Abstract
This cross-sectional study examines the association between chronic musculoskeletal pain and foot reaction time (RT) among older community-living adults. Participants were 307 adults aged 71 years and older in the MOBILIZE Boston Study II. Pain severity, interference, and location were measured by the Brief Pain Inventory and a joint pain questionnaire. With participants seated, simple foot reaction time (SRT) was measured as self-selected foot response time to an intermittent light, and choice foot reaction time (CRT) was measured as response time to the light on the corresponding side of the sensored gait mat. We performed multivariable linear regression to determine associations of pain and foot RT, adjusted for sociodemographic and health characteristics, and serially adjusted for cognitive function (MMSE or Trail Making A). Pain severity and interference were associated with slower SRT (p<0.05). Pain severity and knee pain were associated with slower CRT (p<0.05). Adjustment for cognitive measures had little impact on the pain-RT relationship. This signficant relationship was only observed among participants with less education. These results support the idea that chronic pain may lead to slower foot RT, thus could represent a fall hazard in older adults. Neuromotor mechanisms underlying the pain-fall relationship warrant further investigation.
Perspective:
This study provides insights on the mechanisms underlying the pain-fall relationship. Chronic pain may contribute to slower foot reaction time thus increase fall risk in older adults. This may help inform interventions such as stepping training to reduce fall risk in older adults living with chronic pain.
Keywords: Chronic Pain, Cognition, Epidemiology, Mobility, Falls
Introduction
Chronic musculoskeletal pain affects more than half of older people,31 and poses serious risks leading to mobility decline and falls in older adults.9,20,40,45 It has been reported that musculoskeletal pain and specifically multisite pain are associated with slower gait speed and increased gait variability.8,39 Although studies have examined cognitive factors contributing to falls and several have determined a relationship between pain and falls,16,45 little research has explored mechanisms underlying the relationship between chronic musculoskeletal pain and falls. One possible pathway whereby pain may increase risk for falls is through cognitive and neuromotor effects of pain on mobility.
The capacity to avoid obstacles and respond to hazards (i.e., reaction) is critical to preventing falls.18,26 This “reactive” capacity requires appropriate quick stepping and gripping actions that are dependent on sensorimotor and visuospatial skills, and cognitive function.28 Timing characteristics of protective voluntary stepping are critical for effective balance recovery.35 This capacity of quick response to avoid hazards can be measured by reaction time (RT) which is associated with fall risk.23,24,33 Chronic musculoskeletal pain in older adults, which has been linked to cognitive and neuromotor function, may delay a rapid effective response to a fall hazard, leading to increased fall risk.4,7,15,29
Reaction time is often measured both in the hands and feet. Hand RT, usually tested by a finger pressing a button in response to target stimuli, captures the decision time or primarily cognitive aspects of RT and thus requires only a minimal motor response. Simple hand and foot RT is included in the Physiological Profile Assessment (PPA) as one of the standard tests to assess fall risk among older adults.24 These RT tests are performed using a pedal switch as in the PPA24 or complex technical equipment such as stepping panels, Kinect-based approaches, or an infrared laser device.10,11,23,28
However, foot RT, more so than hand RT, has been found to be associated with risk for falls especially recurrent falls,33,48 possibly because it captures ability to quickly move the lower limbs, an action influenced by both cognitive and neuromotor function.1,23 However, the potential impact of chronic pain on foot RT in older adults has not been examined. In addition, more and more evidence shows that pain may impact cognitive function.7,15,29,52 Thus, we hypothesized that chronic pain would be associated with slower reaction time. As cognitive function has also been linked to physical impairment,36,44 we further hypothesized that cognition may mediate the pain-RT relationship. Thus, the aim of this study is to first examine the association between chronic musculoskeletal pain and foot RT among older adults living in the community, and secondly, to evaluate whether measures of cognitive function may influence the pain-RT relationship.
Methods
This population-based study examines the cross-sectional relationship between chronic musculoskeletal pain and the foot RT among community-dwelling older adults in MOBILIZE Boston Study II (MBS II).
Participant Recruitment
The MBS II was an approximately 6.5 year follow up assessment of the original MOBILIZE Boston Study (MBS) cohort. Details of the original study methods were published previously.21 Briefly, from 2005 to 2008, the MBS recruited 765 adults aged 70 years and older living in community within a 5-mile radius of the Institute for Aging Research at Hebrew SeniorLife. The cohort was randomly selected using city/town lists in Boston and surrounding areas and participants were recruited door-to-door. In addition to age, inclusion criteria were as follows: a) able to speak and understand English, b) expected to live in the area for at least 2 years, and c) able to walk 20 feet without personal assistance. Spouses aged 65 and older of enrollees who were otherwise eligible were also welcomed to participate. Older adults who had terminal disease, severe vision or hearing deficits, or moderate to severe cognitive impairment (Mini-Mental State Examination < 18) were excluded.12 At the start of the MBSII enrollment in November 2011, 531 participants from MBSI were alive and living in the community. In the 4-year enrollment period (2011–2015), 354 older adults participated the MBSII assessment and 310 completed the clinic assessment that included the reaction time testing.
Data collection
The MBSII assessment comprised a 45-minute health interview by trained research assistants and a 3-hour clinical assessment by research nurses that took place at the study clinic at the Institute for Aging Research at Hebrew SeniorLife. Informed consent was obtained at the start of the telephone interview. The study protocol was approved by the Institutional Review Boards at Hebrew SeniorLife and the University of Massachusetts Boston.
