Abstract
Background
Elevated natriuretic peptides (NP) are associated with adverse cerebrovascular conditions including stroke, cerebral small vessel disease, and dementia. However, the mechanisms underlying these associations remain unclear. In this study, we examined the relationship of NT‐proBNP (N‐terminal pro brain NP) and NT‐proANP (N‐terminal pro atrial NP) with cerebrovascular function, measured by cerebral autoregulation.
Methods and Results
We included 154 participants (mean age 56±4 years old) from the CARDIA (Coronary Artery Risk Development in Young Adults) cohort. NT‐proBNP and NT‐proANP were measured in blood samples from the year 25 examination using electrochemiluminescence Immunoassay and enzyme‐linked immunoassay, respectively. Dynamic cerebral autoregulation (dCA) was assessed at the year 30 examination by transcranial Doppler ultrasound, using transfer function analysis (phase and gain) of spontaneous blood pressure and flow velocity oscillations, where lower phase and higher gain reflect less efficient cerebral autoregulation. We used multivariable linear regression models adjusted for demographics, vascular risk factors, and history of kidney and cardiac diseases. Higher NT‐proBNP levels at year 25 were associated with lower phase (β [95% CI]=−5.30 lower degrees of phase [−10.05 to −0.54]) and higher gain (β [95% CI]=0.06 higher cm/s per mm Hg of gain [0.004–0.12]) at year 30. Similarly, higher NT‐proANP levels were associated with lower phase (β [95% CI]=−9.08 lower degrees of phase [−16.46 to −1.70]).
Conclusions
Higher circulating levels of NT‐proBNP and NT‐proANP are associated with less efficient dCA 5 years later. These findings link circulating NP to cerebral autoregulation and may be one mechanism tying NP to adverse cerebrovascular outcomes.
Keywords: autoregulation, brain, cerebrovascular disease, natriuretic peptides
Subject Categories: Clinical Studies, Hemodynamics, Mechanisms
Nonstandard Abbreviations and Acronyms
- dCA
dynamic cerebral autoregulation
- ECLIA
electrochemiluminescence immunoassay
MCA
middle cerebral artery
- MFV
mean flow velocity
- NT‐proANP
N‐terminal pro atrial natriuretic peptide
Clinical Perspective
What Is New?
This study shows that higher levels of plasma natriuretic peptides are associated with less efficient cerebral autoregulation.
The association between natriuretic peptides and cerebral autoregulation is independent of demographic and conventional cardiovascular risk factors.
What Are the Clinical Implications?
Alterations in cerebral autoregulation may underlie the link between natriuretic peptides and adverse cerebrovascular outcomes.
Our findings pave the path for future mechanistic studies to determine the potential diagnostic or therapeutic utility of natriuretic peptides as disease modifying agents in development and progression of cerebrovascular pathologies.
Natriuretic peptides (NP) are a family of endogenous peptides that are abundantly expressed in cardiac myocytes and vascular endothelium. 1 In the periphery, NP not only regulate blood pressure and body fluid homeostasis, but they also inhibit cardiac tissue and blood vessel remodeling. 2 Dysregulated elevation in NP has been linked with adverse systemic and cerebrovascular outcomes. Evidence from epidemiological and clinical studies suggest that higher levels of NP in plasma are linked with a higher risk of stroke, 3 cerebral small vessel disease, 4 subarachnoid hemorrhage, 5 , 6 and cognitive impairment. 7 In line with these observations, studies in mammalian and rodent models have identified the presence of NP receptors in the endothelium of cerebral microvessels. 8 , 9 Recently, it has been shown that NP and their receptors are abundantly present on human cerebral vessels’ smooth muscle cells and endothelium. 10 These are the same cellular components central to cerebral autoregulation; a key physiological mechanism that ensures adequate brain perfusion despite fluctuations in systemic blood pressure. 11 In this study, we examined the relationship between NT‐proBNP (N‐terminal pro brain NP) and NT‐proANP (N‐terminal pro atrial NP) and cerebral autoregulation (dCA) in a bi‐racial community‐dwelling sample of participants from the CARDIA (Coronary Artery Risk Development on Young Adults) cohort. We hypothesized that higher peripheral levels of NP would be associated with less efficient dCA.
Methods
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact‐cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (https://www.cardia.dopm.uab.edu/study‐information/nhlbi‐data‐repository‐data).
