Summary
Motivation is characterized by a willingness to overcome both cognitive and physical effort costs. Impairments in motivation are common in striatal disorders, such as Huntington’s disease (HD), but whether these impairments are isolated to particular domains of behavior is controversial. We ask whether HD differentially affects the willingness of individuals to overcome cognitive versus physical effort. We tested 20 individuals with pre-manifest HD and compared their behavior to 20 controls. Across separate trials, participants made choices about how much cognitive or physical effort they were willing to invest for reward. Our key results were that individuals with pre-manifest HD were less willing than controls to invest cognitive effort but were no different in their overall preference for physical effort. These results cannot be explained by group differences in neuropsychological or psychiatric profiles. This dissociation of cognitive- and physical-effort-based decisions provides important evidence for separable, domain-specific mechanisms of motivation.
Keywords: Huntington’s disease, motivation, apathy, decision making, effort discounting, cognitive effort, physical effort, reward, striatum
Highlights
We examine cognitive and physical effort discounting in pre-manifest HD
Individuals with pre-manifest HD are less cognitively motivated than controls
There are no differences in physical motivation between the two groups
This dissociation is not confounded by neuropsychological or psychiatric factors
Motivational impairments are common in striatal disorders, such as Huntington’s disease (HD). Atkins et al. show that individuals in the pre-manifest stage of HD are less cognitively motivated than controls but equally physically motivated. These results provide empirical support for theories that posit the existence of dissociable subtypes of apathy.
Introduction
A fundamental component of daily life is the willingness to engage in cognitively and physically demanding behavior. Motivation is the process that allows us to overcome effort in pursuit of reward. A growing body of work has implicated the striatum and its connections to the prefrontal cortex as the core of a decision-making network critical to motivated behavior.1, 2, 3, 4, 5, 6 The importance of this network to motivation is exemplified by the frequency of apathy in disorders such as Huntington’s disease (HD), which are characterized by dysfunction to the striatum and its cortical connections. Apathy is a disorder of motivation that can be particularly debilitating and have a significant effect on quality of life, but relatively little is known about its underlying mechanisms.
HD is a progressive neurodegenerative disease whose pathognomonic feature is early striatal cell loss. HD is caused by the expansion of a trinucleotide cytosine-adenine-guanine (CAG) repeat in the huntingtin gene.7, 8, 9 Because it is a highly penetrant, monogenic disease, we can identify asymptomatic gene carriers years before it becomes clinically manifest.9 The prevalence of apathy closely tracks disease progression—rising from 11% in its earliest (pre-manifest) stage to up to 76% in clinically manifest disease.10, 11, 12 The rise in apathy with disease progression mirrors the progressive involvement of corticostriatal pathways, particularly those that have been implicated in facilitating motivation.13,14
Importantly, motivation is not a unitary phenomenon and has been fractionated into subtypes that drive different domains of behavior (e.g., cognitive versus physical effort). A topical controversy has been whether impairments of motivation in one domain are necessarily accompanied by impairments in another (i.e., are “domain general”) or whether such impairments are dissociable across multiple domains (i.e., are “domain specific”). To date, however, no clinical study—in HD or any other patient group—has directly addressed the domain specificity of effort-based decisions. The majority of patient studies have focused on motivation in the physical domain alone,15, 16, 17, 18, 19 with fewer examining motivation in the cognitive domain20,21 and none comparing motivation across both domains within the same individuals. Furthermore, interpreting differences in motivation between patient groups relative to healthy controls can in general be challenging, given that clinical populations are likely to have comorbid motor, cognitive, or psychiatric symptoms that may confound any such differences.22
Here, we asked whether individuals in the pre-manifest stage of HD exhibit dissociable patterns of cognitive and physical motivational deficits compared to healthy controls. Pre-manifest HD offers a unique opportunity to study the distinct consequences of HD on cognitive and physical motivation while minimizing the more profound behavioral and motor effects that accompany advanced disease.23, 24, 25 Importantly, our pre-manifest HD and control groups did not differ across several critical features, including mood, attention, processing speed, and episodic memory, indicating that any differences in motivation between groups could not be due to co-existent clinical features.
To sensitively measure the effect of HD on cognitive and physical motivation, we adopted a neuroeconomic approach to quantify the amount of effort individuals are willing to trade off for a given reward.26, 27, 28, 29 Typically, individuals are averse to investing effort, and effort devalues (or “discounts”) the amount of reward that is available. Effort discounting has proven to be a useful approach to quantifying individual differences in motivation—one that is capable of detecting even subclinical levels of motivational impairment in patient populations.21,30
To disentangle cognitive and physical motivation, we designed two tasks that parametrically varied effort requirements in one domain while holding those in the alternate domain constant. Notably, these two tasks were closely matched in their temporal and demand characteristics. After training participants on both tasks, they were asked to decide how much effort they would be willing to invest in each domain for a given reward. By requiring participants to make separate decisions for the cognitive and physical effort tasks, we could derive separate measurements of motivation for each domain of motivation.
Results
We recruited 20 individuals in the pre-manifest stage of HD. These individuals were genetically confirmed to have ≥38 CAG repeat expansions in the huntingtin gene and had a diagnostic confidence level of <4 on the Unified Huntington’s Disease Rating Scale (UHDRS). We compared their performance to 20 healthy controls, matched for age and gender (Table 1; STAR Methods). Importantly, the groups did not differ across several performance-based cognitive measures, including a standard cognitive screening tool (the Montreal Cognitive Assessment [MoCA]) as well as neuropsychological tests of episodic memory (Hopkins Verbal Learning Test-Revised [HVLT-R]) and attention/psychomotor speed (Symbol Digit Modalities Test [SDMT]). Groups were also matched on scores of clinical anxiety and depression (Hospital Anxiety and Depression Scale)31 and apathy (Apathy Evaluation Scale;32 Dimensional Apathy Scale33).
Table 1.
