Abstract
Introduction
Osteo-arthritis (OA) involves joint degradation and usually pain; with mechanisms poorly understood and few treatment options. There is evidence that the transient receptor potential canonical 5 (TRPC5) mRNA expression is reduced in OA patients’ synovia. Here we examine the profile of TRPC5 in DRG and involvement in murine models of OA.
Design
TRPC5 KO mice were subjected to partial meniscectomy (PMNX) or injected with monoiodoacetate (MIA) and pain-related behaviours were determined. Knee joint pathological scores were analysed and gene expression changes in ipsilateral synovium and dorsal root ganglia (DRG) determined. c-Fos protein expression in the ipsilateral dorsal horn of the spinal cord was quantified.
Results
TRPC5 KO mice developed a discrete enhanced pain-related phenotype. In the MIA model, the pain-related phenotype correlated with c-Fos expression in the dorsal horn and increased expression of nerve injury markers ATF3, CSF1 and galanin in the ipsilateral DRG. There were negligible differences in the joint pathology between WT and TRPC5 KO mice, however detailed gene expression analysis determined increased expression of the mast cell marker CD117 as well as extracellular matrix remodelling proteinases MMP2, MMP13 and ADAMTS4 in MIA-treated TRPC5 KO mice. TRPC5 expression was defined to sensory subpopulations in DRG.
Conclusions
Deletion of TRPC5 receptor signalling is associated with exacerbation of pain-like behaviour in OA which correlates with increased expression of enzymes involved in extracellular remodelling, inflammatory cells in the synovium and increased neuronal activation and injury in DRG. Together, these results identify a modulating role for TRPC5 in OA-induced pain-like behaviours.
Keywords: TRPC5, Osteoarthritis, Inflammation, Synovitis
1. Introduction
OA is sometimes associated with synovitis, which is characterised by increased cellularity and macrophage infiltration [1,2]. The degree of synovitis, detected by MRI, correlates highly with the presence of knee pain [3,4]. With no current cure for OA, the therapeutic approach aims to provide pain relief, however, the existing analgesic options available are poor and ineffective. Thus, there remains an unmet demand for improved therapeutic agents to alleviate joint damage and better pain management for patients suffering from OA. The transient receptor potential (TRP) family of ligand-gated ion channel receptors are non-selective cationic channels [5]; with several of these environmental sensors involved in pain sensation and processing.
We have found that TRPC5 gene expression is reduced in human arthritic OA synovial tissues [6]. TRPC5 is one of seven members of the canonical family, forming functional homo- or heterotetramers with other TRPC channels, TRPC1 and TRPC4 [7,8]. TRPC5 expression was originally found in the brain and later in sensory neurons, where it may act as a cold transducer [9,10]. A potential role for TRPC5 in arthritis was first obtained by Beech's group using TRPC5 and TRPC1 expressing fibroblast-like synoviocytes (FLS) in vitro [11]. Cultured FLS release matrix metalloproteinases (MMP), which are important for tissue remodelling and cartilage degradation in osteoarthritis (OA). The TRPC5 agonist, thioredoxin, inhibited matrix metalloproteinases secretion, in both cultured human and animal FLS. Further, treatment of arthritic patients' FLS with a TRPC5 antibody or with TRPC5 siRNA potentiated MMP2 secretion [11]. These results collectively provide cellular evidence that TRPC5 is protective in rheumatoid arthritis, however, the role of TRPC5 in OA is unknown.
We have previously shown that TRP channels are involved in mediating pain and inflammation in the complete Freund's adjuvant (CFA)-induced model of arthritic inflammatory pain [12,13]. Using this model, we found a protective role for TRPC5 as joint mechanical hyperalgesia, weight bearing and inflammation are exacerbated in mice lacking functional TRPC5 [6]. The primary objective of the present study was to carry out a detailed study of pain-like behaviours in two widely used murine models of OA. These models, the partial meniscectomy (PMNX) and intra-articular monoiodoacetate (MIA) injection are utilised to enable differences in structural pathology and pain behaviour to reflect different aspects of human OA [14]. Both models resulted in exacerbated pain-induced behaviour in the TRPC5 KOs, but with mild pathological changes. This led us to the secondary objective of investigating the mechanisms by which TRPC5 expression may influence the pathophysiology of OA.
2. Methods
Detailed methods are provided as online supplementary material.
2.1. Animals
129S1/SvIm wild type (WT) and TRPC5 knock out (KO) adult male mice (3–5 months, 25–35 g), previously described [6], were used in this study. Food and water were available ad libitum and mice were housed under standard conditions with a 12-h light/dark cycle. Animals were designated experimental numbers, in a randomised manner by an uninformed independent researcher who secured and concealed the allocation until the end of the study. Experiments were performed by investigators blinded to identity of the animals. All procedures were performed according to the UK Animals (Scientific Procedures) Act 1986 and approved by the King's College London Animal Care and Ethics Committees. Further, we adhere to Good Laboratory Practice following the ARRIVE guidelines [15] (see online supplementary material), and principles of the 3Rs (Replacement, Reduction and Refinement).
2.2. Induction of osteoarthritis
The PMNX model was conducted for 63 days as previously described [16]. The MIA model was conducted for 28 days [17]. Behavioural assays were completed at regular intervals (days 0, 7, 10, 14, 21, 28, 35, 42, 49, 56 and 63 in PMXN study and days 0, 3, 7, 14, 21, and 28 in MIA study).
2.3. Joint histology and immunohistological staining
Histological and immunohistological assays were performed as previously described [18,19].
2.4. Quantitative polymerase chain reaction
All primers used are provided on Supplementary Table 1. Briefly, total RNA was extracted from DRG and synovial tissue with RNeasy kit (Qiagen, Manchester, UK) and qPCR was performed using 7900HT Real-Time PCR instrument (Applied Biosystems, Foster City, CA, USA).
2.5. Data analysis
Animals were randomised to experimental groups by an external researcher as described above. Data were tested for normal distribution (Shapiro & Wilk test) and analysed using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). For behavioural analysis, based on our previous experience and data, considering the primary outcome for the von Frey test, in order to achieve an alpha of 0.05 and power of 0.8, and assuming a standard deviation of 0.25, the effect size is 0.3 when 12 animals are used. The number of animals used in the various experiments is reported in the figure legends. Data analysis was performed by two-way repeated measures ANOVA, followed by Bonferroni or Tukey's test with factors considered being time and treatment and genotype. Histological, immunohistochemical, and qPCR data were analysed by two-way ANOVA followed by Tukey's test with factors considered being treatment and genotype. The statistical tests performed, and the numbers of animals used are displayed in the Results section and within the figure legends. All data is presented as mean ± S.D. In all analyses, p < 0.05 was taken to indicate statistical significance.