Study Population
Participants in this study were included from a subsample of the CARDIA cohort. The CARDIA study began in 1985–1986 to investigate cardiovascular risk factors in a cohort of bi‐racial (White/Black) community‐dwelling individuals aged 18 to 30 years (n=5115). 12 The participants were recruited from 4 sites in the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). At the 30th year of follow‐up, participants from the Chicago site were invited to participate in the Cerebral Small Vessels in Motor and Cognitive Decline sub‐study. This ancillary study aimed at investigating vascular biomarkers of cerebral small vessel disease using transcranial Doppler ultrasound. Separate written consent was obtained, and the study was approved by the institutional review board of Northwestern University. Out of a total of 202 participants recruited for the ancillary study, 154 had available blood samples that were collected at the last visit before the transcranial Doppler ultrasound examination. Figure S1 shows the flow diagram of included participants in this study. Characteristics of participants included in this study (n=154) were not significantly different compared to the rest of the Chicago field centers’ participants (n=591), except that those included in this study were more frequently male (Table S1).
NT‐proBNP and NT‐proANP Measurement
NT‐proBNP and NT‐proANP were measured using frozen and stored blood samples (serum and plasma) from the 25th year of follow‐up. The blood draw was performed before 10 am and participants were instructed to be in fasting state. Blood drawing, handling, aliquoting, and transportation have been standardized according to CARDIA protocols. NT‐proBNP was measured using proBNP II electrochemiluminescence immunoassay (ECLIA) on a Cobas e411 chemistry analyzer (Roche Diagnostics, USA). The measurement range is 5.0 to 35 000 pg/mL (0.60–4130 pmol/L) and the inter‐assay coefficient of variation is <5%. NT‐proANP was measured using ELISA (Alpco, Salem, NH, USA), with a standard range 0 to 10 nmol/L and an inter‐assay coefficient of variation <9%. All measurements were performed in the Comprehensive Metabolic Core Lab at Northwestern University, Feinberg School of Medicine.
Cerebral Autoregulation Assessment
At the 30th year of CARDIA follow‐up, participants reported to the Cerebrovascular Laboratory at Northwestern University, Feinberg School of Medicine, for cerebrovascular measures. They were instrumented with a 3‐lead ECG for continuous heart rate recording. Beat‐to‐beat blood pressure monitoring was obtained using a finger photoplethysmographic cuff (Finapres NOVA®, Finapres Medical Systems BV). A 2 MHz digital transcranial Doppler ultrasound transducer (Digi‐LiteTM, Rimed Inc) was used to measure bilateral middle cerebral artery (MCA) blood flow velocities. The MCAs were insonated at a depth of 50 to 65 mm using a Mueller–Moll probe fixation device. Measurements were obtained continuously for 10 minutes while participants were seated upright in a chair. All velocity waveforms derived from a fast fourier transformation of the Doppler signal were digitized at 500 Hz (Windaq, Data Instruments), displayed simultaneously with blood pressure waves, and stored for later off‐line analysis. End tidal CO2 was monitored during dCA measurements to exclude significant fluctuations.
Dynamic Cerebral Autoregulation Quantification
The beat‐to‐beat cycles were determined by detecting the peak of each arterial pressure and cerebral flow velocity waveform. Using a custom program written in MATLAB, mean arterial pressure (MAP) and mean flow velocity (MFV) values were determined from the integrals of each waveform to calculate the systolic and diastolic blood flow velocity within each beat cycle. All MAP and MFV waveforms were visually inspected for artifacts, and only steady‐state data were used for analyses. The frequency‐domain transfer function analyses were used to quantify dCA, as previously described. 13 This method examines the relationship between spontaneous beat‐to‐beat oscillations in MAP and cerebral MFV at rest. 14 Briefly, the power spectral densities of MAP and MFV were estimated using the Welch algorithm of averaging periodograms. The waveforms were linearly detrended, smoothed through a Hanning Window, and transformed through Fast Fourier analysis. The periodograms were averaged across all widows to produce the spectrum estimate. The coherence between MAP and MFV were calculated using the cross‐spectra and autospectra of the data segments. Using a custom MATLAB program, transfer function gain and phase values were calculated using the MAP and MFV signal autospectra over the very low frequency spectra (0.01–0.07 Hz). Since dCA takes about 2 to 10 seconds to engage, frequency domain analyses of dCA are typically studied at the very low frequency range. 14 , 15 The phase and gain values were weighted by their precision in each frequency range to obtain the most accurate data for analysis. Furthermore, since the right and left dCA measures were not significantly different, these measures were averaged to obtain the global values. 16 The transfer function gain quantifies the damping effect of cerebral autoregulation on the magnitude of blood pressure oscillations. A low gain indicates more efficient dCA, whereas a high gain indicates less efficiency. The phase shift represents the temporal difference between MFV oscillations in respect to MAP, and is considered a measure of the time . If the blood pressure and cerebral blood flow oscillations are synchronous, then the phase shift approaches zero degrees, indicating less efficient dCA, whereas a phase shift of 90° indicates more efficient dCA 13 (hence, a lower phase indicates worse dCA).
