Abstract
Background
Endovascular repair has become a viable alternative for aortic pathological features, including those located within the aortic arch. We investigated the anatomic suitability for branched thoracic endovascular repair in patients previously treated with conventional open surgery for aortic arch pathological features.
Methods and Results
Patients who underwent open surgery for aortic arch pathological features at our institution between 2000 and 2018 were included. Anatomic suitability was determined by strict compliance with the anatomic criteria within manufacturers’ instructions for use for each of the following branched thoracic stent grafts: Relay Plus Double‐Branched (Terumo‐Aortic), TAG Thoracic Branch Endoprosthesis (W.L. Gore & Associates), Zenith Arch Branched Device (Cook‐Medical), and Nexus Stent Graft System (Endospan Ltd/Jotec GmbH). Computed tomography angiography images were analyzed with outer luminal line measurements. A total of 377 patients (mean age, 64±14 years; 64% men) were identified, 153 of whom had suitable computed tomography angiography images for measurements. In total, 59 patients (15.6% of the total cohort and 38.6% of the measured cohort) were eligible for endovascular repair using at least one of the devices. Device suitability was 30.9% for thoracic aneurysms, 4.6% for type A dissections, 62.5% for type B dissections, and 28.6% for other pathological features.
Conclusions
The anatomic suitability for endovascular repair of all aortic arch pathological features was modest. The highest suitability rates were observed for thoracic aneurysms and for type B dissections, of which repair included part of the aortic arch. We suggest endovascular repair of arch pathological features should be reserved for high‐volume centers with experience in endovascular arch repair.
Keywords: anatomic suitability, aortic arch, branched stent grafts, cardiothoracic surgery, novel treatment, thoracic endovascular repair, vascular surgery
Subject Categories: Aneurysm, Aortic Dissection, Cardiovascular Surgery, Stent, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CTA
computed tomography angiography
- IFU
instructions for use.
Clinical Perspective
What Is New?
This study is the largest analysis to date that has aimed to evaluate patients’ suitability for endovascular repair of aortic arch pathological features based on aortic arch anatomical features and instructions for use of novel stent grafts.
According to anatomic criteria, 16% of patients treated with open surgery for any aortic arch pathological feature were suitable for endovascular repair.
The most frequent indications that exclude patients from endovascular repair of the aortic arch are a large proximal landing zone diameter in the ascending aorta and type A dissection pathological feature.
What Are the Clinical Implications?
The novel findings are important for optimizing the anatomic screening process for patients with aortic arch pathological features.
Acknowledgement of the frequent anatomic indications that exclude patients from endovascular repair candidacy will provide key information to improve the design and accessibility of stent graft devices.
Traditionally, pathological features of the aortic arch are treated by open surgical approach, and continuous improvements within the domain of cardiothoracic surgery have led to decreased mortality and morbidity rates after open surgery. 1 However, the invasive nature of these procedures combined with advanced cardiovascular disease renders >20% of patients unfit for surgery. 2 , 3 The high rate of patients rejected for open surgery calls for alternative treatment options. Since the introduction of minimally invasive treatments, endovascular solutions have expanded rapidly to treat more complex aortic pathological features. 4 , 5 Thoracic endovascular aortic repair is a viable solution in patients with descending aortic pathological features, including both aneurysms and dissections. 6 , 7 Thoracic endovascular aortic repair combined with adjunct procedures, like debranching techniques, chimneys, fenestrations, and branches, is progressing into the aortic arch while maintaining cerebral perfusion. 5 , 8
Early reports of the use of branched stent grafts for aortic arch pathological features demonstrated low in‐hospital mortality rates and no differences in 5‐year aneurysm‐related mortality. 9 , 10 , 11 , 12 However, stroke rates are considerably high compared with open repair; Tazaki et al presented stroke rates of 11% compared with 2% to 4% after open repair. 13 Furthermore, a high level of expertise is required under specific circumstances, such as emergency surgery and the repair of aortic arch pathological features in zone 0, as evidenced by the high incidence of mortality and stroke. 14 , 15 Because of the aforementioned complication rates and the anatomical restrictions of currently available stent grafts, open surgical repair remains the gold standard for aortic arch pathological features. 16 , 17
The high degree of anatomic complexity within the region of the aortic arch makes it challenging to develop stent grafts suitable for endovascular repair. As anatomic restrictions vary between devices, the previously reported rates of anatomic feasibility for endovascular repair in patients with type A aortic dissection vary between 0% and 50%. 18 , 19 , 20 , 21 Adding to the complexity, the type of aortic arch pathological feature may contribute to changes in aortic anatomical features differently; only 7% of patients with aortic arch aneurysms were suitable for endovascular repair. 22 Also, Stanford type B aortic dissections often require sealing of the stent graft in the aortic arch, covering the left subclavian artery or even extending to the carotid artery or brachiocephalic artery. 23 Consequently, device manufacturers are continuously evolving stent graft designs, expanding the suitability to the aortic arch, and as a result, the rate of anatomic suitability for branched endovascular repair for patients with aortic arch pathological features remains dynamic. 18 , 19 , 24 This study aims to determine the anatomic feasibility of current commercially available branched stent grafts to treat patients with aortic arch pathological features by endovascular means; these patients previously underwent open surgical repair.
