Abstract
A virtual expert roundtable was convened on April 16, 2020, to discuss the evolving landscape of care for treating patients with advanced hepatocellular carcinoma (HCC) and discuss questions related to patient care and treatment selection. This commentary presents highlights from this discussion and provides an expert opinion about approaches to treatment for HCC in the Americas and the European Union. We anticipate that atezolizumab plus bevacizumab will become the standard of care for advanced HCC patients. However, this approach will make decisions regarding the sequencing of treatments for second-line therapies and beyond more challenging. Therapy will require individualization based on patient characteristics and preferences, while insurance coverage decisions and requirements may also impact the options that patients can access. Additional research regarding prognostic and predictive biomarkers is needed to help better identify optimal treatment approaches for specific patient populations. Multidisciplinary tumor boards will continue to play a critical role in guiding treatment selection for individual patients. Atezolizumab plus bevacizumab offers a promising new first-line therapeutic option for patients with advanced HCC, but more research is needed to optimize and individualize patient therapy.
Keywords: hepatocellular carcinoma, immuno-oncologics, biomarkers, patient-reported outcomes
Introduction
The oral multikinase inhibitor sorafenib was the only recommended first-line systemic treatment option for unresectable hepatocellular carcinoma (HCC) based on SHARP trial results in 2007, and lenvatinib was recently added based on the noninferiority results of the REFLECT clinical trial.1–3 Although systemic therapy for HCC has experienced important advancements over the past decade, before 2020, no other treatment demonstrated statistically significant improvement in overall survival (OS) compared with sorafenib (however, lenvatinib did significantly increase time to progression, compared with sorafenib).4,5 In 2020, the results from the IMbrave150 phase 3 clinical trial of the programmed cell death ligand 1 inhibitor atezolizumab plus the vascular endothelial growth factor inhibitor bevacizumab show that this immuno-oncologic (IO) combination statistically significantly improved both OS and progression-free survival compared with sorafenib.5 In March 2020, atezolizumab plus bevacizumab was added as a first-line systemic treatment for HCC in treatment guidelines from the National Comprehensive Cancer Network, followed by FDA approval on May 29, 2020, as first-line therapy for unresectable HCC.1,6 While European Commission decision is pending, on September 17, 2020 European Medicine Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending atezolizumab and bevacizumab for the treatment of adult patients with advanced or unresectable HCC who have not received prior systemic therapy.7 The pivotal phase 3 clinical trial designs as well as efficacy and safety outcomes associated with these three first-line treatment options are summarized in Tables 1 and 2.
Table 1.
Efficacy Outcomes Reported in Phase 3 Clinical Trials of First-Line Treatments for HCC and Product Label Information
| Sorafenib (SHARP Trial)2 | Lenvatinib (REFLECT Trial)3 | Atezolizumab + Bevacizumab (IMbrave150 Trial)5 | |
|---|---|---|---|
| Clinical trial design | Multicenter, randomized, double-blind, placebo-controlled, phase 3 trial | Multicenter, randomized, open-label, phase 3, noninferiority trial | Multicenter, randomized, open-label, phase 3, superiority trial |
| Year of publication | 2008 | 2018 | 2020 |
