ABSTRACT
Background
Soy is commonly consumed in east Asian countries and is suggested to reduce colorectal cancer (CRC) risk. However, results from epidemiologic studies are inconsistent, despite the anti-inflammatory and antiproliferative properties of soy isoflavones and soy protein.
Objective
We evaluated the association between soy isoflavones and soy protein and CRC risk using 4 prospective cohort studies from China and Japan.
Methods
Data were pooled from the Shanghai Women's Health Study (SWHS), Shanghai Men's Health Study (SMHS), Japan Public Health Center–based Prospective Study Cohort 1 (JPHC1), and Cohort 2 (JPHC2). Cox proportional hazards models estimated HRs and corresponding 95% CIs for the association of soy protein and isoflavone intake with CRC risk. The study included 205,060 individuals, among whom 2971 were diagnosed with incident CRC over an average follow-up of 12.7 y.
Results
No statistically significant associations with CRC risk were observed for soy protein or isoflavone intake. No association was observed among ever smokers consuming higher isoflavones (HRisoflavones: 0.83; 95% CI: 0.68, 1.00) and soy protein (HRsoy protein: 0.81; 95% CI: 0.39, 1.10). However, risk reductions were observed among premenopausal women with a body mass index [BMI (kg/m2)] <23.0 at baseline for higher isoflavone (HRisoflavones: 0.58, 95% CI: 0.34, 0.98).
Conclusions
No evidence for an overall reduction in CRC risk by increasing soy food intake (i.e., protein or isoflavones) was observed. However, the association between soy and CRC risk may vary by BMI, smoking, and menopausal status among women. Future investigations are needed to further understand the biologic mechanisms observed.
Keywords: soy, isoflavones, colorectal cancer, cohort, Asian, diet, China, Japan
Introduction
Foods containing soy are commonly consumed in Asian countries, including China and Japan. Tofu, miso, and natto contain high amounts of soy and are integral components of Chinese and Japanese diets. Soybean is rich in protein and other phytochemicals, such as isoflavones, is low in carbohydrates, and is a good source of essential fatty acids in Asian countries (1). In East Asian diets, soybean is also a predominant source of phytoestrogen isoflavones (2). The majority of phytoestrogen intake in Western populations is obtained via lignans; however, in Asian populations high amounts of phytoestrogens are consumed due to the soybean-rich diet. In fact, among Asian populations mean isoflavone intake is almost 10 times higher than that in Western countries (1, 3, 4). Thus, additional studies are important to increase understanding of the effects of this commonly consumed nutritional exposure on cancer outcomes among Asian populations.
Soy protein and isoflavones have been reported to have potential preventive effects on cancer in some in vitro and in vivo experiments (5, 6). Specifically, in studies using human colon cancer cell lines, the peptide lunasin (for which soybean is the primary source) has been reported to reduce cancer cell proliferation and migration (7, 8), regulate the cell cycle (9, 10), and have anti-inflammatory effects (11). Soy proteins contain the highest concentration of lunasin (compared with soy isolates and hydrolyzates); in contrast, soy isoflavones may contain trace amounts of lunasin (10) and may have preventive properties via a different biologic mechanism. As phytoestrogens, isoflavones have a structure similar to that of estrogen and selectively bind to the estrogen receptor (ER), producing an antiestrogenic response in the presence of endogenous estrogen (12, 13) and possibly acting as a preventive agent for hormone-related cancers. In an estrogen-deprived environment, however, phytoestrogens could exert estrogenic effects. There is evidence that estrogen prevents colon tumors in animal models (14–16), in which conversion of estradiol (14, 16) and resulting increased expression of vitamin D receptors (15) has purported protective effects in the colon. Furthermore, isoflavones possess additional anti-inflammatory properties which may also affect cancer development (17). The observed benefits of soy consumption may also be dependent on the presence of certain gut bacteria responsible for converting isoflavones into equol (18), resulting in metabolites with greater anti-inflammatory potential than their precursor isoflavones (19). Among Asians, soy food is typically high in aglycons, which are absorbed faster than glycosides, and as a result may be more easily converted into equol (20, 21). Thus, increasing our understanding of the potential benefits of soy consumption in cancer prevention is important given that soy food is a major component of typical Asian diets, particularly in Chinese and Japanese populations.
Previous epidemiologic studies examining soy food intake among Asians have reported reduced risks for breast cancer (22) and prostate cancer (23). An ∼25% reduction in risk was observed for the association between phytoestrogens and colorectal cancer (CRC) (24); however, the 25% risk reductions were more evident in case–control studies, whereas among cohort studies a null association was observed, suggesting the possibility for differential recall bias in case–control studies. The Shanghai Women's Health Study (SWHS) and the Japan Public Health Center–based Prospective Study Cohort 1 (JPHC1) previously reported results supporting potential CRC risk reductions in men in the highest quantile of isoflavone intake (compared with the lowest) and soy food intake among women (highest quantile compared with the lowest) in Shanghai (25) and Japan (26). However, these previous analyses were based on a short-term follow-up and small numbers of CRC cases, preventing an in-depth evaluation of the association of soy food intake with CRC risk and potential modifiers of this association. Considering that Chinese and Japanese populations have similar soy food intake habits and may have similar long-term effects of soy food intake, we sought to address previous limitations using a large pooled analysis of 4 population-based prospective cohorts, with a long follow-up period, comprising >200,000 individuals from Chinese and Japanese populations.
Methods
Study population
Our study utilized the resources of 4 large population-based cohort studies conducted in China and Japan. The cohort studies from China included the SWHS (n = 73,263) and the Shanghai Men's Health Study (SMHS, n = 61,433). These cohorts were pooled with cohort studies from Japan, which included the JPHC1 (n = 40,993) and the JPHC2 (n = 52,652). Details regarding each study are provided elsewhere (27–29). Briefly, the SWHS and SMHS recruited study participants who were permanent residents of Shanghai in 1997–2000 and 2002–2006, respectively. JPHC1 was initiated in 1990 and participants were recruited from the Iwate, Akita, Nagano, Okinawa, and Tokyo, whereas JPHC2 started in 1993 and participants were recruited from Ibaraki, Niigata, Kochi, Nagasaki, Okinawa, and Osaka. Incident cases of CRC in the Shanghai cohorts were identified during follow-up via annual linkage with the population-based Shanghai Cancer Registry and the Shanghai Vital Statistics Registry. For the Japanese cohorts, CRC cases were identified via surveillance of major local hospitals and population-based cancer registries. Due to unavailable data on cancer incidence, Tokyo area was excluded. Individuals with a prior history of cancer were excluded from all 4 cohorts to estimate incidence of first, primary CRC. The Shanghai cohorts were approved by the institutional review boards for human research in both China and the United States. Similarly, the Japanese cohort study protocols were approved by the institutional review board of the National Cancer Center, Tokyo, Japan.