Covariates
Other covariates were collected using standardized protocols at each CARDIA visit. 17 Body mass index was calculated as weight (kg) divided by height in meters squared. Total cholesterol was determined on a chemistry analyzer using comparable enzymatic procedures (Hitachi 912; Roche Diagnostics). Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or self‐reported use of diabetes mellitus medication. History of hypertension, antihypertensive medication use, and smoking status were self‐reported. History of kidney problems was defined as any self‐reported history of kidney problems, including pyelonephritis, kidney stone, nephritis, glomerulonephritis, or kidney failure. History of cardiac problems was defined as any self‐reported heart problems, including heart attack, heart failure, angina, rheumatic heart disease, mitral valve prolapse, atrial fibrillation, and irregular heartbeat. Missing covariate data (n=2) were replaced with values acquired from a previous exam.
Statistical Analysis
Descriptive measures are reported as mean±SD, proportion, and median with interquartile ranges where applicable. Characteristics of participants across tertiles of NT‐proBNP and NT‐proANP were compared using one‐way ANOVA for continuous variables and chi‐squared test for categorical variables. Multivariable linear regression models were used to assess the association between NP (independent variable) and dCA (dependent variable). Adherence to assumptions of linear regression was examined by visual inspection of the distribution of residuals through histograms and normal probability plots. Ordinary least square linear regression models with restricted cubic spline were used to test for non‐linear associations. All analyses were performed in 2 steps: in the first step (model 1), analyses were adjusted for sociodemographic factors: age, sex, and race. In the next step (model 2), vascular risk factors (smoking status, body mass index, total cholesterol level, history of hypertension, and diabetes mellitus history), history of cardiac problems, and renal history were additionally included as confounders. These variables were used as confounders based on previous literature showing their association with NP and/or dCA. 18 , 19 , 20 , 21 To further test the effect of these vascular risk factors on the results, we performed additional sensitivity analyses by stratifying participants to those with and without vascular risk factors and testing for interaction. The P values for interaction were calculated using linear regression models by adding an interaction term produced by multiplying levels of NP to vascular risk factors. In an extra analysis, to account for the time difference between NP and dCA measures, we calculated the time interval between year‐25 and year‐30 examinations and added it to the linear regressions models, which did not materially change the associations. All statistical analyses were conducted with SPSS (version 25.0) and R (version 3.5.1) software, and a P<0.05 was considered as statistically significant.
Results
Table 1 presents the characteristics of participants. The mean age of our participants was 56 years old; a total of 69 (45%) participants were female and 70 (46%) were Black. The socio‐demographic and vascular risk factors were not different across NT‐proBNP tertiles, except that females had higher NT‐proBNP levels (Table S2). Participants in the highest tertile of NT‐proANP were older (Table S3). The NT‐proBNP and NT‐proANP levels ranged from 5 to 273.5 pg/mL and from 0.18 to 3.01 nmol/L, respectively.
Table 1.
