Abstract
Background
Arterial stiffness is an independent risk factor for cardiovascular disease and can be beneficially influenced by physical activity. However, it is not clear how an individual’s physical activity pattern over a week is associated with arterial stiffness. Therefore, we examined the associations of the amount and pattern of higher intensity physical activity with arterial stiffness.
Methods and Results
Data from the Maastricht Study (n=1699; mean age: 60±8 years, 49.4% women, 26.9% type 2 diabetes mellitus) were used. Arterial stiffness was assessed by carotid‐to‐femoral pulse wave velocity and carotid distensibility. The amount (continuous variable as h/wk) and pattern (categorical variable) of higher intensity physical activity were assessed with the activPAL3. Activity groups were: inactive (<75 min/wk), insufficiently active (75–150 min/wk), weekend warrior (>150 min/wk in ≤2 sessions), and regularly active (>150 min/wk in ≥3 sessions). In the fully adjusted model (adjusted for demographic, lifestyle, and cardiovascular risk factors), higher intensity physical activity was associated with lower carotid‐to‐femoral pulse wave velocity (amount: β = −0.05, 95% CI, −0.09 to −0.01; insufficiently active: β = −0.33, 95% CI, −0.55 to −0.11; weekend warrior: β = −0.38, 95% CI, −0.64 to −0.12; and regularly active: β = −0.46, 95% CI, −0.71 to −0.21 [reference: inactive]). These associations were stronger in those with type 2 diabetes mellitus. There was no statistically significant association between higher intensity physical activity with carotid distensibility.
Conclusions
Participating in higher intensity physical activity was associated with lower carotid‐to‐femoral pulse wave velocity, but there was no difference between the regularly actives and the weekend warriors. From the perspective of arterial stiffness, engaging higher intensity physical activity, regardless of the weekly pattern, may be an important strategy to reduce the risk of cardiovascular disease, particularly in individuals with type 2 diabetes mellitus.
Keywords: accelerometry, arterial stiffness, cardiovascular disease, physical activity, type 2 diabetes mellitus
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise
Nonstandard Abbreviations and Acronyms
- cfPWV
carotid‐to‐femoral pulse wave velocity
- DC
distensibility coefficient
- HR
heart rate
- T2DM
type 2 diabetes mellitus
1.
1. Clinical Perspective
1.1. What Is New
We tested for the first time the association between accelerometry‐derived amount and pattern of higher intensity physical activity and arterial stiffness in a large population‐based study.
1.2. What Are the Clinical Implications?
Participating in higher intensity physical activity was associated with lower carotid‐to‐femoral pulse wave velocity, but there was no difference between the regularly actives (those who spread their activity over the week) and the weekend warriors (those who conduct the majority of their activity in 1 or 2 sessions per week)
In individuals with type 2 diabetes mellitus, the association between higher intensity physical activity and carotid‐to‐femoral pulse wave velocity was stronger.
Engaging in higher intensity physical activity, regardless of the weekly pattern, may be an important strategy to reduce the risk of cardiovascular disease, particularly in individuals with type 2 diabetes mellitus.
2.
Arterial stiffness is an independent risk factor for cardiovascular disease (CVD) 1 , 2 and can be positively influenced by physical activity, 3 , 4 , 5 , 6 , 7 , 8 possibly via improvement of endothelial function. 1 , 9 , 10 , 11 , 12 , 13
International physical activity guidelines recommend at least 150 minutes per week of moderate‐to‐vigorous physical activity divided over several days. 14 , 15 However, it is unclear whether the pattern of moderate‐to‐vigorous physical activity sessions is important in lowering arterial stiffness and eventually CVD risk. So‐called weekend warriors are people who conduct a significant part of the recommended weekly amount of moderate‐to‐vigorous physical activity in only 1 or 2 weekly sessions, whereas other people spread this more regularly over the week. In the literature on physical activity patterns, data from several studies have shown contradictory results on the effect of different physical activity patterns on CVD health and mortality. 4 , 16 , 17 In addition, physical activity in these studies was assessed through interviews and questionnaires, 4 , 17 and only 1 study reported objectively measured pattern data by an accelerometer. 16 More objectively measured data are needed to identify whether different patterns of physical activity are equally beneficial in lowering arterial stiffness in individuals.
In light of this, we investigated the association between objectively measured amounts and patterns of higher intensity physical activity, ie, weekend warriors and regularly actives, and arterial stiffness, as assessed by carotid‐to‐femoral pulse wave velocity (cfPWV) and the carotid distensibility coefficient (DC).
3. Methods
The data that support the findings of this study are available from the corresponding author and the Maastricht Study management team (research.dms@mumc.nl) upon reasonable request for researchers who meet the criteria for access to confidential data.
3.1. Study Population
We used data from the Maastricht Study, an observational prospective population‐based cohort study. The rationale and methodology have been described previously. 18
In brief, the study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes mellitus (T2DM), and is characterized by an extensive phenotyping approach. Eligible participants were individuals between 40 and 75 years of age and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status, with an oversampling of individuals with T2DM, for reasons of efficiency. This study included cross‐sectional data from 3451 participants, who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months.
The study was approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare, and Sports of the Netherlands (Permit No. 131088‐ 105234‐PG). All participants gave written informed consent.
4. Measurements
4.1. Arterial Stiffness Measurements
All measurements were done by trained vascular technicians unaware of the participants’ clinical or DM status, in a dark, quiet temperature‐controlled room (21–23°C). Participants were asked to refrain from smoking and drinking coffee, tea, or alcoholic beverages 3 hours prior to the study. Participants were allowed to have a light meal (breakfast and/or lunch). All measurements were performed in the supine position after 10 minutes of rest. Talking or sleeping was not allowed during the examination. During the vascular measurements (approximately 45 minutes), brachial systolic, diastolic, and mean arterial pressure (MAP) were determined every 5 minutes with an oscillometric device (Accutorr Plus; Datascope Inc, Montvale, NJ). The mean MAP and heart rate (HR) of these measurements were used in the statistical analysis. A 3‐lead ECG was recorded continuously during the measurements to facilitate automatic signal processing.