Analytic cohort
All variables relevant to the analysis were harmonized prior to pooling. After pooling, a total of 228,341 participants were included in the project. Person-years of follow-up for each participant were calculated by counting the number of years on study, starting at completion of the FFQ (baseline for Shanghai cohorts and at 5-y follow-up for Japanese cohorts) and censored using the following criteria (whichever came first): 1) date of CRC diagnosis (event); 2) date of other cancer diagnosis; 3) date of death; 4) date lost to follow-up; or 5) date of study end. Twelve individuals were missing data on the last follow-up date and thus were excluded from the analysis.
Additional exclusions were made to create the analytic cohort for our study. First, to account for potential reverse causation, those with >1 y of follow-up time were excluded from the analysis (n = 2189). Individuals without dietary data (n = 1040, only in JPHC1 and JPHC2), and those with total caloric intake 3 SDs above or below the log-transformed cohort- and sex-specific means (n = 1819) were further excluded. We also excluded women who were current users of hormone replacement therapy or unknown users (n = 5776), had existing diabetes (n = 12,376), or were diagnosed with familial adenomatous polyposis at baseline (n = 69, only SWHS) given that these conditions could potentially influence soy intake. After exclusions, the final analytic cohort included a total of 205,060 individuals. Details regarding exclusions by study cohort are provided in Supplemental Table 1.
Soy intake
In the Shanghai cohorts, diet was assessed at baseline using a quantitative FFQ with 11 specific items dedicated to assessing soy foods. Specific nutrient intake was calculated using values obtained from the Chinese Food Composition Tables (25). In the Japanese cohorts, among those aged 45–74 y, self-administered 138-item FFQs were conducted in 5-y follow-up surveys, which were considered our baseline FFQ values and the start of follow-up for the Japanese cohorts. Specific values for dietary isoflavone and soy protein intake were calculated for each cohort using values from food composition tables in Japan (30–32). Additional details regarding the assessment of soy intake in the JPHC1 and JPHC2 cohorts can be found elsewhere (26). Dietary assessments of soy using these questionnaires were previously validated, and in comparison with 24-h recalls, the correlation coefficients for soy foods ranged from 0.48 to 0.54 (33–36). In our analysis presented here, we focused on soy isoflavone and soy protein intake as a proxy for overall soy food intake. Quartiles based on the distribution of isoflavone (milligrams per day) and soy protein (grams per day) intakes were analyzed using cohort- and sex-specific cut points (Supplemental Table 2).
Statistical analysis
Cox proportional hazards models were used to estimate adjusted HRs and 95% CIs for CRC incidence. No violation of the proportional hazards assumption was observed when interactions between the exposure and time were examined. Schoenfeld residuals were used to evaluate proportional hazards in the multivariate models. Statistically significant correlations between covariate-specific Schoenfeld residuals and person-years of follow-up were identified for physical activity, vegetable intake, red meat intake (only among females), and caloric intake (only in JPCH1, SWHS, and SMHS). Thus, these variables were adjusted for using a multiplicative interaction with person-years of follow-up as a covariate in relevant models.
Time on study was calculated for each study participant as mentioned above and used as the time scale in the analysis. All models were adjusted for the baseline covariates age (continuous), energy (i.e., kilocalories), and cohort. We additionally conducted a multivariate model in which the following additional confounders at baseline (identified using a directed acyclic graph) were included: smoking (current, former, or never), alcohol consumption [heavy intake (>28 g/d in men and >14 g/d in women), moderate (≤28 g/d in men and ≤14 g/d in women), and no intake], physical activity (cohort- and sex-specific quartiles of MET-hours per week), BMI (<18.5, 18.5–22.9, 23–27.49, and ≥27.5 kg/m2), family history of CRC (yes or no), red meat intake (g/d), folate intake (μg/d), vegetable intake (g/d), and menopausal status for women (premenopausal or postmenopausal). For the multivariate models, a complete case analysis was used in which any missing covariate information was excluded from the models. The percentage of missing data was low for the covariates with missing data (i.e., physical activity 6.8%, alcohol consumption 1%, BMI 1%, and smoking status 2.3%). Given low amounts of missing data, we imputed the cohort-specific median values. Results from the multivariate models via a complete case analysis approach and via imputed values for missing covariates were nearly identical. The final analytic sample size for the multivariate models was 186,755. In addition to estimating HRs and 95% CIs for quartiles of dietary isoflavone and soy protein, we also assessed the association using a continuous variable and estimated HRs representing 1 SD intake of isoflavone (i.e., 29.4 mg/d) and soy protein intake (i.e., 6.6 g/d) presented in Supplemental Table 2. The shape of the dose–response curve was also evaluated using restricted cubic splines (Supplemental Figure 1). Effect measure modification by age, smoking status, BMI, and menopausal status was also examined. Statistically significant differences were evaluated on the multiplicative scale using nested models for the interaction term. All analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute Inc.).
Results
The pooled analysis of 4 cohorts included a total of 205,060 individuals with a mean follow-up time of 12.7 y after assessment of soy intake. A total of 2971 cases of incident CRC were diagnosed over the follow-up period. The pooled analysis consisted of participants who were nearly 55 y old on average at baseline (Table 1). Smoking and alcohol intake were more prevalent among males than females in all cohorts. Nearly 60% of Shanghai men reported to be current smokers, which was higher than among Japanese men (∼41–47%). Japanese men consumed more alcohol (>70 g/d) than Shanghai men (34 g/d) on average. Approximately 62% of the women in the pooled cohort were postmenopausal. The median daily intakes of isoflavone and soy protein intake were nearly 30 mg and 8 g per d, respectively, and were similar across the 4 cohorts. Details regarding cohort-specific cutpoint values for isoflavone and soy protein intake are provided in Supplemental Table 2.
TABLE 1.
