Abstract
Background
Coronary artery disease remains a major cause of death despite better outcomes of ST‐segment–elevation myocardial infarction (STEMI). We aimed to analyze data from the Ruti‐STEMI registry of in‐hospital, 28‐day, and 1‐year events in patients with STEMI over the past 3 decades in Catalonia, Spain, to assess trends in STEMI prognosis.
Methods and Results
Between February 1989 and December 2017, a total of 7589 patients with STEMI were admitted consecutively. Patients were grouped into 5 periods: 1989 to 1994 (period 1), 1995 to 1999 (period 2), 2000 to 2004 (period 3), 2005 to 2009 (period 4), and 2010 to 2017 (period 5). We used Cox regression to compare 28‐day and 1‐year STEMI mortality and in‐hospital complication trends across these periods. Mean patient age was 61.6±12.6 years, and 79.3% were men. The 28‐day all‐cause mortality declined from period 1 to period 5 (10.4% versus 6.0%; P<0.001), with a 40% reduction after multivariable adjustment (hazard ratio [HR], 0.6; 95% CI, 0.46–0.80; P<0.001). One‐year all‐cause mortality declined from period 1 to period 5 (11.7% versus 9.0%; P=0.001), with a 24% reduction after multivariable adjustment (HR, 0.76; 95% CI, 0.60–0.98; P=0.036). A significant temporal reduction was observed for in‐hospital complications including postinfarct angina (−78%), ventricular tachycardia (−57%), right ventricular dysfunction (−48%), atrioventricular block (−45%), pericarditis (−63%), and free wall rupture (−53%). Primary ventricular fibrillation showed no significant downslope trend.
Conclusions
In‐hospital STEMI complications and 28‐day and 1‐year mortality rates have dropped markedly in the past 30 years. Reducing ischemia‐driven primary ventricular fibrillation remains a major challenge.
Keywords: prognosis, ST‐segment–elevation myocardial infarction, STEMI complications, STEMI mortality
Subject Categories: Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- ICCU
intensive cardiac care unit
Clinical Perspective
What Is New?
The Ruti‐STEMI is a population‐based registry that included consecutive patients with ST‐segment–elevation myocardial infarction in a Mediterranean cohort over the past 3 decades.
Most in‐hospital ST‐segment–elevation myocardial infarction complications have decreased by 50%.
Twenty‐eight‐day case fatality declined 40% mainly because of the reduction in early acute‐phase mortality in both anterior‐ and inferior‐wall ST‐segment–elevation myocardial infarction; 1‐year all‐cause mortality also decreased 25% from 1989 to 2017, though in the past decade mortality rates have remained quite stable, mainly driven by cardiogenic shock.
What Are the Clinical Implications?
Furthermore, there are still few targets to improve: Ischemia‐driven primary ventricular fibrillation rates have not substantially changed over time.
Some mechanical complications (ventricular septal rupture and papillary muscle rupture) also remain without changes in the past 3 decades.
The first description of myocardial infarction is attributed to James B. Herrick in 1912. 1 The century that followed has seen major changes in medicine, and myocardial infarction has been at the forefront. Galvez‐Montón et al 2 divided the history of myocardial infarction into three eras: an initial era of mainly clinical observation and description of pathophysiology (1912–1950s); a second era centered on coronary care units, the discovery of new drugs, and the development of surgical alternatives (1960s–1980s); and the most recent era of interventional cardiology, focused on invasive cardiology and devices (1980s to present).
ST‐segment–elevation myocardial infarction (STEMI) management has undergone an impressive transformation in recent times because of the establishment of regional and national reperfusion networks and use of newer evidence‐based drugs. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Some studies used only in‐hospital 3 or short‐term outcomes, 5 , 7 , 11 , 12 while others tracked the evolution of acute coronary syndrome outcomes in the last quarter of the 20th century, before percutaneous reperfusion was widely implemented. 7 , 9 , 10 Some studies have used data for nonconsecutive patients enrolled in cross‐sectional registries with defined inclusion and exclusion criteria. 11
Accordingly, the aim of this study using data from the Ruti‐STEMI registry was to conduct a population‐based analysis of trends in 28‐day and 1‐year mortality and in‐hospital complications over the past 3 decades. Our goal was to provide a contemporary real‐life perspective of STEMI prognosis in a Mediterranean region.
Methods
Study Population
The Ruti‐STEMI registry is a prospective population‐based registry maintained from February 1989 to December 2017 and including all consecutive patients with STEMI serving a stable and well‐defined geographic area of ≈850 000 inhabitants in the northern metro area of Barcelona in Catalonia, Spain (Figure 1). During the 30‐year period, the healthcare physical structure has remained stable, with only 1 university hospital with an intensive cardiac care unit (ICCU) and 4 community hospitals that refer patients with STEMI to the ICCU (Figure 1). Several organizational changes have occurred during the registry period. Until the year 2000, reperfusion therapy was mainly performed with fibrinolysis; between 2000 and 2009, primary percutaneous coronary intervention (PCI) was performed only during working hours. A major change in June 2009 was the establishment of the Codi IAM STEMI network, intended as a reperfusion network that prioritizes primary PCI for all patients with STEMI 24 hours a day, 7 days a week. The setup of the Codi IAM network, including the territorial organization and available resources, has been described previously. 13 , 14
Figure 1. Map of the University and community hospitals.
Geographical distribution of the Ruti‐STEMI population‐based registry, in northern Barcelona metro area.
Definitions of myocardial infarction and standard of care were based on current guidelines available during the study life span. 15 , 16 , 17 , 18 STEMI was defined as ST‐segment elevation of ≥1 mm in at least 2 contiguous leads (in V2–V3 ≥2 mm was required) in any location in the index or qualifying ECG.