4.1.1. Carotid‐to‐femoral pulse Wave Velocity
cfPWV was determined according to recent guidelines 19 with the use of applanation tonometry (SphygmoCor; Atcor Medical, Sydney, Australia). Pressure waveforms were determined at the right common carotid and right common femoral arteries. Difference in the time of pulse arrival from the R‐wave of the ECG between the 2 sites (transit time) was determined with the intersecting tangents algorithm. The pulse wave travel distance was calculated as 80% of the direct straight distance (measured with an infantometer) between the 2 arterial sites. The median of 3 consecutive cfPWV (defined as traveled distance/transit time) recordings was used in the analyses.
4.1.2. Carotid Arterial Elastic Properties
4.1.2.1. Data Acquisition
Measurements were done at the left common carotid (10 mm proximal to the carotid bulb), the right common femoral (10–20 mm proximal to the flow divider), and the right brachial (20 mm proximal to the antecubital fossa) arteries, with the use of an ultrasound scanner equipped with a 7.5‐MHz linear probe (MyLab 70; Esaote Europe B.V., Maastricht, the Netherlands). This setup enables the measurement of diameter, distension, and intima‐media thickness as described previously. 20 , 21 Briefly, during the ultrasound measurements a B‐mode image on the basis of 19 M‐lines was depicted onscreen, and an online echo‐tracking algorithm showed real‐time anterior and posterior arterial wall displacements. The M‐mode recordings were composed of 19 simultaneous recordings at a frame rate of 498 Hz. The distance between the M‐line recording positions was 0.96 mm; thus, a total segment of 18.24 mm of each artery was covered by the scan plane. For offline processing, the radiofrequency signal was fed into a dedicated personal‐computer–based acquisition system (ART.LAB; Esaote Europe B.V.) with a sampling frequency of 50 MHz. Data processing was performed in MATLAB (version 7.5; MathWorks, Natick, MA). The distension waveforms were obtained from the radiofrequency data with the use of a wall track algorithm. 20 The median diameter and distension of 3 measurements were used in the analyses.
4.1.2.2. Statistical Analysis
The local arterial elastic property was quantified by calculating the following indices 22 :
The DC represents arterial stiffness.
where D is the arterial diameter; ΔD is the distension; and PP is the brachial pulse pressure (calculated as systolic minus diastolic blood pressure).
4.1.3. Reproducibility
Reproducibility was assessed in 12 individuals (6 men, women; 60.8±6.8 years; 6 T2DM) who were examined by 2 observers at 2 occasions spaced 1 week apart. The intra‐observer and inter‐observer intraclass correlation coefficients were for cfPWV 0.87 and 0.69; and for carotid DC 0.85 and 0.73.
4.2. Physical activity measurements: Accelerometry data
Daily activity levels were measured using the activPAL3 physical activity monitor (PAL Technologies, Glasgow, UK). The activPAL3 is a small (53×35×7 mm), lightweight (15 g) triaxial accelerometer that records movement in the vertical, anteroposterior, and mediolateral axes, and also determines posture (sitting or lying, standing, and stepping) based on acceleration information. The device was attached directly to the skin on the front of the right thigh with transparent 3M Tegaderm tape, after the device had been waterproofed using a nitrile sleeve. Participants were asked to wear the accelerometer for 8 consecutive days, without removing it at any time. To avoid inaccurately identifying nonwear time, participants were asked not to replace the device once removed. Data were uploaded using the activPAL software and processed using customized software written in MATLAB R2013b (MathWorks). 23 Data from the first day were excluded from the analysis because participants performed physical function tests at the research center after the device was attached. In addition, data from the final wear day providing ≤14 waking hours of data were excluded from the analysis. Participants were included if they provided at least 6 valid days (≥10 hours of waking data per day).
We calculated the amount of higher intensity physical activity as the time, in hours per week, spent in higher intensity physical activity, defined as stepping time with a step frequency >110 steps per minute. Weekly activity pattern categories based on higher intensity physical activity were defined as: inactive, <75 minutes of higher intensity physical activity per week; insufficiently active, 75 to 150 minutes of higher intensity physical activity per week; and sufficiently active, ≥150 minutes of higher intensity physical activity per week. The sufficiently active category was further subdivided into weekend warrior and regularly active participants. In accordance with previous research, 16 weekend warriors were defined as participants who did ≥50% of the weekly higher intensity physical activity on only 1 or 2 days. 16 Regularly active participants were participants who did their higher intensity physical activity in ≥3 days. Thus, we defined 4 groups: (1) inactive (<75 minutes of higher intensity physical activity per week), (2) insufficiently active (75–150 minutes of higher intensity physical activity per week), (3) weekend warrior (≥150 minutes of higher intensity physical activity per week with more than 50% of the higher intensity physical activity in 1 or 2 days), and (4) regularly active (≥150 minutes of higher intensity physical activity per week in ≥3 days). Also, we assessed the variation of higher intensity physical activity per week per individual as a continuous weekly pattern variable by calculating the coefficient of variation (SD/mean).