Baseline characteristics of the participating cohorts from China and Japan1
| JPHC1 | JPHC2 | SMHS | SWHS | ||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Men | Women | Men | Women | Men | Women | Total |
| Cohort profile | |||||||
| Participants, n | 17,014 | 18,718 | 21,239 | 23,461 | 56,786 | 67,842 | 205,060 |
| FFQ survey | 1995–1996 | 1995–1996 | 1998–1999 | 1998–1999 | 2001–2006 | 1996–2000 | 1995–2006 |
| Follow-up time, y2 | 15.5 ± 5.9 | 16.4 ± 3.6 | 12.4 ± 3.8 | 13.3 ± 3.1 | 9.2 ± 1.8 | 13.6 ± 2.3 | 12.7 ± 3.7 |
| CRC cases, n | 473 | 323 | 512 | 337 | 561 | 765 | 2971 |
| Age, y | 54.5 ± 6.0 | 54.6 ± 5.9 | 58.6 ± 8.6 | 58.9 ± 8.7 | 54.9 ± 9.6 | 52.1 ± 9.0 | 54.8 ± 8.9 |
| Current smoker, % | 46.8 | 4.4 | 41.5 | 5.2 | 59.7 | 2.3 | 26.5 |
| Regular alcohol drinker,3 % | 75.1 | 17.4 | 70.5 | 17.8 | 33.3 | 2.0 | 27.1 |
| Physical activity,4 MET-h/wk | 33.9 [15.4] | 31.9 [7.2] | 31.9 [10.8] | 31.8 [7.2] | 53.8 [44.1] | 100.9 [56.9] | 48.9 [60.3] |
| BMI, kg/m2 | 23.5 [3.6] | 23.4 [4.0] | 23.3 [3.9] | 23.1 [4.0] | 23.7 [4.0] | 23.6 [4.5] | 23.5 [4.1] |
| Family CRC history, % | 0.9 | 0.8 | 1.5 | 1.4 | 2.1 | 2.2 | 1.8 |
| Postmenopausal women, % | — | 72.8 | — | 76.0 | — | 53.6 | 61.6 |
| Dietary intake | |||||||
| Total energy, kcal/d | 2216 [937] | 1857 [798] | 2005 [867] | 1740 [753] | 1873 [620] | 1642 [493] | 1795 [680] |
| Isoflavone, mg/d | 38.3 [37.1] | 39.1 [36.6] | 30.1 [32.9] | 30.8 [33.9] | 31.0 [26.8] | 25.1 [24.6] | 29.9 [29.3] |
| Soy protein, g/d | 7.9 [6.9] | 7.9 [6.8] | 6.1 [6.0] | 6.1 [6.1] | 9.5 [7.6] | 7.4 [6.7] | 7.7 [7.1] |
| Red meat, g/d | 47.7 [54.1] | 41.3 [49.5] | 41.3 [46.9] | 36.9 [41.6] | 54.4 [47.9] | 43.8 [39.2] | 45.7 [45.5] |
| Vegetables, g/d | 179 [165] | 217 [185] | 159 [154] | 193 [167] | 306 [215] | 261 [192] | 244 [203] |
| Folate, μg/d | 372 [243] | 405 [261] | 335 [229] | 379 [252] | 324 [145] | 276 [127] | 318 [177] |
Data presented are means ± SDs for normally distributed variables, or medians [IQRs] for variables with a skewed distribution, frequency, or proportion (%). CRC, colorectal cancer; JPHC, Japan Public Health Center–based Prospective Study; MET-h, metabolic equivalent hours; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Mean time from enrollment to the date of diagnosis of CRC (event) or censoring due to diagnosis of other cancer, death, lost to follow-up, or end of study.
Including heavy (>28 g/d in men and >14 g/d in women) and moderate (>0 to ≤28 g/d in men or >0 to ≤14 g/d in women) alcohol drinkers.
Mean MET-h per week among participants who reported >0 MET-h/wk (n = 191,044).
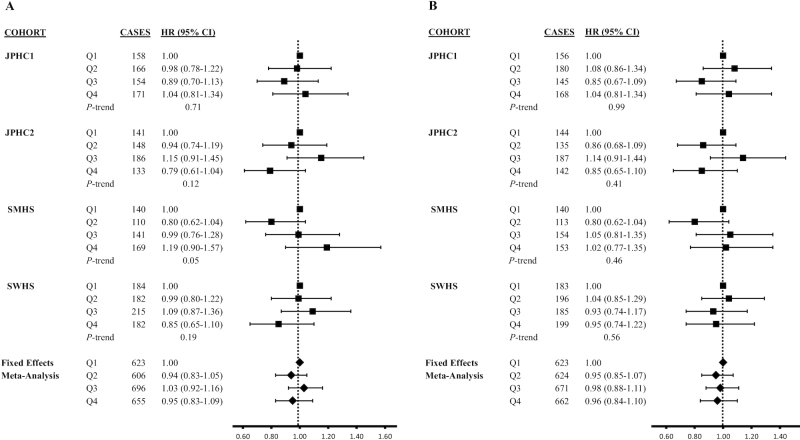
Overall, there were no statistically significant associations of soy isoflavone or soy protein intake with CRC risk in the pooled analysis or when these variables were stratified by sex (Table 2). Although no association was observed, an increased hazard for CRC was suggested for higher intake of isoflavones, as indicated by the P-trend (HR: 1.19; 95% CI: 0.90, 1.57; P-trend = 0.05). However, other associations from the cohort-specific analyses were not statistically significant (Figure 1). We further noted a potential U-shape for soy isoflavones in relation to CRC risk, as indicated by the higher risk observed in the third quartile.
TABLE 2.
