Abstract
Background
The prevalence of thoracic aortic aneurysms (TAA) in patients with known abdominal aortic aneurysms (AAA) is not well known and understudied. Our aim was to conduct a systematic review and meta‐analysis of the overall prevalence of synchronous and metachronous TAA (SM‐TAA) in patients with a known AAA and to understand the characteristics of this sub‐population.
Methods and Results
We searched MEDLINE, EMBASE, and CENTRAL (Cochrane Central Register of Controlled Trials) from inception to November 2019 for all population‐based studies reporting on the prevalence of SM‐TAAs in a cohort of patients with AAA. Article screening and data extraction were performed by 2 authors and data were pooled using a random‐effects model of proportions using Freeman‐Tukey double arcsine transformation. The main outcome was the prevalence of SM‐TAAs in patients with AAAs. Secondary outcomes were the prevalence of synchronous TAAs, metachronous TAAs, prevalence of TAAs in patients with AAA according to the anatomic location (ascending, arch, and descending) and the differences in prevalence of these aneurysms according to sex and risk factors. Six studies were included. The pooled‐prevalence of SM‐TAA in AAA patients was 19.2% (95% CI, 12.3–27.3). Results revealed that 15.2% (95% CI, 7.1–25.6) of men and 30.7% (95% CI, 25.2–36.5) of women with AAA had an SM‐TAA. Women with AAA had a 2‐fold increased risk of having an SM‐TAA than men (relative risk [RRs], 2.16; 95% CI, 1.32–3.55). Diabetes mellitus was associated with a 43% decreased risk of having SM‐TAA (RRs, 0.57; 95% CI, 0.41–0.80).
Conclusions
Since a fifth of AAA patients will have an SM‐TAA, routine screening of SM‐TAA and their clinical impact should be more thoroughly studied in patients with known AAA.
Keywords: abdominal aortic aneurysms, meta‐analysis, metachronous aortic aneurysms, synchronous aortic aneurysms, thoracic aortic aneurysms
Subject Categories: Aneurysm, Vascular Disease, Cardiovascular Disease, Epidemiology, Women
Nonstandard Abbreviations and Acronyms
- CENTRAL
Cochrane Central Register of Controlled Trials
- SM‐TAA
synchronous and metachronous thoracic aortic aneurysms
- TAA
thoracic aortic aneurysms
Clinical Perspective
What Is New?
We found that 19.2% of patients with abdominal aortic aneurysm have a synchronous or metachronous thoracic aortic aneurysm.
Women have a 2‐fold higher risk of having a synchronous or metachronous abdominal and thoracic aortic aneurysm.
Diabetes mellitus is associated with a 43% decreased risk of having a synchronous or metachronous abdominal and thoracic aortic aneurysm.
What Are the Clinical Implications?
Routine screening of synchronous/metachronous thoracic aortic aneurysms and their clinical impact should be more thoroughly studied in patients with known abdominal aortic aneurysms.
Differences in prevalence found between men and women might explain, in part, why women have worse outcomes following abdominal aortic aneurysm repair.
Aneurysmal disease is known nowadays to be a systemic, multifactorial, and unpredictable condition with different causes, behaviors and presentations which increase the complexity of its diagnosis, treatment, and outcomes. 1 , 2
Abdominal aortic aneurysms (AAA) are by far the most common and studied aneurysms and screening strategies have shown to be effective, reducing the incidence in aneurysm rupture rate and improvement of care in a cost‐effective fashion. 3
The presence of synchronous and metachronous aneurysms has been well described in the literature. 4 However, in contrast to synchronous peripheral aneurysms which are frequently screened and reported, the incidence and behavior of synchronous/metachronous thoracic aortic aneurysms (SM‐TAA) is underappreciated. 4
In a previous study in our center, we found that 18.9% of patients submitted to Thoracic Endovascular Aortic Repair, had a synchronous or metachronous AAA. 5 However, thoracic aortic aneurysms (TAA) in general, do not have a screening program to date and the incidence and prevalence of these aneurysms is underappreciated. Since rupture of TAAs in patients with a known AAA or following an AAA repair has been described, 4 , 6 , 7 better understanding of the prevalence of these synchronous/metachronous TAAs in patients with a known AAA might be crucial for patients, physicians, and policy makers.
Our aim was to estimate the overall prevalence of synchronous and metachronous TAAs in patients with a known AAA.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines 8 were followed for reporting and design of this systematic review. The authors declare that all supporting data are available within the article (and its online supplementary files).
Eligibility Criteria
We included all cohort studies, either prospective or retrospective, reporting on the prevalence of thoracic aortic aneurysms in a population of patients with known abdominal aortic aneurysm, irrespective of the diagnostic method used. We accepted the definition of AAA or TAA provided by the studies. We defined synchronous aneurysms as occurring concomitantly and metachronous aneurysms if the diagnosis of a new TAA occurred 2 years after the initial AAA diagnosis and no TAA was present initially.
