Abstract
Despite reductions in smoking rates in the general population, little is known about recent smoking trends among people living with HIV (PLWH). We compared the risk for smoking and temporal trends in smoking among PLWH and the general population in the Philadelphia metropolitan area between 2009 and 2014. We used weighted logistic regression to assess the relation between HIV and smoking, and examined temporal smoking trends. The adjusted odds ratio (OR) for smoking comparing PLWH to the general population was 1.80 (95% CI 1.55-2.09) after adjusting for socio-economic, demographic, and mental health diagnosis variables. Smoking prevalence decreased in both the PLWH and general populations during the study period, and we did not observe a significant difference in rates of decline between groups (P = 0.54). Despite overall progress in smoking cessation, a disparity persisted in smoking rates between PLWH and the general population, with and without adjustment for socio-economic, demographic, and mental health variables. Further research is needed to understand the mechanisms linking HIV and tobacco use in order to inform public health efforts to reduce smoking among PLWH.
Keywords: Smoking, smoking cessation, tobacco use, HIV infection
Introduction
Cigarette smoking is the leading preventable cause of death in the United States, resulting in 480,000 deaths annually (1). In recent years, the prevalence of cigarette smoking has declined significantly in the general US population, from 20.9% in 2005 to 15.5% in 2016 (2). Multi-level factors have likely contributed to this trend, including implementation of population-based interventions such as smoke-free laws, anti-smoking media campaigns, and increased availability and use of tobacco cessation treatment (3). However, disparities in tobacco use persist by geographic region, race/ethnicity, and socioeconomic status (4).
Among people living with HIV (PLWH), rates of tobacco use are two to three times higher than the general population in the United States (5). Estimates of smoking prevalence among PLWH reach 40 to 70% in some studies (6). Smoking among PLWH increases the risk for cancer, cardiovascular disease, and respiratory diseases (7, 8). Furthermore, in settings where anti-retroviral therapy (ART) is readily available, HIV-positive smokers may now lose more lifeyears to smoking than to HIV infection (9).
Despite overall progress in smoking cessation in the general population, little is known about smoking trends among PLWH. Given elevated baseline rates of smoking among PLWH, the effect of smoking cessation programs may have a more dramatic effect among PLWH, particularly given targeted efforts to reduce smoking among PLWH. On the other hand, prior research has indicated that PLWH may have lower quit rates compared to uninfected individuals, but did not compare trends over time (5, 10). Therefore, it is unknown whether PLWH are making proportionate gains in smoking cessation compared with the general population. In addition, given numerous potential confounding and/or mediating variables between HIV and smoking, it is unclear whether HIV infection may be an independent risk factor for smoking. Therefore, our primary aim was to compare the risk for smoking between PLWH and the general population, while controlling for socio-economic, demographic, and mental health variables that are associated with both smoking and HIV infection. Our secondary aim was to examine and compare temporal trends in smoking between PLWH and the general population in a large US city.
Methods
We used two health surveillance surveys to investigate cigarette smoking in the general population and among PLWH in the Philadelphia metropolitan area. The PLWH population was represented by annual population-based surveys (2009-2014) from the Philadelphia Medical Monitoring Project (MMP), which sampled persons receiving care from HIV medical facilities. The MMP used a three-stage sampling process with a probability proportion-to-size sampling design, as has been described elsewhere (11). The general population was represented by the Public Health Management Corporation’s Southeastern Pennsylvania Household Health Survey (HHS) in 2008, 2010, 2012, and 2014 (12). The HHS used a random-digit telephone survey across five counties in the Philadelphia metropolitan area stratified by 54 service areas.
The MMP data included self-reported survey data collected via a standardized survey by trained interviewers, as well as data collected via medical record abstraction. Characteristics included age at survey, sex at birth, race/ethnicity, highest education level, poverty status, health insurance status, incarceration in the past 12 months, housing status, and alcohol and injection drug use. Mental health diagnosis in the MMP was dichotomized as any diagnosis of clinical depression, anxiety disorder, bipolar disorder, psychosis in the medical record, or a score of 10 or greater in the Patient Health Questionnaire-8 administered during the survey, which has been previously validated as a marker of current depression (13). The HHS data included only self-reported survey data collected via a standardized survey by trained interviewers including age at survey, sex at birth, race/ethnicity, highest education level, poverty level, health insurance status, and mental health diagnosis (defined as any diagnosis of clinical depression, anxiety disorder, bipolar disorder, psychosis).