Foot reaction time testing was performed by 2 experienced research assistants, including one biomechanical engineer, trained in the scripted protocol and use of the sensored gait mat (GAITRite, CIR Systems Inc., Franklin, NJ) with the PKMAS software (Protokinetics, Havertown, PA). To measure foot RT, each participant completed 10 trials of each of the two tests: Simple Reaction Time (SRT) and Choice Reaction Time (CRT). At the start of the testing, participants were seated in a straight-backed chair, with their feet placed flat on the sensored mat. For the SRT, they were instructed to pick up their foot, whichever they could move fastest, and tap a blue dot on the mat in front of them in response to a randomly intermittent light on the right side of the mat. For the CRT, participants were instructed to pick up the foot that was on the same side as the light fixture showing a randomly intermittent light on either side of the mat. Participants performed 3–4 practice rounds before each test began. The RT for each trial was calculated as the difference between the time the light turns on and the initiation of the movement to lift the correct foot off the mat. We used the average time of each set of 10 trials for SRT and CRT in the analyses. Our measure of foot reaction time using the GAITRite is similar to the method used to assess swing time, where the instrumentation detects the moment the foot leaves contact with the mat, a well-validated GAITRite measure.6,54 Importantly, the intermittent light is wired directly to the mat instrumentation so the timing of the foot response to the light is embedded in the GAITRite equipment, synced with the mat sensor software, and not dependent on the actions of the tester. For the safety of our participants, because of their advanced age and frailty, participants performed the tests in a seated position.
Chronic pain was assessed according to pain location and distribution, overall pain severity, and pain interference. During the interview, chronic musculoskeletal pain location was assessed using the 13-item joint pain questionnaire, assessing pain lasting 3 or more months in the previous year and present in the past month in hands/wrists, shoulders, back, hip, knees, and feet.20 Pain distribution was classified as: 1) no pain, 2) pain in a single site, and 3) pain in 2 or more musculoskeletal sites. The majority of older adults who have pain suffer from multisite pain.31 This classification of pain distribution based on the joint pain questionnaire is strongly associated with incident disability and fall risk in older adults.9,20,47 Also, recent analyses using this measure support that multisite pain is similar to other geriatric syndromes in its impact on the older population.47 Pain severity was assessed using the average rating of the 4-item Brief Pain Inventory (BPI) subscale which uses a 0–10 numeric rating scale measuring pain intensity in the past week, with 0 referring to no pain and 10 indicating “severe or excruciating pain, as bad as you can imagine”.5 The BPI Pain Interference subscale score is the average of 7 items measuring pain interference with general activity, mood, walking, normal work including housework, relations with other people, sleep, and enjoyment of life, rated on a numeric rating scale where 0 refers to “not at all interferes” and 10 indicates, “completely interferes”.5,17
Sociodemographic characteristics including age, gender, education, and race were collected in the baseline MBS interview. Height and weight were measured during the MBS II clinic visit and body mass index (BMI) was calculated as weight in kilograms divided by height in squared meters, categorized using standard NHLBI cut points for obesity (≥30) and overweight (25 to 29). Presence of mobility difficulty was based on self-reported difficulty with walking ¼ mile and climbing stairs.37 Mini-Mental State Exam (MMSE) which is a global cognitive function test was measured in the home interview.12 Trail-making Test-part A (TMT-A), a validated timed test to assess attention and processing speed, requires participants to draw a line to connect randomly positioned numbers on a page in ascending order as quickly as possible.34 Chronic conditions including osteoarthritis, peripheral arterial disease, diabetes and peripheral neuropathy were assessed using disease algorithms, described previously.21 Physician diagnosis of other chronic conditions including heart disease and stroke were self-reported. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale-Revised (CESD-R) questionnaire.22 Vision deficit was determined by the 10-foot distant vision test using the Good-Lite Chart™.57 Standing balance was measured using 4 timed stands: feet side by side, semi-tandem, tandem, and one-leg stands.14 Fall history, or self-reported number of falls in the past year, was also collected in the health interview. A fall was defined as “any event where any part of your body above your ankle hit the floor or other lower surface”. This included falls that might have occurred on stairs. Use of daily analgesic medications and psychiatric medications was determined using the brown bag method, with medications recorded from medication containers during the clinic visit. Fear of falling was measured using Tinetti’s Falls Efficacy Scale (FES).49 The FES is a 10-item scale to assess the degree of confidence in doing daily activities without falling from 1 to 10, with the 1 meaning “not confident at all” and the 10 meaning “extremely confident”. Participants were identified as having fear of falling if the total score was lower than 90.
Statistical Analysis
For this analysis, we excluded 3 participants who had Parkinson’s disease, which could interfere with RT test performance. Thus, the final sample for the analysis was 307 older adults. We used descriptive analyses, presenting means and standard deviations for continuous measures and percentages for categorical measures, for sociodemographic characteristics, chronic conditions, fall risk factors, pain characteristics, and foot RT. The distribution and missingness of all variables were checked. We recoded outliers for the foot RT measures to the ninety-ninth percentile. Independent t-tests, pairwise t-tests, and one-way ANOVA tests were used to assess group differences of sociodemographic characteristics, fall risk factors, and medical conditions according to foot RT, to characterize the study population and to evaluate potential confounders of the pain-RT relationship. The BPI severity and interference scores were used both as continuous terms, and as categories (pain severity clinical cut-points, <1, 1–3.99, ≥4) or tertiles (pain interference cut-points, 0, <1.5, ≥1.5) in the analysis. While established cutpoints for pain interference are not available, the clinical cutpoints for pain severity are consistent with previous studies.56
Generalized Linear Models (GLM) were used to examine the trends of foot RT according to ordinal groupings of pain characteristics. Multivariable linear regression models were performed to evaluate associations between pain characteristics and foot RT adjusted for potential confounders. Pain severity, pain interference, pain distribution, lower body pain count, and individual pain sites (i.e., pain in back, hip, knee, and feet) were independent variables in separate models. Sociodemographic characteristics, chronic conditions, and daily analgesic use were added as covariates to the models because they have previously been found to be associated with chronic pain.20,32,51 We did not adjust for osteoarthritis, depression, peripheral artery disease, or peripheral neuropathy because these conditions are potentially on a causal pathway leading to pain and slowed RT; this would represent an over-adjustment for pain in the models. In addition, we evaluated potential effects of cognitive measures (MMSE or TMT-A) on the pain-RT relationship. In a final model, we additionally adjusted for fall risk factors (balance impairment, use of psychoactive medication, and fall self-efficacy), to determine their impacts on the pain-RT relationships. There were no more than 3% missing values for any of the variables in the models and we did not use imputation for missing values. We repeated the models using a log-transformation of the RT variables but the results were unchanged, thus we presented the models using the non-transformed outcomes. In a final step, we performed a stratified analysis to examine the pain-RT relationship by education level, a potential moderator of the pain-RT relationship. The significance level was alpha=0.05. Analyses were performed using SAS software 9.4 (SAS Institute, Cary, NC).