Methods
The data, methods used in the analysis, and materials used to conduct the research will be made available to other researchers for purposes of reproducing the results or replicating the procedure on reasonable request. The Amsterdam University Medical Centre, location Vrije Universiteit, Ethics Committee approved the following protocol. All participants gave their informed consent to preoperative computed tomography angiography (CTA) diagnostic imaging. Informed consent was not required for the retrospective analysis, as assessed by the local ethics commission.
Patients with thoracic aortic pathological features who underwent open surgical or a hybrid treatment between 2000 and 2018 at the departments of vascular surgery and cardiothoracic surgery at the Amsterdam University Medical Centre, location Vrije Universiteit, Amsterdam, the Netherlands, were included. Within the study period, no endovascular repair of the aortic arch was performed at our institution. Patients were excluded on the basis of the presence of: descending thoracic aneurysms treatable with a sufficient seal zone using conventional thoracic endovascular aortic repair, stenotic aortic lesions, vascular abnormalities, traumatic lesions, aortitis, endocarditis, mycotic ulcers, combined treatment for aortic root aneurysms and primary valve insufficiency, coronary artery disease requiring surgical revascularization, and secondary interventions after earlier sternotomy. Patients meeting the inclusion criteria were included if they had ascending, arch, or proximal descending thoracic aortic pathological features with insufficient seal zone in the proximal descending aorta for conventional endovascular repair (sealing needed in the aortic arch).
Suitability was determined according to anatomic criteria derived from the instructions for use (IFU) of 4 currently available branched stent grafts for aortic arch pathological features, including the Relay Plus Double‐Branch (Terumo Aortic, Sunrise, FL), TAG Thoracic Branch Endoprosthesis (W.L. Gore & Associates Inc, Flagstaff, AZ), Zenith Arch Branched Device (Cook, Bloomington, IN), and the Nexus Aortic Arch Stent Graft System (Endospan Ltd, Herzliya, Israel), distributed by JOTEC GmbH (Hechingen, Germany) (Figure 1).
Figure 1. Overview of the 4 aortic arch branched stent grafts reviewed.
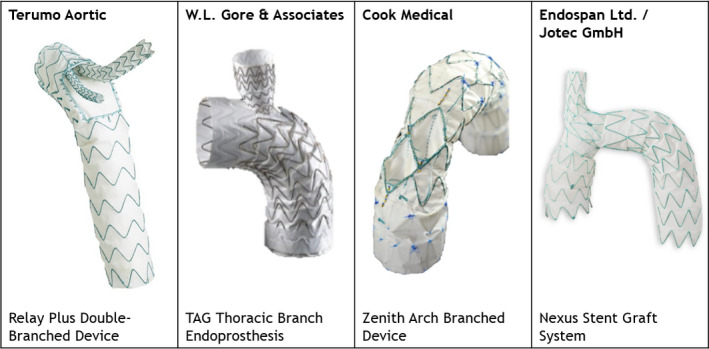
Images are courtesy of Terumo Aortic, W.L. Gore & Associates, Cook Medical, and Endospan Ltd/Jotec.
To determine suitability, preoperative CTA images were gathered for patients who received open repair. If preoperative imaging was not available or of poor quality, patients were excluded. Furthermore, in the case of type A dissections or thoracic aortic aneurysms, patients were deemed unsuitable if the operation reports described valve insufficiency or coronary artery involvement requiring intervention (valve replacement or reimplantation of coronary arteries into the vascular prosthesis).
Preoperative CTAs were analyzed for suitability with use of postimaging software Horos 3.3 (Purview, Annapolis, MD). Imaging analysis was performed on the basis of an outer luminal line from the aortic root to the descending aorta, manually created from a curved multiplanar reconstruction after seed point placement (Figure 2A). According to the IFU, diameter and length measurements were performed perpendicular to the outer luminal line and straight multiplanar reconstruction (Figure 2B). Clock‐face orientation angle measurements of supra‐aortic vessels were performed within an orthogonal plane along the outer luminal line (Figure 2C). The developed 3‐dimensional workstation measurement protocol encompassed anatomical variables from all IFU guidelines.
Figure 2. Creation of the outer lumen line and diameter, length, and clock face.
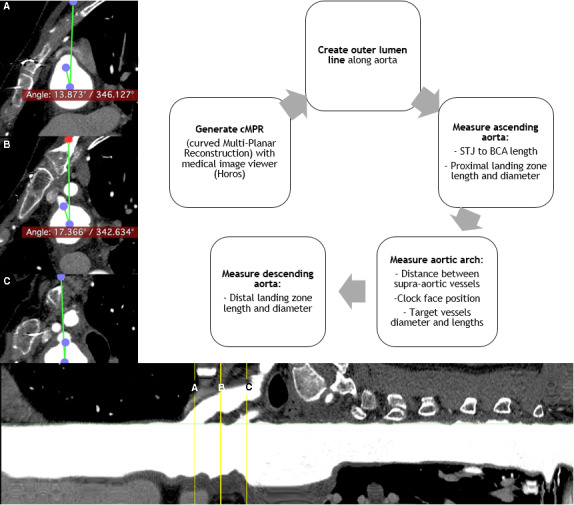
Angle measurements at the left subclavian artery (A), left common carotid artery (B), and brachiocephalic trunk (C). BCA indicates brachiocephalic artery; cMPR, curved multiplanar reconstruction; and STJ, sinotubular junction.
