Abstract
Background
The red blood cell (RBC) storage lesion is a series of morphological, functional, and metabolic changes that RBCs undergo following collection, processing, and refrigerated storage for clinical use. Since the biochemical attributes of the RBC unit shifts with time, transfusion of older blood products may contribute to cardiac complications, including hyperkalemia and cardiac arrest. We measured the direct effect of storage age on cardiac electrophysiology and compared it with hyperkalemia, a prominent biomarker of storage lesion severity.
Methods and Results
Donor RBCs were processed using standard blood‐banking techniques. The supernatant was collected from RBC units, 7 to 50 days after donor collection, for evaluation using Langendorff‐heart preparations (rat) or human induced pluripotent stem cell–derived cardiomyocytes. Cardiac parameters remained stable following exposure to “fresh” supernatant from red blood cell units (day 7: 5.8±0.2 mM K+), but older blood products (day 40: 9.3±0.3 mM K+) caused bradycardia (baseline: 279±5 versus day 40: 216±18 beats per minute), delayed sinus node recovery (baseline: 243±8 versus day 40: 354±23 ms), and increased the effective refractory period of the atrioventricular node (baseline: 77±2 versus day 40: 93±7 ms) and ventricle (baseline: 50±3 versus day 40: 98±10 ms) in perfused hearts. Beating rate was also slowed in human induced pluripotent stem cell–derived cardiomyocytes after exposure to older supernatant from red blood cell units (−75±9%, day 40 versus control). Similar effects on automaticity and electrical conduction were observed with hyperkalemia (10–12 mM K+).
Conclusions
This is the first study to demonstrate that “older” blood products directly impact cardiac electrophysiology, using experimental models. These effects are likely caused by biochemical alterations in the supernatant from red blood cell units that occur over time, including, but not limited to hyperkalemia. Patients receiving large volume and/or rapid transfusions may be sensitive to these effects.
Keywords: cardiac electrophysiology, hyperkalemia, red blood cell storage lesion
Subject Categories: Electrophysiology, Animal Models of Human Disease, Cardiovascular Surgery, Basic Science Research, Physiology
Nonstandard Abbreviations and Acronyms
- AS‐1 or AS‐3
additive solution‐1 or additive solution‐3
- AVNERP
atrioventricular node effective refractory period
- hiPSC‐CM
human induced pluripotent stem cell–derived cardiomyocytes
- KH
Krebs‐Henseleit media
- MEA
microelectrode array
- RBC
red blood cell
- SNRT
sinus node recovery time
- sRBC
supernatant from red blood cell units
- VERP
ventricular effective refractory period
- WBCL
Wenckebach cycle length
Clinical Perspective
What Is New?
This study is the first to demonstrate that “older” red blood cell products (as compared with “fresh” products) may directly impact myocardial automaticity and electrical conduction, using experimental cardiac models.
What Are the Clinical Implications?
Transfusion‐induced hyperkalemic cardiac arrest is a recognized complication of transfusions; our study suggests that cardiac complications could be linked to high potassium concentrations in red blood cells units that are close to expiration.
More than 13 million whole blood and red blood cell (RBC) units are transfused in the United States each year, with cardiac surgical procedures accounting for ≈20% of all blood transfusions. 1 , 2 , 3 , 4 , 5 , 6 , 7 Many cardiac procedures mandate the use of blood and blood products in the preoperative, intraoperative, and postoperative period, particularly with infant and pediatric patients for cardiopulmonary bypass circuitry priming. 3 , 8 Despite the frequency, transfusion of blood and blood products is not without risk. 9 , 10 Transfusion of RBCs in particular has been associated with increased morbidity and mortality, prolongation of hospital stay, and several different cardiac complications. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Many investigators have suggested that RBC transfusion complications are caused by the transfusion of RBCs close to their expiration (42 days), wherein the effects of the RBC storage lesion can contribute to the pathobiology of adverse reactions. 12 , 25 These pathobiological changes include clearance of storage‐damaged RBCs, aberration of nitric oxide metabolism, trapping of RBCs by macrophages resulting in oxidative damage and impaired oxygen delivery, and an increase in circulating nontransferrin bound iron. 24 , 26 , 27 , 28 Briefly, over time, stored RBCs are depleted of ATP, which alters the RBC cell membrane, resulting in hemolysis, the formation of RBC microvesicles, release of intracellular iron, decreased nontransferrin bound iron, and the release of free hemoglobin. Furthermore, the pH and electrolyte composition of the RBC unit also changes because of continued anaerobic metabolism and dysfunction of cation transporters. The latter includes impairment of Na+/K+ ATPase, 29 which leads to a progressive increase of extracellular [K+] in the RBC unit supernatant. 