| Clinical trial population | Advanced-stage HCC (confirmed by pathological analysis; patients were not eligible for or had disease progression after surgical or locoregional therapies), ≥1 untreated target lesion that could be measured in one dimension, according to RECIST; Child-Pugh class A; ECOG performance status score ≤2 |
Unresectable HCC (confirmed histologically or cytologically, or clinically according to AASLD criteria); ≥1 measurable target lesions based on modified (mRECIST), BCLC stage B or C categorization; Child-Pugh class A; and an ECOG performance status score of 0 or 1 |
Locally advanced, metastatic or unresectable HCC (or both), confirmed histologically or cytologically, or clinically according to AASLD criteria; measurable disease, defined by RECIST 1.1, that was not amenable to curative or locoregional therapies or that had progressed thereafter; Child-Pugh class A; ECOG performance status score of 0 or 1; and adequate hematologic and organ function |
| Total randomized patients at baseline according to BCLC staging system, % | BCLC stage B: 17 BCLC stage C: 82 BCLC stage D: <1 |
BCLC stage B: 21 BCLC stage C: 79 |
BCLC stage A: 3 BCLC stage B: 16 BCLC stage C: 82 |
| Exclusion criteria | Previously received molecularly targeted therapies or any other systemic treatment |
≥50% liver occupation, obvious invasion of the bile duct, or invasion at the main portal vein; or if they had received previous systemic therapy for hepatocellular carcinoma | Previous systemic therapy for liver cancer; history of autoimmune disease, coinfection with hepatitis B or hepatitis C virus, and untreated or incompletely treated esophageal or gastric varices with bleeding or high risk of bleeding |
| Treatments | 400 mg sorafenib orally twice daily or matching placebo | Lenvatinib 12 mg/day (for bodyweight ≥60 kg) or 8 mg/day (for bodyweight <60 kg); or sorafenib 400 mg orally twice daily | Atezolizumab 1200 mg plus bevacizumab 15 mg/kg bodyweight intravenously every 3 weeks; or sorafenib 400 mg orally twice daily |
| Median OS, months (95% CI) | Sorafenib 10.7 (9.4–13.3) Placebo 7.9 (6.8–9.1) HR 0.69 (0.55 to 0.87) P <0.001 |
Lenvatinib 13.6 (12.1–14.9) Sorafenib 12.3 (10.4–13.9) HR 0.92 (0.79–1.06) P = NS |
Atezolizumab-bevacizumab NE Sorafenib 13.2 (10.4–NE) HR 0.58 (0.42–0.79) P <0.001 |
| Progression-free survival, months (95% CI) | — | Lenvatinib 7.4 (6.9–8.8) Sorafenib 3.7 (3.6–4.6) HR 0.66 (0.57−0.77) P <0.0001 |
Atezolizumab-bevacizumab 6.8 (5.7–8.3) Sorafenib 4.3 (4.0–5.6) HR 0.59 (0.47–0.76) P <0.001 |
| 1-year survival, % (95% CI) | Sorafenib 44 Placebo 33 Relative risk reduction 31% |
— | Atezolizumab-bevacizumab 67.2 (61.3–73.1) Sorafenib 54.6 (45.2–64.0) |
| Time to progression (months) | — | Lenvatinib 8.9 (7.4–9.2) Sorafenib 3.7 (3.6–5.4) HR 0.63 (0.53–0.73) P <0.0001 |
— |
| Time to symptomatic progression (months) | Sorafenib 4.1 Placebo 4.9 HR 1.08 (95% CI 0.88 to 1.31) P = 0.77 |
— | — |
| Time to radiologic progression (months) | Sorafenib 5.5 Placebo 2.8 HR 0.58 (95% CI, 0.45 to 0.74) P <0.001 |
— | — |
| HCC label indicationa | Unresectable HCC | For the first-line treatment of patients with unresectable HCC | Atezolizumab in combination with bevacizumab for the treatment of patients with unresectable or metastatic HCC who have not received prior systemic therapy |
Notes: aBased on the information reported in the lenvatinib USPI dated November 2020, the sorafenib USPI dated July 2020, the bevacizumab USPI dated October 2020, and the atezolizumab USPI dated November 2020.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; mRECIST, modified RECIST; NE, could not be evaluated; NS, not significant; OS, overall survival; RECIST, Response Evaluation Criteria in Solid Tumors; —, not reported; USPI, United States Package Insert.
Table 2.