Summary of participant demographics means (SD)
| Healthy Controls | Pre-manifest HD | Group Difference | |
|---|---|---|---|
| N | 20 | 20 | n.s. |
| Age (years) | 50.8 (12.8) | 46.2 (12.8) | p = 0.27 |
| Gender (M:F) | 8:12 | 6:14 | p = 0.51 |
| Handedness (R:L) | 20:0 | 19:1 | p = 1.0 |
| Apathy Evaluation Scalea | 27.6 (6.4) | 27.4 (5.7) | p = 0.94 |
| Dimensional Apathy Scale – totalb | 22.6 (6.7) | 19.7 (8.3) | p = 0.29 |
| - Executive | 5.7 (3.4) | 4.35 (4.1) | p = 0.17 |
| - Initiation | 8.4 (3.3) | 7.20 (3.6) | p = 0.22 |
| - Emotional | 8.5 (3.0) | 8.15 (3.6) | p = 0.99 |
| Hospital Anxiety and Depression Scalec | |||
| - Anxiety | 5.00 (3.5) | 4.75 (3.2) | p = 0.84 |
| - Depression | 2.95 (2.8) | 2.15 (2.4) | p = 0.28 |
| Montreal Cognitive Assessmentd | 27.3 (1.9) | 27.2 (2.4) | p = 0.83 |
| Hopkins Verbal Learning Test – R | |||
| - Total recall | 27.0 (3.2) | 26.6 (4.3) | p = 0.76 |
| - Delayed recall | 9.00 (2.2) | 8.33 (2.2) | p = 0.13 |
| - Discrimination index | 10.5 (2.0) | 10.8 (1.7) | p = 0.58 |
| Symbol Digit Modalities Test | 56.6 (12.4) | 53.6 (13.2) | p = 0.48 |
| CAG repeats | N/A | 41.3 (1.9) | N/A |
| [38–45] | |||
| Total functional capacity | N/A | 12.9 (0.3) | N/A |
| [12–13] | |||
| Disease burden score | N/A | 254 (82.7) | N/A |
| [147–435] | |||
| Total motor score (UHDRS)e | N/A | 1.35 (0.44) | N/A |
| [0–4] | |||
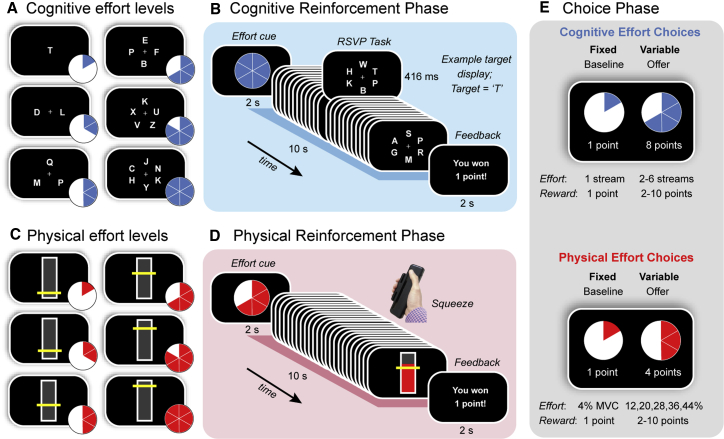
Participants were tested in a single session, during which they completed an effort-based decision-making task, followed by a cognitive test battery. The overall structure of the decision-making task was similar to a previous study examining cognitive- and physical-effort-based decisions in healthy adults (see STAR Methods).5 This task was divided into three phases (Figure 1). The first two (“reinforcement”) phases involved training participants on both a cognitively effortful task (Figures 1A and 1B)21 and a physically effortful task (Figures 1C and 1D),5 in counterbalanced order. Within each task, we parametrically varied effort demands over six levels in the target domain (e.g., cognitive) while keeping those in the other (e.g., physical) constant. In the cognitive effort task, participants had to attend to between one and six streams of rapidly changing letters for a target letter, “T.” In the physical effort task, participants had to exert one of six levels of force on a hand-held dynamometer, quantified as proportions of each participant’s individually calibrated maximum voluntary contraction (MVC).
Figure 1.
Task Design
Participants were first trained on (A and B) a cognitively effortful task and (C and D) a physically effortful task before (E) indicating their preference for investing effort for reward.
(A) The cognitive effort task required participants to monitor one to six RSVP streams for a target letter (“T”).
(B) Each trial began with a blue pie chart indicating the number of streams they had to monitor on that trial. After completing each effort level, participants received feedback on their performance. Each trial lasted 10 s.
(C) The physical effort task required participants to sustain variable amounts of force on a hand-held dynamometer, with the target levels of force defined as a function of each individual’s maximum voluntary contraction (MVC) (4%, 12%, 20%, 28%, 36%, and 44%).
(D) Each trial began with a red pie chart indicating the amount of force they had to apply on that trial. Trial durations were identical to those for the cognitive effort task (10 s). At the conclusion of each trial, participants received feedback on their performance.
(E) The choice phase required participants to decide how much effort they were willing to invest for reward. The choice was always between a fixed baseline option (the lowest level of effort for the lowest reward; one point) and a variable high-effort/high-reward offer (higher levels of effort; rewards of two to ten points). Separate choices were made for cognitive and physical effort.
Finally, to examine participants’ willingness to exert cognitive and physical effort, the reinforcement phases were followed by a critical “choice” phase. In this phase, participants revealed their preference between a fixed, low-effort/low-reward baseline option and a variable, high-effort/high-reward offer. The fixed baseline option was always the option to exert the lowest amount of effort for the lowest reward (one point). In contrast, the variable offer was the option to exert a higher amount of effort (levels 2–6) for a greater reward (2–10 points). Each choice was always between two options in the same domain, which allowed us to separate individuals’ willingness to exert cognitive and physical effort (Figure 1E).
First, we present data from the reinforcement phases, which allowed us to confirm that (1) our cognitive and physical effort manipulations were effective in manipulating task load (i.e., higher levels of effort were objectively more challenging than low levels) and (2) that participants had the capacity to complete all levels of effort, regardless of increasing load (to exclude the possibility that subsequent choices could be influenced by task success).
Task Performance Did Not Differ between Groups
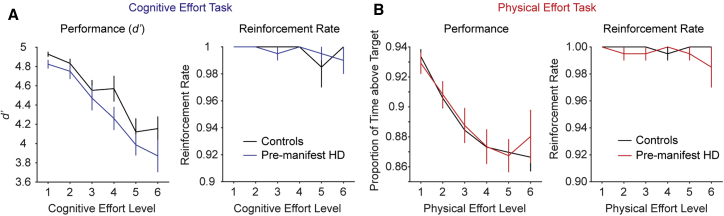
We quantified performance in the cognitive effort task in terms of target detection sensitivity, d’ (Z(hits) – Z(false alarms)), and in the physical effort task as the proportion of time that the generated force was maintained above the target effort level. Using two-way repeated-measures ANOVAs on each of these variables as a function of group (pre-manifest HD, controls) and effort level (1–6), we found that, for each task, performance decreased with increasing effort (Figures 2A and 2B, left panels). Importantly, however, there were no group differences in performance in either the cognitive or physical effort tasks (cognitive: effort F(3.5, 134) = 36.4, p < 0.001; group, F(1, 38) = 2.17, p = 0.15; interaction, F(3.5, 134) = 0.75, p = 0.54); physical: effort, F(2.1, 79.3) = 33.4, p < 0.001; group, F(1, 38) = 0.04, p = 0.85; interaction, F(2.1, 79.3) = 0.56, p = 0.58). These data confirm that our cognitive and physical manipulations were successful in increasing task demands for the respective tasks.