4.3. Covariates
Covariates, which were extracted from a questionnaire, included sex, age, level of education, smoking status, alcohol consumption, energy intake, Greek Mediterranean Diet score, mobility limitation, and history of CVD. Level of education was categorized into low, medium, and high; smoking status was categorized into never, former, and current smoker. Alcohol consumption was categorized into nonconsumers, low consumers (≤7 glasses per week for women and ≤14 glasses per week for men), and high consumers (>7 glasses per week for women and >14 glasses per week for men). Energy intake and dietary habits were obtained from a validated food frequency questionnaire and calculated as the mean energy intake (kcal) per day and adherence to Greek Mediterranean Diet score. Mobility limitation was obtained from the 36‐Item Short Form Health Survey questionnaire and was defined as having difficulty walking 500 m or climbing up a flight of stairs. Prevalent CVD was defined as a self‐reported history of myocardial infarction, cerebrovascular infarction or hemorrhage, or percutaneous artery angioplasty of, or vascular surgery on, the coronary, abdominal, peripheral, or carotid arteries. The use of lipid‐modifying, antihypertensive (β‐blockers, calcium channel blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and nonloop diuretics) and glucose‐lowering medication was assessed during a medication interview. 18 Body mass index, triglycerides, and total cholesterol‐to‐high‐density‐lipoprotein–cholesterol ratio were determined as described elsewhere. 18 T2DM was defined according to the World Health Organization 2006 criteria. 24
4.4. Statistical Analysis
All data were analyzed using IBM SPSS software version 25.0 for Windows (IBM Corp, Armonk, NY). Characteristics of the total study population and according to higher intensity physical activity patterns were summarized as mean (SD) or as percentages. The variables, total to‐high‐density‐lipoprotein–cholesterol ratio and triglycerides had a skewed distribution and were described using the median (interquartile range).
Associations between the amount (continuous) and pattern (categorical) of higher intensity physical activity and cfPWV and carotid DC were examined with the use of multivariable linear regression models. Model 1 was adjusted for age, sex, and DM status; model 2 was additionally adjusted for MAP and HR during vascular measurements; and model 3 was additionally adjusted for history of CVD, level of education, mobility limitations (yes/no), triglycerides, total‐to‐high‐density‐lipoprotein–cholesterol ratio, lipid‐modifying medication, antihypertensive medication (β‐blockers, calcium channel blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and nonloop diuretics separately), smoking status, alcohol use, and body mass index. Several sensitivity analyses were performed: We (1) adjusted for renin‐angiotensin system inhibitors separately instead of all antihypertensive medication, (2) additionally adjusted for adherence to a Greek Mediterranean Diet and kcal, and (3) adjusted for 24‐hour MAP and HR instead of MAP and HR obtained during vascular measurements. Interaction analyses were performed for sex and DM status. A P value <0.05 for interaction was considered statistically significant.
5. Results
From the initial 3451 participants, we excluded 41 participants with other types of diabetes mellitus than T2DM, 1200 with accelerometer measurements <6 days (of whom 673 did not receive an activPAL because of logistics), 483 with missing cfPWV or carotid DC, and 69 who had other missing data. In total, 1699 participants were included in the present analysis (Figure 1). We compared the baseline characteristics of the included and excluded populations and found that the characteristics were similar (Table S1).
Figure 1. Flow diagram of the study populations with available data.
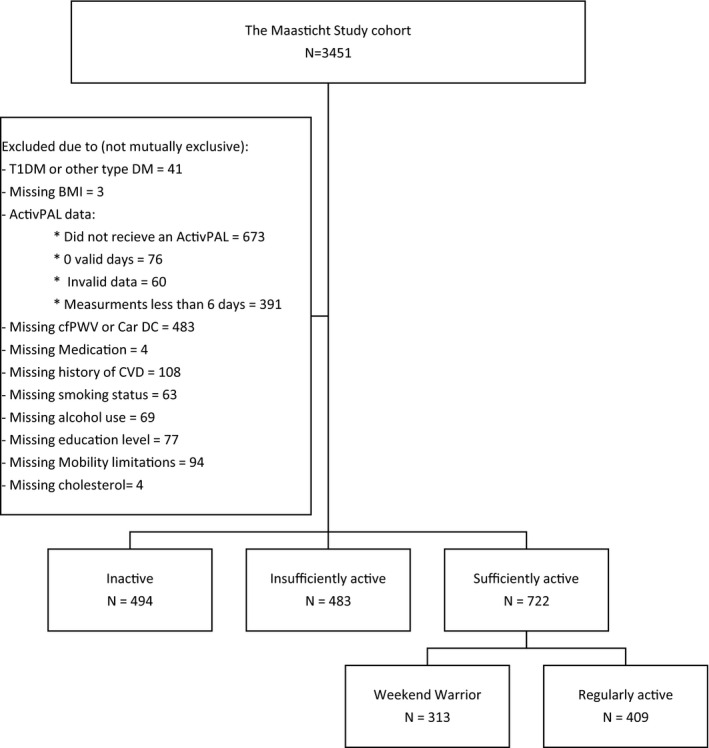
BMI indicates body mass index; Car DC, carotid distensibility coefficient; cfPWV, carotid‐to‐femoral pulse wave velocity; CVD, cardiovascular disease; and T1DM, type 1 diabetes mellitus.
Table 1 shows that participants in the inactive group, as compared with the more active participants, were older, had a higher MAP and HR during vascular measurements, had a higher body mass index, more often had a history of CVD and T2DM, and more often had mobility limitations.
Table 1.