Suitability after debranching procedures using the Thoracic Branch Endoprosthesis (Gore) was investigated in patients unsuitable for proximal stent graft placement in zone 2 or 1. The distribution of aortic arch zones is detailed in Figure 3. In patients with distal arch pathological features, the proximal landing zone assessment was reconsidered according to the IFU, after moving proximally by zone until suitability was achieved or an inadequate zone 0 landing was observed. For pathological features in zone 2 or 3, the landing zone length was measured between the distal portion of the branch target vessel and the central luminal point of the vessel proximal to the target vessel. For example, if the targeted vessel was the left subclavian artery, the length between the distal portion of the left subclavian artery and central luminal point of the left common carotid artery was measured, as the proximal end of the endoprosthesis consists of partially uncovered stent apices.
Figure 3. Aortic arch zone distribution and Stanford classification of the ascending aorta and brachiocephalic artery (zone 0), left common carotid artery (zone 1), left subclavian artery (zone 2), and descending aorta (zone 3).
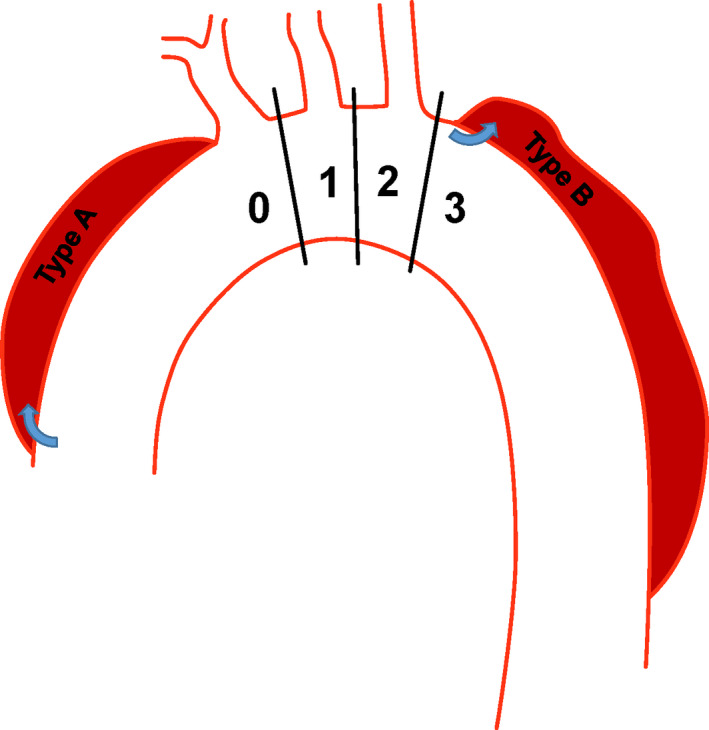
Type A dissections commence from the ascending aorta involving the arch arteries. Type B dissections commence distal to the left subclavian artery.
Statistical Analysis
Data were analyzed with SPSS 26.0 (SPSS Inc, Chicago, IL) statistical software for statistical analysis. Anatomic measurement sets were tested for normality using the Shapiro‐Wilk test. Frequencies and descriptive statistics were calculated. Those with a normal distribution were expressed as a mean±SD, and those deviating from a normal distribution were expressed as a median (range). Measures of frequency (suitability and exclusions) are described as an overall number (percentage).
Results
Our search identified 913 patients who underwent treatment for thoracic aortic pathological features; the screening process is depicted in Figure 4. After selection of potential arch pathological features, 377 patients were included. The average age of patients was 64±14 years, 64% were men, and 50% of patients were treated in an emergency setting; further baseline characteristics are displayed in Table 1.
Figure 4. Flowchart of the patient selection and measurement outcome.
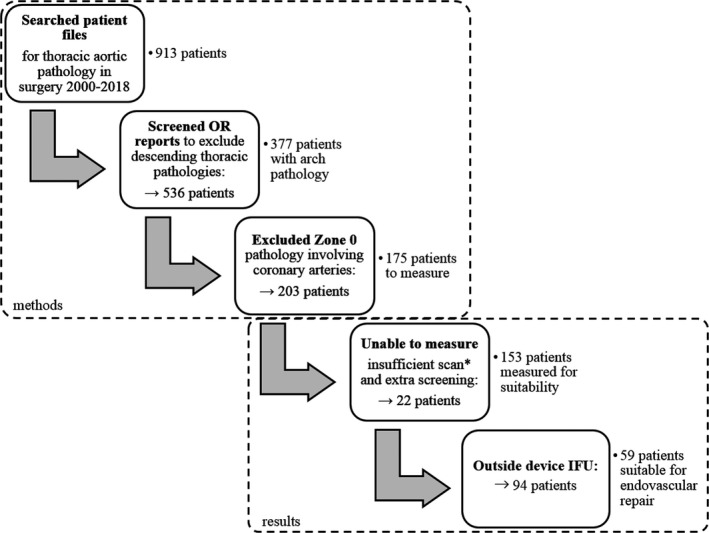
*Insufficient scan: supra‐aortic arteries outside field of view. IFU indicates instructions for use; and OR, operation report.
