Abstract
Penicillium, one of the most common fungi occurring in a diverse range of habitats, has a worldwide distribution and a large economic impact on human health. Hundreds of the species belonging to this genus cause disastrous decay in food crops and are able to produce a varied range of secondary metabolites, from which we can distinguish harmful mycotoxins. Some Penicillium species are considered to be important producers of patulin and ochratoxin A, two well-known mycotoxins. The production of these mycotoxins and other secondary metabolites is controlled and regulated by different mechanisms. The aim of this review is to highlight the different levels of regulation of secondary metabolites in the Penicillium genus.
Keywords: Penicillium, secondary metabolism, regulation, virulence, control of gene expression, transcription factors
1. Introduction
Studies have estimated the existence of at least 2.2–3.8 million fungal species on Earth, from which only around 10% have been isolated and described [1,2]. Penicillium, one of the most common fungi in a various range of habitats, has a worldwide distribution and a large economic impact on human life. This genus is of great importance in numerous and diverse fields, such as food spoilage, biotechnology, plant pathology, and medicine [3,4], and currently contains 483 accepted species [5]. Several of these species, classified as pre- and post-harvest pathogens, can lead to catastrophic decay in food crops, as described by Frisvad and Samson [6], Pitt and Hocking [7], and Samson et al. [8]. Penicillium can also produce a varied range of secondary metabolites, including several harmful mycotoxins [9], antibacterial [10,11,12,13,14] and antifungal compounds [15], immunosuppressants, and cholesterol-lowering agents [16,17,18,19]. The most iconic example of a drug of fungal origin is penicillin, the first antibiotic substance in history [20].
The biosynthesis of several secondary metabolites, such as mycotoxins, depends on several environmental cues including the substrate, pH, temperature, water activity, interrelationships with other microorganisms, and the interactions of these different factors in the natural environment [21,22,23].
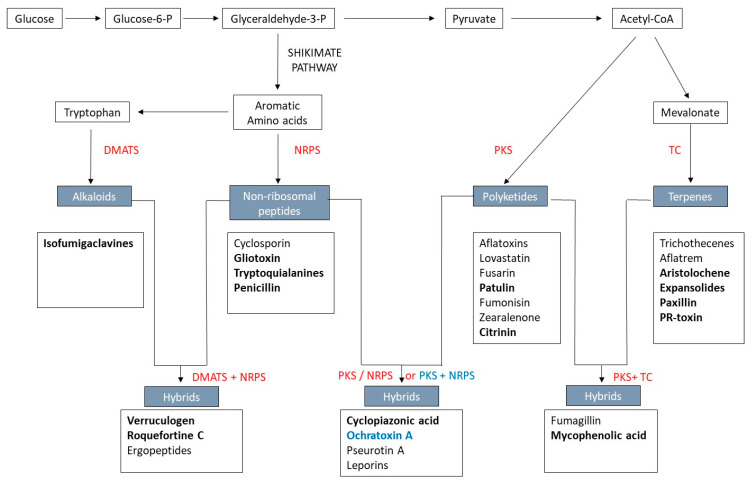
Secondary metabolites are products of enzymatic cascades starting when backbone enzymes such as polyketide synthases (PKSs), non-ribosomal peptide synthetases (NRPSs), terpene cyclases (TCs), and dimethylallyl tryptophan synthases (DMATSs) catalyze, respectively, the rearrangement or condensation of simple primary metabolites, such as acetyl-CoA, amino acids, or isoprene units, resulting in more complex secondary metabolites [24]. Different metabolic pathways can lead to their formation (Figure 1). Fungal secondary metabolites are classified into five categories according to their structures and their precursors: polyketides, cyclic terpenes, non-ribosomal peptides, indole alkaloids, and hybrids (Figure 1) [25]. Other enzymes named tailoring enzymes are also needed and interfere in the catalysis of subsequent reactions in the biosynthetic pathways of mycotoxins. The structural diversity of mycotoxins results from the variety of chemical reactions (cyclization, aromatization, glycosylation, hydroxylation, methylation, acetylation, and epoxidation) involved in their biosynthesis [26] and leads to their broad spectrum of biological properties and functions. The combined involvement of backbone enzymes in the same biosynthesis pathway infinitely broadens this structural diversity of secondary metabolites. This diversity is also enriched by the infrequent existence of crosstalk between different biosynthetic pathways [27].
Figure 1.
Biosynthetic pathways of secondary metabolites. In grey boxes, the typical backbone of secondary metabolites. In grey, the main mycotoxins produced by these pathways. In red, the enzymes associated with each pathway. In blue, separate PKS and NRPS are involved in ochratoxin A (OTA) biosynthesis; NRPS: non-ribosomal peptide synthetase, PKS: polyketide synthase, TC: terpene cyclase, DMATS: dimethylallyl tryptophan synthase. In bold, mycotoxins produced by Penicillium species.
Enzymes are activated at the same time, and the newly synthesized intermediates are consecutively metabolized by the following enzymes. This phenomenon is possible due to the cluster organization of the genes encoding the enzymes involved in the biosynthesis of the metabolites in the same chromosomal region. These genes are often co-activated by a specific transcription factor (TF) located within the clusters [28]. Based on bioinformatics analysis and other studies, it was proven that fungal genomes exhibit different and numerous predicted secondary metabolite clusters. A recent review estimated the number of fungal biosynthetic gene clusters (BGCs) at several million [29]. For the two well-known genera of Aspergillus and Penicillium alone, which contain 446 and 483 species, respectively [5], the number of non-redundant clusters is approximately 25,000. In filamentous fungi, the activation of specific TFs and the resulting production of fungal secondary metabolites is controlled at a higher hierarchical level by global TFs. Understanding the mechanisms underlying mycotoxin biosynthesis contributes to defining/identifying strategies or mechanisms to regulate them and reduce their production [30].
Numerous studies have focused on regulators impacting the formation of secondary metabolites in Aspergillus, Penicillium, and Fusarium, but few reviews have explored the complex and multi-layered regulation of fungal secondary metabolism [31,32,33,34]. While several excellent articles reviewed the different regulatory mechanisms known for Aspergillus [29,35,36,37], this review aims to deepen the understanding of the regulation of secondary metabolism in Penicillium and highlight all the regulatory mechanisms that can occur.
2. Regulation of Secondary Metabolism
For the synthesis of any secondary metabolite, the regulation of its cluster involves a number of factors for activation or repression. This regulation occurs at different levels. Most secondary metabolite clusters have genes encoding TFs that act directly on all other genes located within the cluster. Expression of these internal regulators also depends on other, more global TFs encoded by genes unrelated to the BGCs, which are themselves under the control of different physiological and/or environmental stimuli. An adaptation to a specific environment may also result in the biosynthesis of a certain secondary metabolite. This biosynthesis is connected and regulated by different signaling transduction pathways. Finally, epigenetic regulation, including modification of the chromatin and nucleosome structure, can yield transcriptional control and impact secondary metabolite synthesis extensively [38]. In the following section, the different regulatory systems studied in Penicillium will be discussed.
2.1. Specific Transcription Factors/Cluster-Specific Regulators
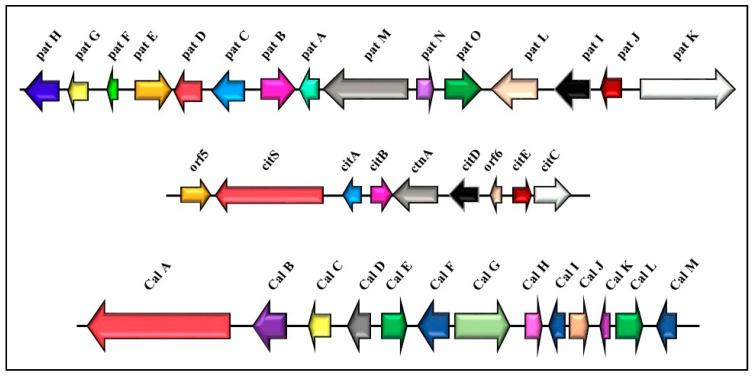
Gene clusters involved in secondary metabolite biosynthesis often include a gene encoding a TF that specifically acts and modulates the expression of the other genes in that cluster (e.g., patL, calC, and ctnA in patulin, calbistrin, and citrinin biosynthetic pathways, respectively). This gene has a switching role within the cluster (Figure 2). The TFs regulate gene expression by binding specifically to the promoters of the genes involved.
Figure 2.
Gene clusters of the patulin biosynthesis pathway (the first one at the top) (15 genes, 40 kb) [39] and the citrinin biosynthesis pathway (the middle group) (nine genes, 22 kb) [40,41] in Penicillium expansum; cluster of the calbistrin biosynthesis pathway (the third one at the bottom) (13 genes, 35kb) in Penicillium decumbens [42].
Several studies comparing TF sequences have shown that these TFs can be classified into different families based on the similarities in their protein sequences. We can distinguish between zinc finger proteins, proteins called helix-turn-helix, and leucine zippers [43]. Nevertheless, almost 90% of the potential gene clusters involved in the synthesis of fungal polyketides belong to the family of zinc finger TFs (Cys2His2, Cys4, or Zn(II)2Cys6) [43,44,45]. Proteins of the Zn(II)2Cys6 family are found exclusively in fungi and yeasts [46], and the C6 type zinc finger DNA binding protein motif (Cys6) is frequently encountered in TFs. Cys6 has been identified on more than 80 proteins found mainly in fungi [43] and is generally considered a transcriptional activator (Table 1). Only in Saccharomyces cerevisiae are the zinc finger proteins (ARGR2, LEU3, and UME6) activators and repressors [47,48,49,50]. Subsequently, the number of proteins belonging to the Zn(II)2Cys6 family has increased significantly due to the number of fungal genomes that have since been sequenced. Numerous examples of Zn(II)2Cys6 TFs identified as being involved in the secondary metabolism of fungi genera other than Penicillium have been largely described in the literature. As examples, we can quote AflR (aflatoxins), Bik5 (bikaverin), and CtnA (citrinin) for Aspergillus, Fusarium, and Monascus, respectively (Table 1).
Table 1.
Examples of identified Zn(II)2Cys6 and leucine zipper transcription factor (TF) involvement in secondary metabolism in fungi; adapted and updated from Yin and Keller [46].
| TF | Biosynthetic Gene Cluster | TF Family | Species | References |
|---|---|---|---|---|
| AflR | Aflatoxin/Sterigmatocystin | Zn(II)2Cys6 |
Aspergillus flavus
Aspergillus nidulans Aspergillus parasiticus |
[51,52,53,54,55] |
| AsaR | Aspergillic Acid | Zn(II)2Cys6 | Aspergillus flavus | [56] |
| GliZ | Gliotoxin | Zn(II)2Cys6 | Aspergillus fumigatus Penicillium lilacinoechinulatum | [57,58] |
| XanC | Xanthocillin | Basic Leucine zipper | Aspergillus fumigatus | [59] |
| FapR | Fumagillin/Pseurotin | Zn(II)2Cys6 | Aspergillus fumigatus | [60] |
| ZEB2 | Zearalenone | Basic Leucine zipper | Fusarium graminearum | [61] |
| SimL | Cyclosporine | Basic Leucine Zipper | Tolypocladium inflatum | [62] |
| OtaR1 | Ochratoxin A | Basic Leucine zipper | Aspergillus carbonarius Aspergillus ochraceus Aspergillus westerdijkiae Penicillium nordicum | [63] |
| SirZ | Sirodesmin PL | Zn(II)2Cys6 | Leptosphaeria maculans | [58] |
| MlcR | Compactin | Zn(II)2Cys6 | Penicillium citrinum | [64] |
| Bik5 | Bikaverin | Zn(II)2Cys6 | Fusarium fujikuroi | [65] |
| DEP6 | Depudecin | Zn(II)2Cys6 | Alternaria brassicicola | [66] |
|
ZFR1 FUM21 |
Fumonisin | Zn(II)2Cys6 | Fusarium verticillioides | [67,68] |
| CTB8 | Cercosporin | Zn(II)2Cys6 | Cercospora nicotianae | [69] |
| GIP2 | Aurofusarin | Zn(II)2Cys6 | Gibberella zeae | [70] |
| CtnA | Citrinin | Zn(II)2Cys6 |
Monascus purpureus
Monascus ruber Penicillium expansum |
[40,41,71] |
| LovE | Lovastatin | Zn(II)2Cys6 | Aspergillus terreus | [72,73] |
| ApdR | Aspyridone | Zn(II)2Cys6 | Aspergillus nidulans | [74] |
| CtnR | Asperfuranone | Zn(II)2Cys6 | Aspergillus nidulans | [75] |
| MdpE |
Monodictyphenone/ Emodin Analogs |
Zn(II)2Cys6 | Aspergillus nidulans | [76] |
| Cmr1p | Melanin | Zn(II)2Cys6 | Colletotrichum lagenarium | [77] |
| Pig1p | Melanin | Zn(II)2Cys6 | Magnaporthe grisea | [77] |
| GsfR1 | Griseofulvin | Zn(II)2Cys6 | Penicillium griseofulvum | [78] |
| MokH | Monacolin K | Zn(II)2Cys6 | Monascus pilosus | [79] |
| CalC | Calbistrin | Zn(II)2Cys6 | Penicillium decumbens | [42] |
| CnsN | Communesins | Zn(II)2Cys6 | Penicillium expansum | [80] |
| Orf2 | Varicidin A and B | Zn(II)2Cys6 | Penicillium variabile | [81] |
| Orf10 | PR-Toxin | Zn(II)2Cys6 |
Penicillium chrysogenum
Penicillium roqueforti |
[82,83] |
| MacR | Macrophorin | Zn(II)2Cys6 | Penicillium terrestris | [84] |
| PatL | Patulin | Zn(II)2Cys6 | Penicillium expansum | [85] |
| SorR1 SorR2 |
Sorbicillin | Zn(II)2Cys6 | Penicillium chrysogenum | [86] |
| TqaK | Tryptoquialanines | Basic leucine zipper | Penicillium aethiopicum Penicillium digitatum | [87,88] |
| Sol4 | Solanapyrone | Zn(II)2Cys6 | Ascochyta rabiei | [89] |
| RolP | Leucinostatin | Zn(II)2Cys6 | Paecilomyces lilacinus | [90] |
Gliotoxin, a secondary fungal metabolite belonging to the class of epipolythiodioxopiperazines (ETPs) and characterized by the presence of a sulfur-bridged dioxopiperazine ring [91], is produced by some Aspergillus and Penicillium species, such as Penicillium lilacinoechinulatum [92], a strain of this species was misidentified as Penicillium terlikowskii in a study by Waring et al. [92,93]. Within its cluster, a Zn(II)2Cys6 finger transcription regulator, GliZ, was identified to be responsible for gliotoxin induction and regulation. A mutation of the gliZ (∆gliZ) gene in Aspergillus fumigatus resulted in the loss of gliotoxin production, while overexpression of gliZ increased the production of gliotoxin [94,95]. In P. lilacinoechinulatum, a homologous gene is present in the genome, but the heterologous complementation of the A. fumigatus ∆gliZ mutant with PlgliZ failed to restore gliotoxin production [58]. The mlcR gene encoding a putative 50.2-kDa protein characterized by a Zn(II)2Cys6 DNA-binding domain was shown to be involved in the regulation and biosynthesis of ML-236B (compactin) in Penicillium citrinum [96].