Both the MMP and HHS data were obtained from complex multi-year survey design where the probability of selection was proportional to the population density within each sampling stratum. In each yearly dataset, every subject was assigned a survey sampling weight to account for project area size and survey nonresponse. For each survey, analyses that pooled data across all years used and assigned each subject an overall sampling weight, calculated as the original weight over the number of years of analysis (six for MMP and four for HHS) (14). Sampling weights were standardized so that they summed to their respective population sample sizes.
The primary outcome variable for analysis was current smoking status. Current smoking status was determined by participants who reported yes to both of the following questions: (1) "Have you smoked at least 100 cigarettes in your entire life?" (MMP and HHS) and (2) “How often do you smoke cigarettes now?” (MMP: daily, weekly, monthly, less than monthly; HHS: Everyday, Some days). Non-current smokers included former smokers.
We assumed that data collected through MMP and HHS were independent samples with separate design variables and weights. We retained subjects’ yearly and overall sampling weights from their original survey. We included individual-level data from both datasets and the following socio-demographic characteristics: age, sex at birth, race/ethnicity, education, poverty level, health insurance status, and mental health diagnosis.
Data Analysis
We conducted univariable and multivariable weighted logistic regression to examine demographic and clinical factors associated with being a current smoker. In our adjusted analyses, we controlled for age, sex, race/ethnicity, education, poverty, insurance, and mental health diagnosis. We used weighted logistic regression to examine linear trends in smoking rates from 2009 to 2014. In order to compare differences in the rate of change of smoking between PLWH and the general population, we used an interaction term between HIV status and year in the model. These models were fit using the SURVEYLOGISTIC procedure in SAS.
For sensitivity analysis, we refit the models using a propensity score matched dataset. This dataset included HIV-positive individuals matched to individuals from the general population using a 1-to-1 propensity score nearest neighbor matching method with the MatchIt package in R (R Core development team, 2019; Vienna, Austria). The propensity score was defined as the conditional probability of being in the HIV-positive cohort given observed covariates. Propensity scores for each subject without missing covariate information were calculated using the covariates age, sex, race/ethnicity, education, poverty, insurance status, and mental health diagnosis.
Statistical analyses were conducted using R software version 3.6.0 (R Core development team, 2019; Vienna, Austria) and SAS, Version 9.4 (SAS Institute Inc., 2013; Cary, NC) and statistical significance was set at P = 0.05 or less.
Results
A total of 1,295 PLWH and 40,079 individuals in the general population were available in the respective surveys. Nine PLWH in the MMP dataset (0.7%) and 223 (0.6%) in the HHS dataset had missing primary outcome data and were excluded. The remaining 1,286 and 39,856 individuals had sampling weights to create our final weighted sample of 9,750 in the PLWH population and 3,048,906 in the general population (Table 1). The PLWH population was predominately male (68%), Non-Hispanic Black (62%), at least 40 years of age (75%), and roughly half of PLWH had incomes below the federal poverty level (51%) and had a mental health diagnosis (49%). In comparison, the general population was predominantly female (54%), Non-Hispanic White (67%), had a larger range in age, and most were at or above the federal poverty level (88%) and did not have a mental health diagnosis (83%). Unweighted population characteristics are shown in Appendix Table 1, and additional characteristics of the PLWH population are shown in Appendix Table 2.