There were no date or language restrictions. Animal studies were not included.
Papers were excluded if they did not specify the specific number of SM‐TAA. If the same population was described in 2 papers 1 of them was excluded to not duplicate events. Papers were also excluded if the studied population (the denominator) included patients without AAA.
Information Sources and Search Method
We searched EMBASE, MEDLINE, and CENTRAL (Cochrane Central Register of Controlled Trials), from inception to November 2019. We also cross‐checked references and consulted specialists for additional potential studies. The search strategy is detailed in Table S1.
The search results were cross‐checked, and duplicates were eliminated.
Study Selection, Data Collection Process, and Synthesis
Two authors (R.G.M. and A.L.) independently screened the titles and abstracts that yielded from the search. Full‐text papers were also independently assessed by both authors and disagreements were resolved by consulting with a third author (G.D.).
After the final search result, data were independently extracted by 2 authors (R.G.M. and A.L.) using a pre‐design report form and uploaded onto a table sheet after cross‐checking.
Data retrieved included: study publication data (authors, date, journal), population studied (years of the study and respective centers), study site, number of participants with known AAA, number of patients with SM‐TAA and AAA, demographics (age, sex, risk factors), location of the TAA, diagnostic methods used, and definitions of TAA and AAA used.
The main outcome of interest was the overall prevalence of SM‐TAA in patients with known AAA. The prevalence was defined as the number of existing TAAs at the time of the study in the population of patients with known AAA.
The secondary outcomes of interest included the prevalence of synchronous TAAs; the prevalence of metachronous TAAs; the prevalence of SM‐TAA according to the anatomic location and the difference in the prevalence of SM‐TAA and AAA according to sex and other risk factors (smoking, hypertension, diabetes mellitus, family history of AAA/TAA, hyperlipidemia, and chronic obstructive pulmonary disease).
Statistical Analysis
We used the Open‐Meta (Analyst) Software 9 for quantitative analysis and to derive forest plots, when appropriate.
The results yielded by the data extraction were expressed in percentages for the prevalence of SM‐TAA. The total number of individuals with known AAA was used as the denominator and the number of patients with SM‐TAA and AAA as the numerator.
A random‐effects model was used to pool the data to account for the heterogeneity of the included studies. 9 The random‐effects model of DerSimonian and Laird was used by default as this approach is the simplest and most commonly used method for fitting the random effects model and is particularly useful for larger samples. 10 Freeman‐Tukey transformation (double arcsine transformation) was used to adjust the data set to estimate the frequency of the events, limiting the CI among 0% to 100%. 11 For subgroup sex analysis, we dichotomized the prevalence data and used male sex as the reference group and for subgroup risk factor analysis we used the group with AAA‐only as the reference group, results were reported using risk ratios [RRs] and 95% CIs. Statistical heterogeneity was assessed using I2, which was defined as low (25%), moderate (50%), or high (75%) according to Higgins and Thompson. 12
Sensitivity analysis was performed according to the diagnostic method used (computed tomography [CT] scan or magnetic resonance/magnetic resonance‐angiogram versus transthoracic echocardiography), to the definition for AAA used (only patients admitted for AAA repair versus all patients with an abdominal aortic diameter ≥3 cm) and type of patients included (both men and women versus only women).
Risk of Bias
We adapted and used the Critical Appraisal Skills Program cohort study checklist to assess for risk of bias, in which we categorized 9 items as having high, unclear, or low risk of bias. 13 Two authors (R.G.M. and A.L.) independently assessed each included paper. The overall risk of bias for each study was divided as high‐ or low‐risk, with high‐risk studies considered those in which at least 2 items were assessed at a high risk of bias, or where >3 items were rated as unclear.
Results
Included Studies
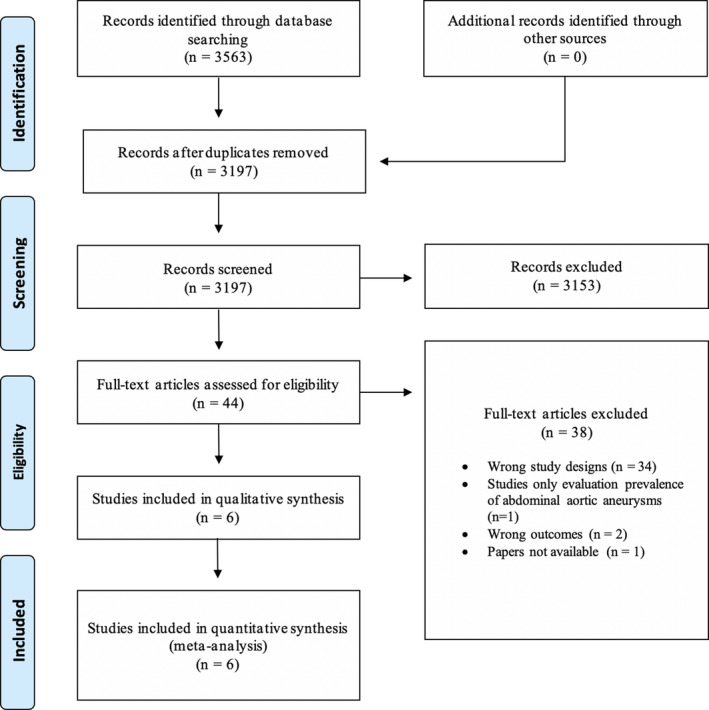
The search yielded 3563 papers which resulted in 3197 articles after duplicates were removed. After title and abstract screening, 44 papers were included in the full‐text assessment. Of these, 6 articles were included in the review. 4 , 14 , 15 , 16 , 17 , 18 The reasons for exclusion of the remainder 38 full‐text articles assessed are detailed in Figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram.