Another gene encoding PatL, a specific TF in Penicillium expansum, was shown to affect patulin production [85]. The protein encoded by this gene has two conserved domains, one of which encodes a Cys6 DNA binding site and the other of which was found in the TFs of the superfamily of zinc finger TFs. Orthologous genes of patL involved in the patulin metabolic pathway were found in other filamentous fungi genomes, such as Penicillium griseofulvum, Penicillium paneum, Penicillium vulpinum, Penicillium carneum, Penicillium antarcticum [97], and Aspergillus clavatus [98]. Sometimes, BGCs such as the sorbicillin gene cluster can contain two genes encoding TFs. In this example, SorR1, a Zn(II)2Cys6 factor, acts as an activator for the expression of all genes located within the cluster. The second zinc finger TF (SorR2) controls the expression of the sorR1 gene [86]. Few cases of TFs belonging to the basic leucine zipper (bZIP) family have been reported to act as specific TFs of secondary metabolite pathways. These TFs include ZEB2, SimL, and OtaR1. The latter TF is present in the OTA cluster in Aspergillus ochraceus, Aspergillus westerdijkiae, Aspergillus carbonarius, and Penicillium nordicum. Its inactivation in A. ochraceus leads to the complete inhibition of OTA production [63]. SimL regulates the production of the well-known immunosuppressant drug cyclosporine [62]. The transcripts of genes located within the zearalenone gene cluster were not detected when the zeb2 gene encoding bZIP was deleted [61,99]. TqaK, another gene encoding a bZIP protein, was reported to be located inside the tryptoquialanine gene cluster in Penicillium aethiopicum. The deletion of tqaK led to tryptoquialanine production equal to only one-twentieth that of the parental strain [87]. An orthologous gene is also present in the genome of Penicillium digitatum, another tryptoquialanine-producing species. Thus far, OtaR1 and TqaK are the only bZIP proteins identified to be directly involved in secondary metabolite biosynthesis in Penicillium.
2.2. Environmental Signals and Associated Regulators
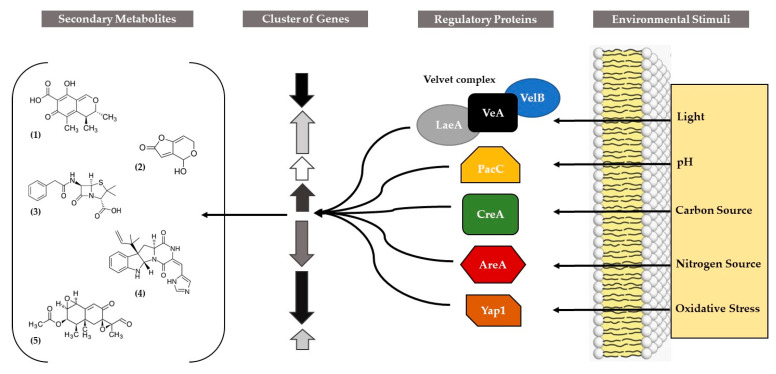
In the previous section, we reviewed specific TFs described in Penicillium that are cluster-specific. However, numerous regulatory elements affected by environmental cues modulate the expression of fungal secondary metabolite clusters and do not reside within the cluster itself. They are considered to be global regulators (Figure 3). Among them, CreA, AreA, Nmc, PacC, Skn7, Yap1, VeA, LaeA, BrlA, PcRFX1, PcFKH1, Pcz1, and NsdD have been mentioned and are discussed in the following.
Figure 3.
Global regulatory proteins involved in the regulation of gene clusters involved in the production of various secondary metabolites in Penicillium (1) citrinin, (2) patulin, (3) penicillin G, (4) roquefortine C, and (5) PR-toxin, adapted from Brakhage [45].
In the fungal kingdom, the synthesis of secondary metabolites is often a response to environmental or ecological changes and is dependent on the developmental stage of the producing species. The activation of a biosynthetic pathway is influenced by the composition of the substrate on which the fungus grows—in particular, the carbon source and the nitrogen source. Glucose and other assimilable sugars can suppress secondary metabolite pathways mediated by CreA, a protein displaying two Cys2His2 zinc finger domains. For example, the biosynthesis of penicillin in Penicillium chrysogenum was shown to be largely regulated by glucose, sucrose and, to a lesser extent, by other sugars (maltose, fructose, and galactose). Cepeda-García et al. [100] showed clear evidence of the involvement of the CreA factor in the catabolic repression of penicillin biosynthesis and the expression of the pcbAB gene, encoding the first enzyme of the penicillin pathway in P. chrysogenum. The authors applied an RNAi strategy attenuating creA gene expression. Transformants expressing small interfering RNAs for creA showed greater production of penicillin. By contrast, a recent study showed that the deletion of creA in P. expansum strains leads to the absence of patulin production in apples [101], although expression of the pat genes is increased. Regarding the nitrogen source, a similar regulatory mechanism called nitrogen metabolite repression exists in Ascomycetes. For instance, a concentration of ammonium above 40 mM caused a repression in the expression of uidA, a promoterless gene for β-glucuronidase in Escherichia coli, when fused to the promoters of pcbAB (acvA) and pcbC, two genes encoding the two first enzymes of the penicillin pathway in P. chrysogenum [102]. In P. griseofulvum (formerly P. urticae), the production of patulin was also affected when ammonium ions were added to the culture medium [103]. On the other hand, the presence of 30 mM ammonium chloride results in a significant decrease in isoepoxydon dehydrogenase (idh) and 6-methylsalicylic synthase (6-msas) transcripts, key genes in the pathways of patulin biosynthesis [104,105]. This nitrogen metabolite repression is mediated by AreA, a Cys2Cys2-type zinc finger TF [31]. This regulatory factor binds to the intergenic region of acvA-pcbC [106] in response to nitrogen and mediates the regulation of penicillin biosynthesis in P. chrysogenum [107]. The idh (patN) and 6-msas (patK) genes interact with the NrfA protein, an orthologue of the AreA protein in P. griseofulvum, through several putative GATA sites located on their promoter. The nmc gene, encoding the AreA orthologous factor, has been characterized in Penicillium roqueforti. This protein, which displays at least 94% identity with that of homologous fungal proteins (AreA in Aspergillus) [108], is induced and upregulated by nitrogen starvation, but no data regarding its impact on P. roqueforti secondary metabolites have already been published.
Another well-known environmental stimulus that induces or represses the secondary metabolism in filamentous fungi is the pH of the substrate. This regulation is mediated by PacC, the key factor of pH fungal regulation [109]. This TF displays three putative Cys2His2 zinc fingers [110]. In the genus Aspergillus, a neutral to alkaline extra-cellular pH is required for the activation of PacC via two proteolytic steps [111]. These steps are mediated by the pal (palA, palB, palC, palF, and palI) pathway [109]. The final mature form of this protein activates the expression of genes expressed under alkaline conditions and, by contrast, represses the transcription of genes expressed under acidic conditions. Many examples of PacC’s involvement in the regulation of biosynthetic pathways in Aspergillus or Fusarium species have been reported in the literature [65,112,113]. Suárez and Peñalva [114] showed that Penicillium pacC transcript levels were higher under alkaline than acidic growth conditions and elevated in later stages of growth. The level of the pcb transcripts followed the same trend, leading to increased production of penicillin under alkaline pH. Barad et al. [115] also studied the link between ammonia accumulation, the activation of pacC, and the synthesis of patulin in P. expansum. The authors concluded that an accumulation of ammonia during nutritional limitation in P. expansum could lead to a modification of the ambient environmental pH, a signal for the activation of pacC, as well as other alkaline induced genes leading to an accumulation of secondary metabolites, such as patulin.
Barad et al. [116] analyzed the role of PacC in the regulation of D-gluconic acid (GLA) production and patulin accumulation in P. expansum. On the one hand, their results showed that GLA production plays a role in the activation of patulin production. On the other hand, this study, based on the characterization of pacC-RNAi mutants of P. expansum, concluded that PacC plays a key role in the regulation of GLA accumulation via the transcriptional regulation of gox2, the most important gene involved in GLA production in P. expansum. This regulation of GLA production through PacC largely affects patulin accumulation in the mutants. A recent publication reported that the production of patulin is completely inhibited in the null mutant Pe∆pacC strain when grown at pH > 6.0 [117]. In P. digitatum, PacC was reported to regulate the expression of genes encoding polygalacturonase PG2 and pectin lyase PNL1, enzymes both involved in the degradation of the citrus cell wall [118].
Osmotic and oxidative stress are considered to be other environmental cues to which filamentous fungi should respond in order to survive. Most of the relevant knowledge comes from the yeast S. cerevisiae and the fungal genus Aspergillus. Skn7, a TF involved in the osmotic and oxidative stress responses in S. cerevisiae [119], has also been identified in Talaromyces (formerly Penicillium) marneffei. The gene skn7 from the latter was used to complement a skn7-disrupted strain of S. cerevisiae and seemed to be involved in the oxidative stress response in the yeast [120]. This result indicates the highly conserved nature of skn7 between the two organisms. Montibus et al. [121] suggested that skn7 could be involved in the regulation of fungal secondary metabolism. A recent study seemed to confirm this hypothesis since the deletion of Afskn7 resulted in a drastic decrease in aflatoxin B1 production in Aspergillus flavus [122]. Yap1, another TF, coordinates the interplay between oxidative stress and secondary metabolism. In Aspergillus parasiticus, the deletion mutant ∆yap1 exhibited an increase in aflatoxin production [123]. The same team later reported that the suppression of the yap1 orthologous gene led to increased OTA accumulation in Aspergillus ochraceus [124]. In T. marneffei, the mutant ∆yapA, a yap1 orthologous gene, was found to be sensitive to oxidative chemicals such as H2O2 or menadione and featured growth, germination, and conidiation delays [125]. For the genus Penicillium, the only works on orthologues Skn7 and Yap1 are mentioned above; their roles in the secondary metabolism of T. marneffei have not yet been investigated.
The development of filamentous fungi and their ability to produce secondary metabolites is largely influenced by light, as well. A velvet complex has been described in Aspergillus nidulans, and the VeA (velvet A) factor has been widely studied, as well as many proteins that seem to interact with it, such as VelB (velvet-like B), VosA (viability of spores A), VelC (velvet-like C), and the non-velvet protein LaeA (loss of aflR expression A), a methyltransferase involved in chromatin remodeling [126]. Depending on the fungal species, VeA is involved in different physiological processes, such as development, asexual and sexual reproduction, secondary metabolism, and virulence. The regulation mediated by this factor depends particularly on light. VeA was first characterized in A. nidulans, whose gene encodes a protein of 573 amino acids with a conserved domain at the N-terminus [127] and a nuclear localization sequence (NLS) [128]. At its C-terminus, a PEST domain (rich in proline (P), glutamic acid (E), serine (S), and threonine (T)) is present [129]. This PEST domain is also found in VeA orthologous proteins in A. parasiticus, A. fumigatus, and Neurospora crassa [130].