Descriptive Characteristics of the Study Population (n=1699)
| Characteristics |
Total (n=1699) |
Inactive (n=494) |
Insufficiently Active (n=483) |
Weekend Warrior (n=313) |
Regularly Active (n=409) |
P Value |
|---|---|---|---|---|---|---|
| Age (y) | 60.45 (7.88) | 62.67 (7.84) | 60.73 (7.88) | 59.43 (7.38) | 58.22 (7.56) | <0.01 |
| Sex (% male) | 50.6 | 70.6 | 46.4 | 48.2 | 31.8 | <0.01 |
| Education level (%) | <0.01 | |||||
| Low | 32.5 | 38.1 | 32.3 | 30.0 | 28.1 | |
| Medium | 28.6 | 27.1 | 31.1 | 24.0 | 31.1 | |
| High | 38.8 | 34.8 | 36.6 | 46.0 | 40.8 | |
|
Mean arterial pressure (mm Hg) |
96.71 (10.27) | 97.27 (10.44) | 97.46 (10.17) | 96.59 (10.17) | 95.25 (10.12) | <0.01 |
| Mean heart rate (bpm) | 62 (9) | 64 (10) | 63 (9) | 60 (9) | 61 (8) | <0.01 |
| Smoking status (%) | <0.01 | |||||
| Current | 11.9 | 17.6 | 9.5 | 6.7 | 11.7 | |
| Former | 53.4 | 53.4 | 55.5 | 55.0 | 49.6 | |
| Never | 34.7 | 28.9 | 35.0 | 38.3 | 38.6 | |
| Alcohol consumption (%) | <0.01 | |||||
| None | 17.3 | 21.7 | 16.8 | 11.8 | 16.9 | |
| Low | 56.5 | 56.1 | 59.4 | 59.4 | 51.3 | |
| High | 26.2 | 22.3 | 23.8 | 28.8 | 31.8 | |
| Mobility limitations (%) | <0.01 | |||||
| Yes | 19.8 | 33.8 | 17.8 | 10.5 | 12.2 | |
| No | 80.2 | 66.2 | 82.2 | 89.5 | 87.8 | |
| BMI (kg/m2) | 26.77 (4.16) | 28.50 (4.63) | 26.82 (3.86) | 25.79 (3.54) | 25.36 (3.55) | <0.01 |
| History of CVD (%) | 16.2 | 23.3 | 18.2 | 10.5 | 9.8 | <0.01 |
| DM status (%) | <0.01 | |||||
| Yes | 26.9 | 46.6 | 26.5 | 15.7 | 12.2 | |
| No | 73.1 | 53.4 | 73.5 | 84.3 | 87.8 | |
| Antihypertensive medication use (%) | 40.3 | 57.3 | 39.8 | 30.7 | 27.6 | <0.01 |
| Lipid‐modifying medication use (%) | 36.7 | 53.0 | 35.6 | 27.5 | 25.2 | <0.01 |
| Glucose‐lowering medication use (%) | 21.0 | 37.4 | 21.1 | 11.5 | 8.1 | <0.01 |
| Total cholesterol to high‐density‐lipoprotein–cholesterol ratio | 3.40 (2.78‐4.23) | 3.63 (3.00‐4.39) | 3.43(2.81‐4.33) | 3.28 (2.70‐4.07) | 3.18 (2.56‐3.91) | <0.01 |
| Triglycerides (mmol/L) | 1.21 (0.89–1.70) | 1.41 (1.07–2.01) | 1.25 (0.92–1.70) | 1.09 (0.81–1.53) | 1.05 (0.80–1.43) | <0.01 |
| cfPWV (m/s) | 9.05 (2.16) | 9.85 (2.40) | 9.08 (2.08) | 8.66 (1.82) | 8.36 (1.87) | <0.01 |
| Carotid DC (103/kPa) | 14.29 (4.98) | 13.53 (4.60) | 14.09 (4.80) | 15.00 (5.41) | 14.90 (5.16) | <0.01 |
| Valid days (n) | 6.65 (0.48) | 6.64 (0.48) | 6.65 (0.48) | 6.56 (0.50) | 6.72 (0.45) | <0.01 |
| Higher‐intensity physical activity (min/wk) | 158.55 (124.86) | 41.66 (19.13) | 111.99 (22.03) | 259.19 (109.10) | 277.71 (116.82) | <0.01 |
Values are means (SD) or median (Q1–Q3), unless stated otherwise. BMI indicates body mass index; cfPWV, carotid‐to‐femoral pulse‐wave velocity; CVD, cardiovascular disease; DC, distensibility coefficient; and DM, diabetes mellitus.
Table 2 shows a statistically significant association between the amount (continues, h/wk) of higher intensity physical activity and cfPWV after full adjustment for demographic, lifestyle, and cardiovascular risk factors (model 3) (regression coefficient β = −0.05 m/s; 95% CI, −0.09 to −0.01). Regarding the pattern (categorical), compared to the inactives as the reference group, in the fully adjusted model (model 3), cfPWV was statistically significantly lower in the insufficiently actives (β = −0.33 m/s; 95% CI, −0.55 to −0.11), weekend warriors (β = −0.38m/s; 95% CI, −0.64 to −0.12) and regularly actives (β = −0.46m/s; 95% CI, −0.71 to −0.21). As shown in Table 2, age, sex, and DM status (model 2) are major confounders.
Table 2.
Associations of Higher‐Intensity Physical Activity Amount and Pattern With cfPWV
| Model | Amount | Pattern | |||
|---|---|---|---|---|---|
| Total Higher Intensity Physical Activity (h/wk) | Inactive | Insufficiently Active | Weekend Warrior | Regularly Active | |
| Crude | −0.22 (−0.27 to –0.17)* | Reference | −0.77 (−1.03 to −0.50)* | −1.19 (−1.48 to −0.89)* | −1.48 (−1.76 to −1.21)* |
| 1 | −0.10 (−0.14 to −0.05)* | Reference | −0.35 (−0.59 to −0.10)* | −0.55 (−0.83 to −0.27)* | −0.66 (−0.93 to −0.39)* |
| 2 | −0.07 (−0.11 to −0.03)* | Reference | −0.39 (−0.60 to −0.17)* | −0.46 (−0.72 to −0.21)* | −0.54 (−0.78 to −0.29)* |
| 3 | −0.05 (−0.09 to −0.01)* | Reference | −0.33 (−0.55 to −0.11)* | −0.38 (−0.64 to −0.12)* | −0.46 (−0.71 to −0.21)* |
Regression results are presented as unstandardized coefficient βs (95% CIs). The associations in model 1 are adjusted for age, sex, and diabetes mellitus status. The associations in model 2 are additionally adjusted for mean arterial pressure and heart rate. The associations in model 3 are additionally adjusted for history of cardiovascular disease, level of education, mobility limitation (yes/no), triglycerides, total‐to‐high‐density‐lipoprotein–cholesterol ratio, use of lipid‐modifying medication, use of antihypertensive medication, smoking, alcohol consumption, and body mass index. cfPWV indicates carotid‐to‐femoral pulse wave velocity.