30 , 31 Consequently, rapid or large‐volume transfusions of RBC units with elevated potassium levels can predispose patients to hyperkalemia, conduction abnormalities, and cardiac arrest. 14 , 15 , 18 , 19 , 22 , 32 Although the incidence of transfusion‐associated hyperkalemia is poorly defined and potentially underreported, 14 Raza et al. noted elevated K+ levels in >70% of adult trauma patients following transfusion, 18 and Livingston et al. observed hyperkalemia in 18% to 23% of pediatric trauma patients following transfusion. 33 Transfusion‐associated hyperkalemia resulting in cardiac arrest is a recognized complication of massive transfusion in children, with a mean serum [K+] level of 9.2±1.8 mM in patients who experienced cardiac arrest. 14 Some investigators suggest that the risk factors for transfusion‐associated hyperkalemia resulting in cardiac arrest include the volume and rate of transfusion, storage age, and irradiation of RBCs, but the perceived risk and reason for such cardiac complications remain actively debated. 14 , 31 , 34 , 35
Chronological storage age is one of the key factors that influence RBC quality and storage lesion severity. 29 , 30 , 36 Despite this, blood banks often employ a “first‐in, first‐out” approach to reduce blood product waste and maintain an inventory supply to support emergency transfusions. Indeed, it is estimated that 10% to 20% of RBC units are transfused after 35 days of refrigerated storage, or near their 42‐day expiration date. 37 Some investigators have recommended a reduction in the maximum allowable storage time for RBCs because of quality concerns. 18 , 24 , 27 , 38 , 39 , 40 , 41 Several clinical studies have raised concerns about the effects of the RBC storage lesion 14 , 15 , 20 , 22 , 23 , 42 , 43 ; however, the direct impact of RBC quality on cardiac health outcomes remains unclear. Identifying a mechanistic relationship between RBC quality and adverse cardiac end points has been hindered in the clinical setting by confounding factors, including disease diagnosis, age, rate/site of infusion, volume of transfusion per unit time, number of transfusions, bypass and cross‐clamp time, secondary complications from surgery, and concomitant medication administration. Recent randomized clinical trials have demonstrated that transfusion with fresh blood (1–10 days storage duration) does not decrease the risk of mortality compared with standard practice (2–3 weeks storage duration). 44 , 45 , 46 , 47 , 48 However, considerably less is known about the risk of transfusing RBCs near expiration (35–42 days), or the impact on secondary end points including cardiac complications. 35 , 49 , 50 , 51
We aimed to address clinical concerns of bradycardia and cardiac arrest by investigating the direct relationship between RBC storage age and myocardial function using experimental models. We hypothesized that electrical conduction would be impaired in cardiac models exposed to the supernatant of “old” RBC (sRBC) units close to expiration as compared with “fresh” units, in part because of elevated extracellular potassium that can alter the myocardial resting membrane potential. 22 , 52 , 53 , 54 To test this hypothesis, electrophysiology parameters were measured using both an intact, isolated rat heart preparation and human induced pluripotent stem cell–derived cardiomyocytes (hiPSC‐CM). Cardiac end points were measured at baseline, and again after exposure to sRBC collected from “fresh” (day 7 postdonor collection), “old” (day 30–40), or “expired” units (day 50). We compared these results with those observed with hyperkalemia, a primary biomarker of RBC storage lesion severity. 29 , 30 , 36
Methods
Data that support the findings of this study are available from the corresponding author upon reasonable request. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Children’s National Research Institute, and followed the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
RBC Sample Preparation
RBC units (300±50 mL) from healthy donors were obtained from the American Red Cross or Children’s National Blood Donor Center. All units were type O blood, sickle‐negative, nonirradiated, collected using standard single‐donor needle methods, and stored in additive preservative solution (AS‐1 or AS‐3) according to standards of the American Academy Blood Banking requirements and the US Food and Drug Administration. 55 Single RBC units were stored at 4 to 6°C in a research‐grade, temperature‐monitored refrigerator according to standards. 55 RBC units underwent gentle centrifugation (4°C, 20 minutes, 2000g; Haemonetics) using accumulated centrifugal effect value of 6.5 × 107 to separate and collect the supernatant (sRBC) 7 to 50 days postdonor collection; sRBC samples were used for subsequent experiments. Experiments were designed to study the impact of RBC storage lesion on cardiac electrophysiology, by comparing end points after exposure to “fresh” sRBC (7 days postdonor collection), “old” sRBC (30–40 days), or “expired” sRBC (50 days).