In our present analysis, patients were stratified by years of admission into 5 periods: 1989 to 1994 (period 1), 1995 to 1999 (period 2), 2000 to 2004 (period 3), 2005 to 2009 (period 4), and 2010 to 2017 (period 5).
All study procedures were in accordance with the ethical standards outlined in the Declaration of Helsinki. Patients provided written consent for use of their clinical data for research purposes. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Outcomes
The aim of the study was to analyze in‐hospital STEMI complications and trends in 28‐day and 1‐year case fatality over the past 3 decades. The primary end points were 28‐day and 1‐year all‐cause mortality. Mortality was curated from patient health records or by direct phone contact with patients or relatives and double‐verified by the Catalan and Spanish health system databases.
Secondary end points included changes in in‐hospital STEMI complications during the 5 studied periods: angina, reinfarction, primary ventricular fibrillation (VF) and tachycardia, atrioventricular block, atrial fibrillation/flutter, ventricular septum or papillary muscle or free wall rupture, pericarditis, right ventricular dysfunction, and maximum Killip–Kimball class. The definitions of these complications were standardized and remained stable during the study period.
Statistical Analysis
Categorical variables are expressed with frequency and percentages and continuous variables as mean±SD. Statistical differences between groups were compared using the chi‐squared and Student t test or analysis of variance including linear trend analysis. Departures from normality were evaluated by normal QQ plots. Multivariate analysis was performed with logistic regression or proportional Cox regression models (backward conditional stepwise method), with the following covariates: age, sex, cardiovascular risk factors, previous acute myocardial infarction, peripheral artery disease, Killip–Kimball class, and reperfusion therapies. Assumption of linearity of continuous variables (logistic regression and Cox) and proportionality (Cox) was tested. Trend curves were graphically fitted using polynomial regression, as they provide better fits to the nonlinear data. Period of admission was treated as a continuous measure for trend testing. Probability values <0.05 from 2‐sided tests were considered to indicate statistical significance. All analyses were performed using the software Statistics SPSS 21 (IBM, Armonk, NY) and STATA version 13.0 (StataCorp, College Station, TX).
Results
A total of 7589 consecutive patients with STEMI were included in the Ruti‐STEMI registry. The mean age was 61.6±12.6 years, and 79.3% were men. Relative to the year of admission, patients were grouped into the 5 periods defined above: 1989 to 1994, n=1337; 1995 to 1999, n=960; 2000 to 2004, n=1059; 2005 to 2009, n=1535; and 2010 to 2017, n=2698. Baseline demographic and clinical characteristics of each group are shown in Table 1. Prevalence in dyslipidemia and hypertension increased over the 3 decades, but peripheral disease and previous acute coronary syndrome showed a significant declining trend. The use of reperfusion therapy increased over time, with a relative change of 114%, the use of primary PCI tripled between period 3 and period 5, while fibrinolysis evolved to negligible levels. Use of coronary angiography at any time during the index admission increased to 97% in 2017.
Table 1.
Demographic Characteristics, Medical Therapies, and Management of Patients With STEMI Among the 5 Periods
| Characteristics* |
Period 1 1989–1994 (N=1337) |
Period 2 1995–1999 (N=960) |
Period 3 2000–2004 (N=1059) |
Period 4 2005–2009 (N=1535) |
Period 5 2010–2017 (N=2698) |
P for Trend |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 61.9 (13.8) | 61.8 (12.4) | 62.2 (11.8) | 62.5 (13.1) | 62.1 (12.9) | 0.481 |
| Men, % | 77.0 | 77.9 | 80.4 | 78.1 | 76.9 | <0.001 |
| Smoker, % | 46.2 | 43.2 | 38.1 | 39.8 | 45.0 | <0.001 |
| Hypertension, % | 45.8 | 46.9 | 49.5 | 52.3 | 54.2 | <0.001 |
| Dyslipidemia, % | 39.5 | 39.8 | 50.2 | 56.6 | 58.4 | <0.001 |
| Diabetes mellitus, % | 25.1 | 27.5 | 31.1 | 27.3 | 24.5 | <0.001 |
| Peripheral disease, % | 9.9 | 10.0 | 11.6 | 12.7 | 7.1 | 0.005 |
| Stroke, % | … | … | … | … | 2.3 | … |
| Kidney disease, % | … | … | … | … | 4.8 | … |
| Previous AMI | 19.7 | 17.6 | 17.5 | 15.8 | 12.0 | <0.001 |
| Killip–Kimball class* | <0.001 | |||||
| I | 73.4 | 74.7 | 77.1 | 79.4 | 81.2 | |
| II | 10.8 | 10.1 | 9.8 | 8.8 | 10.4 | |
