Abstract
Background
Underrepresentation of older people in clinical trials remains. This study aimed to examine the inclusion of older people and associated safety and efficacy reports from clinical trials of new molecular entities for cardiovascular disease indications since commencement of the US Food and Drug Administration Drug Trial Snapshot (DTS) Program. The DTS provides concise information on participants included in clinical trials supporting US Food and Drug Administration approval of new drugs.
Methods and Results
A cross‐sectional analysis between January 1, 2015 and April 30, 2019 of DTS data including approval date, indication, number of trials and participants, age distribution, efficacy, and safety statements was conducted. Participation‐to‐prevalence ratio (PPR) was used to describe representation of older participants in trials relative to disease population. Efficacy and safety statements regarding age were compared with drug prescribing information. A total of 72 079 participants from 10 DTS reports were identified and 39 625 (55.0%) were aged ≥65 years old. Overall, 63.6% of cardiovascular disease DTS reports were representative of people aged ≥65 years old for specific cardiovascular disease conditions. Underrepresentation was observed in 4 DTS: 2 for heart failure (PPR 0.48 and 0.62), 1 for pulmonary arterial hypertension (PPR 0.72), and 1 for venous thromboembolism (PPR 0.38). Participants in clinical trials for new drugs for the treatment of atrial fibrillation (PPR 0.99 and 1.21) and hypercholesterolemia (PPR 0.84 and 0.97) were reflective of the older population for these diseases. An increased risk of adverse events in older participants was reported in 40% DTS safety statements but no differences were reported in the drug product information.
Conclusions
Despite the fact that >60% of cardiovascular disease trial participants for new molecular entities included in the DTS program were representative of the older population in real‐world clinical practice, concerns remain for conditions including heart failure or venous thromboembolism. Drug product information safety statements regarding age differences in adverse events were not reflective of trial findings. An increased directive is needed to facilitate the generation of real‐world evidence and appropriate reporting within drug product information for these potentially at‐risk patient populations.
Keywords: cardiovascular disease, clinical trials, older population, real‐world evidence
Subject Categories: Epidemiology, Aging
Nonstandard Abbreviations and Acronyms
- DTS
drug trial snapshot
- FDA
Food and Drug Administration
- PPR
participation‐to-prevalence ratio
Clinical Perspective
What Is New?
There are concerns that older people aged ≥65 years old are underrepresented in cardiovascular disease drug clinical trials, despite the high prevalence of cardiovascular disease in this population group.
What Are the Clinical Implications?
While >60% of participants included in cardiovascular disease drug trials of new molecular entities since the drug trial snapshot program were reflective of the proportion of older people in real‐world clinical practice, concerns remain for studies in patients with heart failure and venous thromboembolism.
Comparison of reporting of safety statements showed that while an increased risk of adverse events in older participants was reported in 40% of drug trial snapshot safety statements, no differences by age were reported in the drug product information.
An increased directive is needed to facilitate the generation of real‐world evidence and appropriate reporting of risk of adverse events by age within the drug product information for these potentially at‐risk patient populations.