Results
MBSII participants who did not complete the foot RT tests (n=44) were older than the RT test completers (mean ages 88.6 ± 5.1 years and 84.0 ± 4.4 years, respectively; p < 0.001) and had fewer years of education (30% versus 57% were college graduates, respectively; p < 0.001). There were no gender and race differences. The study sample was nearly two-thirds female (64.5%) with an average age of 84 ± 4.4 years, ranging from 71 to 101 years. Two hundred and forty-four (79.5%) participants were white and 47 (15.3%) were African American. More than half of participants (57%) were college graduates (Table 1).
Table 1.
Foot reaction time according to sociodemographic characteristics, cognitive function, and fall risk factors, 307 participants aged 71 years and older, MOBILIZE Boston Study II, 2011–2015.
| Characteristics | Total | SRTa (seconds) | CRTa (seconds) | ||
|---|---|---|---|---|---|
| N (%) | Mean (SD) | p-valuec | Mean (SD) | p-valuec | |
| Total | 307 | 0.245 (0.057) | 0.323 (0.085) | ||
| Age (years) | 0.02 | 0.02 | |||
| <80 | 38 (12.4) | 0.235 (0.04) | 0.310 (0.07) | ||
| 80–84 | 150 (48.9) | 0.246 (0.06) | 0.316 (0.08) | ||
| 85–89 | 78 (25.4) | 0.236 (0.04) | 0.323 (0.10) | ||
| >90 | 41 (13.4) | 0.268 (0.09) | 0.361 (0.09) | ||
| Gender | 0.51 | 0.96 | |||
| Male | 109 (35.5) | 0.243 (0.05) | 0.323 (0.09) | ||
| Female | 198 (64.5) | 0.247(0.06) | 0.323 (0.09) | ||
| Race | |||||
| White | 244 (79.5) | 0.243 (0.06) | - | 0.318 (0.08) | - |
| Black | 47 (15.3) | 0.258 (0.07) | 0.10 | 0.361 (0.11) | 0.002 |
| Other | 16 (5.2) | 0.239 (0.03) | 0.76 | 0.293 (0.05) | 0.25 |
| Educationb | 0.002 | 0.002 | |||
| < Coll. grad. | 131 (42.8) | 0.257 (0.06) | 0.340 (0.09) | ||
| Coll. grad. | 175 (57.2) | 0.237 (0.05) | 0.310 (0.08) | ||
| Body mass indexb | 0.91 | 0.07 | |||
| < 25 | 116 (38.5) | 0.244 (0.06) | 0.318 (0.09) | ||
| 25 – 29 | 122 (40.5) | 0.247 (0.05) | 0.317 (0.07) | ||
| > 30 | 63 (20.9) | 0.246 (0.06) | 0.345 (0.10) | ||
| MMSE | 0.005 | <0.001 | |||
| < 24 | 56 (18.2) | 0.271 (0.08) | 0.381 (0.11) | ||
| > 24 | 251 (81.8) | 0.240 (0.05) | 0.311 (0.07) | ||
| Trail Making Ab | <0.001 | <0.001 | |||
| 1st tertile | 101 (33.7) | 0.233 (0.04) | 0.296 (0.05) | ||
| 2nd tertile | 100 (33.3) | 0.235 (0.04) | 0.315 (0.07) | ||
| 3rd tertile | 99 (33.0) | 0.259 (0.06) | 0.345 (0.10) | ||
| Vision deficitb | 0.18 | 0.60 | |||
| Yes | 55 (18.2) | 0.253 (0.07) | 0.325 (0.08) | ||
| No | 248 (81.9) | 0.242 (0.05) | 0.319 (0.08) | ||
| Mobility difficulty | 0.02 | 0.03 | |||
| Yes | 144 (46.9) | 0.254 (0.06) | 0.334 (0.09) | ||
| No | 163 (53.1) | 0.238 (0.05) | 0.313 (0.08) | ||
| Balance impairmentb | 0.003 | <0.001 | |||
| Yes | 127 (41.5) | 0.257 (0.06) | 0.352 (0.10) | ||
| No | 179 (58.5) | 0.237 (0.05) | 0.302 (0.07) | ||
| Daily analgesic use | 0.97 | 0.62 | |||
| Yes | 84 (27.4) | 0.245 (0.04) | 0.319 (0.08) | ||
| No | 223 (72.6) | 0.246 (0.06) | 0.325 (0.09) | ||
| Psychiatric medication use | 0.02 | 0.06 | |||
| Yes | 59 (19.2) | 0.260 (0.07) | 0.342 (1.0) | ||
| No | 248 (80.8) | 0.242 (0.05) | 0.319 (0.08) | ||
| Fall in the past yearb | 0.04 | 0.75 | |||
| > 2 | 60 (43.1) | 0.251 (0.07) | 0.328 (0.09) | ||
| 0 or 1 | 246 (56.9) | 0.244 (0.05) | 0.322 (0.08) | ||
| Fear of fallingb | 0.02 | <0.001 | |||
| Yes | 64 (21.1) | 0.260 (0.07) | 0.356 (0.12) | ||
| No | 240 (79.0) | 0.242 (0.05) | 0.314 (0.07) | ||
Simple and choice foot reaction time, SRT, CRT; higher numbers indicate slower foot reaction time.