Figure 2.
Performance in the Cognitive and Physical Effort Tasks (Mean ± 1 SEM)
(A) In the cognitive effort task, target detection sensitivity (left panel) and reinforcement rates (right panel) were identical across groups. Controls are shown in black and pre-manifest HD in blue.
(B) In the physical effort task, pre-manifest HD and controls did not differ in the proportion of time they were able to maintain their grip over the target effort level (left panel), which was reflected in identical reinforcement rates between groups (right panel). Controls are shown in black and pre-manifest HD in red.
To ensure that subsequent effort-based decisions were based on the aversiveness of each effort level and not the probability of being able to successfully accomplish them, we next verified that the ability of participants to perform each effort level to the required threshold (their reinforcement rates) was at ceiling (Figures 2A and 2B, right panels). The two-way group × effort ANOVA on reinforcement rates revealed no significant main effects or interactions in either the cognitive or physical tasks (cognitive: group, F(1, 38) = 0.04, p = 0.84; effort, F(5, 190) = 1.19, p = 0.32; group × effort, F(5, 190) = 0.82, p = 0.54; physical: group, F(1, 38) = 1.39, p = 0.25; effort, F(5, 190) = 0.48, p = 0.79; group × effort, F(5, 190) = 0.88, p = 0.49). Together, these data confirm that (1) our tasks effectively increased cognitive and physical loads, (2) both groups were capable of performing each effort level to the required threshold, and (3) there were no significant differences between groups in their ability to perform the tasks or to be rewarded at each level of effort.
Cognitive, but Not Physical, Motivation Was Lower in Individuals with Pre-manifest HD versus Controls
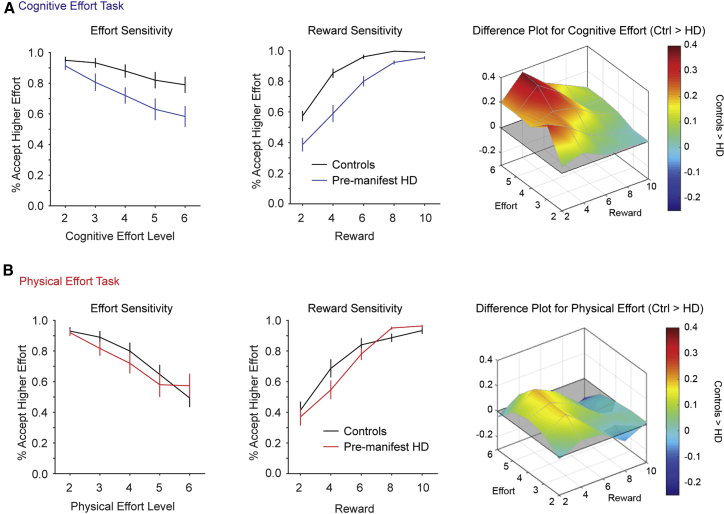
The critical question in this study was whether the pre-manifest HD group differed from controls in their willingness to exert cognitive or physical effort for reward (Figure 3). We examined participants’ choices using a mixed-model ANOVA, with the between-subjects factor of group and within-subjects factors of domain (cognitive and physical), effort (2–6), and reward (2–6).
Figure 3.
Choices in the Cognitive and Physical Effort Tasks (Mean ± 1 SEM)
Acceptance rates for the higher-effort/higher-reward offer are plotted as a function of effort (left column) and reward (center column). Difference plots illustrate choice differences between controls and pre-manifest HD across the two-dimensional effort-reward space (right column). Red indicates greater motivation in controls than pre-manifest HD.
(A) For cognitive-effort-based choices, pre-manifest HD was less willing to accept the higher-effort/higher-reward offers.
(B) For the physical-effort-based choices, decisions were similar between groups.
See also Figures S1 and S2.
The key result was that the pre-manifest HD group accepted significantly fewer effortful offers compared to controls but only for the cognitive effort task and not the physical effort task. This was captured in the two significant higher order interactions involving group: group × domain (F(1, 38) = 4.83; p = 0.03) and group × domain × effort (F(2.1, 80.2) = 3.60; p = 0.03). Decomposing these interactions with Bonferroni-corrected pairwise comparisons indicated that controls were more motivated than individuals with pre-manifest HD. However, this was only the case for the cognitive task and at higher levels of cognitive effort (level 2, p = 0.27; levels 3–6, p ≤ 0.014). Importantly, there were no differences between the groups for any level of the physical effort task (all p ≥ 0.15). The other interactions involving group were not significant (group × effort, F(2.36, 89.78) = 1.86, p = 0.15; group × reward, F(1.58, 60.1) = 2.50, p = 0.10; group × domain × reward, F(2.94, 112) = 0.96, p = 0.41; group × effort × reward, F(4.54, 172) = 0.63, p = 0.66; group × domain × effort × reward, F(8.33, 327) = 0.84, p = 0.57).
The remaining significant main effects and interactions (i.e., not involving group) simply reflected the well-established phenomenon of effort discounting across the entire cohort. The main effects of effort and reward were significant, and this was qualified by a significant effort × reward interaction (effort [F(2.36, 89.7) = 68.1; p < 0.001]; reward [F(1.58, 60.1) = 68.0; p < 0.001]; effort × reward [F(4.54, 172.4) = 12.5; p < 0.001]). This interaction was driven by effort discounting being steepest at the lowest levels of reward and minimal at the highest levels of reward (reflecting the tendency to accept all offers when rewards were high). In addition, the main effect of domain was significant and interacted significantly with effort and reward (domain [F(1, 38) = 6.41, p = 0.016]; domain × effort [F(2.11, 80.2) = 5.55, p = 0.005]; domain × reward [F(2.94, 111.9) = 4.39, p = 0.006]; domain × effort × reward [F(8.33, 317) = 1.90, p = 0.057]). Decomposing these interactions indicated that, overall across both groups, effort discounting was more pronounced for the physical relative to the cognitive domain, with lower acceptance rates for the physical relative to the cognitive effort task at the higher levels of effort (levels 2–4, p ≥ 0.22; levels 5–6, p ≤ 0.02).
In sum, our key result was that the pre-manifest HD group was significantly less cognitively motivated than controls and that choices for the physical effort task did not differ between the two groups.