Study Characteristics and Demographic Data
Overall, 3333 patients with known AAA were included. Of these, 610 were found to have an SM‐TAA.
Definitions of both AAA and TAA varied across studies (Table 1). Four papers 4 , 15 , 16 , 17 defined AAA as an increase in the aortic diameter ≥30 mm and 2 papers 14 , 18 did not give precise definition of AAA. However, both of these later studies included patients who were undergoing elective AAA repair, so we can assume the definition to be a diameter of, at least 50 mm. 14 , 18 , 19 The diagnostic method of TAA also varied: CT in 4 studies 15 , 16 , 17 , 18 ; CT, magnetic resonance, or magnetic resonance‐angiogram in one, 4 and transthoracic echocardiography in another. 14 Specific demographic data, including risk factors, for all participants in both groups (synchronous/metachronous and only AAA patients) were only detailed in 2 studies. 4 , 16 Mean age in the total cohort varied between 72.5 and 75 years. Most patients in the cohort were men, except for the paper by Wallinder et al 17 which only analyzed female patients (Table 2).
Table 1.
Study Details Including Cohort Origin and Definitions
| Study | Years of the Study | Location | Cohort Origin | Definition of AAA | Definition of TAA | Methods of Diagnosing TAA |
|---|---|---|---|---|---|---|
| Larsson et al, 2011 15 | 2004–2008 | Stockholm, Sweden (Karolinska University Hospital) | Patients with AAA diagnosis attending the outpatient clinic | Diameter ≥30 mm |
Women: AAD >42 mm, DAD >33 mm; Men: AAD >47 mm and DAD >37 mm |
CT |
| Chaer et al, 2012 4 | 2000–2008 | Pittsburgh, Pennsylvania, USA (University of Pittsburgh) | Electronic medical record | Diameter >50% normal diameter or diameter ≥30 mm | Diameter >30 mm | CT, MR, or MR angiogram |
| Takigawa et al, 2012 18 | 2001–2006 | Yokosuba, Japan (Cardiovascular Center) | Undergoing elective graft replacement for asymptomatic infrarenal AAA | NR | Diameter >40 mm | CT |
| Agricola et al, 2013 14 | 2013 | Milan, Italy (San Raffaele Hospital) | Patients with AAA who underwent TE before surgery | NR |
Women: AAD >42 mm, AArchD >32; Men: AAD >47 mm, AArchD >37 mm |
TE |
| Wallinder et al, 2018 17 | 2013 | Uppsala and Västernorrland, Sweden | Swedish national vascular registry | Diameter ≥30 mm | Diameter ≥42 (ascending); D ≥33 (descending) | CT |
| Dombrowski et al, 2019 16 | 2007–2017 | Michigan, USA (Beaumont Health System) | Radiology reports of AAA | Diameter ≥30 mm | Diameter ≥40 mm | CT |
AAA indicates abdominal aortic aneurysms; AAD, ascending aortic diameter; AArchD, aortic arch diameter; CT, computed tomography; DAD, descending aortic diameter; MR, magnetic resonance; NR, not reported; TAA, thoracic aortic aneurysm; and TE, transthoracic echocardiography.
Table 2.