Sample sizes do not add to 307 due to missing values.
Trend (ordinal variables) and pairwise p-values from unadjusted Generalized Linear Models (GLM); for race, pairwise comparisons with Whites.
The average foot RT was 0.245 ± 0.057 seconds for SRT and 0.323 ± 0.085 seconds for CRT (Table 1). Older age was associated with slower foot RT in both SRT (p=0.02) and CRT (p=0.02). We did not observe differences in SRT according to sex or race. However, African Americans had slower CRT than other race groups (p=0.002). College graduates had shorter RT than those without college education in both SRT and CRT (p=0.002). Poorer cognitive performance and mobility difficulty were associated with both slower SRT and CRT. Participants who reported recurrent falls (≥2 falls) in the past year had slower SRT (p=0.04) but not CRT. Other known fall risk factors including balance impairment, use of psychiatric medications, and fear of falling were associated with foot reaction time (Table 1). Medical conditions associated with foot RT included osteoarthritis, diabetes, peripheral artery disease, and heart disease (Table 2).
Table 2.
Foot reaction time according to medical conditions, 307 participants aged 71 years and older, MOBILIZE Boston Study II.
| Total | SRTa (seconds) | CRTa (seconds) | |||
|---|---|---|---|---|---|
| N (%) | Mean (SD) | p-valueb | Mean (SD) | p-valueb | |
| Osteoarthritisc | |||||
| Neither site | 200 (70.2) | 0.243 (0.05) | - | 0.315 (0.07) | - |
| Knee only | 30 (10.5) | 0.254 (0.06) | 0.34 | 0.348 (0.09) | 0.05 |
| Hand only | 40 (14.0) | 0.242 (0.05) | 0.89 | 0.327 (0.09) | 0.43 |
| Hand & Knee | 15 (5.3) | 0.288 (0.10) | 0.004 | 0.384 (0.14) | 0.003 |
| Diabetes mellitus | 0.04 | 0.01 | |||
| Yes | 35 (11.4) | 0.264 (0.06) | 0.358 (0.08) | ||
| No | 272 (88.6) | 0.243 (0.06) | 0.318 (0.09) | ||
| Depressive symptoms | 0.18 | 0.89 | |||
| Yes | 39 (12.7) | 0.257 (0.06) | 0.325 (0.07) | ||
| No | 268 (87.3) | 0.244 (0.06) | 0.323 (0.09) | ||
| Peripheral artery disease | <0.001 | 0.05 | |||
| Yes | 35 (11.4) | 0.276 (0.08) | 0.350 (0.10) | ||
| No | 272 (88.6) | 0.241 (0.05) | 0.320 (0.08) | ||
| Peripheral neuropathyc | 0.61 | 0.47 | |||
| Yes | 58 (19.8) | 0.249 (0.05) | 0.331 (0.07) | ||
| No | 235 (80.2) | 0.244 (0.06) | 0.322 (0.09) | ||
| Heart disease | 0.09 | 0.01 | |||
| Yes | 127 (41.4) | 0.252 (0.07) | 0.338 (0.09) | ||
| No | 180 (58.6) | 0.241 (0.05) | 0.313 (0.08) | ||
| Stroke | 0.96 | 0.28 | |||
| Yes | 24 (7.8) | 0.246 (0.06) | 0.341 (0.08) | ||
| No | 283 (92.2) | 0.245 (0.06) | 0.321 (0.09) | ||
Simple and choice foot reaction time, SRT, CRT.
Unadjusted Generalized Linear Model (GLM) using ordinal variables, except for osteoarthritis where the p-value was for pairwise comparisons to the first category of variable (neither site).
Sample sizes do not add to 307 due to missing values.
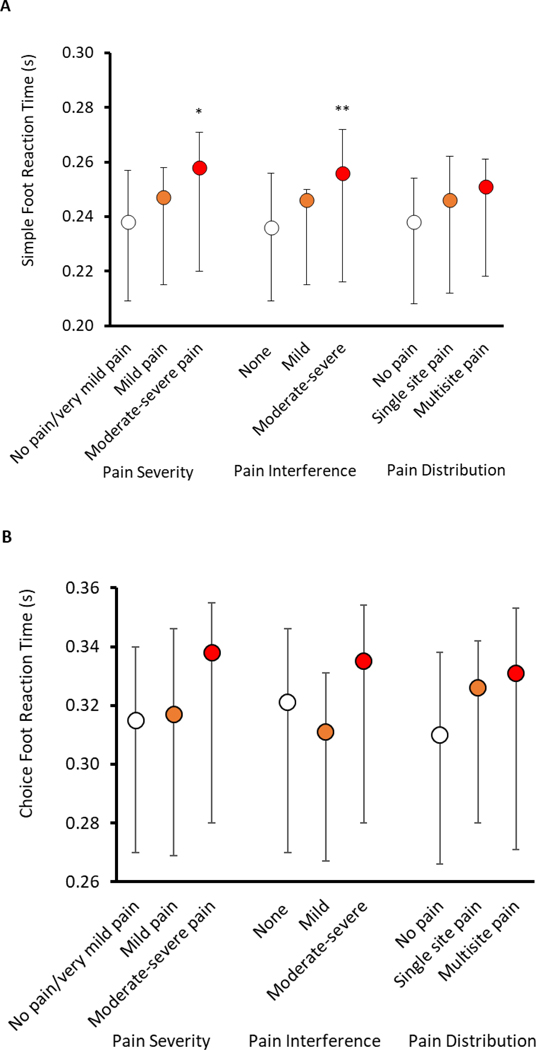
Participants with more severe pain or more pain interference had significantly slower SRT (p<0.05) than their peers with none or mild pain (Fig. 1A; Supplemental Table). We did not observe the same significant trends in the relation between pain characteristics and CRT (Fig. 1B; Supplemental Table). However, in pairwise analysis, older adults with moderate-to-severe pain had slower CRT compared to those with none/very mild pain and those with mild pain (p=0.03 and p=0.04, respectively). We did not find significant associations between pain distribution and SRT or CRT.