Association between dietary soy food intake and CRC risk in a pooled analysis of cohorts from China and Japan1
| Q1 (low) | Q2 | Q3 | Q4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Cases | HR | Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | P-trend |
| Dietary isoflavone intake | |||||||||
| All participants | |||||||||
| Age-, energy-, and cohort-adjusted | 722 | 1.00 | 701 | 0.96 (0.87, 1.07) | 789 | 1.05 (0.95, 1.17) | 759 | 0.96 (0.86, 1.07) | 0.59 |
| Multivariate2,3 | 625 | 1.00 | 609 | 0.94 (0.83, 1.05) | 697 | 1.07 (0.95, 1.20) | 658 | 0.97 (0.86, 1.11) | 0.68 |
| Men4 | |||||||||
| Age-, energy-, and cohort-adjusted | 387 | 1.00 | 349 | 0.91 (0.78, 1.05) | 396 | 1.01 (0.88, 1.17) | 414 | 1.01 (0.87, 1.18) | 0.49 |
| Multivariate2,5 | 324 | 1.00 | 295 | 0.86 (0.73, 1.01) | 339 | 0.96 (0.81, 1.13) | 356 | 1.01 (0.85, 1.21) | 0.41 |
| Women | |||||||||
| Age-, energy-, and cohort-adjusted | 335 | 1.00 | 352 | 1.06 (0.91, 1.23) | 393 | 1.16 (0.99, 1.35) | 345 | 0.99 (0.84, 1.16) | 0.81 |
| Multivariate2,6 | 299 | 1.00 | 311 | 1.00 (0.85, 1.17) | 357 | 1.10 (0.93, 1.30) | 299 | 0.87 (0.72, 1.05) | 0.18 |
| Dietary soy protein intake | |||||||||
| All participants | |||||||||
| Age-, energy-, and cohort-adjusted | 719 | 1.00 | 726 | 0.99 (0.89, 1.10) | 754 | 1.00 (0.90, 1.11) | 772 | 0.96 (0.86, 1.07) | 0.49 |
| Multivariate2,3 | 626 | 1.00 | 625 | 0.96 (0.85, 1.07) | 673 | 1.01 (0.90, 1.14) | 665 | 0.98 (0.86, 1.12) | 0.67 |
| Men7 | |||||||||
| Age-, energy-, and cohort-adjusted | 384 | 1.00 | 351 | 0.91 (0.79, 1.05) | 404 | 1.03 (0.89, 1.19) | 407 | 0.99 (0.85, 1.15) | 0.70 |
| Multivariate2,5 | 323 | 1.00 | 293 | 0.86 (0.73, 1.01) | 353 | 1.00 (0.85, 1.18) | 345 | 0.99 (0.83, 1.18) | 0.65 |
| Women | |||||||||
| Age-, energy-, and cohort-adjusted | 335 | 1.00 | 375 | 1.11 (0.95, 1.29) | 350 | 1.02 (0.87, 1.19) | 365 | 1.03 (0.88, 1.21) | 0.95 |
| Multivariate2,6 | 300 | 1.00 | 331 | 1.06 (0.90, 1.24) | 318 | 0.95 (0.80, 1.12) | 317 | 0.93 (0.77, 1.12) | 0.29 |
Based on the cohort- and sex-specific quartiles. Please see Supplemental Table 2 for specific cutpoint values for each cohort. CRC, colorectal cancer; Q, quartile.
Adjusted for age, total energy, cohort, smoking status, alcohol consumption, physical activity, BMI, family history of colorectal cancer, intake of red meat, vegetable intake, folate intake, and menopausal status in women.
Complete case analysis based on total sample size of n = 186,755 with 2589 colorectal cancer cases.
P for multiplicative interaction was 0.15 for isoflavone intake using likelihood ratio test from nested models.
Complete case analysis based on total sample size of n = 85,916 with 1314 colorectal cancer cases among men.
Complete case analysis based on total sample size of n = 100,204 with 1266 colorectal cancer cases among women.
P for multiplicative interaction was 0.14 for soy protein intake using likelihood ratio test from nested models.
FIGURE 1.
Association between dietary soy isoflavone (mg/d; A) and soy protein (g/d; B) intake and colorectal cancer risk by study cohorts and fixed-effects meta-analysis. P-heterogeneity for soy isoflavone intake was 0.60, 0.45, and 0.14, for Q2, Q3, and Q4, respectively. P-heterogeneity for soy protein intake was 0.22, 0.33, and 0.71, for Q2, Q3, and Q4, respectively. The cohort- and sex-specific quartiles were used (see Supplemental Table 2 for specific cutpoint values for each cohort). Complete case analyses (based on total sample size of n = 186,755 with 2589 colorectal cancer cases) were adjusted for age, total energy, sex, smoking status, alcohol consumption, physical activity, obesity status, family history of colorectal cancer, intake of red meat, vegetable intake, folate intake, and menopausal status in women. JPHC1, Japan Public Health Center–based Prospective Study Cohort 1; JPHC2, Japan Public Health Center–based Prospective Study Cohort 2; Q, quartile; SWHS, Shanghai Women's Health Study.
In Table 3, data are shown for our examination of potential effect measure modification by median age, smoking status, menopausal status, and cancer site. For both isoflavone and soy protein intake, there was an indication of a reduced hazard of CRC among ever smokers consuming the high isoflavones (HRQ4 vs. Q1: 0.83; 95% CI: 0.68, 1.00) and soy protein (HRQ4 vs. Q1: 0.81; 95% CI: 0.67, 0.99) compared with ever smokers with lower intake. Reduced hazards of similar magnitudes were observed among women who were premenopausal at baseline for intakes of both isoflavone (HRQ4 vs. Q1: 0.81; 95% CI: 0.63, 1.04; P-trend = 0.03) and soy protein (HRQ4 vs. Q1: 0.87; 95% CI: 0.68, 1.12), though the associations did not achieve statistical significance. Furthermore, no statistically significant differences between strata were observed on the multiplicative scale.
TABLE 3.
