Abstract
Adipocytokines are hormonally active molecules that are believed to play a key role in the regulation of crucial biological processes in the human body. Numerous experimental studies established significant alterations in the adipokine secretion patterns throughout pregnancy. The exact etiology of various gestational complications, such as gestational diabetes, preeclampsia, and fetal growth abnormalities, needs to be fully elucidated. The discovery of adipokines raised questions about their potential contribution to the molecular pathophysiology of those diseases. Multiple studies analyzed their local mRNA expression and circulating protein levels. However, most studies report conflicting results. Several adipokines such as leptin, resistin, irisin, apelin, chemerin, and omentin were proposed as potential novel early markers of heterogeneous gestational complications. The inclusion of the adipokines in the standard predictive multifactorial models could improve their prognostic values. Nonetheless, their independent diagnostic value is mostly insufficient to be implemented into standard clinical practice. Routine assessments of adipokine levels during pregnancy are not recommended in the management of both normal and complicated pregnancies. Based on the animal models (e.g., apelin and its receptors in the rodent preeclampsia models), future implementation of adipokines and their receptors as new therapeutic targets appears promising but requires further validation in humans.
Keywords: adiponectin, apelin, chemerin, gestational diabetes, irisin, leptin, omentin, preeclampsia, resistin, visfatin
1. Introduction
According to the newest data, 30.5% of European women are overweight (body mass index (BMI) > 25 kg/m2), and a stunning 15.9% of them are diagnosed with obesity (BMI > 30 kg/m2), which forces obstetricians to face with the state of an obesity epidemic and obesity-related diseases among their patients [1]. Moreover, the pregnancy itself is connected with increased insulin resistance and promotes the worsening of multiple metabolic parameters. The exact pathophysiological mechanism of numerous pregnancy complications such as gestational hypertension, preeclampsia (PE), gestational diabetes (GDM), or fetal growth abnormalities is quite elaborate, however, its incidence among obese patients is significantly elevated [2,3,4].
Obesity is a pathological condition considered to be mainly associated with bad lifestyle habits—high caloric intake and the lack of physical activity—leading to morphological changes in the structure of adipose tissue [5]. Excessive body weight promotes adipocyte hypertrophy and the occurrence of the local cellular hypoxia, which together contribute to the development of the state of chronic low-grade inflammation [6]. This results in increased migration and the presence of immune cells, such as macrophages and lymphocytes, in pathologically re-modelled adipose tissue due to increased adipocyte apoptosis promoted by hypoxia [7]. The increased infiltration of immune cells exposed to persistent hypoxia and increased oxidative stress destroys the balance between secretion of anti- and pro-inflammatory cytokines [8]. In response to the presence of those stressors, the synthesis of various adipocyte-specific molecules, such as leptin and adiponectin, is altered compared to lean individuals. It has been found that adipokine secretion patterns are strictly linked with the excessive body weight, percentage of hormonally active adipose tissue, and inflammatory status [9]. However, these changes can be reversed through a reduction of both subcutaneous and visceral fat levels [10,11].
Adipokines belong to the group of protein hormones and cytokines secreted by adipocytes, immune cells, fibroblasts, and other hormone-secreting cells originating from the adipose tissue. Adipocytes release hundreds of signaling molecules that are responsible for the regulation of the local and systemic cellular activities through their autocrine, paracrine, and endocrine activity [12,13]. It has been established that adipokines play a key role in the regulation of many crucial processes in the human body, like glucose and lipid metabolism, insulin sensitivity, appetite, immune response, and inflammation [14]. Due to their engagement in these processes, adipokines may be treated as potential targets for novel therapeutic strategies in numerous medical conditions [15].
A dysregulation in adipokine production is known as a characteristic feature of obesity and occurs in a variety of obesity-related diseases. Furthermore, those abnormalities are treated as partial causative factors in the pathogenesis of several metabolic, inflammatory, and neoplastic diseases [16]. The abnormal leptin-to-adiponectin ratio is often observed in test results of individuals diagnosed with impaired glucose tolerance, dyslipidemias, and high blood pressure [17,18]. Without any predominant changes in daily habits and diet, those conditions lead straight to the development of the metabolic syndrome [19]. Patients affected by metabolic syndrome are often diagnosed with type 2 diabetes and advanced atherosclerosis, which makes them more susceptible to micro- and macro-vascular diseases and puts them at risk of death from cardiovascular events like stroke or heart attack [20].
While there are still multiple possible hypotheses regarding the mechanisms of pregnancy-related complications, such as gestational hypertension, preeclampsia, gestational diabetes, or fetal growth abnormalities, the discovery of the adipose-derived hormones sheds new light on those conditions. Current studies tried to analyze the contribution of adipocyte-specific peptides to the pathogenesis of those complications. Moreover, it seems essential to assess the possible benefits of routine adipokines concentration measurements performed in patients with uncomplicated pregnancies. Those assessments could establish their utility as potential early markers of gestational complications. Standard measurements of adipokine levels in a few mentioned high-risk pregnancy complications may also influence the choice of proper treatment strategies. Finally, adipokines open promising perspectives for the invention of new drugs that could be used during gestation.
2. Scope and Methodology
This review was compiled to analyze the potential clinical applications of adipokine measurements in early detection and further management strategies of a variety of gestational complications. A list of analyzed adipokines was restricted to relatively well-characterized molecules that included leptin, adiponectin, visfatin, resistin, irisin, omentin, chemerin, and apelin. Every adipokine is characterized in a separate section. Gestational complications were limited to those which are thought to be even partially associated with disturbances in patient’s metabolic status—maternal complications and fetal abnormalities that might be related to maternal metabolic diseases. Analyzed conditions include obesity, gestational diabetes, gestational hypertension, preeclampsia, fetal growth restriction (FGR), and fetal macrosomia. To establish a list of relevant references, PubMed database was searched from the first records until September 2020 using the following formulas: (“an adipokine” + “a pregnancy complication”)—from those listed above.
3. Clinical Applications of Adipokines Measurements in Various Pathologies
3.1. Leptin
Leptin is a peptide hormone secreted mainly by the white adipose tissue, with its most important functions including regulation of energy homeostasis, metabolism, and neuroendocrine function [21]. Leptin was reported to be elevated even in physiological pregnancy, steadily increasing throughout its course [22]. Leptin was the first described adipokine linking the adipose tissue with reproduction [23]. This protein is also expressed by the placental tissue and fetal adipose tissue. Ninety-five percent of leptin produced in the placenta is delivered into maternal circulation where it promotes excessive weight gain during pregnancy [24,25,26]. Hence, leptin is often considered to be responsible for pregnancy-associated energy balance changes [27]. Furthermore, reproductive functions, such as embryo implantation and development, have also been found to be influenced by its expression. In turn, mouse model studies reported infertility caused by leptin deficiency, with the affected animals rescued by exogenous administration of this protein [28]. Leptin was also found to stimulate gonadotropin hormone expression, as well as act as a puberty permissive factor under the influence of steroid hormones [29]. It was further reported to affect ovarian function, linking follicular alterations with obesity [30]. Additionally, a significant role of leptin was suggested in embryo implantation, as the protein and its receptors are expressed in blastocysts and can be extracted from embryo-conditioned media [31,32].
3.1.1. Gestational Diabetes Mellitus (GDM)
GDM is defined as maternal hyperglycemia and glucose intolerance of varying degrees of severity which occur for the first time during pregnancy. The diagnosis is based on fasting glucose and oral glucose tolerance test results [4]. GDM patients’ placentas were characterized by increased expression of leptin and leptin receptors, with this protein suggested as a first-trimester prognostic factor for this condition [33]. Furthermore, leptin was suggested to control fetal homeostasis in response to hyperinsulinemia [34,35]. Cord blood leptin levels also seem to be dependent on maternal glucose, providing a possible explanation to the predisposition of GDM newborns to obesity [36]. Moreover, increased leptin gene expression in GDM placentas compared to controls correlated with pro-inflammatory cytokine production, resulting in chronic inflammation and further amplification of leptin production [37]. It was reported that insulin induces leptin expression in cells of the trophoblast through an increase in activity of leptin promoter, as those two hormones share several signaling pathways (e.g., MAPK, PI3K, and JAK2/STAT-3) [38]. Moreover, a placental crosstalk between leptin and insulin signaling was demonstrated, manifesting through increased STAT-3, MAPK 1/3, and PKB pathway activity (Figure 1) [39]. A study evaluating plasma levels of leptin also showed its increase in plasma of patients with GDM [40]. This was confirmed in further studies, which showed a statistically significant increase of venous blood and serum levels of this protein in women affected by GDM compared to healthy controls [22,40]. These results were confirmed in a recent publication, which furthermore suggests that while the overall baseline concentration of circulating leptin is higher in GDM patients, a smaller increase in the levels of this protein can be observed throughout the course of pregnancy [22].
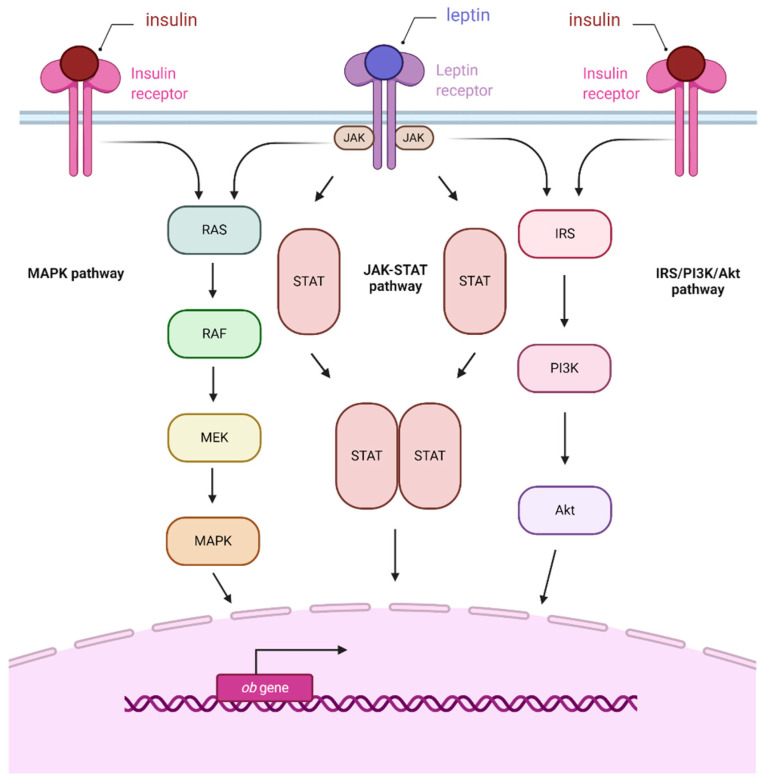
Figure 1.
Pictorial diagram of insulin and leptin signaling in placenta which includes 3 major signaling pathways, namely JAK-STAT, MAPK, and IRS/PI3K/Akt. Abbreviations: Akt—protein kinase B; IRS—insulin receptor substrate; JAK—janus kinase; MAPK—mitogen-activated protein kinase; MEK—mitogen-activated protein kinase kinase; PI3K—phosphatidylinositol 3-kinase; RAF—RAF kinase; RAS—small GTPase; STAT—signal transducer and activator of transcription (according to References [38,39]) (Created with BioRender.com).
Overall, while the available studies suggest that there is an increase in leptin levels in GDM patients compared to controls, possible explanations and implications of that occurrence are not sufficiently explored. Hence, there is an understandable lack of clinical studies targeting leptin in the treatment of GDM.
3.1.2. Preeclampsia
The American College of Obstetrics and Gynecology defines PE as a pregnancy complication which occurs after 20 weeks of gestation. The current criteria of PE includes elevated blood pressure with the presence of other severe features with or without proteinuria [41], and in severe cases, widespread organ failure. It is a major contributor to maternal as well as fetal morbidity and mortality [41]. The etiology of PE is probably heterogeneous and not completely clarified, but it is believed that the disease starts in the first trimester as a placental condition resulting in insufficient placental perfusion, followed by a maternal syndrome with endothelial dysfunction and hypertension [42].
Multiple studies suggest an increase in leptin serum levels in PE patients, as well as elevated levels of this protein in preeclamptic placentas [43,44,45]. However, the same correlation was not observed in pre-term PE [45]. Hence, while the connection between BMI and PE has not yet been defined, leptin has been among the proposed factors that could facilitate that link [45]. Two suggested dependencies either indicated leptin increase as a sign of placental stress, such as preeclampsia-associated hypoxia, or note its increase even before the onset of the disease, with the possibility of its use as a prognostic factor [46]. Another explanation, basing on the proangiogenic ability of leptin, suggests its role in response to under-perfusion of PE placenta, supporting neovascularization and improving nutrient delivery [37].
Overall, while the mode of leptin action during preeclampsia is not yet fully determined, multiple studies agree that its levels are elevated in PE patients [21]. Hence, this protein could potentially become a diagnostic-prognostic factor in this condition. However, a recent study reported about decreased levels of this protein in PE-affected obese women compared to normal pregnancy controls [47].
3.1.3. Other Gestational Complications
Apart from PE and GDM, the role of leptin in recurrent miscarriages is an important topic [48]. Women affected by recurrent miscarriage exhibited elevated serum leptin concentration compared to the control group [49]. However, in another study, 5–8 gestation weeks plasma levels in women who subsequently miscarried were found to be lower than among women in which term birth was noted [50]. Finally, the study of Tommaselli et al. found no significant difference in maternal serum leptin concentration, maybe due to the heterogeneity of the miscarriage cases in the study group [51]. Hence, the data on leptin concentration in recurrent miscarriage are highly conflicted, with no consensus that could shed light on the possible involvement of this protein in this condition.
Cord blood leptin levels are positively correlated with neonatal birth weight. Moreover, large-for-gestational-age (LGA) newborns have higher cord blood leptin concentrations in comparison to appropriate-for-gestational-age controls. Thus, it was established that elevated leptin levels promote the increased fetal weight gain [52,53]. Intrauterine growth restriction (IUGR) was also associated with decreased leptin levels in both maternal and fetal blood. While the detected levels of both were lower in IUGR pregnancies, there was no statistically significant relationship between the maternal and fetal concentrations [54]. Furthermore, a comparison between PE and IUGR patients (without PE) showed that the levels of leptin were significantly lower in patients without preeclampsia, showing differences despite similar placental pathology [55].
3.2. Adiponectin
Adiponectin is a 30 kDa adipokine most abundantly secreted by adipocytes, with reported antidiabetic, anti-inflammatory, and cardioprotective functions [56]. Its expression was shown to exhibit significant sexual dimorphism, with males characterized by notably lower levels of this protein than females [57], while its action is exerted through two functionally different receptors, AdipoR1 and AdipoR2. It is one of the hormones that were, in a number of studies, associated with the development of obesity [58]. Its reduction plays a significant role in diseases associated with excessive weight, as it counteracts insulin resistance and inflammation [59]. Hence, administration of recombinant adiponectin was a subject of a number of therapeutic interventions [60]. Furthermore, adiponectin is one of the most studied adipokines within the context of pregnancy. It was found that adiponectin, independently from maternal BMI, modulates the glucose homeostasis, promoting the increased insulin sensitivity [61,62]. Most studies suggest that in normal pregnancies, plasma levels of this protein did not significantly differ between non-pregnant and first trimester pregnant women, decreasing towards the end of pregnancy and falling again postpartum [63]. This inverse correlation with the gestational age of the fetus is not observed in overweight women. This dependency can be explained with the need for maternal pregnancy-related fat deposition and the downregulation of adiponectin expression in response to increasing adipose mass [64,65,66,67]. Hence, despite that singular studies based on a small size sample group question the correlation of adiponectin level with gestation advancement, the general consensus states that concentration of this protein decreases significantly between the first and third trimester of pregnancy. Nevertheless, the physiological range of this protein’s serum levels during gestation is not yet established [63]. Finally, adiponectin was implicated in a range of other diseases, such as hypertension, chronic kidney diseases, atherosclerosis, chronic obstructive pulmonary disease, diabetic retinopathy and cancer, as summarized by a number of existing excellent reviews focused solely on this adipokine [59,60].