Performance Differences Did Not Account for the Lower Cognitive Motivation in Pre-manifest HD
To ensure that the lower cognitive motivation in pre-manifest HD relative to controls was not simply due to a lower capacity of the HD group to perform the cognitive effort task, we performed two control analyses. First, we performed a mixed-effects ANOVA on the acceptance rates for the cognitive effort task (group × effort × reward) while controlling for performance by including each participant’s mean d’ as a covariate. This analysis again showed that the pre-manifest HD group were willing to invest less cognitive effort than controls, particularly for the higher levels of cognitive effort (group × effort interaction, F(1.9, 71.3) = 4.44, p = 0.016, with group differences at levels 3–6 [p ≤ 0.022], but not level 2 [p = 0.246]). Importantly, performance did not have a significant effect on acceptance rates (performance, F(1, 37) = 0.011, p = 0.92; performance × effort, F(1.93, 71.3) = 1.26, p = 0.29; performance × reward, F(1.54, 56.9) = 1.13, p = 0.35; performance × effort × reward, F(5.2, 193.2) = 1.66, p = 0.14).
Second, we quantified the null effect of performance on acceptance rates by performing the Bayesian equivalent of the preceding analysis. We included subject as a random intercept in all models. Thus, the null model for all comparisons was a model including the grand mean but also subject as an additive factor. We compared the null model together with the full model space of all simple effects and their interactions. These model comparisons showed that the data were best fit by a model that did not include performance as a factor (namely, group + effort + reward + group × effort + group × reward + effort × reward). The posterior probability of this model was 0.648. The best-fitting model that incorporated performance as a factor was the third best-fitting model overall, with a probability of 0.145. Comparing these two models revealed a Bayes factor of 4.457 in favor of the former—thus providing substantial evidence38 in favor of the best-fitting model without performance as a factor relative to the best model with performance as a factor.
This result was reaffirmed when we compared the family of models that contained performance to equivalent models stripped of its effect. We computed the model-averaged results for each simple effect (group, effort, reward, and performance) and interaction. Most importantly, the family of models that included performance as a predictor had a posterior inclusion probability of 0.178. This corresponded to an exclusion Bayes factor of 4.627 for performance, again providing substantial evidence for excluding it as a predictor. Together, these analyses indicate that the lower cognitive motivation in pre-manifest HD relative to controls is unlikely to have been driven by any group differences in the ability to perform the cognitive effort task.
Computational Models of Choice Confirmed the Dissociation between Cognitive and Physical Effort
Finally, to allow for comparisons between our data and those from previous studies on effort discounting, we applied computational models of effort discounting to choice data from both groups.21,28,39,40 This analysis allowed us to capture individual differences in the preference for cognitive and physical effort, with model comparisons revealing a pattern of results that complemented the dissociation between cognitive and physical motivation noted above. Details of these analyses are presented in the Supplemental Information (Figures S1 and S2).
Discussion
This study examined motivation across multiple domains in pre-manifest HD. Our key findings were that cognitive and physical motivation were differentially affected in individuals with pre-manifest HD relative to healthy controls. The pre-manifest HD group had clear motivational deficits in the cognitive domain, as demonstrated by their lower willingness to exert cognitive effort for reward. In contrast, decisions in the physical domain were unaffected by disease. Importantly, these differences were not confounded by age, gender, neuropsychological or neuropsychiatric issues, or the capacity to successfully perform each task. These data provide empirical support for frameworks proposing that cognitive- and physical-effort-based decisions are dissociable and inform current debates on the role of domain-general versus domain-specific corticostriatal pathways in motivated behavior.13,41,42
Apathy is commonly described as a syndrome in which motivation is diminished across multiple domains.33,43, 44, 45, 46 A recent approach to investigating motivation has been through effort-discounting paradigms, which quantify the amount of effort individuals are willing to exert in return for reward.15,18,21,47 Such paradigms provide a platform to test a critical assumption of prevailing multidimensional theories of apathy—that motivational deficits should be dissociable across separate domains of effort. Here, we provide strong evidence in favor of such theories by demonstrating a selective involvement of cognitive over physical motivation in pre-manifest HD. Beyond its theoretical implications, this result stresses the clinical importance of recognizing the heterogeneity of apathy, particularly with a view to developing potential treatments that are targeted to the affected domain/s.
A key advantage of focusing on HD in the pre-manifest stage was that it allowed us to examine motivation in individuals who were otherwise very closely matched to their healthy counterparts. Several previous studies that have examined cost-benefit decision making in clinical populations have found differences in patterns of effort discounting but in the setting of baseline differences in their neuropsychological or neuropsychiatric profiles. It can therefore become difficult to determine the relationship between group differences in effort discounting and issues with mood or clinical apathy. Here, the HD and control groups did not differ in their ratings of depression or clinical apathy, neuropsychological measures of episodic memory or processing speed, or their ability to successfully perform the cognitive or physical tasks. Thus, our data indicate that motivational impairments can occur independently of comorbid neuropsychiatric issues, neuropsychological disturbance,23,24 or an inability to perform the tasks themselves. Rather, the group differences in motivation most likely reflected a primary motivational deficit.
Our finding that cognitive motivation is more significantly impacted in the early stages of HD could potentially be explained by the characteristic progression of HD pathology. Striatal atrophy in HD typically proceeds along a dorsomedial-to-ventrolateral gradient.48, 49, 50, 51 In pre-manifest disease, the most consistent finding is dorsal striatal atrophy, which is detectable up to 20 years before diagnosis.7, 8, 9,52,53 In addition, other subcortical regions, such as the amygdala, are variably affected in early HD.8,54,55 As the disease advances, there is increasing cortical involvement, particularly of frontal cortical areas.56,57 This progressive corticostriatal dysfunction is believed to underpin the cognitive and behavioral phenotype of HD,58, 59, 60, 61, 62, 63 as well as the rising prevalence of apathy as the disease advances.14,25
Importantly, however, recent studies have suggested that components of these corticostriatal pathways may be differentially sensitive to specific motivational domains. For example, areas that are typically affected earlier in HD (e.g., the dorsal striatum and amygdala) have been more selectively implicated in cognitive motivation. The dorsal striatum has been proposed as an important node specifically in the development of cognitive apathy.13 This is supported by data showing that deactivating or lesioning the rodent dorsal striatum impairs the allocation of cognitive,64 but not physical,65, 66, 67, 68 effort. Furthermore, the dorsal striatum has been implicated in decisions to exert cognitive over physical effort,1 which is consistent with its broader role in behavioral flexibility and cognitive control.69, 70, 71 In addition, other subcortical areas affected in pre-manifest HD—such as the amygdala—play a unique role in cognitive versus physical motivation.5,72
In contrast, those networks typically affected later in the course of HD (e.g., the ventral striatum and its prefrontal connections)73 have been implicated in physical or domain-general motivation. For example, deactivating the rodent nucleus accumbens disrupts physical-effort-based decisions,65, 66, 67, 68 although the impact on cognitive-effort-based decisions is less clear.64 Similarly, human imaging studies have implicated the ventral striatum and its prefrontal connections in the valuation of both physical1,74 and cognitive effort costs.1,2,75,76 Taken together, the spatiotemporal progression of HD could therefore lead to differential effects on motivation in the cognitive and physical domains, with pathways affected earlier in the course of HD preferentially involved in cognitive relative to physical motivation.