Stinnett et al. [128] studied the intracellular localization of VeA. This study demonstrated that this localization is dependent on light. In the dark, VeA is mainly located in the nucleus, whereas in the presence of light, VeA is mainly found in the cytoplasm. In the veA1 mutant [131], VeA is mostly found in the cytoplasm independently of light. In this mutant, the presence of a mutation on the transcription initiation codon led to a truncated protein where the first 36 amino acids were missing and, therefore, did not have a functional NLS, thus explaining the cytoplasmic localization of VeA. In the same study, it was demonstrated that the transfer of VeA into the nucleus depends on the importin α KapA and that a functional NLS is essential to allow the interaction of these two proteins.
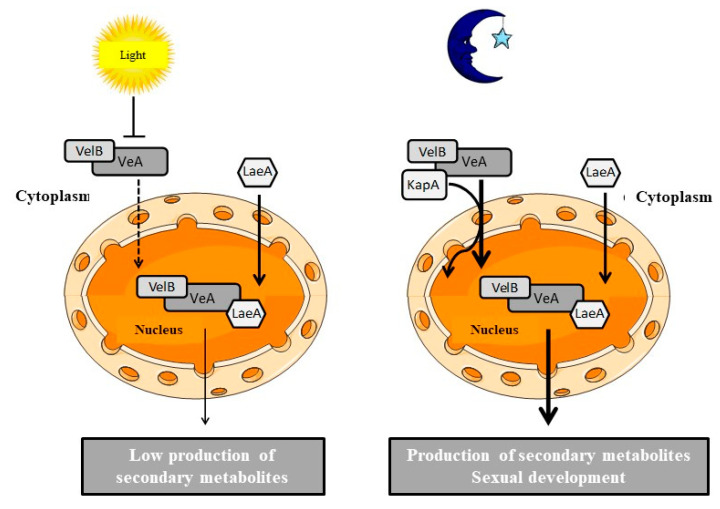
To identify the proteins interacting with VeA, Bayram et al. [127] used the Tandem Affinity Purification (TAP) technique from a strain of A. nidulans expressing a VeA protein coupled to a TAP-tag at the C-terminus. In the dark, the proteins VelB, LaeA, and importin α KapA interact with VeA. Conversely, only VelB interacts with VeA in the presence of light. Using the yeast two-hybrid technique, these analyses confirmed the interactions of VeA–VelB and VeA–LaeA; however, no interaction was demonstrated between LaeA and VelB, suggesting that VeA acts as a bridge between these two proteins. In addition, fluorescence assays showed that the VeA–LaeA interaction occurs in the nucleus, while VeA and VelB interact in the nucleus and the cytoplasm. LaeA is located in the nucleus, and its interaction with VeA is nuclear. VelB must, therefore, be able to enter the nucleus despite the absence of NLS in its sequence. Bayram et al. [127] demonstrated that VeA helps VelB to enter the nucleus to form the velvet complex.
The results obtained in the various studies allowed Bayram et al. [127] to propose a mechanism (Figure 4) that coordinates the regulation of sexual development and the production of secondary metabolites in A. nidulans. In the dark, the VelB/VeA/LaeA complex controls and induces the epigenetic activity of LaeA, which consequently controls the expression of the genes of the clusters responsible for the synthesis of the secondary metabolites. In the presence of light, this interaction decreases because VeA is retained in the cytoplasm, and LaeA has low activity.
Figure 4.
Operating model of the velvet complex in Aspergillus nidulans adapted from Bayram et al. [127]. In the presence of light, VeA is retained in the cytoplasm (-----), and LaeA has low activity. In the dark, VeA coupled to VelB is transported in the nucleus by the importin α KapA (——), and the velvet complex is formed with LaeA to activate the production of secondary metabolites and sexual development.
Despite its strong conservation among different fungal species, VeA has different roles, reflecting the diversity of fungal development patterns. Therefore, veA has a role in the regulation of secondary metabolism. The expression of genes involved in the synthesis of secondary metabolites is affected by VeA [132,133,134,135]. Kato et al. [132] demonstrated that in A. nidulans, VeA regulates the expression of genes involved in sterigmatocystin synthesis. Indeed, VeA is necessary for the expression of aflR, which encodes the TF specific to the biosynthetic pathway of this mycotoxin [136]. Similarly, the veA gene is required for the transcription of aflR and aflJ, another gene coding for a TF, also located within the aflatoxin/sterigmatocystin cluster in A. flavus [137,138]. Other studies revealed that VeA is needed for the synthesis of other secondary metabolites, such as cyclopiazonic acid and aflatrem in A. flavus [133], penicillin in A. nidulans [132], or trichothecenes in Fusarium graminearum [139]. In this last study, FgVe1 was shown to be a positive regulator of the virulence of F. graminearum. In Fusarium verticillioides, FvVe1 is necessary not only for the production of fumonisins but also for the infection of corn plants by the fungus [140]. In P. chrysogenum, veA controls penicillin biosynthesis [141]. Recently, it was shown that the disruption of veA in P. expansum quasi-totally altered patulin and citrinin production when the fungus was grown on the usual mycological media (Malt Extract Agar and Potato Dextrose Agar). This decrease in production is explained by a drastic decrease in the expression of patulin and citrinin genes [142]. This finding was confirmed in vivo, as no patulin was detected when the null mutant was developed in apples. In the same study, an analysis of the impact of VeA on the expression of all secondary metabolism backbone genes in P. expansum was performed from the genome of the d1 strain, including PKS, NRPS, terpene synthase, and DMATS genes. The expression analysis showed a positive or negative regulation of 15/35 backbone genes and supports the hypothesis that P. expansum’s secondary metabolism is modulated by the transcriptional regulator factor VeA. In a recent study, Li et al. [143] assessed the involvement of the proteins VeA, VelB, VelC, and VosA, belonging to the velvet family, in the regulation of patulin biosynthesis in P. expansum. The absence of VeA and VelB blocked the production of the mycotoxin, whereas the absence of VelC caused a drastic decrease in patulin production. In contrast, deletion of the vosA gene had no effect on the capacity of the fungus to synthesize patulin. These findings suggest the lack of involvement of VosA in the biosynthesis of patulin in P. expansum in contrast to the other three proteins (VeA, VelB, and VelC) of the velvet complex.
Baba et al. [144] also showed through gene deletion that veA plays critical roles in the production of the hypocholesterolemic lovastatin analogue compactin (ML-236B) in P. citrinum by controlling the expression of mlcR, the pathway-specific activator gene for compactin biosynthesis.
It was also shown that different components of the velvet complex may play opposite roles in the regulation of secondary metabolism. In P. chrysogenum, PcVelC, together with the velvet PcVeA (orthologue of VeA in P. chrysogenum) and the methyltransferase PcLaeA, induced penicillin production, and, in contrast, PcVelB acted as a repressor [141,145].
Under the conditions tested by Kosalkova et al. [146], LaeA controlled some secondary metabolism gene clusters in P. chrysogenum. Its overexpression resulted in a four-fold increase in pcbC and penDE expression, leading to a 25% increase in gene expression in penicillin biosynthesis, while its suppression significantly reduced the expression of these genes. In contrast, the absence of an expression level difference (∆laeA vs. wild type (WT)) for the rpt gene involved in the second step of the roquefortine biosynthetic pathway suggests that PclaeA does not regulate the biosynthesis of roquefortine C. The regulation of the secondary metabolism of P. expansum by laeA was investigated from two cultures on different media [147]. Of the 54 backbone genes examined, many appeared to be positively regulated by laeA, such as those involved in the biosynthesis of roquefortine C, an unknown ETP-like metabolite, and patulin. In Penicillium oxalicum, it has been shown that the putative methyltransferase LaeA controls, among other things, the expression of some secondary metabolic gene clusters [148]. However, the cluster predicted to be involved in roquefortine C/ meleagrin/oxaline biosynthesis was not affected by the suppression of the laeA gene in P. oxalicum. The difference observed between these studies regarding laeA regulation of the genes involved in the biosynthesis of roquefortine C could be due to the species used and the medium tested.
Zhu et al. [149] demonstrated the role of laeA in secondary metabolism regulation, conidial production, and stress responses in P. digitatum. The deletion of PdlaeA resulted in decreased expression of various secondary metabolite gene clusters, including the Tq cluster involved in tryptoquialanine biosynthesis. Deletion of this gene also affected the expression of several regulators of conidiation, including BrlA. A comparison between the WT and the null mutant Pd∆laeA strains revealed increased sensitivity of the null mutant strain under alkaline conditions. The loss of PdlaeA had no significant effect on the virulence of the null mutant strain. This work showed the involvement of laeA in the biosynthesis of several secondary metabolites, as well as the development and the adaptation of P. digitatum to its environment.
Yu et al. [150] showed that the overexpression of LaeA in the Penicillium dipodomyis marine-derived strain YJ-11 leads not only to morphological but also metabolic changes. Overexpression mutants displayed the ability to produce several sorbicillinoids, two of which are new compounds, as well as four known sorbicillin analogues. These results indicate that LaeA plays a key role in the activation of cryptic genes that are silent under normal laeA expression.
Kumar et al. [151] showed the effects of the intrinsic factors of apples in modulating patulin accumulation and on laeA and pat gene expression in apples colonized by P. expansum. The authors used two apple varieties, Golden Delicious and Granny Smith, which have similar total soluble solid value profiles at the time of ripening but different pH values and malic acid concentrations. These factors differentially affected the expression of LaeA along with the expression of the patulin cluster genes and, therefore, patulin accumulation. To understand the complexity of these interactions, in vitro studies were performed. These studies proved that sucrose and malic acid concentrations and pH are all involved, in association with chlorogenic acid and epicatechin, in a complex interaction system that modulates the regulation and production of patulin.
Penicillium brocae HDN-12-143 is a fungus isolated from marine sediments that has strong potential for the biosynthesis of secondary metabolites. Wang et al. [152] studied the effect of overexpression of the laeA gene on the secondary metabolism of P. brocae. This overexpression revealed that four compounds could be isolated, including fumigatin chlorohydrin and a new polyketide compound, iso-fumigatin chlorohydrin. In summary, the results indicate that LaeA can suppress or activate the expression of gene clusters and that its overexpression can induce the production of new secondary metabolites.
In Aspergillus, VeA is responsible for the activation or repression of general genes such as brlA [134,153]. BrlA is a C2H2-type zinc finger TF which is part of the central regulatory pathway (CRP) controlling the expression of genes specific to asexual reproduction. The conformation of brlA is complicated and comprises two overlapping transcription units, brlAα and brlAβ [154]. Expression of the brlA gene was studied in P. oxalicum strains, initially identified as Penicillium decumbens, by Qin et al. [155], and the expression levels of 7/28 gene clusters of secondary metabolism were regulated in a ∆brlA deletion strain. The cluster involved in the roquefortine C/meleagrin/oxaline biosynthetic pathway was downregulated. In a P. chrysogenum brlA-deficient mutant, the production of penicillin V was not affected, whereas a reduction of almost 99% was determined via HPLC analysis accompanied by a drastic downregulation of the expression of penicillin biosynthetic genes in a stuA-deficient strain [156]. Moreover, the deletion of laeA reduced the conidiation in P. oxalicum, and the expression of brlA was downregulated [148]. A recent study in P. expansum showed that the brlA gene not only affects the stage of conidiation of the fungus but also affects the biosynthesis of secondary metabolites. Zetina-Serrano et al. [157] showed that the suppression of brlA results in a strain devoid of conidia and that the production of communesins and derivatives was drastically decreased, whereas the production of chaetoglobosins and derivatives increased. Neither patulin nor citrinin production was affected by the suppression of brlA.
PcRFX1 is a TF that was characterized in P. chrysogenum by Domínguez-Santos et al. [158]. PcRFX1 is the orthologue of the regulatory proteins CPCR1 and RFxA in Acremonium chrysogenum and T. marneffei, respectively. Knockdown and overexpression techniques of the Pcrfx1 gene have proven that PcRFX1 regulates pcbAB, pcbC, and penDE transcription and thereby controls penicillin biosynthesis. PcRFX1 was also suggested to be involved in the control of the pathways of primary metabolism.
PcFKH1, another TF of the winged-helix family, also positively regulates penicillin biosynthesis in P. chrysogenum by binding to the pcbC promoter, interacting with the promoter region of the penDE gene and controlling other genes such as phlA and ppt encoding phenylacetyl CoA ligase and phosphopantetheinyl transferase [159].