| III | 8.0 | 8.5 | 7.6 | 5.6 | 2.5 | |
| IV | 7.3 | 6.4 | 5.5 | 5.8 | 6.0 | |
| AMI location | <0.001 | |||||
| Anterior wall | 42.6 | 41.7 | 46.5 | 49.3 | 45.2 | |
| Inferior wall | 57.4 | 58.3 | 53.5 | 50.3 | 54.8 | |
| Medications | ||||||
| Aspirin, % | 91.7 | 92.9 | 93.2 | 96.6 | 97.8 | <0.001 |
| Clopidogrel, % | … | … | 25.4 | 78.6 | 89.3 | <0.001 |
| Ticagrelor, % | … | … | … | … | 2.5 | … |
| Prasugrel, % | … | … | … | … | 12.4 | … |
| GPIIb/IIIa inh. % | … | 0.1 | 12.2 | 38.1 | 25.1 | <0.001 |
| Heparin, % | 59.3 | 64.9 | 71.9 | 64.5 | 71.2 | <0.001 |
| Low‐molecular‐weight heparin, % | 3.8 | 4.4 | 30.7 | 44.0 | 30.7 | <0.001 |
| β‐Blockers, % | 51.0 | 74.2 | 82.7 | 83.7 | 81.3 | <0.001 |
| Statins, % | 1.6 | 0.1 | 26.3 | 75.6 | 88.0 | <0.001 |
| ACE inhibitor/ARB, % | 20.5 | 26.0 | 41.0 | 54.2 | 54.8 | <0.001 |
| Lidocaine, % | 22.9 | 15.8 | 7.6 | 6.0 | 2.4 | <0.001 |
| Amiodarone, % | 7.9 | 8.6 | 7.5 | 6.9 | 4.9 | <0.001 |
| Reperfusion, % | 42.8 | 54.7 | 57.7 | 78.9 | 91.9 | <0.001 |
| Fibrinolysis, % | 100 | 100 | 66.7 | 22.1 | 0.9 | |
| Primary PCI, % | 0 | 0 | 33.3 | 77.9 | 99.1 | |
| Rescue PCI, % | 0 | 0 | 0.7 | 4.6 | 0.9 | |
| Time onset‐reperfusion min, median (IQR) | … | … | 170 (138) | 240 (210) | 185 (170) | <0.001 |
| Coronary angiography | 3.1 | 5.2 | 27.3 | 70.6 | 97 | <0.001 |
| CABG, % | 1.2 | 0.2 | 3.0 | 2.9 | 1.1 | <0.001 |
| PAC, % | 5.1 | 4.8 | 3.5 | 14.6 | 1.5 | <0.001 |
| IABP, % | … | 0.2 | 1.7 | 3.0 | 2.8 | <0.001 |
| LVAD, % | … | … | … | … | 0.9 | … |
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; GPIIB/IIIa inh, glycoprotein IIb/IIIa inhibitors; IABP, intra‐aortic balloon pump; IQR, interquartile range; LVAD, left ventricular assist device (Impella CP); PAC, pulmonary artery catether; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Maximum Killip–Kimball class.
The use of evidence‐based treatments during the first 48 hours from admission increased gradually over the 3‐decade period. Early use of β‐blockers increased 59%, use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers increased 167%, and statins use increased from 1.6% to 88.0%. Likewise, use of antithrombotic medications during the first 48 hours of admission changed markedly, with increasing early use of aspirin (6.6% relative increase) and clopidogrel, which use was 2.5‐fold higher from period 3 to period 5; in period 5, ticagrelor and prasugrel were incorporated. Moreover, the use of low‐molecular‐weight heparin and glycoprotein IIb/IIIa inhibitors peaked in period 4.
In‐hospital STEMI complications were prospectively registered during these 3 decades (Table 2), and most declined significantly. Postinfarct angina decreased 78%; sustained ventricular tachycardia 57%; atrioventricular block 45%, free wall rupture 153%, acute‐phase atrial fibrillation/flutter 27%; pericarditis 63%, and right ventricular dysfunction 48%. Trends in most relevant in‐hospital complications over the 5 periods are shown in Figure 2.
Table 2.
In‐Hospital Prognosis and Mortality
|
Period 1 1989–1994 (N=1337) |
Period 2 1995–1999 (N=960) |
Period 3 2000–2004 (N=1059) |
Period 4 2005–2009 (N=1535) |
Period 5 2010–2017 (N=2698) |
P for Trend | |
|---|---|---|---|---|---|---|
| Angina, % | 9.8 | 10.3 | 8.9 | 6.0 | 2.1 | <0.001 |
| Reinfarction, % | 1.3 | 1.5 | 2.1 | 1.7 | 1.7 | 0.686 |
| Primary VF, % | 7.6 | 6.9 | 6.9 | 6.6 | 6.8 | 0.114 |
| VT, % | 8.7 | 7.7 | 4.4 | 7.8 | 3.7 | <0.001 |
| AV block, % | 9.7 | 12.5 | 6.2 | 5.8 | 5.3 | <0.001 |
| AFib/flutter, % | 8.4 | 11.9 | 8.5 | 7.6 | 6.1 | <0.001 |
| VS rupture, % | 0.7 | 0.8 | 0.9 | 0.6 | 0.5 | 0.609 |
| PM rupture, % | 0.3 | 0.5 | 0.4 | 0.7 | 0.3 | 0.066 |
| FW rupture, % | 1.9 | 2.1 | 1.4 | 0.7 | 0.9 | 0.002 |
| Pericarditis, % | 7.6 | 3.6 | 2.8 | 2.1 | 2.8 | <0.001 |
| RV dysfunction, % | 9.6 | 12.1 | 6.9 | 5.1 | 5.0 | <0.001 |
| ACCU LoS, d | 5.0 | 5.5 | 4.7 | 3.3 | 2.4 | <0.001 |
| ACCU mortality, % | 8.9 | 8.1 | 5.8 | 3.8 | 4.2 | <0.001 |
| Anterior wall AMI | 11.2 | 11.0 | 6.1 | 4.3 | 4.7 | <0.001 |
| Inferior wall AMI | 8.9 | 8.1 | 5.8 | 3.4 | 3.8 | <0.001 |
| 28‐d mortality, % | 10.4 | 9.9 | 7.3 | 5.1 | 6.0 | <0.001 |
| 1‐y mortality, % | 11.7 | 13.4 | 10.5 | 8.7 | 9.0 | <0.001 |
ACCU indicates acute cardiovascular care unit; AFib, atrial fibrillation; AV, atrioventricular; FW, free‐wall; LoS, length of stay; PM, papillary muscle; RV, right ventricle; VF, ventricular fibrillation; VS, ventricular septum; and VT, sustained ventricular tachycardia.
Figure 2. Trends in STEMI in‐hospital complications among periods.