3.2.1. Gestational Diabetes Mellitus
Adiponectin levels are decreased in GDM patients, regardless of their weight, BMI, or insulin resistance [37,68,69,70,71]. Moreover, some studies suggest that adiponectin pre-pregnancy and early pregnancy measurements could act as a predictive factor for the later development of GDM, with some of them including a combination of other maternal factors to improve prediction rate [72,73,74,75,76]. The results of a meta-analysis conducted by Ilidromiti et al. suggest that in combination with maternal characteristics, blood adiponectin, as a known predictor of insulin resistance status, could indeed be used to evaluate the risk of GDM [77]. Furthermore, decreased adiponectin mRNA levels were also detected in placental tissue of GDM patients [78]. A decrease in adiponectin levels can also be observed in circulation of fetuses born out of GDM pregnancies, compared to the controls of the same gestational age, independent of the fetal birth weight [79].
Overall, the results of adiponectin studies are relatively uniform, with the levels of this protein in maternal blood generally accepted as a good potential prognostic/diagnostic factor for GDM.
3.2.2. Preeclampsia
Adiponectin receptor’s mRNA and protein are upregulated in preeclampsia, while adipokine itself shows downregulation in placental tissue [80,81]. These levels were also found to correlate positively with the expression of p-STAT5 and inversely with p-p38 (both factors affecting the function of placental trophoblasts), implicating the role of adiponectin in preeclampsia [82]. Ramsay et al. found a significant rise in adiponectin concentration in patients affected by PE compared to controls, suggesting that this increase is tied to higher adipocyte secretion [83]. These results were confirmed by multiple later studies, even those correcting for hematocrit [84,85,86,87]. However, several different studies reported significantly lower levels of circulating adiponectin in preeclamptic patients compared to normal pregnancy [88,89,90]. A proposed explanation of this status is the BMI differences of patients, as those with BMI > 25 and preeclampsia exhibited significantly decreased blood concentration of adiponectin. Similarly, normal-weight woman affected with the same disease showed a major increase in circulating levels of this protein [91]. However, it must be noted that this correlation might be non-functional in overweight women affected by preeclampsia, due to their increased insulin and adiponectin resistance, while in the same way representing the physiological response of the normal-weight women to impaired placental perfusion [64]. This assumption is supported by a recent study published by Thagaard et al., that compared normal and preeclamptic obese pregnant women, confirming that the former were characterized by a significantly lower expression of adiponectin, suggesting that lack of this protein might play a role in the pathogenesis of the disease [47]. Moreover, a recent study evaluating the usefulness of serum adiponectin quantification for prediction of PE concluded that adiponectin levels were the best prognostic factor (as compared to visfatin, resistin, and leptin), when corrected for BMI, age, parity, and family history of PE and diabetes [92]. In summary, the mechanism of adiponectin action in preeclampsia is not yet well known, but it appears that lower levels of this protein might play a certain role in overweight and obese women, with a less likely involvement in normal weight pregnancy.
3.3. Visfatin
Visfatin is a protein produced predominantly by the visceral adipose tissue, with its tissue and circulation levels significantly increasing in obesity [93]. Visfatin levels are, also, higher in pregnant compared to non-pregnant controls [94]. During normal pregnancy, the plasma concentration of this protein changes, reaching a peak between 19 and 26 weeks of gestation. In normal pregnancies of obese women, visfatin levels were unchanged [95]. Decreased placental visfatin expression was associated with poor glycemic control and macrosomia in women with type 1 diabetes [96]. However, the physiological range of visfatin in normal pregnancy remains undetermined, with the variation between studies mostly dependent on the characteristics of the studied group [97].
3.3.1. Gestational Diabetes Mellitus
Similarly, in the case of GDM, the results concerning visfatin levels in affected and control patients are conflicting [98]. Akturk et al. in their study of plasma visfatin levels of women between 33 and 39 weeks of gestation, concluded that the concentration of this protein drops in GDM patients compared to healthy controls [99]. Several other studies, on the contrary, reported significantly elevated visfatin levels in GDM pregnancies [100,101,102]. A meta-analysis of 26 original studies conducted by Zhang et al. concluded that the level of this protein is significantly increased in obese mothers affected by GDM, in comparison to controls. However, they also noted that this finding is most probably associated with extreme maternal weight, rather than with GDM itself [103]. Moreover, Mazaki-Tovi et al. determined that maternal GDM and fetal hypertrophy are independently associated with high plasma visfatin levels, indicating its potential usefulness as a diagnostic/prognostic factor for GDM screening [104]. Another study positively correlated umbilical cord visfatin levels with fetal birth weight, indicating its potential role in fetal growth regulation [105]. Taraqi et al. found an inverse correlation between visfatin levels and birth weight in patients with higher than normal BMI [106]. Furthermore, higher levels of this protein were associated with IUGR (defined as birth weight ≤ 3rd customized centile), especially during the third trimester of pregnancy [107,108].
3.3.2. Preeclampsia
The visfatin levels in preeclampsia remain relatively unknown [70]. Fasshauer et al. reported increased serum levels of visfatin in PE patients compared to healthy controls [109]. On the contrary, Hu et al. reported a notable decrease in the levels of this protein among pregnant women with and without PE in the third trimester of pregnancy [110]. The differing results among several studies may be related to the characteristics of the study groups [111,112,113]. Nevertheless, a recent study, evaluating the usefulness of a number of adipokines in PE prediction, concluded that while visfatin levels (reported as higher in PE patients than in controls) are a weaker predictor than, e.g., adiponectin, it was, nonetheless, a significant prognostic factor when corrected for BMI [92].
A significant number of studies tie visfatin to normal and pathological pregnancies, but their results are often conflicting. Furthermore, the gaps in knowledge about the exact function of this protein in physiological, as well as pathological pregnancy, calls for more research before it can be fully recommended as a diagnostic/prognostic factor [98].
3.4. Resistin
Resistin is a pro-inflammatory adipokine that is predominantly expressed by mononuclear cells, as well as adipocytes and most importantly, placental trophoblastic cells during pregnancy [114,115]. Resistin impairs glucose uptake by adipocytes, increases plasma glucose concentration, and thus decreases insulin sensitivity [114]. It is also known as a factor inducing the production of inflammatory cytokines and cell adhesion molecules [116]. The plasma resistin levels in pregnant women at term are significantly higher than those in age-matched non-pregnant controls. Because, adipose resistin expression remains unchanged during pregnancy, it suggests that the placenta is likely a major source of resistin in the maternal circulation [79,115]. Moreover, high resistin level could further affect the placental transfer of glucose by decreasing the expression of trophoblastic cell surface glucose transporters, GLUT-1 [117]. Some authors linked the high resistin concentrations with the increase in insulin resistance during the latter half of pregnancy, which may suggest its potential indirect role in the regulation of fetal growth. Those effects could be caused by increased insulin secretion and its pro-growth activity [118].
3.4.1. Gestational Diabetes Mellitus
Even though experimental findings suggest that resistin might be involved in the diabetes pathophysiology and glucose homeostasis, its role in the development of insulin resistance and GDM in pregnancy remains unclear [119,120]. Despite the fact that several studies attempted to assess the concentrations of circulating resistin in GDM, there appears to be a significant discrepancy between the results. It is concluded by most studies that the differences in concentrations between women with GDM and pregnant controls are insignificant [75]. Besides, three independent prospective studies did not find any association between resistin and increased GDM risks [75,76,121]. However, significant differences in levels depending on trimesters have been reported by other studies. Chen et al. and Nien et al. reported that resistin level remains stable in the first and second trimesters and increases in the third trimester [122,123]. In contrast, Cortelazzi et al. found a progressive decline in resistin level between 10 and 41 weeks [79]. However, there was the substantial heterogeneity between the studies. Lobo et al. in their study revealed that significantly higher plasma resistin levels, between 11 and 13 weeks of pregnancy, existed in participants, who later developed GDM as compared to their healthy control counterparts [120].
Interestingly, even though Lappas et al. did not detect any characteristic differences in the release of resistin while comparing GDM patients with pregnant controls, the study showed that several inflammatory mediators and hormones modulate resistin release, including insulin which stimulates the release of resistin from the human placenta [124]. These controversial results may be attributed to not correlating different gestational ages, lack of adjustment for BMI, and other confounders such as smoking, maternal age, and parity.
Kuzmicki et al. discovered that significantly higher plasma resistin between 11 and 13 weeks of pregnancy existed in participants, who later developed GDM as compared to their healthy control counterparts, but this finding is contrary to a report of Megia et al., which indicated that GDM is associated with lower resistin levels [125,126].
Importantly, common and sometimes inevitable phenomena during pregnancy such as increased BMI and substantial weight gain are known to cause changes in the metabolism and gene expression of adipocytes, which results in increased lipolysis and release of pro-inflammatory cytokines that recruit and activate macrophages. Macrophages in turn produce large amounts of proinflammatory mediators, including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, and resistin, that therefore create a positive feedback loop since an insulin-resistant state will be induced and amplified by these inflammatory mediators. Owing to this fact, the hypothesis of the inflammatory pathway having a noticeable effect on glucose regulation during pregnancy is consistent with the potential role of this same pathway in the development of GDM, since pregnancy is an insulin-resistant state [119].
Factors such as advanced maternal age, obesity, family history of diabetes mellitus, history of a poor obstetric outcome, as well as abnormal lipid levels, especially triglycerides, are said to contribute to the development of GDM. Thus, the etiology of GDM is very multilayered, and we should not overlook and underestimate any of those factors.
In summary, it is too early to draw solid conclusions due to the heterogeneity between various studies and the numerous contradictory results. There is a need for more well-designed, high-quality studies to clarify the possible implications of differences in resistin levels in GDM patients compared with healthy pregnant women with similar characteristics. Only then will it be possible to gain a better understanding of the role of resistin in the pathophysiology of GDM.
3.4.2. Preeclampsia
Data concerning the changes in serum resistin levels in preeclampsia are both limited and conflicting. Women with preeclampsia very often have to give birth significantly earlier (37.0 (33–41) weeks) than healthy controls (40.0 (38–41) weeks), and fetal birth weights are significantly lower in the preeclamptic group than in the healthy third-trimester individuals [127]. Higher resistin concentrations in women affected by PE have previously been reported by Haugen et al., however, after controlling for insulin resistance (IR), such findings lost their significance, probably due to unchanged resistin placental gene expression. Higher levels of resistin, in that case, should be attributed to altered renal function in preeclamptic patients [128].
Nanda et al. demonstrated that maternal serum resistin concentrations are increased, at 11 to 13 weeks’ gestation, in pregnancies that subsequently developed PE [121]. Seol et al. discovered that there was a marked elevation in serum resistin levels in women with preeclampsia compared to women with normal pregnancies. However, there were no significant differences in placental expression between normal pregnancies and those complicated with preeclampsia [129]. Therefore, according to those results, increased serum resistin levels in women with preeclampsia might not be assumed to be related to placental production.
In contrast to the findings of Nanda et al. and Seol et al., the circulating resistin levels were found to be lower in preeclamptic women than those noted in healthy pregnancies by Cortelazzi et al. and Chen et al. Smaller size of the placenta and subsequent reduced placental resistin production could possibly be the reason for those findings. Finally, Hendler et al. failed to find a difference in serum resistin concentrations between pregnant women with and without PE [91].
Chen et al. have also reported that resistin was detected in the trophoblast culture supernatant, which confirms the production of resistin by the human placenta. Therefore, it may be hypothesized that the decrease in resistin levels in women with PE is due to the smaller size of the placenta, but we could not exclude the possibility of lower expression of resistin by the placenta in preeclampsia, as well as the PE responsibility for an exaggeration of insulin resistance [114].
It is important to note that in the control and PE pregnancies, there were not any significant correlations between serum resistin and maternal weight, maternal BMI, maternal age, or birth weight, which suggests that the maternal serum concentration of resistin is not defined by the metabolic status of the mother [127].
In short, resistin concentrations in PE may be increased, decreased, or not significantly changed in comparison to those in healthy controls. Such significant discrepancies between these studies arise probably from the heterogeneity of studied populations, different definitions of PE, and the complexity of the disease itself. Therefore, the exact role of resistin in PE still remains unclear.
3.4.3. Other Gestational Complications
The relatively high concentrations of resistin in umbilical plasma samples might suggest a potential role of resistin in the control of fetal energy homeostasis, and intrauterine deposition of adipose tissue, as reported by Cortelazzi et al. and Briana et al. [79,130]. As a result, high circulating resistin levels may enhance hepatic gluconeogenesis, and as a consequence, reduce the incidence of neonatal hypoglycemia. Some factors that affect normal childbirth such as inordinately fat or large fetus may be avoided by negative feedback signaling from adipocytes via limiting excessive proliferation and accumulation of fat tissue in the infant. This adaptive response not only decreases the risk of fetal macrosomia but also may possibly ensure the survival of the newborn by providing adequate glucose amount for central nervous system utilization and subsequently, prevent hypoglycemia at birth with all of its negative consequences [79,130].
However, this result has been contradicted by Wang et al., who displayed a decrease in resistin concentrations both in the maternal and umbilical serum as opposed to the control group. Furthermore, umbilical resistin levels, BMI, and neonatal birth weight show a negative and statistically significant correlation between each other. This supports the notion that resistin has a direct effect on regulation of fetal development and may be related to the occurrence of fetal macrosomia [131].
Kim et al. found evidence of resistin having an inhibitory effect on adipose conversion. Accordingly, it is speculated that resistin may be a feedback regulator of adipogenesis and a signal to decrease and restrict adipose tissue formation [132]. Thus, the lower resistin level discovered by Wang et al. may lose the inhibitory effect on adipogenesis and conversely result in excess adipose tissue formation in fetus. One should keep in mind, however, that a decline of resistin may entail an increase in insulin sensitivity, which results in a much greater glucose uptake in muscle and adipose tissue. Hence, the above-mentioned mechanisms are intertwined with one another and may potentially provide a valuable insight into the relationship of low resistin levels with fetal macrosomia [131].
Umbilical cord blood concentrations of resistin might differ between IUGR fetuses and AGA controls. Briana et al. challenged this hypothesis by demonstrating no significant differences in cord blood resistin concentrations between IUGR cases and AGA controls [130]. Briana’s results suggest that resistin may not be directly involved in the regulation of insulin sensitivity and adipogenesis in the perinatal period.
Furthermore, altered regulation of adipocytokines secretion in the IUGR pregnancies and macrosomic fetuses may be predictive of the occurrence of metabolic diseases in adulthood. A deeper understanding of how early programming of adipose tissue influences postnatal metabolic pathways involved in the development of obesity will support the establishment of more effective preventive strategies and therapeutic approaches to slow down the worldwide epidemic of type 2 diabetes and obesity [130].
3.5. Irisin
Irisin is a novel secreted myokine, encoded by the fibronectin type III domain-containing protein 5 (FNDC5) precursor gene. Functional studies of irisin demonstrated that its metabolic role was to mediate exercise-related energy expenditure by turning white adipose tissue (WAT) into brown adipose tissue (BAT) in response to activation of the peroxisome proliferator-activated receptor-gamma coactivator-1 α (PGC-1α) [133]. Irisin is cleaved and secreted mostly from skeletal muscle after exercise, but low levels may also be found in the pancreas, liver, and adipose tissue [133,134,135,136]. Irisin is expressed in the female reproductive system, including the ovary, as well as in the placenta and in neonatal cord blood serum [137,138].
In healthy eumenorrheic women, serum irisin levels vary throughout the menstrual cycle, being higher in the luteal phase than in the follicular phase, with an approximately 26% increase of these levels. The rise of irisin in the luteal phase suggests that it might be involved in the ovulation cycle. Furthermore, serum irisin levels were found to rise during normal pregnancy. Additionally, irisin levels are higher in middle and late pregnancy with respect to early pregnancy in healthy women, with an increase in levels of approximately 16% and 21%, respectively [137,139].