Figure 1.
Medians and interquartile ranges of Simple (A) and Choice (B) Foot Reaction Time according to pain characteristics in MOBILIZE Boston Study II. *Generalized Linear Models (GLM), test of trend of foot reaction time according to categories of pain severity (cutpoints: <1, 1–3.99, ≥4), pain interference (cutpoints: 0, <1.5, ≥1.5), and pain distribution, p-value < 0.05, **p-value < 0.01.
After adjusting for sociodemographic and health characteristics, pain severity was associated with slower SRT and CRT (Model 1, p=0.04 and p=0.03, respectively) (Table 3). Pain interference was associated with SRT (p=0.04) but not CRT after adjusting for sociodemographic and health characteristics. In pain site-specific analyses, we found that knee pain was strongly and consistently associated with CRT but not SRT (p=0.01). Other individual sites of pain (back, hip, and feet) were not associated with SRT or CRT (data not shown). To evaluate the role of cognitive factors on the pathway from pain to RT, we separately adjusted for measures of cognitive functioning, and found that the MMSE and TMT-A had only modest impacts on the relationships. Specifically, adjusting for MMSE modestly attenuated the relationship of pain severity and interference with SRT, while adjusting for the TMT-A primarily attenuated the pain interference-SRT association. In an additional model (Model 4), we adjusted for known fall risk factors which diminished the observed relationships between pain and reaction time, except for the association between knee pain and choice reaction time.
Table 3.
Association between pain characteristics and foot reaction time, 307 participants aged 71 years and older, MOBILIZE Boston Study II.
| Separate Models | Model 1a | Model 2b (Model 1 + MMSE) | Model 3c (Model 1 + TMT-A) | Model 4d (Model 1 + balance impairment, psychiatric medication use, and fear of falling) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | |
| SRT | ||||||||||||
| Pain severity | 0.0042 | 0.0020 | 0.036 | 0.0039 | 0.0020 | 0.049 | 0.0041 | 0.0018 | 0.022 | 0.0032 | 0.0020 | 0.114 |
| Pain interference | 0.0040 | 0.0020 | 0.041 | 0.0035 | 0.0019 | 0.068 | 0.0032 | 00018 | 0.070 | 0.0026 | 0.0020 | 0.209 |
| Lower body pain count | 0.0043 | 0.0044 | 0.328 | 0.0037 | 0.0044 | 0.391 | 0.0047 | 0.0039 | 0.231 | 0.0019 | 0.0045 | 0.667 |
| Knee pain | 0.0141 | 0.0075 | 0.060 | 0.0134 | 0.0074 | 0.069 | 0.0122 | 0.0065 | 0.063 | 0.0114 | 0.0076 | 0.137 |
| CRT | ||||||||||||
| Pain severity | 0.0062 | 0.0029 | 0.034 | 0.0057 | 0.0028 | 0.047 | 0.0067 | 0.0026 | 0.012 | 0.0037 | 0.0029 | 0.203 |
| Pain interference | 0.0039 | 0.0029 | 0.177 | 0.0030 | 0.0028 | 0.287 | 0.0036 | 0.0026 | 0.179 | 0.0011 | 0.0029 | 0.707 |
| Lower body pain count | 0.0124 | 0.0064 | 0.056 | 0.0116 | 0.0063 | 0.067 | 0.0132 | 0.0058 | 0.023 | 0.0068 | 0.0065 | 0.298 |
| Knee pain | 0.0288 | 0.0109 | 0.009 | 0.0280 | 0.0106 | 0.009 | 0.0299 | 0.0097 | 0.002 | 0.0229 | 0.0109 | 0.036 |
Model 1 Generalized Linear Model adjusted for age, gender, race, education, diabetes, heart disease, and daily analgesic use.
Model 2 included all variables from model 1 and Mini-Mental State Exam (MMSE) score.
Model 3 included all variables from model 1 and Trail Making Test-part A (TMT-A); 7 participants did not complete the TMT-A and are not included in the model.
Model 4 included all variables from model 1 and balance impairment, psychiatric medication use, and fear of falling.
When we examined the relationships according to education level, we found that among older adults with less education (not college graduates), pain severity and pain interference were strongly associated with SRT, after adjusting for demographic characteristics, chronic conditions, and analgesic use (both p=0.01) (Table 4). Knee pain was associated with slower CRT among participants who were not college graduates (p=0.01). Among college graduates, none of the pain characteristics were associated with SRT or CRT.
Table 4.