One question that remains is the extent to which our findings may generalize to other forms of cognitive motivation. Prominent theoretical frameworks argue that dopamine and the basal ganglia play a central role in the allocation of effort in any cognitive domain that is capacity limited.40,77 Data in support of such theories have been derived from multiple paradigms, which have manipulated effort in terms of perceptual demand, attention, working memory, and arithmetic and across multiple species, including rodents and humans.1,2,21,78, 79, 80, 81 These data predict that, relative to controls, the aversion of the pre-manifest HD group to attentional load should generalize to other types of cognitive effort, but it remains for future studies to empirically confirm this prediction.
The issue of whether cognitive- and physical-effort-based decisions are dissociable is central to current neurobiological theories of motivation. Here, we showed that the willingness of individuals to invest cognitive and physical effort is differentially affected in HD, prior to the clinical onset of motor disease. These data exemplify the utility of neuroeconomic paradigms in providing sensitive measurements of motivated behavior and provide broad support for frameworks that posit separable, domain-specific mechanisms of motivation.
Limitations of Study
The absence of neuroimaging data in our study limits the extent to which we can ascribe our behavioral dissociation to dysfunction within a specific network. Furthermore, our study only tested a cohort of individuals with pre-manifest HD at a single time point. An important focus of future work should be to combine longitudinal neuroimaging studies in HD with behavioral tasks that are sensitive to different motivational subtypes. Such studies will provide valuable information on how changes in corticostriatal pathways over time drive the development and progression of apathy across different domains of behavior.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| Analyzed reinforcement and choice data for cognitive and physical effort tasks, in the control and pre-manifest HD groups | This paper | N/A |
| Software and Algorithms | ||
| MATLAB | Mathworks, USA | https://www.mathworks.com |
| Psychtoolbox | psychtoolbox.org | http://psychtoolbox.org |
| Presentation | Neurobehavioral Systems | https://www.neurobs.com |
| JASP | University of Amsterdam | https://jasp-stats.org |
Resource Availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, Trevor Chong (trevor.chong@monash.edu).
Materials availability
This study did not generate unique reagents or other materials.
Data and Code availability
The datasets supporting the current study are available from the corresponding author on request.
Experimental Model and Subject Details
We recruited 20 individuals in the pre-manifest stage of HD. These individuals were genetically confirmed to have ≥ 38 CAG repeat expansions in the huntingtin gene, and had a diagnostic confidence level of < 4 on the Unified Huntington’s Disease Rating Scale (UHDRS). We compared their performance to 20 healthy controls, matched for age and gender (Table 1). Exclusion criteria included a history of neurological disease (other than HD, in the case of the pre-manifest group), major traumatic brain injury, cerebrovascular accident, or substance abuse. Participants were recruited from our internal research database, the Calvary Bethlehem Hospital in Melbourne, and the wider community. This study received approval from the Monash University Human Research Committee and all participants provided informed consent in accordance with the Declaration of Helsinki.
We assessed cognition using several performance-based measures, including: a standard cognitive screening tool (the Montreal Cognitive Assessment, MoCA), as well as neuropsychological tests of episodic memory (Hopkins Verbal Learning Test – Revised, HVLT-R), and attention/psychomotor speed (Symbol Digit Modalities Test, SDMT). We used the self-reported Hospital Anxiety and Depression Scale31 to measure depressive symptoms. Apathy was assessed using the Apathy Evaluation Scale (AES), which provides a total apathy score32, and the Dimensional Apathy Scale (DAS), which separates apathy into ‘Executive’, ‘Initiation’ and ‘Emotional’ subtypes33. Importantly, the pre-manifest HD and control groups did not differ in any of these measures (Table 1).
Method Details
Participants were tested in a single session, during which they completed an effort-based decision-making task, followed by the battery of cognitive tests. The overall structure of the decision-making task was similar to a previous study examining cognitive and physical effort-based decisions in healthy adults5. The task was divided into three phases (Figure 1). The first two (‘Reinforcement’) phases involved training participants on both a cognitively effortful task (Figures 1A and 1B)21 and a physically effortful task (Figures 1C and 1D)5, in counterbalanced order. Within each task, we parametrically varied demands in the target domain (e.g., cognitive), while keeping those in the other (e.g., physical) constant. The Reinforcement phases were followed by a final ‘Choice’ phase, during which participants were asked to choose between a fixed low-effort/low-reward option, and a variable high-effort/high-reward offer (Figure 1E). These decisions allowed us to quantify the willingness of individuals to exert distinct types of effort.
Reinforcement phases
Cognitive Effort Task. For the cognitive effort task, we utilized a rapid serial visual presentation (RSVP) paradigm, previously described by McGuigan et al.21 Participants were required to monitor a series of rapidly changing letters (Arial, 26-point font, Figures 1A and 1B) and press a button whenever they detected the target letter, ‘T’. We parametrically manipulated cognitive demand by increasing the number of letter streams from one to six. In the least effortful condition (Level 1), a single stream was presented at the central fixation point. In the more effortful conditions (Levels 2-6), between two to six streams were positioned equiangularly and equidistantly from fixation. The target letter could appear randomly in any stream, and the timing of the target stimuli was pseudorandom such that they could not appear in consecutive stimulus frames (to avoid an attentional blink). Each effort level comprised 24 stimulus frames, each of which lasted 416 ms, for a total trial duration of 10 s.
Each trial of the Reinforcement phase commenced with a blue pie chart, which acted as a cue to indicate the level of cognitive effort required on that trial. Participants then completed the required level of effort, after which they received feedback with regards to their success. They were rewarded with one point if they were able to complete each trial above a threshold level of performance (more than one hit; fewer than three false alarms); otherwise they received no points. Participants were instructed that their task was to maximize the number of points won. Participants completed two blocks of 30 trials (i.e., 10 trials per effort level, randomly allocated), with an opportunity to rest after each block. These experimental blocks were preceded by a practice block of 12 trials (two per effort level). Responses were registered on a Cedrus button box, and the task was implemented on Presentation software (Neurobehavioral Systems).