Safety Outcomes Reported in Phase 3 Clinical Trials of First-Line Treatments for HCC and US Product Label Informationa
| Sorafenib (SHARP Trial)2 | Lenvatinib (REFLECT Trial)3 | Atezolizumab + bevacizumab (IMbrave150 Trial)5 | |
|---|---|---|---|
| Adverse events overall incidence, % | Sorafenib: 80 Placebo: 52 |
Lenvatinib: 99 Sorafenib: 99 |
Atezolizumab + bevacizumab: 98 Sorafenib: 99 |
| Most common adverse events (>10%) | Sorafenib: diarrhea, fatigue, hand-foot skin reaction, rash or desquamation, alopecia, anorexia, nausea Placebo: fatigue, rash or desquamation, diarrhea, nausea |
Lenvatinib: Palmar-plantar erythrodysethesia, diarrhea, hypertension, decreased appetite, decreased weight, fatigue, proteinuria, dysphoria, nausea, abdominal pain, decreased platelet count, elevated aspartate aminotransferase, hypothyroidism, vomiting, constipation, rash, increased blood bilirubin Sorafenib: Palmar-plantar erythrodysethesia, diarrhea, hypertension, decreased appetite, decreased weight, fatigue, alopecia, proteinuria, dysphoria, nausea, abdominal pain, decreased platelet count, elevated aspartate aminotransferase, constipation, rash, increased blood bilirubin |
Atezolizumab + bevacizumab: hypertension, fatigue, proteinuria, aspartate aminotransferase increase, pruritis, diarrhea, decreased appetite, pyrexia, alanine aminotransferase increase, constipation, blood bilirubin increase, rash, abdominal pain, nausea, cough, infusion-related reaction, weight decrease, platelet count decrease Sorafenib: hypertension, fatigue, aspartate aminotransferase increase, diarrhea,decreased appetite, constipation, blood bilirubin increase, rash, abdominal pain, nausea, platelet count decrease, asthenia, alopecia, palmar-plantar erythrodysesthesia syndrome |
| Adverse events leading to dose reduction/withdrawal, % | Sorafenib: 26/11 Placebo: 7/5 |
Lenvatinib: 37/9 Sorafenib: 38/7 |
Atezolizumab + bevacizumab: 49.5b/15.5 Sorafenib: 60.9b/10.3 |
| Adverse events of grade 3 or 4, % | not reported | Lenvatinib: 57c Sorafenib: 49c |
Atezolizumab + bevacizumab: 56.5 Sorafenib: 55.1 |
| Adverse events of grade 5, % | not reported | not reported | Atezolizumab + bevacizumab: 4.6 Sorafenib: 5.8 |
| Serious adverse events, % | not reported | Lenvatinib: 18 Sorafenib: 10 |
Atezolizumab + bevacizumab: 38 Sorafenib: 31 |
| Label contraindications (US) | Patients with known severe hypersensitivity to sorafenib or any other component Sorafenib in combination with carboplatin and paclitaxel is contraindicated in patients with squamous cell lung cancer |
None | Atezolizumab: None Bevacizumab: None |
| Label warnings (US) | Cardiovascular events, bleeding, hypertension, dermatologic toxicities, gastrointestinal perforation, QT prolongation, drug-induced liver injury, embryo-fetal toxicity, impairment of TSH suppression in DTC | Hypertension, cardiac dysfunction, ATE, hepatotoxicity, renal failure or impairment, proteinuria, diarrhea, fistula formation and gastrointestinal perforation, QT interval prolongation, hypocalcemia, hemorrhagic events, RPLS, impairment of TSH/hormone suppression/thyroid dysfunction, impaired wound healing, embryo-fetal toxicity |
Atezolizumab: immune-mediated pneumonitis, immune-mediated hepatitis, immune-mediated colitis, immune-mediated endocrinopathies, hypophysitis, thyroid disorders, adrenal insufficiency, type 1 diabetes mellitus, infections, infusion-related reactions, embryo-fetal toxicity Bevacizumab: gastrointestinal perforations and fistula, surgery and wound healing complications, hemorrhage, ATE, VTE, hypertension, hypertensive crisis or hypertensive encephalopathy, PRES, renal injury and proteinuria, infusion-related reactions, embryo-fetal toxicity, ovarian failure, CHF |
Notes: aBased on the information reported in the lenvatinib USPI dated November 2020, the sorafenib USPI dated July 2020, the bevacizumab USPI dated October 2020, and the atezolizumab USPI dated November 2020. bReported as dose modification or interruption. cThis percentage corresponds to adverse events of grade ≥3.
Abbreviations: ATE, arterial thromboembolic events; CHF, congestive heart failure; DTC, differentiated thyroid cancer; HCC, hepatocellular carcinoma; PRES, posterior reversible encephalopathy syndrome; RPLS, reversible posterior leukoencephalopathy syndrome; TSH, thyroid-stimulating hormone; VTE, venous thromboembolic events.