Despite efforts over the past decade by regulatory authorities to increase the external validity of randomized clinical trials (RCTs), concerns remain with regard to the exclusion or underrepresentation of certain populations, including older people (≥65 years old) and those with comorbidity. 1 As a consequence, the evidence base included in current treatment guidelines is often not reflective of "real‐world" patients treated in clinical practice. This has led to the applicability of treatment guidelines to older people being questioned. 2 Age‐related changes in physiology and associated effects on medication pharmacokinetics and pharmacodynamics, together with the increasing prevalence of multimorbidity, polypharmacy, and frailty, mean that the results obtained from RCTs in younger people cannot necessarily be extrapolated to the older population. 3 , 4 , 5
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide and the prevalence increases with age. 6 In the United States, 36.6% (n=92 100 000) of the adult population has CVD and this increases to 69% and 85%, for adults aged 60 to 79 and ≥80 years old, respectively. 6 Previous studies have reported exclusion of older people with CVD from RCTs. 7 , 8 An analysis of 839 clinical trials between 2006 and 2015 reported that 53% (n=446) explicitly excluded older adults aged ≥65 years old; the estimated proportion of participants aged ≥65 years old was 42.5% and for those ≥75 years old it was 12.3%. 7 Of the 22 late‐breaking abstracts of the 2011 American Heart Association Meeting, 36% (n=8) did not include older adults aged >60 to 80 years old. 8
There is an increased recognition globally of the need to provide real‐world evidence for the safety and efficacy of medicines. In 2012, the US Food and Drug Administration (FDA) Safety and Innovation Act directed the FDA to formulate an action plan to improve participation, data quality, and transparency in clinical trials. As part of this response, the FDA implemented the Drug Trials Snapshots (DTS) Program in 2015, where demographic data from participants (sex, race, and age) from RCTs of new molecular entities that were approved by the FDA are publicly reported, together with statements on efficacy and safety by these subgroups. 9 This study aimed to examine the inclusion of older people in RCTs of new molecular entities for CVD indications approved by the FDA since the inception of the DTS program. It also aimed to compare the age‐specific efficacy and safety statements in the DTS with those included in the available drug prescribing information.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. As publicly available data were used, approval from the institutional review board was not required for this study.
DTS data between January 1, 2015 and April 30, 2019 (date of data extraction) available from the FDA website (https://www.fda.gov/drugs/informationondrugs/ucm412998.htm) were obtained for all new molecular entities for cardiovascular (including antithrombotic) medications approved by the FDA. Specifically, the date of approval, indication, total number of participants, number of trials, age of participants (median, range, and proportion) <65 years old, ≥65 years old, and ≥75 years old (where available), and age‐specific safety and efficacy summaries were extracted.
The participation‐to‐prevalence ratio (PPR) was used to describe the representation of participants aged ≥65 and ≥75 years old in a clinical trial relative to their representation in the disease population. 10 For each new molecular entity identified, each CVD indication was examined. This was calculated by dividing the proportion of participants aged ≥65 years old in the clinical trial by the total proportion of people aged ≥65 years old in the specific CVD populations, obtained from high‐quality population‐based studies. 11 , 12 , 13 , 14 , 15 , 16 , 17 This was also done for people aged ≥75 years where data were available. 11 , 13 , 14 , 17 A PPR close to 1.0 indicates that the proportion of people aged ≥65 years old in the clinical trials are comparable to that of the disease population, a PPR <0.8 indicates that the older population is underrepresented, and >1.2 indicates that they are overrepresented. 10 The average age of individuals within the specific disease populations was also reported. 12 , 13 , 16 , 18 , 19 , 20
Prescribing information from the package insert (also referred to as the drug product label) for each of the identified cardiovascular medications was obtained. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Data regarding efficacy and safety statements specifically pertaining to age and/or geriatric statements were abstracted and compared with the DTS information.
Results
A total of 72 079 participants from 10 DTS reports for CVD were identified, which included 9 new molecular entities approved by the FDA. The specific CVD conditions included atrial fibrillation (AF) (n=1 oral anticoagulant), prevention of venous thromboembolism (VTE) (n=2 oral anticoagulant), AF/and or VTE, (n=1 reversal of oral anticoagulant), heart failure (HF) (n=2), acute coronary syndrome (n=1), hypercholesterolemia (n=2), and pulmonary arterial hypertension (n=1). Edoxaban had 2 DTS reports because it is indicated for both AF and VTE. Of the 72 079 participants enrolled in the clinical trials 39 625 (55.0%) were ≥65 years old (Table 1). 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The proportion of participants aged ≥65 years old ranged from 17.9% to 90.0% with a median or average age ranging from 48 to 77 years old.
Table 1.
