggests the UK Quality and Outcomes Framework (QOF) encouraged GPs to prioritise care that was easily measurable [60], and that the US Medicare 30-Day Readmissions Penalty prioritised avoiding readmissions to the detriment of mortality [61]. These can interfere with an RCT’s ability to detect the influence of an intervention, and should therefore be accounted for in future trials.
In light of this, we offer the following observations and recommendations to strengthen future SMP studies:
-
•
Measure intermediate outcomes, like gains in knowledge and skills. By measuring only downstream outcomes, the mechanisms by which an SMP may lead to those outcomes is obscured. For instance, SMPs aim to change patient behaviour by providing education and skills training. Appropriate intermediate outcomes would flow directly from the learning objectives of those programmes. Intermediate indicators of behaviour change, such as smoking cessation or adherence to medication or exercise regimes may also be useful. In the absence of behaviour change, it would then be possible to know whether this is due to an ineffective programme or other factors.
-
•
Especially in a complex, real world environment, every effort must be made to reduce sources of variation, and control for those that do exist so that the intervention effect can be assessed accurately. Randomised controlled trials operate on the assumption that the distribution of treatment and control groups are unbiased, and that therefore all individual-level characteristics aside from the intervention will cancel out [59]. Any change in characteristics after randomisation is assumed to be due to the treatment (which has been delivered as prescribed) – these are strong assumptions outside a laboratory setting. Under these conditions, establishing causality requires some procedure to match and control for covariates and address attrition during statistical analysis.
-
•
Sample sizes should reflect the magnitude of effect that can realistically be anticipated from the intervention. We recognise the challenges associated with recruiting participants for real-world interventions; convenience sampling is oftentimes the only realistic option. However, conducting studies that are under-powered to detect an effect reduces the confidence that any effect demonstrated is not an artefact. In extreme cases, this can be seen as a waste of time and resources. In a more moderate sense, it contributes to confusion about best practices.
The inconclusive results in this review may call into question the enthusiasm for the ability of SMPs to improve chronic disease management. However, a lack of statistical significance does not necessarily imply a lack of effect, merely that the effect is uncertain. The observed outcome could be due to: 1) environmental or patient-level factors masking an effect on the measured endpoints; 2) study design choices that prevent optimal statistical analysis; or 3) perhaps SMPs being truly ineffective at changing healthcare utilisation. In light of the paucity of evidence that this behaviour change intervention modifies healthcare use, it is worth going back to the basics, by thinking first about the (implicit or explicit) theoretical basis underpinning self-management and SMPs, followed by the mechanism(s) by which SMPs may promote change, and finally a programme design that builds rigorously upon them.
A study could for instance hypothesise that SMPs will reduce hospitalisations by helping patients understand the circumstances under which they can self-treat at home. The primary outcome of this study would be change in hospitalisation rate. The programme curriculum would centre on discriminating between varying degrees of deterioration, and appropriate self-treatment protocols. The study would measure the direct outcomes of the training modules, such as comprehension or successful demonstration of treatment protocols. Refinements to the SMP could then be targeted to the root causes of any observed problems, and unfruitful hypotheses can be ruled out if necessary.
4.2. Limitations
A limitation of this study is that the analysis of programme design, implementation, and results were secondary – we collected data as reported by the study authors. Fidelity to the study protocol is presumed, but not observed; similarly, we do not know the intentions of either the programme designers or the participants in the study. An in-depth understanding of each programme’s application of behaviour change techniques would require ethnographic or other qualitative work [8].
Second, we used a vote-counting method to analyse our primary outcomes. The vote-counting approach has known limitations [24], however we found the method to be appropriate here because no standard outcome measure was reported across studies. Of the 16 studies considered for meta-analysis across four outcomes, only four studies consistently reported metrics in such a way that they could be standardised or otherwise compared with the others; meta-analysis of each outcome of interest would have pooled fewer than five studies. Combined with the complexity of SMP programme design, the number and degree of sources of variation would make statistical comparisons of these outcomes inappropriate.
Finally, the expectation that a successful SMP will lead to reductions in healthcare utilisation in all cases is neither realistic nor desirable. It is reasonable to expect that increases in certain types of utilisation may offset decreases in others. For example, medication use may understandably rise as patients begin initiating treatment for themselves [37,50]. In other cases, SM education may lead patients to identify changes in symptoms more readily and increase service use in response [36]. Whilst a more appropriate use of services often implies a reduction in utilisation, this relationship is difficult to parse, and must be interpreted carefully.