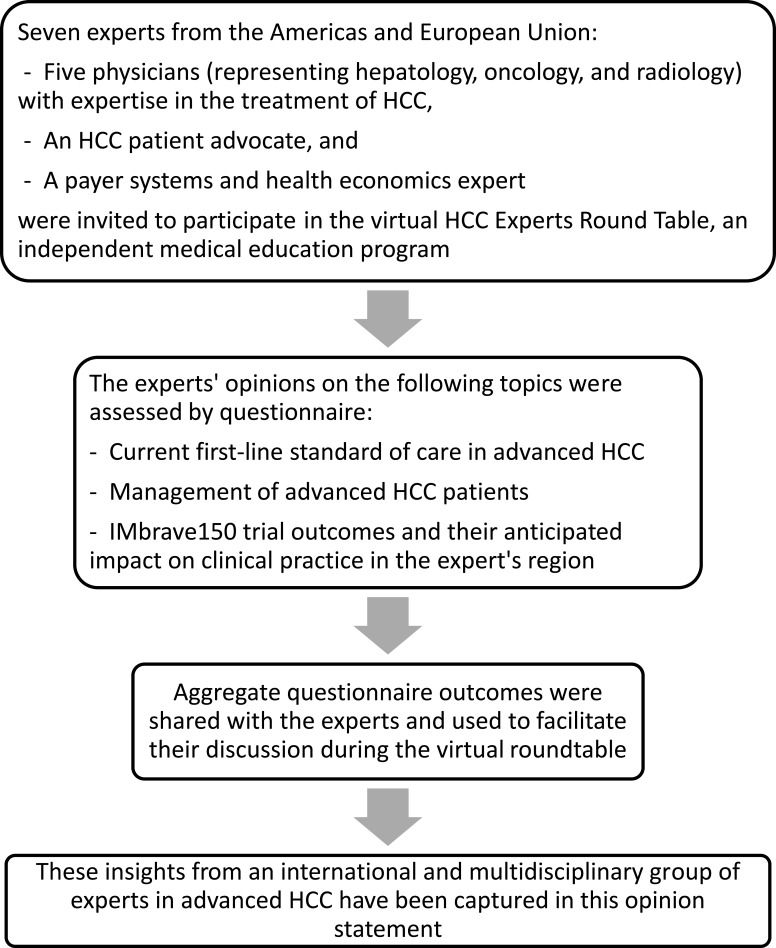
A virtual expert roundtable including five physicians with expertise in the treatment of HCC (LK, LGF, ARH, JR, PRG), an HCC patient advocate (AW), and payer systems and health economics expert (YZ) was convened on April 16, 2020 and facilitated by COR2ED, an independent medical education company. In advance of the expert roundtable, COR2ED designed a questionnaire to collect participants’ expert opinion on topics including the current first-line standard of care in advanced HCC, the management of advanced HCC patients, and IMbrave150 trial outcomes and their anticipated impact on clinical practice. COR2ED compiled, anonymized, and shared the outcomes with the expert participants prior to the roundtable. During the expert roundtable, these outcomes were presented without bias, in keeping with the independent nature of the program. The aggregate results of the questionnaire formed the basis for all expert discussion during the virtual roundtable facilitated by COR2ED (Figure 1). This commentary presents highlights from this HCC experts roundtable and provides a multidisciplinary expert opinion regarding treatment approaches in the Americas and European Union for advanced HCC in the first-line setting.
Figure 1.
Development of multidisciplinary expert opinion on the evolving treatment landscape of advanced hepatocellular carcinoma in the first-line setting.
What are the Key Clinical Considerations Facing Selection of First-Line Treatment of HCC in the Americas and European Union?
Prior to the results from IMbrave150, the multikinase inhibitor sorafenib was generally considered the standard of care for the first-line treatment of advanced HCC.4 While lenvatinib, another multikinase inhibitor, is equally supported in treatment guidelines, it is not as widely used as a first-line agent by all treating physicians. We believe that this discrepancy may be partially related to more limited availability and insurance coverage in different countries and regions for lenvatinib.8,9 Additionally, physicians’ long-term experience using sorafenib and associated comfort level, combined with the noninferiority of lenvatinib compared with sorafenib in the REFLECT trial, has likely influenced prescribing decisions.3,8
When evaluating new drugs for the treatment of unresectable HCC, improved OS is the primary endpoint in pivotal clinical trials. In the SHARP trial, the median OS for sorafenib was 10.7 months, which was significantly longer than the median OS of 7.9 months for placebo.2 In the REFLECT trial, the median OS for lenvatinib was 13.6 months, which was noninferior to the median OS for sorafenib of 12.3 months.3 The modest improvement in median OS associated with these agents (approximately 3 months) left an unmet need for more effective first-line treatment options for patients with HCC.