Indicates statistical significance (P<0.05).
As shown in Table 3, there were no statistically significant associations between higher intensity physical activity amount and patterns and carotid DC after adjustment for age, sex, DM status, MAP, and HR (model 2); and after additional adjustments for history of CVD, level of education, mobility limitation, triglycerides, total‐ to high‐density‐lipoprotein–cholesterol ratio, use of lipid‐modifying medication, use of antihypertensive medication, smoking behavior, alcohol consumption, and body mass index (model 3).
Table 3.
Associations of Higher Intensity Physical Activity Amount and Pattern With Carotid DC
| Model | Amount | Pattern | |||
|---|---|---|---|---|---|
| Total Higher Intensity Physical Activity (h/wk) | Inactive | Insufficiently Active | Weekend Warrior | Regularly Active | |
| Crude | 0.20 (0.09–0.32)* | Reference | 0.56 (−0.07 to 1.18) | 1.47 (0.77 to 2.17)* | 1.37 (0.72 to 2.02)* |
| 1 | 0.05 (−0.06 to 0.16) | Reference | 0.08 (−0.50 to 0.66) | 0.52 (−0.14 to 1.17) | 0.24 (−0.39 to 0.87) |
| 2 | −0.03 (−0.13 to 0.07) | Reference | 0.14 (−0.38 to 0.66) | 0.24 (−0.35 to 0.84) | −0.11 (−0.68 to 0.46) |
| 3 | −0.08 (−0.18 to 0.02) | Reference | 0.08 (−0.44 to 0.60) | 0.07 (−0.55 to 0.68) | −0.33 (−0.91 to 0.26) |
Regression results are presented as unstandardized coefficient βs (95% CIs). The associations in model 1 are adjusted for age, sex and diabetes mellitus status. The associations in model 2 are additionally adjusted for mean arterial pressure and heart rate. The associations in model 3 are additionally adjusted for history of cardiovascular disease, level of education, mobility limitation (yes/no), triglycerides, total‐to‐high‐density‐lipoprotein–cholesterol ratio, use of lipid‐modifying medication, use of antihypertensive medication, smoking, alcohol consumption, and body mass index. DC indicates distensibility coefficient.
Indicates statistical significance (P<0.05).
As shown in Table 4, there were no statistically significant associations between coefficient of variation of physical activity and cfPWV and carotid DC in the fully adjusted model (model 3).
Table 4.
Associations of Coefficient of Variation of Higher Intensity Physical Activity With cfPWV and Carotid DC
| Model | ||
|---|---|---|
| cfPWV | Crude | 0.24 (−0.13 to 0.61) |
| 1 | −0.06 (−0.39 to 0.27) | |
| 2 | −0.01 (−0.30 to 0.29) | |
| 3 | 0.03 (−0.26 to 0.32) | |
| Carotid DC | Crude | 0.15 (−0.70 to 0.99) |
| 1 | 0.39 (−0.38 to 1.15) | |
| 2 | 0.24 (−0.45 to 0.93) | |
| 3 | 0.20 (−0.48 to 0.89) |
Regression results are presented as unstandardized coefficient βs (95% CIs). The associations in model 1 are adjusted for age, sex, and diabetes mellitus status. The associations in model 2 are additionally adjusted for mean arterial pressure and heart rate. The associations in model 3 are additionally adjusted for history of cardiovascular disease, level of education, mobility limitation (yes/no), triglycerides, total‐to‐high‐density‐lipoprotein–cholesterol ratio, use of lipid‐modifying medication, use of antihypertensive medication, smoking, alcohol consumption, and body mass index. cfPWV indicates carotid‐to‐femoral pulse wave velocity; and DC, distensibility coefficient.
There was no statistically significant interaction with sex and higher intensity physical activity amount or pattern or coefficient of variation with regard to cfPWV and carotid DC (data not shown). Associations of higher intensity physical activity amount and pattern, with cfPWV, but not with carotid DC (data not shown), were stronger in those with T2DM in the fully adjusted model (Table 5; only cfPWV; p interaction <0.05). In stratified analyses, higher intensity physical activity patterns, but not the amount, were associated with lower cfPWV in those without T2DM in the fully adjusted model (model 3; Table 5). Associations were stronger in those with T2DM in the fully adjusted model (model 3; amount [h/wk]: β = −0.15m/s, 95% CI, −0.28 to −0.02; insufficiently active: β = −0.61 m/s, 95% CI, −1.10 to −0.13; weekend warrior: β = −0.56 m/s, 95% CI, −1.24 to −0.12; and regularly active: β = −0.90 m/s, 95% CI, −1.57 to −0.22).
Table 5.