Proportion of Participants Aged ≥65 and ≥75 Years Old in CVD DTS of Clinical Trials Between September 2015 to April 2019
| Drug and Date FDA Approval | Indication | Number of Trials/(Total N) | Specific CVD | Age Participants, N (%) | Disease Population | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median Age (Range) | 18–65 y | ≥65 y | ≥75 y* | Average Age | % ≥65 y† | % ≥75 y‡ | ||||
|
Edoxaban January 8, 2015 |
Prevention of stroke in patients with AF | 1 (21 026) | AF | 72 (25–96) | 5483 (26.1) | 15 543 (73.9) | 8432 (40.1) | 73.5 | 74.4% | 50.0% |
|
Edoxaban January 8, 2015 |
VTE prophylaxis in patients with prior VTE | 1 (8240) | VTE | 57 (18–106) | 5536 (67.2) | 2704 (32.8) | N/A | 62.7 | 85.7% | … |
|
Ivabradine April 15, 2015 |
Reduce hospitalization from worsening HF | 1 (6505) | HF | 60 (19–92) | 4031 (62.0) | 2474 (38.0) | 722 (11.1) | 76.7 | 78.7% | 56.9% |
|
Cangrelor June 22, 2015 |
Prevention of coronary artery thrombosis in patients undergoing PCI | 1 (11 145) | ACS | 64 (24–95) | 5795 (52.0) | 5350 (48.0) | 2008 (18.0) | 66.0 | 58.4% | 35.5% |
|
Sacubitril/Valsartan July 7, 2015 |
Treatment of HF | 1 (8442) | HF | 64 (18–96) | 4299 (50.9) | 4143 (49.1) | 1563 (18.5) | 76.7 | 78.7% | 56.9% |
|
Alirocumab July 24, 2015 |
Treatment of certain patients with high cholesterol | 9 (3752) | HCL | 60 (18–89) | 2549 (68.0) | 1203 (32.0) | N/A | 60.9 | 33.2%§ | … |
| Evolocumab August 27, 2015 | Treatment of certain patients with high cholesterol | 8 (4177) | HCL | 58 (18–80) | 3107 (72.0) | 1160 (28.0) | N/A | 60.9 | 33.2%§ | … |
| Idarucizumab October 16, 2015 | Reversal of oral anticoagulant (dabigatran) | 1 (123) | AF or VTE|| | 77 (48–93) | 12 (10.0) | 111 (90.0) | N/A |
73.5 (AF) 62.7 (VTE) |
74.4–85.7% | … |
|
Selexipag December 21, 2015 |
Treatment of PAH | 1 (1156) | PAH | 48.1 (15.4)¶ | 946 (82.1) | 206 (17.9) | N/A | 51.9 | 24.9% | … |
|
Betrixaban June 23, 2017 |
Prevention of VTE | 1 (7513) | VTE | 77 (40–103) | 782 (10.4) | 6731 (89.6) | 4740 (63.8) | 62.7 | 85.7% | 71.1%# |
ACS indicates acute coronary syndrome; AF, atrial fibrillation; CVD, cardiovascular disease; DTS, drug trial snapshot; FDA, US Food and Drug Administration; HCL, hypercholesterolemia; HF, heart failure; N/A, not available; PAH, pulmonary arterial hypertension; PCI, percutaneous coronary intervention; and VTE, venous thromboembolism.
Data only available for people aged ≥75 years old in 5 of the DTS.
The proportion of people aged ≥65 years old within the disease population was calculated by dividing the prevalence of the disease in people aged ≥65 years old by the total disease population prevalence obtained from referenced large high‐quality population‐based studies.
The proportion of people aged ≥75 years old within the disease population was calculated by dividing the prevalence of the disease in people aged ≥75 years old by the total disease population prevalence obtained from referenced large high‐quality population‐based studies.
Based on total % of participants aged ≥60 years old from National Center for Health Statistics/National Health and Nutrition Examination Survey 2015 to 2016 survey of adults aged ≥20 years old with high total cholesterol levels defined as ≥240 mg/dL 15 .
Calculated for AF or VTE, respectively.
Mean (SD).
Population‐based data for the proportion of people with VTE aged ≥70 years old.