3. Results
3.1. Effect of TRPC5 deletion in the chronic PMNX model
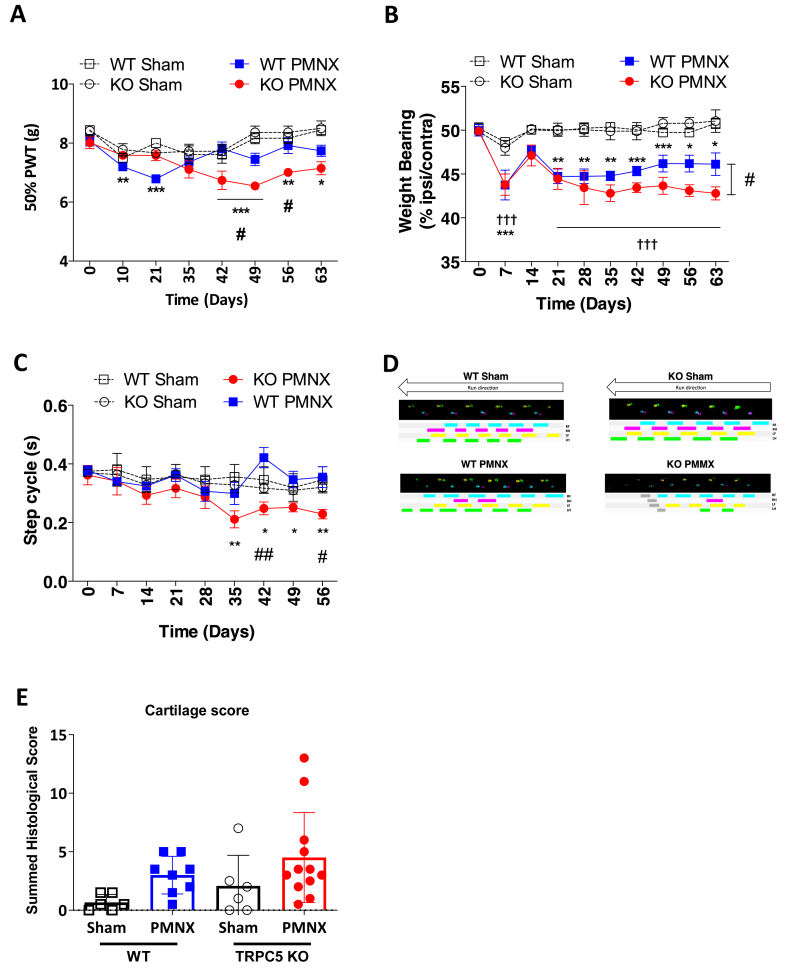
Initially, we used a surgical model of osteoarthritis, the PMNX model. Partial destabilisation of the medial meniscus in WT mice was associated with transient (day 10 and day 21) referred mechanical hypersensitivity (Fig. 1A) as well as a decrease in the ipsilateral hindpaw weight bearing (Fig. 1B), but no change in step cycle was observed throughout the duration of the study (Fig. 1C and D). Changes in ipsilateral weight bearing were first reduced at day 7 post-PMNX compared to WT sham, then subsided at day 14 to reappear at day 21 and maintained lower than WT Sham group during the remainder of the study (Fig. 1B). A similar lowering of ipsilateral weight bearing was observed in TRPC5 KO mice subjected to PMNX (Fig. 1B). At day 63 after PMNX, the percentage of weight borne in the ipsilateral hindpaw was lower in the TRPC5 KO PMNX group than in the WT PMNX group (Fig. 1B). Further, in TRPC5 KO mice subjected to PMNX, the mechanical thresholds and step cycle were lower than the sham control group, from days 42 and 35, respectively (Fig. 1A and C). Other parameters in catwalk analysis showed similar trends (Supplementary Fig. 1).
Fig. 1.
Mechanical hyperalgesia and spontaneous gait deficits following PMNX-induced OA in TRPC5 KO mice. (A) Mechanical thresholds measured as paw withdrawal thresholds (PWT) after PMNX or sham surgery to WT or TRPC5 KO mice. (B) Time-course of weight bearing distribution between ipsilateral and contralateral hind paws, following PMNX surgery. (C) Time-course of the ipsilateral hind-paw step cycle. (D) Real-time representative paw prints of WT and TRPC5 KO mice following sham or PMNX surgery (day 56). Values are mean ± S.E.M., n = 5–6. †††p < 0.001 KO PMNX vs KO sham, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs day 0; #p < 0.05, ##p < 0.01 vs WT PMNX, as determined by two-way ANOVA and Bonferroni post hoc tests. (E) Quantitative scoring of osteoarthritis joint pathology using OARSI score after Safranin O staining. Values are mean ± S.D. n = 6–12. Two-way ANOVA and Tukey's post hoc tests. Each point in the dot plot represents an individual animal.
At the end of the study, the knee joints were collected and analysed for cartilage degradation, which is associated with the PMNX pain phenotype. Overall a non-significant cartilage loss was found in the PMNX groups (0.67 ± 0.68 vs 3 ± 1.60, p = 0.42, WT saline vs WT MIA and 2.08 ± 2.61 vs 4.5 ± 3.83; p = 0.32, TRPC5 KO vs TRPC5 KO MIA) (Fig. 1E).
3.2. Effect of TRPC5 deletion in the monoiodoacetate (MIA) model of osteoarthritis
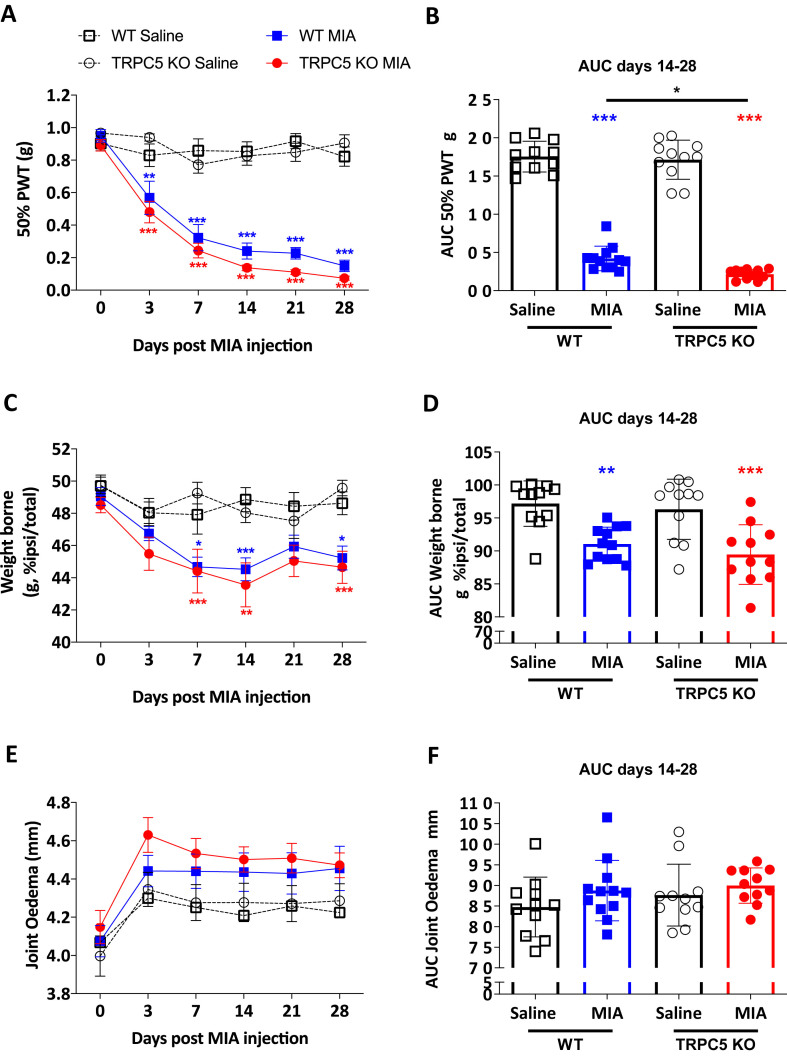
To study processes involved in the protective mechanism of TRPC5, MIA was injected in the joint to induce cartilage degradation and behaviours were followed for 28 days. In MIA-injected WT mice, ipsilateral mechanical thresholds (Fig. 2A and B) and hindpaw weight bearing (Fig. 2C and D) were lower than in saline-injected WT mice, from day 3 and 7 after injection up to 28 days (Fig. 2A,B,C and D). In TRPC5 KO mice, both ipsilateral mechanical thresholds and weight bearing asymmetries were lower than TRPC5 KO treated with saline, as early as day 3 and 7 post injection, respectively, and such difference continued up to day 28 after MIA injection (Fig. 2A and C). However, despite a trend for TRPC5 KO mice thresholds to be lower than WT MIA-treated mice, there was no difference between these groups (Fig. 2A and C). The MIA model typically consists of two phases; an initial stage driven by inflammation, and a second phase demonstrating neuropathic pain mechanisms in addition to inflammatory processes, which collectively drive the pain [20]. Although there was no difference in AUC analysis of mechanical thresholds and weight bearing changes from days 0–14, between MIA-treated WT and TRPC5 KO (data not shown), TRPC5 KO mice had lower mechanical thresholds from days 14–28 (0.42 ± 0.16 vs 0.22 ± 0.06; p = 0.04, WT MIA vs TRPC5 KO MIA) (Fig. 2B) but no weight bearing differences (91.06 ± 2.55 vs 89.45 ± 4.51; p = 0.75, WT MIA vs TRPC5 KO MIA) (Fig. 2D). No detectable changes were observed in ipsilateral knee joint diameter over the duration of the study, on any of the experimental groups (Fig. 2E and F).