Table 1:
Characteristics of the PLWH and general survey weighted populations with known smoking status over all survey years from the Medical Monitoring Project (MMP) and HHS Household Health Survey (HHS) respectively.
| Characteristic | PLWH MMP N =9,750 n (%) |
General HHS N =3,048,906 n (%) |
||
|---|---|---|---|---|
| Nonsmoker N=5,315 (54.5) |
Smoker N=4,435 (45.5) |
Non-smoker N = 2,468,928 (81.0) |
Smoker N = 579,978 (19.0) |
|
| Age (years) | ||||
| 18-29 | 647 (12.2) | 411 (9.3) | 397,300 (16.1) | 114,305 (19.7) |
| 30-39 | 759 (14.3) | 644 (14.5) | 368,528 (14.9) | 102,238 (17.6) |
| 40-49 | 1,338 (25.2) | 1,604 (36.2) | 377,963 (15.3) | 105,542 (18.2) |
| >49 | 2,571 (48.4) | 1,776 (40.0) | 1,305,864 (52.9) | 255,233 (44.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 19,274 (0.8) | 2,660 (0.5) |
| Sex at birth | ||||
| Male | 3,748 (70.5) | 2,888 (65.1) | 1,117,690 (45.3) | 290,251 (50.0) |
| Female | 1,567 (29.5) | 1,532 (34.5) | 1,351,238 (54.7) | 289,727 (50.0) |
| Other/Unknown | 0 (0.0) | 15 (0.3) | 0 (0.0) | 0 (0.0) |
| Race/Ethnicity | ||||
| Non-Hispanic white | 1,211 (22.8) | 757 (17.1) | 1,661,701 (67.3) | 350,292 (60.4) |
| Non-Hispanic black | 3,127 (58.8) | 2,963 (66.8) | 459,642 (18.6) | 155,200 (26.8) |
| Hispanic/Latino | 825 (15.5) | 570 (12.9) | 170,859 (6.9) | 43,014 (7.4) |
| Other/Unknown | 152 (2.9) | 145 (3.3) | 176,726 (7.2) | 31,472 (5.4) |
| Education | ||||
| Less than high school | 1,071 (20.1) | 1,459 (32.9) | 149,475 (6.1) | 76,739 (13.2) |
| High school/GED | 1,749 (32.9) | 1,602 (36.1) | 647,008 (26.2) | 243,575 (42.0) |
| More than high school | 2,495 (46.9) | 1,375 (31.0) | 1,659,360 (67.2) | 257,525 (44.4) |
| Unknown | 0 (0.00) | 0 (0.0) | 13,085 (0.5) | 2,139 (0.4) |
| Poverty level | ||||
| At or above federal | 2,944 (55.4) | 1,545 (34.8) | 2,231,057 (90.4) | 457,035 (78.8) |
| Below federal | 2,150 (40.5) | 2,812 (63.4) | 237,206 (9.6) | 122,579 (21.1) |
| Unknown | 221 (4.2) | 78 (1.8) | 666 (0.0) | 364 (0.1) |
| Health insurance status | ||||
| Not insured | 345 (6.5) | 256 (5.8) | 123,132 (5.0) | 59,080 (10.2) |
| Insured | 4,963 (93.4) | 4,179 (94.2) | 2,295,129 (93.0) | 494,915 (85.3) |
| Unknown | 7 (0.1) | 0 (0.0) | 50,667 (2.0) | 25,983 (4.5) |
| Mental health diagnosis | ||||
| No | 3,086 (58.1) | 1,849 (41.7) | 2,123,661 (86.0) | 408,600 (70.5) |
| Yes | 2,229 (41.9) | 2,586 (58.3) | 335,694 (13.6) | 168,565 (29.1) |
| Unknown | 0 (0.0) | 0 (0.0) | 9,573 (0.4) | 2,813 (0.5) |
Overall, an estimated 45.5% (95% CI 42.6, 48.3) and 19.0% (95% CI 17.6, 20.5) of PLWH and of the general population in the Philadelphia metropolitan area reported current smoking, respectively. Among current smokers, 86.3% (95% CI 83.7, 88.9) of PLWH and 70.4% (95% CI 68.6, 72.1) of the general population reported smoking daily. In unadjusted analyses, PLWH were 3.55 (95% CI 3.06, 4.12) times as likely to report current smoking compared to the general population. In analyses adjusting for age, sex, race/ethnicity, education, poverty, health insurance, and mental health diagnoses, PLWH were 1.80 (95% CI 1.55, 2.09) times more likely to be a current smoker compared with the general population. When we removed mental health diagnoses from the model, PLWH were 2.29 (95% CI 1.98, 2.64) times more likely to be a current smoker compared with the general population. In a sensitivity analysis using propensity-score matched data, we obtained attenuated, but still statistically significant elevated rates of smoking for PLWH compared to the general population with an OR 1.41 (95% CI 1.21, 1.76).