Study Data
| Study | No. of Participants | Mean Age, y (SD) | Men/Women, n (%) | Patients With TAA (n) | Synchronous; Metachronous TAA (n) | Location of the Aneurysm—Asc; Desc; Arch (n) | TAAs—Men/Women, n (%) |
|---|---|---|---|---|---|---|---|
| Larsson et al, 2011 15 | 354 | 74 (NR) | 274 (77.4)/80 (22.6) | 100 | 100; NR | 12; 94; 6 | 62 (62)/38 (38) |
| Chaer et al, 2012 4 | 1082 | 74.6 (9) | 724 (66.9)/358 (33.1) | 253 | 117; 136 | NR | 143 (68.5)/105 (41.5) |
| Takigawa et al, 2012 18 | 157 | 72.7 (7.5) | 128 (82)/29 (18) | 13 | 13; NR | 2; 8; 3 | NR |
| Agricola et al, 2013 14 | 1305 | NR | 1034 (79.2)/271 (20.8) | 137 | 137; NR | 52; NR; 85 | 66 (48)/71 (52) |
| Wallinder et al, 2018 17 | 217 | 75 (NR) | 0 (0)/217 (100) | 67 | NR; NR | 8; 53; 2 | NR/67 (100) |
| Dombrowski et al, 2019 16 | 218 | 74 (NR) | 136 (62.4)/82 (37.6) | 40 | 40; NR | 19; 13; 8 | 20 (50)/20 (50) |
AAA indicates abdominal aortic aneurysms; Arch, aortic arch; Asc, ascending thoracic aorta; CT, computed tomography; Desc, descending thoracic aorta; NR, not reported; and TAA, thoracic aortic aneurysm.
Only 1 paper 4 provided with individualized data for both synchronous and metachronous TAAs, 4 papers 14 , 15 , 16 , 18 only reported on synchronous TAAs and another included both synchronous and metachronous TAAs but did not report the specific number of each. 17
Three papers provided the indications for chest imaging: in the Agricola et al 14 study the reason was to measure the ascending aorta and aortic arch diameter and in the other 2 studies the majority of patients performed a chest CT for non‐aortic related problems, mostly for pulmonary indications (74% in Chaer et al 4 and 68% in Dombrowski et al 16 ). Information on specific aortic diameter of the TAAs was only available in 2 studies. 4 , 17 In the Wallinder et al 17 study, one ascending aortic aneurysm had ≥60 mm and 14 descending aortic aneurysms had ≥55 mm leading to 13 of the patients having undergone repair. In the Chaer et al 4 study they reported that 61 of 253 patients underwent repair, ruptured, or had a TAA >55 mm, and 13 patients died from a ruptured TAA. Mortality from TAA was not described in any other paper.
Risk of Bias
Overall, the risk of bias was considered to be high. The main source of risk of bias was the absence of adjusting for key risk factors, which occurred in all studies, such as age, smoking, and hypertension. Additional sources of risk of bias were: the selective recruitment of patients undergoing AAA repair in the studies by Agricola et al 14 and Takigawa et al, 18 rather than including every patient with AAA (ie, diameter ≥30 mm); and in the way the outcome (prevalence of TAA) was measured in the study by Agricola et al, 14 which used transthoracic echocardiography only (Figure S1).
Prevalence of Synchronous/Metachronous TAA in Patients With Known AAA
Overall, we found that 19.2% of patients with AAA had an SM‐TAA (95% CI, 12.3–27.3; I2=96%; 6 studies; 3333 participants)—Figure 2.
Figure 2. Forest plot analyzing the prevalence of synchronous/metachronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
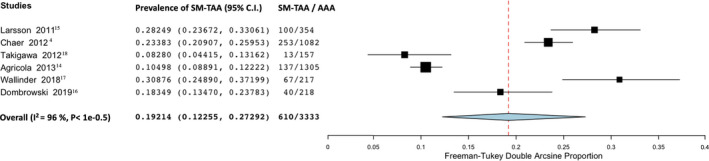
AAA indicates abdominal aortic aneurysm; and SM‐TAA, synchronous/metachronous thoracic aortic aneurysm.
Individualized Prevalence of Synchronous and Metachronous TAA in Patients With Known AAA
The prevalence of synchronous TAA in patients with known AAA was 14.6% (95% CI, 0.09–20.9; I2=94%; 5 studies;, 3116 participants)—Figure 3. Only the study from Chaer et al 4 specified the number of metachronous TAAs in patients with known AAA and found a prevalence of 12.7%.
Figure 3. Forest plot analyzing the prevalence of synchronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
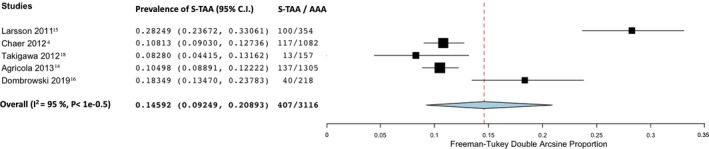
AAA indicates abdominal aortic aneurysm; and S‐TAA, synchronous thoracic aortic aneurysm.
Prevalence of Synchronous/Metachronous TAA in Patients With Known AAA According to Anatomic Location
The prevalence of SM‐TAA in patients with known AAA according to the anatomic location of the TAA was 4% in the ascending thoracic aorta (95% CI, 2.4–5.7; I2=69%; 5 studies; 2251 participants)—Figure 4; 14.1% in the descending thoracic aorta (95% CI, 4.7–27.3; I2=96%; 4 studies; 946 participants)—Figure 5 and 2.8% involving the aortic arch (95% CI, 0.9–5.5; I2=87%; 5 studies; 2251 participants)—Figure 6.