Associations Between Higher Intensity Physical Activity Amount and Pattern and cfPWV, Stratified by DM Status
| Model | Amount | Pattern | ||||
|---|---|---|---|---|---|---|
| Total Higher Intensity Physical Activity (h/wk) | Inactive | Insufficiently Active | Weekend Warrior | Regularly Active | ||
| Without T2DM | ||||||
| cfPWV | Crude | −0.11 (−0.16 to −0.06)* | Reference | −0.24 (−0.53 to 0.06) | −0.60 (−0.92 to −0.28)* | −0.86 (−1.16 to −0.56)* |
| 1 | −0.07 (−0.12 to −0.02)* | Reference | −0.07 (−0.34 to 0.21) | −0.33 (−0.62 to −0.03)* | −0.44 (−0.72 to −0.15)* | |
| 2 | −0.05 (−0.09 to −0.01)* | Reference | −0.15 (−0.40 to 0.10) | −0.30 (−0.56 to −0.03)* | −0.35 (−0.61 to −0.10)* | |
| 3 | −0.03 (−0.08 to 0.01) | Reference | −0.11 (−0.36 to 0.14) | −0.22 (−0.50 to 0.05) | −0.30 (−0.56 to −0.03)* | |
| T2DM | ||||||
| cfPWV | Crude | −0.32 (−0.46 to −0.17)* | Reference | −1.04 (−1.55 to −0.52)* | −1.20 (−1.93 to −0.46)* | −1.52 (−2.26 to −0.79)* |
| 1 | 0.24 (−0.38 to −0.10)* | Reference | −0.80 (−1.30 to −0.30)* | −0.94 (−1.64 to −0.23)* | −1.07 (−1.78 to −0.36)* | |
| 2 | −0.18 (−0.31 to −0.05)* | Reference | −0.71 (−1.16 to −0.25)* | −0.71 (−1.35 to −0.07)* | −0.88 (−1.53 to −0.24)* | |
| 3 | −0.15 (−0.28 to −0.02)* | Reference | −0.61 (−1.10 to −0.13)* | −0.56 (−1.24 to 0.12) | −0.90 (−1.57 to −0.22)* | |
Regression results are presented as unstandardized coefficient βs (95% CIs). The associations in model 1 are adjusted for age and sex. The associations in model 2 are additionally adjusted for mean arterial pressure and heart rate. The associations in model 3 are additionally adjusted for history of cardiovascular disease, level of education, mobility limitation (yes/no), triglycerides, total‐to‐high‐density‐lipoprotein–cholesterol ratio, use of lipid‐modifying medication, use of antihypertensive medication, smoking, alcohol consumption, and body mass index. cfPWV indicates carotid‐to‐femoral pulse wave velocity; and T2DM, type 2 diabetes mellitus.
Indicates statistical significance (P<0.05).
5.1. Additional Analyses
Several sensitivity analyses were performed. First, we adjusted for renin‐angiotensin system inhibitors instead of all antihypertensive medication in model 3. Associations between higher intensity physical activity amount and patterns and cfPWV remained similar in model 3: β = −0.05 m/s, 95% CI, −0.09 to −0.01 for amount (h/wk); β = −0.34 m/s, 95% CI, −0.56 to −0.11 for insufficiently actives; β = −0.38 m/s, 95% CI, −0.64 to −0.12 for weekend warriors; and β = −0.47 m/s, 95% CI, −0.72 to −0.22 for regularly actives. Second, we added adherence to Greek Mediterranean Diet score and kcal to model 3. Again, results for cfPWV remained similar: β = −0.04 m/s, 95% CI, −0.09 to 0.00 for amount (h/wk); β = −0.34 m/s, 95% CI, −0.57 to −0.11 for insufficiently actives; β = −0.38 m/s, 95% CI, −0.65 to −0.11 for weekend warriors; and β = −0.43 m/s, 95% CI, −0.69 to −0.17 for regularly actives. Finally, we replaced MAP and HR during vascular measurements by MAP and HR from 24‐hour blood pressure measurements (in model 2, not shown). This resulted in similar findings for the association with cfPWV in model 3: β = −0.05 m/s, 95% CI, −0.10 to 0.00 for amount (h/wk); β = −0.33 m/s, 95% CI, −0.58 to −0.08 for insufficiently actives; β = −0.39 m/s, 95% CI, −0.69 to −0.10 for weekend warriors; and β = −0.51 m/s, 95% CI, −0.79 to −0.23 for regularly actives.
6. Discussion
This study on the associations between objectively measured higher intensity physical activity (further referred to as physical activity) amount in hours per week and patterns (categorical) and arterial stiffness, had 2 main findings. First, more physical activity was associated with lower cfPWV. Compared with the inactives, the insufficiently actives, regularly actives, and the weekend warriors all had a significantly lower cfPVW. No significant differences in cfPWV between the regularly actives and weekend warriors, nor with coefficient of variation of higher intensity physical activity over the week, were observed suggesting that the distribution of activity over the week does not matter. In addition, the association between physical activity and cfPWV was stronger in individuals with T2DM than in those without T2DM. Second, neither the amount of physical activity nor physical activity patterns was significantly associated with carotid DC.
Our findings on cfPWV are novel in 2 respects. First, previous studies have been small, 25 , 26 and have used questionnaires to evaluate the amount of physical activity, 4 whereas the current study was population‐based, used objective measures of physical activity, and evaluated not only amount of physical activity, but also physical activity patterns, which is an extension of previously conducted research. 27 Second, we observed a stronger association of physical activity with cfPWV in individuals with T2DM compared with individuals without T2DM.
The mechanisms underlying the association between physical activity and arterial stiffness are thought to include reduced vascular oxidative stress, increased endothelial nitric oxide production by shear stress, and augmented blood flow (during exercise), resulting in increased (bioavailability of) nitric oxide and improved vessel wall homeostasis. 9 , 10 , 11 In addition, physical activity may mitigate sympathetic hyperactivity and improve sympatho‐vagal balance, 28 which may result in less arterial stiffness. 29 The association between amount of physical activity and cfPWV was stronger in individuals with T2DM than in those without T2DM. In individuals with T2DM, oxidative stress and inflammation are known to be increased, resulting in higher arterial stiffness in T2DM. 30 This does not necessarily explain the interaction between physical activity and DM status, and this finding (which we regard as hypothesis‐generating) requires confirmation.