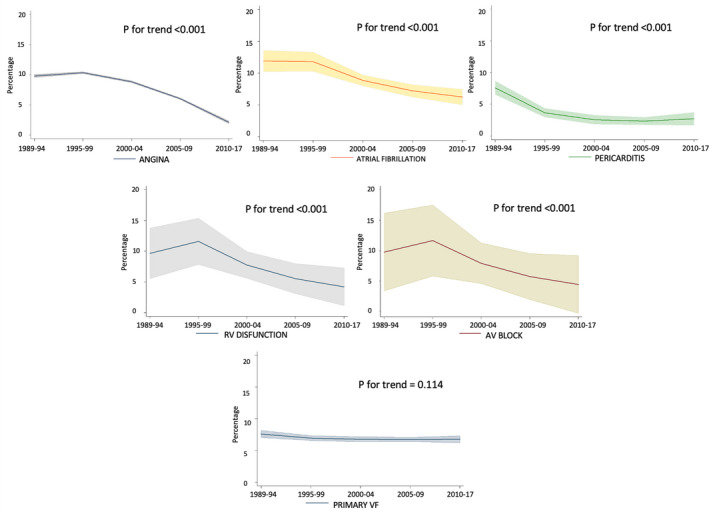
Angina (top left); atrial fibrillation (top middle); pericarditis (top right); right ventricular (RV) dysfunction (middle left); atrioventricular block (middle right); primary ventricular fibrillation (VF; bottom). Shaded regions represents 95% CI. AV indicates atrioventricular; and STEMI, ST‐segment–elevation myocardial infarction.
Acute heart failure complicating STEMI also showed a downslope trend over time. Patients with maximum Killip–Kimball class III‐IV (pulmonary edema or cardiogenic shock) showed a 45% reduction from period 1 to period 5 (15.3% versus 8.5%, respectively; P<0.001), with 81.2% of patients in Killip–Kimball I in the period 5 group. Ventricular septal rupture and papillary muscle rupture, both infrequent mechanical complications of STEMI, remained unchanged at <1% throughout the study period. Of note, primary VF was the only prevalent acute‐phase complication that showed no significant downslope trend over the past 3 decades (7.6% in period 1 versus 6.8% in period 5, respectively; P=0.114).
The remarkable reductions in STEMI complications observed during the study period allowed for a significant reduction in ICCU length of stay, from 5.0 days in period 1 to 2.4 days in period 5 (P<0.001). Early mortality, defined as occurring in the ICCU, was cut by half during the study period, from 8.9% to 4.2% (percentage change, −4.7% [95% CI, −2.9% to −6.3%]), 11.2% versus 4.7% for anterior‐wall acute myocardial infarction and 8.9% versus 3.8% for inferior‐wall infarcts; all P for trend <0.001) (Figure 3).
Figure 3. Trends in changes in 28‐day case fatality related to infarct location between periods.
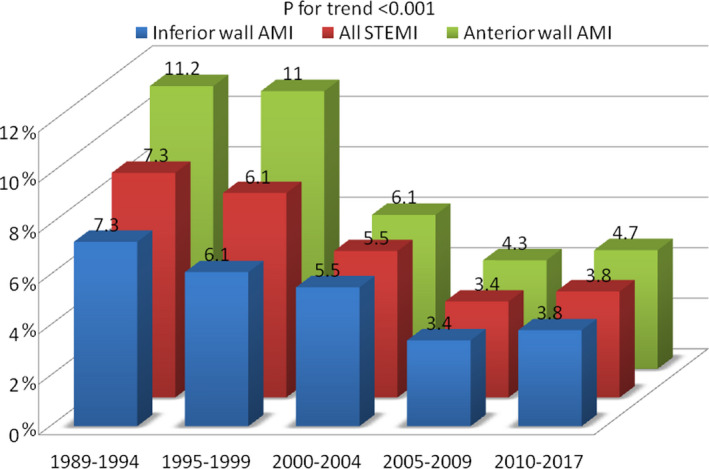
Inferior wall AMI (blue), anterior wall AMI (green), all STEMI (red). AMI indicates acute myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
Short‐term 28‐day STEMI mortality showed a progressive downslope trend over the past 3 decades, from 10.4% in period 1 to 6.0% in period 5 (P<0.001) (Table 2; Figure 4A). After multivariable adjustment by age, sex, hypertension, diabetes mellitus, dyslipidemia, peripheral disease, previous acute myocardial infarction, Killip–Kimball class, and reperfusion therapy, 28‐day case‐fatality rates declined by 40% over time (hazard ratio [HR], 0.60; 95% CI, 0.46–0.80; P<0.001; Table 3). Figure 5 illustrates 28‐day mortality by study period stratified according to maximum Killip–Kimball class. Mortality benefits over time were significant across all Killip–Kimball strata, and especially so in patients with Killip–Kimball class IV, despite 59% and 50% of all 28‐day deaths were attributable to patients with Killip–Kimball class IV in periods 4 and 5, respectively. This benefit was mainly observed in the early acute phase, during ICCU admission (Figure 6).
Figure 4. Kaplan–Meier curves among periods.
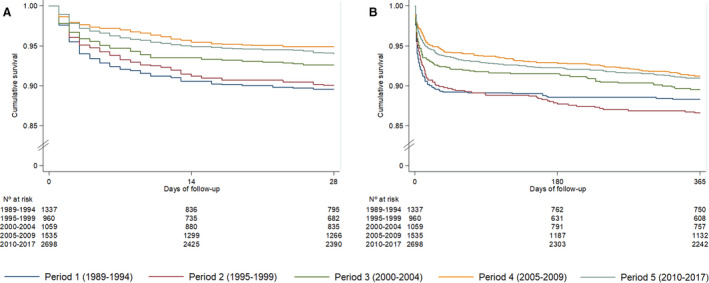
A, 28‐day case fatalities and (B) 1‐year all‐cause mortality among patients with STEMI during the 5 periods: period 1 (dark blue), period 2 (brown), period 3 (green), period 4 (orange) and period 5 (light blue). STEMI indicates ST‐segment–elevation myocardial infarction.