Association between dietary soy food intake and CRC incidence in a pooled analysis of cohorts from China and Japan, stratified by baseline CRC risk factors and by CRC site1
| Q1(low) | Q2 | Q3 | Q4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Cases | HR | Cases | HR (95% CI)2,3 | Cases | HR (95% CI)b,c | Cases | HR (95% CI)b,c | P-trend | P-heterogeneity4 |
| Dietary isoflavone intake | ||||||||||
| Median age, y | ||||||||||
| ≤53 | 197 | 1.00 | 176 | 0.91 (0.74, 1.12) | 203 | 1.13 (0.92, 1.38) | 156 | 0.98 (0.79, 1.23) | 0.78 | 0.68 |
| >53 | 428 | 1.00 | 433 | 0.92 (0.80, 1.06) | 494 | 1.00 (0.87, 1.15) | 502 | 0.93 (0.80, 1.08) | 0.75 | |
| Smoking status | ||||||||||
| Ever | 240 | 1.00 | 209 | 0.80 (0.66, 0.96) | 249 | 0.92 (0.77, 1.11) | 237 | 0.83 (0.68, 1.00) | 0.69 | 0.15 |
| Never | 385 | 1.00 | 400 | 1.01 (0.87, 1.16) | 448 | 1.13 (0.98, 1.30) | 421 | 1.03 (0.88, 1.21) | 0.77 | |
| Menopausal status | ||||||||||
| Premenopausal | 152 | 1.00 | 152 | 1.02 (0.81, 1.28) | 166 | 1.05 (0.83, 1.32) | 146 | 0.81 (0.63, 1.04) | 0.03 | 0.68 |
| Postmenopausal | 147 | 1.00 | 159 | 1.02 (0.81, 1.28) | 191 | 1.21 (0.96, 1.51) | 153 | 0.93 (0.72, 1.19) | 0.81 | |
| Cancer site | ||||||||||
| Colon | 370 | 1.00 | 384 | 0.97 (0.84, 1.13) | 422 | 1.05 (0.91, 1.23) | 421 | 1.01 (0.85, 1.19) | 0.81 | 0.80 |
| Rectal | 217 | 1.00 | 206 | 0.93 (0.76, 1.13) | 243 | 1.09 (0.89, 1.33) | 208 | 0.92 (0.73, 1.15) | 0.62 | |
| Dietary soy protein intake | ||||||||||
| Median age, y | ||||||||||
| ≤53 | 201 | 1.00 | 185 | 0.97 (0.79, 1.18) | 182 | 1.01 (0.82, 1.24) | 164 | 1.04 (0.84, 1.30) | 0.99 | 0.77 |
| >53 | 425 | 1.00 | 440 | 0.94 (0.82, 1.07) | 491 | 0.98 (0.85, 1.12) | 501 | 0.92 (0.79, 1.07) | 0.63 | |
| Smoking status | ||||||||||
| Ever | 238 | 1.00 | 214 | 0.82 (0.68, 0.99) | 250 | 0.94 (0.78, 1.12) | 233 | 0.81 (0.67, 0.99) | 0.62 | 0.09 |
| Never | 388 | 1.00 | 411 | 1.04 (0.90, 1.20) | 423 | 1.03 (0.89, 1.19) | 432 | 1.05 (0.90, 1.23) | 0.75 | |
| Menopausal status | ||||||||||
| Premenopausal | 152 | 1.00 | 165 | 1.09 (0.87, 1.36) | 143 | 0.88 (0.69, 1.12) | 156 | 0.87 (0.68, 1.12) | 0.07 | 0.40 |
| Postmenopausal | 148 | 1.00 | 166 | 1.07 (0.86, 1.34) | 175 | 1.10 (0.88, 1.38) | 161 | 0.98 (0.77, 1.26) | 0.67 | |
| Cancer site | ||||||||||
| Colon | 385 | 1.00 | 385 | 0.94 (0.81, 1.09) | 406 | 0.95 (0.82, 1.10) | 421 | 0.95 (0.80, 1.12) | 0.66 | 0.67 |
| Rectal | 204 | 1.00 | 221 | 1.07 (0.88, 1.30) | 233 | 1.12 (0.92, 1.38) | 216 | 1.04 (0.83, 1.31) | 0.80 | |
Based on the cohort- and sex-specific quartiles. Please see Supplemental Table 2 for specific cutpoint values for each cohort. CRC, colorectal cancer; Q, quartile.
Adjusted for age, sex, total energy, cohort, smoking status, alcohol consumption, physical activity, BMI, family history of colorectal cancer, intake of red meat, vegetable intake, folate intake, and menopausal status in women.
Complete case analysis based on total sample size of 186,755 with 2589 colorectal cancer cases.
Calculated using the likelihood ratio test for nested models for the multiplicative interaction term. P for overall heterogeneity was calculated to determine differences by cancer site.
In Table 4, data for further analysis stratified by BMI demonstrate an observed a statistically significant linear trend with increasing intake among those in the highest category of BMI (i.e., ≥27.5), with an ∼40% increased hazard for CRC observed among those consuming high amounts of isoflavones (HRQ4 vs. Q1: 1.39; 95% CI: 0.98, 1.97) or soy protein (HRQ4 vs. Q1: 1.42; 95% CI: 1.01, 2.00). Among premenopausal women, there was an indication for reduced hazards for isoflavone (HRQ4 vs. Q1: 0.58; 95% CI: 0.34, 0.98) among premenopausal women with BMI <23.0. Increased CRC hazards were also observed among men and postmenopausal women; however, the estimates were imprecise due to fewer observed CRC events in these subgroups. This interaction was also examined using continuous measure by estimating HRs for 1-SD increases in isoflavone and soy protein intake in Supplemental Table 3. Similar increased hazards were observed in subgroups of individuals with higher BMIs (≥27.5).
TABLE 4.
Evaluation of potential interaction between soy food intake and BMI on CRC risk among women (pre- and postmenopausal) and men: a pooled analysis of data from 4 cohorts conducted in China and Japan1
| Q1 (low) | Q2 | Q3 | Q4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Cases | HR | Cases | HR (95% CI)2,3 | Cases | HR (95% CI)b,c | Cases | HR (95% CI)b,c | P-trend | P-heterogeneity4 |
| Dietary isoflavone intake | ||||||||||
| All participants, BMI | ||||||||||
| <23.0 | 260 | 1.00 | 245 | 0.90 (0.75, 1.08) | 273 | 1.05 (0.87, 1.26) | 222 | 0.82 (0.67, 1.02) | 0.24 | 0.88 |
| 23.0 to 27.49 | 284 | 1.00 | 285 | 0.90 (0.76, 1.07) | 319 | 0.96 (0.81, 1.14) | 327 | 0.92 (0.76, 1.11) | 0.73 | |
| ≥27.5 | 79 | 1.00 | 76 | 1.01 (0.73, 1.39) | 104 | 1.38 (1.00, 1.90) | 106 | 1.39 (0.98, 1.97) | 0.03 | |
| Premenopausal women, BMI | ||||||||||
| <23.0 | 60 | 1.00 | 49 | 0.97 (0.65, 1.44) | 57 | 1.08 (0.71, 1.63) | 34 | 0.58 (0.34, 0.98) | 0.16 | 0.65 |