Our study showed no clear differences in the association of physical activity with cfPWV between weekend warriors and regularly actives. Moreover, the insufficiently actives had a significantly lower cfPWV compared with the inactives, suggesting that any physical activity is better than none. Further, the coefficient of variation as a continuous measure of variation of higher intensity physical activity over the week was not associated with cfPWV. So, the variation over the week is not important in the light of cfPWV. Previous studies have generally shown inverse associations of physical activity with other health outcomes such as metabolic syndrome, mortality, and CVD, regardless of physical activity pattern, 4 , 16 , 31 and these findings are broadly consistent with ours. In contrast, 1 study, in which physical activity was assessed by questionnaire, showed that in men with at least 1 cardiovascular risk factor, the mortality risk was lower in regularly actives than in weekend warriors. 17
We did not find an association of physical activity (or its patterns) with carotid DC. Prior data on the association between physical activity and carotid DC, whether based on observational data 32 , 33 , 34 or on randomized controlled trials, 35 , 36 have not been consistent. 32 , 33 , 34 , 35 , 36 Regional arterial stiffening (eg, carotid versus aortic) is known to be affected differently by risk factors, and this may be true also for physical activity, 37 although the mechanisms for such differences remain poorly understood.
This study has several strengths. We used posture‐based accelerometry data to measure physical activity. In addition, waterproof attachment ensured 24‐hour accelerometer data and prevented problems with the registration of nonwear time. This is the most reliable method to objectively measure physical activity. Additionally, the sample size of the study was large, and many potential confounders could be accounted for. Our study also had some limitations. First, this study was cross‐sectional in design, which makes it difficult to draw conclusions on causality. Second, as the accelerometer was worn for 1 week, it could be argued that this may not reflect habitual behavior in all subjects. However, in practice, this appears not to be an issue. 38 Third, as we analyzed associations with higher intensity physical activity based on step frequency as a measure of moderate‐to‐vigorous physical activity, which may be less precise than using acceleration data to determine intensity levels. Fourth, the study population consisted only of White participants, which limits the generalizability of our findings.
6.1. Perspectives
This large population‐based study showed that more physical activity was associated with lower cfPWV (ie, aortic stiffness), but not with carotid DC. There was no difference in cfPWV between the regularly actives and the weekend warriors; so from the perspective of arterial stiffness, the amount is of greater consequence than the pattern of higher intensity physical activity, especially in individuals with T2DM. Future research should aim to understand the biological pathway underlying the stronger association between physical activity and arterial stiffness in those with T2DM.
7. Sources of Funding
This study was supported by the European Regional Development Fund via OP‐Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (CVC; Maastricht), CARIM School for Cardiovascular Diseases (Maastricht), CAPHRI Care and Public Health Research Institute (Maastricht), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht), Stichting Annadal (Maastricht), and the Health Foundation Limburg (Maastricht), and by unrestricted grants from Janssen‐Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), and Sanofi‐Aventis Netherlands B.V. (Gouda, the Netherlands). This project was partly funded through a European Foundation for the Study of Diabetes award supported by AstraZeneca.
8. Disclosures
None.
Supporting information
Table S1
J Am Heart Assoc 2020;9:e05584 DOI: 10.1161/JAHA.120.017502.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017502
For Sources of Funding and Disclosures, see page 9.
References
- 1. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H. European Network for Non‐invasive Investigation of Large A. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;2588–2605. [DOI] [PubMed] [Google Scholar]
- 2. Mikael LR, Paiva AMG, Gomes MM, Sousa ALL, Jardim P, Vitorino PVO, Euzebio MB, Sousa WM, Barroso WKS. Vascular aging and arterial stiffness. Arq Bras Cardiol. 2017;253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all‐cause mortality: The hoorn study. J Am Coll Cardiol. 2014;1739–1747. [DOI] [PubMed] [Google Scholar]
- 4. O'Donovan G, Lee IM, Hamer M, Stamatakis E. Association of "weekend warrior" and other leisure time physical activity patterns with risks for all‐cause, cardiovascular disease, and cancer mortality. JAMA Intern Med. 2017;335–342. [DOI] [PubMed] [Google Scholar]
- 5. Lanier JB, Bury DC, Richardson SW. Diet and physical activity for cardiovascular disease prevention. Am Fam Physician. 2016;919–924. [PubMed] [Google Scholar]
- 6. Lachman S, Boekholdt SM, Luben RN, Sharp SJ, Brage S, Khaw KT, Peters RJ, Wareham NJ. Impact of physical activity on the risk of cardiovascular disease in middle‐aged and older adults: Epic norfolk prospective population study. Eur J Prev Cardiol. 2018;200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashor AW, Lara J, Siervo M, Celis‐Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2014;9:e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasankari V, Husu P, Vaha‐Ypya H, Suni J, Tokola K, Halonen J, Hartikainen J, Sievanen H, Vasankari T. Association of objectively measured sedentary behaviour and physical activity with cardiovascular disease risk. Eur J Prev Cardiol. 2017;1311–1318. [DOI] [PubMed] [Google Scholar]
- 9. Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;797–812. [DOI] [PubMed] [Google Scholar]
- 10. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation. 2010;1221–1238. [DOI] [PubMed] [Google Scholar]