Fig. 2.
MIA-induced mechanical hypersensitivity is exacerbated in TRPC5 KO mice. (A) Mechanical thresholds measured as paw withdrawal thresholds (PWT) after intra-articular injection of monoiodoacetate (MIA) (1 mg/mouse) or saline (10 μl/mouse) to WT or TRPC5 KO mice. Values are mean ± S.E.M., n = 11–12 mice/group. ∗∗p < 0.01; ∗∗∗p < 0.001, versus saline group; two-way repeated measures (RM) ANOVA, post hoc Tukey's test. (B) Areas under the curve (AUC) for ipsilateral PWT from days 14–28 after MIA injection. Values are mean ± S.D.∗p < 0.05, ∗∗∗p < 0.001, vs saline control group or vs WT MIA, two-way ANOVA, post hoc Tukey test. Each point in the dot plot represents an individual animal. (C) Time-course of weight bearing distribution between ipsilateral and contralateral hind paws, following MIA injection. ∗p < 0.05, ∗∗p < 0.01 ∗∗∗p < 0.001, versus saline group; two-way RM ANOVA, post hoc Tukey's test. (D) AUC for weight bearing distribution. Values are mean ± S.D. ∗∗p < 0.01, ∗∗∗p < 0.001, versus saline group; two-way ANOVA, post hoc Tukey's test. Each point in the dot plot represents an individual animal. (E) Time-course of ipsilateral knee joint diameter, following MIA injection. Values are mean ± S.E.M., two-way RM ANOVA, post hoc Tukey's test. (F) Areas under the curve for joint diameter. Values are mean ± S.D. two-way ANOVA, post hoc Tukey test. Each point in the dot plot represents an individual animal.
3.3. Similar joint pathology in MIA-treated WT and TRPC5 KO mice
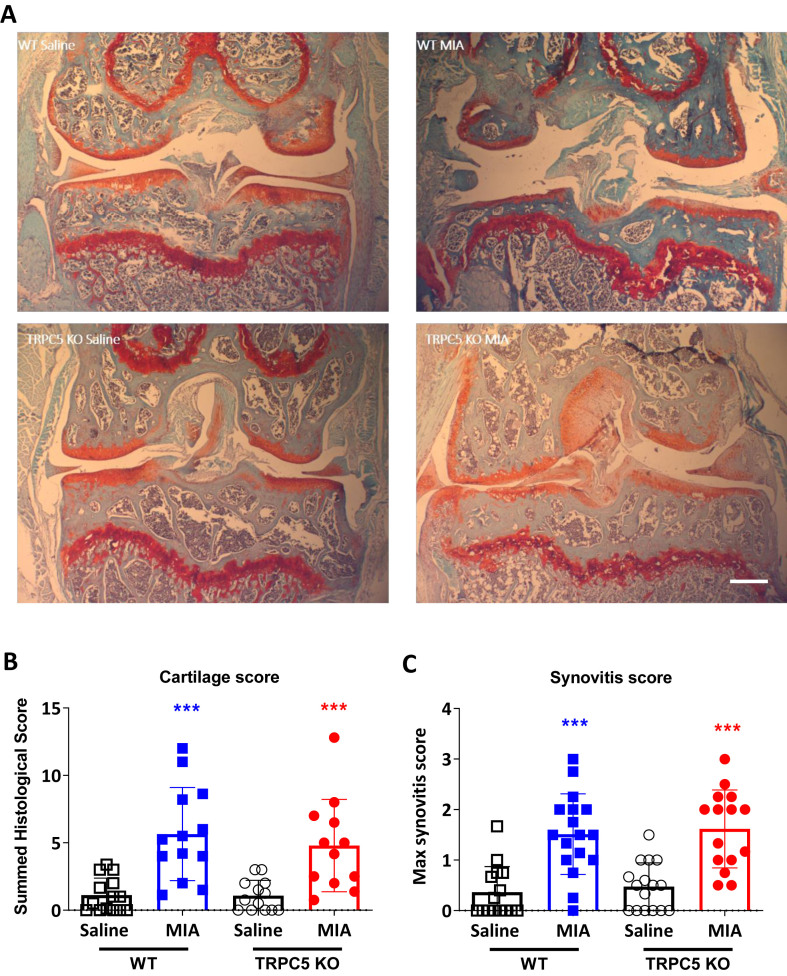
To determine whether there were differences between TRPC5 KO and WT in the progression of cartilage loss, knee joints were collected and cartilage proteoglycans were stained using Safranin-O. H histopathological changes were quantified using OARSI recommended scoring system (Fig. 3A) [18]. Intraarticular MIA injection was associated with articular cartilage loss and reduced Safranin-O staining in the joints in both WT and TRPC5 KO mice (1.12 ± 1.27 vs 5.65 ± 3.46; p = 0.0002, WT Saline vs WT MIA and 1.08 ± 1.14 vs 4.80 ± 3.43; p = 0.003, TRPC5 KO Saline vs TRPC5 KO MIA). No difference was observed between WT and TRPC5 KO mice (5.65 ± 3.46 vs 4.80 ± 3.43; p = 0.84, WT MIA vs TRPC5 KO MIA) (Fig. 3A and B). Likewise, MIA treatment resulted in synovial lining grade expansion in the joints of both WT and TRPC5 KO mice, but no differences could be observed between the groups (0.36 ± 0.50 vs 1.51 ± 0.8; p < 0.0001, WT Saline vs WT MIA; 0.47 ± 0.47 vs 1.62 ± 0.77; p < 0.0001, TRPC5 KO Saline vs TRPC5 KO MIA and 1.51 ± 0.8 vs 1.62 ± 0.77; p = 0.97, WT MIA vs TRPC5 KO MIA) (Fig. 3C).
Fig. 3.
Intra-articular monosodium iodoacetate (MIA) causes cartilage degradation within the mouse knee joint. (A) Safranin O staining of articular cartilage from saline and 1 mg/mouse MIA knee joints of WT and TRPC5 KO mice, 28 days after injection. Scale bar = 100 μm (B). OARSI score of ipsilateral knee joints, 28 days after MIA or saline injection. Values are mean ± S.D., n = 13–17. ∗∗∗p < 0.001 vs saline group; two-way ANOVA and Tukey's post hoc test. Each point in the dot plot represents an individual animal. (C) Synovitis score in ipsilateral knee joints, 28 days after MIA or saline injection. according to Mapp et al., 2008. Values are mean ± S.D., n = 13–17. ∗∗∗p < 0.001 vs saline group; two-way ANOVA and Tukey's post hoc test. Each point in the dot plot represents an individual animal.