| 23.0– 27.49 | 63 | 1.00 | 76 | 1.11 (0.78, 1.57) | 80 | 0.99 (0.68, 1.43) | 76 | 0.76 (0.50, 1.15) | 0.05 | |
| ≥27.5 | 29 | 1.00 | 27 | 0.91 (0.52, 1.57) | 29 | 0.85 (0.47, 1.52) | 36 | 0.83 (0.43, 1.58) | 0.59 | |
| Postmenopausal women, BMI | ||||||||||
| <23.0 | 68 | 1.00 | 72 | 0.98 (0.69, 1.37) | 75 | 1.03 (0.72, 1.47) | 67 | 0.99 (0.67, 1.49) | 0.79 | 0.67 |
| 23.0–27.49 | 64 | 1.00 | 72 | 1.03 (0.73, 1.46) | 90 | 1.25 (0.88, 1.76) | 70 | 0.93 (0.63, 1.39) | 0.87 | |
| ≥27.5 | 15 | 1.00 | 15 | 1.02 (0.49, 2.12) | 26 | 1.75 (0.87, 3.50) | 16 | 1.26 (0.55, 2.90) | 0.17 | |
| Men, BMI | ||||||||||
| <23.0 | 132 | 1.00 | 124 | 0.88 (0.68, 1.13) | 141 | 1.04 (0.81, 1.35) | 121 | 0.91 (0.68, 1.21) | 0.73 | 0.45 |
| 23.0–27.49 | 157 | 1.00 | 137 | 0.78 (0.61, 0.98) | 149 | 0.82 (0.64, 1.04) | 181 | 0.98 (0.76, 1.26) | 0.62 | |
| ≥27.5 | 35 | 1.00 | 34 | 0.90 (0.55, 1.48) | 49 | 1.39 (0.87, 2.23) | 54 | 1.53 (0.93, 2.52) | 0.03 | |
| Dietary soy protein intake | ||||||||||
| All participants, BMI | ||||||||||
| <23.0 | 262 | 1.00 | 249 | 0.92 (0.77, 1.10) | 267 | 1.01 (0.84, 1.21) | 222 | 0.82 (0.66, 1.01) | 0.19 | 0.87 |
| 23.0–27.49 | 280 | 1.00 | 297 | 0.95 (0.81, 1.13) | 310 | 0.93 (0.78, 1.11) | 328 | 0.93 (0.77, 1.13) | 0.58 | |
| ≥27.5 | 81 | 1.00 | 78 | 1.03 (0.75, 1.42) | 94 | 1.18 (0.85, 1.62) | 112 | 1.42 (1.01, 2.00) | 0.02 | |
| Premenopausal women, BMI | ||||||||||
| <23.0 | 60 | 1.00 | 55 | 1.04 (0.71, 1.52) | 48 | 0.87 (0.57, 1.34) | 37 | 0.66 (0.39, 1.10) | 0.27 | 0.44 |
| 23.0–27.49 | 63 | 1.00 | 81 | 1.21 (0.86, 1.71) | 72 | 0.88 (0.60, 1.29) | 79 | 0.80 (0.53, 1.20) | 0.04 | |
| ≥27.5 | 29 | 1.00 | 29 | 0.92 (0.53, 1.59) | 23 | 0.66 (0.36, 1.21) | 40 | 1.00 (0.53, 1.86) | 0.90 | |
| Postmenopausal women, BMI | ||||||||||
| <23.0 | 69 | 1.00 | 75 | 1.04 (0.74, 1.45) | 69 | 0.90 (0.63, 1.30) | 70 | 1.01 (0.68, 1.49) | 0.94 | 0.83 |
| 23.0–27.49 | 64 | 1.00 | 74 | 1.07 (0.76, 1.50) | 87 | 1.13 (0.80, 1.59) | 71 | 0.93 (0.63, 1.37) | 0.74 | |
| ≥27.5 | 15 | 1.00 | 17 | 1.18 (0.58, 2.40) | 20 | 1.35 (0.66, 2.77) | 20 | 1.47 (0.67, 3.26) | 0.11 | |
| Men, BMI | ||||||||||
| <23.0 | 133 | 1.00 | 119 | 0.86 (0.67, 1.11) | 151 | 1.10 (0.85, 1.42) | 115 | 0.85 (0.64, 1.14) | 0.46 | 0.39 |
| 23.0–27.49 | 153 | 1.00 | 142 | 0.81 (0.64, 1.03) | 151 | 0.85 (0.66, 1.08) | 178 | 0.99 (0.76, 1.28) | 0.64 | |
| ≥27.5 | 37 | 1.00 | 32 | 0.80 (0.49, 1.31) | 51 | 1.27 (0.80, 2.02) | 52 | 1.38 (0.85, 2.25) | 0.05 | |
Based on the cohort- and sex-specific quartiles. Please see Supplemental Table 2 for specific cutpoint values for each cohort. . CRC, colorectal cancer.
Adjusted for age, total energy, cohort, smoking status, alcohol consumption, physical activity, BMI, family history of CRC, intake of red meat, vegetable intake, fte, a intakend menopausal status in women.
Complete case analysis based on total sample size of n = 185,482 with 2568 CRC cases.
Estimated by likelihood ratio test to compare the models with and without a multiplicative interaction term.
Discussion
In this pooled analysis of 4 large population-based cohorts conducted among Chinese and Japanese individuals, we observed 42% hazard reductions for CRC among premenopausal women with low BMI (<23.0) consuming higher amounts relative to lower amounts of isoflavones.
Few previous studies have been conducted to examine associations between soy foods and CRC incidence. Previous studies among Asian populations examined both soy and isoflavone intake in relation to CRC risk (37–41); however, the results from these prior studies are inconsistent. The majority of the previous studies were case–control in design and reported risk reductions ranging from ∼6% to ∼29% (37, 38, 40, 41) for higher intakes of soy foods and/or isoflavones in relation to CRC incidence. It is possible that many of these case–control designs could be biased away from the null due to differential recall of dietary exposures. Two previous studies (39, 42), which were not included in our pooled analysis, examined soy intake among Asian populations using a prospective cohort design. Oba et al. conducted a prospective population-based cohort study among a total of ∼30,000 men and women in Japan who were followed for an average of 7 y, during which 213 individuals developed colon cancer. Soy intake was assessed at baseline using an FFQ, and Oba et al. reported nearly halving of the risk of colon cancer among women consuming highest tertile of soy product consumption compared with the lowest (HRT3 vs. T1: 0.56, 95% CI: 0.34, 0.92) (39). Similarly, Butler et al. examined the association between both isoflavones and soy foods in relation to CRC incidence among a Singaporean population-based cohort including more than 60,000 men and women who were followed for nearly 10 y (42). No association was observed for CRC individual intakes of isoflavones (HRQ4 vs. Q1: 0.95, 95% CI: 0.79, 1.13) and soy foods (HRQ4 vs. Q1: 0.95, 95% CI: 0.78, 1.16) also assessed at baseline via FFQ among a population of Singaporean Chinese individuals.