Physical Effort Task. In the physical effort task, participants were required to exert one of six levels of force on a hand-held dynamometer (SS25LA, BIOPAC systems, USA) using their dominant hand (Figures 1C and 1D) – an approach similar to the physical effort task described in Chong et al.5 At the beginning of the experiment, we determined individuals’ maximum voluntary contraction (MVC), which was defined as the maximum of three consecutive squeezes. To standardize effort requirements across participants, we defined the target effort levels for each individual as a function of their own MVC (4, 12, 20, 28, 36, 44%). Target levels were visually depicted as a horizontal yellow line on a vertical bar, and participants received real-time visual feedback of their applied force.
Each trial in the physical effort task commenced with a red pie chart, which cued the level of physical effort required on that trial. Participants then had to initiate their contraction, and maintain it above the required effort level for at least 50% of the total trial duration (i.e., ≥ 5 of 10 s) to be positively reinforced. Importantly, the physical effort task was identical to the cognitive effort task in terms of the trial durations (10 s per effort level); number of trials per effort level; and overall block structure. The physical effort task was implemented on Psychtoolbox (http://psychtoolbox.org) running in MATLAB (Mathworks, USA).
Choice phase
Participants revealed their preference between a fixed, low-effort/low-reward baseline option, and a variable, high-effort/high-reward offer. We sampled the entire effort-reward space evenly and randomly across both domains over a total of 150 trials. Participants made their selection with a button press, and trials were self-paced. To reduce the impact of fatigue on subsequent decision-making, participants were not required to execute their choices, but simply indicate their preferred option. They were explicitly told that their decisions were hypothetical, in that points did not alter remuneration, but that they should select the option that was most preferable to them. This protocol is consistent with previous studies.3,5,21,39
Quantification and Statistical Analysis
Statistical analyses were performed in MATLAB (Mathworks Inc, USA) and JASP v 0.12.2 (University of Amsterdam). Statistical details of the analyses are presented in the main text and Supplemental Information. For the frequentist analyses, violations to sphericity were addressed with Greenhouse-Geisser correction, and pairwise comparisons were corrected for multiple comparisons with the Bonferroni method. For the Bayesian analyses, we specified a multivariate Cauchy prior on the effects, with a distribution centered around zero and a width parameter of 0.707. Bayes Factors were used to quantify evidence in favor of each hypothesis, and interpreted according to Jeffreys.38
Acknowledgments
The authors wish to express their gratitude to all participants and their families. K.J.A. was supported by the Australian Government Research Training Scheme. T.T.-J.C. is supported by the Australian Research Council (DP 180102383 and DE 180100389), the Judith Jane Mason and Harold Stannett Williams Memorial Foundation, the Brain Foundation, the Rebecca L. Cooper Medical Research Foundation, and the Society for Mental Health Research. S.C.A. is supported by a fellowship from the Huntington’s Disease Society of America. J.C.S. is supported by the National Health and Medical Research Council of Australia.
Author Contributions
All authors were involved in study design. K.J.A. collected the data. K.J.A. and T.T.-J.C. analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 30, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xcrm.2020.100152.
Supplemental Information
References
- 1.Schmidt L., Lebreton M., Cléry-Melin M.L., Daunizeau J., Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 2012;10:e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westbrook A., Lamichhane B., Braver T. The subjective value of cognitive effort is encoded by a domain-general valuation network. J. Neurosci. 2019;39:3934–3947. doi: 10.1523/JNEUROSCI.3071-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skvortsova V., Degos B., Welter M.-L., Vidailhet M., Pessiglione M. A selective role for dopamine in learning to maximize reward but not to minimize effort: evidence from patients with Parkinson’s disease. J. Neurosci. 2017;37:6087–6097. doi: 10.1523/JNEUROSCI.2081-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurniawan I.T., Seymour B., Talmi D., Yoshida W., Chater N., Dolan R.J. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J. Neurophysiol. 2010;104:313–321. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong T.T.-J., Apps M., Giehl K., Sillence A., Grima L.L., Husain M. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 2017;15:e1002598. doi: 10.1371/journal.pbio.1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chau B.K.H., Jarvis H., Law C.-K., Chong T.T.-J. Dopamine and reward: a view from the prefrontal cortex. Behav. Pharmacol. 2018;29:569–583. doi: 10.1097/FBP.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 7.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 8.Tabrizi S.J., Scahill R.I., Owen G., Durr A., Leavitt B.R., Roos R.A., Borowsky B., Landwehrmeyer B., Frost C., Johnson H., TRACK-HD Investigators Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 9.Walker F.O. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 10.van Duijn E., Craufurd D., Hubers A.A., Giltay E.J., Bonelli R., Rickards H., Anderson K.E., van Walsem M.R., van der Mast R.C., Orth M., Landwehrmeyer G.B., European Huntington’s Disease Network Behavioural Phenotype Working Group Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY) J. Neurol. Neurosurg. Psychiatry. 2014;85:1411–1418. doi: 10.1136/jnnp-2013-307343. [DOI] [PubMed] [Google Scholar]
- 11.Craufurd D., Thompson J.C., Snowden J.S. Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- 12.Martinez-Horta S., Perez-Perez J., van Duijn E., Fernandez-Bobadilla R., Carceller M., Pagonabarraga J., Pascual-Sedano B., Campolongo A., Ruiz-Idiago J., Sampedro F., Spanish REGISTRY investigators of the European Huntington’s Disease Network Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s Disease. Parkinsonism Relat. Disord. 2016;25:58–64. doi: 10.1016/j.parkreldis.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Levy R., Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 14.Thompson J.C., Harris J., Sollom A.C., Stopford C.L., Howard E., Snowden J.S., Craufurd D. Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J. Neuropsychiatry Clin. Neurosci. 2012;24:53–60. doi: 10.1176/appi.neuropsych.11030057. [DOI] [PubMed] [Google Scholar]