4.3. Conclusion
As chronic respiratory diseases become increasingly common, demand for programmes that will enable patients to assume more responsibility for their care management will only rise. As it stands, the body of literature on SMPs is large but unstandardized and inconclusive. If we are to glean lessons from it, we must be able to consolidate evidence and replicate previous methods to verify what works. This will require building upon accumulated knowledge through conceptual and theoretical frameworks, and refining those frameworks to reflect the evidence from new studies.
This review highlights the challenges with consolidating evidence because of issues related to data collection and reporting. Authors should be encouraged to follow CONSORT, TIDieR, or other guidelines for reporting results of clinical trials [[25], [26], [27]], so that results can be compared within and across intervention studies. Furthermore recommendations, future research should focus on clarifying the objectives, outcome measures, and programme features that are most relevant for SMPs, based on established behavioural theories and frameworks, such as the TDF.
4.4. Practice implications
Self-management programmes, while intuitively attractive [1,2], have demonstrated limited effectiveness. We conclude this is due to a lack of consistency in the aims and primary outcomes of these programmes, including how they are measured and analysed. The stakes of these measurement challenges are not trivial. As Zarin et al. [62] outline, trials for which study limitations obscure the effectiveness of the interventions are not just research inefficiencies; they argue that these constitute a breach of research ethics by violating the expectation that patients contribute to scientific knowledge by participating. The safety risk associated with entrusting patients to self-manage if they do not possess the knowledge and skills should not be underestimated.
Presently, practices are left with no clear direction on whether to adopt or continue using SMPs. In studies with insignificant results, it is unclear whether the lack of effect is due to: 1) an inability of SMPs to change behaviour; 2) the inability of a particular SMP to change behaviour; 3) patient-level or environmental factors that inhibit behaviour change; or 4) aspects of the study design or conduct that mask the effect.
With finite resources, practices must optimise value for both time and money. SMPs for COPD currently do not reliably demonstrate either. Barring more definitive conclusions from the research, practices who wish to implement SMPs for their patients with COPD would be advised to 1) consider carefully the objectives they hope to achieve; 2) choose or design a programme that is closely tied to those objectives; and 3) collect intermediate outcome measures that can indicate that the programme is working as intended.
Ethics approval and consent to participate
Not applicable, as this is a secondary review of the literature.
Consent for publication
Not applicable, as this is a secondary review of the literature.
Funding
This paper is independent research funded by the National Institute for Health Research (NIHR) Imperial Patient Safety Translational Research Centre (PSTRC-2016-004) with infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC). The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
Availability of data and materials
All data generated or analysed during this study are included in this published article, its supplementary information files, and/or the previously published protocol.
CRediT authorship contribution statement
Katelyn R. Smalley: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Lisa Aufegger: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing, Supervision. Kelsey Flott: Conceptualization, Methodology, Writing - review & editing. Erik K. Mayer: Conceptualization, Methodology, Writing - review & editing, Supervision. Ara Darzi: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank the National Institute for Health Research (NIHR) Imperial Patient Safety Translation Research Centre (PSTRC) for its support of this research.
We thank Jacqueline Cousins and Michael Gainsford (information specialists at Imperial College London) for their support improving the composition of the search terms and procedural aspects of the search strategy, and Gracie Holt for her assistance in conducting the initial article screening for this review.
We also thank 3 anonymous reviewers for their helpful suggestions and feedback.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.pec.2020.08.015.
Contributor Information
Katelyn R. Smalley, Email: k.smalley17@imperial.ac.uk.
Lisa Aufegger, Email: l.aufegger@imperial.ac.uk.
Kelsey Flott, Email: k.flott14@imperial.ac.uk.
Erik K. Mayer, Email: e.mayer@imperial.ac.uk.