Characteristics of Participants
| n=154 | |
|---|---|
| Demographics | |
| Age, y, mean (SD) | 55.7 (3.9) |
| Female, N (%) | 69 (44.8) |
| Black, N (%) | 70 (45.5) |
| Risk factors | |
| SBP, mm Hg, mean (SD) | 120.5 (15.4) |
| DBP, mm Hg, mean (SD) | 74.1 (11.2) |
| Body mass index, kg/m2, mean (SD) | 30.0 (6.0) |
| Total cholesterol, mg/dL, mean (SD) | 191.8 (39.0) |
| Current smoker, N (%) | 22 (14.3) |
| History of hypertension, N (%) | 51 (33.1) |
| History of diabetes mellitus, N (%) | 24 (15.6) |
| History of cardiac problems, N (%) | 18 (11.7) |
| Renal history, N (%) | 12 (7.8) |
| TCD measures | |
| MCA flow velocity, cm/s, mean (SD) | 51.2 (12.1) |
| Phase, degree, mean (SD) | 42.24 (23.97) |
| Gain, cm/s per mm Hg, mean (SD) | 0.70 (0.3) |
| Coherence, mean (SD) | 0.51 (0.12) |
DBP indicates diastolic blood pressure; TCD, transcranial doppler; MCA, middle cerebra artery; SBP, systolic blood pressure; and transcranial Doppler ultrasound.
Table 2 shows the prospective association of NT‐proBNP and NT‐proANP with transfer function measures of dCA. In model 1, each unit (pg/mL) increase in NT‐proBNP was associated with a 0.12° lower phase (indicating less efficient dCA; 95% CI, −0.21 to −0.03) and a 0.001 cm/s per mm Hg higher gain (indicating less efficient dCA; 95% CI, 0.0002 to 0.002). After full adjustments for vascular risk factors, and history of cardiac or renal problems, higher NT‐proBNP remained associated with lower phase and higher gain (Table 2, model 2). Each unit (nmol/L) increase in NT‐proANP was associated with a 9.08° lower phase (indicating less efficient dCA; 95% CI, −16.46 to −1.70) after full adjustments (Table 2, model 2). We did not find a non‐linear association between NT‐proBNP and NT‐proANP in relation to transfer function measures of dCA (all P>0.05) (Figure 1).
Table 2.
Prospective Association Between Natriuretic Peptides and Cerebrovascular Autoregulation
|
NT‐proBNP (pg/mL) Per 1 Unit Increase |
NT‐proANP (nmol/L) Per 1 Unit Increase |
|||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Phase, degree | ||||
| Model 1 | −0.12 (−0.21 to −0.03) | 0.013 | −6.90 (−14.17 to 0.38) | 0.063 |
| Model 2 | −0.10 (−0.20 to −0.01) | 0.030 | −9.08 (−16.46 to −1.70) | 0.016 |
| Gain, cm/s per mm Hg | ||||
| Model 1 | 0.001 (0.0002 to 0.002) | 0.022 | 0.03 (−0.05 to 0.12) | 0.444 |
| Model 2 | 0.001 (0.0004 to 0.003) | 0.008 | 0.04 (−0.05 to 0.13) | 0.386 |
Beta (β) coefficient represents difference in phase (degree) and gain (cm/s per mm Hg), corresponding to 1 unit increase on NT‐proBNP (picograms/milliliter) or NT‐proANP (nanomoles/liter). Model 1 is adjusted for age, sex, and race. Model 2 is adjusted for age, sex, race, smoking status, body mass index, total cholesterol level, history of hypertension, history of diabetes mellitus, history of cardiac problem, and renal history. NT‐proANP indicates N‐terminal pro atrial natriuretic peptide; and NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Figure 1. Linear association between plasma natriuretic peptides and cerebrovascular autoregulation.
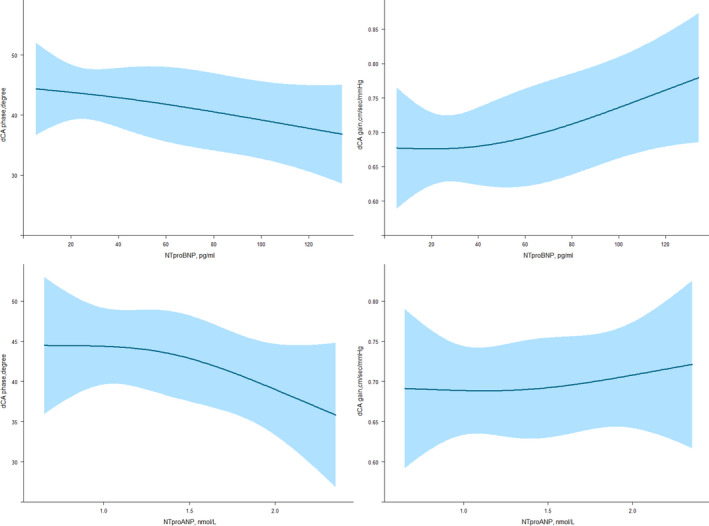
The figure shows fitted restricted cubic splines and 95% CIs, indicating no evidence of non‐linear association between plasma natriuretic peptides (NTproBNP and NTproANP) and measures of cerebral autoregulation (phase and gain). All Pvalues are >0.05. dCA indicates dynamic cerebral autoregulation; NT‐proANP, N‐terminal pro atrial natriuretic peptide; and NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Figure 2 shows the prospective relation of NT‐proBNP and NT‐proANP with transfer function measures of dCA in different subgroups of participants. The association between higher NT‐proBNP and lower phase was stronger in males, Black patients, and those with a history of diabetes mellitus (all P for interaction <0.05). The association of higher NT‐proBNP and higher gain was stronger in Black participants (P for interaction <0.05). The association of NT‐proANP and transfer function measures of dCA was not different among the subgroups. Finally, after exclusion of participants with history of cardiac disease (n=18), NT‐proBNP (β=−0.16; 95% CI, −0.26 to −0.05) and NT‐proANP (β=−11.35; 95% CI, −19.67 to −3.03) remain significantly associated with phase.