The pcz1 gene (Penicillium C6 zinc domain protein 1), encoding a Zn(II)2Cys6 protein and controlling the growth and development processes of the fungus, has also been described in P. roqueforti. It was suggested to participate in the physiological processes in this fungus and plays a key role in regulating its secondary metabolism [160,161]. The silencing of pcz1 in P. roqueforti resulted in the downregulation of the brlA, abaA, and wetA genes of the CRP [160]. In pcz1 downregulated strains, the production of the metabolites roquefortine C, andrastin A, and mycophenolic acid was severely reduced; however, when pcz1 was overexpressed, only mycophenolic acid was overproduced, and levels of roquefortine C and andrastin A were decreased [161].
Finally, the PoxnsdD gene of P. oxalicum was characterized by He et al. [162]. This gene is an orthologue of the nsdD gene (initially isolated in A. nidulans) encoding a GATA-type zinc finger TF that was proven to be involved in the production of secondary metabolites. In the PoxΔnsdD strain, the 230 differentially expressed genes identified covered 69 putative BGCs. Among them, 11 were predicted to produce aspyridone, emericellin, citrinin, leucinostatins, roquefortine C/meleagrin, beauvericin, cytochalasin, malbrancheamide, and viridicatumtoxin.
2.3. Signal Transduction Pathways
In general, fungi present a very dynamic and structured cell wall. During the cell cycle, organisms need to adapt quickly to changes under environmental conditions and imposed stresses and thus regulate the composition and structural organization of their cell wall [163,164,165]. All these factors influence the biosynthesis of secondary metabolites in the fungus. Numerous signaling pathways activate and regulate the growth and differentiation of filamentous fungi and initiate secondary metabolite biosynthesis under specific conditions. These signaling pathways sense and transduce signals external to TFs that, in turn, activate the expression of genes that could be involved in the biosynthesis of certain secondary metabolites. The cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA), calcineurin/calmodulin, TOR, and mitogen-activated protein kinase are the most studied pathways [34]. The production of many secondary metabolites has been associated with one of these transduction signals and specific active molecules. Among the different signaling pathways listed, we focus on those that affect only the secondary metabolism of Penicillium, starting with the cAMP pathway.
2.3.1. cAMP Pathways
Heterotrimeric G proteins are considered to be important components of these signal transduction pathways. They can integrate a variety of signals and then transduce them to downstream signaling cascades. Most filamentous fungi have three Gα proteins belonging to classes I, II, or III [166]. Gα subunits belonging to class I are involved in many aspects related not only to the development of the fungus or its pathogenicity but also its secondary metabolism, which is not the case for Gα subunits of classes II and III. The deletion of genes encoding class II Gα proteins showed negligible effects on fungal metabolism [167,168], while those of class III are involved in fungal development and pathogenicity [169,170,171]. Alterations have been observed in the secondary metabolism of different fungi, including P. chrysogenum [172] and T. marneffei [173]. The pga1 gene, encoding subunits of subgroup I Gα protein in P. chrysogenum, has been shown to affect the production of three secondary metabolites: penicillin, chrysogenin, and roquefortine C. The deletion of pga1 induces a decrease in the production of roquefortine C and penicillin by regulating the expression of pcbAB, pcbC, and penDE, the three structural biosynthetic genes of the penicillin cluster. Chrysogenin biosynthesis is enhanced, and roquefortine and penicillin biosynthesis is upregulated by the presence of a dominant activating pga1 (G42R) allele or a constitutively active Pga1 [172]. Based on a proteomic analysis, Carrasco-Navarro et al. [174] suggested that Pga1 signaling affects penicillin biosynthesis by acting on the primary metabolism pathways that are also involved in cysteine, ATP, and NADPH biosynthesis. They also propose a model for the Pga1-mediated signal transduction pathway.
2.3.2. The Osmostress Response Pathway
Usually, inhibition of the HOG (high osmolarity glycerol) signaling pathway negatively affects the production of metabolites; in other words, challenging osmotic conditions activate the cascade of the HOG MAP kinase signal, thereby activating several osmo-regulated genes or downstream TFs by phosphorylation. In their study, Stoll et al. [175] showed that NaCl induced production of OTA in correlation with the phosphorylation status of the HOG MAP kinase in P. nordicum and Penicillium verrucosum. The activation of HOG phosphorylation and the concomitant OTA biosynthesis suggest a link between the two processes and that this regulation may be mediated by the HOG MAP kinase signal transduction pathway. This was confirmed by inactivating the hog gene in P. verrucosum, making the fungus unable to produce OTA under high NaCl conditions. The biosynthesis of citrinin, another P. verrucosum toxin, was not affected. This could be explained by the subsequent work of Schmidt-Heydt et al. [176], which showed the impact of high oxidative stress conditions on citrinin biosynthesis. Indeed, by increasing Cu2+ concentrations in a growth medium, P. verrucosum shifts the biosynthesis of its secondary metabolism from OTA to citrinin. Increasing amounts of external cAMP reduce citrinin biosynthesis depending on the concentration chosen and suggest that citrinin biosynthesis is regulated by a cAMP/PKA signaling pathway.
2.4. Epigenetic Regulation
As previously discussed, Penicillium species, including other fungi, produce a set of bioactive secondary metabolites that are not essential to their survival. Genes for biosynthesis and the regulation of secondary metabolites in fungi are not evenly distributed over the genomes and tend to be sub-telomerically located [177]. The manipulation of global epigenetic regulators has contributed to the study of many unknown secondary metabolites, and many histone modifications have been associated with the regulation of secondary metabolism gene clusters [178,179]. The epigenetic phenomena that can occur are reversible, and many changes in the gene expression levels of the fungus do not alter the DNA sequence and can occur throughout the fungus life cycle. Fungal epigenetic regulation involves mainly histone modifications, such as methylation, acetylation, and sumoylation. Histone proteins are the primary protein components of chromatin and, through their modifications, regulation can be limited to a specific region of the chromosome and, therefore, affect some genes. This supports the advantage of grouping secondary metabolism genes into clusters. The first involvement of the epigenetic regulation of secondary metabolites described in the literature was that of the A. nidulans histone deacetylase coded by hdaA, an orthologue of the histone deacetylase hdaA1 gene of S. cerevisiae. Deletion of this gene caused the activation of two secondary metabolite gene clusters. In the same paper, treatment of the P. expansum culture with trichostatin A, a histone deacetylase (HDAC) inhibitor, resulted in the overproduction of several non-determined metabolites [179].
In P. chrysogenum, hdaA appears to be a key regulator of the secondary metabolism of the fungus. Deletion of hdaA induced a significant effect on the expression of numerous PKS and NRPS-encoding genes. A downregulation of the NRPS encoded gene associated with the BGC of chrysogine was also observed. This observation was confirmed by Ding et al. [180]. In parallel, transcriptional activation of the BGC of sorbicillinoids occurs, which is associated with the detection of a new compound produced only under these conditions. These results obtained by Guzman-Chavez et al. [181] suggest the existence of crosstalk between BGCs. In a recent study, the disruption of hdaA led to an upregulation of the meleagrin/roquefortine C biosynthesis gene cluster, accompanied by higher meleagrin production [180].
Akiyama et al. [182] investigated the involvement of clr3 in Penicillium brasilianum physiology. Clr3 is a homologue of the class 2 histone deacetylase hda1 in S. cerevisiae. On the one hand, the deletion of clr3 resulted in decreased fungal growth under oxidative stress conditions. In addition, various secondary metabolites, such as austin-related meroterpenoids, brasiliamides, cyclodepsipeptides, and mycotoxins, including verruculogen and penicillic acid, were downregulated in the null mutant ∆clr3 strain. On the other hand, epigenetic modulation was studied using suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, and nicotinamide. These treatments also resulted in reduced secondary metabolite biosynthesis. Together, these findings suggest that clr3 plays a key role in the regulation of secondary metabolism in P. brasilianum.
By growing Penicillium variabile on a maltose medium in the presence of 5-azacytidine (a DNA methyltransferase inhibitor), varitatin A synthesis was induced [183]. In addition, by growing it on a potato-based medium in the presence of SAHA, seven polyketides were induced, including three known wortmannilactones (E, F, and H), as well as new varilactones (A-B) and wortmannilactones (M-N) [184]. In cultures treated with 50 μM of 5-azacytidine, Penicillium citreonigrum formed exudates, which are droplets rich in primary and secondary metabolites, inorganic substances, and proteins/enzymes and are known as guttates. These exudates were very rich in different compounds compared to the control. Indeed, 5-azacytidine induced the formation of six azaphilones (fungal metabolites with diverse biological activities), pencolide, and two new meroterpenes [185]. The addition of 5-azacytidine to the culture medium of Penicillium funiculosum also altered the metabolic profiles of this fungus [186]. Two new prenyleudesmane diterpenoids were extracted from the culture and exhibited cytotoxic and antibacterial activities. Eupenicillium sp. LG41, an endophytic fungus, was exposed to an epigenetic modulation using nicotinamide, a NAD+-dependent HDAC inhibitor [187]. This led to the production of many compounds: eupenicinicols C and D, along with eujavanicol A and eupenicinicol A. El-Hawary et al. [188] showed that cultures of a marine-derived strain of Penicillium brevicompactum exposed to nicotinamide and sodium butyrate result in the production of phenolic metabolites. In the presence of nicotinamide, many compounds, including p-anisic acid, benzyl anisate, syringic acid, and sinapic acid, were isolated and identified. Sodium butyrate also enhanced the production of anthranilic acid and ergosterol peroxide.
In one of the many studies to explore compounds with innovative structures and biological activities from endophytes of ancestral Chinese medicine, Guo et al. [189] used chemical epigenetic manipulation to evaluate the secondary metabolism of the Penicillium herquei strain, recovered from the fruiting body of Cordyceps sinensis. This latter has been used for thousands of years by the Chinese to boost longevity, endurance, and vitality. The DNA methyltransferase inhibitor, 5-aza-2-deoxycytidine, affected the production of secondary metabolites, purifying three previously unpublished polyketides with a pyran-2-one scaffold.
Ying et al. [190] showed that cultures of Penicillium sp. HS-11, isolated from the medicinal plant Huperzia serrata, produced two compounds in the presence of SAHA: 4-epipenicillone B and (R)-(+)-chrysogine, which are both absent under normal laboratory conditions.
The addition of 500 μM of suberoyl bis-hydroxamic acid, a Zn(II)-type or NAD+-dependent HDAC inhibitor, and 100 μM of nicotinamide (an NAD+-dependent HDAC inhibitor) to a culture of Penicillium sp. isolated from leaves of Catharanthus roseus improved the production of citreoviripyrone A and citreomontanin. In addition, nicotinamide enhanced the production of (−)-citreoviridin [191]. Xiong et al. [192] explored the role of the high-mobility group box protein, PoxHmbB, involved in chromatin organization and identified in P. oxalicum. The authors observed that conidiation and hyphae growth were delayed in a mutant PoxΔhmbB strain. PoxhmbB regulated the expression of genes encoding plant biomass-degrading enzymes and other genes involved in conidiation. Although the suppression of the orthologous gene resulted in an absence of sterigmatocystin production in A. nidulans [193], the involvement of this protein in the secondary metabolism of Penicillium has not yet been investigated.
Tannous et al. [194] evaluated the involvement of the epigenetic reader SntB in the pathogenicity and secondary metabolism of P. expansum. Firstly, the results showed that the deletion of sntB caused numerous phenotypic changes in the plant pathogen. In the absence of sntB, P. expansum showed delayed vegetative growth, reduced conidiation, an accelerated germination rate, and decreased virulence in apples. Secondly, the data showed that sntB played a key role in regulating secondary metabolism, especially patulin and citrinin biosynthesis. In addition, the role of sntB in the positive regulation of three TFs of secondary metabolism and virulence (LaeA, CreA, and PacC) was demonstrated. Finally, this study revealed the downregulation of sntB in response to environmental factors such as low temperature and high CO2 levels, conditions to which apples are subjected during storage. These findings suggest a possible method for integrating these epigenetic control strategies to fight post-harvest fruit rot.
Finally, the chromatin regulation of small molecule gene clusters allowed the specific control of secondary metabolism gene clusters and permitted filamentous fungi to modify chemical diversity and successfully exploit environmental resources. Epigenetic regulation is considered a promising strategy for investigating unknown secondary metabolite clusters, particularly because under certain laboratory culture conditions, many clusters can remain silent, making it difficult to elucidate their functions and regulatory mechanisms [195].
3. Conclusions
Fungal secondary metabolism is very broad, and this review focused on metabolism regulated in the Penicillium genus. Given the diversity of secondary metabolites, their key roles as virulence and pathogenicity factors, and their great medical and agricultural interest, further research should be conducted on these metabolites. This review highlighted how the production of these secondary metabolites is controlled and regulated. It discussed the different levels of regulation of secondary metabolites, including specific regulators, global TFs, transduction signaling pathways, and epigenetic regulation, as well as the combination of many different parameters affecting the biosynthesis pathways of metabolites. Many TFs that affect the expression of genes involved in secondary metabolism seem to belong to the category of zinc-binding proteins. LaeA and the velvet complex proteins are considered to be global regulators and are able to control many clusters at the same time. Although much is known about these global TFs and their regulatory proteins, more research is needed to explore the details that link them to the transcription of genes involved in secondary metabolite biosynthetic pathways. This would help us to better understand the molecular mechanisms underlying this complex regulatory network. The analysis of a large number of works related to secondary metabolism regulation in filamentous fungi revealed great complexity. This complexity is suggested by the observation of inter-species differences in the impact of a given TF gene deletion on the same biosynthetic pathway.