General Protocol and Biochemical Analysis
Trauma patients and patients undergoing cardiac surgery or extracorporeal membrane oxygenation can receive large transfusion volumes ≥60% of the patient’s total blood volume. 56 , 57 As examples, an estimated 3% to 8% of civilian trauma patients and 10% of military trauma patients receive large‐volume RBC transfusions, 58 , 59 while >90% of children undergoing cardiopulmonary bypass receive packed RBCs (average 3.4 RBC units for complex procedures). 60 To mimic exposure, we estimated 10% supernatant volume exposure from reconstituted blood (½ volume packed RBCs [20–30% supernatant containing anticoagulant and 70–80% RBCs] and ½ volume plasma). Accordingly, sRBC samples were diluted to 10% volume using Krebs‐Henseleit (KH) buffered media (denoted in mM: 118 NaCl, 3.29 KCl, 1.2 MgSO4, 1.12 KH2PO4, 24 NaHCO3, 10 glucose, 2 C3H3NaO3, 10 HEPES, and 0.33 CaCl). Biochemical analyses were performed on each diluted sRBC sample, using an Epoc® point‐of‐care blood analysis system (Siemens Diagnostics: SMNS10736382) to measure Na+, K+, Ca2+, Cl−, and lactate levels. pH measurements were performed using an Oakton 700 pH meter, and osmolarity was assessed using an Advanced Instruments 3300 micro‐osmometer (Table 1).
Table 1.
Biochemical Analyses of sRBC Diluted to 10% Volume in KH‐Buffered Media
| Parameter (mmol/L) | Days of Storage | ||||
|---|---|---|---|---|---|
| KH Media | 7 | 30–35 | 40 | 50 | |
| pH | 7.38±0.01 | 7.40±0.01 | 7.41±0.01 | 7.40±0.003 | 7.41±0.01 |
| Osmolarity, mOsm/L | 315±0.58 | 314±2.65* | 315±0.86 | 318±1.86 | 319±1.67 |
| K+ | 4.48±0.04 | 5.82±0.16* | 9.28±0.41*,** | 9.28±0.33*,** | 9.65±0.26*,** |
| Lactate | 0.30±0.002 | 0.79±0.96 | 2.76±0.24*,** | 2.42±0.17*,** | 2.98±0.20*,** |
| Cl− | 117.9±0.51 | 117.9±0.23 | 117.6±0.32 | 116.7±0.61 | 119.3±0.33 |
| Ca2+ | 1.70±0.05 | 1.14±0.11* | 0.97±0.10* | 1.10±0.13* | 1.27±0.11* |
| Na+ | 151±0.32 | 149±0.34* | 145±0.45*,** | 145±0.80*,** | 147±0.33*,** |
Storage age was associated with deviations in the electrolyte composition of sRBC samples. Mean±SEM. KH indicates Krebs‐Henseleit media; and sRBC, supernatant from red blood cell units.
*Value differs significantly from control KH buffer, or **Value differs from day 7 sample (P<0.05). n=3–10 per group.
Intact, Whole Heart Preparations
Experiments were conducted using adult, female Sprague‐Dawley rats (>8 weeks old, >200 g, Taconic Biosciences). Animals were housed in conventional rat cages in the Research Animal Facility under standard environmental conditions (12:12 hour light:dark cycle, 64–78°F, 30–70% humidity, free access to reverse osmosis water, corncob bedding, and food [2918 rodent chow, Envigo]). Animals were anesthetized with 3% to 5% isoflurane; the heart was then excised and transferred to a temperature‐controlled (37°C), constant‐pressure (70 mm Hg) Langendorff‐perfusion system for electrophysiology experiments (Figure 1). After isolating and transferring the heart to the perfusion system, excised hearts were perfused with Krebs‐Henseleit buffer bubbled with carbogen (95% oxygen, 5% CO2) throughout the duration of the experiment. 61 Pseudo‐ECGs were continuously recorded in lead II configuration during sinus rhythm; ECG signals were analyzed to quantitate heart rate, atrioventricular conduction (PR interval), ventricular depolarization time (QRS width), ventricular repolarization (QTc), 62 and arrhythmia incidence. 63 , 64 , 65 Biosignals were acquired in iox2 and ECG parameters were analyzed in ecgAUTO (emka Technologies).
Figure 1. Heart preparation and experimental timeline.