3.4. Enhanced dorsal horn neuron activation in the spinal cord and neuronal injury in dorsal root ganglia of TRPC5 KO mice after intra-articular MIA
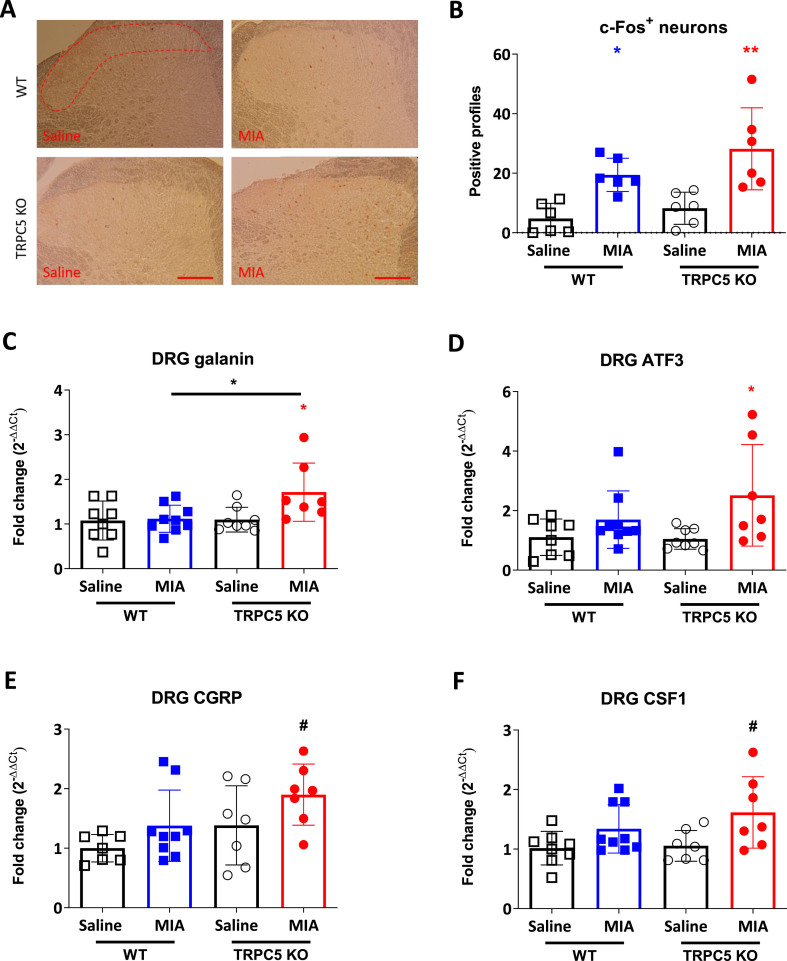
Given the fact that the cartilage degenerative phenotype was more prominent in the MIA model of OA, we investigated this model further. Consistent with an increased afferent input to laminae I and II of the spinal cord in this model [19], there were more c-Fos expressing neurons in ipsilateral dorsal horn compared to saline, 28 days after MIA (4.78 ± 5.1 vs 19.44 ± 5.57; p = 0.029, WT Saline vs WT MIA) (Fig. 4A and B). Furthermore, the number of c-Fos positive cells was higher in TRPC5 KO than WT dorsal horns when each was compared with the respective vehicle group (4.78 ± 5.1 vs 19.44 ± 5.57; p = 0.029, WT Saline vs WT MIA and 8.22 ± 5.39 vs 28.19 ± 13.78; p = 0.0025, TRPC5 KO Saline vs TRPC5 KO MIA) (Fig. 4A and B). However, the difference between WT MIA and KO MIA did not reach significance. No changes in contralateral c-Fos expression were observed in either WT or TRPC5 KO spinal cords (data not shown).
Fig. 4.
Greater dorsal horn neuron activation in the lumbar spinal cord and enhanced neuronal injury in TRPC5 KO mice, 28 days after MIA. (A) c-Fos immunoreactivity in the L3-L5 dorsal horn. Scale bar = 100 μm. (B), Quantification of c-Fos immunoreactivity in laminae I and II 28 days post-MIA (1 mg/mouse) or saline (10 μl/saline) injection. Values are mean ± S.D. ∗p < 0.05, ∗∗p < 0.01, vs saline control, two-way ANOVA, post hoc Tukey test, n = 6. Each point in the dot plot represents an individual animal. (C) mRNA expression in L3-L5 ipsilateral dorsal root ganglia (DRG) of nerve injury markers determined by qPCR in DRG of neuropeptide galanin (C), nerve injury marker ATF3 (D), CGRP (E) and CSF1 (F). Values are mean ± S.D. two-way ANOVA, post hoc Tukey test, n = 7–8; ∗p < 0.05, vs. saline group or vs. WT MIA group; #p < 0.05 vs. WT saline group. Each point in the dot plots represents an individual animal.
Previously in MIA-induced OA, several pathological changes in the dorsal root ganglia (DRG) have been observed [21,22]. Here we analysed expression changes of nerve injury markers in the ipsilateral L3-L5 ganglia. We found that both the neuropeptide galanin and activating transcription factor 3 (ATF3) were significantly upregulated only in TRPC5 KO mice after MIA treatment (galanin: 1.08 ± 0.44 vs 1.12 ± 0.30; p = 0.997, WT Saline vs WT MIA and 81.1 ± 0.28 vs 1.71 ± 0.65 p = 0.05, TRPC5 KO Saline vs TRPC5 KO MIA and ATF3: 1.105 ± 0.61 vs 1.69 ± 0.96; p = 0.63, WT Saline vs WT MIA and 1.046 ± 0.345 vs 2.51 ± 1.70; p = 0.04, TRPC5 KO Saline vs TRPC5 KO MIA) (Fig. 4C and D). Furthermore, calcitonin gene related peptide (CGRP) and colony stimulating factor 1 (CSF1) were increased in ipsilateral DRG from MIA-treated TRPC5 KO mice, when compared with saline treated WT mice (CGRP: 1 ± 0.23 vs 1.89 ± 0.51; p = 0.02, WT Saline vs TRPC5 KO and CSF1: 1.02 ± 0.28 vs 1.61 ± 0.60; p = 0.04, WT Saline vs TRPC5 KO MIA) (Fig. 4E and F). Taken together, our results suggest that TRPC5 depletion is associated with an increased nociceptive phenotype, increased dorsal horn activation and increased neuronal injury, 28 days after MIA injection.
3.5. TRPC5 is expressed in both nociceptive and non-nociceptive dorsal root ganglia
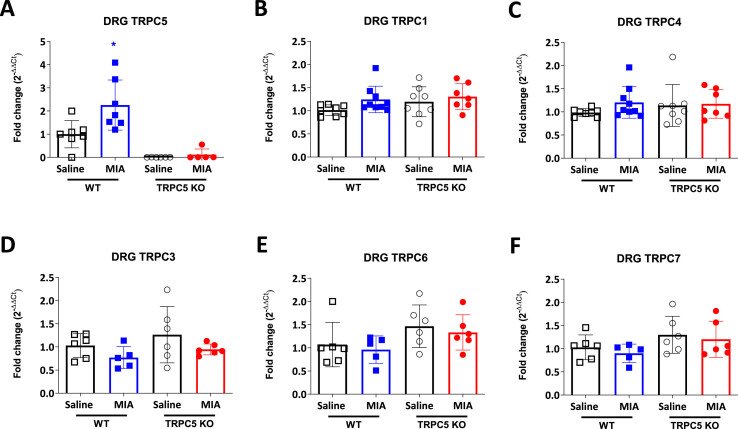
Next, we determined whether TRPC5 and other TRPC channels were expressed in DRG. We confirmed mRNA expression of TRPC5 along with TRPC1 and TRPC4 in mouse DRG (Fig. 5 A, B and C). Interestingly, we found that TRPC5 expression is increased in MIA treated WT mice (1 ± 0.59 vs 2.26 ±. 1.08; p = 0.009, WT Saline vs WT MIA) (Fig. 5A) with no changes in TRPC1 and TRPC4 (TRPC1: 1.013 ± 0.11 vs 1.24 ± 0.28; p = 0.29, WT Saline vs WT MIA and 1.19 ± 0.32 vs 1.30 ± 0.28; p = 0.85, TRPC5 KO Saline vs TRPC5 KO MIA and TRPC4: 0.98 ± 0.08 vs 1.20 ± 0.34; p = 0.52, WT Saline vs WT MIA and 1.14 ± 0.45 vs 1.17 ± 0.30; p = 0.997, TRPC5 KO Saline vs TRPC5 KO MIA) (Fig. 5B and C). At present only TRPC1 and TRPC4 are known to form channels with TRPC5. However, we also looked at the expression of other TRPC channels -TRPC3, TRPC6 and TRPC7 - for the possibility of compensatory mechanisms but observed no expression changes in any of the experimental groups (Fig. 5D, E and F).