Figure 2. Prospective association between natriuretic peptides and cerebrovascular autoregulation stratified by risk factors.
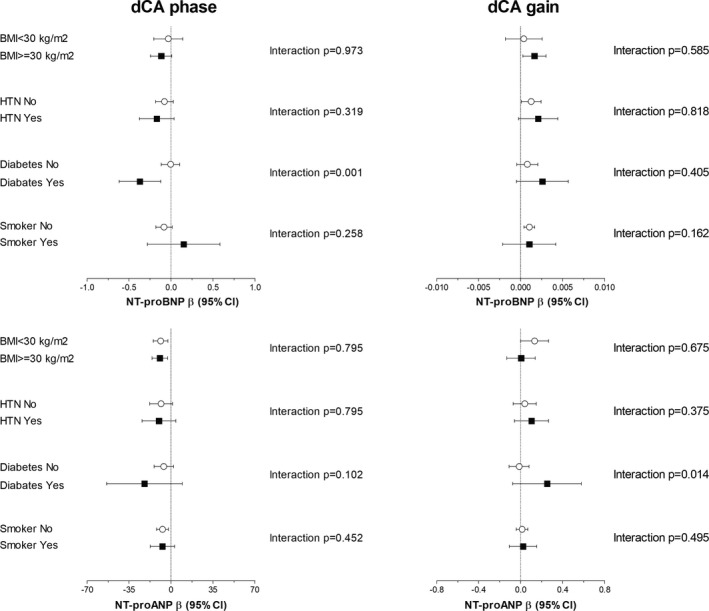
Forest plots showing the relationship between natriuretic peptides and cerebrovascular autoregulation across different risk groups. Analyses were performed in the fully adjusted model (model 2). The β shows unstandardized regression coefficient. BMI indicates body mass index; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NT‐proANP, N‐terminal pro atrial natriuretic peptide; dCA, dynamic cerebral autoregulation; and HTN, hypertension.
Discussion
In this population‐based study of middle age adults, we show that elevated circulating levels of NT‐proBNP and NT‐proANP are associated with less efficient cerebral autoregulation. This association was independent of socio‐demographics and cardiovascular risk factors.
Evidence from neuropathological studies indicate that ANP and BNP are not only present in cardiac myocytes, but also in the central nervous system and its vascular beds. 22 In fact, observations from animal studies suggest that NPs’ receptors are localized in the brain vessels, including in isolated cerebral microvessels and cultured brain capillary endothelium. 2 , 23 For example, Chabrier et al were among the first to demonstrate specific ANP binding sites on bovine cerebral microvessel preparations. 9 , 24 Further in vitro and in vivo experiments have shown the binding of ANP on rats’ brain microvessel endothelium. 8 , 25 In humans, we have recently shown the immunohistochemical staining of ANP, BNP, and their receptors (including NP receptor types A, B, and C [NPR‐A, NPR‐B, and NPR‐C]) in the smooth muscle and endothelium of cerebral vessels. 10 Given these observations, it is plausible that NP contributes to cerebrovascular pathologies through modulation of cerebral hemodynamics. 26 In this context, the present study extends the current knowledge on the roles of NP in cerebrovascular hemodynamics by reporting a close link between elevated systemic NP and less efficient cerebral autoregulation in a cohort of middle‐age adults. To our knowledge, this is the first study to examine this relationship in a midlife cohort without significant cardiovascular disease burden.