Ara Darzi, Email: a.darzi@imperial.ac.uk.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Barlow J., Wright C., Sheasby J., Turner A., Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ. Couns. 2002;48:177–187. doi: 10.1016/S0738-3991%2802%2900032-0. [DOI] [PubMed] [Google Scholar]
- 2.NHS England . 2014. Five Year Forward View. [Google Scholar]
- 3.Richard A.A., Shea K. Delineation of self-care and associated concepts. J. Nurs. Scholarsh. 2011;43 doi: 10.1111/j.1547-5069.2011.01404.x. no-no. [DOI] [PubMed] [Google Scholar]
- 4.Kneale D., Thomas J., Harris K. 2015. Developing and Optimising the Use of Logic Models in Systematic Reviews : Exploring Practice and Good Practice in the Use of Programme Theory in Reviews; pp. 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbeau J., Nault D., Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ. Couns. 2004;52:271–277. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 6.Newham J.J., Presseau J., Heslop-Marshall K., Russell S., Ogunbayo O.J., Netts P., Hanratty B., Kaner E. Features of self-management interventions for people with COPD associated with improved health-related quality of life and reduced emergency department visits: a systematic review and meta-analysis. Int. J. COPD. 2017;12:1705–1720. doi: 10.2147/COPD.S133317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kislov R., Pope C., Martin G.P., Wilson P.M. Harnessing the power of theorising in implementation science. Implement. Sci. 2019;14:1–8. doi: 10.1186/s13012-019-0957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins L., Francis J., Islam R., O’Connor D., Patey A., Ivers N., Foy R., Duncan E.M., Colquhoun H., Grimshaw J.M., Lawton R., Michie S. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017;12:77. doi: 10.1186/s13012-017-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michie S., van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cane J., O’Connor D., Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grol R., Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet (London, England) 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 12.Davies P., Walker A.E., Grimshaw J.M. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement. Sci. 2010;5:14. doi: 10.1186/1748-5908-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bero L.A., Grilli R., Grimshaw J.M., Harvey E., Oxman A.D., Thomson M.A. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317 doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-durra M., Torio M., Cafazzo J.A., Street E. Vol. 17. 2015. pp. 1–13. (The Use of Behavior Change Theory in Internet-Based Asthma Self-Management Interventions : A Systematic Review Corresponding Author). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand M.-A., Stiel M., Boivin J., Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ. Couns. 2008;71:125–135. doi: 10.1016/j.pec.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Colquhoun H.L., Carroll K., Eva K.W., Grimshaw J.M., Ivers N., Michie S., Sales A., Brehaut J.C. Advancing the literature on designing audit and feedback interventions: identifying theory-informed hypotheses. Implement. Sci. 2017;12:117. doi: 10.1186/s13012-017-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French S.D., Green S.E., O’Connor D.A., McKenzie J.E., Francis J.J., Michie S., Buchbinder R., Schattner P., Spike N., Grimshaw J.M. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement. Sci. 2012;7:38. doi: 10.1186/1748-5908-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damschroder L.J. Clarity out of chaos: use of theory in implementation research. Psychiatry Res. 2020;283 doi: 10.1016/j.psychres.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Vestbo J., Hurd S.S., Agustí A.G., Jones P.W., Vogelmeier C., Anzueto A., Barnes P.J., Fabbri L.M., Martinez F.J., Nishimura M., Stockley R.A., Sin D.D., Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 20.2016. What Is COPD? | British Lung Foundation.https://www.blf.org.uk/support-for-you/copd/what-is-it (Accessed 2 August 2018) [Google Scholar]
- 21.Barnes P.J., Burney P.G.J., Silverman E.K., Celli B.R., Vestbo J., Wedzicha J.A., Wouters E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.76. 15076. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence (NICE) 2019. Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management. [PubMed] [Google Scholar]