Table 3.
Multivariable Cox Regression Analyses for 28‐Day Case Fatality and 1‐Year All‐Cause Mortality
| 28‐d Case‐Fatality | 1‐y All‐Cause Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
Model 1 HR (95% CI) |
P Value |
Model 2 HR (95% CI) |
P Value |
Model 1 HR (95% CI) |
P Value |
Model 2 HR (95% CI) |
P Value | |
| 1989–1994 | 1 | … | 1 | … | 1 | … | 1 | … |
| 1995–1999 | 0.92 (0.70–1.21) | 0.56 | 1.08 (0.81–1.43) | 0.602 | 1.07 (0.83–1.36) | 0.612 | 1.21 (0.93–1.57) | 0.150 |
| 2000–2004 | 0.68 (0.51–0.91) | 0.009 | 0.81 (0.60–1.09) | 0.168 | 0.82 (0.64–1.06) | 0.132 | 0.89 (0.68–1.17) | 0.419 |
| 2005–2009 | 0.46 (0.35–0.61) | <0.001 | 0.51 (0.37–0.69) | <0.001 | 0.66 (0.52–0.84) | 0.001 | 0.70 (0.54–0.92) | 0.010 |
| 2010–2017 | 0.55 (0.43–0.69) | <0.001 | 0.61 (0.46–0.80) | <0.001 | 0.69 (0.56–0.86) | 0.001 | 0.76 (0.60–0.98) | 0.036 |
Model 1: Crude 28‐day case fatality or 1‐year all‐cause mortality by period; Model 2: Including as covariates age, sex (women), hypertension, diabetes mellitus, dyslipidemia, peripheral disease, previous AMI, Killip–Kimball class, interaction between age‐Killip and reperfusion therapy. AMI indicates acute myocardial infarction; and HR, hazard ratio.
Figure 5. Twenty‐eight‐day case fatality among periods depending on maximum Killip class during hospital admission.
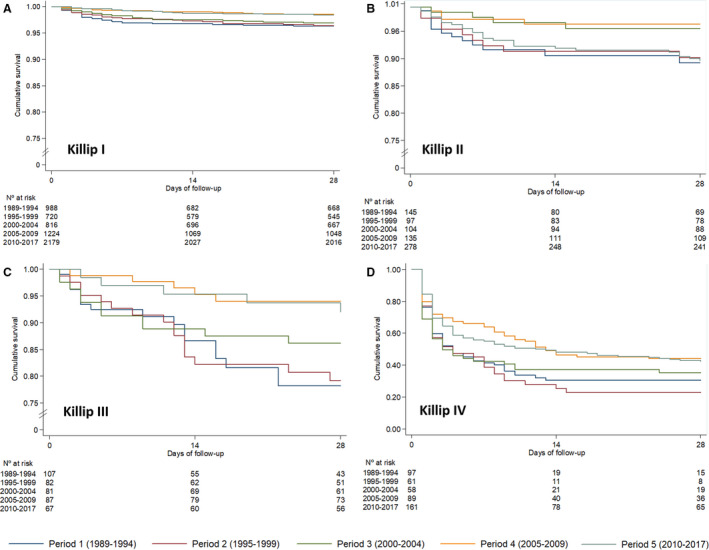
A, Killip I; B, Killip II; C, Killip III; D, Killip IV. Period 1 (dark blue), period 2 (brown), period 3 (green), period 4 (orange) and period 5 (light blue).
Figure 6. Early acute‐phase mortality relative to maximum Killip–Kimball class during intensive cardiac care unit admission.
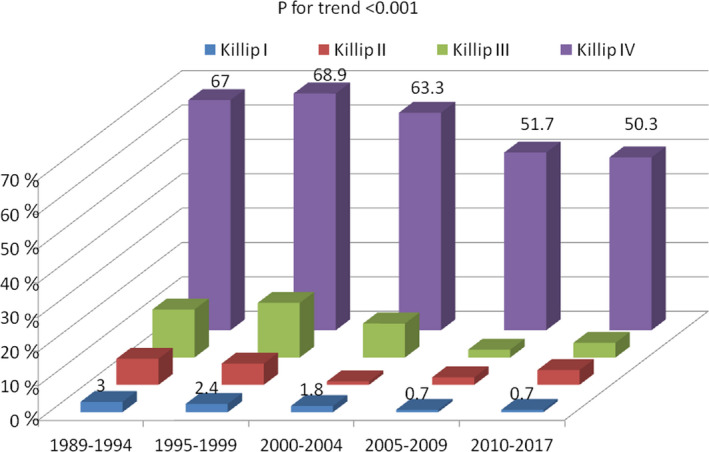
Period 1 (dark blue), period 2 (red), period 3 (green), period 4 (violet) and period 5 (light blue).
Long‐term 1‐year all‐cause STEMI mortality also showed a downslope trend over the past 3 decades, from 11.7% in 1989–1994 to 9.0% in 2010–2017 (P<0.001) (Table 2; Figure 4B). After multivariable adjustment by age, sex, Killip–Kimball class, hypertension, diabetes mellitus, dyslipidemia, peripheral disease, previous acute myocardial infarction, and reperfusion therapy, 1‐year all‐cause mortality decreased by 24% over time (HR, 0.76; 95% CI, 0.60–0.98; P=0.036; Table 3).