Table 1.
Patient Baseline Characteristics
|
Baseline characteristics (n=377) |
No. (%) or Mean (SD) |
|---|---|
| Age, y | 64±14 |
| Men | 240 (64) |
| Tobacco use | 105 (27.8) |
| Nonsmoker | 59 (15.6) |
| Current smoker | 96 (25.4) |
| Former smoker | 118 (31.2) |
|
Diabetes mellitus |
11 (2.9) |
| Unknown | 19 (5.0) |
| Hypertension |
274 (72.5) |
| Unknown | 19 (5.0) |
| Hyperlipidemia |
126 (33.3) |
| Unknown | 20 (5.3) |
| Carotid disease |
28 (7.4) |
| Unknown | 20 (5.3) |
| Coronary disease |
60 (15.9) |
| Unknown | 20 (5.3) |
| Renal failure |
19 (5.0) |
| Unknown | 20 (5.3) |
| Pulmonary disease |
49 (13.0) |
| Unknown | 21 (5.6) |
| ASA score |
40 (10.6) |
| I | 67 (17.7) |
| II | 127 (33.6) |
| III | 71 (18.8) |
| IV | 5 (1.3) |
| V | 61 (16.1) |
ASA indicates American Society of Anesthesiologists.
Pathological features treated within the patient cohort included thoracic aortic aneurysms (29%), type A dissections (62%), type B dissections (4%), and other pathological features (4%). Other pathological features include ruptured thoracic aneurysms, intramural hematomas, vascular transection, Kommerell diverticulum, and penetrating atherosclerotic ulcer; all incidences are reported in Table 2.
Table 2.
Aortic Arch Cohort (n=377) According to Pathological Features and Arch Zone
| Pathological Feature/Arch Zone | Zone 0 | Zone 1 | Zone 2 | Zone 3 | Total Patients, N (%) |
|---|---|---|---|---|---|
| TAA | 46 | 12 | 21 | 31 | 110 (29) |
| Type A dissection | 234 | 3 | 0 | 0 | 237 (63) |
| Type B dissection | 0 | 1 | 6 | 9 | 16 (4) |
| Other* | 5 | 1 | 5 | 3 | 14 (4) |
| Total patients, N (%) | 285 (75) | 17 (4) | 32 (8) | 43 (12) | 377 (100) |
TAA indicates Thoracic Aortic Aneurysm.
Other indicates ruptured thoracic aneurysm, intramural hematoma, vascular transection, Kommerell diverticulum, and penetrating atherosclerotic ulcer.
From the remaining 377 patients, 203 were rendered unsuitable after screening operation reports for valve or coronary artery involvement in zone 0, resulting in 175 patients to measure. Of these 175 patients, 22 were unable to be analyzed because of an insufficient CTA scan with supra‐aortic arteries outside field of view or extra operation report screening. This resulted in a total of 153 patients measured for suitability.
Patient Suitability
In total, 59 patients were suitable for at least one thoracic endovascular aortic stent graft. From the patient cohort with aortic arch pathological features previously treated with open repair (n=377), this results in a percentage of 15.6% suitability. From the measurement cohort (n=153) after the exclusion of pathological features involving the coronary arteries in zone 0 and patients with insufficient imaging, this resulted in a percentage of 38.6% suitability.
All results of patient suitability by pathological features are displayed in Table 3 and by zone in Table 4. For the Relay Plus Double‐Branched (Terumo Aortic) stent graft, 26 of 377 (6.9%) patients fulfilled the IFU anatomic criteria, with the highest suitability by pathological feature of 4 of 16 (25.0%) for type B dissections and 16 of 32 (50%) for zone 2. The rate of suitability for the Thoracic Branch Endoprosthesis (W.L Gore & Associates) stent graft was 46 of 377 (12.2%) with debranching and 28 of 377 (7.4%) without debranching, with the highest suitability by pathological features of 9 of 16 (56.3%) for type B dissections and 17 of 32 (53.1%) for zone 2. Patient suitability for the Zenith Arch Branched Device (Cook Medical) stent graft was 21 of 377 (5.6%), with the highest suitability by pathological features of 4 of 16 (25.0%) for type B dissections and 8 of 32 (25%) for zone 2. Last, for the Nexus (Endospan/Jotec) stent graft, which requires debranching in all cases, 31 of 377 (8.2%) patients were suitable, with the highest suitability by pathological features of 5 of 16 (31.3%) for type B dissections and 13 of 32 (40.6%) for zone 2.
Table 3.
Patient Suitability Result by Arch Pathological Feature in Relation to the Total Arch Pathological Feature Cohort
| Variable |
Thoracic Aneurysm |
Type A Dissection | Type B Dissection | Other |
Patients Suitable |
|---|---|---|---|---|---|
|
Terumo Relay Plus Double‐Branched |
19/110 (17.3) |
Excluded 0/237 (0) |
4/16 (25) |
3/14 (21.4) |
26/377 (6.9)* |
|
Gore TAG Thoracic Branch Endoprosthesis |
28/110 (25.5) |
6/237 (2.5) |
9/16 (56.3) |
3/14 (21.4) |
46/377 (12.2) † |
|
Cook Zenith Arch Branched Device |
13/110 (11.8) |
2/237 (0.8) |
4/16 (25.0) |
2/14 (14.3) |
21/377 (5.5) |
|
Endospan/Jotec Nexus Stent Graft System |
15/110 (13.6) |
8/237 (3.4) |
5/16 (31.3) |
3/14 (21.4) |
31/377 (8.2) ‡ |
| Any device/total cohort |
34/110 (30.9) |
11/237 (4.6) |
10/16 (62.5) |
4/14 (28.6) |
59/377 (15.6) |
| Any device/measured |
34/56 (60.7) |
11/80 (13.8) |
10/11 (90.9) |
4/6 (66.7) |
59/153 (38.6) |
Data are given as number/total (percentage).