Examination of the PPR for disease‐specific representativeness for the 9 new molecular entities identified in our study included 11 CVD indications. This was because edoxaban is indicated for both AF and VTE, and idarucizumab is indicated as a reversal agent for the oral anticoagulant dabigatran, whose indications include both AF and VTE. Overall, 63.6% (7/11) of the indications for the DTS studies for cardiovascular medications were representative of the proportion of people aged ≥65 years old in the specific CVD (Figure). For the identified DTS, participants aged ≥65 years old in RCTs for the conditions acute coronary syndrome, AF, and hypercholesterolemia were similar to the proportion of people aged ≥65 years in the specific disease populations (Figure). Underrepresentation of people aged ≥65 years old was observed for 4 DTS (36.4%): 2 HF DTS, 1 pulmonary arterial hypertension DTS, and 1 VTE DTS (Figure). Data were reported for participants aged ≥75 years old in 5 of the DTS included in this study (Table 1). Three of the DTS were underrepresentative of people aged ≥75 years old: 2 HF DTS and 1 acute coronary syndrome DTS (PPRs 0.20, 0.33, and 0.51, respectively) (Figure).
Figure 1. PPR of CVD RCT participants included in the FDA drug trial snapshots aged ≥65 years old (blue bars) and ≥75 years old (red bars) compared with the proportion aged ≥65 and ≥75 years old, respectively in the specific CVD population for which the drug is indicated.
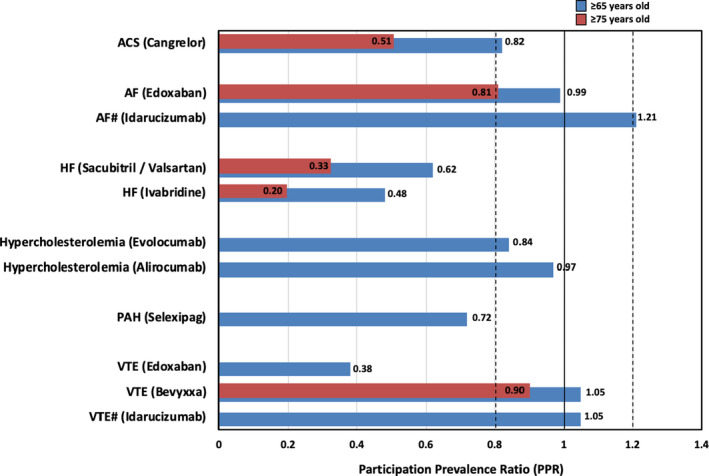
#Reversal of oral anticoagulant (dabigatran) in patients with either AF or VTE. ACS indicates acute coronary syndrome; AF, atrial fibrillation; CVD, cardiovascular disease; FDA, US Food and Drug Administration; HF, heart failure; PAH, pulmonary arterial hypertension; PPR, participation‐to‐prevalence ratio; RCT, randomized clinical trials; and VTE, venous thromboembolism.
All of the DTS efficacy statements reported that medications had similar efficacy in older and younger participants (Table 2). 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Five (50%) of the DTS safety statements reported no differences in the risk of adverse events between younger and older participants treated with the study medication; 4 (40%) reported a higher rate of adverse events in participants aged ≥65 years old; and 1 (10%) concluded that because the majority (90%) of participants were aged ≥65 years old, differences between younger and older participants could not be determined (Table 2). By comparison, none of the product information examined reported differences in either efficacy or safety with age. Seven (70%) specifically stated no differences in the efficacy or safety between younger and older patients using the study medication and the remaining 3 (30%) reported general pharmacokinetic responses only, which were not reported to differ with age (Table 2).
Table 2.