The study of the regulation of secondary metabolite biosynthesis in Penicillium is much less advanced than that in Aspergillus, and some orthologous genes already studied in Aspergillus (including rtfA, cpsA, rmtA, mtfA) should be investigated in Penicillium sp.
Acknowledgments
We thank Selma P. Snini of the University of Toulouse (Toulouse, France) for Figure 4.
Abbreviations
| BGC | Biosynthetic gene cluster |
| bZIP | Basic leucine zipper |
| CRP | Central regulatory pathway |
| DMATS | Dimethylallyl tryptophan synthase |
| ETP | Epipolythiodioxopiperazine |
| HDAC | Histone deacetylase |
| HOG | High osmolarity glycerol |
| NLS | Nuclear localization sequence |
| NRPS | Non-ribosomal peptide synthetase |
| OTA | Ochratoxin A |
| PKS | Polyketide synthase |
| SAHA | Suberoylanilide hydroxamic acid |
| TAP | Tandem Affinity Purification |
| TC | Terpene cyclase |
| TF | Transcription factor |
| WT | Wild Type |
Author Contributions
Writing—original draft preparation, C.E.H.A., C.Z.-S., N.T., S.L., and O.P.; writing—review and editing, A.E.K., A.A., I.P.O., S.L., and O.P. All authors have read and agreed to the published version of the manuscript.
Funding
C.H.A. was supported by a doctoral fellowship funded by the Belgian Research Institute for Agriculture, Fisheries and Food and la Région Occitanie (France) under Grant 15050427. C.Z.-S. was supported by a doctoral fellowship funded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) México, grant number CVU CONACYT 623107. N.T. was supported by a doctoral fellowship funded by The National Council for Scientific Research of Lebanon (CNRS-L). This research was funded by CASDAR AAP RT 2015, grant number 1508, by French National Research Agency, grant numbers ANR-15-CE21-0010-01 NEWMYCO and ANR-17-CE21-0008 PATRISK, and by International Cooperation Program CAPES/COFECUB (project number Sv 947/19).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawksworth D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991;95:641–655. doi: 10.1016/S0953-7562(09)80810-1. [DOI] [Google Scholar]
- 2.Hawksworth D.L., Lücking R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectrum. 2017;5:FUNK-0052-2016. doi: 10.1128/microbiolspec.funk-0052-2016. [DOI] [PubMed] [Google Scholar]
- 3.Cho H.S., Hong S.B., Go S.J. First report of Penicillium brasilianum and P. daleae isolated from soil in Korea. Mycobiology. 2005;33:113–117. doi: 10.4489/MYCO.2005.33.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazioli J.M., Amaral L.D.S., Fill T.P., Rodrigues-Filho E. Insights into Penicillium brasilianum secondary metabolism and its biotechnological potential. Molecules. 2017;22:858–880. doi: 10.3390/molecules22060858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houbraken J., Kocsubé S., Visagie C.M., Yilmaz N., Wang X.C., Meijer M., Kraak B., Hubka V., Samson R.A., Frisvad J.C. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020;95:5–169. doi: 10.1016/j.simyco.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium: A guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–174. [Google Scholar]
- 7.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. Springer; Berlin/Heidelberg, Germany: 2009. Fungi and food spoilage; pp. 243–245. [Google Scholar]
- 8.Samson R.A., Houbraken J., Thrane U., Frisvad J.C., Andersen B. Food and Indoor Fungi. CBS-KNAW; Utrecht, The Netherlands: 2010. 390p [Google Scholar]
- 9.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Mycology. 2004;49:201–241. [Google Scholar]
- 10.Chain E., Florey H.W., Gardner A.D., Heatley N.G., Jennings M.A., Orr-Ewing J., Sanders A.G. Penicillin as a chemotherapeutic agent. Lancet. 1940;236:226–228. doi: 10.1016/S0140-6736(01)08728-1. [DOI] [Google Scholar]
- 11.Abraham E.P., Gardner A.D., Chain E., Heatley N.G., Fletcher C.M., Jennings M.A., Florey H.W., Adelaide M.B. Further observations on penicillin. Lancet. 1941;238:177–189. doi: 10.1016/S0140-6736(00)72122-2. [DOI] [PubMed] [Google Scholar]
- 12.Thom C. Mycology presents penicillin. Mycol. Soc. Am. 1945;37:460–475. doi: 10.1080/00275514.1945.12024006. [DOI] [Google Scholar]
- 13.Rančić A., Soković M., Karioti A., Vukojević J., Skaltsa H. Isolation and structural elucidation of two secondary metabolites from the filamentous fungus Penicillium ochrochloron with antimicrobial activity. Environ. Toxicol. Pharmacol. 2006;22:80–84. doi: 10.1016/j.etap.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Lucas E.M.F., De Castro M.C.M., Takahashi J.A. Antimicrobial properties of sclerotiorin, isochromophilone VI and pencolide, metabolites from a brazilian cerrado isolate of Penicillium sclerotiorum van Beyma. Braz. J. Microbiol. 2007;38:785–789. doi: 10.1590/S1517-83822007000400036. [DOI] [Google Scholar]
- 15.Nicoletti R., Lopez-Gresa M.P., Manzo E., Carella A., Ciavatta M.L. Production and fungitoxic activity of Sch 642305, a secondary metabolite of Penicillium canescens. Mycopathologia. 2007;163:295–301. doi: 10.1007/s11046-007-9015-x. [DOI] [PubMed] [Google Scholar]
- 16.Göhrt A., Zeeck A. Secondary metabolites by chemical screening. 9 decarestrictines, a new family of inhibitors of cholesterol biosynthesis from Penicillium. J. Antibiot. 1992;45:66–73. doi: 10.7164/antibiotics.45.66. [DOI] [PubMed] [Google Scholar]
- 17.Oswald I.P., Coméra C. Immunotoxicity of mycotoxins. Rev. Méd. Vét. 1998;149:585–590. [Google Scholar]
- 18.Rho M.C., Lee H.S., Chang K.T., Song H.Y., Kwon O.E., Lee S.W., Ko J.S., Hong S.G., Kim Y. K Phenylpyropene C, a new inhibitor of Acyl-CoA: Cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 2002;55:211–214. doi: 10.7164/antibiotics.55.211. [DOI] [PubMed] [Google Scholar]
- 19.Kwon O.E., Rho M.C., Song H.Y., Lee S.W., Chung M.Y., Lee J.H., Kim Y.H., Lee H.S., Kim Y.K. Phenylpyropene A and B, new inhibitors of Acyl-CoA: Cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 2002;55:1004–1008. doi: 10.7164/antibiotics.55.1004. [DOI] [PubMed] [Google Scholar]
- 20.Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. Influenzae. Br. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. [DOI] [Google Scholar]
- 21.Geisen R. Molecular monitoring of environmental conditions influencing the induction of ochratoxin A biosynthesis genes in Penicillium nordicum. Mol. Nutr. Food Res. 2004;48:532–540. doi: 10.1002/mnfr.200400036. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Heydt M., Geisen R. A microarray for monitoring the production of mycotoxins in food. Int. J. Food Microbiol. 2007;17:131–140. doi: 10.1016/j.ijfoodmicro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Heydt M., Magan N., Geisen R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008;284:142–149. doi: 10.1111/j.1574-6968.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 24.Khan A.A., Bacha N., Ahmad B., Lutfullah G., Farooq U., Cox R.J. Fungi as chemical industries and genetic engineering for the production of biologically active secondary metabolites. Asian Pac. J. Trop. Biomed. 2014;4:859–870. doi: 10.12980/APJTB.4.2014APJTB-2014-0230. [DOI] [Google Scholar]
- 25.Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism - From biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 26.Boettger D., Hertweck C. Molecular diversity sculpted by fungal PKS-NRPS hybrids. ChemBioChem. 2013;14:28–42. doi: 10.1002/cbic.201200624. [DOI] [PubMed] [Google Scholar]
- 27.Tsunematsu Y., Ishikawa N., Wakana D., Goda Y., Noguchi H., Moriya H., Hotta K., Watanabe K. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat. Chem. Biol. 2013;9:818–825. doi: 10.1038/nchembio.1366. [DOI] [PubMed] [Google Scholar]
- 28.Osbourn A. Secondary metabolic gene clusters: Evolutionary toolkits for chemical innovation. Trends Genet. 2010;26:449–457. doi: 10.1016/j.tig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Keller N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019;17:167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reverberi M., Ricelli A., Zjalic S., Fabbri A.A., Fanelli C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010;87:899–911. doi: 10.1007/s00253-010-2657-5. [DOI] [PubMed] [Google Scholar]
- 31.Tudzynski B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014;5:656. doi: 10.3389/fmicb.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merhej J., Richard-Forget F., Barreau C. Regulation of trichothecene biosynthesis in Fusarium: Recent advances and new insights. Appl. Microbiol. Biotechnol. 2011;91:519–528. doi: 10.1007/s00253-011-3397-x. [DOI] [PubMed] [Google Scholar]
- 33.Brakhage A.A., Spröte P., Al-Abdallah Q., Gehrke A., Plattner H., Tüncher A. Regulation of penicillin biosynthesis in filamentous fungi. Adv. Biochem. Engineer. Biotechnol. 2004;88:45–90. doi: 10.1007/b99257. [DOI] [PubMed] [Google Scholar]
- 34.Macheleidt J., Mattern D.J., Fischer J., Netzker T., Weber J., Schroeckh V., Valiante V., Brakhage A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016;50:371–392. doi: 10.1146/annurev-genet-120215-035203. [DOI] [PubMed] [Google Scholar]
- 35.Alkhayyat F., Yu J.H. Upstream regulation of mycotoxin biosynthesis. Adv. Appl. Microbiol. 2014;86:251–278. doi: 10.1016/B978-0-12-800262-9.00005-6. [DOI] [PubMed] [Google Scholar]
- 36.Lee M.K., Kwon N.J., Lee I.S., Jung S., Kim S.C., Yu J.H. Negative regulation and developmental competence in Aspergillus. Sci. Rep. 2016;6:28874. doi: 10.1038/srep28874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lind A.L., Smith T.D., Saterlee T., Calvo A.M., Rokas A. Regulation of secondary metabolism by the velvet complex is temperature-responsive in Aspergillus. G3. 2016;6:4023–4033. doi: 10.1534/g3.116.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfannenstiel B.T., Keller N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019;37:107345. doi: 10.1016/j.biotechadv.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannous J., El Khoury R., Snini S.P., Lippi Y., El Khoury A., Atoui A., Lteif R., Oswald I.P., Puel O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014;189:51–60. doi: 10.1016/j.ijfoodmicro.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Ballester A.R., Marcet-Houben M., Levin E., Sela N., Selma-Lázaro C., Carmona L., Wisniewski M., Droby S., González-Candelas L., Gabaldón T. Genome, transcriptome, and functional analyses of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol. Plant. Microbe Interact. 2015;28:232–248. doi: 10.1094/MPMI-09-14-0261-FI. [DOI] [PubMed] [Google Scholar]
- 41.He Y., Cox R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016;7:2119–2127. doi: 10.1039/C5SC04027B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grijseels S., Pohl C., Nielsen J.C., Wasil Z., Nygård Y., Frisvad J.C., Nielsen K.F., Workman M., Larsen T.O., Driessen A.J.M., et al. Identification of the decumbenone biosynthetic gene cluster in Penicillium decumbens and the importance for production of calbistrin. Fungal Biol. Biotechnol. 2018;5:18. doi: 10.1186/s40694-018-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todd R.B., Andrianopoulos A. Evolution of a fungal regulatory gene family: The Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet. Biol. 1997;21:388–405. doi: 10.1006/fgbi.1997.0993. [DOI] [PubMed] [Google Scholar]
- 44.MacPherson S., Larochelle M., Turcotte B. A fungal family of transcriptional regulators: The zinc cluster proteins. Microbiol. Mol. Biol. Rev. 2006;70:583–604. doi: 10.1128/MMBR.00015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brakhage A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- 46.Yin W., Keller N.P. Transcriptional regulatory elements in fungal secondary metabolism. J. Microbiol. 2011;49:329–339. doi: 10.1007/s12275-011-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bechet J., Greenson M., Wiame J.M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur. J. Biochem. 1970;12:40–47. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 48.Messenguy F., Dubois E. The yeast ARGRII regulatory protein has homology with various RNases and DNA binding proteins. Mol. Gen. Genet. 1988;211:102–105. doi: 10.1007/BF00338399. [DOI] [PubMed] [Google Scholar]