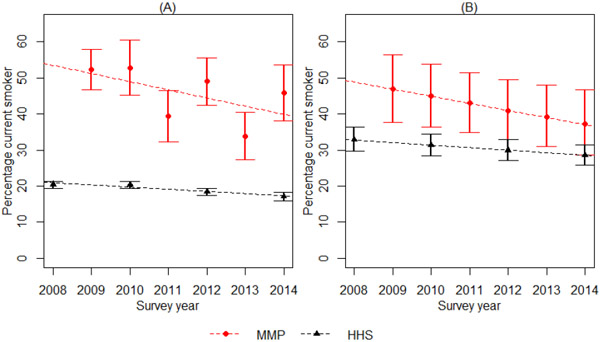
Smoking status declined statistically significantly over time in both groups with the unadjusted OR of smoking per year for PLWH of 0.91 (95% CI 0.85, 0.98) and the general population of 0.96 (95% CI 0.95, 0.98) (Figure 1). Covariate adjusted ORs were similar and still statistically significant: 0.92 (95% CI 0.86, 0.99) and 0.97 (95% CI 0.95, 0.98), in the PLWH population and general population, respectively. Coefficient estimates and standard errors for each covariate are shown in Appendix Table 3. Finally, we compared the change in smoking rates by HIV status. There was no statistically significant difference between the rates of decline in smoking status between PLWH and the general population, as there was no indication of effect modification in the slope of decline by HIV status in either the unadjusted (β = −0.026, P = 0.15) or adjusted (β = −0.012, P = 0.54) analyses.
Figure 1:
Current smoking prevalence estimates and trend by survey year among PLWH and general population
Panel A shows total population estimates from the unadjusted models, and Panel B shows model estimates for a person who is aged 40-49, Male, non-Hispanic black, high school educated, at or above federal poverty, health insured, and has no mental health diagnosis. MMP = Medical Monitoring Project, representing people living with HIV. HHS = Household Health Survey, representing the general population.
Discussion
We found that rates of current smoking among PLWH were persistently higher than the general population even after controlling for known smoking risk factors. We observed commensurate declines in smoking rates in both PLWH and the general population during the study period. Although the rate of decline over the study period was slightly steeper among PLWH compared to the general population, the observed slopes were not statistically different between the two groups.
This study supports evidence that HIV infection itself may be a risk factor for smoking, and smoking cessation interventions specifically tailored for PLWH may be needed. Prior studies have suggested that higher rates of current smoking among PLWH may be related to socio-demographic factors such as higher rates of poverty, racial/ethnic disparities, and educational disparities mental health diagnoses, and other risk factors associated with HIV infection (10, 15). However, we found that after controlling for socio-demographic and mental health co-morbidities, PLWH were still more likely to report smoking compared to the general population. Although we were likely unable to account for all unmeasured confounders, this finding is important because it suggests that these socio-demographic factors alone may not explain higher rates of smoking among PLWH. Potential biological mechanisms that explain higher rates of smoking may be faster rates of nicotine metabolism among HIV-positive smokers (16), which has been shown to be associated with severity of nicotine dependence and greater difficulty quitting (17, 18). For instance, use of the antiretroviral drug efavirenz, has been associated with significantly faster nicotine metabolism among HIV-positive smokers (19). Prior research has also demonstrated that PLWH may have more difficulty quitting smoking compared to the general population, and the efficacy of smoking cessation pharmacotherapy may be diminished among PLWH (20, 21). In addition, psychosocial factors (22), internalized HIV stigma (23), and neurocognitive differences (24) associated with HIV may have a significant impact on ongoing tobacco use. Further research is needed to elucidate the mechanisms linking HIV and smoking, which can help inform smoking cessation therapies for PLWH.