3.5.1. Gestational Diabetes Mellitus
Cui et al. presumes that the low irisin levels may contribute to increased serum glucose, decrease in insulin sensitivity, and increasing insulin resistance after discovering significantly lower levels of irisin in the GDM patient group in comparison with the control pregnant group. Interestingly, this fact may also be supported by animal studies that have shown that exogenous administration of irisin fundamentally decreased blood glucose levels at 60, 90, and 120 min, which is possibly due to irisin’s positive effects on insulin sensitivity and improvement of insulin resistance [140]. Erol et al. also have come to this conclusion after detecting significantly lower circulating levels of irisin in the first trimester and the second trimester in the pregnant woman who subsequently developed GDM, which is suggestive of maternal irisin being possible an effective predictor of the GDM development [141].
A cross-sectional study by Kulhan et al. also showed a lower serum concentration of irisin in pregnant women with GDM when compared with that of pregnant women without GDM. However, the authors additionally discovered that the maternal serum level of irisin has a negative correlation with BMI and a positive correlation with insulin resistance, and was negatively correlated with BMI but positively correlated with insulin resistance [142].
The meta-analysis conducted by Cui et al. found that the irisin levels in the postpartum GDM group varied with the time of collection. No significant difference in circulating irisin was found within 24 h after delivery for GDM and control groups. Then, at two weeks and six weeks postpartum, irisin concentration in the GDM group was significantly lower than in the control group. Subsequently, at three months postpartum, there was no significant difference in blood irisin levels between the GDM group and the control group. However, a follow-up study found that the blood irisin levels were significantly higher for the GDM group than that for the control group, with a median postpartum time of 4 years [140]. Park et al. proposed the concept of compensatory hyperirisinemia, and noted that high levels of irisin were associated with increased risk of metabolic syndrome and cardiovascular disease [143].
Additionally, the meta-analysis by Cui et al. investigates the irisin levels not only in the maternal blood but also in the cord blood and breast milk. Unsurprisingly, the breast milk of the GDM patients had lower concentrations of irisin that of the control group, regardless of colostrum or mature milk [140]. Additionally, Fatima et al. speculated that unfavorable effects on health and lipid regulation may stem from continuous feeding of breast milk with fewer irisin [144]. The remarkable connection between low irisin levels in breast milk and infant weight require further investigation.
3.5.2. Preeclampsia
Garcés et al. showed that irisin levels were lower in pregnant women with preeclampsia compared with normal pregnant women from the early stages of pregnancy. The difference was significant in the third trimester, close to 49% in the levels of this adipomyokine noted in healthy individuals. It is possible that this decrease in serum levels of irisin could be explained in part by the reduction in the contribution of placental and other irisin-secretory tissues in the group of preeclamptic patients. Although the results failed to be significant in this study, the low levels during the first and second trimesters might be a valuable predictor of the development of the disease [137]. In another study, Foda et al. reported that serum irisin levels in normal pregnancies, early in labor, were significantly higher than in mild preeclampsia, which is in agreement with the results of Garcés et al. [137,145].
Interestingly the increased endothelial permeability, in preeclampsia, may be associated with decreased endothelial nitric oxide synthase expression and activity in endothelial cells resulting in vasoconstriction [146]. According to Xiang et al., circulating irisin levels were positively associated with endothelium-dependent vasodilatation in newly diagnosed type 2 diabetic patients without clinical angiopathy, which creates a possible correlation between lowered levels of resistin in the first or second trimester and subsequent development of PE [147].
Foda et al. also compared serum irisin levels in patients with mild PE undergoing vaginal delivery and cesarean section. In patients who experienced vaginal delivery, irisin levels were significantly higher both early in labor and after fetal delivery in comparison to those who underwent cesarean section [145]. Vaginal delivery, like muscular exercise, is associated with a massive release of irisin during the labor from the placenta, or from the uterine and pelvic muscles. These findings are comparable to the previous reports done by Lee et al. and Kurdiova et al. that mention a three-fold increase in serum irisin levels measured immediately following one hour of moderate exercise [135,148]. It could be concluded that labor is a strong stimulus to the release of irisin into the fetal circulation.
In the absence of more evidence, it is only possible to speculate on the mechanism of decreased maternal irisin levels in preeclamptic pregnancies. As to whether alterations in the expression and secretion of irisin-secretory tissues that are compromised in preeclamptic women remains to be elucidated.
3.5.3. Other Gestational Complications
Çaǧlar et al. compared irisin levels in maternal and umbilical cord blood in normal pregnancies and those complicated by FGR. Maternal and umbilical vein irisin levels did not differ significantly between controls and study groups, but umbilical artery irisin levels were significantly lower in the study group. Partly, this finding may be explained by the fact that infants affected by FGR have different body composition with reductions in the levels of body fat and lean mass. One can speculate that it raises the suspicion of low levels of irisin in idiopathic FGR contributing to the pathogenesis of this condition [149]. Furthermore, fetuses with FGR are at higher risk of developing obesity, hypertension, hypercholesterolemia, cardiovascular disorders, glucose intolerance, and type 2 DM at later ages, compared to the general population [150].
Pavlova et al., using a univariate model, discovered a positive association between cord blood irisin concentration and preterm birth occurrence in the studied cohort. A higher irisin level in preterm infants compared with term deliveries was discovered. It could be described by the fact that the onset of labor provides a strong stimulus for the release of irisin into maternal and fetal circulations [145,151], and could increase cord blood irisin level by nearly 40% [152]. In addition, it has been suggested that increased irisin release into cord blood may be caused by temporary uteroplacental ischemia during vaginal delivery, thus leading to fetal stress [145].
Moreover, maternal and neonatal irisin precursor gene FNDC5 polymorphism is associated with preterm birth. Women and neonates bearing the FNDC5 rs726344 GG genotype had a 2.18-fold and 2.24-fold higher chance, for term delivery respectively, compared to both AG and AA genotypes [139].
3.6. Omentin
Omentin was initially described in intestinal Paneth cells and has been implicated in the gut defensive mechanisms against pathogenic bacteria [153]. There are two homologs, omentin-1 that is the major circulating form, and omentin-2. This adipokine is preferentially produced and secreted by visceral adipose tissue (VAT) and is predominantly expressed in VAT stromal vascular cells [153]. Yang et al. noted that in vitro experiments revealed that omentin enhances insulin-stimulated glucose uptake in human adipocytes and presents insulin-sensitizing properties [154]. Omentin is also expressed in the heart, lungs, ovary, and placenta. Omentin-1 was shown to be downregulated by insulin and glucose, resulting in decreased levels in overweight women suffering from polycystic ovary syndrome [155]. Moreover, decreased omentin-1 levels were found in patients suffering from obesity and diabetes [156]. Briana et al. also reported that omentin is detectable in the serum of infants and it is mainly derived from fetal and/or maternal tissues. Thus, a positive correlation between maternal and fetal omentin-1 concentrations was found, implying a transplacental transport of this adipocytokine. In addition, the placenta most probably does not contribute to circulating concentrations of omentin-1, as its concentrations do not decline after placental elimination. It was also demonstrated that there are relatively high concentrations of omentin-1 in umbilical serum samples. Therefore, omentin-1 is hypothesized to play a similar role in energy homeostasis [157]. It is worth keeping in mind that glucose acts as a main source of energy during prenatal development and growth. Insulin in turn is well-known for increasing the uptake of circulating glucose by fetal muscle and adipose tissue. Therefore, despite the fact that there is no available information about a potential role of omentin-1 in fetal growth, enhanced growth-promoting effect through its insulin-sensitizing action may be attributed to high levels of omentin-1 in the fetus.
3.6.1. Gestational Diabetes Mellitus
Overall, a substantial number of studies on the association between concentrations of omentin-1 and DM found contradictory results. For example, in one cohort, similar omentin-1 concentrations between patients suffering from GDM and controls have been observed [158], whereas another cohort showed decreased concentrations in GDM [159].
According to Barker et al. and Franz et al., their prospective case-control studies showed lower omentin-1 levels in obese women, but not in women with GDM [158,160]. Furthermore, they showed a decrease in omentin-1 levels throughout pregnancy, which at first may seem counterintuitive due to the fact that the placenta has been shown to additionally produce omentin-1. Such results may be explained by an increased clearance in the later stage of pregnancy or physiological hemodilution during gestation. However, this needs further evaluation. What is more, De Souza Batista et al. demonstrated that omentin-1 is downregulated by insulin and glucose [161]. It may be assumed that increased insulin resistance during pregnancy could explain decreased omentin-1 levels in the third trimester in both women with GDM and the normoglycemic ones.
Increased risks of obesity, metabolic syndrome, and type 2 diabetes with substantially lower omentin-1 levels and higher serum C-peptide levels in later life have been seen in the offspring of mothers who suffered from GDM [162,163,164]. This could reflect an already altered metabolic state possibly associated with a greater risk of adverse metabolic sequelae in childhood and adult life. Catli et al. supported this statement in their study while demonstrating that omentin-1 is significantly lower in obese children compared to normal-weight ones [165].
Significantly lower omentin-1 concentrations were observed in people with GDM or T2DM than in the controls by Pan et al., whereas no difference was found between the T1DM and controls [166]. Decreased omentin-1 concentrations may be an important indicator of gestational diabetes mellitus and type 2 diabetes mellitus.
The results of the meta-analysis carried out by Sun et al. found that circulating omentin was lower in GDM patients than in controls. This supports the idea of omentin’s chance of being a novel biomarker for the early diagnosis of GDM, which affects many pregnant women [167]. The decreased omentin levels in mothers with GDM, compared with healthy controls, may result from impaired synthesis or release, but the mechanism for this requires further investigation.
3.6.2. Other Gestational Complications
To date, few studies have examined omentin-1 during pregnancy. GDM and omentin levels correlation has been getting the most attention in the last 5 years. Therefore, further investigation is needed to assess the input of omentin into the pathophysiology of other gestational complications.
Šplíchal et al. in their pioneer study concluded that circulating maternal omentin-1 levels were higher in normal spontaneous term births than in preterm births and almost identical in an additional subgroup comparison conducted between preterm births with and without preterm premature rupture of membranes (PPROM) [168]. These results suggest that omentin-1 may play a role in the pathophysiology of preterm birth, but it is not involved in the mechanism of PPROM. Further studies are needed to evaluate the role of omentin-1 in preterm births.
3.7. Chemerin
Chemerin is a hormonally active protein that is suspected to be associated with a range of metabolic, inflammatory, and cardiovascular diseases [169,170]. Chemerin is expressed in various human tissues, and also in the placenta. However, its expression is mainly pronounced in the liver and subcutaneous and visceral adipose tissue. [171,172,173]. Its concentration in peripheral blood correlates well with BMI and obesity [174]. Pregnancy is a state of increased chemerin secretion, which rises throughout the gestation [171,175,176]. Nonetheless, its involvement in the regulation of physiological pregnancy is not fully elucidated yet. However, it is thought that chemerin may play a significant role in the pathophysiology of various gestational complications.
3.7.1. Gestational Diabetes Mellitus
Chemerin is known as a novel adipokine thought to have a substantial impact on glucose and lipid metabolism. That effect occurs especially during the pregnancy when the significant increase in chemerin levels is highly pronounced [171,176]. Surprisingly, there was not any correlation between chemerin levels and fasting glucose, C-peptide, and OGTT test results [177,178]. The analyses of relationships between chemerin, fasting insulin, and HOMA-IR (homeostatic model assessment of insulin resistance) values lead to contradictory results. Whereas some authors did not observe any significant correlations between those parameters [177], the others found that chemerin values were positively markedly correlated with HOMA-IR or insulin levels [172,179,180,181]. Chemerin values were also found to be positively correlated with fatty acid-binding protein 4, interleukin-6, and tumor necrosis factor-α [182].
To analyze its utility in the prediction of GDM, it is crucial to evaluate its blood concentrations in patients with GDM and healthy individuals. Some authors found that chemerin concentrations in peripheral blood are significantly increased throughout the pregnancy in patients with GDM [172,177,179,182,183,184,185]. Whereas some studies reported that chemerin levels are also significantly elevated in the cord blood samples [172,186], the others did not find any differences [187]. Besides the adipose tissue, the expression of chemerin mRNA was also detected in the placental tissue [172,179]. There is a 6- to 24-fold increase in its expression in visceral and subcutaneous adipose tissue, compared with the levels detected in placental tissue samples. It was discovered that the relative chemerin mRNA and protein expression was markedly increased in both placental and adipose tissue samples in diabetic patients [172]. However, Tsiotra et al. did not find any differences in the placental expression of chemerin mRNA. They found significant differences in the expression of chemerin mRNA, in visceral adipose tissue, between the groups of obese-GDM patients and non-obese euglycemic controls. Surprisingly, Li et al. reported that the expression of chemerin mRNA was significantly downregulated in women with GDM, especially in obesity [185]. Other studies did not find any differences in chemerin levels determined in peripheral blood in patients with GDM and healthy controls [176,180,186,187,188,189,190]. Interestingly, Hare et al. noted that the mean third-trimester serum chemerin values, in the healthy control group, were significantly higher than those observed in patients with GDM [191]. Finally, the results of the most recent meta-analysis (20 studies) suggest that there is no difference in circulating chemerin levels in patients with GDM compared with normal pregnancies [167]. The graphical summary of relationships between chemerin concentrations and chemerin mRNA expression according to the diabetic status is presented in Figure 2. A long-term observational study found no differences in chemerin levels 3-years postpartum in women with GDM and controls [192].
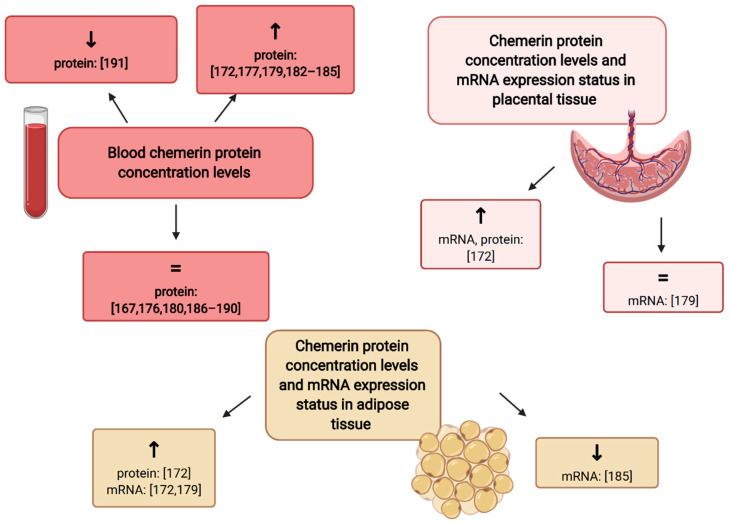
Figure 2.
The graphical summary of data concerning chemerin concentration and chemerin mRNA expression levels in GDM patients as compared to control patients. ↑ increase in parameter level as compared to control [172,177,179,182,183,184,185]; ↓ decrease in parameter level as compared to control [191]; =: no significant alterations observed as compared to control [167,176,180,186,187,188,189,190] (Created with BioRender.com).
Nonetheless, it is noteworthy that first and second trimester multiple regression models revealed that chemerin values are positively associated with the elevated risk of GDM, and along with other parameters, chemerin may be treated as an independent risk factor of GDM [177,182,184,193]. The implementation of the results of chemerin levels measurement improves the predictive values of models used in the prediction of GDM [177,182]. Finally, it is unlikely that its concentration measurement could be used as a single early marker in the prediction of GDM. Performing the ROC (receiver-operator curve) analysis, it was estimated that its capacity to predict GDM was poor or moderate, its accuracy (AUC values) ranged from 0.59 to 0.82 [182,188]. Fatima et al.’s estimated value of chemerin AUC (0.97), with the sensitivity of 96% and specificity of 72%, in the diagnosis of GDM, seems bias compared with other studies [184].
It has been found that the frequency of the genotypes of chemerin rs4721 gene polymorphism was significantly differentiated among patients with GDM in comparison to healthy controls. Both the dominant and co-dominant version of the rs4721 genotype was associated with the increased incidence of GDM [194]. Moreover, some studies have considered the possible clinical applications of chemerin measurements in the treatment of patients with GDM. It was speculated that adipocytokines concentrations assessment could be used in the prediction of a need of drugs administration, for glycemic control, in the patients who used the nutritional therapy. Nevertheless, there was not any significant connection between poor glycemic control results in those patients and the chemerin levels [195].