We observe that the tolerability of sorafenib and lenvatinib is generally acceptable, with a low percentage of patients permanently discontinuing due to toxicity.2,3 Adverse events (AEs) are predictable based on class effects; however, some AEs—including diarrhea, fatigue, decreased appetite, hand and foot reaction, and hypertension—are troublesome for patients and can negatively impact the quality of life. In general, patients who experience certain AEs (ie, dermatologic events, hypertension, and diarrhea) during treatment with sorafenib were reported to be more likely to respond to treatment.10–12 Therefore, in many cases, patients are willing to accept unpleasant AEs if there are an improved survival benefit and management strategies to support patients (eg, dosage reduction) can be employed. Importantly, immune-related AEs are generally broader and may affect multiple organ systems.13 If patients experience severe, clinically relevant immune-related AEs while receiving IO therapies, these agents should be withheld and/or discontinued.13
Available data regarding response to therapy based on HCC etiology suggest that sorafenib provides a greater benefit in patients infected with hepatitis C virus (HCV); this benefit was less pronounced in patients infected with hepatitis B virus (HBV).14,15 It is not known if the consistently improved OS associated with sorafenib in patients with HCV is related to active viral replication or if this effect persists after a sustained viral response is achieved. Of note, lenvatinib was associated with the greatest relative efficacy compared with sorafenib in patients with HBV, but the difference did not reach statistical significance.16 The data regarding efficacy according to viral etiology are based on post hoc analyses. Prospective trials are needed to better understand potential mechanistic differences based on viral etiology before drawing definitive conclusions that would impact treatment decisions.
Additional biomarker research is required to identify subsets of patients most likely to benefit from longer periods of disease control and OS with sorafenib and lenvatinib. In the future, biomarkers may be of particular importance for selecting patients who are most appropriate for IO treatments; however, at present, we have no means to predict response.
What are the Implications of the IMbrave150 Trial Results for Clinical Practice?
The IMbrave150 clinical trial results demonstrated a statistically significant survival advantage of IO treatment with the programmed cell death ligand 1 inhibitor, atezolizumab, plus the vascular endothelial growth factor inhibitor bevacizumab compared with sorafenib as first-line systemic therapy in advanced HCC.5 Because sorafenib has been widely regarded as the standard of care for first-line therapy of unresectable HCC, these results are likely to have a substantial impact on patient care. However, it is important to interpret clinical trial results over time with caution. For example, the median OS associated with sorafenib was 10.7 months in the SHARP trial, 12.3 months in the REFLECT trial, and 13.2 months in the IMbrave150 trial. These differences may be due to improvements in patient care over time as well as differences among inclusion and exclusion criteria in the three studies (Table 1).
Atezolizumab plus bevacizumab remains a new treatment regimen, and clinicians are or will be learning about its safety and potential toxicities in more heterogenous real-world populations in clinical practice. As real-world data based on treatment experience with atezolizumab plus bevacizumab emerge, they will provide important information to guide treatment approaches. In the meantime, several questions arise as atezolizumab plus bevacizumab is integrated into clinical practice and patient care evolves.
In the IMbrave150 trial, the OS for atezolizumab plus bevacizumab was significantly improved compared with sorafenib (P <0.001).5 However, median OS was not reached in the atezolizumab plus bevacizumab treatment arm, making it more complicated to form a complete assessment of efficacy. Nonetheless, a complete data set is not necessarily needed if it is clear from the available data that a treatment provides a survival benefit according to the trial design (ie, internal validity). Furthermore, the ability to assess the validity of OS data can be confounded by the length of follow-up as well as the use of second-line therapies post-progression (especially IO therapies). As more investigational treatments emerge, other treatments—particularly combination therapies for which there is a biological plausibility for improved efficacy compared to monotherapy—may also not reach a median OS during clinical trials. Therefore, alternative measures (eg, progression-free survival or survival at 1 year or 2 years) are needed to interpret survival benefits and communicate information to patients, clinicians, and payers to inform their decisions.
Following FDA approval on May 29, 2020 and the EMA CHMP opinion on September 17, 2020, we anticipate that atezolizumab plus bevacizumab will become the standard of care for patients with advanced HCC. However, this approach will complicate decisions regarding the sequencing of treatments for second-line therapies and beyond. There are no data to define optimal treatment for patients who progress after first-line combination treatments.1 While head-to-head trials are the optimal strategy for assessing comparative efficacy to determine the most effective sequencing, indirect comparisons provide a practical alternative that is increasingly being utilized within the accepted limitations due to heterogeneity across trials. Expert opinion will play an important role in interpreting available evidence and guiding the sequencing of treatment.