The decline in rates of cigarette smoking among PLWH and the general population we observed is similar to previously published literature that examined smoking among PLWH (10). Rates of smoking in PLWH and the general population in our study were comparable to previously reported national rates (5). Our study builds upon these prior data by examining differences in the rates of decline between PLWH and the general population. We did not observe a statistically significant difference in the rates of decline, and observed a persistent disparity in smoking rates between PLWH and the general population during all time points. Smoking cessation interventions that focus specifically on HIV-positive smokers are needed to reduce this disparity and accelerate smoking cessation among PLWH. Novel smoking cessation strategies tailored for PLWH include use of mobile technologies and telehealth counseling (25, 26), targeting the neurocognitive complications of HIV (24), treatment of concomitant substance use disorders (27), and tailoring smoking cessation pharmacotherapies and ART regimens for PLWH (19). Moreover, provider-facing interventions have focused on implementing smoking cessation services across a broad range of clinical settings for PLWH (6, 28).
Several limitations and strengths are worth discussion. Similar to other studies comparing smoking rates in PLWH and the general population, the temporal trend relied on an ecological design. Caution must thus be exercised in making causal inferences based on these findings. Second, our study was limited to a single metropolitan area, thus limiting potential generalizability. However, given observed geographic disparities in smoking rates and variable tobacco-control policies based on region (29), a strength of our study is that it was not subject to potential geographic confounding. Third, PLWH were drawn from the MMP, who may not be representative of all PLWH and may introduce sampling bias with regard to co-morbidities such as tobacco use, mental health diagnoses, and socio-economic status. In addition, PLWH in the MMP were already engaged in HIV care and may not reflect PLWH not on ART. Further research is needed to elucidate the role of ART and viral suppression on smoking cessation among PLWH. Sampling designs were different for the two populations and used different weights. In order to obtain less biased estimates in our joint analysis, we ensured that the sampling weights were standardized such that they sum to their respective population sample size. In addition, there were differences in data collection on certain variables such as mental health between the two surveys, potentially introducing measurement bias. Despite controlling for multiple potential confounding variables, it is likely that there were still unmeasured confounders, such as substance use and housing status, that could not be controlled for in our analysis due to unavailability in the HHS dataset. Nonetheless, a significant strength of our study was that we controlled for confounding of multiple factors in the multivariable analysis, and conducted a sensitivity analysis with propensity score matching, which generated similar results.
In summary, we observed a persistent disparity in smoking rates between PLWH and the general population. This disparity persisted despite overall progress in smoking rates in both groups and after controlling for multiple concomitant factors. This finding supports evidence that HIV itself may be a risk factor for ongoing tobacco use, and highlights the need for further research that explores potential mechanisms linking HIV and smoking. This research can inform smoking cessation interventions specifically tailored for PLWH, in order to reduce the significant morbidity and mortality burden associated with smoking among PLWH.
Supplementary Material
Acknowledgments
Sources of funding: This research was supported by the Centers for Disease Control and Prevention through Cooperative Agreement 6 NU62PS004960 to the Philadelphia Department of Public Health (PI: Kathleen A. Brady) This research was also supported by core services and support from the Penn Center for AIDS Research (P30 AI045008) and the Penn Mental Health AIDS Research Center (P30 MH097488).
Footnotes
Conflicts of Interest: Dr. Gross serves on a Data and Safety Monitoring Board for Pfizer Inc for medication trials unrelated to HIV or smoking.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking--50 Years of Progress: A Report of the Surgeon General. Atlanta; 2014. Available at: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf Accessed October 14, 2019. [Google Scholar]
- 2.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titus AR, Kalousova L, Meza R, Levy DT, Thrasher JF, Elliott MR, et al. Smoke-Free Policies and Smoking Cessation in the United States, 2003-2015. Int J Environ Res Public Health. 2019;16(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Smoking is down, but almost 38 million Americans still smoke. 2018. Available at: https://www.cdc.gov/media/releases/2018/p0118-smoking-rates-declining.html. Accessed October 12, 2019.