In our study, we observed potential differences in the associations between soy and CRC risk when stratified by BMI. A meta-analysis reported that expression of ER-β was reduced in CRC, indicating the potential for ER-β to act as a tumor suppressor (43), and isoflavones have been suggested to preferentially bind to ER-β (44, 45). The reduced hazards observed among premenopausal women consuming higher amounts of soy may reflect increased ER-β transactivation due to the combination of phytoestrogen intake and endogenous estrogen. It has been suggested that increased soy intake among perimenopausal women may help to improve the various symptoms of menopause (46). However, the potential benefit of increased soy intake during the menopausal transition for reducing CRC risk, particularly among women with low BMI, is unclear. The potential preventive role of estrogen exposure in CRC development has been corroborated in population-based studies in which the incidence of CRC was reduced among women using oral contraceptives (47) and those using hormone replacement therapy (48) compared with those who were not. Furthermore, our results indicate that CRC risk reduction among those consuming higher amounts of soy does not persist among those with higher BMI (i.e., ≥27.5), regardless of menopausal status or sex. These results are not intuitive, especially among postmenopausal women, given the potential for soy to exert an estrogenic effect in the presence of low endogenous estrogen. However, increased body fat mass is known to be a source of estrogen production (49), thus it is possible that adipose tissue may be a source of estrogen in postmenopausal women with higher BMI. However, this possibility would not explain our finding seen among overweight men. Thus, it is possible that the combination of high soy intake and high BMI may lead to increased CRC risk via other mechanisms (50–52), for example via increased insulin-like growth factor 1 production, which could nullify a potential benefit of increased estrogen-induced ER-β expression in this subgroup. Finally, although we adjusted for red meat consumption in the pooled analysis, we were unable to adjust for processed meats specifically. Thus, it is possible that potential residual confounding by processed meat consumption could be an issue in our pooled analysis.
Our study has several strengths. First, we examined the association between soy food intake and CRC risk using a pooled cohort of 205,060 individuals with an average follow-up time of 12.7 y, during which nearly 3000 CRC cases were identified. To our knowledge, this is the largest pooled population-based cohort study to examine the association between soy and CRC. However, diet was assessed at baseline, and the average time from baseline assessment to diagnosis in our pooled cohort was 8.5 y. We are unable to account for changes in diet over the follow-up period in this analysis, which influenced our results, especially among those diagnosed with cardiovascular disease over the follow-up period, which could have led to changes in dietary behaviors in these populations. However, it is also possible that the soy and isoflavone intakes in our pooled study were assessed during a biologically plausible etiologic exposure window for CRC, which may be close to a decade (53). Given that most Asians consume soy on a regular basis, and it is considered a dietary staple, drastic changes in soy intake over time may be of lesser concern in this population.
In conclusion, we report reduced risk for CRC among women who were premenopausal and had a low BMI (i.e., <23) at baseline, and who consumed higher amounts soy foods. In contrast, among postmenopausal women and men with higher BMI (i.e., ≥27.5), nearly 30% to 50% increased CRC hazards were observed for those with higher intakes of isoflavone and soy protein compared with those with lower intakes. Future investigations should explore the relationship between soy intake and CRC among those with higher BMI to further understand the mechanisms behind this association. Further exploration of personalized nutrition approaches based on genetic variations and differences in the gut microbiome may help to identify specific groups of individuals that may benefit the most from soy food consumption.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TS, NS, X-OS, and WZ: designed the research; TY, JG, AG, H-LL, MI, GY, TS, Y-BX, and MI: conducted the research; NKK, JJY, and WW: analyzed the data; NKK: wrote the paper; and all authors: read and approved the final manuscript.
Notes
The Shanghai Men's Health Study (SMHS) and the Shanghai Women's Health Study (SWHS) are funded by UM1 CA173640 and UM1 CA182910, respectively. Japan Public Health Center-based Prospective Study (JPHC) 1 and 2 are supported by National Cancer Center Research and Development Fund (23-A-31[toku], 26-A-2, 29-A-4) (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the ‘‘Online Supplementary Material’’ link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: CRC, colorectal cancer; ER, estrogen receptor; JPHC1, Japan Public Health Center–based Prospective Study Cohort 1; JPHC2, Japan Public Health Center–based Prospective Study Cohort 2; MET, metabolic equivalent; Q, quartile; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study
Contributor Information
Nikhil K Khankari, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jae Jeong Yang, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Norie Sawada, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Wanqing Wen, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Taiki Yamaji, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Jing Gao, Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Atsushi Goto, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Hong-Lan Li, Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Motoki Iwasaki, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Gong Yang, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Taichi Shimazu, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Yong-Bing Xiang, Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Manami Inoue, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Xiao-Ou Shu, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Shoichiro Tsugane, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Chuo-ku, Tokyo, Japan.
Wei Zheng, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu J, Bi X, Yu B, Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chun OK, Lee SG, Wang Y, Vance T, Song WO. Estimated flavonoid intake of the elderly in the United States and around the world. J Nutr Gerontol Geriatr. 2012;31:190–205. [DOI] [PubMed] [Google Scholar]
- 4. Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Hsieh C-C, Martínez-Villaluenga C, de Lumen BO, Hernández-Ledesma B. Updating the research on the chemopreventive and therapeutic role of the peptide lunasin: properties of the peptide lunasin. J Sci Food Agric. 2018;98:2070–79. [DOI] [PubMed] [Google Scholar]
- 6. Wan X, Liu H, Sun Y, Zhang J, Chen X, Chen N. Lunasin: a promising polypeptide for the prevention and treatment of cancer. Oncol Lett. 2017;13:3997–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways. Oncol Rep. 2016;36:253–62. [DOI] [PubMed] [Google Scholar]
- 8. Hsieh C-C, Hernández-Ledesma B, de Lumen BO. Soybean peptide lunasin suppresses in vitro and in vivo 7,12-dimethylbenz[a]anthracene-induced tumorigenesis. J Food Sci. 2010;75:H311–6. [DOI] [PubMed] [Google Scholar]