Discussion
In this report, we provide outcomes data from a large population‐based registry of patients with STEMI (N=7589) living in a Mediterranean region in Catalonia from 1989 to 2017. In the Ruti‐STEMI registry, we identified a 40% reduction in 28‐day and 25% reduction in 1‐year STEMI mortality over the past 3 decades, together with a 60% decrease in early mortality (during ICCU hospitalization) with decreases in both anterior (−68%) and inferior (−57%) STEMI mortality. Detailed data during the hospitalization period allowed for a refined characterization of in‐hospital complications. Most STEMI‐driven in‐hospital complications were reduced by 50% or more over the past 3 decades, except for primary VF and some low‐prevalence mechanical complications (papillary muscle and septal rupture), whose incidence remained unaltered (Figure 7).
Figure 7. Trends in STEMI outcomes among 3 decades in a Mediterranean population‐based registry.
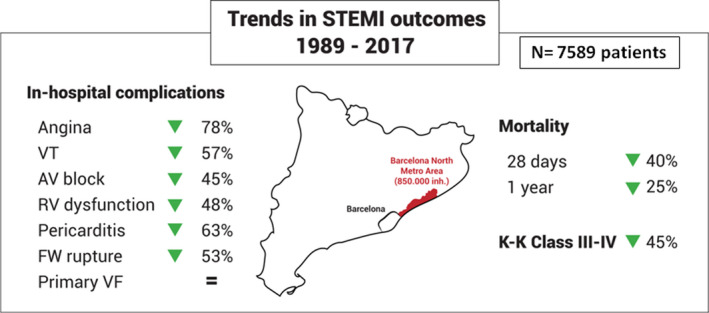
Most in‐hospital STEMI (ST‐segment–elevation myocardial infarction) complications have been reduced above 50%: angina, right ventricular dysfunction, pericarditis, atrioventricular block, ventricular tachycardia (VT), heart failure, and free‐wall (FW) rupture, although primary ventricular fibrillation (VF) remains without changes. Twenty‐eight‐day case fatality declined 40% and 1‐year mortality has been reduced 25% among the past 3 decades. K‐K indicates Killip–Kimball.
The past 3 decades have witnessed a surge of new treatments for STEMI and new organizational networks to prioritize percutaneous reperfusion based on the premise that “time is myocardium.” Several studies, mainly from Northern Europe and the United States, have already yielded data on the short‐ and long‐term impacts of these novel strategies, 5 , 7 , 11 , 19 , 20 although most included a mix of patients with and without STEMI, representing different clinical entities with different management and outcomes. In the Worcester registry, 5 in‐hospital mortality fell from 9.6% to 6.5% from 2001 to 2011, but >64% of their patients were non‐STEMI. Yeh et al 7 reported 30‐day mortality reduction from 10.5% to 7.8% in the US state of California during 1999 to 2008; in that registry, benefit was associated mostly with patients without STEMI, without significant changes among those with STEMI (odds ratio, 0.93; 95% CI, 0.71–1.20). The European Heart Survey, including only STEMI cases, 10 revealed a 30‐day 15% decline in case fatalities from 2000 to 2004, with 6.4% of the mortality decreased in the last period, similar to our data for periods 4 and 5 (covering 2005–2017). The FAST‐MI (French Registry on Acute ST‐Elevation and Non–ST‐Elevation Myocardial Infarction) registries, covering 1995 to 2010, 11 showed a 30‐day 60% reduction in case fatalities after adjustment by patient profile and reperfusion. These authors point to the increase in evidence‐based medical therapies (antithrombotic agents, statins, β‐blockers, and angiotensin‐converting enzyme inhibitors) as one factor in the improved prognosis for patients with STEMI. We observed a similar increase in evidence‐based medical therapies in our study. Nevertheless, the most relevant decline observed in our series also coincides with the implementation of primary PCI, initially during normal working hours (2000–2009) and available 24 hours a day, 7 days a week since 2009.
Data on long‐term prognosis in real‐life STEMI registries are scarce. In this study, we observed a 25% reduction in 1‐year adjusted STEMI mortality over the 3 studied decades. In addition to the above‐reported benefits of acute reperfusion and implementation of newer drugs and devices, we observed a remarkable decline in post‐STEMI heart failure, a major driver of adverse outcomes in the long term. In the Ruti‐STEMI registry, we found a significant increase in Killip–Kimball I infarcts (without heart failure) and a 45% reduction in Killip–Kimball class III to IV STEMIs from period 1 to period 5. Indeed, cardiogenic shock—defined as Killip‐Kimball class IV—declined by 18% over 3 decades, similar to the results of the Worcester registry. 21 In our population, cardiogenic shock developed in 6% of patients with STEMI in period 5, much like the findings reported in the Swedish STEMI registry. 22 These data are in agreement with Bayes‐Genis et al, 23 who previously reported having proportionally fewer patients with heart failure with reduced ejection fraction and more patients with heart failure and midrange ejection fraction since the implementation of the STEMI Codi IAM network in Catalonia. Current evidence supports much better outcomes for patients with reduced versus midrange ejection fraction.