- 49.Strich R., Surosky R.T., Steber C., Dubois E., Messenguy F., Esposito R.E. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994;8:796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- 50.Rubin-Bejerano I., Mandel S., Robzyk K., Kassir Y. Induction of meiosis in Saccharomyces cerevisiae depends on conversion of the transcriptional represssor Ume6 to a positive regulator by its regulated association with the transcriptional activator Ime1. Mol. Cell Biol. 1996;16:2518–2526. doi: 10.1128/MCB.16.5.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown D.W., Yu J.H., Kelkar H.S., Fernandes M., Nesbitt T.C., Keller N.P., Adams T.H., Leonard T.J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang P.K., Bhatnagar D., Cleveland T.E., Bennett J.W. Sequence variability in homologs of the aflatoxin pathway gene aflR distinguishes species in Aspergillus section Flavi. Appl. Environ. Microbiol. 1995;61:40–43. doi: 10.1128/AEM.61.1.40-43.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrlich K.C., Montalbano B.G., Cary J.W. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene. 1999;230:249–257. doi: 10.1016/S0378-1119(99)00075-X. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes M., Keller N.P., Adams T.H. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 1998;28:1355–1365. doi: 10.1046/j.1365-2958.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 55.Yu J.H., Butchko R.A.E., Fernandes M., Keller N.P., Leonard T.J., Adams T.H. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
- 56.Lebar M.D., Cary J.W., Majumdar R., Carter-Wientjes C.H., Mack B.M., Wei Q., Uka V., De Saeger S., Diana Di Mavungu J. Identification and functional analysis of the aspergillic acid gene cluster in Aspergillus flavus. Fungal Genet. Biol. 2018;116:14–23. doi: 10.1016/j.fgb.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Bok J.W., Chung D.W., Balajee S.A., Marr K.A., Andes D., Nielsen K.F., Frisvad J.C., Kirby K.A., Keller N.P. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox E.M., Gardiner D.M., Keller N.P., Howlett B.J. A Zn(II)2Cys6 DNA binding protein regulates the sirodesmin PL biosynthetic gene cluster in Leptosphaeria maculans. Fungal Genet. Biol. 2008;45:671–682. doi: 10.1016/j.fgb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim F.Y., Won T.H., Raffa N., Baccile J.A., Wisecaver J., Rokas A., Schroeder F.C., Keller N.P. Fungal isocyanide synthases and xanthocillin biosynthesis in Aspergillus fumigatus. MBio. 2018;9:e00785-18. doi: 10.1128/mBio.00785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiemann P., Guo C.-J., Palmer J.M., Sekonyela R., Wang C.C.C., Keller N.P. Prototype of an intertwined secondary metabolite supercluster. Proc. Natl. Acad. Sci. USA. 2013;110:17065–17070. doi: 10.1073/pnas.1313258110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J.E., Son H., Lee Y.W. Biosynthetic mechanism and regulation of zearalenone in Fusarium graminearum. JSM Mycotoxins. 2018;68:1–6. doi: 10.2520/myco.68-1-2. [DOI] [Google Scholar]
- 62.Yang X., Feng P., Yin Y., Bushley K., Spatafora J.W., Wang C. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. MBio. 2018;9:e01211-18. doi: 10.1128/mBio.01211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Wang L., Wu F., Liu F., Wang Q., Zhang X., Selvaraj J.N., Zhao Y., Xing F., Yin W.-B., et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018;84:e01009-18. doi: 10.1128/AEM.01009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abe Y., Suzuki T., Ono C., Iwamoto K., Hosobuchi M., Yoshikawa H. Molecular cloning and characterization of an ML-236B (compactin) biosynthetic gene cluster in Penicillium citrinum. Mol. Genet. Genomics. 2002;267:636–646. doi: 10.1007/s00438-002-0697-y. [DOI] [PubMed] [Google Scholar]
- 65.Wiemann P., Willmann A., Straeten M., Kleigrewe K., Beyer M., Humpf H.U., Tudzynski B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009;72:931–946. doi: 10.1111/j.1365-2958.2009.06695.x. [DOI] [PubMed] [Google Scholar]
- 66.Wight W.D., Kim K.H., Lawrence C.B., Walton J.D. Biosynthesis and role in virulence of the histone deacetylase inhibitor depudecin from Alternaria brassicicola. Mol. Plant-Microbe Interact. 2009;22:1258–1267. doi: 10.1094/MPMI-22-10-1258. [DOI] [PubMed] [Google Scholar]
- 67.Brown D.W., Butchko R.A.E., Busman M., Proctor R.H. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot. Cell. 2007;6:1210–1218. doi: 10.1128/EC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flaherty J.E., Woloshuk C.P. Regulation of fumonisin biosynthesis in Fusarium verticillioides by a zinc binuclear cluster-type gene, ZFR1. Appl. Environ. Microbiol. 2004;70:2653–2659. doi: 10.1128/AEM.70.5.2653-2659.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H., Lee M.H., Daub M.E., Chung K.R. Molecular analysis of the cercosporin biosynthetic gene cluster in Cercospora nicotianae. Mol. Microbiol. 2007;64:755–770. doi: 10.1111/j.1365-2958.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- 70.Kim J.E., Jin J., Kim H., Kim J.C., Yun S.H., Lee Y.W. GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl. Environ. Microbiol. 2006;72:1645–1652. doi: 10.1128/AEM.72.2.1645-1652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu T., Kinoshita H., Nihira T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007;73:5097–5103. doi: 10.1128/AEM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X., Li H.M. Cloning and bioinformatic analysis of lovastatin biosynthesis regulatory gene lovE. Chin. Med. J. 2009;122:1800–1805. doi: 10.3760/cma.j.issn.0366-6999.2009.15.016. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy J., Auclair K., Kendrew S.G., Park C., Vederas J.C., Hutchinson C.R. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science. 1999;284:1368–1372. doi: 10.1126/science.284.5418.1368. [DOI] [PubMed] [Google Scholar]
- 74.Bergmann S., Schümann J., Scherlach K., Lange C., Brakhage A.A., Hertweck C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007;3:213–217. doi: 10.1038/nchembio869. [DOI] [PubMed] [Google Scholar]
- 75.Chiang Y.M., Szewczyk E., Davidson A.D., Keller N., Oakley B.R., Wang C.C.C. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. J. Am. Chem. Soc. 2009;131:2965–2970. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiang Y.M., Szewczyk E., Davidson A.D., Entwistle R., Keller N.P., Wang C.C.C., Oakley B. Characterization of the Aspergillus nidulans monodictyphenone gene cluster. Appl. Environ. Microbiol. 2010;76:2067–2074. doi: 10.1128/AEM.02187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuji G., Kenmochi Y., Takano Y., Sweigard J., Farrall L., Furusawa I., Horino O., Kubo Y. Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and Pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol. Microbiol. 2000;38:940–954. doi: 10.1046/j.1365-2958.2000.02181.x. [DOI] [PubMed] [Google Scholar]
- 78.Valente S., Cometto A., Piombo E., Meloni G.R., Ballester A.R., González-Candelas L., Spadaro D. Elaborated regulation of griseofulvin biosynthesis in Penicillium griseofulvum and its role on conidiation and virulence. Int. J. Food Microbiol. 2020;328:108687. doi: 10.1016/j.ijfoodmicro.2020.108687. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y.I.P., Yuan G.F., Hsieh S.Y., Lin Y.U.S., Wang W.Y.I., Liaw L.I.L., Tseng C.P. Identification of the mokh gene encoding transcription factor for the upregulation of monacolin k biosynthesis in Monascus pilosus. J. Agric. Food Chem. 2010;58:287–293. doi: 10.1021/jf903139x. [DOI] [PubMed] [Google Scholar]
- 80.Lin H.C., Chiou G., Chooi Y.H., McMahon T.C., Xu W., Garg N.K., Tang Y. Elucidation of the concise biosynthetic pathway of the communesin indole alkaloids. Angew. Chem. 2015;54:3004–3007. doi: 10.1002/anie.201411297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan D., Jamieson C.S., Ohashi M., Tang M.C., Houk K.N., Tang Y. Genome-mined Diels-Alderase catalyzes formation of the cis-octahydrodecalins of varicidin A and B. J. Am. Chem. Soc. 2019;141:769–773. doi: 10.1021/jacs.8b12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hidalgo P.I., Ullán R.V., Albillos S.M., Montero O., Fernández-Bodega M.Á., García-Estrada C., Fernández-Aguado M., Martín J.F. Molecular characterization of the PR-toxin gene cluster in Penicillium roqueforti and Penicillium chrysogenum: Cross talk of secondary metabolite pathways. Fungal Genet. Biol. 2014;62:11–24. doi: 10.1016/j.fgb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Hidalgo P.I., Poirier E., Ullán R.V., Piqueras J., Meslet-Cladière L., Coton E., Coton M. Penicillium roqueforti PR toxin gene cluster characterization. Appl. Microbiol. Biotechnol. 2017;101:2043–2056. doi: 10.1007/s00253-016-7995-5. [DOI] [PubMed] [Google Scholar]
- 84.Tang M.C., Cui X., He X., Ding Z., Zhu T., Tang Y., Li D. Late-stage terpene cyclization by an integral membrane cyclase in the biosynthesis of isoprenoid epoxycyclohexenone natural products. Org. Lett. 2017;19:5376–5379. doi: 10.1021/acs.orglett.7b02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snini S.P., Tannous J., Heuillard P., Bailly S., Lippi Y., Zehraoui E., Barreau C., Oswald I.P., Puel O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant. Pathol. 2016;17:920–930. doi: 10.1111/mpp.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guzmán-Chávez F., Salo O., Nygård Y., Lankhorst P.P., Bovenberg R.A.L., Driessen A.J.M. Mechanism and regulation of sorbicillin biosynthesis by Penicillium chrysogenum. Microb. Biotechnol. 2017;10:958–968. doi: 10.1111/1751-7915.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao X., Chooi Y.H., Ames B.D., Wang P., Walsh C.T., Tang Y. Fungal indole alkaloid biosynthesis: Genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 2011;133:2729–2741. doi: 10.1021/ja1101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marcet-Houben M., Ballester A.R., de la Fuente B., Harries E., Marcos J.F., González-Candelas L., Gabaldón T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genomics. 2012;13:646. doi: 10.1186/1471-2164-13-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim W., Park J.J., Gang D.R., Peever T.L., Chena W. A novel type pathway-specific regulator and dynamic genome environments of a solanapyrone biosynthesis gene cluster in the fungus Ascochyta rabiei. Eukaryot. Cell. 2015;14:1102–1113. doi: 10.1128/EC.00084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang F., Abdelnabby H., Xiao Y. The Zn(II)2Cys6 putative transcription factor is involved in the regulation of leucinostatin production and pathogenicity of the nematophagous fungus Paecilomyces lilacinus. Can. J. Plant. Pathol. 2015;3:342–352. doi: 10.1080/07060661.2015.1065437. [DOI] [Google Scholar]
- 91.Gardiner D.M., Waring P., Howlett B.J. The epipolythiodioxopiperazine (ETP) class of fungal toxins: Distribution, mode of action, functions and biosynthesis. Microbiology. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- 92.Waring P., Eichner R.D., Tiwari-Palni U., Müllbacher A. Gliotoxin-E: A new biologically active epipolythiodioxopiperazine isolated from Penicillium terlikowskii. Aust. J. Chem. 1987;40:991–997. doi: 10.1071/CH9870991. [DOI] [Google Scholar]
- 93.Patron N.J., Waller R.F., Cozijnsen A.J., Straney D.C., Gardiner D.M., Nierman W.C., Howlett B.J. Origin and distribution of epipolythiodioxopiperazine (ETP) gene clusters in filamentous ascomycetes. BMC Evol. Biol. 2007;7:174–188. doi: 10.1186/1471-2148-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cramer R.A., Gamcsik M.P., Brooking R.M., Najvar L.K., Kirkpatrick W.R., Patterson T.F., Balibar C.J., Graybill J.R., Perfect J.R., Abraham S.N., et al. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell. 2006;5:972–980. doi: 10.1128/EC.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schoberle T.J., Nguyen-Coleman C.K., Herold J., Yang A., Weirauch M., Hughes T.R., McMurray J.S., May G.S. A novel C2H2 transcription factor that regulates gliA expression interdependently with GliZ in Aspergillus fumigatus. PLoS Genet. 2014;10:e1004336. doi: 10.1371/journal.pgen.1004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abe Y., Ono C., Hosobuchi M., Yoshikawa H. Functional analysis of mlcR, a regulatory gene for ML-236B (compactin) biosynthesis in Penicillium citrinum. Mol. Genet. Genomics. 2002;268:352–361. doi: 10.1007/s00438-002-0755-5. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen J.C., Grijseels S., Prigent S., Ji B., Dainat J., Nielsen K.F., Frisvad J.C., Workman M., Nielsen J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017;2:17044. doi: 10.1038/nmicrobiol.2017.44. [DOI] [PubMed] [Google Scholar]