DTS Efficacy and Safety Statements by Age for CVD Indications and Corresponding Prescribing Information
| Drug; Indication (Comparator) | DTS Efficacy Statement | DTS Safety Statement | Prescribing Information |
|---|---|---|---|
| Edoxaban; Prevention of stroke in patients with AF (warfarin) | Edoxaban similarly effective in patients >75 and <75 y old |
Subgroup analysis for major bleeding was conducted for ages <65, ages 65 to 74, and ≥75 y old. Risk of major bleeding similar across these 3 age groups when compared with those treated with warfarin |
Geriatric: In clinical trials efficacy and safety of edoxaban in elderly (≥65 y old) and younger patients were similar. Age: In a population pharmacokinetic analysis, after taking renal function and body weight into account, age had no additional clinically significant effect on edoxaban pharmacokinetics |
| Edoxaban; VTE prophylaxis in patients with prior VTE (warfarin) | Edoxaban was similarly effective in patients >65 and <65 y old |
Subgroup analyses for major bleeding were conducted for age <65 and >65 y old. The risk of major bleeding increased with age for edoxaban |
Geriatric: In clinical trials, efficacy and safety of edoxaban in elderly (≥65 y old) and younger patients were similar. Age: In a population pharmacokinetic analysis, after taking renal function and body weight into account, age had no additional clinically significant effect on edoxaban pharmacokinetics |
| Ivabradine; Reduce hospitalization from worsening HF (placebo) | Ivabradine similarly effective in patients across age groups studied | Risk of high blood pressure in patients treated with ivabradine is higher as age increases |
Geriatric: No pharmacokinetic differences have been observed in elderly (≥65 y) or very elderly (≥75 y) patients compared with the overall population. However, ivabradine has only been studied in a limited number of patients ≥75 y old. Age: No pharmacokinetic differences (AUC or Cmax) have been observed between elderly (≥65 y old) or very elderly (≥75 y old) patients and the overall patient population |
| Cangrelor; Prevention of coronary artery clot in patients undergoing PCI (clopidogrel) | Cangrelor similarly effective in all age groups studied |
More bleeding observed in patients ≥65 y old treated with cangrelor compared with younger patients treated with cangrelor. In the trial all patients (all ages) treated with cangrelor had more bleeding compared with clopidogrel |
Geriatric/Age: Pharmacokinetics not affected by sex, age, renal status, or hepatic function. No dose adjustment is needed for these factors |
| Sacubitril/Valsartan; Treatment of HF (enalapril) | Sacubitril/Valsartan worked similarly in all age groups studied | Risk of low blood pressure (hypotension) was higher in patients ≥65 y old compared with patients ≤65 y old | Geriatric: No relevant pharmacokinetic differences have been observed in elderly (≥65 y old) or very elderly (≥75 y old) patients compared with the overall population |
| Alirocumab; Treatment of certain patients with high cholesterol (placebo) | Alicrocumab worked similarly in all age groups studied | Risk of side effects was similar in all age groups studied |
Geriatric: No overall differences in safety or effectiveness observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Age: Age, body weight, sex, race, and creatinine clearance were found not to significantly influence alirocumab pharmacokinetics |
| Evolocumab; Treatment of certain patients with high cholesterol (placebo) | Evolocumab worked similarly in all age groups studied | Risk of side effects was similar among age groups studied |
Geriatric: No overall differences in safety or effectiveness observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Age: The pharmacokinetics of evolocumab were not affected by age, sex, race, or creatinine clearance, across all approved populations |
| Idarucizumab; Reversal of oral anticoagulant dabigatran (placebo) | Most patients in the trial were ≥65 y old. Idarucizumab worked similarly among all ages studied |
Most patients in the trial were ≥65 y old. Differences between patients <65 and >65 y old could not be determined |
Geriatric: No overall differences in safety or effectiveness observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Age: Age had no clinically important effect on systemic exposure of idarucizumab based on population pharmacokinetic analyses in healthy volunteers |
| Selexipag; Treatment of PAH (placebo) | Selexipag worked similarly in patients below and >65 y old—Slowed progression of disease and reduced PAH hospitalizations | Risk of side effects was similar in patients <65 and above >65 y old |
Geriatric: No overall differences observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity cannot be ruled out. Age: The pharmacokinetic variables (Cmax and AUC) were similar in adult and elderly subjects ≤75 y old. There was no effect of age on pharmacokinetics of selexipag and active metabolite in patients with PAH |
| Betrixaban; Prevention of VTE (enoxaparin) | Betrixaban worked similarly in patients above and <65 y old—VTE events | Occurrence of bleeding was similar in patients above and <65 y old | Geriatric: Of the total number of patients in the clinical study, 90% were ≥65 y old, while 68.6% were ≥75 y old. No clinically significant differences in safety or effectiveness were observed between older and younger patients |
AF indicates atrial fibrillation; AUC, area under the curve; Cmax, maximum concentration of drug; CVD, cardiovascular disease; DTS, drug trial snapshot; HF, heart failure; PAH, pulmonary arterial hypertension; PCI, percutaneous coronary intervention; and VTE, venous thromboembolism.