Regarding in‐hospital complications, our data are in line with previously reported studies. 24 , 25 As an example, in our last period, high‐grade atrioventricular block appeared in 5.3% of patients STEMI, similar to the 6% reported in the recent European Heart Survey. 24 The widespread use of primary PCI also underlies the reduction in some mechanical complications, mainly free‐wall rupture. 26 Of note, in our series, we found no reduction in ventricular septal rupture or papillary muscle rupture, in contrast with findings in a recent study of elderly acute myocardial infarction patients. 26 Ventricular septal rupture still developed in 0.5% of patients, similar to the 0.6% reported in a recent Swiss registry. 27
There are reasons for optimism but not for complacency in the reported trends. There are (at least) 2 issues of concern. On one hand, no reduction in mortality was achieved in the past decade, very likely attributable to acute cardiogenic shock mortality. Indeed, cardiogenic shock is the challenge of the present decade if we wish to improve our performance in acute STEMI. On the other hand, a major finding of the Ruti‐STEMI registry is the lack of reduction in ischemia‐driven primary VF over the past 3 decades. In our registry, primary VF occurred in 7.6% of patients in period 1 and in 6.8% in period 5, without statistical differences over time. Similar trends in primary VF and malignant arrhythmias have been reported previously in other population cohorts. 28 The widespread use of reperfusion therapies has probably reduced in‐hospital primary VF, but a recent report indicated that 75% of all primary VFs occurred out of hospital. 29 The STEMI reperfusion network prioritizes emergent attention to cardiac patients with a prompt transfer, which allows more patients to arrive alive at the hospital. Patients have more probabilities to arrive alive at the hospital after an out‐of‐hospital cardiac arrest attributable to PVF after the onset of primary PCI reperfusion networks. In the clinical treatment of patients with STEMI, the current standard is based on the premise that primary VF is benign in terms of long‐term prognosis if the patient survives to discharge. However, recent registry data challenge this impression, suggesting that primary VF is an independent predictor of short‐ and long‐term mortality. 29 In sum, our data emphasize the need to seek novel out‐of‐the‐box strategies for preventing and managing primary VF. Ischemia could be speculated to be a second hit for primary VF in a myocardium with existing susceptibility from genetic or inflammatory determinants of arrhythmia, offering potential targets for risk stratification.
Study Limitations
We acknowledge the lack of valid prospective all‐comers echocardiography data from the start of the registry, precluding us from providing individual patient ejection fraction. This lack is a major limitation of most, if not all, real‐life registries. Second, a historical cohort study covering 3 decades implies changes in some definitions of cardiovascular risk factors (diabetes mellitus, hypertension, and hypercholesterolemia), which may have affected their prevalence across periods. Moreover, a better knowledge of coronary risk factors may have also affected treatment, that is, different management of hypertension or dyslipidemia over time. The definition of myocardial infarction has also changed over time, as have the biomarkers used for myocyte injury. Nevertheless, the essential diagnostic criteria for STEMI remained the presence of symptoms and compatible ECG findings. Injury biomarkers are not needed to initiate treatment in STEMI. In patients undergoing primary PCI for STEMI, the type of stent (bare metal or drug‐eluting stent) is not available in this registry. Finally, although follow‐up data were double‐verified through the Catalan and Spanish health system databases, we cannot exclude potential sources of error in the early stages. If this were the case, the number of events in early stages would be even greater and the reduction over time still higher.
Conclusions
The results of the prospective all‐comers Ruti‐STEMI registry over 3 decades in a well‐defined Mediterranean area indicate that 28‐day STEMI case fatality declined by 40%, and 1‐year all‐cause mortality declined by 24% from 1989 to 2017. In‐hospital complications also decreased by ≥50%, although primary VF and some mechanical complications showed stable rates over time. Generalization of reperfusion therapy, with the widespread use of primary PCI more recently, and the use of evidence‐based medical therapies have been the main drivers underlying the progressive decline in most STEMI complications. Tackling ischemia‐driven primary VF requires imagination and divergent thinking because everything that has been done so far has been essentially futile.
Appendix
The RUTI‐STEMI Investigators: Maria José Martínez‐Membrive, Elisabeth Zamora, Angel Caballero, Cinta Llibre, Oriol Rodríguez‐Leor, Eduard Fernandez‐Nofrerias, Omar Abdul‐Jawad, Victoria Vilalta.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
The authors thank Drs Antoni Curós and Jordi Serra for their vision and perseverance in data collection and their invaluable clinical work during these 3 decades. The authors also thank Susanna Tello, Marta Cabañero, and Leny Franco (from Institut Hospital del Mar d’Investigacions Mèdiques) and Sandra Rios and Pilar Gomariz (from Germans Trias Hospital) for their roles in data management and performing database maintenance. We also express special gratitude to all doctors, residents, and nurses who participated in the Ruti‐STEMI registry over the past 3 decades.
J. Am. Heart Assoc. 2020;9:e017159 DOI: 10.1161/JAHA.120.017159.
Dr Teresa Oliveras is currently located at the Department of Medicine, Autonomous University of Barcelona.
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Cosme García‐García, Email: cosmecg7@gmail.com.