Fig. 5.
TRPC5 mRNA is expressed in dorsal root ganglions. Real-time PCR analysis of (A) TRPC5, (B) TRPC1, (C) TRPC4, (D) TRPC3, (E) TRPC6 and (F) TRPC7 expression in ipsilateral L3-L5 DRG, 28 days post-MIA (1 mg/mouse) or saline (10 μl/saline) injection. Expression was normalised to housekeeping genes, β2 macroglobulin (B2M), HPRT and β-actin. Values are mean ± S.D. n = 7–8; ∗p < 0.05, vs. WT Saline group. Two-way ANOVA, post hoc Tukey test. Each point in the dot plots represents an individual animal.
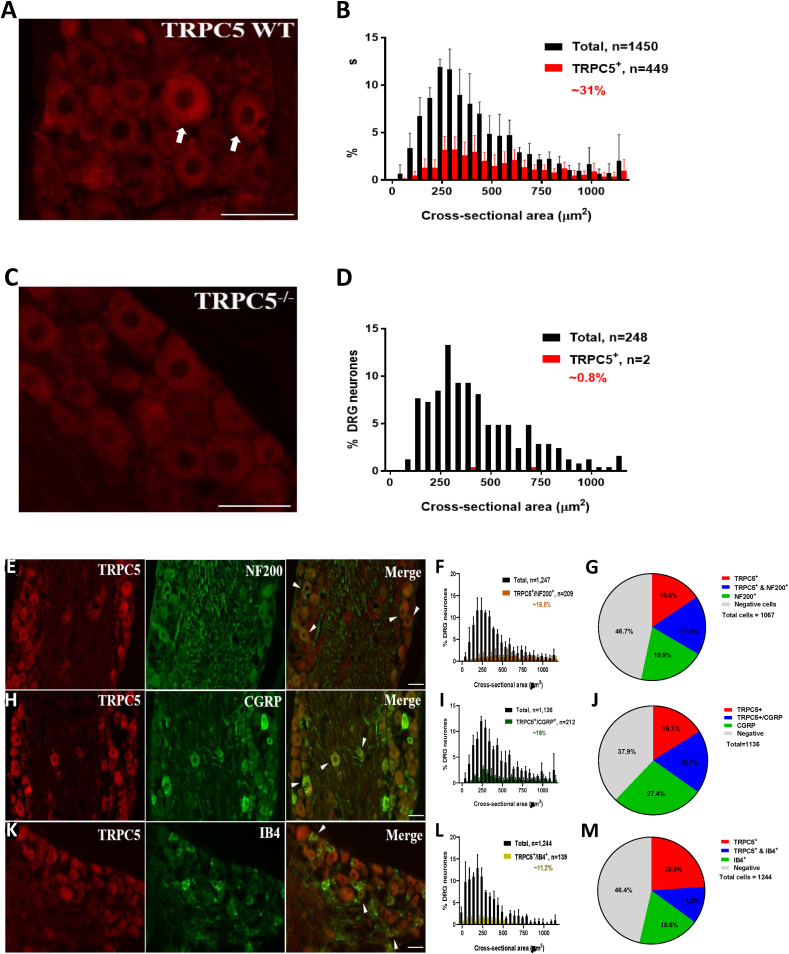
To further characterise the population of primary sensory neurons that express TRPC5, we analysed DRG sections, after single immunostaining with the anti-TRPC5 antibody. We found that 30.96% (448 out of 1450) of neurons in TRPC5 WT mice exhibited TRPC5 immunostaining (Fig. 6A and B). No TRPC5 immunostaining was seen in cells other than primary sensory neurons (Fig. 6A) and negligible staining could be detected in DRG sections from TRPC5 KO mice (Fig. 6C and D). Primary sensory neurons are both morphologically and functionally heterogeneous; they consist of small diameter nociceptive and large diameter non-nociceptive cells, which possess myelinated and unmyelinated processes, respectively [23]. TRPC5 immunopositive neurons appeared along the entire size range (Fig. 6A). The high molecular weight neurofilament, NF200 is regarded as a specific marker for large myelinated neurons, [24]. 17.9 ± 0.73% of the TRPC5 immunopositive cells were immunostained with the anti-NF200 antibody (Fig. 6E, F and G). By comparison, half of the nociceptive primary sensory neurons produce and release various neuropeptides, including CGRP, whereas the other half of the cells express binding sites for the Griffonia simplicifolia isolectin IB4 [25,26]. While 18.7 ± 3.38% of the TRPC5 immunopositive cells expressed CGRP (Fig. 6H, I and J), 11.2 ± 0.54% of the TRPC5 immunopositive neurons bound IB4 (Fig. 6K, L and M).
Fig. 6.
TRPC5 protein is expressed in dorsal root ganglions. (A) An anti-TRPC5 antibody (Alomone Labs) staining of WT DRG. Positive profiles can be seen as diffuse cytoplasmic staining. Scale bar = 50 μm (B) Cell size distribution of TRPC5-immunopositive (red bars) and total analysed cells (black bars) in WT sensory neurons. Results shown as mean ± S.D. (C) TRPC5 antibody failed to produce any detectable staining in DRG tissue from TRPC5 KO mice. Scale bar = 50 μm (D) Cell size distribution of TRPC5-immunopositive (red bars) and total analysed cells (black bars) in TRPC5 KO sensory neurons. Results shown as mean ± S.D. (E) Combined immunolabeling was produced by using the anti-TRPC5 antibody with an antibody raised against the 200 kD neurofilament NF200, with an antibody raised against (H) CGRP or (K) with biotinylated IB4. Scale bars = 50 μm. (F) Cell size distribution of cells co-expressing TRPC5 and NF200 (orange bars), (I) CGRP (green bars) or (L) IB4 (yellow bars) and total analysed cells (black bars) in wild type sensory neurons. Pie charts showing the average proportions of TRPC5 immunopositive neurons alone (red), co-expressing (blue) markers (G) or NF200, (J) CGRP and (M) IB4 immunopositive neurons alone (green).