Figure 4. Forest plot analyzing the prevalence of synchronous/metachronous ascending thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
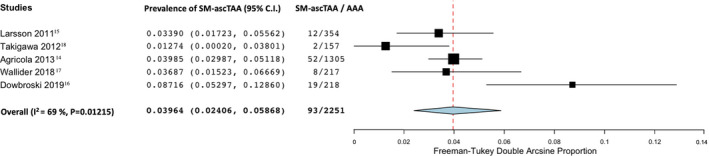
AAA indicates abdominal aortic aneurysm; and SM‐ascTAA: synchronous/metachronous ascending thoracic aortic aneurysm.
Figure 5. Forest plot analyzing the prevalence of synchronous/metachronous descending thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
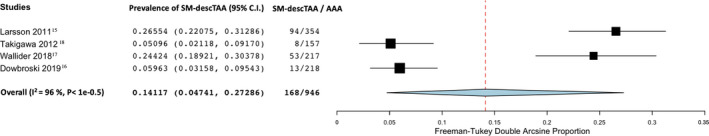
AAA indicates abdominal aortic aneurysm; and SM‐descTAA, synchronous/metachronous descending thoracic aortic aneurysm.
Figure 6. Forest plot analyzing the prevalence of synchronous/metachronous thoracic aortic arch aneurysm in patients with known abdominal aortic aneurysm.
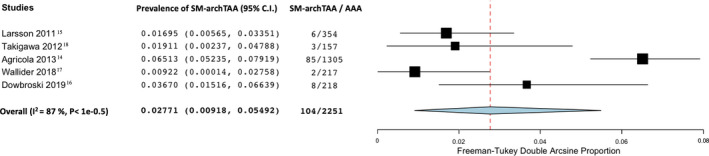
AAA indicates abdominal aortic aneurysm; and SM‐archTAA: synchronous/metachronous arch thoracic aortic aneurysm.
Prevalence and Risk of Synchronous/Metachronous TAA in Patients With Known AAA According to Sex
We found that 15.2% of male patients with AAA had an SM‐TAA (95% CI, 7.1–25.6; I2=97%; 4 studies; 2168 participants)—Figure 7. In female patients, the prevalence was higher: 30.7% of female patients with AAA had an SM‐TAA (95% CI; 25.2–36.5; I2=71%; 5 studies; 1008 participants)—Figure 8.
Figure 7. Forest plot analyzing the prevalence in men of synchronous/metachronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
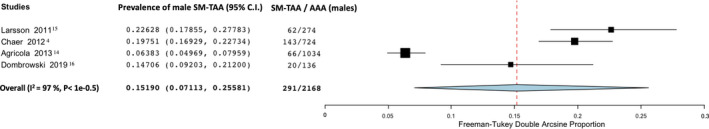
AAA indicates abdominal aortic aneurysm; and SM‐TAA, synchronous/metachronous thoracic aortic aneurysm.
Figure 8. Forest plot analyzing the prevalence in women of synchronous/metachronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.
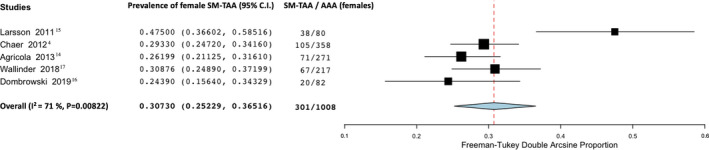
AAA indicates abdominal aortic aneurysm; and SM‐TAA, synchronous/metachronous thoracic aortic aneurysm.
Comparing both groups, women with AAA had a 2‐fold increased risk of having SM‐TAA compared with men with AAA (RRs, 2.16; 95% CI, 1.32–3.55; I2=90%; 4 studies; 2168 participants)—Figure 9.
Figure 9. Forest plot analyzing relative risk of female sex comparing with male sex on the prevalence of synchronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.

AAA indicates abdominal aortic aneurysm; and SM‐TAA, synchronous/metachronous thoracic aortic aneurysm.
Risk of Synchronous/Metachronous TAA in Patients With Known AAA According to Other Risk Factors
Information on risk factors in both groups (SM‐TAA and only AAA patients) was only available in 2 studies. 4 , 16 Overall, diabetes mellitus was associated with a 43% decreased risk of having SM‐TAA and AAA (RRs, 0.57; 95% CI, 0.41–0.80; I2=0%; 2 studies; 1007 participants)—Figure 10. All other identified risk factors: smoking; hypertension; chronic obstructive pulmonary disease; family history of aortic aneurysm; and hyperlipidemia were not associated with an increased or decreased risk of having an SM‐TAA and AAA (Table 3).