Chemerin concentrations could also be measured in different body fluids. Okten et al. performed an analysis of adipocytokines concentrations in saliva. Chemerin levels in women with GDM were found to be markedly elevated in comparison to healthy controls [178]. Furthermore, chemerin concentrations in maternal milk were increased compared with values determined in peripheral blood. Mothers with GDM had significantly higher levels in their milk compared with controls [196].
In summary, the potential role of chemerin levels assessments, in gestational diabetes mellitus, was mainly restricted to the prediction of GDM development. Even though some studies suggested its possible usefulness in that indication, its predictive value is insufficient to be applied in standard clinical practice.
3.7.2. Preeclampsia
Even though the pathogenesis of PE is still not fully elucidated, the disturbances in chemerin secretion are suspected to be associated with its increased incidence. Xu et al. reported that first trimester chemerin serum values were significantly elevated in patients who developed preeclampsia after 20 weeks of pregnancy. They proposed chemerin as an independent risk factor of preeclampsia, with the estimated sensitivity of 87.8% and specificity of 75.7%, in the prediction of preeclampsia (threshold value > 183.5 ng/mL, estimated AUC—0.85) [197]. Due to that fact, chemerin may be regarded as a potential early marker of preeclampsia. The patients with preeclampsia had also markedly increased second and third trimester chemerin blood levels compared with normotensive individuals [181,198,199,200]. Furthermore, it was noted that the chemerin concentrations correlated with the severity of the pathology. Patients with severe preeclampsia had significantly higher chemerin blood levels in comparison to those with mild preeclampsia or healthy control group [181,197,199]. Both chemerin mRNA and protein expression were substantially increased in placental tissue samples from patients with preeclampsia [200]. The ROC analysis revealed that chemerin levels assessment has high sensitivity—95.5% and specificity—95.7% in the diagnosis of preeclampsia (threshold > 252 ng/mL, AUC—0.98) [181]. The chemerin levels were found to be significantly correlated with maternal blood pressure values, renal function parameters (proteinuria, creatinine), hepatic parameters (ALT, AST), and other adipokines (leptin and adiponectin) [181,198,199,200]. Maternal obesity additionally promotes increased chemerin production in the preeclamptic pregnancies [201]. Furthermore, the neonatal parameters (birth weight and Apgar score) were found to be related to maternal chemerin levels [181]. Long-term observations discovered that chemerin levels remain significantly elevated, for at least 6 months, after the pregnancy in patients diagnosed with preeclampsia [198].
Those results suggest that chemerin may play a role in the development of preeclampsia. Nonetheless, the possible molecular mechanism of its activity is still unknown.
3.7.3. Other Gestational Complications
Chemerin levels are independently associated with fetal growth and neonatal birth weight [184,202]. Furthermore, it was found that chemerin concentrations in both mixed arteriovenous and arterial cord blood samples were markedly elevated in LGA newborns in comparison to those with normal birth weight [202,203]. In contrast, Van Poppel et al. did not observe any correlation between birth weight and chemerin concentrations in neither amniotic fluid nor cord blood [186]. Interestingly, the analysis of chemerin concentrations in discordant twins revealed that neonates with SGA (small for gestational age) had significantly lower chemerin cord levels than their siblings [202]. The follow-up study performed on the group of SGA newborns demonstrated that newborns with slow catch-up growth had significantly higher chemerin levels, measured at 3 months of age, compared with those with normal catch-up growth [204].
To analyze the regulatory activity of chemerin in the maintenance of early pregnancy, Yang et al. assessed the expression of chemerin and its receptors in the decidua tissues from women with spontaneous and planned selective abortions. They discovered the significantly increased chemerin expression and downregulated expression of its receptors (chemokine-like receptor-1 (CMKLR1)) in patients with spontaneous abortions [205]. The intrauterine injection of chemerin receptors’ antagonist resulted in a higher rate of embryo resorption in mice and lower ERK1/2 phosphorylation rate in uterine tissue [205]. Interestingly, they also described the association between the pre-pregnancy serum chemerin levels and the risk of spontaneous abortion in women with polycystic ovary syndrome (PCOS). According to their results, diminished chemerin levels, in patients with PCOS, may be treated as an independent risk factor of spontaneous abortion [206].
Finally, it was found that prenatal exposure to tobacco may alter the chemerin gene expression in neonates. Reynolds et al. observed a significant increase in chemerin mRNA expression and the reduction in chemerin DNA methylation in the group of newborns born to smoking mothers [207].
3.8. Apelin
Apelin is a peptide hormone widely expressed in various human tissues. Apelin and its receptors (APJ) are thought to be involved in the regulation of numerous physiological processes [208], making the Apelin/APJ axis a promising target for implementing novel therapeutic strategies for a range of metabolic and cardiovascular diseases [209,210]. It was reported that the state of physiological pregnancy is associated with a significant reduction in apelin secretion [211]. Both the possible applications of apelin measurements and its potential as a new therapeutic strategy during pregnancy will be considered in this section.
3.8.1. Gestational Diabetes Mellitus
It has been reported that apelin levels, measured in the peripheral blood samples, were significantly elevated in the group of GDM patients and rise throughout their pregnancy [212,213]. However, some authors did not find any differences in apelin levels measured in the maternal plasma or umbilical cord blood [213,214,215]. The expression of apelin mRNA in subcutaneous adipose tissue, visceral adipose tissue, and placental tissue samples was not altered in women with GDM as compared to healthy individuals [214]. The placental apelin mRNA expression and apelin protein abundance were significantly higher (10-fold to 20-fold) than those determined in adipose tissue [214,216]. Furthermore, its expression markedly correlated with the expression of its receptors [214]. On the contrary, the others noted that apelin levels in the peripheral or cord blood samples were lower in patients with GDM [215,217,218]. Also, the apelin concentrations in human breast milk were significantly lower among diabetic patients [218]. However, the results of a reliable meta-analysis suggest that there are no differences in apelin levels in GDM patients and healthy individuals [167]. The summary of data concerning apelin concentration and apelin mRNA expression levels in GDM patients as compared to control patients is shown in Table 1. The ROC analysis revealed that apelin has a lower negative predictive value as a screening tool to exclude GDM as compare with leptin and adiponectin [217]. Most studies did not find any correlations between apelin and BMI and other metabolic parameters such as fasting glucose, insulin, glycated hemoglobin, HOMA-IR, and lipids [213,214,215,216,217]. However, Guo et al. reported that apelin values, in GDM patients, were negatively correlated with total cholesterol levels [212].
Table 1.
The summary of data concerning apelin concentration and apelin mRNA expression levels in GDM patients as compared to control patients.
| Characteristics of the Study Group | Measured Data Type | Parameter Level as Compared to Control | References |
|---|---|---|---|
| Analysis of 20 studies concerning apelin serum concentration levels in 1493 GDM and 1488 control patients | protein concentration | not altered | [167] |
| Apelin peripheral blood concentration levels in 79 GDM and 80 control patients in the second trimester, as well as in 87 GDM and 88 control patients in the third trimester | protein concentration | increased | [212] |
| Apelin maternal serum concentration levels in 30 GDM and 30 control patients |
protein concentration | increased | [213] |
| Apelin cord blood concentration levels in 30 GDM and 30 control patients |
protein concentration | not altered | [213] |
| Apelin plasma concentration levels in 101 GDM and 101 control patients between 24 and 32 weeks of gestation, as well as in 20 GDM and 16 control patients at term | protein concentration | not altered | [214] |
| Apelin mRNA expression levels in SAT, VAT, and placental tissue in 20 GDM and 16 control patients at term | mRNA expression | not altered | [214] |
| Apelin maternal blood concentration levels in 24 GDM and 21 control patients | protein concentration | not altered | [215] |
| Apelin cord blood concentration levels in 24 GDM and 21 control patients | protein concentration | decreased | [215] |
| Apelin serum concentration levels in 127 GDM and 109 control patients (during pregnancy), as well as 30 GDM and 20 control patients (post-partum) | protein concentration | decreased | [217] |
| Apelin serum concentration levels in 10 GDM and 10 control patients | protein concentration | decreased | [218] |
| Apelin breast milk concentration levels in 10 GDM and 10 control patients | protein concentration | decreased | [218] |
Abbreviations: GDM—gestational diabetes mellitus; SAT—subcutaneous adipose tissue; VAT—visceral adipose tissue.
The follow-up observational study found that apelin levels were significantly lower among women who experienced GDM in previous pregnancy in comparison to healthy controls. Interestingly, they noted that apelin levels were inversely associated with fasting glucose, OGTT results, and carotid intima-media thickness (IMT) [219]. IMT is a parameter applied in the prediction of the risk of cardiovascular events [220]. Those outcomes support the hypothesis that the reduction in apelin secretion in women with the history of GDM may play a role in their higher susceptibility to the development of T2D and its cardiovascular consequences [221].
In summary, the results of the studies focused on apelin measurements are inconsistent. Nonetheless, based on the currently available clinical date, it is unlikely that apelin could be applied as a marker of glucose intolerance and insulin resistance. It should be highlighted that the highly pronounced local placental apelin expression illustrates its potential para- and autocrine fetoplacental activity during gestation which requires further investigations.
3.8.2. Preeclampsia
It has been established that serum apelin levels were significantly decreased among patients with preeclampsia [222,223,224,225]. Moreover, alternations in apelin levels were also identified between the groups of patients diagnosed with mild and severe preeclampsia [222,226]. Similar differences were also observed in the samples of umbilical cord blood [226]. The placental expression of apelin and its receptor gene mRNA, as well as their protein products, were decreased in samples of placental tissue in preeclamptic women [227,228]. On the contrary, some authors report significantly higher serum apelin levels among preeclamptic women or no difference in apelin levels in patients with mild and severe preeclampsia [227,229,230]. However, there is a strong negative correlation between blood pressure values and apelin levels [222], and a reverse correlation with BMI [224]. The ROC analyses revealed that apelin has poor reliability as a diagnostic tool in the exclusion of preeclampsia—sensitivity (61–64%) and specificity (61–73%, AUC (0.63–0.67)) [224].
The immunostaining of placental tissue samples illustrated that the expression of apelin and its receptors (known as APJ receptors) in term placenta was highly pronounced especially in the cytoplasm of syncytiotrophoblast cells of the chorionic villi, while its presence during the early gestation was mainly detected at the cellular membranes of syncytiotrophoblasts and extravillous trophoblast cells, which may suggest their active involvement in the process of early placentation [227]. It was discovered that apelin present in the chorionic villi exists in various molecular isoforms. However, the pyroglutamate apelin-13 ((Pyr1)-apelin-13) is the most predominant form [228]. The potential molecular mechanism of apelin activity and its contribution to the development of preeclampsia may be elucidated via the effects caused by the stimulation of its cellular receptors. It was proposed that deficiency in the placental elabela (ELA) secretion (a protein, ligand of the APJ) may be liable for the abnormal early placentation and as a consequence could lead to the development of PE in the murine model [231]. It was established that ELA has a capacity to increase the trophoblast invasiveness in vitro [232]. The pathogenesis of preeclampsia is also believed to be connected with the vascular endothelial dysfunction [233]. The study performed on the population of human umbilical vein endothelial cells revealed that the treatment with ELA solutions improved their viability, migration, and tube formation abilities, which may be beneficial for the patients with features of PE. Those effects were said to be invoked by the upregulation of the APJ expression and the activation of PI3K/Akt pathways [234]. Interestingly it was noted that elabela blood levels, mRNA, and protein expressions in placental tissue are also decreased in patients with late-onset PE [235]. In contrast, Para et al. found ELA levels to be significantly increased in women with late-onset PE in comparison to healthy individuals [236]. Nonetheless, it is noteworthy that the other observations performed in humans did not reveal any differences in circulating ELA levels and its placental mRNA expression in preeclamptic pregnant women and healthy individuals [237]. Finally, the potential role of ELA-APJ axis in the pathophysiology of human disease remains unclear.
Based on those promising early findings, Yamaleyeva et al. assessed the efficacy and safety of (Pyr1)-apelin-13 administration in transgenic rats with preeclamptic features. Rats treated with apelin derivatives have significantly lower blood pressure, normalized heart rate variability, and increased ejection fraction. Moreover, apelin administration resulted in decreased proteinuria and improved the renal histopathological findings (lower renal cortical collagen deposition and expression of oxidative stress markers), as compared with the control group without any substantial adverse effects [238]. Furthermore, Wang et al. reported that apelin administration in preeclamptic rats reduced the mean maternal blood pressure and the levels of oxidative stress indicators. Notably, they also observed a significant increase in mean fetal weight, embryo survival rate, and the expression of endothelial nitric oxide synthase [239].
Therefore, those experimental studies made the first step in the development of a novel therapeutic strategy for women with preeclampsia. Nonetheless, the clinical translation of those animal outcomes requires further validations.
3.8.3. Other Gestational Complications
Apelin levels measured in neonates were positively correlated with their birth weight, HOMA-IR, and fasting insulin levels [240]. Furthermore, LGA neonates had markedly increased circulating apelin levels. Interestingly, LGA newborns of diabetic mothers have significantly elevated apelin concentrations compared with LGA infants of mothers with normal glucose tolerance [240]. However, there was not any significant correlation between apelin concentrations in maternal circulation and neonatal birth weight [215,216,241], whereas the others found an inverse correlation between those parameters [213]. Vaughan et al. noted that obese mothers delivered newborns with significantly increased birth weight in comparison to those with normal BMI. However, they did not find any relationship between maternal obesity and apelin levels [216]. Furthermore, it was reported that there was not any association between late-pregnancy maternal apelin levels and the incidence of adverse perinatal outcomes (SGA, FGR, preterm deliveries, and admission to neonatal intensive care units) in either preeclamptic patients or healthy pregnant women [224]. In contrast, Van Mieghem et al. reported that they found significantly lower apelin levels in pregnancies complicated with fetal growth restriction [242]. However, the study groups were relatively small. Finally, there is not enough available data to analyze the potential contribution of differences in apelin levels to the development of the fetal growth abnormalities.
Ratana et al. reported that they observed significantly lower apelin mRNA and protein expression in fetal membranes in patients after the spontaneous labor in comparison to tissues collected after the cesarean section. Moreover, they found the transfection of apelin siRNA into the primary amnion cells promoted the interleukin (IL)-1β-induced release of pro-inflammatory cytokines (IL-6, IL-8) and pro-labor mediators (prostaglandin E2, prostaglandin F2α) [243]. Their findings could suggest the possible involvement of downregulation in apelin expression in the induction of the spontaneous rupture of membranes and vaginal delivery. Furthermore, it was discovered that apelin has the capacity to inhibit spontaneous and oxytocin-induced uterine contractility [244]. The authors speculated that generally higher apelin concentrations in obese individuals might be responsible for the reduction of uterine contractility in vivo, which put them at increased risk multiple labor complications [244,245]. It was proposed that prolonged fasting could lead to elevation of circulating apelin levels during gestation [246].
4. Future Perspectives
It is unlikely that biomarkers such as leptin, resistin, irisin, apelin, chemerin, and omentin will be implemented as novel, reliable, independent early markers of various gestational pathologies because their independent predictive values are generally insufficient. The majority of pregnancy-related complications, including GDM, pregnancy-induced hypertension, or preeclampsia, always occur in the middle or late pregnancy. Thus, there is a possibility that the analyses of adipokines’ concentrations performed, at the early stage of gestation, together with the assessments of standard maternal risk factors of GDM, PE, etc., could improve the early prediction and prevention of those pregnancy complications. Their combined predictive values should be investigated in further studies. Nonetheless, there is an uncertainty whether the alternations in these serum markers happen before or after the clinical manifestation of those diseases. Importantly, it should be highlighted that such factors as advanced maternal age, obesity, family history of diabetes mellitus, history of a poor obstetric outcome, as well as abnormal lipid levels, especially triglycerides, all together are said to contribute to the development of GDM. Thus, the etiology of GDM and other gestational pathologies is multilayered, and we should not overlook and underestimate any of them.
Whereas the recent human studies focused mainly on the analyses of adipokines’ concentrations in peripheral blood and local expression of their encoded genes in both placental and adipose tissue, the animal studies shed new light on the elucidation of their biological properties analyzing the expression of their receptors and intracellular consequences of its stimulation (for example, the Apelin/APJ axis in relation to PE in mice). Further studies in that matter could establish novel targets for innovative therapeutic strategies in various gestational complications in humans.