The observed link between NP and dCA can be explained in different ways. Elevated NP and impaired dCA may both reflect vascular damage that share a common cause. Previous work shows that higher circulating NP levels strongly associate with cardiovascular mortality, 27 cardiovascular diseases, 28 , 29 and vascular risk factors. 30 , 31 , 32 , 33 Similarly, cerebral autoregulation has been shown to be impaired in patients with hypertension 34 and diabetes mellitus. 35 In line with these observations, we found that the association between NP and dCA was stronger in those with a history of diabetes mellitus. However, adjustment of our analyses for measured vascular risk factors did not essentially change the association between NP and dCA. Nevertheless, we cannot rule out the effect of residual confounding and unmeasured risk factors. Alternatively, our findings could be attributed to autonomic dysfunction. The sympathetic nervous system is known to activate secretion of NP, and autonomic failure has been associated with altered dCA. Therefore, elevated NP and impaired dCA may reflect an epi‐phenomenon representing early signs of autonomic dysregulation, activating secretion of NP 36 and altering dCA 37 in parallel.
It is widely accepted that dCA is a multifactorial phenomenon involving myogenic, autonomic, and metabolic mechanisms. 11 Specifically, the myogenic response arises from the vascular smooth muscle activities in response to intravascular pressure changes, and it is crucial for maintaining vascular resistance. Furthermore, the endothelium also plays critical roles in the regulation of vascular tone and cerebral autoregulation through production of vasoactive mediators, such NO. 11 Previous work in experimental models shows that all NP exert their vasoactive properties by modulating intracellular calcium (Ca2+) concentration in the brain. In particular, NP activate production of intracellular cyclic guanosine monophosphate (cGMP), which in turn reduces Ca2+ concentrations along with the activity of Ca2+‐activated potassium (K+) channels and adenosine triphosphate (ATP) sensitive K+ channels, thereby affecting smooth muscle responses and changing vessel diameter. 26 NP also stimulate the production of nitric oxide and exert vasodilation by affecting nitric oxide synthase (NOS) production. 26 , 38 On the other hand, ANP is able to modulate the autonomic nervous system by affecting cardiac baroreceptor nerve endings and inhibiting sympathetic ganglionic neurotransmission. 36 Given these local effects of NP on cerebral vessels, it is also possible that a long‐lasting unregulated elevation in NP may hamper the response of smooth muscle and endothelium of cerebral vessels to intravascular pressure changes, thereby affecting cerebral vascular tone and autoregulation. Our data show that higher plasma NT‐proBNP and NT‐proANP were both associated with less efficient dCA. Although the systemic effects of ANP and BNP have been extensively studied, their local impact on the brain vasculature and the interaction between systemic and central NP remains largely unexplored. It is worth noting that the third member of the NP family, C‐type NP (CNP), is found to have higher concentrations in the central nervous system than in the systemic circulation. 22 Therefore, future studies on humans are needed to investigate the local effects of NP on cerebrovascular function.
The strength of this study includes a well‐characterized cohort of bi‐racial middle age adults with extensive phenotyping of vascular profile that enabled us to correct for several potential confounders. Furthermore, to the best of our knowledge, this is the first study to examine the association between NP and cerebral autoregulation. Our study also has limitations. One important limitation is the 5 year time lag between NP and dCA measurements. Without knowing the longitudinal association between NP and dCA, our findings should be interpreted with caution. While MCA is considered the largest vascular territory in the brain, and hence a reasonable measure of global dCA, 16 it is important to also note that the inability to acquire dCA in all vascular territories simultaneously is a limitation in all such studies. It should also be noted that NP were measured using stored blood samples. While using stored samples is a well‐established approach in cohort studies and previous studies have shown stability of NP measures, 39 , 40 obtaining fresh blood samples might decrease the chance of random errors and results should be further explored using fresh blood samples. Moreover, we measured NT‐proANP, and more recent work suggests that mid‐regional pro ANP may be a more stable marker of ANP, so this marker should be explored in the future. Finally, we would like to emphasize that this is an observational study and causality cannot be inferred. A better understanding of the underpinning mechanisms discussed relies on future experimental models, as well as on interventional studies and collaborative effort across basic, translational, and clinical investigators.