- 5.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–44. [DOI] [PubMed] [Google Scholar]
- 6.Pacek LR, Cioe PA. Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV. Curr HIV/AIDS Rep. 2015;12(4):413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacek LR, Crum RM. A Review of the Literature Concerning HIV and Cigarette Smoking: Morbidity and Mortality, Associations with Individual- and Social-Level Characteristics, and Smoking Cessation Efforts. Addict Res Theory. 2015;23(1):10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy KP, Kong CY, Hyle EP, Baggett TP, Huang M, Parker RA, et al. Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States. JAMA Intern Med. 2017;177(11):1613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–34. [DOI] [PubMed] [Google Scholar]
- 10.Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009-2014. Prev Med. 2018;111:231–4. [DOI] [PubMed] [Google Scholar]
- 11.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD. Clinical and behavioral characteristics of adults receiving medical care for HIV infection --- Medical Monitoring Project, United States, 2007. MMWR Surveill Summ. 2011;60(11):1–20. [PubMed] [Google Scholar]
- 12.Public Health Management Corporation. Community Health Data Base. Public Health Management Coorporation; 2018. Available at: https://chdbdataportal.phmc.org/. Accessed November 4, 2019. [Google Scholar]
- 13.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect. 2009;114(1):163–73. [DOI] [PubMed] [Google Scholar]
- 14.Iachan R, Johnson CH, Harding RL, Kyle T, Saavedra P, Frazier EL, et al. Design and Weighting Methods for a Nationally Representative Sample of HIV-infected Adults Receiving Medical Care in the United States-Medical Monitoring Project. Open AIDS J. 2016;10:164–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regan S, Meigs JB, Grinspoon SK, Triant VA. Determinants of Smoking and Quitting in HIV-Infected Individuals. PLoS One. 2016;11(4):e0153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashare RL, Thompson M, Leone F, Metzger D, Gross R, Mounzer K, et al. Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS. 2019;33(6):1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allenby CE, Boylan KA, Lerman C, Falcone M. Precision Medicine for Tobacco Dependence: Development and Validation of the Nicotine Metabolite Ratio. J Neuroimmune Pharmacol. 2016;11(3):471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota T, Nakajima-Taniguchi C, Fukuda T, Funamoto M, Maeda M, Tange E, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006;6(2):115–9. [DOI] [PubMed] [Google Scholar]
- 19.Schnoll RA, Thompson M, Serrano K, Leone F, Metzger D, Frank I, et al. Brief Report: Rate of Nicotine Metabolism and Tobacco Use Among Persons With HIV: Implications for Treatment and Research. J Acquir Immune Defic Syndr. 2019;80(2):e36–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashare RL, Thompson M, Serrano K, Leone F, Metzger D, Frank I, et al. Placebocontrolled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug Alcohol Depend. 2019;200:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercié P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV. 2018;5(3):e126–e35. [DOI] [PubMed] [Google Scholar]
- 22.Webb MS, Vanable PA, Carey MP, Blair DC. Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. J Behav Med. 2007;30(5):371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamarel KE, Finer Z, Resnicow K, Green-Jones M, Kelley E, Jadwin-Cakmak L, et al. Associations Between Internalized HIV Stigma and Tobacco Smoking Among Adolescents and Young Adults Living with HIV: The Moderating Role of Future Orientations. AIDS Behav. 2020;24(1):165–72. [DOI] [PubMed] [Google Scholar]
- 24.Harrison JD, Dochney JA, Blazekovic S, Leone F, Metzger D, Frank I, et al. The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV. J Neurovirol. 2017;23(4):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4(4):Cd006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2012;14(1):106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnall R, Carcamo J, Porras T, Huang MC, Webb Hooper M. Use of the Phase-Based Model of Smoking Treatment to Guide Intervention Development for Persons Living with HIV Who Self-Identify as African American Tobacco Smokers. Int J Environ Res Public Health. 2019;16(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledgerwood DM, Yskes R. Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis. Nicotine Tob Res. 2016;18(12):2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.