As atezolizumab plus bevacizumab is expected to become first-line therapy for advanced HCC, it is unclear whether sorafenib and lenvatinib will become second-line therapies or if current second-line therapies (eg, regorafenib, cabozantinib, ramucirumab [approved as second-line for patients with alpha fetoprotein of ≥400 ng/mL], nivolumab, pembrolizumab, and the combination of nivolumab and ipilimumab)1,17 will be used in patients who progress on atezolizumab plus bevacizumab. Furthermore, additional investigational therapies may emerge that will need to be incorporated into the treatment paradigm for HCC.
The appropriate time to switch to secondary and tertiary therapies is another important decision when treating HCC. Minor progression or stable disease could lead to a continuation of the current therapy, whereas a lack of clinical benefit (eg, decline of liver function from cancer progression, worsening clinical symptoms from cancer progression, or appearance of distant metastasis) or unacceptable toxicity are generally accepted as outcomes that indicate the need to switch therapies. Individual clinical response patterns including decline in tumor markers, improvement of performance status, or change in Child-Pugh class may also help guide the timing for treatment switches and the selection of subsequent treatments.
Importantly, radiological progression does not necessarily signify short-term treatment failure or poor prognosis.18,19 Further, data illustrating relationships between patterns of progression and outcomes are lacking. Development of models that stratify patients with HCC upon progression and allow identification of different prognostic subgroups associated with specific patterns of radiological progression (eg, intrahepatic vs extrahepatic progression) would help inform the decision-making process. A more complex, “dismal” endpoint that accounts for radiological progression, pattern of spread, growth rate, impact on liver condition, and prognostic and predictive biomarkers was proposed but requires validation.19
To improve OS—which remains the main objective in systemic treatment for HCC—it is key to identify clinically relevant events of tumor progression (eg, malignant portal vein thrombosis) that can lead to rapid deterioration and disqualify patients for subsequent treatment approaches. Therefore, treatment approaches that are most likely to delay clinically relevant disease progression while preserving liver function are needed to preserve future options. Tumor boards are expected to play an increasingly essential role in guiding treatment selection for individual patients.
Utilizing agents with different mechanisms of action (eg, a tyrosine kinase inhibitor and an IO therapy) may be an ideal approach, and clinical trials are underway investigating various combinations.20 Treatment decisions may also be individualized based on the potential for interactions with comorbid patient conditions (eg, hypertension) as well as the pattern of their response to first-line therapy (eg, length of response, toxicities). Finally, the accessibility of treatment options within a patient’s country or insurance plan may constrain the set of feasible combinations or sequencing choices at the individual patient level.
What Patient Characteristics May Inform Treatment Selection with Atezolizumab Plus Bevacizumab?
The majority of patients enrolled in IMbrave150 were Barcelona Clinic Liver Cancer (BCLC) stage C (81% in the sorafenib arm and 82% in the atezolizumab plus bevacizumab arm) and a smaller proportion were stage B (16% in the sorafenib arm and 15% in the atezolizumab plus bevacizumab arm).5 Post hoc analyses showed that the OS and progression-free survival hazard ratios favoring atezolizumab plus bevacizumab were greatest for stage C patients.21 Post hoc analyses also indicated a possible benefit in patients with HBV or HCV who were treated with atezolizumab plus bevacizumab compared with sorafenib.21
Experts remain divided regarding whether atezolizumab plus bevacizumab is an appropriate first-line therapy for BCLC stage B patients. Intra-arterial therapies, specifically transarterial chemoembolization (TACE) is the currently endorsed therapy for patients who are stage B.22 However, TACE was developed at a time when systemic therapy of HCC was unavailable. Some experts believe that starting systemic treatment earlier may delay the development of metastatic disease.23 Due to potential concerns for graft rejection in patients receiving IO treatments, the possibility that a patient may be downstaged to be eligible for transplant or resection should also be considered when selecting systemic treatment options. Individual patient characteristics will likely inform whether patients who are stage B should attempt systemic therapy rather than TACE.23 Future clinical trials that assess systemic therapy options in intermediate stage disease are needed.
What Real-World Factors Influence the Selection of First-Line Therapies?
Information about patients’ preferences and experiences as well as payer coverage decisions can have an important influence on clinical decision-making for individual patients.
Patient Preferences and Patient-Reported Outcomes
In our experience, extending life and returning to regular activities are generally the most pressing concerns for patients. However, there is typically a trade-off between length of life and quality of life. Patients are often willing to delay a return to activity if it will result in extending their life in a meaningful way, which is subjective and can vary. Patients may prefer the convenience of oral therapies that they can self-administer at home, instead of infusions, particularly if they must travel a significant distance to an infusion center.