- 11. Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S. Circulating endothelial and progenitor cells: evidence from acute and long‐term exercise effects. World J Cardiol. 2012;312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the framingham heart study. Circulation. 2010;505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weggemans RM, Backx FJG, Borghouts L, Chinapaw M, Hopman MTE, Koster A, Kremers S, van Loon LJC, May A, Mosterd A, et al. The 2017 dutch physical activity guidelines. Int J Behav Nutr Phys Act. 2018;58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for americans. JAMA. 2018;2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiroma EJ, Lee IM, Schepps MA, Kamada M, Harris TB. Physical activity patterns and mortality: The weekend warrior and activity bouts. Med Sci Sports Exerc. 2019;35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr. The "weekend warrior" and risk of mortality. Am J Epidemiol. 2004;636–641. [DOI] [PubMed] [Google Scholar]
- 18. Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RM, Stehouwer CD. The maastricht study: An extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;439–451. [DOI] [PubMed] [Google Scholar]
- 19. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace‐Raso FU, Protogerou AD, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;445–448. [DOI] [PubMed] [Google Scholar]
- 20. Hermeling E, Reesink KD, Kornmann LM, Reneman RS, Hoeks AP. The dicrotic notch as alternative time‐reference point to measure local pulse wave velocity in the carotid artery by means of ultrasonography. J Hypertens. 2009;2028–2035. [DOI] [PubMed] [Google Scholar]
- 21. Willekes C, Hoeks AP, Bots ML, Brands PJ, Willigers JM, Reneman RS. Evaluation of off‐line automated intima‐media thickness detection of the common carotid artery based on m‐line signal processing. Ultrasound Med Biol. 1999;57–64. [DOI] [PubMed] [Google Scholar]
- 22. Reneman RS, Meinders JM, Hoeks AP. Non‐invasive ultrasound in arterial wall dynamics in humans: What have we learned and what remains to be solved. Eur Heart J. 2005;960–966. [DOI] [PubMed] [Google Scholar]
- 23. van der Berg JD, Willems PJ, van der Velde JH, Savelberg HH, Schaper NC, Schram MT, Sep SJ, Dagnelie PC, Bosma H, Stehouwer CD, et al. Identifying waking time in 24‐h accelerometry data in adults using an automated algorithm. J Sports Sci. 2016;1867–1873. [DOI] [PubMed] [Google Scholar]
- 24. Organization WH . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a who/idf consultation. 2006.
- 25. O'Donovan C, Lithander FE, Raftery T, Gormley J, Mahmud A, Hussey J. Inverse relationship between physical activity and arterial stiffness in adults with hypertension. J Phys Act Health. 2014;272–277. [DOI] [PubMed] [Google Scholar]
- 26. Aoyagi Y, Park H, Kakiyama T, Park S, Yoshiuchi K, Shephard RJ. Yearlong physical activity and regional stiffness of arteries in older adults: The nakanojo study. Eur J Appl Physiol. 2010;455–464. [DOI] [PubMed] [Google Scholar]
- 27. Andersson C, Lyass A, Larson MG, Spartano NL, Vita JA, Benjamin EJ, Murabito JM, Esliger DW, Blease SJ, Hamburg NM, et al. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle‐aged adults. J Am Heart Assoc. 2015;9:e001528 DOI: 10.1161/JAHA.114.001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ino‐Oka E, Sekino H, Ohtaki Y, Inooka H. Effects of daily physical activity level on the degree of sympathetic tone. Intern Med. 2009;19–24. [DOI] [PubMed] [Google Scholar]
- 29. Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;979–984. [DOI] [PubMed] [Google Scholar]
- 30. Funck KL, Laugesen E, Hoyem P, Fleischer J, Cichosz SL, Christiansen JS, Hansen TK, Poulsen PL. Low physical activity is associated with increased arterial stiffness in patients recently diagnosed with type 2 diabetes. Am J Hypertens. 2016;882–888. [DOI] [PubMed] [Google Scholar]
- 31. Metzger JS, Catellier DJ, Evenson KR, Treuth MS, Rosamond WD, Siega‐Riz AM. Associations between patterns of objectively measured physical activity and risk factors for the metabolic syndrome. Am J Health Promot. 2010;161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palve KS, Pahkala K, Magnussen CG, Koivistoinen T, Juonala M, Kahonen M, Lehtimaki T, Ronnemaa T, Viikari JS, Raitakari OT. Association of physical activity in childhood and early adulthood with carotid artery elasticity 21 years later: The cardiovascular risk in young finns study. J Am Heart Assoc. 2014;9:e000594 DOI: 10.1161/JAHA.113.000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parsons TJ, Sartini C, Ellins EA, Halcox JPJ, Smith KE, Ash S, Lennon LT, Wannamethee SG, Lee IM, Whincup PH, et al. Objectively measured physical activity, sedentary time and subclinical vascular disease: Cross‐sectional study in older british men. Prev Med. 2016;194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boss HM, van der Graaf Y, Visseren FLJ, Van den Berg‐Vos RM, Bots ML, de Borst GJ, Cramer MJ, Kappelle LJ, Geerlings MI. Physical activity and characteristics of the carotid artery wall in high‐risk patients‐the smart (second manifestations of arterial disease) study. J Am Heart Assoc. 2017;9:e005143 DOI: 10.1161/JAHA.116.005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magalhaes JP, Melo X, Correia IR, Ribeiro RT, Raposo J, Dores H, Bicho M, Sardinha LB. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: A 1‐year randomized controlled trial. Cardiovasc Diabetol. 2019;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slivovskaja I, Ryliskyte L, Serpytis P, Navickas R, Badariene J, Celutkiene J, Puronaite R, Ryliskiene K, Cypiene A, Rinkuniene E, et al. Aerobic training effect on arterial stiffness in metabolic syndrome. Am J Med. 2018;148–155. [DOI] [PubMed] [Google Scholar]
- 37. Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: Determinants of discrepancies. Hypertension. 2006;371–376. [DOI] [PubMed] [Google Scholar]
- 38. Aguilar‐Farias N, Martino‐Fuentealba P, Salom‐Diaz N, Brown WJ. How many days are enough for measuring weekly activity behaviours with the activpal in adults? J Sci Med Sport. 2019;684–688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