Device instructions for use exclude type A dissection; 26 of 144 (18.1%) with type A dissection excluded.
Including debranching of supra‐aortic arch arteries. Without debranching: 28 of 377 (7.4%).
With debranching of supra‐aortic arch arteries for all patients.
Table 4.
Patient Suitability Result by Zone
| Variable | Zone 0 | Zone 1 | Zone 2 | Zone 3 |
Patients Suitable |
|---|---|---|---|---|---|
|
Terumo Relay Plus Double‐Branched |
0/285 (0) |
1/17 (5.9) |
16/32 (50.0) |
9/43 (20.9) |
26/377 (6.9)* |
|
Gore TAG Thoracic Branch Endoprosthesis |
7/285 (2.5) |
3/17 (17.6) |
17/32 (53.1) |
19/43 (44.2) |
46/377 (12.2) † |
|
Cook Zenith Arch Branched Device |
2/285 (0.7) |
3/17 (17.6) |
8/32 (25.0) |
8/43 (18.6) |
21/377 (5.5) |
|
Endospan/Jotec Nexus Stent Graft System |
7/285 (2.5) |
3/17 (17.6) |
13/32 (40.6) |
8/43 (18.6) |
31/377 (8.2) ‡ |
| Any device/total cohort |
11/285 (3.9) |
5/17 (29.4) |
23/32 (71.9) |
20/43 (46.5) |
59/377 (15.6) |
| Any device/measured |
11/95 (11.6) |
5/7 (71.4) |
23/28 (82.1) |
20/23 (87.0) |
59/153 (38.6) |
Data are given as number/total (percentage).
Device instructions for use exclude type A dissection; 26 of 144 (18.1%) with type A dissection excluded.
Including debranching of supra‐aortic arch arteries. Without debranching: 28 of 377 (7.4%).
With debranching of supra‐aortic arch arteries for all patients.
The 2 pathological features with the highest suitability rates for any device were type B dissections (62.5%) and thoracic aneurysms (30.9%). The 2 zones with highest suitability for any device were zone 2 (71.9%) and zone 3 (83.3%).
Measurement Results
The diameter, length, and angle measurement results are displayed in Table 5. An overview of exclusion rates is detailed in absolute numbers and percentages. The bolded percentages indicate measurement locations with exclusion rates >30%. For the Relay Plus Double‐Branched (Terumo Aortic) stent graft, 2 anatomic parameters resulted in an exclusion rate >30%: the proximal landing zone (zone 0+60 mm), which excluded 32%; and the distal left common carotid artery diameter, with an exclusion rate of 33%. For the Thoracic Branch Endoprosthesis (W.L Gore & Associates) stent graft, the proximal brachiocephalic artery diameter excluded 37% and the mid brachiocephalic artery to distal left common carotid artery excluded 78%. For the Zenith Arch Branched Device (Cook Medical) stent graft, the proximal landing zone (zone 0–total) diameter excluded 54%. For the Nexus (Endospan/Jotec) stent graft, the proximal landing zone (zone 0–brachiocephalic artery) diameter excluded 65% and the distal brachiocephalic artery diameter excluded 43%.
Table 5.