Figure 10. Forest plot analyzing relative risk of diabetes mellitus on the prevalence of synchronous thoracic aortic aneurysm in patients with known abdominal aortic aneurysm.

AAA indicates abdominal aortic aneurysm; DM, diabetes mellitus; RR, relative risk; and SM‐TAA, synchronous/metachronous thoracic aortic aneurysm.
Table 3.
Relative Risk of Having a Synchronous TAA and AAA Compared With Patients With AAA Only Across the Different Risk Factors
| Risk Factor | Relative Risk |
|---|---|
| Diabetes mellitus | 0.57 (95% CI, 0.41–0.80; I2=0%; 2 studies; 1007 participants) |
| Smoking | 0.97 (95% CI, 0.85–1.10; I2=62%; 2 studies; 1007 participants) |
| Hypertension | 1.01 (95% CI, 0.94–1.01; I2=70%; 2 studies; 1007 participants) |
| Hyperlipidemia | 1.10 (95% CI, 0.89–1.3; I2=58%; 2 studies; 1007 participants) |
| COPD | 1.09 (95% CI, 0.93–1.28; I2=0%; 2 studies; 1007 participants) |
| Family history of AAA/TAA | 2.37 (95% CI, 0.97–5.77; I2=35%; 2 studies; 1007 participants) |
AAA indicates abdominal aortic aneurysm; COPD, chronic obstructive pulmonary disease; and TAAs, thoracic aortic aneurysms.
Sensitivity Analysis
To analyze the impact of using different diagnostic methods (namely transthoracic echocardiography), different definitions for AAA and only including female patients in the study, we performed a sensitivity analysis evaluating these effects (Table 4). Excluding the paper from Agricola et al, 14 which used transthoracic echocardiography as the diagnostic method to screen for TAAs and screened only for ascending and arch aneurysms, showed an increase in the prevalence (19.2% versus 21.5%). However, when analyzing according to the anatomic location the prevalence remained the same on ascending TAAs and was lower on arch TAAs (2.8% versus 1.9%). Also, when we only included papers using a definition of ≥30 mm 4 , 15 , 16 , 17 for AAAs the prevalence of SM‐TAA also increased (19.2% versus 25%).
Table 4.
Sensitivity Analysis
| Synchronous/Metachronous TAA in Patients With Known AAA | Prevalence (%) |
|---|---|
| Overall Prevalence of SM‐TAA in Patients With Known AAA | 19.2 (95% CI, 12.3–27.3; I2=96%; 6 studies; 3333 participants) |
| Studies using CT and/or MR for the diagnosis of TAA | 21.5 (95% CI, 15.4–28.2; I2=90%; 5 studies; 2028 participants) |
| Studies using TE for the diagnosis of TAA | 10.5 (95% CI, 8.9–12.2, 1 study; 1305 participants) |
| Using AAA definition of a diameter ≥30 mm | 25.0 (95% CI, 20.6–29.7; I2=76%; 4 studies; 1871 participants) |
| Including only AAA patients admitted for surgery | 10.2 (95% CI, 8.6–11.8; I2=0%; 2 studies; 1462 participants) |
| Including both male and female patients | 17.2 (95% CI, 10.2–25.5; I2=96%, 4 studies, 3116 participants) |
| Studies only analyzing female patients | 30.9 (95% CI, 24.9–37.2) |
| Overall Prevalence of Snchronous/Metachronous Ascending TAAs in Patients With Known AAA | 4% (95% CI, 2.4–5.7; I2=69%; 5 studies; 2251 participants) |
|---|---|
| Studies using CT and/or MR for the diagnosis of ascending TAA | 4% (95% CI, 1.7–7.0; I2=76%; 4 studies; 946 participants) |
| Overall Prevalence of Synchronous/Metachronous Arch TAAs in Patients With Known AAA | 2.8% (95% CI, 0.9–5.5; I2=87%; 5 studies; 2251 participants) |
|---|---|
| Studies using CT and/or MR for the diagnosis of arch TAA | 1.9% (95% CI, 1.0–3.1; I2=23%; 4 studies; 946 participants) |
AAA indicates abdominal aortic aneurysm; CT, computed tomography; MR, magnetic resonance; SM‐TAA, synchronous/metachronous thoracic aortic aneurysms; TAAs, thoracic aortic aneurysms; and TE, transthoracic echocardiography.
Discussion
The main findings of this review were: (1) the overall prevalence of SM‐TAA in patients with a known AAA was 19.2% (95% CI, 12.3–27.3) and (2) the prevalence of SM‐TAA was higher in women—30.7% (95% CI, 25.2–36.5) versus 15.2% (95% CI, 7.1–25.6)—with a 2‐fold increase in risk.
These findings are surprising as almost a fifth of every patient with an AAA will have a TAA and that number increases to almost a third in women.