the Ruti‐STEMI Investigators:
Maria José Martínez‐Membrive, Elisabeth Zamora, Angel Caballero, Cinta Llibre, Oriol Rodríguez‐Leor, Eduard Fernandez‐Nofrerias, Omar Abdul‐Jawad, and Victoria Vilalta
References
- 1. Herrick JB. Clinical features of sudden obstruction of the coronary arteries. JAMA. 1912;1757–1765. [PubMed] [Google Scholar]
- 2. Gálvez‐Montón C, Ordoñez‐Llanos J, de Luna AB, Bayes‐Genis A. One hundred years of myocardial infarction. Eur Heart J. 2012;2888–2891. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade long trends (2001–2011) in the incidence and hospital death rates associated with the in‐hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velagapudi P, Kolte D, Ather K, Khera S, Gupta T, Gordon PC, Aronow HD, Kirtane AJ, Abbott JD. Temporal trends and factors associated with prolonged length of stay in patients with ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2018;185–191. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg RJ, Tisminetzky M, Tran HV, Yarzebski J, Lessard D, Gore JM. Decade long trends (2001–2011) in the incidence rates of initial acute myocardial infarction. Am J Cardiol. 2019;206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;2388–2398. [DOI] [PubMed] [Google Scholar]
- 7. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;2155–2165. [DOI] [PubMed] [Google Scholar]
- 8. Goldberg R, Yarzebski J, Lessard D, Gore JM. A two‐decade (1975 to 1995) long experience in the incidence, in‐hospital and long‐term case‐fatality rates of acute myocardial infarction: a community‐wide perspective. J Am Coll Cardiol. 1999;1533–1539. [DOI] [PubMed] [Google Scholar]
- 9. Gil M, Marrugat J, Sala J, Gil M, Marrugat J, Sala J, Masiá R, Elosua R, Albert X, Pena A, et al. Relationship of therapeutic improvements and 28‐day case fatality in patients hospitalized with acute myocardial infarction between 1978 and 1993 in the REGICOR study, Gerona, Spain. Circulation. 1999;1767–1773. [DOI] [PubMed] [Google Scholar]
- 10. Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, Filippatos G, Gitt A, Hasdai D, Hasin Y, Marrugat J, et al.; Euro Heart Survey Investigators . The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;2285–2293. [DOI] [PubMed] [Google Scholar]
- 11. Puymirat E, Simon T, Steg PG, Schiele F, Gueret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, et al; USIK USIC 2000 Investigators; FAST MI Investigators . Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;998–1006. [DOI] [PubMed] [Google Scholar]
- 12. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, et al. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet. 2014;1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosch X, Curós A, Argimon JM, Faixedas M, Figueras J, Jiménez‐Fàbrega FX, Masià R, Mauri J, Tresserras R. Model of primary percutaneous intervention in Catalonia. Rev Esp Cardiol. 2011;1034–1040. [Google Scholar]
- 14. Carrillo X, Fernandez-Nofrerias E, Rodriguez-Leor O, Oliveras T, Serra J, Mauri J, Curos A, Rueda F, García-García C, Tresserras R, et al; Codi IAM Investigators . Early ST elevation myocardial infarction in non‐capable percutaneous coronary intervention centres: in situ fibrinolysis vs. percutaneous coronary intervention transfer. Eur Heart J. 2016;37:1034–1040. [DOI] [PubMed] [Google Scholar]
- 15. Gunnar RM, Bourdillon PD, Dixon DW, Fuster V, Karp RB, Kennedy JW, Klocke FK, Passamani ER, Pitt B, Rapaport E, et al. ACC/AHA guidelines for the early management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on assessment of diagnostic and therapeutic cardiovascular procedures. Circulation. 1990;664–707. [DOI] [PubMed] [Google Scholar]
- 16. Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, et al. Management of acute myocardial infarction in patients presenting with ST‐segment elevation. Task Force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2003;28–66. [DOI] [PubMed] [Google Scholar]
- 17. Steg PG, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2012;2569–2619. [DOI] [PubMed] [Google Scholar]
- 18. Ibañez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;119–177. [DOI] [PubMed] [Google Scholar]
- 19. Sala C, Grau M, Masia R, Vila J, Subirana I, Ramos R, Aboal J, Sureda A, Brugada R, Marrugat J, et al. Trends in Q‐wave acute myocardial infarction case fatality from 1978 to 2007 and analisys of the effectiveness of different treatments. Am Heart J. 2011;444–450. [DOI] [PubMed] [Google Scholar]
- 20. Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty‐two year trends in incidence of myocardial infarction, CHD mortality, and case‐fatality in four US communities, 1987 to 2008. Circulation. 2012;1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty‐year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population‐based perspective. Circulation. 2009;1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Redfors B, Angerås O, Råmunddal T, Dworeck C, Haraldsson I, Ioanes D, Petursson P, Libungan B, Odenstedt J, Stewart J, et al. 17‐year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int J Cardiol. 2015;256–262. [DOI] [PubMed] [Google Scholar]
- 23. Bayes‐Genis A, García‐Garcia C, de Antonio M, Fernandez‐Nofrerías E, Domingo M, Zamora E, Moliner P, Lupón J. Impact of a “stent for life” initiative on post‐ST elevation myocardial infarction heart failure: a 15 year heart failure clinic experience. ESC Heart Fail. 2018;101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh SM, FitzGerald G, Yan AT, Brieger D, Fox KA, López‐Sendón J, Yan RT, Eagle KA, Steg PG, Budaj A, et al. High grade atrioventricular block in acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. Eur Heart J. 2015;976–983. [DOI] [PubMed] [Google Scholar]
- 25. Kundu A, O’Day K, Shaikh AY, Lessard D, Saczynski JS, Yarzebski J, Darling C, Thabet R, Akhter M, Floyd KC, et al. Relation of atrial fibrillation in acute myocardial infarction to in‐hospital complications and early hospital readmission. Am J Cardiol. 2016;1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puerto E, Viana‐Tejedor A, Martínez‐Sellés M, Domínguez‐Pérez L, Moreno G, Martín‐Asenjo R, Bueno H. Temporal trends in mechanical complications of acute myocardial infarction in the elderly. J Am Coll Cardiol. 2018;959–966. [DOI] [PubMed] [Google Scholar]
- 27. Lanz J, Wyss D, Räber L, Stortecky S, Hunziker L, Blöchlinger S, Reineke D, Englberger L, Zanchin T, Valgimigli M, et al. Mechanical complications in patients with ST‐segment elevation myocardial infarction: a single centre experience. PLoS One. 2019;9:e0209502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson CA, Yarzebski J, Goldberg RJ, Lessard D, Gore JM, Dalen JE. Changes over time in the incidence and case‐fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: perspectives from the Worcester Heart Attack Study. Am Heart J. 2000;1014–1022. [DOI] [PubMed] [Google Scholar]
- 29. García‐García C, Oliveras T, Rueda F, Pérez‐Fernández S, Ferrer M, Serra J, Labata C, Vila J, Carrillo X, Rodríguez‐Leor O, et al. Primary ventricular fibrillation in the primary percutaneous coronary intervention ST‐segment elevation myocardial infarction era (from the “Codi IAM” Multicenter Registry). Am J Cardiol. 2018;529–536. [DOI] [PubMed] [Google Scholar]