Association between pain characteristics and foot reaction time according to education level, 307 participants aged 71 years and older, MOBILIZE Boston Study II.
| Separate Modelsb | Not college graduate (n=131) | College graduate (n=175) | ||||
|---|---|---|---|---|---|---|
| B | SE B | p | B | SE B | p | |
| SRTa | ||||||
| Pain severity | 0.0082 | 0.0031 | 0.009 | 0.0004 | 0.0026 | 0.880 |
| Pain interference | 0.0076 | 0.0030 | 0.012 | 0.0001 | 0.0026 | 0.959 |
| Knee pain | 0.0208 | 0.0129 | 0.110 | 0.0079 | 0.0090 | 0.379 |
| CRTa | ||||||
| Pain severity | 0.0075 | 0.0041 | 0.073 | 0.0060 | 0.0041 | 0.143 |
| Pain interference | 0.0094 | 0.0039 | 0.018 | −0.0011 | 0.0041 | 0.786 |
| Knee pain | 0.0460 | 0.0168 | 0.007 | 0.0164 | 0.0141 | 0.246 |
Simple and choice foot reaction time, SRT, CRT.
Generalized Linear Models with SRT and CRT as the outcomes and in separate models, each pain characteristic as the independent variable, adjusted for age, gender, race, diabetes, heart disease, and daily analgesic use stratified by education level.
Discussion
In this study, we found that chronic pain is associated with foot reaction time. Pain severity is associated with both SRT and CRT, pain interference is associated with SRT only, and knee pain is associated with CRT only. Further adjustment for cognitive performance resulted in a modest attenuation of the associations of pain severity and pain interference with reaction time. Although adjusting for attention did not alter the pain severity-SRT relationship, it did modestly attenuate the pain interference-SRT association. Adjusting for other fall risk factors, potentially on the pathway from pain to slowed reaction time, diminished the association in all but the knee pain relation to choice reaction time. In the examination of the role of education level, the pain-RT relationship is evident only among older adults who are not college graduates, suggesting a protective effect of education. These results need to be considered in view of the proposed cognitive and neuromotor pathway through which pain contributes to slower foot RT, and thereby may increase fall risk in older adults.
In several recent studies, global measures of chronic pain have been found to contribute to the onset of mobility limitations and disability in older adults.2,8,9,41,42 Previously, selected sites of chronic pain such as back, foot, and knee pain were reported to contribute to mobility and balance problems.2,19,25,27,55 Global pain severity and multisite pain are associated with higher rates of falls in older adults.20,32,46 The short-term effects of pain severity on fall risk have also been observed in the MBS cohort, where global chronic pain severity measured in a given month predicts falls in the subsequent month.20 Impaired foot RT may be one of several mechanisms that underlie the pain-mobility-falls pathway. In older adults, engagement in physical activity is important for physical and neurocognitive functioning.13 Pain in older adults, primarily musculoskeletal in origin, can lead to reduced activity and fear of falling, further slowing reaction time and interfering with balance and mobility, thereby increasing fall risk in this population.53
Previous studies have found that the differences in RT can discriminate fallers from nonfallers.10,11,28,33 Although the RT tests have been performed using various approaches, slower foot CRT has consistently been found to be associated with fall risk,10,23,28 and provides a composite measure that encompasses both neuropsychological and sensorimotor factors.23 Reaction time testing is generally viewed as cognitively demanding and our findings support this idea, reflected in the modest attenuation of the pain-RT associations with adjustment for cognitive performance. Previously, Weiner and colleagues found that neuropsychological performance mediated the relationship between chronic pain intensity and physical function.55 Studies, including the MBS, have found that people with chronic pain had poorer performance in tests of cognitive function, both in measures of global cognitive function as well as in measures of attention, memory, executive function, and reasoning ability.7,15,29,51 Pain may disrupt mobility by interfering with central neural control pathways that support the automaticity of walking, which are already compromised in old age.53 Pain requires greater use of executive locomotor control strategies for safe walking, a departure from the automaticity of lower body mobility.4 It is possible that our measure of foot RT is capturing executive locomotor control and is less influenced by other domains of cognitive function.
The MBS cohort has a relatively higher education level than general U.S. population.9 Our data show that higher education is strongly associated with faster foot RT in older adults. Older adults with less education seemed most vulnerable to the effects of pain on foot RT. Evidence has shown that education has an important protective role in cognitive abilities in late adulthood.43 Again, the underlying mechanisms remain unclear. A number of theories support that higher education and lifelong cognitive activities provide a neural or cognitive pool of resources.30 Also, advanced education is associated with slower cognitive aging and faster reaction time in adults.50 Thus, highly educated older people might have better ability to perform foot RT through the availability of enhanced neural resources, thus overcoming to some extent the detrimental impact of chronic pain. In addition, older adults with higher education level may have better coping skills to reduce the impacts of pain on mobility.3,38 Attention to this apparent vulnerability of older adults with low education may prove to be important in fall prevention approaches with older adults with chronic pain.
In terms of foot RT measurements, we used a new method to detect initial movement time when participants were seated in a chair. Most studies measured stepping RT in a standing position, which captures not only cognitive and neuromotor aspects of RT but also places demand on balance and mobility. This measure can be influenced by physical impairments that may not be related to RT per se.23,28,33 The seated position that we used in the MBS allowed for a measurement that more specifically reflects cognitive and neuromotor aspects of RT. Also, of note, testing foot RT from a seated position is easier and safer for older adults who have joint pain and poor lower extremity strength or balance.