In summary, our findings link elevated circulating levels of NP with less efficient cerebral autoregulation measured 5 years later, and this suggests that cerebrovascular dysregulation may be a potential mechanism linking elevated NP with adverse brain and cerebrovascular outcomes. Our observations have the potential to facilitate collaborative initiatives bridging basic, translational, and clinical efforts to advance our mechanistic understanding of the link between NP and cerebrovascular dysregulation, with an eye towards developing novel therapeutic targets.
Sources of Funding
This work was partially supported by a grant from CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018‐28 & 2012‐06 Heart Brain Connection). The Cerebral Small Vessel in Motor and Cognitive Decline study is supported by National Institutes of Health (NIH, R01NS085002). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This article has been reviewed by CARDIA for scientific content.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
We would like to thank the Comprehensive Metabolic Core Lab at Northwestern University Feinberg School of Medicine for performing NT‐proBNP and NT‐proANP measurements.
(J. Am. Heart Assoc. 2020;9:e018203 DOI: 10.1161/JAHA.120.018203.)
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Potter LR, Abbey‐Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate‐dependent signaling functions. Endocr Rev. 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 2. Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93. [DOI] [PubMed] [Google Scholar]
- 3. Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N‐terminal pro‐B‐type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vilar‐Bergua A, Riba‐Llena I, Penalba A, Cruz LM, Jimenez‐Balado J, Montaner J, Delgado P. N‐terminal pro‐brain natriuretic peptide and subclinical brain small vessel disease. Neurology. 2016;87:2533–2539. [DOI] [PubMed] [Google Scholar]
- 5. Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997;349:245–249. [DOI] [PubMed] [Google Scholar]
- 6. Sviri GE, Feinsod M, Soustiel JF. Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage. Clinical and TCD correlations. Stroke. 2000;31:118–122. [DOI] [PubMed] [Google Scholar]
- 7. van der Velpen IF, Feleus S, Bertens AS, Sabayan B. Hemodynamic and serum cardiac markers and risk of cognitive impairment and dementia. Alzheimers Dement. 2017;13:441–453. [DOI] [PubMed] [Google Scholar]
- 8. Ermisch A, Ruhle HJ, Kretzschmar R, Baethmann A. On the blood‐brain barrier to peptides: specific binding of atrial natriuretic peptide in vivo and in vitro. Brain Res. 1991;554:209–216. [DOI] [PubMed] [Google Scholar]
- 9. Chabrier PE, Roubert P, Braquet P. Specific binding of atrial natriuretic factor in brain microvessels. Proc Natl Acad Sci USA. 1987;84:2078–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahinrad S, Bulk M, van der Velpen I, Mahfouz A, van Roon‐Mom W, Fedarko N, Yasar S, Sabayan B, van Heemst D, van der Weerd L. Natriuretic peptides in post‐mortem brain tissue and cerebrospinal fluid of non‐demented humans and Alzheimer's disease patients. Front Neurosci. 2018;12:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR Jr, Liu K, Orden S, Pirie P, et al. Recruitment in the coronary artery disease risk development in young adults (CARDIA) study. Control Clin Trials. 1987;8:68S–73S. [DOI] [PubMed] [Google Scholar]
- 13. Nakagawa K, Serrador JM, LaRose SL, Sorond FA. Dynamic cerebral autoregulation after intracerebral hemorrhage: a case‐control study. BMC Neurol. 2011;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Claassen JA, Meel‐van den Abeelen AS, Simpson DM, Panerai RB; International Cerebral Autoregulation Research N . Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab. 2016;36:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008;8:42–59. [DOI] [PubMed] [Google Scholar]
- 16. Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, Guttmann CR, Fisher ND, Lipsitz LA, Sorond FA. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab. 2014;34:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 18. Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bettencourt P. NT‐proBNP and BNP: biomarkers for heart failure management. Eur J Heart Fail. 2004;6:359–363. [DOI] [PubMed] [Google Scholar]