Screening strategies for AAA with an abdominal ultrasound have shown to be effective, reducing the incidence in aneurysm rupture rate and improvement of care in a cost‐effective fashion. 3 Contrary to AAAs, implementing a screening strategy for TAAs is difficult, since for an accurate diagnosis, a chest CT is usually necessary. Transthoracic echocardiography might find some but certainly not all TAAs, especially in the descending thoracic aorta. We found this in our sensitivity analysis: the prevalence increased (19.2% versus 21.5%) when we excluded the study from Agricola et al. 14 In fact, in this latter study, 14 which used only transthoracic echocardiography to screen for TAAs, the prevalence described for synchronous TAAs in patients with known AAA was only 10.5%, which is probably because of the fact that descending TAAs were not screened (Table 4).
The absence of a timely diagnosis has led to a lot of TAAs passing undiagnosed until a complication occurs, such as rupture, which is usually fatal. 20 , 21 A TAA diagnosed before rupture is a potential life saved and this is usually made accidentally. We found that 19.2% of AAAs have an SM‐TAA, this means that screening every patient with a known AAA with a chest CT might be useful. Moreover, death attributable to rupture of other aneurysms, such as TAAs, is already a problem recognized in long‐term follow‐up of AAA repair. 4 , 6 , 7 , 22 , 23
Currently, there are no clear indications in the current Society of Vascular Surgery guidelines 19 about full aortic imaging when an AAA is diagnosed. Although more research is needed to demonstrate cost‐effectiveness and the real impact of these SM‐TAA, we believe TAA screening in patients with a known AAA should probably become common practice since 19.2% is not negligible.
The difference found between male and female sex is striking. It has been known that women have a lower threshold for aneurysm rupture and have worse outcomes after AAA repair, even when operated at smaller diameters. 24 , 25 No one has yet clearly understood why this occurs. The higher prevalence of synchronous/metachronous thoracic and abdominal aortic aneurysms in women, might indicate that aneurysm disease has a different pathophysiology in the female sex, with a different and probably more aggressive and systemic behavior than in men. This might be one of the causes for these worse long‐term outcomes found in women. Understanding this fact might lead us to increase our diagnostic suspicion for synchronous and metachronous TAAs and lead us to better surveillance and ultimately improved care.
Because of lack of data, we were not able to analyze in‐depth other risk factors which might be predictive of higher prevalence of SM‐TAA. We found diabetes mellitus to be negatively associated with the risk of having SM‐TAA and AAA, however, this data were only available in 2 studies, 4 , 16 which limits our findings. In the paper from Chaer et al 4 positive predictors of SM‐TAA were Black race; family history of TAA; hypertension; and obesity; and negative predictors were diabetes mellitus, infra‐renal location of the AAA; and smoking.
This review has some limitations, the difference in study designs on diagnostic method and definitions of both AAA and TAAs brings some clinical and possibly statistical heterogeneity to our results in general. The fact that the data retrieved was not age‐standardized also limits our findings, and explains, at least, partially, the statistical heterogeneity. The lack of data about risk factors in both groups (synchronous and AAA‐only patients), such as smoking or hypertension, limited our analysis on possible confounding or predictive factors for the presence of SM‐TAA. Other important data that were not available across all studies was the size of the TAAs found, the number of TAAs that ruptured, and their mortality before repair, which limits our analysis about risk and the prognosis of these aneurysms.
Further studies are needed to understand the clinical behavior of synchronous and metachronous TAAs in AAA patients (including their morbidity and mortality), what is the true impact of screening patients with an AAA with a chest CT, to assess the cost‐effectiveness of such a screening program and to understand their relationship with the female sex.
A feasible observational study would be to compare 2 time periods: pre‐ and post‐SM‐TAA screening with a chest CT in all patients with a known AAA to address the impact of screening in the number of treatable/near treatable TAAs and the number of preventable ruptured TAAs and deaths. Also, using large registry data would be useful to identify other clinical characteristics that might be more common in patients with synchronous or metachronous aortic aneurysms.
CONCLUSIONS
This meta‐analysis increases the evidence about the presence of synchronous/metachronous TAA in patients with known AAA. The higher prevalence of these aneurysms in women is striking and might explain one of the aspects why worse outcomes on follow‐up of AAA are observed in women and shows the importance of aortic screening in women.
To improve short‐ and long‐term outcomes after AAA repair, the authors recommend that routine screening of synchronous and metachronous TAAs and their clinical impact should be more thoroughly studied, since 19.2% of AAAs with an SM‐TAA is not a negligible number.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1
Figure S1
Acknowledgments
Gouveia e Melo and Silva Duarte conceived the idea for the protocol and made the main contribution to planning and preparation of timelines for completion. Gouveia e Melo and Lopes analyzed all papers, extracted the data, and analyzed the risk of bias. Gouveia e Melo performed the statistical analysis. Caldeira and Alves analyzed the data and confirmed the statistical analysis. Gouveia e Melo designed the tables and wrote the first draft of the manuscript, which was then reviewed and amended by Alves, Caldeira, Fernandes e Fernandes, and Pedro. All authors then approved the final written manuscript. Gouveia e Melo is the guarantor for the work.