In some ways, the consistent association between knee pain and CRT was not surprising. For the SRT test, participants could self-select which leg to move but in CRT, participants were instructed to use the leg which corresponded to the light on either side of the mat, forcing them to use the leg with a more painful knee. Knee pain may have a specific impact on the extension of knee joint needed for the RT test performance while seated, thus slowing the initiation of foot movement. It is unclear whether this could reflect the contribution of knee pain to fall risk because the additional impact of weight bearing on the ability to move the knee quickly in response to a fall hazard was not captured in our measure. In the MBS as well as in the NHATS populations, knee pain was not an independent predictor of falls except as part of a multisite pain condition.20,32 In the present study, 80% of people with knee pain reported pain in at least one other site. Our previous study found that participants with polyarticular pain had poorer physical performance, impaired mobility, and an increased risk for falls than those without pain.20 More specifically, for each site of joint pain found to contribute to fall risk, increased risk was only found when polyarticular pain was present in comparisons to persons without pain. Pain in older adults is primarily multisite thus attributing fall risk to individual pain sites rather than the overall burden of pain may not have clinical relevance.
Our findings need to be considered in light of the study limitations. As this is a cross-sectional study, the temporal relation between chronic pain and RT cannot be confirmed based on our findings. Also, the MBS cohort had a somewhat higher education level than the general older population, which could limit the generalizability of the study results.9 Our study sample had limited racial/ethnic diversity, though it was reflective of the older population within the geographic area of the study.21 Another limitation is that the seated reaction time protocol that we used was not validated against other measures of reaction time. Nonetheless, the testing was performed by trained research assistants following a scripted protocol using the well-validated instrumentation of the GAITRite walkway. In the future, a longitudinal study is needed to investigate the relationship between chronic pain and foot RT. Further consideration of other factors including sleep quality and cognitive and neuromuscular factors that might play a role in this relationship is also warranted. To our knowledge, this study is the first to examine the relationship between characteristics of chronic pain and foot RT, a known mobility risk factor for falls among older adults.
In conclusion, chronic musculoskeletal pain is associated with slower foot reaction time, which may be part of the pathway through which pain leads to falls in older adults. Prospective studies examining the role of RT in the pain-falls relationship and in the possible contribution to recurrent falls are needed. A better understanding of the pathway could lead to interventions that potentially could improve foot RT among older adults living with chronic pain as part of a physical therapy or exercise-based intervention to reduce fall risk.
Supplementary Material
Highlights.
Chronic pain is associated with slower foot reaction time in older adults
Education may play a protective role in the impacts of pain on mobility
Chronic pain may contribute to falls through a cognitive and neuromotor pathway
Acknowledgements
We thank the MOBILIZE Boston research staff and study participants for their effort and dedication.
Disclosures: The MOBILIZE Boston study was supported by the National Institute on Aging, NIH: Research Nursing Home Program Project #P01AG004390, and Research Grant #R01AG041525.
Footnotes
The authors do not have any conflict of interest to declare.
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR: Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology 23:500–508, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Buchman AS, Shah RC, Leurgans SE, Boyle PA, Wilson RS, Bennett DA: Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care Res (Hoboken) 62:1287–1293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano A, Mayo A, Ventimiglia M: Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. J Pain 7:459–468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark DJ: Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci 9:1–13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleeland CS, Ryan KM: Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129–138, 1994 [PubMed] [Google Scholar]
- 6.Cutlip RG, Mancinelli C, Huber F, DiPasquale J: Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture 12:134–138, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Eccleston C: Chronic pain and attention: a cognitive approach. Br J Clin Psychol 33:535–547, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Eggermont LHP, Bean JF, Guralnik JM, Leveille SG: Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function.Gerontol A Biol Sci Med Sci 64:763–770, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggermont LHP, Leveille SG, Shi L, Kiely DK, Shmerling RH, Jones RN, Guralnik JM,Bean JF: Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the elderly boston study. Am Geriatr Soc 62:1007–1016, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejupi A, Brodie M, Gschwind YJ, Schoene D, Lord S, Delbaere K: Choice stepping reaction time test using exergame technology for fall risk assessment in older people. Conf Proc IEEE Eng Med Biol Soc 2014:6957–6960, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Ejupi A, Gschwind YJ, Brodie M, Zagler WL, Lord SR, Delbaere K: Kinect-based choice reaching and stepping reaction time tests for clinical and in-home assessment of fall risk in older people: a prospective study. Eur Rev aging Phys Act 13:2, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198, 1975 [DOI] [PubMed] [Google Scholar]
- 13.Gajewski PD, Falkenstein M: Physical activity and neurocognitive functioning in aging - a condensed updated review. Eur Rev aging Phys Act 13:1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB: Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl Med 332:556–561, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins DM, Martin AM, Baker DG, Vasterling JJ, Risbrough V: The relationship between chronic pain and neurocognitive function: a systematic review. Clin J Pain 34:262–275, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CL, Nagamatsu LS, Davis JC, Liu-Ambrose T: Examining the relationship between specific cognitive processes and falls risk in older adults: a systematic review. Osteoporos Int 23:2409–2424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS: Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin Pain 20:309–318, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lajoie Y, Gallagher SP: Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr 38:11–26, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Leveille SG, Guralnik JM, Ferrucci L, Hirsch R, Simonsick E, Hochberg MC: Foot pain and disability in older women. Am J Epidemiol 148:657–665, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, Kiel DP, Lipsitz LA, Bean JF: Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 302:2214–2221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, Kang HG, Samelson EJ, Gagnon M, Freeman M, Lipsitz LA: The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr 8:16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB: Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12:277–287, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lord SR, Fitzpatrick RC: Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol A Biol Sci Med Sci 56:M627–M632, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Lord SR, Menz HB, Tiedemann A: A physiological profile approach to falls risk assessment and prevention. Phys Ther 83:237–252, 2003 [PubMed] [Google Scholar]