5. Conclusions
Numerous experimental studies established significant alterations in adipokine secretion patterns throughout the normal and high-risk pregnancies. Nonetheless, most studies have conflicting results potentially due to differences in inclusion criteria (age, BMI, or comorbidity, etc.). The alterations in the secretion patterns of the analyzed adipokines illustrate their potential role in the development of physiological adaptations to pregnancy and delivery. Nevertheless, the underlying mechanisms of adipokine involvement in the pathophysiology of pregnancy-related diseases remain unclear. Maternal obesity often independently affects the expression of multiple adipokines in the adipose and placental tissue. Therefore, the relationships between the analyzed adipocytokines and pregnancy-related complications should be interpreted separately referring to maternal pre-pregnancy BMI and gestational weight gain. Several adipokines were proposed as potential novel early markers of heterogeneous gestational complications. Nonetheless, their prognostic value is mostly insufficient to be implemented into standard clinical practice. Routine assessments of adipokine levels during pregnancy is currently not recommended in the management of both normal and complicated pregnancies. Based on the animal models, the future application of adipokines and their placental receptors as new therapeutic targets for pharmacological interventions seems promising but requires further validations in humans.
Author Contributions
Conceptualization, P.G., R.S., M.J., and E.W.-O.; methodology, P.G. and R.S.; software, M.J.; writing—original draft preparation, P.G., R.S., M.J., and K.A.; writing—review and editing, P.M., B.K., and E.W.-O.; visualization, R.S., M.J., and R.B.; supervision, B.K. and E.W.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marques A., Peralta M., Naia A., Loureiro N., De Matos M.G. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur. J. Public Health. 2018;28:295–300. doi: 10.1093/eurpub/ckx143. [DOI] [PubMed] [Google Scholar]
- 2.Roberts J.M., Bell M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 2013;99 doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts J.M., Bodnar L.M., Patrick T.E., Powers R.W. The role of obesity in preeclampsia. Pregnancy Hypertens. 2011;1 doi: 10.1016/j.preghy.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano P.M. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J. Dev. Orig. Health Dis. 2010;1:208–215. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslam D.W., James W.P.T. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro R., Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010;2010 doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y., Sun Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revelo X.S., Luck H., Winer S., Winer D.A. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocr. Pathol. 2014;25:93–101. doi: 10.1007/s12022-013-9288-1. [DOI] [PubMed] [Google Scholar]
- 9.Cao H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014;220:T47–T59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvani M., Scarfone A., Granato L., Mora E.V., Nanni G., Castagneto M., Greco A.V., Manco M., Mingrone G. Restoration of Adiponectin Pulsatility in Severely Obese Subjects after Weight Loss. Diabetes. 2004;53:939–947. doi: 10.2337/diabetes.53.4.939. [DOI] [PubMed] [Google Scholar]
- 11.Reinehr T., Roth C., Menke T., Andler W. Adiponectin before and after weight loss in obese children. J. Clin. Endocrinol. Metab. 2004;89:3790–3794. doi: 10.1210/jc.2003-031925. [DOI] [PubMed] [Google Scholar]
- 12.Makki K., Froguel P., Wolowczuk I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013;2013 doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karastergiou K., Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol. Cell. Endocrinol. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 15.El Husseny M.W.A., Mamdouh M., Shaban S., Ibrahim Abushouk A., Zaki M.M.M., Ahmed O.M., Abdel-Daim M.M. Adipokines: Potential Therapeutic Targets for Vascular Dysfunction in Type II Diabetes Mellitus and Obesity. J. Diabetes Res. 2017;2017 doi: 10.1155/2017/8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancuso P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh N., Naruse M., Usui T., Tagami T., Suganami T., Yamada K., Kuzuya H., Shimatsu A., Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 18.Frühbeck G., Catalán V., Rodríguez A., Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supriya R., Tam B.T., Yu A.P., Lee P.H., Lai C.W., Cheng K.K., Yau S.Y., Chan L.W., Yung B.Y., Sheridan S., et al. Adipokines demonstrate the interacting influence of central obesity with other cardiometabolic risk factors of metabolic syndrome in Hong Kong Chinese adults. PLoS ONE. 2018;13:e0201585. doi: 10.1371/journal.pone.0201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelesidis T., Kelesidis I., Chou S., Mantzoros C.S. Narrative Review: The Role of Leptin in Human Physiology: Emerging Clinical Applications. Ann. Intern. Med. 2010;152:93. doi: 10.7326/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao W.Q., He J.R., Shen S.Y., Lu J.H., Kuang Y.S., Wei X.L., Qiu X. Maternal circulating leptin profile during pregnancy and gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2020;161:108041. doi: 10.1016/j.diabres.2020.108041. [DOI] [PubMed] [Google Scholar]
- 23.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 24.Houseknecht K.L., Portocarrero C.P. Leptin and its receptors: Regulators of whole-body energy homeostasis. Domest. Anim. Endocrinol. 1998;15:457–475. doi: 10.1016/S0739-7240(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 25.Iciek R., Wender-Ozegowska E., Zawiejska A., Mikolajczak P., Mrozikiewicz P.M., Pietryga M., Brazert J. Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J. Physiol. Pharmacol. 2013;64:579–585. [PubMed] [Google Scholar]
- 26.Lepercq J., Challier J.C., Guerre-Millo M., Cauzac M., Vidal H., Hauguel-De Mouzon S. Prenatal leptin production: Evidence that fetal adipose tissue produces leptin. J. Clin. Endocrinol. Metab. 2001;86:2409–2413. doi: 10.1210/jcem.86.6.7529. [DOI] [PubMed] [Google Scholar]
- 27.Hoggard N., Haggarty P., Thomas L., Lea R. Leptin expression in placental and fetal tissues: Does leptin have a functional role? Biochem. Soc. Trans. 2001;29:57. doi: 10.1042/bst0290057. [DOI] [PubMed] [Google Scholar]
- 28.González R.R., Simón C., Caballero-Campo P., Norman R., Chardonnens D., Devoto L., Bischof P. Leptin and reproduction. Hum. Reprod. Update. 2000;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- 29.Jin L., Zhang S., Burguera B.G., Couce M.E., Osamura R.Y., Kulig E., Lloyd R.V. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson C., Lindell K., Svensson E., Bergh C., Lind P., Billig H., Carlsson L.M.S., Carlsson B. Expression of Functional Leptin Receptors in the Human Ovary. J. Clin. Endocrinol. Metab. 1997;82:4144–4148. doi: 10.1210/jc.82.12.4144. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura K., Sato N., Fukuda J., Kodama H., Kumagai J., Tanikawa H., Nakamura A., Tanaka T. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology. 2002;143:1922–1931. doi: 10.1210/endo.143.5.8818. [DOI] [PubMed] [Google Scholar]
- 32.Cervero A., Horcajadas J.A., Domínguez F., Pellicer A., Simón C. Leptin system in embryo development and implantation: A protein in search of a function. Reprod. Biomed. Online. 2005;10:217–223. doi: 10.1016/S1472-6483(10)60943-1. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Pérez A., Maymó J.L., Gambino Y.P., Guadix P., Dueñas J.L., Varone C.L., Sánchez-Margalet V. Activated translation signaling in placenta from pregnant women with gestational diabetes mellitus: Possible role of leptin. Horm. Metab. Res. 2013;45:436–442. doi: 10.1055/s-0032-1333276. [DOI] [PubMed] [Google Scholar]
- 34.Lepercq J., Cauzac M., Lahlou N., Timsit J., Girard J., Auwerx J., Mouzon S.H. De Overexpression of placental leptin in diabetic pregnancy: A critical role for insulin. Diabetes. 1998;47:847–850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- 35.Uzelac P.S., Li X., Lin J., Neese L.D., Lin L., Nakajima S.T., Bohler H., Lei Z. Dysregulation of Leptin and Testosterone Production and Their Receptor Expression in the Human Placenta with Gestational Diabetes Mellitus. Placenta. 2010;31:581–588. doi: 10.1016/j.placenta.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Côté S., Gagné-Ouellet V., Guay S.P., Allard C., Houde A.A., Perron P., Baillargeon J.P., Gaudet D., Guérin R., Brisson D., et al. PPARGC1α gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin. Epigenetics. 2016;8:72. doi: 10.1186/s13148-016-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miehle K., Stepan H., Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin. Endocrinol. Oxf. 2012;76:2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Pérez A., Maymó J., Gambino Y., Guadix P., Dueñas J.L., Varone C., Sánchez-Margalet V. Insulin enhances leptin expression in human trophoblastic cells. Biol. Reprod. 2013;89:20. doi: 10.1095/biolreprod.113.109348. [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Pérez A., Guadix P., Maymó J., Dueñas J.L., Varone C., Fernández-Sánchez M., Sánchez-Margalet V. Insulin and Leptin Signaling in Placenta from Gestational Diabetic Subjects. Horm. Metab. Res. 2015;48:62–69. doi: 10.1055/s-0035-1559722. [DOI] [PubMed] [Google Scholar]
- 40.Kautzky-Willer A., Pacini G., Tura A., Bieglmayer C., Schneider B., Ludvik B., Prager R., Waldhäusl W. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44:164–172. doi: 10.1007/s001250051595. [DOI] [PubMed] [Google Scholar]
- 41.Rana S., Lemoine E., Granger J., Karumanchi S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 42.Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 43.Song Y., Gao J., Qu Y., Wang S., Wang X., Liu J. Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin. Chim. Acta. 2016;458:133–137. doi: 10.1016/j.cca.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 44.Kalinderis M., Papanikolaou A., Kalinderi K., Vyzantiadis T.A., Ioakimidou A., Tarlatzis B.C. Serum levels of leptin and IP-10 in preeclampsia compared to controls. Arch. Gynecol. Obstet. 2015;292:343–347. doi: 10.1007/s00404-015-3659-4. [DOI] [PubMed] [Google Scholar]
- 45.Taylor B.D., Ness R.B., Olsen J., Hougaard D.M., Skogstrand K., Roberts J.M., Haggerty C.L. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension. 2015;65:594–599. doi: 10.1161/HYPERTENSIONAHA.114.03979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chrelias G., Makris G.M., Papanota A.M., Spathis A., Salamalekis G., Sergentanis T.N., Rizos D., Karakitsos P., Chrelias C. Serum inhibin and leptin: Risk factors for pre-eclampsia? Clin. Chim. Acta. 2016;463:84–87. doi: 10.1016/j.cca.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Thagaard I.N., Hedley P.L., Holm J.C., Lange T., Larsen T., Krebs L., Christiansen M. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019;15:78–83. doi: 10.1016/j.preghy.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Pérez-Pérez A., Toro A., Vilariño-García T., Maymó J., Guadix P., Dueñas J.L., Fernández-Sánchez M., Varone C., Sánchez-Margalet V. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018;22:716–727. doi: 10.1111/jcmm.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zidan H.E., Rezk N.A., Alnemr A.A.A., Moniem M.I.A. Interleukin-17 and leptin genes polymorphisms and their levels in relation to recurrent pregnancy loss in Egyptian females. Immunogenetics. 2015;67:665–673. doi: 10.1007/s00251-015-0876-8. [DOI] [PubMed] [Google Scholar]
- 50.Laird S.M., Quinton N.D., Anstie B., Li T.C., Blakemore A.I.F. Leptin and leptin-binding activity in women with recurrent miscarriage: Correlation with pregnancy outcome. Hum. Reprod. 2001;16:2008–2013. doi: 10.1093/humrep/16.9.2008. [DOI] [PubMed] [Google Scholar]
- 51.Tommaselli G.A., Di Spiezio Sardo A., Di Carlo C., Bifulco G., Cerrota G., Cirillo D., Greco E., Nappi C. Do serum leptin levels have a role in the prediction of pregnancy outcome in case of threatened miscarriage? Clin. Endocrinol. Oxf. 2006;65:772–775. doi: 10.1111/j.1365-2265.2006.02665.x. [DOI] [PubMed] [Google Scholar]
- 52.Marchini G., Fried G., Östlund E., Hagenäs L. Plasma leptin in infants: Relations to birth weight and weight loss. Pediatrics. 1998;101:429–432. doi: 10.1542/peds.101.3.429. [DOI] [PubMed] [Google Scholar]
- 53.Shaarawy M., El-Mallah S.Y. Leptin and gestational weight gain: Relation of maternal and cord blood leptin to birth weight. J. Soc. Gynecol. Investig. 1999;6:70–73. doi: 10.1177/107155769900600204. [DOI] [PubMed] [Google Scholar]
- 54.Yildiz L., Avci B., Ingeç M. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clin. Chem. Lab. Med. 2002;40:1114–1117. doi: 10.1515/cclm.2002.195. [DOI] [PubMed] [Google Scholar]
- 55.Laivuori H., Gallaher M.J., Collura L., Crombleholme W.R., Markovic N., Rajakumar A., Hubel C.A., Roberts J.M., Powers R.W. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol. Hum. Reprod. 2006;12:551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 56.Ohashi K., Ouchi N., Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–2142. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Daniele A., Cammarata R., Masullo M., Nerone G., Finamore F., D’Andrea M., Pilla F., Oriani G. Analysis of Adiponectin Gene and Comparison of Its Expression in Two Different Pig Breeds. Obesity. 2008;16:1869–1874. doi: 10.1038/oby.2008.275. [DOI] [PubMed] [Google Scholar]
- 58.Kadowaki T., Yamauchi T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 59.Fang H., Judd R.L. Comprehensive Physiology. Volume 8. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2018. Adiponectin Regulation and Function; pp. 1031–1063. [DOI] [PubMed] [Google Scholar]
- 60.Nigro E., Scudiero O., Monaco M.L., Palmieri A., Mazzarella G., Costagliola C., Bianco A., Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacroix M., Battista M.C., Doyon M., Ménard J., Ardilouze J.L., Perron P., Hivert M.F. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care. 2013;36:1577–1583. doi: 10.2337/dc12-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catalano P.M., Hoegh M., Minium J., Huston-Presley L., Bernard S., Kalhan S., Hauguel-De Mouzon S. Adiponectin in human pregnancy: Implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 63.Mazaki-Tovi S., Kanety H., Pariente C., Hemi R., Wiser A., Schiff E., Sivan E. Maternal serum adiponectin levels during human pregnancy. J. Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 64.Engin A. Advances in Experimental Medicine and Biology. Volume 960. Springer; New York, NY, USA: 2017. Adiponectin-resistance in obesity; pp. 415–441. [DOI] [PubMed] [Google Scholar]
- 65.Nien J.K., Mazaki-Tovi S., Romero R., Erez O., Kusanovic J.P., Gotsch F., Pineles B.L., Gomez R., Edwin S., Mazor M., et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Straughen J.K., Trudeau S., Misra V.K. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr. Diabetes. 2013;3:e84. doi: 10.1038/nutd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutaj P., Sibiak R., Wirlstlein P., Wender-Ożegowska E. Determinants of adiponectin levels during pregnancy in women with type 1 diabetes mellitus. Pol. Arch. Intern. Med. 2020;130:252. doi: 10.20452/pamw.15227. [DOI] [PubMed] [Google Scholar]