Number of Patient Exclusions Based on Diameter, Length, and Angle Measurements of the Aortic Arch and Supra‐Aortic Vessels
| Aortic Diameters, mm* |
Mean±SD or Median (Range) |
Exclusions, N (%) | |||
|---|---|---|---|---|---|
|
Relay Plus Double‐Branch Device (Terumo) |
TAG Thoracic Branch Endo‐Prosthesis (Gore) |
Zenith Arch Branched Device (Cook) |
Nexus Stent Graft System (Endospan/ Jotec) |
||
| Proximal LZ (zero point) (n=59) | 36.1 (25.6–74.9) | 11 (19) | – | – | – |
| Proximal LZ (zone 0+45 mm) (n=18)† | 35.4±3.8 | 0 (0) | – | – | – |
| Proximal LZ (zone 0+60 mm) (n=38)† | 42.3 (30.7–81.3) | 12 (32) | – | – | – |
| Proximal LZ (zone 0–total) (n=76) | 38.4 (26.0–81.3) | – | 10 (18) | 41 (54) | – |
| Proximal LZ (zone 0–BCA) (n=153) | 44.5±11.4 | – | – | – | 100 (65) |
| Proximal LZ (zone 1) (n=23)† | 34.2±3.7 | – | 0 (0) | – | – |
| Proximal LZ (zone 2) (n=22) | 32.7±4.1 | – | 0 (0) | – | – |
| Proximal BCA (n=76) | 17.5 (12.7–30.3) | 9 (15) | 21 (37)‡ | 8 (11) | – |
| Distal BCA+25 mm (n=57)§ | 14.1 (10.8–21.1) | 1 (2) | – | – | – |
| Distal BCA (n=153) | 18.8±4.6 | – | – | – | 67 (43) |
| Distal LCCA (n=75)|| | 7.5±1.2 | 19 (33) | 0 (0) | – | – |
| Maximal LSA (n=22) | 13.4±2.1 | – | 1 (1) | – | – |
| Aortic lengths, mm¶ | |||||
| STJ to zero point (n=76) | 96.3±22.5 | 3 (5) | 0 (0)|| | 17 (22) | – |
| Zero point to distal LCCA (n=59) | 38.1±6.2 | 10 (17) | – | – | – |
| Mid BCA to distal LCCA (n=23)‡ | 20.0±3.9 | – | 18 (78) | – | – |
| Distal BCA to distal LCCA (n=23)‡ | 12.4±3.7 | – | 20 (87) | – | – |
| Mid LCCA to distal LSA (n=22) | 28.3±4.8 | – | 3 (14) | – | – |
| Distal LCCA to distal LSA (n=22) | 23.4±4.7 | – | 3 (14) | – | – |
| BCA ostium to bifurcation (n=76) | 33.5 (14.5–62.9) | 2 (3) | 5 (9)|| | – | – |
|
LSA to first branch of LSA (n=22) STJ to BCA (n=153) |
38.7±10.1 102.6±27.4 |
– – |
3 (14) – |
– – |
– 0 (0) |
| Distal BCA LZ length (n=153) | 34.7±12.8 | – | – | – | 11 (7) |
| Branch clock positions/angles (degrees)¶ | |||||
| BCA clock position (n=76) | 3.8 (−43.3 to 19.1) | – | 1 (0) | 7 (9) | – |
| LCCA clock position (n=76) | 13.5±14.5 | – | 1 (0) | 2 (3) | – |
| LSA clock position (n=76) | 7.1±16.7 | – | – | – | – |
| BCA angle to aortic arch (n=165) | 141.0±23.3 | – | – | – | 30 (18) |
BCA indicates brachiocephalic artery; LCCA, left common carotid artery; LSA, left subclavian artery; LZ, landing zone; and STJ, sinotubular junction.
Measurements composed of 110 TAAs, 253 aortic dissections, of which 12 were chronic dissections and the rest acute, and 14 other pathological features.
A total of 56 of 59 measured; 3 patients were ineligible for either 45‐ or 60‐mm proximal length device because of insufficient BCA–zero point length.
A total of 23 of 30 measured; 7 patients were excluded from zone 1 proximal LZ length measurements because of proximity of BCA.
A total of 57 of 59 measured; 2 patients did not have sufficient BCA length to measure 25 mm distally.
A total of 75 of 76 (58/59 Relay Branch eligible) patients measured; 1 patient had a stenosis of LCCA.
A total of 57 of 76 patients were subjected to zone 0 assessment with the TAG Thoracic Branch Endoprosthesis (Gore).
Discussion
In this study, we investigated the suitability for endovascular repair in patients with aortic arch pathological features previously treated by open surgical repair. We found that 59 of 377 (16%) of our patients treated with open surgery for any aortic pathological feature were suitable for endovascular repair. When excluding zone 0 pathological features involving coronary arteries and isolated descending thoracic pathological features, 59 of 153 (39%) patients were found to be suitable for endovascular repair.
The implications of these findings suggest that the percentage of patients benefiting from an endovascular procedure for arch pathological features may be limited. The complexity of anatomic features included in the IFU for branched devices results in a substantial degree of incompatibility for these grafts. Valvular insufficiency, the proximity of the pathological features in regard to the valve or coronary arteries, and complex configuration at the branches are the main reasons to exclude patients for endovascular repair. From this perspective, the added value of this expensive procedure is controversial. The increased risk of stroke described by Tazaki et al, resulting from branched endovascular repair in the aortic arch, raises the question whether we should proceed in the development of multibranched highly complex endovascular devices. 13 , 25 Yet, with the advancement of stent grafts in the recent years and expected learning curve, better results might be achieved in the near future, as described by Spear et al. 12 However, because the exposure to these procedures is ambiguous, open repair for arch pathological features still remains the gold standard. Endovascular repair with use of multibranched stent grafts in the aortic arch should be reserved for patients unfit to endure invasive sternotomy and might be better restricted to high‐volume centers with extensive experience in endovascular arch repair.
The aforementioned complexity of the procedure can be reduced with the use of a single branched stent graft and adjunctive debranching procedures. This adds to the invasive nature of the procedure; however, it increases suitability rates in our cohort.
If urgent repair is required, off‐the‐shelf single branched grafts provide the highest compatibility rates in patients because of the variety of sizes of main and branch grafts. The modular design of the Thoracic Branched Endoprosthesis (Gore) allows for extensive variability in its configuration and thus allowed for the highest rate of suitability within the current study (46/377; 12.2%). Custom‐made stent grafts experience longer production times, which impairs the use in acute or semiacute cases; and even if pathological feature allows for longer production times, a large ascending aortic diameter excludes endovascular repair in most cases. Fujimura et al reported the proximal landing zone diameter of the ascending aorta as the main limitation of the custom Zenith Arch Branched Device (Cook Medical), with only 24% of patients with type A dissection meeting the 24‐ to 38‐mm requirement. 18 Along with production times, the cost associated with custom‐made grafts dismisses these devices as a therapy of first choice.