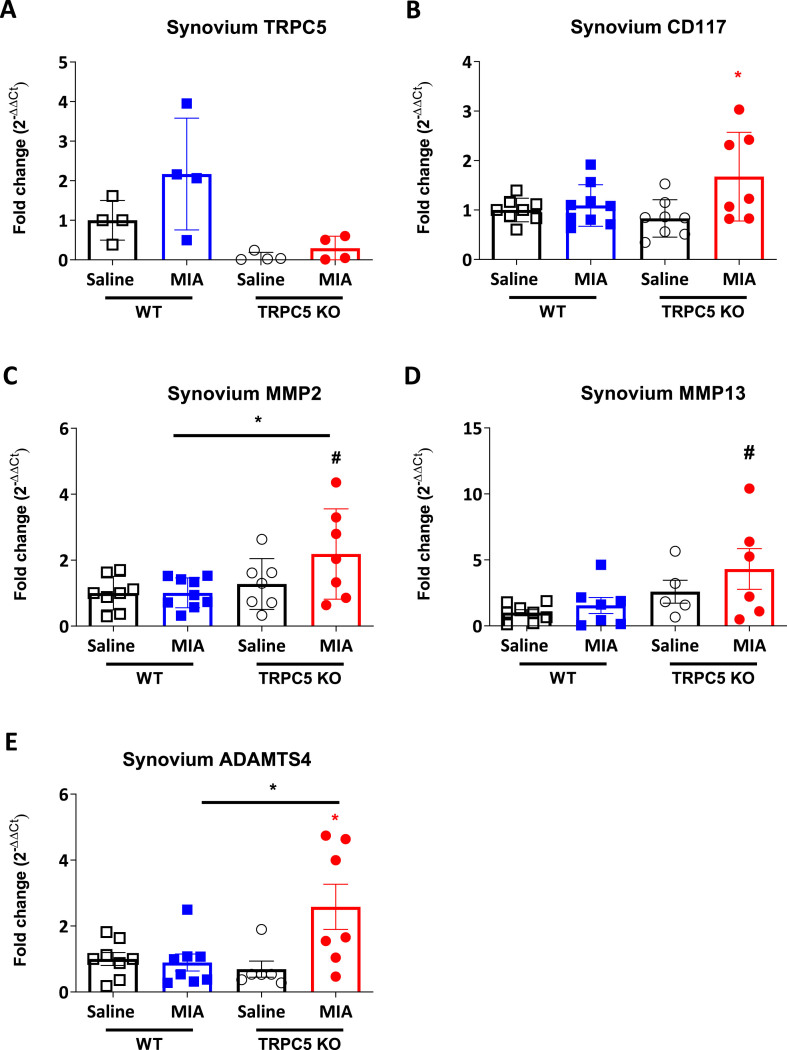
3.6. Upregulation of metalloproteinase expression in the synovium of TRPC5 KO mice after intra-articular MIA
We were interested in exploring novel neuro-immune mechanisms that would involve the TRPC5 signalling in the joint. As TRPC5 has been proposed to be expressed in synoviocytes [11], we investigated the mRNA expression of inflammatory markers and extracellular remodelling enzymes in the synovium 28 days after MIA injection. We confirmed TRPC5 expression in the WT synovium (Fig. 7A) and observed a significant induction in the expression of the mast cell marker CD117 in the ipsilateral synovium of TRPC5 KO mice (1 ± 0.24 vs 1.09 ± 0.42; p = 0.98, WT Saline vs WT MIA and 0.83 ± 0.38 vs 1.67 ± 0.90; p = 0.02, TRPC5 KO Saline vs TRPC5 KO MIA) (Fig. 7B). Similarly, we observed a potentiated induction in the expression of enzymes MMP2, MMP13 and a disintegrin metalloproteinase with thrombospondin motifs 4 (ADAMTS4) in TRPC5 KO mice compared with WT mice, suggesting augmented extracellular remodelling activity (MMP2: 1.01 ± 0.45 vs 2.19 ± 1.27; p = 0.04, WT MIA vs TRPC5 KO MIA; 1 ± 0.66 vs 4.31 ± 3.78; p = 0.05, WT Saline vs TRPC5 KO MIA and ADAMTS4: 0.89 ± 0.73 vs 2.58 ± 1.81; p = 0.02, WT MIA vs TRPC5 KO MIA) (Fig. 7C, D and E). Collectively the data show that mice lacking TRPC5 expression show an increased OA pain-related behaviour, nerve injury in the dorsal root ganglia and enhanced expression of extracellular remodelling enzymes in the synovium.
Fig. 7.
Enhanced extracellular matrix remodelling in TRPC5 KO mice 28 days following MIA-induced osteoarthritis. Real-time PCR analysis of (A) TRPC5 (B) CD117, (C) MMP2, (D) MMP13 and (E) ADAMTS4 expression in ipsilateral synovium, 28 days post-MIA (1 mg/mouse) or saline (10 μl/saline) injection. Expression was normalised to housekeeping genes, β2 macroglobulin, HPRT and β-actin. Values are mean ± S.D. n = 7–8; ∗p < 0.05, vs. saline group or vs. WT MIA group; #p < 0.05 vs. WT saline group. Two-way ANOVA, post hoc Tukey test. Each point in the dot plots represents an individual animal.
4. Discussion
We identify a novel pathway by which TRPC5 participates in pain signalling in OA. Using two distinct murine models of OA we show that deletion of TRPC5 is associated with modest signs of elevated mechanical allodynia and referred pain. The behavioural differences correlated with an enhancement in markers of extracellular matrix remodelling in TRPC5 KO mice despite an absence of overt histological signs of differences in cartilage degradation and synovitis between the two genotypes (Fig. 8). The findings also indicate that increased neuronal signalling is associated with the enhanced pain sensation in TRPC5 KO mice. This is in keeping with activation and sensitisation of sensory afferents in the joint which in turn facilitate noxious signalling from the OA joint to the dorsal horn of the spinal cord. We provide information that TRPC5 is expressed in the DRG as well as in synovial tissues.
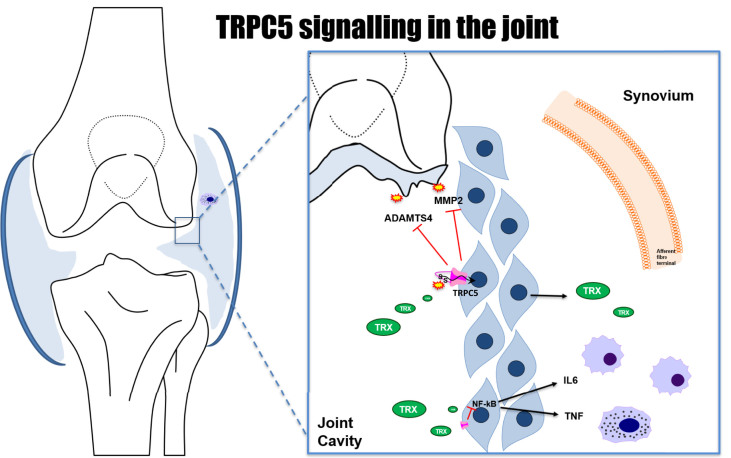
Fig. 8.
Proposed model describing the pathways through which TRPC5 in the knee joint modulates pain and inflammation in OA. Reduced thioredoxin (TRX) which is found to be elevated in the synovial fluid of human arthritis, acts through disulfide bridges, stimulating TRPC5 channels in synoviocytes [11], resulting in the inhibition of release of MMPs and ADAMTS4, related to tissue remodelling and progression of arthritis. Cytokines released from macrophages and mast cells and other mediators can induce the release of MMPs from synovial lining fibroblasts.
Here, using the PMNX and intra-articular MIA injection models, we discovered that weight bearing deficits and hindpaw withdrawal thresholds were exacerbated in TRPC5 KO mice in both models, although the magnitude of that difference was bigger in the PMNX model. This is in line with our previous findings concerning the role of TRPC5 in inflammatory joint arthritis [6] and with the involvement of inflammation in pain behaviour in the PMNX model [14]. We chose to analyse mechanisms involved in the mouse MIA model to further understand the influence of TRPC5 on nociceptive signalling.
Structural changes in the cartilage and surrounding tissues are typically present in human OA often associating with pain severity despite the lack of association with specific OA lesions [27,28]. Here, we found histological signs of cartilage degradation in the MIA model, consistent with previous reports [14]. MIA-induced pathological changes were similar between WT and TRPC5 KO mice, suggesting that TRPC5 does not have a major role in the development of OA-related histopathological changes.
There is increasing evidence of synovitis in human OA [1,2]. Likewise, in the MIA model, the presence of chronic synovitis is characterised by synovial hyperplasia and immune cell infiltration [19,29]. We previously found that in the CFA-induced joint inflammation, TRPC5 participates in an anti-inflammatory pathway, with TRPC5 KO mice displaying increased signs of synovitis [6]. Although in the present study, MIA resulted in increased synovial lining thickness, both in WT and TRPC5 KO mice, significance was not reached. This may be, in part, a consequence of the milder nature of inflammation in the MIA model, compared with the robust synovitis induced by CFA [6].