- 25.Makris UE, Paul TM, Holt NE, Latham NK, Ni P, Jette A, Leveille SG, Bean JF: The relationship among neuromuscular impairments, chronic back pain, and mobility in older adults. PM R 8:738–747, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIlroy WE, Maki BE: Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol A Biol Sci Med Sci 51:M289–M296, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Messier SP, Glasser JL, Ettinger WHJ, Craven TE, Miller ME: Declines in strength and balance in older adults with chronic knee pain: a 30-month longitudinal, observational study. Arthritis Rheum 47:141–148, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Nishiguchi S, Yamada M, Uemura K, Matsumura T, Takahashi M, Moriguchi T, Aoyama T: A novel infrared laser device that measures multilateral parameters of stepping performance for assessment of fall risk in elderly individuals. Aging Clin Exp Res 25:311–316, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Oosterman JM, Derksen LC, van Wijck AJM, Veldhuijzen DS, Kessels RPC: Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain 27:70–75, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Park DC, Bischof GN: The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci 15:109–119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel KV, Guralnik JM, Dansie EJ, Turk DC: Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain 154:2649–2657, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel KV, Phelan EA, Leveille SG, Guralnik JM, Turk DC: High prevalence of falls, fear of falling, and impaired balance among older adults with pain in the U.S.: findngs from the 2011 National Health and Aging Trends Study. J Am Geriatr Soc 62:1844–1852, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pijnappels M, Delbaere K, Sturnieks DL, Lord SR: The association between choice stepping reaction time and falls in older adults--a path analysis model. Age Ageing 39:99–104, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8:271–276, 1958 [Google Scholar]
- 35.Rogers MW, Johnson ME, Martinez KM, Mille M-L, Hedman LD: Step training improves the speed of voluntary step initiation in aging. J Gerontol A Biol Sci Med Sci 58:46–51, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman AB: Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology 24:8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Rosow I, Breslau N: A Guttman health scale for the aged. J Gerontol 21:556–559, 1966 [DOI] [PubMed] [Google Scholar]
- 38.Roth RS, Geisser ME: Educational achievement and chronic pain disability: mediating role of pain-related cognitions. Clin J Pain 18:286–296, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Sawa R, Doi T, Misu S, Saito T, Sugimoto T, Murata S, Asai T, Yamada M, Ono R: The severity and number of musculoskeletal pain associated with gait in community-dwelling elderly individuals. Gait Posture 54:242–247, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Schepker CA, Leveille SG, Pedersen MM, Ward RE, Kurlinski LA, Grande L, Kiely DK, Bean JF: Effect of pain and mild cognitive impairment on mobility. J Am Geriatr Soc 64:138–143, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah RC, Buchman AS, Boyle PA, Leurgans SE, Wilson RS, Andersson GB, Bennett DA: Musculoskeletal pain is associated with incident mobility disability in community-dwelling elders. J Gerontol A Biol Sci Med Sci 66:82–88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soldato M, Liperoti R, Landi F, Finne-Sovery H, Carpenter I, Fialova D, Bernabei R, Onder G: Non malignant daily pain and risk of disability among older adults in home care in Europe. Pain 129:304–310, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Stern Y: Cognitive reserve. Neuropsychologia 47:2015–2028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stijntjes M, Aartsen MJ, Taekema DG, Gussekloo J, Huisman M, Meskers CGM, De Craen AJM, Maier AB: Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. J Gerontol A Biol Sci Med Sci 72:662–668, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Stubbs B, Binnekade T, Eggermont L, Sepehry AA, Patchay S, Schofield P: Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Arch Phys Med Rehabil 95:175–187.e9, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Stubbs B, Schofield P, Binnekade T, Patchay S, Sepehry A, Eggermont L: Pain is associated with recurrent falls in community-dwelling older adults: evidence from a systematic review and meta-analysis. Pain Med 15:1115–1128, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Thapa S, Shmerling RH, Bean JF, Cai Y, Leveille SG: Chronic multisite pain: evaluation of a new geriatric syndrome. Aging Clin Exp Res 31:1129–1137, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tijsma M, Vister E, Hoang P, Lord SR: A simple test of choice stepping reaction time for assessing fall risk in people with multiple sclerosis. Disabil Rehabil 39:601–607, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Tinetti ME, Richman D, Powell L: Falls efficacy as a measure of fear of falling. J Gerontol 45:239–243, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Tun PA, Lachman ME: Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Dev Psychol 44:1421–1429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Leeuw G, Eggermont LHP, Shi L, Milberg WP, Gross AL, Hausdorff JM, Bean JF, Leveille SG: Pain and cognitive function among older adults living in the community. Gerontol A Biol Sci Med Sci 71:398–405, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Leeuw G, Leveille SG, Dong Z, Shi L, Habtemariam D, Milberg W, Hausdorff JM, Grande L, Gagnon P, McLean RR, Bean JF: Chronic pain and attention in older community-dwelling adults. J Am Geriatr Soc 66:1318–1324, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.VanSwearingen JM, Studenski SA: Aging, motor skill, and the energy cost of walking: implications for the prevention and treatment of mobility decline in older persons. J Gerontol A Biol Sci Med Sci 69:1429–1436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster KE, Wittwer JE, Feller JA: Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture 22:317–321, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S: The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med 7:60–70, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Woo A, Lechner B, Fu T, Wong CS, Chiu N, Lam H, Pulenzas N, Soliman H, DeAngelis C, Chow E: Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med 4:176–183, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Wood KM, Edwards JD, Clay OJ, Wadley VG, Roenker DL, Ball KK: Sensory and cognitive factors influencing functional ability in older adults. Gerontology 51:131–141, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.