- 20. Shekhar S, Liu R, Travis OK, Roman RJ, Fan F. Cerebral autoregulation in hypertension and ischemic stroke: a mini review. J Pharm Sci Exp Pharmacol. 2017;2017:21–27. [PMC free article] [PubMed] [Google Scholar]
- 21. Vianna LC, Deo SH, Jensen AK, Holwerda SW, Zimmerman MC, Fadel PJ. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2015;308:H681–H687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao LH, Yang XL. Natriuretic peptides and their receptors in the central nervous system. Prog Neurogibol. 2008;84:234–248. [DOI] [PubMed] [Google Scholar]
- 23. Mahinrad S, de Craen AJM, Yasar S, van Heemst D, Sabayan B. Natriuretic peptides in the central nervous system: novel targets for cognitive impairment. Neurosci Biobehav Rev. 2016;68:148–156. [DOI] [PubMed] [Google Scholar]
- 24. Chabrier PE, Roubert P, Plas P, Braquet P. Blood‐brain barrier and atrial natriuretic factor. Can J Physiol Pharmacol. 1988;66:276–279. [DOI] [PubMed] [Google Scholar]
- 25. Steardo L, Nathanson JA. Brain barrier tissues: end organs for atriopeptins. Science. 1987;235:470–473. [DOI] [PubMed] [Google Scholar]
- 26. Guo S, Barringer F, Zois NE, Goetze JP, Ashina M. Natriuretic peptides and cerebral hemodynamics. Regul Pept. 2014;192–193:15–23. [DOI] [PubMed] [Google Scholar]
- 27. Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M, et al. Racial differences in plasma levels of N‐terminal pro‐B‐type natriuretic peptide and outcomes: the reasons for geographic and racial differences in stroke (REGARDS) study. JAMA Cardiol. 2018;3:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 29. Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B‐type natriuretic peptides and cardiovascular risk: systematic review and meta‐analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. [DOI] [PubMed] [Google Scholar]
- 30. Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of nt‐probnp in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. 2004;27:1929–1935. [DOI] [PubMed] [Google Scholar]
- 31. van Hateren KJ, Landman GW, Kleefstra N, Groenier KH, Struck J, Navis GJ, Bakker SJ, Houweling ST, van der Meer K, Bilo HJ. The midregional fragment of pro‐A‐type natriuretic peptide, blood pressure, and mortality in a prospective cohort study of patients with type 2 diabetes (ZODIAC‐25). Diabetes Care. 2013;36:1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krauser DG, Lloyd‐Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, Chen A, Tung R, Januzzi JL Jr. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a proBNP investigation of dyspnea in the emergency department (PRIDE) substudy. Am Heart J. 2005;149:744–750. [DOI] [PubMed] [Google Scholar]
- 33. Alyan O, Kacmaz F, Ozdemir O, Maden O, Topaloglu S, Ozbakir C, Metin F, Karadede A, Ilkay E. Effects of cigarette smoking on heart rate variability and plasma N‐terminal pro‐B‐type natriuretic peptide in healthy subjects: is there the relationship between both markers? Ann Noninvasive Electrocardiol. 2008;13:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strandgaard S, Paulson OB. Cerebral blood flow and its pathophysiology in hypertension. Am J Hypertens. 1989;2:486–492. [DOI] [PubMed] [Google Scholar]
- 35. Mankovsky BN, Piolot R, Mankovsky OL, Ziegler D. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabet Med. 2003;20:119–126. [DOI] [PubMed] [Google Scholar]
- 36. Luchner A, Schunkert H. Interactions between the sympathetic nervous system and the cardiac natriuretic peptide system. Cardiovasc Res. 2004;63:443–449. [DOI] [PubMed] [Google Scholar]
- 37. Blaber AP, Bondar RL, Stein F, Dunphy PT, Moradshahi P, Kassam MS, Freeman R. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692. [DOI] [PubMed] [Google Scholar]
- 38. Costa MD, Bosc LV, Majowicz MP, Vidal NA, Balaszczuk AM, Arranz CT. Atrial natriuretic peptide modifies arterial blood pressure through nitric oxide pathway in rats. Hypertension. 2000;35:1119–1123. [DOI] [PubMed] [Google Scholar]
- 39. Cauliez B, Guignery J, Marinier S, Mariau I, Lavoinne A. Two‐year stability of NT‐proBNP in frozen samples using the Roche Elecsys system. Ann Clin Biochem. 2008;45:318–319. [DOI] [PubMed] [Google Scholar]
- 40. Downie PF, Talwar S, Squire IB, Davies JE, Barnett DB, Ng LL. Assessment of the stability of N‐terminal pro‐brain natriuretic peptide in vitro: implications for assessment of left ventricular dysfunction. Clin Sci (Lond). 1999;97:255–258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