Discussion
Almost two thirds (63.6%) of participants included in DTS clinical trials used as the basis for approval of new molecular entities for CVD were representative of older people, for whom the medication is indicated. Previous studies have reported exclusion of older people from CVD RCTs, with only 32% to 42% of people including those aged ≥65 years old. 7 , 8 , 30 The increased representativeness of older people observed in this evaluation of relatively recent clinical trials may in part be because of an increased recognition of the disparity between clinical trial participants and "real‐world" patient populations over the past decade. In addition, policies such as the action plan to improve participation in clinical trials, in response to the 2012 FDA Safety and Innovation Act, 31 may have also contributed. However, given the increased prevalence of CVD, multimorbidity, frailty, and risk of adverse drug events, there is a clear need to better understand medication risks and benefits in older people likely to receive these medications in clinical practice.
Based on the findings from this study, concerns remain, especially for the newly approved HF medications. None of the clinical trials participants included within the DTS were representative of the older population with HF, especially when our analysis was limited to people aged ≥75 years old. The average age of people with HF is 76.7 (SD 12.6) years old, and the majority (≈80%) are aged ≥65 years old, whose care is complicated by multimorbidity and increased frailty, potentially contributing to their exclusion from clinical trials. 13 , 32 Similar concerns with regard to underrepresentation of women in HF clinical trials have also been reported. 10 Another study where we observed an underrepresentation of older people was the RCT of edoxaban for prevention of VTE recurrence in patients who have had a VTE. The average age of participants in this study was 55.8 years old but aimed to include patients with a broad range of venous thromboembolic manifestations from limited proximal deep‐vein thrombosis to severe pulmonary embolism, and therefore potentially increasing the likelihood of a younger study cohort. 33 The lack of patients aged ≥65 years old from the selexipag study for the treatment of pulmonary arterial hypertension is reflective of the age distribution of this condition; data from 11 pulmonary arterial hypertension registries worldwide report a mean age range of 45 to 65 years old. 34
There were no reported differences in efficacy between younger (<65 years old) and older (≥65 years old) people as reported in the CVD DTS program, which was consistent with that reported in the corresponding product information. In contrast, comparison of the safety statements made in DTS and product information found inconsistencies between the two. While 40% of the DTS reported an increased risk of adverse events in the older participants using the study medication compared with younger participants using the study medication, no differences between age groups were reported in the product information. It is unclear as to the reasons for these differences. In some instances, the increased risk of adverse events in the older population was similar for both the study medication and the comparator (eg, older people treated with edoxaban compared with warfarin). However, as a mandated regulatory document and despite the product information having specific section headings of geriatrics and/or age, it is clear that the reporting of age‐associated differences in both efficacy and safety (as observed in the clinical trials) is required to facilitate safe and effective prescribing in the older population. While the DTS explicitly states that it should not be solely used to make prescribing decisions and is not a substitute for the prescribing information, 9 it is clear that improved reporting of risk of adverse events and harms for the older population in the product information reflective of clinical trial data is warranted. Other considerations are the inclusion of a geriatric rating within the product information that rates the risk of harm in the older population (evidence based), similar to the pregnancy ratings, together with the establishment of a Geriatric Advisory Panel/Committee similar to the Pediatric Advisory Committee in the FDA, 35 to oversee geriatric research.
While the study results are encouraging in terms of increased participation of older people in clinical trials, more is needed to ensure that the older people included are truly representative of those seen in clinical practice (ie, multimorbidity and/or frailty, rather than older people with a CVD indication and few non‐CVD comorbidities). While we were unable to examine this in the current study, the inclusion of explicit or implicit inclusion criteria based on the presence of multimorbidity and/or frailty will be more informative. Mandating such criteria by the FDA (where appropriate) may facilitate representativeness of older people reflective of clinical practice included as trial participants. Furthermore, while the proportion of older people included in a clinical trial may in fact be representative of the disease population, the trial may not be adequately powered to provide efficacy and safety data for the older subgroup.