When focusing on our cohort, 30.9% of patients with thoracic aneurysms possess suitable aortic anatomical features to be treated endovascularly as well as 62.5% of patients with type B dissections extending to the subclavian artery. These percentages are high compared with the total suitability for all arch pathological features. This is attributable to specific device IFUs limiting the scope by which certain pathological features may be treated. Aneurysms and type B dissections needing sealing in the aortic arch are permitted in all cases, meanwhile treatment of type A dissections is contraindicated within the IFU of certain endoprostheses, such as the Relay Plus Double‐Branched (Terumo Aortic) device. As there was a high percentage of type A dissections in our cohort (63%), this contributed to the lower total suitability for all arch pathological features. When focusing on thoracic aortic aneurysms and type B dissections, endovascular surgery seems promising given the relatively high suitability rates when following strict IFUs.
The prominent limitation of this study was the retrospective nature. We reviewed a cohort of patients with a wide variety of pathological features by any means of open cardiovascular surgery involving the aorta. This introduces bias in the cohort because these patients have passed preoperative screening. Although open repair of aortic arch pathological features remains the gold standard, the invasive nature combined with the typical state of advanced cardiovascular disease render a large proportion of patients (>20% of acute type A dissections) ineligible for surgery. 2 Of interest, therefore, is the patient cohort that was not accepted for surgery and deemed unfit. These patients could benefit the most from a less invasive procedure, which avoids a thoracotomy and the use of cardiopulmonary bypass. A future study is proposed in which patients with arch pathological features that were declined for surgery are tested on thoracic stent graft IFU suitability. Also, when assessing patient suitability, we upheld strict compliance with the anatomic criteria within manufacturer IFUs. However, given the novelty of the stent grafts, the IFUs are under continuous development. Currently, during the preoperative phase, manufacturers may be consulted on a patient with challenging anatomical features and the standard IFU can be expanded with the addition of specific customizations, indicating a possible larger patient group that can be treated endovascularly in nonemergent settings. Furthermore, we only performed measurements on the aortic arch because of a lack of abdominal CTA and we were unable to analyze the femoral access. Therefore, it is possible that a patient with suitable aortic anatomical features can be deemed ineligible for endovascular surgery because of inaccessible vascular access sites. In addition, 21 patients with suitable pathological features had insufficient CTA scans for measurement analysis because of supra‐aortic arteries outside the field of view. Although this may have negatively influenced the overall suitability of the cohort, the current study sample is the largest anatomic feasibility assessment for endovascular repair among patients with aortic arch pathological features to date.
The use of branched thoracic stent grafts is still in its infancy, yielding small cohorts with relatively high stroke rates. 11 , 13 , 15 Future studies should focus on the clinical outcomes of endovascular repair of thoracic pathological features in patients deemed unfit for conventional thoracic aortic surgery.
To conclude, on the basis of anatomic suitability, endovascular repair of the aortic arch is feasible. However, in our cohort, stent graft suitability for all pathological features was modest. The highest suitability rates were observed for thoracic aortic aneurysms and type B dissections requiring seal in the aortic arch. The feasibility of endovascular repair may be increased by expanding device offerings to accommodate a wider range of proximal landing zone and branch diameters.
Sources of Funding
None.
Disclosures
None.
Acknowledgements
We thank the companies Terumo Aortic, W.L. Gore & Associates, Cook Medical, and Endospan Ltd/Jotec GmbH for providing their latest instructions for use.