- 15.Chong T.T., Bonnelle V., Veromann K.R., Juurmaa J., Taba P., Plant O., Husain M. Dissociation of reward and effort sensitivity in methcathinone-induced Parkinsonism. J. Neuropsychol. 2018;12:291–297. doi: 10.1111/jnp.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt L., d’Arc B.F., Lafargue G., Galanaud D., Czernecki V., Grabli D., Schüpbach M., Hartmann A., Lévy R., Dubois B., Pessiglione M. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- 17.Cléry-Melin M.L., Schmidt L., Lafargue G., Baup N., Fossati P., Pessiglione M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE. 2011;6:e23178. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann M.N., Hager O.M., Reimann A.V., Chumbley J.R., Kirschner M., Seifritz E., Tobler P.N., Kaiser S. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr. Bull. 2015;41:503–512. doi: 10.1093/schbul/sbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barch D.M., Treadway M.T., Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J. Abnorm. Psychol. 2014;123:387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold J.M., Kool W., Botvinick M.M., Hubzin L., August S., Waltz J.A. Cognitive effort avoidance and detection in people with schizophrenia. Cogn. Affect. Behav. Neurosci. 2015;15:145–154. doi: 10.3758/s13415-014-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuigan S., Zhou S.-H., Brosnan M.B., Thyagarajan D., Bellgrove M.A., Chong T.T.-J. Dopamine restores cognitive motivation in Parkinson’s disease. Brain. 2019;142:719–732. doi: 10.1093/brain/awy341. [DOI] [PubMed] [Google Scholar]
- 22.Chong T.T.-J., Husain M. The role of dopamine in the pathophysiology and treatment of apathy. Prog. Brain Res. 2016;229:389–426. doi: 10.1016/bs.pbr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Baudic S., Maison P., Dolbeau G., Boissé M.F., Bartolomeo P., Dalla Barba G., Traykov L., Bachoud-Lévi A.C. Cognitive impairment related to apathy in early Huntington’s disease. Dement. Geriatr. Cogn. Disord. 2006;21:316–321. doi: 10.1159/000091523. [DOI] [PubMed] [Google Scholar]
- 24.Paulsen J.S., Ready R.E., Hamilton J.M., Mega M.S., Cummings J.L. Neuropsychiatric aspects of Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 2001;71:310–314. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson J.C., Snowden J.S., Craufurd D., Neary D. Behavior in Huntington’s disease: dissociating cognition-based and mood-based changes. J. Neuropsychiatry Clin. Neurosci. 2002;14:37–43. doi: 10.1176/jnp.14.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Bonnelle V., Manohar S., Behrens T., Husain M. Individual differences in premotor brain systems underlie behavioral apathy. Cereb. Cortex. 2016;26:807–819. doi: 10.1093/cercor/bhv247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong T.T.-J., Bonnelle V., Husain M. Quantifying motivation with effort-based decision-making paradigms in health and disease. Prog. Brain Res. 2016;229:71–100. doi: 10.1016/bs.pbr.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Pessiglione M., Vinckier F., Bouret S., Daunizeau J., Le Bouc R. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain. 2018;141:629–650. doi: 10.1093/brain/awx278. [DOI] [PubMed] [Google Scholar]
- 29.Castrellon J.J., Seaman K.L., Crawford J.L., Young J.S., Smith C.T., Dang L.C., Hsu M., Cowan R.L., Zald D.H., Samanez-Larkin G.R. Individual differences in dopamine are associated with reward discounting in clinical groups but not in healthy adults. J. Neurosci. 2019;39:321–332. doi: 10.1523/JNEUROSCI.1984-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong T.T.-J., Bonnelle V., Manohar S., Veromann K.-R., Muhammed K., Tofaris G.K., Hu M., Husain M. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex. 2015;69:40–46. doi: 10.1016/j.cortex.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Marin R.S., Biedrzycki R.C., Firinciogullari S. Reliability and validity of the apathy evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 33.Radakovic R., Abrahams S. Developing a new apathy measurement scale: Dimensional Apathy Scale. Psychiatry Res. 2014;219:658–663. doi: 10.1016/j.psychres.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Naarding P., Janzing J.G., Eling P., van der Werf S., Kremer B. Apathy is not depression in Huntington’s disease. J. Neuropsychiatry Clin. Neurosci. 2009;21:266–270. doi: 10.1176/jnp.2009.21.3.266. [DOI] [PubMed] [Google Scholar]
- 35.Mason S., Barker R.A. Rating apathy in Huntington’s disease: Patients and companions agree. J. Huntingtons Dis. 2015;4:49–59. [PubMed] [Google Scholar]
- 36.Santangelo G., D’Iorio A., Piscopo F., Cuoco S., Longo K., Amboni M., Baiano C., Tafuri D., Pellecchia M.T., Barone P., Vitale C. Assessment of apathy minimising the effect of motor dysfunctions in Parkinson’s disease: a validation study of the dimensional apathy scale. Qual. Life Res. 2017;26:2533–2540. doi: 10.1007/s11136-017-1569-6. [DOI] [PubMed] [Google Scholar]
- 37.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 38.Jeffreys H. Third Edition. Oxford University; 1961. Theory of Probability. [Google Scholar]
- 39.Chong T.T.-J., Apps M.A.J., Giehl K., Hall S., Clifton C.H., Husain M. Computational modelling reveals distinct patterns of cognitive and physical motivation in elite athletes. Sci. Rep. 2018;8:11888. doi: 10.1038/s41598-018-30220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westbrook A., Braver T.S. Cognitive effort: a neuroeconomic approach. Cogn. Affect. Behav. Neurosci. 2015;15:395–415. doi: 10.3758/s13415-015-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radakovic R., Abrahams S. Multidimensional apathy: evidence from neurodegenerative disease. Curr. Opin. Behav. Sci. 2018;22:42–49. [Google Scholar]
- 42.Chong T.T.-J. Updating the role of dopamine in human motivation and apathy. Curr. Opin. Behav. Sci. 2018;22:35–41. [Google Scholar]
- 43.Marin R.S. Apathy: a neuropsychiatric syndrome. J. Neuropsychiatry Clin. Neurosci. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 44.Chong T.T.-J. Definition: apathy. Cortex. 2020;128:326–327. doi: 10.1016/j.cortex.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Robert P., Lanctôt K.L., Agüera-Ortiz L., Aalten P., Bremond F., Defrancesco M., Hanon C., David R., Dubois B., Dujardin K. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry. 2018;54:71–76. doi: 10.1016/j.eurpsy.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Sockeel P., Dujardin K., Devos D., Denève C., Destée A., Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2006;77:579–584. doi: 10.1136/jnnp.2005.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Culbreth A., Westbrook A., Barch D. Negative symptoms are associated with an increased subjective cost of cognitive effort. J. Abnorm. Psychol. 2016;125:528–536. doi: 10.1037/abn0000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douaud G., Behrens T.E., Poupon C., Cointepas Y., Jbabdi S., Gaura V., Golestani N., Krystkowiak P., Verny C., Damier P. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009;46:958–966. doi: 10.1016/j.neuroimage.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Kassubek J., Juengling F.D., Kioschies T., Henkel K., Karitzky J., Kramer B., Ecker D., Andrich J., Saft C., Kraus P. Topography of cerebral atrophy in early Huntington’s disease: a voxel based morphometric MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75:213–220. [PMC free article] [PubMed] [Google Scholar]