- 68.Ranheim T., Haugen F., Staff A.C., Braekke K., Harsem N.K., Drevon C.A. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet. Gynecol. Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 69.Thyfault J.P., Hedberg E.M., Anchan R.M., Thorne O.P., Isler C.M., Newton E.R., Dohm G.L., DeVente J.E. Gestational diabetes is associated with depressed adiponectin levels. J. Soc. Gynecol. Investig. 2005;12:41–55. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Bhograj A., Suryanarayana K., Nayak A., Murthy N., Dharmalingam M., Kalra P. Serum adiponectin levels in gestational diabetes mellitus. Indian J. Endocrinol. Metab. 2016;20:752–755. doi: 10.4103/2230-8210.192909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Worda C., Leipold H., Gruber C., Kautzky-Willer A., Knöfler M., Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2004;191:2120–2124. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 72.Weerakiet S., Lertnarkorn K., Panburana P., Pitakitronakorn S., Vesathada K., Wansumrith S. Can adiponectin predict gestational diabetes? Gynecol. Endocrinol. 2006;22:362–368. doi: 10.1080/09513590600818919. [DOI] [PubMed] [Google Scholar]
- 73.Williams M.A., Qiu C., Muy-Rivera M., Vadachkoria S., Song T., Luthy D.A. Plasma Adiponectin Concentrations in Early Pregnancy and Subsequent Risk of Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2004;89:2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 74.Savvidou M., Nelson S.M., Makgoba M., Messow C.M., Sattar N., Nicolaides K. First-trimester prediction of gestational diabetes mellitus: Examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59:3017–3022. doi: 10.2337/db10-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Georgiou H.M., Lappas M., Georgiou G.M., Marita A., Bryant V.J., Hiscock R., Permezel M., Khalil Z., Rice G.E. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008;45:157–165. doi: 10.1007/s00592-008-0037-8. [DOI] [PubMed] [Google Scholar]
- 76.Lain K.Y., Daftary A.R., Ness R.B., Roberts J.M. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin. Endocrinol. Oxf. 2008;69:407–411. doi: 10.1111/j.1365-2265.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 77.Iliodromiti S., Sassarini J., Kelsey T.W., Lindsay R.S., Sattar N., Nelson S.M. Accuracy of circulating adiponectin for predicting gestational diabetes: A systematic review and meta-analysis. Diabetologia. 2016;59:692–699. doi: 10.1007/s00125-015-3855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J., Tan B., Karteris E., Zervou S., Digby J., Hillhouse E.W., Vatish M., Randeva H.S. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 79.Cortelazzi D., Corbetta S., Ronzoni S., Pelle F., Marconi A., Cozzi V., Cetin I., Cortelazzi R., Beck-Peccoz P., Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin. Endocrinol. Oxf. 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 80.Adu-Gyamfi E.A., Fondjo L.A., Owiredu W.K.B.A., Czika A., Nelson W., Lamptey J., Wang Y., Ding Y. The role of adiponectin in placentation and preeclampsia. Cell Biochem. Funct. 2020;38:106–117. doi: 10.1002/cbf.3458. [DOI] [PubMed] [Google Scholar]
- 81.Weiwei T., Haiyan Y., Juan C., Xiaodong W., Weibo C., Rong Z. Expressions of adiponectin receptors in placenta and their correlation with preeclampsia. Reprod. Sci. 2009;16:676–684. doi: 10.1177/1933719109334258. [DOI] [PubMed] [Google Scholar]
- 82.Dong G., Tian Y., Li X. Adiponectin Participates in Preeclampsia by Regulating the Biological Function of Placental Trophoblasts through P38 MAPK-STAT5 Pathway. Iran. J. Public Health. 2018;47:1838–1844. [PMC free article] [PubMed] [Google Scholar]
- 83.Ramsay J.E., Jamieson N., Greer I.A., Sattar N. Paradoxical Elevation in Adiponectin Concentrations in Women with Preeclampsia. Hypertension. 2003;42:891–894. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 84.Naruse K., Yamasaki M., Umekage H., Sado T., Sakamoto Y., Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 85.Lu D., Yang X., Wu Y., Wang H., Huang H., Dong M. Serum adiponectin, leptin and soluble leptin receptor in pre-eclampsia. Int. J. Gynecol. Obstet. 2006;95:121–126. doi: 10.1016/j.ijgo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 86.Takemura Y., Osuga Y., Koga K., Tajima T., Hirota Y., Hirata T., Morimoto C., Harada M., Yano T., Taketani Y. Selective increase in high molecular weight adiponectin concentration in serum of women with preeclampsia. J. Reprod. Immunol. 2007;73:60–65. doi: 10.1016/j.jri.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 87.Nien J.K., Mazaki-Tovi S., Romero R., Erez O., Kusanovic J.P., Gotsch F., Pineles B.L., Gomez R., Edwin S., Mazor M., et al. Adiponectin in severe preeclampsia. J. Perinat. Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ouyang Y., Chen H., Chen H. Reduced plasma adiponectin and elevated leptin in pre-eclampsia. Int. J. Gynecol. Obstet. 2007;98:110–114. doi: 10.1016/j.ijgo.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 89.Mazaki-Tovi S., Romero R., Vaisbuch E., Kusanovic J.P., Erez O., Gotsch F., Chaiworapongsa T., Than N.G., Kim S.K., Nhan-Chang C.L., et al. Maternal serum adiponectin multimers in preeclampsia. J. Perinat. Med. 2009;37:349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herse F., Youpeng B., Staff A.C., Yong-Meid J., Dechend R., Rong Z. Circulating and uteroplacental adipocytokine concentrations in preeclampsia. Reprod. Sci. 2009;16:584–590. doi: 10.1177/1933719109332828. [DOI] [PubMed] [Google Scholar]
- 91.Hendler I., Blackwell S.C., Mehta S.H., Whitty J.E., Russell E., Sorokin Y., Cotton D.B. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 2005;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 92.Bawah A.T., Yeboah F.A., Nanga S., Alidu H., Ngala R.A. Serum adipocytokines and adiposity as predictive indices of preeclampsia. Clin. Hypertens. 2020;26:19. doi: 10.1186/s40885-020-00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sethi J.K., Vidal-Puig A. Visfatin: The missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morgan S.A., Bringolf J.B., Seidel E.R. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008;29:1382–1389. doi: 10.1016/j.peptides.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 95.Mazaki-Tovi S., Romero R., Kusanovic J.P., Vaisbuch E., Erez O., Than N.G., Chaiworapongsa T., Nhan-Chang C.L., Pacora P., Gotsch F., et al. Maternal visfatin concentration in normal pregnancy. J. Perinat. Med. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iciek R., Brazert M., Wender-Ozegowska E., Pietryga M., Brazert J. Low placental visfatin expression is related to impaired glycaemic control and fetal macrosomia in pregnancies complicated by type 1 diabetes. J. Physiol. Pharmacol. 2018;69:61–66. doi: 10.26402/jpp.2018.1.06. [DOI] [PubMed] [Google Scholar]
- 97.Wnuk A., Stangret A., Wątroba M., Płatek A.E., Skoda M., Cendrowski K., Sawicki W., Szukiewicz D. Can adipokine visfatin be a novel marker of pregnancy-related disorders in women with obesity? Obes. Rev. 2020;21:e13022. doi: 10.1111/obr.13022. [DOI] [PubMed] [Google Scholar]
- 98.Radzicka S., Pietryga M., Iciek R., Brazert J. The role of visfatin in the pathogenesis of gestational diabetes (GDM) Ginekol. Pol. 2018;89:518–521. doi: 10.5603/GP.a2018.0088. [DOI] [PubMed] [Google Scholar]
- 99.Akturk M., Altinova A.E., Mert I., Buyukkagnici U., Sargin A., Arslan M., Danisman N. Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. J. Endocrinol. Investig. 2008;31:610–613. doi: 10.1007/BF03345611. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira A.F.A., Rezende J.C., Vaikousi E., Akolekar R., Nicolaides K.H. Maternal Serum Visfatin at 11–13 Weeks of Gestation in Gestational Diabetes Mellitus. Clin. Chem. 2011;57:609–613. doi: 10.1373/clinchem.2010.159806. [DOI] [PubMed] [Google Scholar]
- 101.Lewandowski K.C., Stojanovic N., Press M., Tuck S.M., Szosland K., Bienkiewicz M., Vatish M., Lewinski A., Prelevic G.M., Randeva H.S. Elevated serum levels of visfatin in gestational diabetes: A comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 102.Brink H.S., van der Lely A.J., van der Linden J. The potential role of biomarkers in predicting gestational diabetes. Endocr. Connect. 2016;5:R26–R34. doi: 10.1530/EC-16-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W., Zhao D., Meng Z., Wang H., Zhao K., Feng X., Li Y., Dun A., Jin X., Hou H. Association between circulating visfatin and gestational diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018;55:1113–1120. doi: 10.1007/s00592-018-1188-x. [DOI] [PubMed] [Google Scholar]
- 104.Mazaki-Tovi S., Romero R., Kusanovic J.P., Vaisbuch E., Erez O., Than N.G., Chaiworapongsa T., Nhan-Chang C.L., Pacora P., Gotsch F., et al. Visfatin in human pregnancy: Maternal gestational diabetes vis-à-vis neonatal birthweight. J. Perinat. Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cekmez F., Pirgon O., Tanju A., Ipcioglu O.M. Cord plasma concentrations of visfatin, adiponectin and insulin in healthy term neonates: Positive correlation with birthweight. Int. J. Biomed. Sci. 2009;5:257–260. [PMC free article] [PubMed] [Google Scholar]
- 106.Taraqi A.S.M., Tehranian N., Aghoozi M.F., Yousefi S., Kazemnejad A., Roudbaneh S.P., Esmaeilzadeh M.S. Visfatin as a predictor for growth of fetus and infant. Turk. J. Obstet. Gynecol. 2018;15:80–86. doi: 10.4274/tjod.48091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garg M., Thamotharan M., Dai Y., Lagishetty V., Matveyenko A.V., Lee W.N.P., Devaskar S.U. Glucose intolerance and lipid metabolic adaptations in response to intrauterine and postnatal calorie restriction in male adult rats. Endocrinology. 2013;154:102–113. doi: 10.1210/en.2012-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dessì A., Pravettoni C., Cesare Marincola F., Schirru A., Fanos V. The biomarkers of fetal growth in intrauterine growth retardation and large for gestational age cases: From adipocytokines to a metabolomic all-in-one tool. Expert Rev. Proteom. 2015;12:309–316. doi: 10.1586/14789450.2015.1034694. [DOI] [PubMed] [Google Scholar]
- 109.Fasshauer M., Waldeyer T., Seeger J., Schrey S., Ebert T., Kratzsch J., Lossner U., Bluher M., Stumvoll M., Faber R., et al. Serum levels of the adipokine visfatin are increased in pre-eclampsia. Clin. Endocrinol. Oxf. 2008;69:69–73. doi: 10.1111/j.1365-2265.2007.03147.x. [DOI] [PubMed] [Google Scholar]
- 110.Hu W., Wang Z., Wang H., Huang H., Dong M. Serum visfatin levels in late pregnancy and pre-eclampsia. Acta Obstet. Gynecol. Scand. 2008;87:413–418. doi: 10.1080/00016340801976012. [DOI] [PubMed] [Google Scholar]
- 111.Zulfikaroglu E., Isman F., PayaslI A., KIlIc S., Kucur M., DanIsman N. Plasma visfatin levels in preeclamptic and normal pregnancies. Arch. Gynecol. Obstet. 2010;281:995–998. doi: 10.1007/s00404-009-1192-z. [DOI] [PubMed] [Google Scholar]
- 112.Kim S.-C., Park M.J., Joo B.-S., Joo J.-K., Suh D.-S., Lee K.-S. Decreased expressions of vascular endothelial growth factor and visfatin in the placental bed of pregnancies complicated by preeclampsia. J. Obstet. Gynaecol. Res. 2012;38:665–673. doi: 10.1111/j.1447-0756.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- 113.Zorba E., Vavilis D., Venetis C.A., Zournatzi V., Kellartzis D., Tarlatzis B.C. Visfatin serum levels are increased in women with preeclampsia: A case-control study. J. Matern. Neonatal Med. 2012;25:1668–1673. doi: 10.3109/14767058.2012.657275. [DOI] [PubMed] [Google Scholar]
- 114.Chen D., Dong M., Fang Q., He J., Wang Z., Yang X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin. Sci. 2005;108:81–84. doi: 10.1042/CS20040225. [DOI] [PubMed] [Google Scholar]
- 115.Yura S., Sagawa N., Itoh H., Kakui K., Nuamah M.A., Korita D., Takemura M., Fujii S. Resistin is expressed in the human placenta. J. Clin. Endocrinol. Metab. 2003;88:1394–1397. doi: 10.1210/jc.2002-011926. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz D.R., Lazar M.A. Human resistin: Found in translation from mouse to man. Trends Endocrinol. Metab. 2011;22:259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Di Simone N., Di Nicuolo F., Marzioni D., Castellucci M., Sanguinetti M., D’Lppolito S., Caruso A. Resistin modulates glucose uptake and glucose transporter-1 (GLUT-1) expression in trophoblast cells. J. Cell. Mol. Med. 2009;13:388–397. doi: 10.1111/j.1582-4934.2008.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Estienne A., Bongrani A., Reverchon M., Ramé C., Ducluzeau P.H., Froment P., Dupont J. Involvement of novel adipokines, chemerin, visfatin, resistin and apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int. J. Mol. Sci. 2019;20:4431. doi: 10.3390/ijms20184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hivert M.F., Manning A.K., McAteer J.B., Dupuis J., Fox C.S., Cupples L.A., Meigs J.B., Florez J.C. Association of variants in RETN with plasma resistin levels and diabetes-related traits in the framingham offspring study. Diabetes. 2009;58:750–756. doi: 10.2337/db08-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lobo T.F., Torloni M.R., Gueuvoghlanian-Silva B.Y., Mattar R., Daher S. Resistin concentration and gestational diabetes: A systematic review of the literature. J. Reprod. Immunol. 2013;97:120–127. doi: 10.1016/j.jri.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 121.Nanda S., Poon L.C.Y., Muhaisen M., Acosta I.C., Nicolaides K.H. Maternal serum resistin at 11 to 13 weeks’ gestation in normal and pathological pregnancies. Metabolism. 2012;61:699–705. doi: 10.1016/j.metabol.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Chen D., Fang Q., Chai Y., Wang H., Huang H., Dong M. Serum resistin in gestational diabetes mellitus and early postpartum. Clin. Endocrinol. Oxf. 2007;67:208–211. doi: 10.1111/j.1365-2265.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 123.Nien J.K., Mazaki-Tovi S., Romero R., Kusanovic J.P., Erez O., Gotsch F., Pineles B.L., Friel L.A., Espinoza J., Goncalves L., et al. Resistin: A hormone which induces insulin resistance is increased in normal pregnancy. J. Perinat. Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lappas M., Yee K., Permezel M., Rice G.E. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J. Endocrinol. 2005;186:457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 125.Kuzmicki M., Telejko B., Szamatowicz J., Zonenberg A., Nikolajuk A., Kretowski A., Gorska M. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol. Endocrinol. 2009;25:258–263. doi: 10.1080/09513590802653825. [DOI] [PubMed] [Google Scholar]
- 126.Megia A., Vendrell J., Gutierrez C., Sabaté M., Broch M., Fernández-Real J.M., Simón I. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur. J. Endocrinol. 2008;158:173–178. doi: 10.1530/EJE-07-0671. [DOI] [PubMed] [Google Scholar]
- 127.Christiansen M., Hedley P.L., Placing S., Wøjdemann K.R., Carlsen A.L., Jørgensen J.M., Gjerris A.C., Shalmi A.C., Rode L., Sundberg K., et al. Maternal serum resistin is reduced in first trimester preeclampsia pregnancies and is a marker of clinical severity. Hypertens. Pregnancy. 2015;34:422–433. doi: 10.3109/10641955.2014.913615. [DOI] [PubMed] [Google Scholar]