If treatment appears to be futile, the focus should shift to supporting the patient to live in the way that is most meaningful. For example, a patient may have a goal to live long enough to see a certain life event (such as a wedding or birth) and be willing to tolerate AEs to potentially achieve that goal. In other cases, palliation of symptoms may be a more acceptable approach that allows patients to engage in role functions (eg, parenting) to the fullest extent possible for as long as possible.
Patient-reported outcomes (PROs) data can help clinicians select treatments that will align with individual patient goals. PROs are intended to assess the benefit of a treatment option more broadly in terms of outcomes that are important to patients and have recently become an important focus in clinical trials and subsequent regulatory and payer approval. PRO data from the IMbrave150 trial were collected with the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire for Cancer instrument.24 These PRO data indicated that, compared with sorafenib, treatment with atezolizumab plus bevacizumab resulted in clinically meaningful delays in deterioration in patient-reported quality of life, physical functioning, and role functioning.24 Atezolizumab plus bevacizumab also delayed deterioration in key HCC-related patient-reported symptoms, including appetite loss, diarrhea, fatigue, and pain.24
Payer Coverage Decisions
The cost-effectiveness of available treatments is another important consideration, and payer coverage of therapies impacts patient access. When making coverage decisions, payers must weigh the benefits of interventions against the cost and seek to equitably allocate scarce resources to best meet the needs of the entire population they cover.
When evaluating new therapies, payers carefully weigh any increase in cost against the magnitude of the additional benefit provided. We anticipate that the criteria for evaluating benefits of atezolizumab plus bevacizumab may be based on efficacy, tolerability, effects on quality of life assessed by PROs, ability to manage AEs, downstaging opportunities, and route of administration (ie, oral vs infusion), and include the impact of these factors on treatment adherence and satisfaction. Additionally, factors including the size of the eligible patient population, degree of additional survival benefit, health insurance setup, and competing financial pressures will likely inform coverage decisions and application of utilization management strategies.
What is the Role of Tumor Boards in Selecting Treatments for Individual Patients?
A multidisciplinary approach is critical in the treatment of patients with HCC, and multidisciplinary tumor boards (MTBs) are essential for remaining abreast of emerging information and applying this information to real-world clinical care when selecting treatment approaches for individual patients.
The membership of an MTB should include all appropriate specialists and can be customized to address individual patient needs. Core members of a tumor board could call upon other specialists to participate as needed based on patient characteristics and circumstances. For example, a social worker could be invited to the MTB when appropriate to help with designing patient interventions and supports. Specialists who can advise the treatment team about the management of AEs—such as dermatologists to address hand and foot reactions or immunologists to address the management of immune-related AEs—could also help the MTB customize treatment approaches. As biomarkers that can inform treatment decisions are identified, inclusion of specialists with an expertise for interpreting this information will also be important.
Summary
The treatment of advanced HCC is rapidly evolving and treatment decisions are becoming more complex as clinicians aim to appropriately apply innovations to patient care. Compared to 15 years ago, we now can offer multiple treatment options that provide meaningful improvement in survival. The improved OS and PFS associated with atezolizumab plus bevacizumab, compared with sorafenib, indicate that this therapy will play an important role in clinical practice. However, treatment choices should be individualized based on patient characteristics and preferences. Additionally, third-party payer coverage for emerging oncologic therapies will have an important impact on patient access to treatment options.
In the future, biomarkers that can be identified before therapeutic decisions would be useful, enabling individualized treatment. Because HCC is a rapidly evolving field, it remains critical that patients are cared for by multidisciplinary teams that remain abreast of new developments and can apply these developments to clinical practice in the real world.
Acknowledgments
The HCC Experts Round Table was supported by an Independent Educational Grant from Roche to COR2ED GmbH, an independent medical education company (Bottmingen, Basel-Land, Switzerland). Roche did not have a role in the design and conduct of the HCC Experts Round Table; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication. Medical writing support was provided by Judy Lofton, MS, of AlphaBioCom, LLC (King of Prussia, PA, USA) and was funded by COR2ED through an Independent Educational Grant from Roche.
Funding Statement
Supported by: COR2ED (Bottmingen, Basel-Land, Switzerland) through an Independent Educational Grant from Roche.