- 23.Smalley K.R., Aufegger L., Flott K., Holt G., Mayer E.K., Darzi A. Which behaviour change techniques are most effective in improving healthcare utilisation in COPD self-management programmes? A protocol for a systematic review. BMJ Open Respir. Res. 2019;6 doi: 10.1136/bmjresp-2018-000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.J. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. Page, V. Welch, (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019) 2019; www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 25.Hoffmann T., Glasziou P., Boutron I., Milne R., Perera R., Moher D., Altman D., Barbour V., Macdonald H., Johnston M., Lamb S., Dixon-Woods M., McCulloch P., Wyatt J., Chan A., Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348 doi: 10.1136/bmj.g1687. http://www.bmj.com/content/348/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 26.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 30.Blackstock F.C., Webster K.E., McDonald C.F., Hill C.J., B. F.C, W. K.E, M. C.F Comparable improvements achieved in chronic obstructive pulmonary disease through pulmonary rehabilitation with and without a structured educational intervention: a randomized controlled trial. Respirology. 2014;19:193–202. doi: 10.1111/resp.12203. [DOI] [PubMed] [Google Scholar]
- 31.Bourbeau J., Julien M., Maltais F., Rouleau M., Beaupre A., Begin R., Renzi P., Nault D., Borycki E., Schwartzman K., Singh R., Collet J.-P. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch. Intern. Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 32.Bucknall C.E., Miller G., Lloyd S.M., Cleland J., McCluskey S., Cotton M., Stevenson R.D., Cotton P., McConnachie A. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:16. doi: 10.1136/bmj.e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casas A., Troosters T., Garcia-Aymerich J., Roca J., Hernandez C., Alonso A., del Pozo F., de Toledo P., Anto J.M., Rodriguez-Roisin R., Decramer M., Members of the C Project, Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur. Respir. J. 2006;28:123–130. doi: 10.1183/09031936.06.00063205. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=16611656 [DOI] [PubMed] [Google Scholar]
- 34.Dewan N.A., Rice K.L., Caldwell M., Hilleman D.E. Economic evaluation of a disease management program for chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2011;8:153–159. doi: 10.3109/15412555.2011.560129. [DOI] [PubMed] [Google Scholar]
- 35.Dritsaki M., Johnson-Warrington V., Mitchell K., Singh S., Rees K., D. M, J.-W. V, M. K, S. S An economic evaluation of a self-management programme of activity, coping and education for patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2016;13:48–56. doi: 10.1177/1479972315619578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan V.S., Gaziano J.M., Lew R., Bourbeau J., Adams S.G., Leatherman S., Thwin S.S., Huang G.D., Robbins R., Sriram P.S., Sharafkhaneh A., Mador M.J., Sarosi G., Panos R.J., Rastogi P., Wagner T.H., Mazzuca S.A., Shannon C., Colling C., Liang M.H., Stoller J.K., Fiore L., Niewoehner D.E. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann. Intern. Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 37.Farmer A., Williams V., Velardo C., Shah S.A., Yu L.M., Rutter H., Jones L., Williams N., Heneghan C., Price J., Hardinge M., Tarassenko L. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomized controlled trial. J. Med. Internet Res. 2017;19:e144. doi: 10.2196/jmir.7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadoury M.-A., Schwartzman K., Rouleau M., Maltais F., Julien M., Beaupre A., Renzi P., Begin R., Nault D. Self-management reduces both short- and long-term hospitalisation in COPD. Eur. Respir. J. 2005;26:853–857. doi: 10.1183/09031936.05.00093204. [DOI] [PubMed] [Google Scholar]
- 39.Gallefoss F., Bakke P.S., G. F, Gallefoss F., Bakke P.S. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir. Med. 2000;94:279–287. doi: 10.1053/rmed.1999.0749. [DOI] [PubMed] [Google Scholar]
- 40.Gallefoss F., Bakke P.S., G. F, Gallefoss F., Bakke P.S. Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD - A 1-year follow-up randomized, controlled trial. Respir. Med. 2002;96:424–431. doi: 10.1053/rmed.2002.1293. [DOI] [PubMed] [Google Scholar]
- 41.Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Educ. Couns. 2004;52:259–266. doi: 10.1016/S0738-3991%2803%2900100-9. [DOI] [PubMed] [Google Scholar]
- 42.Johnson-Warrington V., Rees K., Gelder C., Morgan M.D., Singh S.J. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. Int. J. COPD. 2016;11:1161–1169. doi: 10.2147/COPD.S91253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khdour M.R., Kidney J.C., Smyth B.M., McElnay J.C. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br. J. Clin. Pharmacol. 2009;68:588–598. doi: 10.1111/j.1365-2125.2009.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khdour M.R., Agus A.M., Kidney J.C., Smyth B.M., McElnay J.C., Crealey G.E. Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD. Int. J. Clin. Pharm. 2011;33:665–673. doi: 10.1007/s11096-011-9524-z. [DOI] [PubMed] [Google Scholar]