Study Limitations
This study only included new molecular entities approved by the FDA since the implementation of the DTS program and does not reflect all CVD drug studies submitted to the FDA, including those that were not approved. The calculation of the PPR is potentially limited by the availability of high‐quality and current epidemiological data on the age distributions within each CVD area; however, this was minimized by the inclusion of contemporary large population‐based studies in this analysis. Furthermore, data were limited on the proportion of people aged ≥75 years old in the DTS, with only 5 studies including this. We only focused on age‐specific factors of participants identified in CVD DTS, and other factors such as sex, concomitant medications, renal function, frailty, and genetic variations may contribute when examining the efficacy and safety of medications.
Conclusions
While our study showed an encouraging increase in the proportion of older people participating in CVD clinical trials for new molecular entities, more can be done to facilitate the inclusion of participants reflective of "real‐world" clinical practice. An increased directive for this when designing and conducting clinical trials will facilitate the generation of the much‐needed evidence base. Additionally, accurate reporting of increased risks of adverse events in the older population within the drug product information may potentially facilitate appropriate prescribing and reduce the likelihood of harm for these at‐risk patient populations.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc 2020;9:e016936 DOI: 10.1161/JAHA.120.016936.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Watts G. Why the exclusion of older people from clinical research must stop. BMJ. 2012;9:e3445. [DOI] [PubMed] [Google Scholar]
- 2. Scott IA, Guyatt GH. Cautionary tales in the interpretation of clinical studies involving older persons. Arch Intern Med. 2010;587–595. [DOI] [PubMed] [Google Scholar]
- 3. Mangoni AA, Jackson SHD. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caughey GE, Roughead EE. Multimorbidity research challenges: where to go from here? J Comorbidity. 2011;8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;1376–1386. [DOI] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;e67–e492. [DOI] [PubMed] [Google Scholar]
- 7. Bourgeois FT, Orenstein L, Ballakur S, Mandl KD, Ioannidis JPA. Exclusion of elderly people from randomized clinical trials of drugs for ischemic heart disease. J Am Geriatr Soc. 2017;2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green P, Maurer MS, Foody JM, Forman DE, Wenger NK. Representation of older adults in the late‐breaking clinical trials; American Heart Association 2011 Scientific Sessions. J Am Coll Cardiol. 2012;869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration . Drug trial snapshots. 2015. Available at: https://www.fda.gov/drugs/informationondrugs/ucm412998.htm. Accessed April 30, 2019.
- 10. Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell T‐Y, Geller RJ, Elahi M, Temple RJ, Woodcock J. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol. 2018;1960–1969. [DOI] [PubMed] [Google Scholar]
- 11. Davis RC, Hobbs FDR, Kenkre JE, Roalfe AK, Iles R, Lip GY, Davies MK. Prevalence of atrial fibrillation in the general population and in high‐risk groups: the ECHOES study. Europace. 2012;1553–1559. [DOI] [PubMed] [Google Scholar]
- 12. Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011;217–220. [DOI] [PubMed] [Google Scholar]
- 13. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018;572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexander KP, Roe MT, Chen AY, Lytle BL, Pollack CV Jr, Foody JM, Boden WE, Smith SC Jr, Gibler WB, Ohman EM, et al. Evolution in cardiovascular care for elderly patients with non‐ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005;1479–1487. [DOI] [PubMed] [Google Scholar]
- 15. Carroll MD, Fryar CD, Nguyen DT. High Total and Low High‐Density Lipoprotein Cholesterol in Adults: United States, 2015–2016. NCHS Data Brief, No 290. Hyattsville, MD: National Center for Health Statistics; 2017. Available at: https://www.cdc.gov/nchs/data/databriefs/db290.pdf. Accessed June 25, 2019. [Google Scholar]