(J Am Heart Assoc 2020;9:e017468 DOI: 10.1161/JAHA.120.017468.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017468
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg. 2008;1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guirguis‐Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;2219–2238. [DOI] [PubMed] [Google Scholar]
- 4. Chaer RA, Vasoncelos R, Marone LK, Al‐Khoury G, Rhee RY, Cho JS, Makaroun MS. Synchronous and metachronous thoracic aneurysms in patients with abdominal aortic aneurysms. J Vasc Surg. 2012;1261–1265. [DOI] [PubMed] [Google Scholar]
- 5. Garrido P, Pedro LM, Fernandes RF, Silvestre L, Sousa G, Martins C, Fernandes JF. Endovascular treatment of synchronous and metachronous aneurysms of the thoracic aorta. Is there an increase risk in the procedural risk? Angiol Cir Vasc. 2016;226–233. [Google Scholar]
- 6. Clouse WD, Marone LK, Davison JK, Dorer DJ, Brewster DC, LaMuraglia GM, Cambria RP. Late aortic and graft‐related events after thoracoabdominal aneurysm repair. J Vasc Surg. 2003;254–261. [DOI] [PubMed] [Google Scholar]
- 7. Plate G, Hollier LA, O’Brien P, Pairolero PC, Cherry KJ, Kazmier FJ. Recurrent aneurysms and late vascular complications following repair of abdominal aortic aneurysms. Arch Surg. 1985;590–594. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Jos K, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;1–15. [Google Scholar]
- 10. Dersimonian R, Laird N. Meta‐analysis in clinical trials. Stat Med. 1986;177–188. [DOI] [PubMed] [Google Scholar]
- 11. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;974–978. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;1539–1558. [DOI] [PubMed] [Google Scholar]
- 13. Critical Appraisal Skills Programme . CASP (cohort study) checklist. [online]. 2018. Available at: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf. Accessed April 25, 2020.
- 14. Agricola E, Slavich M, Tufaro V, Fisicaro A, Oppizzi M, Melissano G, Bertoglio L, Marone E, Civilini E, Margonato A, et al. Prevalence of thoracic ascending aortic aneurysms in adult patients with known abdominal aortic an aneurysm: an echocardiographic study. Int J Cardiol. 2013;3147–3148. [DOI] [PubMed] [Google Scholar]
- 15. Larsson E, Vishnevskaya L, Kalin B, Granath F, Swedenbor J, Hultgren R. High frequency of thoracic aneurysms in patients with abdominal aortic aneurysms. Ann Surg. 2011;180–184. [DOI] [PubMed] [Google Scholar]
- 16. Dombrowski D, Long G, Chan J, Brown O. Screening chest computed tomography is indicated in all patients with abdominal aortic aneurysm. Ann Vasc Surg. 2019;190–195. [DOI] [PubMed] [Google Scholar]
- 17. Wallinder J, Georgiou A, Wanhainen A, Björck M. Prevalence of synchronous and metachronous aneurysms in women with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2018;435–440. [DOI] [PubMed] [Google Scholar]
- 18. Takigawa M, Yoshimuta T, Akutsu K, Takeshita S, Yokoyama N. Prevalence and predictors of coexistent silent atherosclerotic cardiovascular disease in patients with abdominal aortic aneurysm without previous symptomatic cardiovascular diseases. Angiology. 2012;380–385. [DOI] [PubMed] [Google Scholar]
- 19. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;2–77. [DOI] [PubMed] [Google Scholar]
- 20. Bickerstaff LK, Pairolero PC, Hollier LH, Melton J, Van Pennen HJ, Cherry KJ, Joyce JW, Lie JT. Thoracic aortic aneurysms: a population‐based study. Surgery. 1982;1103–1108. [PubMed] [Google Scholar]
- 21. Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;2611–2618. [DOI] [PubMed] [Google Scholar]
- 22. Goodney PP, Tavris D, Lee L, Gross T, Fisher ES, Finlayson SRG. Causes of late mortality after endovascular and open surgical repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2010;1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Schaik TG, Yeung KK, Verhagen HJ, Bruin JL, Van Sambeek MRHM, Bam R, Zeebregts CJ, Van Herwaarden JA, Blankensteijn JD; DREAM trial participants . Long‐term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;1379–1389. [DOI] [PubMed] [Google Scholar]
- 24. Deery SE, Soden PA, Zettervall SL, Shean KE, Bodewes TCF, Pothof AB, Lo RC, Schermerhorn ML. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo CR, Schermerhorn ML. Abdominal aortic aneurysms in women. J Vasc Surg. 2016;839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