- 50.Vonsattel J.P., Myers R.H., Stevens T.J., Ferrante R.J., Bird E.D., Richardson E.P.J., Jr. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Vonsattel J.P.G. Huntington disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:55–69. doi: 10.1007/s00401-007-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cepeda C., Wu N., André V.M., Cummings D.M., Levine M.S. The corticostriatal pathway in Huntington’s disease. Prog. Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgiou-Karistianis N., Gray M.A., Domínguez D J.F., Dymowski A.R., Bohanna I., Johnston L.A., Churchyard A., Chua P., Stout J.C., Egan G.F. Automated differentiation of pre-diagnosis Huntington’s disease from healthy control individuals based on quadratic discriminant analysis of the basal ganglia: the IMAGE-HD study. Neurobiol. Dis. 2013;51:82–92. doi: 10.1016/j.nbd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Misiura M.B., Ciarochi J., Vaidya J., Bockholt J., Johnson H.J., Calhoun V.D., Paulsen J.S., Turner J.A., PREDICT-HD Investigators & Working Group Apathy is related to cognitive control and striatum volumes in prodromal Huntington’s disease. J. Int. Neuropsychol. Soc. 2019;25:462–469. doi: 10.1017/S1355617719000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahveninen L.M., Stout J.C., Georgiou-Karistianis N., Lorenzetti V., Glikmann-Johnston Y. Reduced amygdala volumes are related to motor and cognitive signs in Huntington’s disease: The IMAGE-HD study. Neuroimage Clin. 2018;18:881–887. doi: 10.1016/j.nicl.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabrizi S.J., Langbehn D.R., Leavitt B.R., Roos R.A., Durr A., Craufurd D., Kennard C., Hicks S.L., Fox N.C., Scahill R.I., TRACK-HD investigators Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosas H.D., Liu A.K., Hersch S., Glessner M., Ferrante R.J., Salat D.H., van der Kouwe A., Jenkins B.G., Dale A.M., Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 58.Gray M.A., Egan G.F., Ando A., Churchyard A., Chua P., Stout J.C., Georgiou-Karistianis N. Prefrontal activity in Huntington’s disease reflects cognitive and neuropsychiatric disturbances: the IMAGE-HD study. Exp. Neurol. 2013;239:218–228. doi: 10.1016/j.expneurol.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Harrington D.L., Smith M.M., Zhang Y., Carlozzi N.E., Paulsen J.S., PREDICT-HD Investigators of the Huntington Study Group Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J. Neurol. Neurosurg. Psychiatry. 2012;83:612–619. doi: 10.1136/jnnp-2011-301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paulsen J.S. Cognitive impairment in Huntington disease: diagnosis and treatment. Curr. Neurol. Neurosci. Rep. 2011;11:474–483. doi: 10.1007/s11910-011-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stout J.C., Paulsen J.S., Queller S., Solomon A.C., Whitlock K.B., Campbell J.C., Carlozzi N., Duff K., Beglinger L.J., Langbehn D.R. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25:1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papoutsi M., Labuschagne I., Tabrizi S.J., Stout J.C. The cognitive burden in Huntington’s disease: pathology, phenotype, and mechanisms of compensation. Mov. Disord. 2014;29:673–683. doi: 10.1002/mds.25864. [DOI] [PubMed] [Google Scholar]
- 63.Lawrence A.D., Sahakian B.J., Robbins T.W. Cognitive functions and corticostriatal circuits: insights from Huntington’s disease. Trends Cogn. Sci. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- 64.Silveira M.M., Tremblay M., Winstanley C.A. Dissociable contributions of dorsal and ventral striatal regions on a rodent cost/benefit decision-making task requiring cognitive effort. Neuropharmacology. 2018;137:322–331. doi: 10.1016/j.neuropharm.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Cousins M.S., Sokolowski J.D., Salamone J.D. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol. Biochem. Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- 66.Farrar A.M., Segovia K.N., Randall P.A., Nunes E.J., Collins L.E., Stopper C.M., Port R.G., Hockemeyer J., Müller C.E., Correa M., Salamone J.D. Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166:1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 67.Font L., Mingote S., Farrar A.M., Pereira M., Worden L., Stopper C., Port R.G., Salamone J.D. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl.) 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nunes E.J., Randall P.A., Podurgiel S., Correa M., Salamone J.D. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci. Biobehav. Rev. 2013;37(9 Pt A):2015–2025. doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Gruber A.J., McDonald R.J. Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front. Behav. Neurosci. 2012;6:50. doi: 10.3389/fnbeh.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grahn J.A., Parkinson J.A., Owen A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Devan B.D., Hong N.S., McDonald R.J. Parallel associative processing in the dorsal striatum: segregation of stimulus-response and cognitive control subregions. Neurobiol. Learn. Mem. 2011;96:95–120. doi: 10.1016/j.nlm.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Hosking J.G., Cocker P.J., Winstanley C.A. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology. 2014;39:1558–1567. doi: 10.1038/npp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dogan I., Eickhoff S.B., Schulz J.B., Shah N.J., Laird A.R., Fox P.T., Reetz K. Consistent neurodegeneration and its association with clinical progression in Huntington’s disease: a coordinate-based meta-analysis. Neurodegener. Dis. 2013;12:23–35. doi: 10.1159/000339528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Croxson P.L., Walton M.E., O’Reilly J.X., Behrens T.E.J., Rushworth M.F.S. Effort-based cost-benefit valuation and the human brain. J. Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Botvinick M.M., Huffstetler S., McGuire J.T. Effort discounting in human nucleus accumbens. Cogn. Affect. Behav. Neurosci. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobryakova E., Jessup R.K., Tricomi E. Modulation of ventral striatal activity by cognitive effort. Neuroimage. 2017;147:330–338. doi: 10.1016/j.neuroimage.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Botvinick M., Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu. Rev. Psychol. 2015;66:83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- 78.Hosking J.G., Floresco S.B., Winstanley C.A. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–1015. doi: 10.1038/npp.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Westbrook A., van den Bosch R., Määttä J.I., Hofmans L., Papadopetraki D., Cools R., Frank M.J. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science. 2020;367:1362–1366. doi: 10.1126/science.aaz5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vassena E., Silvetti M., Boehler C.N., Achten E., Fias W., Verguts T. Overlapping neural systems represent cognitive effort and reward anticipation. PLoS ONE. 2014;9:e91008. doi: 10.1371/journal.pone.0091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schouppe N., Demanet J., Boehler C.N., Ridderinkhof K.R., Notebaert W. The role of the striatum in effort-based decision-making in the absence of reward. J. Neurosci. 2014;34:2148–2154. doi: 10.1523/JNEUROSCI.1214-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the current study are available from the corresponding author on request.