(J Am Heart Assoc. 2020;9:e016695 DOI: 10.1161/JAHA.120.016695.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Sundt TM 3rd, Orszulak TA, Cook DJ, Schaff HV. Improving results of open arch replacement. Ann Thorac Surg. 2008;787–796. [DOI] [PubMed] [Google Scholar]
- 2. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;897–903. [DOI] [PubMed] [Google Scholar]
- 3. Girsowicz E, Georg Y, Lefebvre F, Lejay A, Thaveau F, Roy C, Ohana M, Chakfe N. Anatomical study of healthy aortic arches. Ann Vasc Surg. 2017;179–189. [DOI] [PubMed] [Google Scholar]
- 4. Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent‐grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;1729–1734. [DOI] [PubMed] [Google Scholar]
- 5. Vallabhajosyula P, Szeto WY. Current paradigms in aortic arch repair: striking the balance between open surgery and endovascular repair. J Thorac Cardiovasc Surg. 2015;1399–1400. [DOI] [PubMed] [Google Scholar]
- 6. Czerny M, Rylski B, Morlock J, Schrofel H, Beyersdorf F, Saint Lebes B, Meyrignac O, Mokrane F, Lescan M, Schlensak C, et al. Orthotopic branched endovascular aortic arch repair in patients who cannot undergo classical surgery. Eur J Cardiothorac Surg. 2018;1007–1012. [DOI] [PubMed] [Google Scholar]
- 7. Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, et al. Endovascular repair of type B aortic dissection: long‐term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;407–416. [DOI] [PubMed] [Google Scholar]
- 8. Mazzaccaro D, Sciarrini M, Nano G. Analysis of origin of the supra‐aortic trunks from the aortic arch. J Vasc Surg. 2018;399–408. [DOI] [PubMed] [Google Scholar]
- 9. Haulon S, Greenberg RK, Spear R, Eagleton M, Abraham C, Lioupis C, Verhoeven E, Ivancev K, Kolbel T, Stanley B, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;1709–1716. [DOI] [PubMed] [Google Scholar]
- 10. Kawatou M, Minakata K, Sakamoto K, Nakatsu T, Tazaki J, Higami H, Uehara K, Yamazaki K, Inoue K, Kimura T, et al. Comparison of endovascular repair with branched stent graft and open repair for aortic arch aneurysm. Interact Cardiovasc Thorac Surg. 2017;246–253. [DOI] [PubMed] [Google Scholar]
- 11. Patel HJ, Dake MD, Bavaria JE, Singh MJ, Filinger M, Fischbein MP, Williams DM, Matsumura JS, Oderich G. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the gore thoracic branch endoprosthesis. Ann Thorac Surg. 2016;1190–1198. [DOI] [PubMed] [Google Scholar]
- 12. Spear R, Haulon S, Ohki T, Tsilimparis N, Kanaoka Y, Milne CP, Debus S, Takizawa R, Kolbel T. Editor's choice ‐ subsequent results for arch aneurysm repair with inner branched endografts. Eur J Vasc Endovasc Surg. 2016;380–385. [DOI] [PubMed] [Google Scholar]
- 13. Tazaki J, Inoue K, Higami H, Higashitani N, Toma M, Saito N, Kawatou M, Kimura T. Thoracic endovascular aortic repair with branched Inoue Stent Graft for arch aortic aneurysms. J Vasc Surg. 2017;1340–1348.e5. [DOI] [PubMed] [Google Scholar]
- 14. Andrasi TB, Grossmann M, Zenker D, Danner BC, Schondube FA. Supra‐aortic interventions for endovascular exclusion of the entire aortic arch. J Vasc Surg. 2017;281–297.e2. [DOI] [PubMed] [Google Scholar]
- 15. Hiraoka A, Chikazawa G, Tamura K, Totsugawa T, Sakaguchi T, Yoshitaka H. Clinical outcomes of different approaches to aortic arch disease. J Vasc Surg. 2015;88–95. [DOI] [PubMed] [Google Scholar]
- 16. Lioupis C, Abraham CZ. Results and challenges for the endovascular repair of aortic arch aneurysms. Perspect Vasc Surg Endovasc Ther. 2011;202–213. [DOI] [PubMed] [Google Scholar]
- 17. Moulakakis KG, Dalainas I, Kakisis J, Mylonas S, Liapis CD. Endovascular treatment versus open repair for abdominal aortic aneurysms: the influence of fitness in decision making. Int J Angiol. 2013;9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujimura N, Kawaguchi S, Obara H, Yoshitake A, Inoue M, Otsubo S, Kitagawa Y, Shimizu H. Anatomic feasibility of next‐generation stent grafts for the management of type A aortic dissection in Japanese patients. Circ J. 2017;1388–1394. [DOI] [PubMed] [Google Scholar]
- 19. Huang C, Zhou M, Liu Z, Huang D, Ran F, Wang W, Zhang M, Liu C, Liu C, Qiao T, et al. Computed tomography‐based study exploring the feasibility of endovascular treatment of type A aortic dissection in the Chinese population. J Endovasc Ther. 2014;707–713. [DOI] [PubMed] [Google Scholar]
- 20. Moon MC, Greenberg RK, Morales JP, Martin Z, Lu Q, Dowdall JF, Hernandez AV. Computed tomography‐based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. J Vasc Surg. 2011;942–949. [DOI] [PubMed] [Google Scholar]
- 21. Sobocinski J, O'Brien N, Maurel B, Bartoli M, Goueffic Y, Sassard T, Midulla M, Koussa M, Vincentelli A, Haulon S. Endovascular approaches to acute aortic type A dissection: a CT‐based feasibility study. Eur J Vasc Endovasc Surg. 2011;442–447. [DOI] [PubMed] [Google Scholar]
- 22. Sonesson B, Landenhed M, Dias N, Kristmundsson T, Ingemansson R, Koul B, Malina M, Resch T. Anatomic feasibility of endovascular reconstruction in aortic arch aneurysms. Vascular. 2015;17–20. [DOI] [PubMed] [Google Scholar]
- 23. Czerny M, Rylski B, Beyersdorf F. Thoracic endovascular aortic repair for uncomplicated type B aortic dissection. Curr Opin Cardiol. 2016;606–610. [DOI] [PubMed] [Google Scholar]
- 24. Milewski RK, Szeto WY, Pochettino A, Moser GW, Moeller P, Bavaria JE. Have hybrid procedures replaced open aortic arch reconstruction in high‐risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2010;590–597. [DOI] [PubMed] [Google Scholar]
- 25. Anderson J, Nykamp M, Remund T, Kelly P. Complete endovascular debranching of the aortic arch: a report of two cases. Vascular. 2015;310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]