Disclosure
LK declares serving as a speaker, consultant or advisory board member for Bayer, Eisai, Merck, Exelixis, and BMS; and reports personal fees from Eisai, Gilead, Merck, Exelixis, Bayer, and PeeerView, and research support from Target HCC, during the conduct of the study. ARH reports grants from Genentech and Merck, outside the submitted work, speaker’s bureau for BMS and Bayer. JR reports receiving lecture fees and a travel grant from Bayer during the conduct of the study. AWW declares serving as a consultant for AstraZeneca, Genentech, and Eisai; and serving as CEO and Co-founder of Cancer University, a for-profit, social-benefit, health-tech startup; and reports grants from AstraZeneca, Bristol-Myers Squibb, Eisai, Exelixis, and Genentech, outside the submitted work. PG declares financial relationships with Bayer, BMS, MSD, Merck, SIRTEX, AstraZeneca, Sillajen, Lilly, Roche, Novartis, Ipsen; and reports grants from Roche, grants and personal fees from Bayer. The authors report no other potential conflicts of interest for this work.
References
- 1.National Comprehensive Cancer Network. NCCN guidelines version 1.2020. Hepatocellular carcinoma. 2020:1–151. [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 4.Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol. 2018;7:17. doi: 10.1186/s40164-018-0109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. [DOI] [PubMed] [Google Scholar]
- 6.FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma; 2020. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma. Accessed June26, 2020.
- 7.European Medicines Agency. Tecentriq. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/tecentriq-2. Accessed October19, 2020.
- 8.Personeni N, Pressiani T, Rimassa L. Lenvatinib for the treatment of unresectable hepatocellular carcinoma: evidence to date. J Hepatocell Carcinoma. 2019;6:31–39. doi: 10.2147/JHC.S168953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kircher SM, Meeker CR, Nimeiri H, et al. The parity paradigm: can legislation help reduce the cost burden of oral anticancer medications? Value Health. 2016;19(1):88–98. doi: 10.1016/j.jval.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Gonzalez A, Sanduzzi-Zamparelli M, Sapena V, et al. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2019;49(5):482–491. doi: 10.1111/apt.15088 [DOI] [PubMed] [Google Scholar]
- 11.Bettinger D, Schultheiss M, Knuppel E, Thimme R, Blum HE, Spangenberg HC. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2012;56(2):789–790. doi: 10.1002/hep.25637 [DOI] [PubMed] [Google Scholar]
- 12.Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36(4):319–324. doi: 10.1097/COC.0b013e3182468039 [DOI] [PubMed] [Google Scholar]
- 13.Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 2017;69(4):687–699. doi: 10.1002/art.40043 [DOI] [PubMed] [Google Scholar]
- 14.Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized Phase III trials. J Clin Oncol. 2017;35(6):622–628. [DOI] [PubMed] [Google Scholar]
- 15.Yeh ML, Huang CF, Huang CI, et al. The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment. Kaohsiung J Med Sci. 2019;35(10):624–632. doi: 10.1002/kjm2.12105 [DOI] [PubMed] [Google Scholar]
- 16.Park J, Cho J, Lim JH, Lee MH, Kim J. Relative efficacy of systemic treatments for patients with advanced hepatocellular carcinoma according to viral status: a systematic review and network meta-analysis. Target Oncol. 2019;14(4):395–403. doi: 10.1007/s11523-019-00651-7 [DOI] [PubMed] [Google Scholar]
- 17.Cyramza prescribing information Indianapolis, IN: Eli Lilly and Company; 2020. [Google Scholar]
- 18.Reig M, Rimola J, Torres F, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58(6):2023–2031. doi: 10.1002/hep.26586 [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16(10):617–630. doi: 10.1038/s41575-019-0179-x [DOI] [PubMed] [Google Scholar]
- 20.El Dika I, Makki I, Abou-Alfa GK. Hepatocellular carcinoma, novel therapies on the horizon. Chin Clin Oncol. 2020;9:8. doi: 10.21037/cco-20-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn RS, Qin S, Ikeda M, et al. Supplement to: atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 22.Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M, Ikeda M, Ueshima K, et al. Response evaluation criteria in cancer of the liver version 5 (RECICL 2019 revised version). Hepatol Res. 2019;49(9):981–989. doi: 10.1111/hepr.13394 [DOI] [PubMed] [Google Scholar]
- 24.Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes from the phase 3 IMbrave150 trial of atezolizumab + bevacizumab versus sorafenib as first-line treatment for patients with unresectable hepatocellular carcinoma. Abstract 476. In: 2020 Gastrointestinal Cancers Symposium; January24, 2020; San Francisco, CA, USA; 2020. [Google Scholar]