- 98.Artigot M.P., Loiseau N., Laffitte J., Mas-Reguieg L., Tadrist S., Oswald I.P., Puel O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology. 2009;155:1738–1747. doi: 10.1099/mic.0.024836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim Y.T., Lee Y.R., Jin J., Han K.H., Kim H., Kim J.C., Lee T., Yun S.H., Lee Y.W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol. Microbiol. 2005;58:1102–1113. doi: 10.1111/j.1365-2958.2005.04884.x. [DOI] [PubMed] [Google Scholar]
- 100.Cepeda-García C., Domínguez-Santos R., García-Rico R.O., García-Estrada C., Cajiao A., Fierro F., Martín J.F. Direct involvement of the CreA transcription factor in penicillin biosynthesis and expression of the pcbAB gene in Penicillium chrysogenum. Appl. Genet. Mol. Biotechnol. 2014;98:7113–7124. doi: 10.1007/s00253-014-5760-1. [DOI] [PubMed] [Google Scholar]
- 101.Tannous J., Kumar D., Sela N., Sionov E., Prusky D., Keller N.P. Fungal attack and host defence pathways unveiled in near-avirulent interactions of Penicillium expansum creA mutants on apples. Mol. Plant. Pathol. 2018;19:2635–2650. doi: 10.1111/mpp.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng B., Friedlin E., Marzluf G.A. A reporter gene analysis of penicillin biosynthesis gene expression in Penicillium chrysogenum and its regulation by nitrogen and glucose catabolite repression. Appl. Environ. Microbiol. 1994;60:4432–4439. doi: 10.1128/AEM.60.12.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ellis. C.M. Ph.D. Thesis. University of Calgary; Calgary, AB, Canada: 1996. Regulation of Polyketide Gene Expression: The Isolation and Function of Nitrogen Regulatory Factor NRFA from Penicillium urticae. [DOI] [Google Scholar]
- 104.Fedeshko R.W. Ph.D. Thesis. University of Calgary; Calgary, AB, Canada: 1992. Polyketide Enzymes and Genes. [DOI] [Google Scholar]
- 105.Rollins M.J., Gaucher G.M. Ammonium repression of antibiotic and intracellular proteinase production in Penicillium urticae. Appl. Microbiol. Biotechnol. 1994;41:447–455. doi: 10.1007/BF00212256. [DOI] [PubMed] [Google Scholar]
- 106.Haas H., Marzluf G.A. NRE, the major nitrogen regulatory protein of Penicillium chrysogenum, binds specifically to elements in the intergenic promoter regions of nitrate assimilation and penicillin biosynthetic gene clusters. Curr. Genet. 1995;28:177–183. doi: 10.1007/BF00315785. [DOI] [PubMed] [Google Scholar]
- 107.Martín J.F. Molecular control of expression of penicillin biosynthesis genes in fungi: Regulatory proteins interact with a bidirectional promoter region. J. Bacteriol. 2000;182:2355–2362. doi: 10.1128/JB.182.9.2355-2362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gente S., Poussereau N., Fèvre M. Isolation and expression of a nitrogen regulatory gene, nmc, of Penicillium roqueforti. FEMS Microbiol. Lett. 1999;175:291–297. doi: 10.1111/j.1574-6968.1999.tb13633.x. [DOI] [PubMed] [Google Scholar]
- 109.Peñalva M.A., Arst H.N. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 2004;58:425–451. doi: 10.1146/annurev.micro.58.030603.123715. [DOI] [PubMed] [Google Scholar]
- 110.Tilburn J., Sarkar S., Widdick D.A., Espeso E.A., Orejas M., Mungroo J., Peñalva M.A., Arst H.N. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mingot J.M., Espeso E.A., Díez E., Peñalva M. Ambient pH signaling regulates nuclear localization of the Aspergillus nidulans PacC transcription factor. Mol. Cell Biol. 2001;21:1688–1699. doi: 10.1128/MCB.21.5.1688-1699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merhej J., Richard-Forget F., Barreau C. The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet. Biol. 2011;48:275–284. doi: 10.1016/j.fgb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y., Liu F., Wang L., Wang Q., Selvaraj J.N., Zhao Y., Wang Y., Xing F., Liu Y. The pH-signaling transcription factor AopacC regulates ochratoxin A biosynthesis in Aspergillus ochraceus. J. Agric. Food Chem. 2018;66:4394–4401. doi: 10.1021/acs.jafc.8b00790. [DOI] [PubMed] [Google Scholar]
- 114.Suárez T., Peñalva M.A. Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol. Microbiol. 1996;20:529–540. doi: 10.1046/j.1365-2958.1996.5421065.x. [DOI] [PubMed] [Google Scholar]
- 115.Barad S., Espeso E.A., Sherman A., Prusky D. Ammonia activates pacC and patulin accumulation in an acidic environment during apple colonization by Penicillium expansum. Mol. Plant. Pathol. 2016;17:727–740. doi: 10.1111/mpp.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barad S., Horowitz S.B., Kobiler I., Sherman A., Prusky D. Accumulation of the mycotoxin patulin in the presence of gluconic acid contributes to pathogenicity of Penicillium expansum. Mol. Plant-Microbe Interact. 2014;27:66–77. doi: 10.1094/MPMI-05-13-0138-R. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y., Li B., Xu X., Zhang Z., Tian S. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum. Environ. Microbiol. 2018;20:4063–4078. doi: 10.1111/1462-2920.14453. [DOI] [PubMed] [Google Scholar]
- 118.Zhang T., Sun X., Xu Q., Candelas L.G., Li H. The pH signaling transcription factor PacC is required for full virulence in Penicillium digitatum. Appl. Microbiol. Biotechnol. 2013;97:9087–9098. doi: 10.1007/s00253-013-5129-x. [DOI] [PubMed] [Google Scholar]
- 119.Morgan B.A., Banks G.R., Toone M.W., Raitt D., Kuge S., Johnston L.H. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997;16:1035–1044. doi: 10.1093/emboj/16.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao C., Liu W., Li R. Penicillium marneffei SKN7, a novel gene, could complement the hypersensitivity of S. cerevisiae skn7 disruptant strain to oxidative stress. Mycopathologia. 2009;168:23–30. doi: 10.1007/s11046-009-9192-x. [DOI] [PubMed] [Google Scholar]
- 121.Montibus M., Pinson-Gadais L., Richard-Forget F., Barreau C., Ponts N. Coupling of transcriptional response to oxidative stress and secondary metabolism regulation in filamentous fungi. Crit. Rev. Microbiol. 2015;41:295–308. doi: 10.3109/1040841X.2013.829416. [DOI] [PubMed] [Google Scholar]
- 122.Zhang F., Xu G., Geng L., Lu X., Yang K., Yuan J., Nie X., Zhuang Z., Wang S. The stress response regulator AflSkn7 influences morphological development, stress response, and pathogenicity in the fungus Aspergillus flavus. Toxins. 2016;8:202. doi: 10.3390/toxins8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reverberi M., Zjalic S., Ricelli A., Punelli F., Camera E., Fabbri C., Picardo M., Fanelli C., Fabbri A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: A role for the ApyapA gene. Eukaryot. Cell. 2008;7:988–1000. doi: 10.1128/EC.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reverberi M., Gazzetti K., Punelli F., Scarpari M., Zjalic S., Ricelli A., Fabbri A.A., Fanelli C. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl. Microbiol. Biotechnol. 2012;95:1293–1304. doi: 10.1007/s00253-012-3985-4. [DOI] [PubMed] [Google Scholar]
- 125.Dankai W., Pongpom M., Youngchim S., Cooper C.R., Vanittanakom N. The yapA encodes bZip transcription factor involved in stress tolerance in pathogenic fungus Talaromyces marneffei. PLoS ONE. 2016;11:e0163778. doi: 10.1371/journal.pone.0163778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bayram Ö., Braus G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 127.Bayram Ö., Krappmann S., Ni M., Bok J.W., Helmstaedt K., Valerius O., Braus-Stromeyer S., Kwon N.J., Keller N.P., Yu J.H., et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 128.Stinnett S.M., Espeso E.A., Cobeño L., Araújo-Bazán L., Calvo A.M. Aspergillus nidulans VeA subcellular localization is dependent on the importin α carrier and on light. Mol. Microbiol. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- 129.Kim H.S., Han K.Y., Kim K.J., Han D.M., Jahng K.Y., Chae K.S. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:72–80. doi: 10.1016/S1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 130.Bayram O., Krappmann S., Seiler S., Vogt N., Braus G.H. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet. Biol. 2008;45:127–138. doi: 10.1016/j.fgb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 131.Käfer E. Origins of translocations in Aspergillus nidulans. Genetics. 1965;52:217–232. doi: 10.1093/genetics/52.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kato N., Brooks W., Calvo A.M. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duran R.M., Cary J.W., Calvo A.M. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- 134.Calvo A.M. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 135.Cary J.W., Calvo A.M. Regulation of Aspergillus mycotoxin biosynthesis. Toxin Rev. 2008;27:347–370. doi: 10.1080/15569540802373999. [DOI] [Google Scholar]
- 136.Payne G.A., Nystrom G.J., Bhatnagar D., Cleveland T.E., Woloshuk C.P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl. Environ. Microbiol. 1993;59:156–162. doi: 10.1128/AEM.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meyers D.M., Obrian G., Du W.L., Bhatnagar D., Payne G.A. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 1998;64:3713–3717. doi: 10.1128/AEM.64.10.3713-3717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du W., Obrian G.R., Payne G.A. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit. Contam. 2007;24:1043–1050. doi: 10.1080/02652030701513826. [DOI] [PubMed] [Google Scholar]
- 139.Merhej J., Urban M., Dufresne M., Hammond-Kosack K.E., Richard-Forget F., Barreau C. The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum. Mol. Plant. Pathol. 2012;13:363–374. doi: 10.1111/j.1364-3703.2011.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Myung K., Zitomer N.C., Duvall M., Glenn A.E., Riley R.T., Calvo A.M. The conserved global regulator VeA is necessary for symptom production and mycotoxin synthesis in maize seedlings by Fusarium verticillioides. Plant. Pathol. 2012;61:152–160. doi: 10.1111/j.1365-3059.2011.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoff B., Kamerewerd J., Sigl C., Mitterbauer R., Zadra I., Kürnsteiner H., Kück U. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot. Cell. 2010;9:1236–1250. doi: 10.1128/EC.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El Hajj Assaf C., Snini S.P., Tadrist S., Bailly S., Naylies C., Oswald I.P., Lorber S., Puel O. Impact of veA on the development, aggressiveness, dissemination and secondary metabolism of Penicillium expansum. Mol. Plant. Pathol. 2018;19:1971–1983. doi: 10.1111/mpp.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li B., Chen Y., Zong Y., Shang Y., Zhang Z., Xu X., Wang X., Long M., Tian S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019;21:1124–1139. doi: 10.1111/1462-2920.14542. [DOI] [PubMed] [Google Scholar]
- 144.Baba S., Kinoshita H., Nihira T. Identification and characterization of Penicillium citrinum VeA and LaeA as global regulators for ML-236B production. Curr. Genet. 2012;58:1–11. doi: 10.1007/s00294-011-0359-x. [DOI] [PubMed] [Google Scholar]
- 145.Kopke K., Hoff B., Bloemendal S., Katschorowski A., Kamerewerd J., Kück U. Members of the Penicillium chrysogenum velvet complex play functionally opposing roles in the regulation of penicillin biosynthesis and conidiation. Eukaryot. Cell. 2013;12:299–310. doi: 10.1128/EC.00272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kosalková K., García-Estrada C., Ullán R.V., Godio R.P., Feltrer R., Teijeira F., Mauriz E., Martín J.F. The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie. 2009;91:214–225. doi: 10.1016/j.biochi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 147.Kumar D., Barad S., Chen Y., Luo X., Tannous J., Dubey A., Matana N.G., Tian S., Li B., Keller N., et al. LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol. Plant. Pathol. 2017;18:1150–1163. doi: 10.1111/mpp.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X., Zhu Y., Bao L., Gao L., Yao G., Li Y., Yang Z., Li Z., Zhong Y., Li F.-L., et al. Putative methyltransferase LaeA and transcription factor CreA are necessary for proper asexual development and controlling secondary metabolic gene cluster expression. Fungal Genet. Biol. 2016;94:32–46. doi: 10.1016/j.fgb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 149.Zhu C., Wang Y., Hu X., Lei M., Wang M., Zeng J., Li H., Liu Z., Zhou T., Yu D. Involvement of LaeA in the regulation of conidia production and stress responses in Penicillium digitatum. J. Basic Microbiol. 2020;60:82–88. doi: 10.1002/jobm.201900367. [DOI] [PubMed] [Google Scholar]