Mouse knee joints are innervated by primary afferents whose cell bodies reside in the L3-L5 DRG [30]. To investigate the nociceptive pathways involved in MIA-induced joint pain, we studied gene expression changes of pain associated genes by real-time qPCR. We found that CGRP expression was upregulated in DRG from TRPC5 KO mice. Consistent with that, MIA injection in the knee is associated with an increased release of peptides from primary afferent fibres and intrathecal CGRP antagonists can reverse established MIA-induced referred allodynia [31,32]. Neuronal injury secondary to peripheral inflammation in the MIA model is suggested to play a role in pain phenotype [33]. In keeping, with a neuropathic component to human OA pain [34], we show an increased expression of nerve injury marker ATF3, the neuropeptide galanin and CSF1. ATF3 was chosen as a marker of neuronal injury, as described in MIA-treated rats [35], confirms a neuropathic involvement in the pain [22,36]. Blocking joint inflammation in the early stage of the model prevents joint nerve damage in the latter stage of the disease [33], thus we hypothesise that exacerbation of inflammation in TRPC5 KO mice leads to a more pronounced expression of nerve injury markers.
The expression of galanin in DRG is increased in nerve injury and arthritis [37,38]; but to our knowledge upregulation of galanin expression has never been shown in models of OA despite evidence of involvement in nociception at spinal levels [39]. CSF1 is a cytokine involved in macrophage accumulation in arthritic synovia and also pain signalling with dorsal horn macrophages critical for the initiation and maintenance of neuropathic pain [40]. Interestingly, a recent study showed that activation of TRPC5 may inhibit the M1 polarization of macrophages by regulating Akt/IκB/NF-κB signalling pathways [41], suggesting a role of TRPC5 in macrophage regulation.
The TRPC5 receptor is expressed in DRG with a suggested role in cold detection [9]. However, it's localisation to sensory neuron subtypes is unclear. We confirmed TRPC5 expression in DRG along with TRPC1 and TRPC4, which are able to interact and oligomerise with TRPC5 [42] and show for the first time that MIA led to TPRC5 upregulation, 28 days after injection, but not TRPC1 and TRPC4. Previously, in an animal model of nerve injury, where a greater loss of small sized cells occurs, TRPC5 expression was shown to decrease, suggesting that TRPC5, found in DRG may participate in nociceptive signalling [43]. We found that approximately 30% of the CGRP+ sensory neurons involved in pain-processing, are also positive for TRPC5 with TRPC5 being localised to small-to-medium sized neurons.
Primary afferent fibres in the joint project to several spinal cord segments and terminate in both the superficial and deeper laminae, where they synapse with dorsal horn neurons [44]. Pathological changes in the joint lead to increase firing of joint afferents, resulting dorsal horn neurons to become hyperexcitable [45]. Intraarticular MIA injection can induce dorsal horn activation at both early and late phases of the model [19,46] and that facilitation of spinal neuronal responses correlate with changes in the hindpaw mechanical withdrawal thresholds [47]. Further, TRPC5 expressing fibres have been found in lamina I and II of the spinal cord [9], where nociceptive primary sensory neurons terminate, suggesting a role for TRPC5 in spinal cord nociceptive processing. Here, after 28 days, we observed an increased number of c-Fos expressing neurons in laminas I and II of MIA-treated TRPC5 KO mice, consistent with the increased mechanical allodynia, and with the increased expression of nerve injury markers in TRPC5 KO mice DRG.
Human OA is associated with synovitis, characterised by increased synovial cellularity and macrophage infiltration [1,2]. Further, the degree of synovitis detected by MRI correlates with the presence of knee pain, even in patients without radiographic signs of OA [3,4]. Finally, local anti-inflammatory interventions such as intra-articular injection of corticosteroids and oral or topical application of nonsteroidal anti-inflammatory drugs [48] are beneficial for some patients. Combined, this suggests an important contribution from inflammation to OA pain. In the present study we found marked signs of synovial lining expansion in the knee joint following MIA treatment, but did not observe discernible differences, between WT and TRPC5 KO mice. Our previous work in CFA-induced joint inflammatory pain, revealed that TRPC5 KO mice showed elevated inflammation and mediators. To look into the inflammation in more detail we investigated gene expression changes of inflammatory mediators in the MIA-induced OA synovium extracts. The main proteinases responsible for the breakdown of the extracellular matrix components are MMPs and ADAMTSs [49,50]. Interestingly, joint levels of MMP2, MMP13 and ADAMTS4 are elevated in the joints of OA patients [51,52] and shown to be released from lining synoviocytes [11]. Here we found that MMP2 and MMP13, along with ADAMTS4 gene expression are elevated in the joints of TRPC5 KO mice. A study showing that TRPC5 activation in cultured human fibroblast-like synoviocytes, by reduced thioredoxin (found to be elevated in synovial fluid of arthritic patients) results in the inhibition of secretion of MMPs, supports our gene expression findings [11]. We therefore propose that in the OA joint, TRPC5 receptor activation in synoviocytes inhibits MMP release. These MMPs participate both in cartilage degradation and extracellular matrix breakdown as well as orchestrate cell migration and infiltration in the tissue of mast cells and other immune cells (Fig. 8). Future studies employing unbiased transcriptomic approaches would help further, to explore the joint pathways by which TRPC5 participates in joint pathology in OA.
To conclude, we show that TRPC5 deletion in two models of OA is associated with a modest increase in pain-related phenotype and activation of the pain neuroaxis. Whilst there is a lack of overt differences in cartilage and synovium pathology between WT and TRPC5 KO joints, more detailed gene expression analysis revealed considerable changes in extracellular remodelling pathways and mast cell numbers. These novel findings indicate the ability of TRPC5 to influence signalling relevant to synovium biology. This advances knowledge of molecular mechanisms by which synovitis and pain may be modulated in OA.
Author contributions
JSV, KA and SDB designed the research, JSV, KA, SB, PK, NA and FA performed experiments and collected and analysed the data. XK, DT and FA helped with the blinding. All authors participated in the interpretation of the data. JSV and SDB wrote the paper. All authors read and approved the submitted manuscript.
Role of the funding source
This work was supported by Versus Arthritis (ARUK21524). Versus arthritis did not participate in the study design, collection, analysis and interpretation of data nor in the writing of the manuscript and in the decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgements
The authors would like to thank Professor David E. Clapham (Howard Hughes Medical Institute, Boston Children's Hospital, Harvard Medical School) for kindly gifting WT and TRPC5 KO mice breeding pairs used in this study. The authors would also like to thank Mr Carl Hobbs and Mr Tom Pitcher for technical assistance with these studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100119.
Contributor Information
João de Sousa Valente, Email: joao.de_sousa_valente@kcl.ac.uk.
Khadija M. Alawi, Email: khadija.k.alawi@kcl.ac.uk.
Patrik Keringer, Email: patrik.keringer@aok.pte.hu.
Sabah Bharde, Email: sabahbharde1@gmail.com.
Faseeha Ayaz, Email: faseeha.ayaz@ccc.ox.ac.uk.
Nurjahan Saleque, Email: nurjahan.saleque@kcl.ac.uk.
Xenia Kodji, Email: xenia.m.kodji@kcl.ac.uk.
Dibesh Thapa, Email: dibesh.thapa@kcl.ac.uk.
Fulye Argunhan, Email: fulye.argunhan@kcl.ac.uk.