- 128.Haugen F., Ranheim T., Harsem N.K., Lips E., Staff A.C., Drevon C.A. Increased plasma levels of adipokines in preeclampsia: Relationship to placenta and adipose tissue gene expression. Am. J. Physiol. Endocrinol. Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 129.Seol H.J., Oh M.J., Yeo M.K., Kim A., Lee E.S., Kim H.J. Comparison of serum levels and the placental expression of resistin between patients with preeclampsia and normal pregnant women. Hypertens. Pregnancy. 2010;29:310–317. doi: 10.3109/10641950902849850. [DOI] [PubMed] [Google Scholar]
- 130.Briana D.D., Malamitsi-Puchner A. The role of adipocytokines in fetal growth. Ann. N. Y. Acad. Sci. 2010;1205:82–87. doi: 10.1111/j.1749-6632.2010.05650.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang J., Shang L.X., Dong X., Wang X., Wu N., Wang S.H., Zhang F., Xu L.M., Xiao Y. Relationship of adiponectin and resistin levels in umbilical serum, maternal serum and placenta with neonatal birth weight. Aust. N. Z. J. Obstet. Gynaecol. 2010;50:432–438. doi: 10.1111/j.1479-828X.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 132.Kim K.H., Lee K., Moon Y.S., Sul H.S. A Cysteine-rich Adipose Tissue-specific Secretory Factor Inhibits Adipocyte Differentiation. J. Biol. Chem. 2001;276:11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 133.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K., Gulseth H.L., Birkeland K.I., Jensen J., Drevon C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 135.Kurdiova T., Balaz M., Vician M., Maderova D., Vlcek M., Valkovic L., Srbecky M., Imrich R., Kyselovicova O., Belan V., et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E., Mantzoros C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garcés M.F., Peralta J.J., Ruiz-Linares C.E., Lozano A.R., Poveda N.E., Torres-Sierra A.L., Eslava-Schmalbach J.H., Alzate J.P., Sánchez Á.Y., Sanchez E., et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J. Clin. Endocrinol. Metab. 2014;99:2113–2119. doi: 10.1210/jc.2013-4127. [DOI] [PubMed] [Google Scholar]
- 138.Piya M.K., Harte A.L., Sivakumar K., Tripathi G., Voyias P.D., James S., Sabico S., Al-Daghri N.M., Saravanan P., Barber T.M., et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014;306:E512–E518. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- 139.Salem H., Yatchenko Y., Anosov M., Rosenfeld T., Altarescu G., Grisaru-Granovsky S., Birk R. Maternal and neonatal irisin precursor gene FNDC5 polymorphism is associated with preterm birth. Gene. 2018;649:58–62. doi: 10.1016/j.gene.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 140.Cui L., Qiao T., Xu F., Li Z., Chen T., Su H., Chen G., Zhang L., Xu D., Zhang X. Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: A systematic review and meta-analysis. Cytokine. 2020;126:154924. doi: 10.1016/j.cyto.2019.154924. [DOI] [PubMed] [Google Scholar]
- 141.Erol O., Erkal N., Ellidağ H.Y., İsenlik B.S., Aydın Ö., Derbent A.U., Yılmaz N. Irisin as an early marker for predicting gestational diabetes mellitus: A prospective study. J. Matern. Neonatal Med. 2016;29:3590–3595. doi: 10.3109/14767058.2016.1142967. [DOI] [PubMed] [Google Scholar]
- 142.Kulhan N.G., Kulhan M., Turkler C., Ata N., Kiremitli T., Kiremitli S. Could serum levels of irisin be used in gestational diabetes predicting? Taiwan. J. Obstet. Gynecol. 2019;58:434–437. doi: 10.1016/j.tjog.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 143.Park K.H., Zaichenko L., Brinkoetter M., Thakkar B., Sahin-Efe A., Joung K.E., Tsoukas M.A., Geladari E.V., Huh J.Y., Dincer F., et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013;98:4899–4907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fatima S.S., Khalid E., Ladak A.A., Ali S.A. Colostrum and mature breast milk analysis of serum irisin and sterol regulatory element-binding proteins-1c in gestational diabetes mellitus. J. Matern. Neonatal Med. 2019;32:2993–2999. doi: 10.1080/14767058.2018.1454422. [DOI] [PubMed] [Google Scholar]
- 145.Foda A.A., Foda E.A. Effects of delivery on maternal & neonatal irisin levels in normal and preeclamptic pregnant women. Pregnancy Hypertens. 2017;10:226–229. doi: 10.1016/j.preghy.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y., Gu Y., Zhang Y., Lewis D.F. Evidence of endothelial dysfunction in preeclampsia: Decreased endothelial nitric oxide synthase expression is associated with increased cell permeability in endothelial cells from preeclampsia. Am. J. Obstet. Gynecol. 2004;190:817–824. doi: 10.1016/j.ajog.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 147.Xiang L., Xiang G., Yue L., Zhang J., Zhao L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014;235:328–333. doi: 10.1016/j.atherosclerosis.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 148.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C., Perron R.M., Werner C.D., Phan G.Q., Kammula U.S., et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Çaǧlar M., Göksu M., Isenlik B.S., Yavuzcan A., Yilmaz M., Üstün Y., Aydin S., Kumru S. Irisin in idiopathic foetal growth restriction. J. Endocrinol. Investig. 2014;37:619–624. doi: 10.1007/s40618-014-0078-5. [DOI] [PubMed] [Google Scholar]
- 150.Ergaz Z., Avgil M., Ornoy A. Intrauterine growth restriction—Etiology and consequences: What do we know about the human situation and experimental animal models? Reprod. Toxicol. 2005;20:301–322. doi: 10.1016/j.reprotox.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 151.Pavlova T., Zlamal F., Tomandl J., Hodicka Z., Gulati S., Bienertova-Vasku J. Irisin maternal plasma and cord blood levels in mothers with spontaneous preterm and term delivery. Dis. Markers. 2018;2018 doi: 10.1155/2018/7628957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hernandez-Trejo M., Garcia-Rivas G., Torres-Quintanilla A., Laresgoiti-Servitje E. Relationship between irisin concentration and serum cytokines in mother and newborn. PLoS ONE. 2016;11:e0165229. doi: 10.1371/journal.pone.0165229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Komiya T., Tanigawa Y., Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem. Biophys. Res. Commun. 1998;251:759–762. doi: 10.1006/bbrc.1998.9513. [DOI] [PubMed] [Google Scholar]
- 154.Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C., Shuldiner A.R., Fried S.K., McLenithan J.C., Gong D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 155.Tan B.K., Adya R., Farhatullah S., Lewandowski K.C., O’Hare P., Lehnert H., Randeva H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome ex vivo in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 156.Tan B.K., Adya R., Randeva H.S. Omentin: A Novel Link Between Inflammation, Diabesity, and Cardiovascular Disease. Trends Cardiovasc. Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 157.Briana D.D., Boutsikou M., Baka S., Gourgiotis D., Marmarinos A., Liosi S., Hassiakos D., Malamitsi-Puchner A. Omentin-1 and vaspin are present in the fetus and neonate, and perinatal concentrations are similar in normal and growth-restricted pregnancies. Metabolism. 2011;60:486–490. doi: 10.1016/j.metabol.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 158.Barker G., Lim R., Georgiou H.M., Lappas M. Omentin-1 Is Decreased in Maternal Plasma, Placenta and Adipose Tissue of Women with Pre-Existing Obesity. PLoS ONE. 2012;7:e42943. doi: 10.1371/journal.pone.0042943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lewandowski K., Nadel I., Lewinski A., Bienkiewicz M., Tan B., Randeva H.S., Cypryk K. Positive correlation between serum omentin and thrombospondin-1 in gestational diabetes despite lack of correlation with insulin resistance indices. Ginekol. Pol. 2010;81:907–912. [PubMed] [Google Scholar]
- 160.Franz M., Polterauer M., Springer S., Kuessel L., Haslinger P., Worda C., Worda K. Maternal and neonatal omentin-1 levels in gestational diabetes. Arch. Gynecol. Obstet. 2018;297:885–889. doi: 10.1007/s00404-018-4652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Souza Batista C.M., Yang R.Z., Lee M.J., Glynn N.M., Yu D.Z., Pray J., Ndubuizu K., Patil S., Schwartz A., Kligman M., et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 162.Silverman B.L., Rizzo T., Green O.C., Cho N.H., Winter R.J., Ogata E.S., Richards G.E., Metzger B.E. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40:121–125. doi: 10.2337/diab.40.2.S121. [DOI] [PubMed] [Google Scholar]
- 163.Fetita L.S., Sobngwi E., Serradas P., Calvo F., Gautier J.F. Review: Consequences of fetal exposure to maternal diabetes in offspring. J. Clin. Endocrinol. Metab. 2006;91:3718–3724. doi: 10.1210/jc.2006-0624. [DOI] [PubMed] [Google Scholar]
- 164.Philipps L.H., Santhakumaran S., Gale C., Prior E., Logan K.M., Hyde M.J., Modi N. The diabetic pregnancy and offspring BMI in childhood: A systematic review and meta-analysis. Diabetologia. 2011;54:1957–1966. doi: 10.1007/s00125-011-2180-y. [DOI] [PubMed] [Google Scholar]
- 165.Catli G., Anik A., Abaci A., Kume T., Bober E. Low omentin-1 levels are related with clinical and metabolic parameters in obese children. Exp. Clin. Endocrinol. Diabetes. 2013;121:595–600. doi: 10.1055/s-0033-1355338. [DOI] [PubMed] [Google Scholar]
- 166.Pan X., Kaminga A.C., Wen S.W., Acheampong K., Liu A. Omentin-1 in diabetes mellitus: A systematic review and meta-analysis. PLoS ONE. 2019;14:e0226292. doi: 10.1371/journal.pone.0226292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun J., Ren J., Zuo C., Deng D., Pan F., Chen R., Zhu J., Chen C., Ye S. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: A systematic review and meta-Analysis. Lipids Health Dis. 2020;19 doi: 10.1186/s12944-020-01209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Šplíchal Z., Zlámal F., Máchal J., Lipková J., Pavlová T., Hodická Z., Ventruba P., Vašků A., Bienertová-Vašků J. Comparison of maternal omentin-1 levels and genetic variability between spontaneous term and preterm births. J. Matern. Neonatal Med. 2018;31:1689–1695. doi: 10.1080/14767058.2017.1323530. [DOI] [PubMed] [Google Scholar]
- 169.Helfer G., Wu Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018;238:R79–R94. doi: 10.1530/JOE-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaur J., Mattu H.S., Chatha K., Randeva H.S. Chemerin in human cardiovascular disease. Vascul. Pharmacol. 2018;110 doi: 10.1016/j.vph.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 171.Kasher-Merona M., Mazaki-Tovia S., Barhod E., Hemi R., Haas J., Gat I., Zilberberg E., Yinon Y., Karasik A., Kanety H. Chemerin concentrations in maternal and fetal compartments: Implications for metabolic adaptations to normal human pregnancy. J. Perinat. Med. 2014;42 doi: 10.1515/jpm-2013-0166. [DOI] [PubMed] [Google Scholar]
- 172.Liang Z., Zhou M., Xu X.K., Qu F., Chen D. Is Chemerin associated with gestational diabetes mellitus? An evidence-based clinical research from Chinese women. J. Obstet. Gynaecol. Lahore. 2018;38:482–487. doi: 10.1080/01443615.2017.1385596. [DOI] [PubMed] [Google Scholar]
- 173.Stojek M. The role of chemerin in human disease. Postepy Hig. Med. Dosw. 2017;71:110–117. doi: 10.5604/01.3001.0010.3795. [DOI] [PubMed] [Google Scholar]
- 174.Buechler C., Feder S., Haberl E.M., Aslanidis C. Chemerin isoforms and activity in obesity. Int. J. Mol. Sci. 2019;20:1128. doi: 10.3390/ijms20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Garces M.F., Sanchez E., Ruíz-Parra A.I., Rubio-Romero J.A., Angel-Müller E., Suarez M.A., Bohórquez L.F., Bravo S.B., Nogueiras R., Diéguez C., et al. Serum chemerin levels during normal human pregnancy. Peptides. 2013;42:138–143. doi: 10.1016/j.peptides.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 176.Ebert T., Gebhardt C., Scholz M., Schleinitz D., Blüher M., Stumvoll M., Kovacs P., Fasshauer M., Tönjes A. Adipocytokines are not associated with gestational diabetes mellitus but with pregnancy status. Cytokine. 2020;131:155088. doi: 10.1016/j.cyto.2020.155088. [DOI] [PubMed] [Google Scholar]
- 177.Francis E.C., Li M., Hinkle S.N., Cao Y., Chen J., Wu J., Zhu Y., Cao H., Kemper K., Rennert L., et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: A longitudinal study in a multiracial cohort. BMJ Open Diabetes Res. Care. 2020;8:e001333. doi: 10.1136/bmjdrc-2020-001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Okten S.B., Bildacı T.B. Salivary Leptin and Chemerin; a novel way of gestational diabetes screening. Gynecol. Endocrinol. 2020;36:1116–1118. doi: 10.1080/09513590.2020.1749999. [DOI] [PubMed] [Google Scholar]
- 179.Tsiotra P.C., Halvatsiotis P., Patsouras K., Maratou E., Salamalekis G., Raptis S.A., Dimitriadis G., Boutati E. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157–166. doi: 10.1016/j.peptides.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 180.Pfau D., Stepan H., Kratzsch J., Verlohren M., Verlohren H.J., Drynda K., Lössner U., Blüher M., Stumvoll M., Fasshauer M. Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Horm. Res. Paediatr. 2010;74:56–61. doi: 10.1159/000282114. [DOI] [PubMed] [Google Scholar]
- 181.Cetin O., Kurdoglu Z., Kurdoglu M., Sahin H.G. Chemerin level in pregnancies complicated by preeclampsia and its relation with disease severity and neonatal outcomes. J. Obstet. Gynaecol. Lahore. 2017;37 doi: 10.1080/01443615.2016.1233947. [DOI] [PubMed] [Google Scholar]
- 182.Wang X., Liu J., Wang D., Zhu H., Kang L., Jiang J. Expression and correlation of Chemerin and FABP4 in peripheral blood of gestational diabetes mellitus patients. Exp. Ther. Med. 2019;19:710–716. doi: 10.3892/etm.2019.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang J., Chi H., Xiao H., Tian X., Wang Y., Yun X., Xu Y. Interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) single nucleotide polymorphisms (SNPs), inflammation and metabolism in gestational diabetes mellitus in inner mongolia. Med. Sci. Monit. 2017;23:4149–4157. doi: 10.12659/MSM.903565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fatima S.S., Alam F., Chaudhry B., Khan T.A. Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. J. Matern. Neonatal Med. 2017;30 doi: 10.1080/14767058.2016.1199671. [DOI] [PubMed] [Google Scholar]
- 185.Li X.M., Ji H., Li C.J., Wang P.H., Yu P., Yu D.M. Chemerin expression in Chinese pregnant women with and without gestational diabetes mellitus. Ann. Endocrinol. Paris. 2015;76:19–24. doi: 10.1016/j.ando.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 186.Van Poppel M.N.M., Zeck W., Ulrich D., Schest E.C., Hirschmugl B., Lang U., Wadsack C., Desoye G. Cord blood chemerin: Differential effects of gestational diabetes mellitus and maternal obesity. Clin. Endocrinol. Oxf. 2014;80:65–72. doi: 10.1111/cen.12140. [DOI] [PubMed] [Google Scholar]
- 187.Barker G., Lim R., Rice G.E., Lappas M. Increased chemerin concentrations in fetuses of obese mothers and correlation with maternal insulin sensitivity. J. Matern. Neonatal Med. 2012;25:2274–2280. doi: 10.3109/14767058.2012.686540. [DOI] [PubMed] [Google Scholar]
- 188.Ueland T., Michelsen A.E., Aukrust P., Henriksen T., Bollerslev J., Lekva T. Adipokines and macrophage markers during pregnancy—Possible role for sCD163 in prediction and progression of gestational diabetes mellitus. Diabetes. Metab. Res. Rev. 2019;35 doi: 10.1002/dmrr.3114. [DOI] [PubMed] [Google Scholar]