- 150.Yu J., Han H., Zhang X., Ma C., Sun C., Che Q., Gu Q., Zhu T., Zhang G., Li D. Discovery of two new sorbicillinoids by overexpression of the global regulator LaeA in a marine-derived fungus Penicillium dipodomyis YJ-11. Mar. Drugs. 2019;17:446. doi: 10.3390/md17080446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar D., Tannous J., Sionov E., Keller N., Prusky D. Apple intrinsic factors modulating the global regulator, LaeA, the patulin gene cluster and patulin accumulation during fruit colonization by Penicillium expansum. Front. Plant. Sci. 2018;9:1–13. doi: 10.3389/fpls.2018.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L., Zhang X., Zhang K., Zhang X., Zhu T., Che Q., Zhang G., Li D. Overexpression of global regulator PbrlaeA leads to the discovery of new polyketide in fungus Penicillium brocae HDN-12-143. Front. Chem. 2020;8:1–7. doi: 10.3389/fchem.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ahmed Y.L., Gerke J., Park H.S., Bayram Ö., Neumann P., Ni M., Dickmanns A., Kim S.C., Yu J.H., Braus G.H., et al. The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-κB. PLoS Biol. 2013;11:e1001750. doi: 10.1371/journal.pbio.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Han S., Adams T.H. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genomics. 2001;266:260–270. doi: 10.1007/s004380100552. [DOI] [PubMed] [Google Scholar]
- 155.Qin Y., Bao L., Gao M., Chen M., Lei Y., Liu G., Qu Y. Penicillium decumbens BrlA extensively regulates secondary metabolism and functionally associates with the expression of cellulase genes. Appl. Microbiol. Biotechnol. 2013;97:10453–10467. doi: 10.1007/s00253-013-5273-3. [DOI] [PubMed] [Google Scholar]
- 156.Sigl C., Haas H., Specht T., Pfaller K., Kürnsteiner H., Zadra I. Among developmental regulators, StuA but not BrlA is essential for penicillin V production in Penicillium chrysogenum. Appl. Environ. Microbiol. 2011;77:972–982. doi: 10.1128/AEM.01557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zetina-Serrano C., Rocher O., Naylies C., Lippi Y., Oswald I.P., Lorber S., Puel O. The brlA gene deletion reveals that patulin biosynthesis is not related to conidiation in Penicillium expansum. Int. J. Mol. Sci. 2020;21:6660. doi: 10.3390/ijms21186660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Domínguez-Santos R., Martín J.F., Kosalková K., Prieto C., Ullán R.V., García-Estrada C. The regulatory factor PcRFX1 controls the expression of the three genes of β-lactam biosynthesis in Penicillium chrysogenum. Fungal Genet. Biol. 2012;49:866–881. doi: 10.1016/j.fgb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 159.Domínguez-Santos R., García-Estrada C., Kosalková K., Prieto C., Santamarta I., Martín J.F. PcFKH1, a novel regulatory factor from the forkhead family, controls the biosynthesis of penicillin in Penicillium chrysogenum. Biochimie. 2015;115:162–176. doi: 10.1016/j.biochi.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 160.Gil-Durán C., Rojas-Aedo J.F., Medina E., Vaca I., García-Rico R.O., Villagrán S., Levicán G., Chávez R. The pcz1 gene, which encodes a Zn(II)2Cys6 protein, is involved in the control of growth, conidiation, and conidial germination in the filamentous fungus Penicillium roqueforti. PLoS ONE. 2015;10:e0120740. doi: 10.1371/journal.pone.0120740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rojas-Aedo J.F., Gil-Durán C., Goity A., Vaca I., Levicán G., Larrondo L.F., Chávez R. The developmental regulator Pcz1 affects the production of secondary metabolites in the filamentous fungus Penicillium roqueforti. Microbiol. Res. 2018;212–213:67–74. doi: 10.1016/j.micres.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 162.He Q.-P., Zhao S., Wang J.-X., Li C.-X., Yan Y.-S., Wang L., Liao L.-S., Feng J.-X. Transcription factor NsdD regulates the expression of genes involved in plant biomass-degrading enzymes, conidiation, and pigment biosynthesis in Penicillium oxalicum. Appl. Environ. Microbiol. 2018;84:e01039-18. doi: 10.1128/AEM.01039-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klis F.M., Boorsma A., De Groot P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- 164.Ruiz-Herrera J., Elorza M.V., Valentín E., Sentandreu R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006;6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 165.Munro C.A., Selvaggini S., De Bruijn I., Walker L., Lenardon M.D., Gerssen B., Milne S., Brown A.J.P., Gow N.A.R. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007;63:1399–1413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bölker M. Sex and crime: Heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 1998;25:143–156. doi: 10.1006/fgbi.1998.1102. [DOI] [PubMed] [Google Scholar]
- 167.Liu S., Dean R.A. G protein subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 1997;10:1075–1086. doi: 10.1094/MPMI.1997.10.9.1075. [DOI] [PubMed] [Google Scholar]
- 168.Gronover C.S., Kasulke D., Tudzynski P., Tudzynski B. The role of G protein alpha subunits in the infection process of the gray mold fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 2001;14:1293–1302. doi: 10.1094/MPMI.2001.14.11.1293. [DOI] [PubMed] [Google Scholar]
- 169.Chang M.H., Chae K.S., Han D.M., Jahng K.Y. The GanB Gα-protein negatively regulates asexual sporulation and plays a positive role in conidial germination in Aspergillus nidulans. Genetics. 2004;167:1305–1315. doi: 10.1534/genetics.103.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Doehlemann G., Berndt P., Hahn M. Different signalling pathways involving a Gα protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 2006;59:821–835. doi: 10.1111/j.1365-2958.2005.04991.x. [DOI] [PubMed] [Google Scholar]
- 171.Hu Y., Liu G., Li Z., Qin Y., Qu Y., Song X. G protein-cAMP signaling pathway mediated by PGA3 plays different roles in regulating the expressions of amylases and cellulases in Penicillium decumbens. Fungal Genet. Biol. 2013;58–59:62–70. doi: 10.1016/j.fgb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 172.García-Rico R.O., Fierro F., Martín J.F. Heterotrimeric Gα protein Pga1 of Penicillium chrysogenum controls conidiation mainly by a cAMP-independent mechanism. Biochem. Cell Biol. 2008;86:57–69. doi: 10.1139/O07-148. [DOI] [PubMed] [Google Scholar]
- 173.Zuber S., Hynes M.J., Andrianopoulos A. G-protein signaling mediates asexual development at 25 °C but has no effect on yeast-like growth at 37 °C in the dimorphic fungus Penicillium marneffei. Eukaryot. Cell. 2002;1:440–447. doi: 10.1128/EC.1.3.440-447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carrasco-Navarro U., Vera-Estrella R., Barkla B.J., Zúñiga-León E., Reyes-Vivas H., Fernández F.J., Fierro F. Proteomic analysis of the signaling pathway mediated by the heterotrimeric Ga protein Pga1 of Penicillium chrysogenum. Microb. Cell Fact. 2016;15:1–17. doi: 10.1186/s12934-016-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stoll D., Schmidt-Heydt M., Geisen R. Differences in the regulation of ochratoxin A by the HOG pathway in Penicillium and Aspergillus in response to high osmolar environments. Toxins. 2013;5:1282–1298. doi: 10.3390/toxins5071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schmidt-Heydt M., Stoll D., Schütz P., Geisen R. Oxidative stress induces the biosynthesis of citrinin by Penicillium verrucosum at the expense of ochratoxin. Int. J. Food Microbiol. 2015;192:1–6. doi: 10.1016/j.ijfoodmicro.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 177.Strauss J., Reyes-Dominguez Y. Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet. Biol. 2011;48:62–69. doi: 10.1016/j.fgb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bok J.W., Keller N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shwab E.K., Bok J.W., Tribus M., Galehr J., Graessle S., Keller N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell. 2007;6:1656–1664. doi: 10.1128/EC.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ding Z., Zhou H., Wang X., Huang H., Wang H., Zhang R., Wang Z., Han J. Deletion of the histone deacetylase HdaA in endophytic fungus Penicillium chrysogenum Fes1701 induces the complex response of multiple bioactive secondary metabolite production and relevant gene cluster expression. Molecules. 2020;25:3657. doi: 10.3390/molecules25163657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guzman-Chavez F., Salo O., Samol M., Ries M., Kuipers J., Bovenberg R.A.L., Vreeken R.J., Driessen A.J.M. Deregulation of secondary metabolism in a histone deacetylase mutant of Penicillium chrysogenum. Microbiologyopen. 2018;7:1–15. doi: 10.1002/mbo3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akiyama D.Y., Rocha M.C., Costa J.H., Malavazi I., Fill T.P. The histone deacetylase clr3 regulates secondary metabolite production and growth under oxidative stress conditions in Penicillium brasilianum. BioRxiv. 2020:1–27. doi: 10.1101/2020.05.01.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.He X., Zhang Z., Chen Y., Che Q., Zhu T., Gu Q., Li D. Varitatin A, a highly modified fatty acid amide from Penicillium variabile cultured with a DNA methyltransferase inhibitor. J. Nat. Prod. 2015;78:2841–2845. doi: 10.1021/acs.jnatprod.5b00742. [DOI] [PubMed] [Google Scholar]
- 184.He X., Zhang Z., Che Q., Zhu T., Gu Q., Li D. Varilactones and wortmannilactones produced by Penicillium variabile cultured with histone deacetylase inhibitor. Arch. Pharm. Res. 2018;41:57–63. doi: 10.1007/s12272-017-0982-2. [DOI] [PubMed] [Google Scholar]
- 185.Wang X., Filho J.G.S., Hoover A.R., King J.B., Ellis T.K., Powell D.R., Cichewicz R.H. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an atlantic-forest-soil-derived Penicillium citreonigrum. J. Nat. Prod. 2010;73:942–948. doi: 10.1021/np100142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu D.Z., Liang B.W., Li X.F., Liu Q. Induced production of new diterpenoids in the fungus Penicillium funiculosum. Nat. Prod. Commun. 2014;9:607–608. doi: 10.1177/1934578X1400900502. [DOI] [PubMed] [Google Scholar]
- 187.Li G., Kusari S., Golz C., Laatsch H., Strohmann C., Spiteller M. Epigenetic modulation of endophytic Eupenicillium sp. LG41 by a histone deacetylase inhibitor for production of decalin-containing compounds. J. Nat. Prod. 2017;80:983–988. doi: 10.1021/acs.jnatprod.6b00997. [DOI] [PubMed] [Google Scholar]
- 188.El-Hawary S.S., Sayed A.M., Mohammed R., Hassan H.M., Zaki M.A., Rateb M.E., Mohammed T.A., Amin E., Abdelmohsen U.R. Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicompactum. Mar. Drugs. 2018;16:253. doi: 10.3390/md16080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo D.L., Qiu L., Feng D., He X., Li X.H., Cao Z.X., Gu Y.C., Mei L., Deng F., Deng Y. Three new ɑ-pyrone derivatives induced by chemical epigenetic manipulation of Penicillium herquei, an endophytic fungus isolated from Cordyceps sinensis. Nat. Prod. Res. 2020;34:958–964. doi: 10.1080/14786419.2018.1544974. [DOI] [PubMed] [Google Scholar]
- 190.Ying Y.M., Li L., Yu H.F., Xu Y.L., Huang L., Mao W., Tong C.P., Zhang Z.D., Zhan Z.J., Zhang Y. Induced production of a new polyketide in Penicillium sp. HS-11 by chemical epigenetic manipulation. Nat. Prod. Res. 2020 doi: 10.1080/14786419.2019.1709190. [DOI] [PubMed] [Google Scholar]
- 191.Asai T., Luo D., Yamashita K., Oshima Y. Structures and biomimetic synthesis of novel α-pyrone polyketides of an endophytic Penicillium sp. in Catharanthus roseus. Org. Lett. 2013;15:1020–1023. doi: 10.1021/ol303506t. [DOI] [PubMed] [Google Scholar]
- 192.Xiong Y.R., Zhao S., Fu L.H., Liao X.Z., Li C.X., Yan Y.S., Liao L.S., Feng J.X. Characterization of novel roles of a HMG-box protein PoxHmbB in biomass-degrading enzyme production by Penicillium oxalicum. Appl. Microbiol. Biotechnol. 2018;102:3739–3753. doi: 10.1007/s00253-018-8867-y. [DOI] [PubMed] [Google Scholar]
- 193.Karácsony Z., Gácser A., Vágvölgyi C., Scazzocchio C., Hamari Z. A dually located multi-HMG-box protein of Aspergillus nidulans has a crucial role in conidial and ascospore germination. Mol. Microbiol. 2014;94:383–402. doi: 10.1111/mmi.12772. [DOI] [PubMed] [Google Scholar]
- 194.Tannous J., Barda O., Luciano-Rosario D., Prusky D.B., Sionov E., Keller N.P. New insight into pathogenicity and secondary metabolism of the plant pathogen Penicillium expansum through deletion of the epigenetic reader SntB. Front. Microbiol. 2020;11:1–13. doi: 10.3389/fmicb.2020.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Berg M.A.V.D., Albang R., Albermann K., Badger J.H., Daran J.-M., Driessen A.J.M., Garcia-Estrada C., Fedorova N.D., Harris D.M., Heijne W.H.M., et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008;26:1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]