- 45.Koff P.B., Jones R.H., Cashman J.M., Voelkel N.F., Vandivier R.W. Proactive integrated care improves quality of life in patients with COPD. Eur. Respir. J. 2009;33:1031–1038. doi: 10.1183/09031936.00063108. [DOI] [PubMed] [Google Scholar]
- 46.Martin I.R., Mcnamara D., Sutherland F.R., Tilyard M.W., Taylor D.R. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron. Respir. Dis. 2004;1:191–195. doi: 10.1191/1479972304cd047oa. [DOI] [PubMed] [Google Scholar]
- 47.McGeoch G.R.B., Willsman K.J., Dowson C.A., Town G.I., Frampton C.M., McCartin F.J., Cook J.M., Epton M.J. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology. 2006;11:611–618. doi: 10.1111/j.1440-1843.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell K.E., Johnson-Warrington V., Apps L.D., Bankart J., Sewell L., Williams J.E., Rees K., Jolly K., Steiner M., Morgan M., Singh S.J. A self-management programme for COPD: A randomised controlled trial. Eur. Respir. J. 2014;44:1538–1547. doi: 10.1183/09031936.00047814. [DOI] [PubMed] [Google Scholar]
- 49.Rea H., McAuley S., Stewart A., Lamont C., Roseman P., Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern. Med. J. 2004;34:608–614. doi: 10.1111/j.1445-5994.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 50.Rice K.L., Dewan N., Bloomfield H.E., Grill J., Schult T.M., Nelson D.B., Kumari S., Thomas M., Geist L.J., Beaner C., Caldwell M., Niewoehner D.E. Disease management program for chronic obstructive pulmonary disease a randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010;182:890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 51.Rose L., Istanboulian L., Carriere L., Thomas A., Lee H.-B., Rezaie S., Shafai R., Fraser I. Program of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (PIC COPD+): a randomised controlled trial. Eur. Respir. J. 2018;51 doi: 10.1183/13993003.01567-2017. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Nieto J.M., Andujar-Espinosa R., Bernabeu-Mora R., Hu C., Galvez-Martinez B., Carrillo-Alcaraz A., Alvarez-Miranda C.F., Meca-Birlanga O., Abad-Corpa E. Efficacy of a self-management plan in exacerbations for patients with advanced COPD. Int. J. COPD. 2016;11:1939–1947. doi: 10.2147/COPD.S104728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trappenburg J.C.A.A., Monninkhof E.M., Bourbeau J., Troosters T., Schrijvers A.J.P.P., Verheij T.J.M.M., Lammers J.-W.W.J. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: A multicentre randomised controlled trial. Thorax. 2011;66:977–984. doi: 10.1136/thoraxjnl-2011-200071. [DOI] [PubMed] [Google Scholar]
- 54.Wakabayashi R., Motegi T., Yamada K., Ishii T., Jones R.C.C.M., Hyland M.E., Gemma A., Kida K. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatr. Gerontol. Int. 2011;11:422–430. doi: 10.1111/j.1447-0594.2011.00696.x. [DOI] [PubMed] [Google Scholar]
- 55.W. L.H, Z. Y, C. L.Y, Z. L, L.H.O, Zhang Y.M., Wang A.O.- The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease. Clin. Respir. J. 2020;14:148–157. doi: 10.1111/crj.13112. [DOI] [PubMed] [Google Scholar]
- 56.Ranganathan P., Pramesh C.S., Aggarwal R. Common pitfalls in statistical analysis: intention-to-treat versus per-protocol analysis. Perspect. Clin. Res. 2016;7:144–146. doi: 10.4103/2229-3485.184823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai J.-W., Chen X.-X., Liu S., Yu L., Xu J.-F. Smoking cessation affects the natural history of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:3323–3328. doi: 10.2147/COPD.S150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephenson J., Imrie J. Why do we need randomised controlled trials to assess behavioural interventions? BMJ. 1998;316:611–613. doi: 10.1136/bmj.316.7131.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deaton A., Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018;210:2–21. doi: 10.1016/j.socscimed.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roland M., Olesen F. Can pay for performance improve the quality of primary care? BMJ. 2016;354 doi: 10.1136/bmj.i4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdul-Aziz A.A., Hayward R.A., Aaronson K.D., Hummel S.L. Association between medicare hospital readmission penalties and 30-day combined excess readmission and mortality. JAMA Cardiol. 2017;2:200–203. doi: 10.1001/jamacardio.2016.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarin D.A., Goodman S.N., Kimmelman J. Harms from uninformative clinical trials. JAMA. 2019;322:813–814. doi: 10.1001/jama.2019.9892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article, its supplementary information files, and/or the previously published protocol.