Susan D. Brain, Email: sue.brain@kcl.ac.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J., Huang Z., Yu X., Zhou L., Pei F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019;46:36–44. doi: 10.1016/j.cytogfr.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Hunter D.J., Jin X., Ding C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthr. Cartil. 2018;26:165–174. doi: 10.1016/j.joca.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Baker K., Grainger A., Niu J., Clancy M., Guermazi A., Crema M., et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 2010;69:1779–1783. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilius B., Mahieu F., Karashima Y., Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem. Soc. Trans. 2007;35:105–108. doi: 10.1042/BST0350105. [DOI] [PubMed] [Google Scholar]
- 6.Alawi K.M., Russell F.A., Aubdool A.A., Srivastava S., Riffo-Vasquez Y., Baldissera L., Jr., et al. Transient receptor potential canonical 5 (TRPC5) protects against pain and vascular inflammation in arthritis and joint inflammation. Ann. Rheum. Dis. 2017;76:252–260. doi: 10.1136/annrheumdis-2015-208886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer M., Plant T.D., Obukhov A.G., Hofmann T., Gudermann T., Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 8.Strubing C., Krapivinsky G., Krapivinsky L., Clapham D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann K., Lennerz J.K., Hein A., Link A.S., Kaczmarek J.S., Delling M., et al. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riccio A., Li Y., Moon J., Kim K.S., Smith K.S., Rudolph U., et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S.Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes E.S., Russell F.A., Spina D., McDougall J.J., Graepel R., Gentry C., et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund's complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- 13.Keeble J., Russell F., Curtis B., Starr A., Pinter E., Brain S.D. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- 14.Mapp P.I., Sagar D.R., Ashraf S., Burston J.J., Suri S., Chapman V., et al. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1336–1345. doi: 10.1016/j.joca.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Knights C.B., Gentry C., Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012;153:281–292. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher T., Sousa-Valente J., Malcangio M. The monoiodoacetate model of osteoarthritis pain in the mouse. J Vis Exp. 2016;16(111) doi: 10.3791/53746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasson S.S., Chambers M.G., Van Den Berg W.B., Little C.B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Sousa-Valente J., Calvo L., Vacca V., Simeoli R., Arevalo J.C., Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr. Cartil. 2018;26:84–94. doi: 10.1016/j.joca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 20.de Sousa Valente J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis. Front. Pharmacol. 2019;10:974. doi: 10.3389/fphar.2019.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira-Gomes J., Adaes S., Sarkander J., Castro-Lopes J.M. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum. 2010;62:3677–3685. doi: 10.1002/art.27713. [DOI] [PubMed] [Google Scholar]
- 22.Thakur M., Rahman W., Hobbs C., Dickenson A.H., Bennett D.L. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy I. In: Anesthetic Pharmacology: Physiologic Principles and Clinical Practice. Evers, Maze, editors. 2004. Sensory processing: primary afferent neurons/DRG; pp. 187–197. [Google Scholar]
- 24.Goldstein M.E., House S.B., Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J. Neurosci. Res. 1991;30:92–104. doi: 10.1002/jnr.490300111. [DOI] [PubMed] [Google Scholar]
- 25.Molliver D.C., Radeke M.J., Feinstein S.C., Snider W.D. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J. Comp. Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 26.Silverman J.D., Kruger L. Lectin and neuropeptide labeling of separate populations of dorsal root ganglion neurons and associated "nociceptor" thin axons in rat testis and cornea whole-mount preparations. Somatosens. Res. 1988;5:259–267. doi: 10.3109/07367228809144630. [DOI] [PubMed] [Google Scholar]
- 27.Torres L., Dunlop D.D., Peterfy C., Guermazi A., Prasad P., Hayes K.W., et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthr. Cartil. 2006;14:1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Walsh D.A., Yousef A., McWilliams D.F., Hill R., Hargin E., Wilson D. Evaluation of a Photographic Chondropathy Score (PCS) for pathological samples in a study of inflammation in tibiofemoral osteoarthritis. Osteoarthr. Cartil. 2009;17:304–312. doi: 10.1016/j.joca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Mapp P.I., Avery P.S., McWilliams D.F., Bowyer J., Day C., Moores S., et al. Angiogenesis in two animal models of osteoarthritis. Osteoarthr. Cartil. 2008;16:61–69. doi: 10.1016/j.joca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Salo P.T., Tatton W.G. Age-related loss of knee joint afferents in mice. J. Neurosci. Res. 1993;35:664–677. doi: 10.1002/jnr.490350609. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch S., Corradini L., Just S., Arndt K., Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain. 2013;154:700–707. doi: 10.1016/j.pain.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Ogbonna A.C., Clark A.K., Gentry C., Hobbs C., Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur. J. Pain. 2013;17:514–526. doi: 10.1002/j.1532-2149.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- 33.McDougall J.J., Muley M.M., Philpott H.T., Reid A., Krustev E. Early blockade of joint inflammation with a fatty acid amide hydrolase inhibitor decreases end-stage osteoarthritis pain and peripheral neuropathy in mice. Arthritis Res. Ther. 2017;19:106. doi: 10.1186/s13075-017-1313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakur M., Dickenson A.H., Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014;10:374–380. doi: 10.1038/nrrheum.2014.47. [DOI] [PubMed] [Google Scholar]
- 35.Ivanavicius S.P., Ball A.D., Heapy C.G., Westwood F.R., Murray F., Read S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 36.McDougall J.J., Albacete S., Schuelert N., Mitchell P.G., Lin C., Oskins J.L., et al. Lysophosphatidic acid provides a missing link between osteoarthritis and joint neuropathic pain. Osteoarthr. Cartil. 2017;25:926–934. doi: 10.1016/j.joca.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Su J., Gao T., Shi T., Xiang Q., Xu X., Wiesenfeld-Hallin Z., et al. Phenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse model. J. Comp. Neurol. 2015;523:1505–1528. doi: 10.1002/cne.23749. [DOI] [PubMed] [Google Scholar]
- 38.Hokfelt T., Wiesenfeld-Hallin Z., Villar M., Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci. Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 39.Xu X., Yang X., Zhang P., Chen X., Liu H., Li Z. Effects of exogenous galanin on neuropathic pain state and change of galanin and its receptors in DRG and SDH after sciatic nerve-pinch injury in rat. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X., Liu H., Hamel K.A., Morvan M.G., Yu S., Leff J., et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat. Commun. 2020;11:264. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao L., Guo G., Qi Y., Xiong Y., Ma X., Wu N., et al. Inhibition of canonical transient receptor potential 5 channels polarizes macrophages to an M1 phenotype. Pharmacology. 2020;105:202–208. doi: 10.1159/000503452. [DOI] [PubMed] [Google Scholar]
- 42.Rubaiy H.N. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019;176:832–846. doi: 10.1111/bph.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staaf S., Oerther S., Lucas G., Mattsson J.P., Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144:187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Woolf C.J., Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Neugebauer V., Lucke T., Schaible H.G. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J. Neurophysiol. 1993;70:1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- 46.Orita S., Ishikawa T., Miyagi M., Ochiai N., Inoue G., Eguchi Y., et al. Percutaneously absorbed NSAIDs attenuate local production of proinflammatory cytokines and suppress the expression of c-Fos in the spinal cord of a rodent model of knee osteoarthritis. J. Orthop. Sci. 2012;17:77–86. doi: 10.1007/s00776-011-0175-7. [DOI] [PubMed] [Google Scholar]
- 47.Sagar D.R., Staniaszek L.E., Okine B.N., Woodhams S., Norris L.M., Pearson R.G., et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–3676. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conaghan P.G., Dickson J., Grant R.L., Guideline Development G. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336:502–503. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy G., Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat. Clin. Pract. Rheumatol. 2008;4:128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 50.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu W., Billinghurst R.C., Pidoux I., Antoniou J., Zukor D., Tanzer M., et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 52.Kevorkian L., Young D.A., Darrah C., Donell S.T., Shepstone L., Porter S., et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.