- 9. Dia VP, de Mejia EG. Lunasin promotes apoptosis in human colon cancer cells by mitochondrial pathway activation and induction of nuclear clusterin expression. Cancer Lett. 2010;295:44–53. [DOI] [PubMed] [Google Scholar]
- 10. Dia VP, Gonzalez de Mejia E. Lunasin induces apoptosis and modifies the expression of genes associated with extracellular matrix and cell adhesion in human metastatic colon cancer cells. Mol Nutr Food Res. 2011;55:623–34. [DOI] [PubMed] [Google Scholar]
- 11. García-Nebot MJ, Recio I, Hernández-Ledesma B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem Toxicol. 2014;65:155–61. [DOI] [PubMed] [Google Scholar]
- 12. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. [DOI] [PubMed] [Google Scholar]
- 13. Phetnoo N, Werawatganon D, Siriviriyakul P. Genistein could have a therapeutic potential for gastrointestinal diseases. Thai J Gastroenterol. 2013;14:120–5. [Google Scholar]
- 14. Guo J-Y, Li X, Browning JD, Rottinghaus GE, Lubahn DB, Constantinou A, Bennink M, MacDonald RS. Dietary soy isoflavones and estrone protect ovariectomized ERalphaKO and wild-type mice from carcinogen-induced colon cancer. J Nutr. 2004;134:179–82. [DOI] [PubMed] [Google Scholar]
- 15. Smirnoff P, Liel Y, Gnainsky J, Shany S, Schwartz B. The protective effect of estrogen against chemically induced murine colon carcinogenesis is associated with decreased CpG island methylation and increased mRNA and protein expression of the colonic vitamin D receptor. Oncol Res. 1999;11:255–64. [PubMed] [Google Scholar]
- 16. Weyant MJ, Carothers AM, Mahmoud NN, Bradlow HL, Remotti H, Bilinski RT, Bertagnolli MM. Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. 2001;61:2547–51. [PubMed] [Google Scholar]
- 17. Paradkar PN, Blum PS, Berhow MA, Baumann H, Kuo S-M. Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett. 2004;215:21–8. [DOI] [PubMed] [Google Scholar]
- 18. Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal slackia isoflavoniconvertens in gnotobiotic rats. J Nutr. 2012;142:40–6. [DOI] [PubMed] [Google Scholar]
- 19. Setchell KDR, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. [DOI] [PubMed] [Google Scholar]
- 21. Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77:1459–65. [DOI] [PubMed] [Google Scholar]
- 22. Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Applegate C, Rowles J, Ranard K, Jeon S, Erdman J. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. 2018;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang R, Botma A, Rudolph A, Hüsing A, Chang-Claude J. Phyto-oestrogens and colorectal cancer risk: a systematic review and dose-response meta-analysis of observational studies. Br J Nutr. 2016;116:2115–28. [DOI] [PubMed] [Google Scholar]
- 25. Yang G, Shu X-O, Li H, Chow W-H, Cai H, Zhang X, Gao Y-T, Zheng W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S, for the Japan Public Health Center-Based Prospective Study Group. Dietary soy and isoflavone intake and risk of colorectal cancer in the Japan Public Health Center-Based Prospective Study. Cancer Epidemiol Biomarkers Prev. 2008;17:2128–35. [DOI] [PubMed] [Google Scholar]
- 27. Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Q et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. [DOI] [PubMed] [Google Scholar]
- 28. Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang Y-B. Cohort profile: the Shanghai Men's Health Study. Int J Epidemiol. 2015;44:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44:777–82. [DOI] [PubMed] [Google Scholar]
- 30. Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8:168–75. [DOI] [PubMed] [Google Scholar]
- 31. Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–50. [DOI] [PubMed] [Google Scholar]
- 32. The council for science and technology, Ministry of Education, Sports, Science and Technology, Japan. Standard tables of food composition in Japan. 5th ed.Tokyo: National Printing Bureau; 2005. [Google Scholar]
- 33. Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao Y-T, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur J Clin Nutr. 2004;58:17–23. [DOI] [PubMed] [Google Scholar]
- 34. Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S, Japan Public Health Center-Based Prospective Study Group . Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomark Prev. 2007;16:538–45. [DOI] [PubMed] [Google Scholar]
- 35. Ishihara J, Sobue T, Yamamoto S, Yoshimi I, Sasaki S, Kobayashi M, Takahashi T, Iitoi Y, Akabane M, Tsugane S et al. Validity and reproducibility of a self-administered food frequency questionnaire in the JPHC Study Cohort II: study design, participant profile and results in comparison with Cohort I. J Epidemiol. 2003;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villegas R, Yang G, Liu D, Xiang Y-B, Cai H, Zheng W, Ou Shu X. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai Men's Health Study. Br J Nutr. 2007;97:993–1000. [DOI] [PubMed] [Google Scholar]
- 37. Budhathoki S, Joshi AM, Ohnaka K, Yin G, Toyomura K, Kono S, Mibu R, Tanaka M, Kakeji Y, Maehara Y et al. Soy food and isoflavone intake and colorectal cancer risk: The Fukuoka Colorectal Cancer Study. Scand J Gastroenterol. 2011;46:165–72. [DOI] [PubMed] [Google Scholar]
- 38. Shin A, Lee J, Lee J, Park MS, Park JW, Park SC, Oh JH, Kim J. Isoflavone and soyfood intake and colorectal cancer risk: a case-control study in Korea. PLoS One. 2015;10:e0143228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oba S, Nagata C, Shimizu N, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S. Soy product consumption and the risk of colon cancer: a prospective study in Takayama, Japan. Nutr Cancer. 2007;57:151–7. [DOI] [PubMed] [Google Scholar]
- 40. Hoshiyama Y, Sekine T, Sasaba T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med. 1993;171:153–65. [DOI] [PubMed] [Google Scholar]
- 41. Huang X-E, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J, Takezaki T, Tajima K. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac J Cancer Prev. 2004;5:419–27. [PubMed] [Google Scholar]
- 42. Butler LM, Wang R, Koh W-P, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer. 2008;99:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niv Y. Estrogen receptor β expression and colorectal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1438–42. [DOI] [PubMed] [Google Scholar]
- 44. Margeat E, Bourdoncle A, Margueron R, Poujol N, Cavaillès V, Royer C. Ligands differentially modulate the protein interactions of the human estrogen receptors alpha and beta. J Mol Biol. 2003;326:77–92. [DOI] [PubMed] [Google Scholar]
- 45. Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–5. [DOI] [PubMed] [Google Scholar]
- 46. Ahsan M. The effect of soy isoflavones on the menopause rating scale scoring in perimenopausal and postmenopausal women: a pilot study. J Clin Diagn Res. 2017; 11:FC13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luan N-N, Wu L, Gong T-T, Wang Y-L, Lin B, Wu Q-J. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control. 2015;26:65–78. [DOI] [PubMed] [Google Scholar]
- 48. Barnes EL, Long MD. Colorectal cancer in women: hormone replacement therapy and chemoprevention. Climacteric. 2012;15:250–5. [DOI] [PubMed] [Google Scholar]
- 49. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–24. [DOI] [PubMed] [Google Scholar]
- 50. Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. [DOI] [PubMed] [Google Scholar]
- 51. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16.. [DOI] [PubMed] [Google Scholar]
- 52. Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. [DOI] [PubMed] [Google Scholar]
- 53. Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.