- 16. Burger CD, Long PK, Shah MR, McGoon MD, Miller DP, Romero AJ, Benton WW, Safford RE. Characterization of first‐time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heit JA, Ashrani A, Crusan DJ, McBane RD, Petterson TM, Bailey K. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost. 2017;390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johansson C, Dahlqvist E, Andersson J, Jansson J‐H, Johansson L. Incidence, type of atrial fibrillation and risk factors for stroke: a population‐based cohort study. Clin Epidemiol. 2017;53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elgendy IY, Mahmoud AN, Wen X, Bavry AA. Meta‐analysis of randomized trials of long‐term all‐cause mortality in patients with non–ST‐elevation acute coronary syndrome managed with routine invasive versus selective invasive strategies. Am J Cardiol. 2017;560–564. [DOI] [PubMed] [Google Scholar]
- 20. Wong ND, Young D, Zhao Y, Nguyen H, Caballes J, Khan I, Sanchez RJ. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low‐density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;1109–1118. [DOI] [PubMed] [Google Scholar]
- 21. Savaysa (edoxaban) [package insert]. Tokyo, Japan: Daiichi Sankyo Co.; 2015. Available at: https://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed June 25, 2019. [Google Scholar]
- 22. Corlanor (ivabradine) [package insert]. Thousand Oaks, CA: Amgen Inc.; 2015. Available at: https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed June 25, 2019. [Google Scholar]
- 23. Kengreal (cangrelor) [package insert]. Cary, NC: Chiesi USA, Inc.; 2015. Available at: https://resources.chiesiusa.com/Kengreal/KENGREAL_US_PI.pdf. Accessed June 25, 2019. [Google Scholar]
- 24. Entresto (sacubitril/valsartan) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015. Available at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/entresto.pdf. Accessed June 25, 2019. [Google Scholar]
- 25. Praluent (alirocumab) [package insert]. Bridgewater, NJ: Sanofi‐aventis U.S.; 2015. Available at: http://products.sanofi.us/praluent/praluent.pdf. Accessed June 25, 2019. [Google Scholar]
- 26. Repatha (evolocumab) [package insert]. Thousand Oaks, CA: Amgen Inc.; 2015. Available at: https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english.pdf. Accessed June 25, 2019. [Google Scholar]
- 27. Praxbind (idarucizumab) [package insert]. Ridgefield, CT: BoehringerIngelheim Pharmaceuticals, Inc.; 2015. Available at: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf. Accessed June 25, 2019. [Google Scholar]
- 28. Uptravi (selexipag) [package insert]. South San Francisco, CA: Actelion Pharmaceuticals US, Inc.; 2015. Available at: https://www.uptravi.com/assets/pdf/UPTRAVI-full-prescribing-information.pdf. Accessed June 25, 2019. [Google Scholar]
- 29. Bevyxxa (betrixaban) [package insert]. South San Francisco, CA: Portola Pharmaceuticals, Inc.; 2017. Available at: https://www.bevyxxa.com/wp-content/uploads/2017/11/bevyxxa-betrixaban-capsules-prescribing-information-pdf.pdf. Accessed June 25, 2019. [Google Scholar]
- 30. Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA From 2010 to 2012. Am J Ther. 2015;435–455. [DOI] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration Safety and Innovation Act . Food and Drug Safety Innovation Act. Public Law 112–144 (07/09/2012). Available at: http://www.gpo.gov/fdsys/pkg/BILLS-112s3187enr/pdf/BILLS-112s3187enr.pdf. Accessed May 1, 2019.
- 32. Caughey GE, Shakib S, Barratt JD, Roughead EE. Use of medicines that may exacerbate heart failure in older adults: therapeutic complexity of multimorbidity. Drugs Aging. 2019;471–479. [DOI] [PubMed] [Google Scholar]
- 33. The Hokusai‐VTE Investigators . Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;1406–1415. [DOI] [PubMed] [Google Scholar]
- 34. Prins KW, Thenappan T. World Health Organization Group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin. 2016;363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Food and Drug Administration . Pediatric Advisory Committee. 2020. Available at: https://www.fda.gov/advisory-committees/committees-and-meeting-materials/pediatric-advisory-committee. Accessed June 14, 2020.