- 189.Guelfi K.J., Ong M.J., Li S., Wallman K.E., Doherty D.A., Fournier P.A., Newnham J.P., Keelan J.A. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism. 2017;75:54–60. doi: 10.1016/j.metabol.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 190.Görkem Ü., Küçükler F.K., Toğrul C., Güngör T. Are adipokines associated with gestational diabetes mellitus? J. Turk. Ger. Gynecol. Assoc. 2016;17:186–190. doi: 10.5152/jtgga.2016.16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hare K.J., Bonde L., Svare J.A., Randeva H.S., Asmar M., Larsen S., Vilsbøll T., Knop F.K. Decreased plasma chemerin levels in women with gestational diabetes mellitus. Diabet. Med. 2014;31:936–940. doi: 10.1111/dme.12436. [DOI] [PubMed] [Google Scholar]
- 192.Mehmood S., Ye C., Connelly P.W., Hanley A.J., Zinman B., Retnakaran R. Rising plasminogen activator inhibitor-1 and hypoadiponectinemia characterize the cardiometabolic biomarker profile of women with recent gestational diabetes. Cardiovasc. Diabetol. 2018;17:133. doi: 10.1186/s12933-018-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang X., Quan X., Lan Y., Ye J., Wei Q., Yin X., Fan F., Xing H. Serum chemerin level during the first trimester of pregnancy and the risk of gestational diabetes mellitus. Gynecol. Endocrinol. 2017;33:770–773. doi: 10.1080/09513590.2017.1320382. [DOI] [PubMed] [Google Scholar]
- 194.Hasanvand Z., Sadeghi A., Rezvanfar M.R., Goodarzi M.T., Rahmannezhad G., Mashayekhi F.J. Association between chemerin rs17173608 and rs4721 gene polymorphisms and gestational diabetes mellitus in Iranian pregnant women. Gene. 2018;649:87–92. doi: 10.1016/j.gene.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 195.Aviram A., Shtaif B., Gat-Yablonski G., Yogev Y. The association between adipocytokines and glycemic control in women with gestational diabetes mellitus. J. Matern. Neonatal Med. 2020;33:177–183. doi: 10.1080/14767058.2018.1487944. [DOI] [PubMed] [Google Scholar]
- 196.Ustebay S., Baykus Y., Deniz R., Ugur K., Yavuzkir S., Yardim M., Kalayci M., Çaglar M., Aydin S. Chemerin and Dermcidin in Human Milk and Their Alteration in Gestational Diabetes. J. Hum. Lact. 2019;35:550–558. doi: 10.1177/0890334419837523. [DOI] [PubMed] [Google Scholar]
- 197.Xu Q.L., Zhu M., Jin Y., Wang N., Xu H.X., Quan L.M., Wang S.S., Li S.S. The predictive value of the first-trimester maternal serum chemerin level for pre-eclampsia. Peptides. 2014;62:150–154. doi: 10.1016/j.peptides.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 198.Stepan H., Philipp A., Roth I., Kralisch S., Jank A., Schaarschmidt W., Lössner U., Kratzsch J., Blüher M., Stumvoll M., et al. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul. Pept. 2011;168:69–72. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 199.Duan D.M., Niu J.M., Lei Q., Lin X.H., Chen X. Serum levels of the adipokine chemerin in preeclampsia. J. Perinat. Med. 2012;40:121–127. doi: 10.1515/jpm.2011.127. [DOI] [PubMed] [Google Scholar]
- 200.Wang L., Yang T., Ding Y., Zhong Y., Yu L., Peng M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2014;48:299–308. doi: 10.1007/s12020-014-0286-y. [DOI] [PubMed] [Google Scholar]
- 201.Turgut A., Ozler A., Goruk N.Y., Tunç S.Y., Sak M.E., Evsen M.S., Evliyaoglu O., Gul T. Serum levels of the adipokines, free fatty acids, and oxidative stress markers in obese and non-obese preeclamptic patients. Clin. Exp. Obstet. Gynecol. 2015;42:473–479. [PubMed] [Google Scholar]
- 202.Mazaki-Tovi S., Kasher-Meron M., Hemi R., Haas J., Gat I., Lantsberg D., Hendler I., Kanety H. Chemerin is present in human cord blood and is positively correlated with birthweight. Am. J. Obstet. Gynecol. 2012;207:412.e1–412.e10. doi: 10.1016/j.ajog.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 203.Boutsikou T., Briana D.D., Boutsikou M., Kafalidis G., Stamati L., Baka S., Hassiakos D., Gourgiotis D., Malamitsi-Puchner A. Cord blood chemerin and obestatin levels in large for gestational age infants. J. Matern. Neonatal Med. 2013;26:123–126. doi: 10.3109/14767058.2012.728648. [DOI] [PubMed] [Google Scholar]
- 204.Léniz A., Portillo M.P., Fernández-Quintela A., Macarulla M.T., Sarasua-Miranda A., del Hoyo M., Díez-López I. Has the adipokine profile an influence on the catch-up growth type in small for gestational age infants? J. Physiol. Biochem. 2019;75:311–319. doi: 10.1007/s13105-019-00684-6. [DOI] [PubMed] [Google Scholar]
- 205.Yang X., Yao J., Wei Q., Ye J., Yin X., Quan X., Lan Y., Xing H. Role of chemerin/CMKLR1 in the maintenance of early pregnancy. Front. Med. 2018;12:525–532. doi: 10.1007/s11684-017-0577-9. [DOI] [PubMed] [Google Scholar]
- 206.Yang X., Quan X., Lan Y., Wei Q., Ye J., Yin X., Ji Z., Xing H., Yang Y. Serum chemerin level in women with PCOS and its relation with the risk of spontaneous abortion. Gynecol. Endocrinol. 2018;34:864–867. doi: 10.1080/09513590.2018.1462316. [DOI] [PubMed] [Google Scholar]
- 207.Reynolds L.J., Chavan N.R., DeHoff L.B., Preston J.D., Maddox H.F., O’Brien J.M., Armstrong D.A., Marsit C.J., Pearson K.J. Smoking during pregnancy increases chemerin expression in neonatal tissue. Exp. Physiol. 2019;104:93–99. doi: 10.1113/EP087307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Antushevich H., Wójcik M. Review: Apelin in disease. Clin. Chim. Acta. 2018;483:241–248. doi: 10.1016/j.cca.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 209.Hu H., He L., Li L., Chen L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol. Genet. Metab. 2016;119:20–27. doi: 10.1016/j.ymgme.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 210.Zhong J.C., Zhang Z.Z., Wang W., McKinnie S.M.K., Vederas J.C., Oudit G.Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1942–1950. doi: 10.1016/j.bbadis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 211.Kourtis A., Gkiomisi A., Mouzaki M., Makedou K., Anastasilakis A.D., Toulis K.A., Gerou S., Gavana E., Agorastos T. Apelin levels in normal pregnancy. Clin. Endocrinol. Oxf. 2011;75:367–371. doi: 10.1111/j.1365-2265.2011.04061.x. [DOI] [PubMed] [Google Scholar]
- 212.Guo Y.Y., Li T., Liu H., Tang L., Li Y.C., Hu H.T., Su Y.F., Lin Y., Wang Y.Y., Li C., et al. Circulating levels of Elabela and Apelin in the second and third trimesters of pregnancies with gestational diabetes mellitus. Gynecol. Endocrinol. 2020;36:890–894. doi: 10.1080/09513590.2020.1739264. [DOI] [PubMed] [Google Scholar]
- 213.Aslan M., Celik O., Celik N., Turkcuoglu I., Yilmaz E., Karaer A., Simsek Y., Celik E., Aydin S. Cord blood nesfatin-1 and apelin-36 levels in gestational diabetes mellitus. Endocrine. 2012;41:424–429. doi: 10.1007/s12020-011-9577-8. [DOI] [PubMed] [Google Scholar]
- 214.Telejko B., Kuzmicki M., Wawrusiewicz-Kurylonek N., Szamatowicz J., Nikolajuk A., Zonenberg A., Zwierz-Gugala D., Jelski W., Laudański P., Wilczynski J., et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2010;87:176–183. doi: 10.1016/j.diabres.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 215.Oncul M., Tuten A., Erman H., Gelisgen R., Benian A., Uzun H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013;104:527–535. [PubMed] [Google Scholar]
- 216.Vaughan O.R., Powell T.L., Jansson T. Apelin is a novel regulator of human trophoblast amino acid transport. Am. J. Physiol. Endocrinol. Metab. 2019;316:E810–E816. doi: 10.1152/ajpendo.00012.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boyadzhieva M., Atanasova I., Zacharieva S., Kedikova S. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. J. Endocrinol. Investig. 2013;36:944–949. doi: 10.3275/8968. [DOI] [PubMed] [Google Scholar]
- 218.Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–2240. doi: 10.1016/j.peptides.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 219.Akinci B., Celtik A., Tunali S., Genc S., Yuksel F., Secil M., Ozcan M.A., Bayraktar F. Circulating apelin levels are associated with cardiometabolic risk factors in women with previous gestational diabetes. Arch. Gynecol. Obstet. 2014;289:787–793. doi: 10.1007/s00404-013-3070-y. [DOI] [PubMed] [Google Scholar]
- 220.Cobble M., Bale B. Carotid intima-media thickness: Knowledge and application to everyday practice. Postgrad. Med. 2010;122:10–18. doi: 10.3810/pgm.2010.01.2091. [DOI] [PubMed] [Google Scholar]
- 221.Farahvar S., Walfisch A., Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev. Endocrinol. Metab. 2019;14:63–74. doi: 10.1080/17446651.2018.1476135. [DOI] [PubMed] [Google Scholar]
- 222.Temur M., Yilmaz Ö., Taşgöz F.N., Kume T. The evaluation of serum apelin levels in patients complicated with preeclampsia. J. Matern. Neonatal Med. 2020 doi: 10.1080/14767058.2020.1814238. [DOI] [PubMed] [Google Scholar]
- 223.Sattar Taha A., Zahraei Z., Al-Hakeim H.K. Serum apelin and galectin-3 in preeclampsia in Iraq. Hypertens. Pregnancy. 2020;39:379–386. doi: 10.1080/10641955.2020.1777300. [DOI] [PubMed] [Google Scholar]
- 224.Gürlek B., Yılmaz A., Durakoğlugil M.E., Karakaş S., Kazaz İ.M., Önal Ö., Şatıroğlu Ö. Evaluation of serum apelin-13 and apelin-36 concentrations in preeclamptic pregnancies. J. Obstet. Gynaecol. Res. 2020;46:58–65. doi: 10.1111/jog.14137. [DOI] [PubMed] [Google Scholar]
- 225.Bortoff K.D., Qiu C., Runyon S., Williams M.A., Maitra R. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens. Pregnancy. 2012;31:398–404. doi: 10.3109/10641955.2012.690054. [DOI] [PubMed] [Google Scholar]
- 226.Deniz R., Baykus Y., Ustebay S., Ugur K., Yavuzkir Ş., Aydin S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J. Obstet. Gynaecol. Lahore. 2019;39:907–912. doi: 10.1080/01443615.2019.1572727. [DOI] [PubMed] [Google Scholar]
- 227.Inuzuka H., Nishizawa H., Inagaki A., Suzuki M., Ota S., Miyamura H., Miyazaki J., Sekiya T., Kurahashi H., Udagawa Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens. Pregnancy. 2013;32:410–421. doi: 10.3109/10641955.2013.813535. [DOI] [PubMed] [Google Scholar]
- 228.Yamaleyeva L.M., Chappell M.C., Bridget Brosnihan K., Anton L., Caudell D.L., Shi S., McGee C., Pirro N., Gallagher P.E., Taylor R.N., et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2015;309:E852–E860. doi: 10.1152/ajpendo.00272.2015. [DOI] [PubMed] [Google Scholar]
- 229.Simsek Y., Celik O., Yilmaz E., Karaer A., Dogan C., Aydin S., Ozer A. Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J. Matern. Neonatal Med. 2012;25:1705–1708. doi: 10.3109/14767058.2011.660221. [DOI] [PubMed] [Google Scholar]
- 230.Kucur M., Tuten A., Oncul M., Acikgoz A.S., Yuksel M.A., Imamoglu M., Ekmekci O.B., Yilmaz N., Madazli R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens. Pregnancy. 2014;33:467–475. doi: 10.3109/10641955.2014.944709. [DOI] [PubMed] [Google Scholar]
- 231.Ho L., Van Dijk M., Chye S.T.J., Messerschmidt D.M., Chng S.C., Ong S., Yi L.K., Boussata S., Goh G.H.Y., Afink G.B., et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science. 2017;357:707–713. doi: 10.1126/science.aam6607. [DOI] [PubMed] [Google Scholar]
- 232.Georgiadou D., Boussata S., Ranzijn W.H.M., Root L.E.A., Hillenius S., bij de Weg J.M., Abheiden C.N.H., de Boer M.A., de Vries J.I.P., Vrijkotte T.G.M., et al. Peptide hormone ELABELA enhances extravillous trophoblast differentiation, but placenta is not the major source of circulating ELABELA in pregnancy. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tomimatsu T., Mimura K., Matsuzaki S., Endo M., Kumasawa K., Kimura T. Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors. Int. J. Mol. Sci. 2019;20:4246. doi: 10.3390/ijms20174246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang X., Liang G., Guo Q., Cai W., Zhang X., Ni J., Tao Y., Niu X., Chen S. ELABELA improves endothelial cell function via the ELA–APJ axis by activating the PI3K/Akt signalling pathway in HUVECs and EA.hy926 cells. Clin. Exp. Pharmacol. Physiol. 2020;1440:1953–1964. doi: 10.1111/1440-1681.13382. [DOI] [PubMed] [Google Scholar]
- 235.Zhou L., Sun H., Cheng R., Fan X., Lai X.S., Deng C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am. J. Physiol. Endocrinol. Metab. 2019;316:E773–E781. doi: 10.1152/ajpendo.00383.2018. [DOI] [PubMed] [Google Scholar]
- 236.Para R., Romero R., Gomez-Lopez N., Tarca A.L., Panaitescu B., Done B., Hsu R., Pacora P., Hsu C.D. Maternal circulating concentrations of soluble Fas and Elabela in early- and late-onset preeclampsia. J. Matern. Neonatal Med. 2020 doi: 10.1080/14767058.2020.1716720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pritchard N., Kaitu’u-Lino T.J., Gong S., Dopierala J., Smith G.C.S., Charnock-Jones D.S., Tong S. ELABELA/APELA Levels Are Not Decreased in the Maternal Circulation or Placenta among Women with Preeclampsia. Am. J. Pathol. 2018;188:1749–1753. doi: 10.1016/j.ajpath.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yamaleyeva L.M., Brosnihan K.B., Elsangeedy E., McGee C., Shi S., Caudell D., Miller C., Varagic J., Bader M., Dechend R., et al. Systemic Outcomes of (Pyr1)-Apelin-13 Infusion at Mid-Late Pregnancy in a Rat Model with Preeclamptic Features. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-44971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang C., Liu X., Kong D., Qin X., Li Y., Teng X., Huang X. Apelin as a novel drug for treating preeclampsia. Exp. Ther. Med. 2017;14:5917–5923. doi: 10.3892/etm.2017.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cekmez F., Canpolat F.E., Pirgon O., Çetinkaya M., Aydinoz S., Suleymanoglu S., Ipcioglu O.M., Sarici S.U. Apelin, vaspin, visfatin and adiponectin in large for gestational age infants with insulin resistance. Cytokine. 2011;56:387–391. doi: 10.1016/j.cyto.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 241.Malamitsi-Puchner A., Gourgiotis D., Boutsikou M., Baka S., Hassiakos D., Briana D.D. Circulating apelin concentrations in mother/infant pairs at term. Acta Paediatr. Int. J. Paediatr. 2007;96:1751–1754. doi: 10.1111/j.1651-2227.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 242.Van Mieghem T., Doherty A., Baczyk D., Drewlo S., Baud D., Carvalho J., Kingdom J. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod. Sci. 2016;23:1037–1043. doi: 10.1177/1933719116630422. [DOI] [PubMed] [Google Scholar]
- 243.Lim R., Barker G., Riley C., Lappas M. Apelin is decreased with human preterm and term labor and regulates prolabor mediators in human primary amnion cells. Reprod. Sci. 2013;20:957–967. doi: 10.1177/1933719112472741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hehir M.P., Morrison J.J. The adipokine apelin and human uterine contractility. Am. J. Obstet. Gynecol. 2012;206:359.e1–359.e5. doi: 10.1016/j.ajog.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 245.Carpenter J.R. Intrapartum management of the obese gravida. Clin. Obstet. Gynecol. 2016;59:172–179. doi: 10.1097/GRF.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 246.Caglayan E.K., Engin-Ustun Y., Sari N., Gocmen A.Y., Polat M.F. The effects of prolonged fasting on the levels of adiponectin, leptin, apelin, and omentin in pregnant women. J. Obstet. Gynaecol. Lahore. 2016;36:555–558. doi: 10.3109/01443615.2015.1103716. [DOI] [PubMed] [Google Scholar]