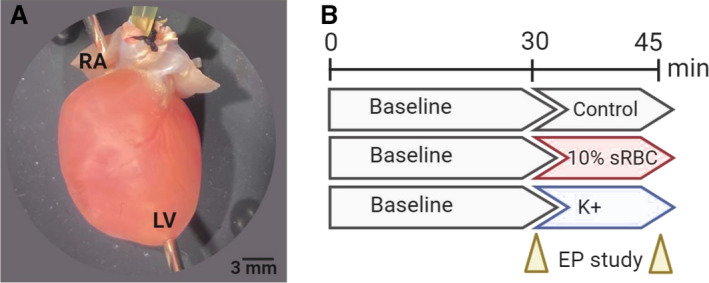
A, Isolated, intact rodent heart with retrograde Langendorff‐perfusion via an aortic cannula. Pacing electrodes were attached to the RA and apex of the LV to perform an EP. B, Experimental timeline included 30‐minutes perfusion with KH media, containing 4.5 mM K+ (control), which commenced with an EP protocol. Thereafter, the media remained unchanged (control), supplemented with 10% sRBC, or supplemented with increasing potassium concentrations. The EP study was repeated again after 15–20 minutes, and results were compared with baseline. Timeline created using biorender.com. EP indicates electrophysiology study; KH, Krebs‐Henseleit media; LV, left ventricle; RA, right atria; and sRBC, supernatant from red blood cell units.
Electrophysiology Measurements
To further investigate cardiac electrophysiology, a pacing protocol was implemented using stimulation electrodes positioned on the right atrium and the apex of the left ventricle (Figure 1). 63 , 65 A Bloom Classic electrophysiology stimulator (Fisher Medical) was set at a pacing current 1.5× the minimum pacing threshold (1–2 mA) with 1‐ms monophasic pulse width. Sinus node recovery time (SNRT) was assessed by applying a pacing train of 150 ms (S1−S1) to the right atrium and measuring the time delay until the next spontaneous sinoatrial node–mediated activity. To determine the Wenckebach cycle length (WBCL), an S1–S1 pacing interval was applied to the right atrium; the pacing cycle length was decremented stepwise to pinpoint the shortest interval that resulted in 1:1 atrioventricular conduction. Next, an S1–S2 pacing interval was applied to the right atrium to determine the atrioventricular nodal effective refractory period (AVNERP). An S1–S2 pacing interval was applied to the left ventricle to find the shortest coupling interval that resulted in 1:1 ventricular depolarization, signifying the ventricular effective refractory period (VERP).
Experimental Timeline and Treatment Groups
Isolated, intact hearts were perfused with KH media for 30 minutes, followed by implementation of electrophysiology pacing protocols (“baseline”). Hearts were then perfused for another 15 to 20 minutes, with either KH media alone (control), media supplemented with 10% sRBC (7–50 days postdonor collection), or media supplemented with elevated potassium concentrations (6–12 mM KCl). Electrophysiology protocols were performed a second time to determine the effects of sRBC treatment or hyperkalemia on electrical conduction (Figure 1). This protocol allowed each animal to serve as its own control, and account for experimental or animal variability.
hiPSC‐CM Preparation and Microelectrode Array Recordings
hiPSC‐CM (iCell, Cellular Dynamics International) are commonly used as a model to detect drug‐induced changes in cardiac electrophysiology, including use in the comprehensive in vitro proarrhythmia assay initiative. 66 , 67 This cell line contains a mixed population of cardiomyocytes with nodal, atrial, and ventricular‐like electrophysiological properties that resemble native cardiomyocytes. 68 hiPSC‐CM were plated onto fibronectin‐coated microelectrode arrays (MEA 24, Axion Biosystems), at a density of 30 000 cells per well. hiPSC‐CM were maintained under standard cell culture conditions (37ºC, 5% CO2). hiPSC‐CM formed a confluent contracting monolayer (40–60 beats per minute) and MEA recordings were performed 7 to 10 days after plating to measure the spontaneous beating rate. hiPSC‐CM were equilibrated in the MEA system for 15 minutes, and then the spontaneous beating rate was recorded (“baseline”) using an integrated MEA system (Maestro Edge, Axion Biosystems) with temperature and gas control (37ºC, 5% CO2). Cardiomyocytes were then treated for 5 minutes with iCell maintenance media (control), media supplemented with 10% sRBC (7 to 40 days postdonor collection), or media supplemented with elevated potassium concentrations (9–12 mM). Spontaneous beating rate was also recorded 1 hour posttreatment and after washout. To account for cell plating variability, each treated cardiomyocyte monolayer was compared to baseline. 69
Statistical Analysis
Results are reported as mean±SEM. Data normality was assessed by Shapiro‐Wilk testing (GraphPad Prism). For sRBC measurements, end points were measured before and after treatment within the same heart (baseline versus treatment) and statistical analysis was performed using either a 2‐tailed paired t test (passed normality test) or Wilcoxon matched‐pairs signed rank test (failed normality test). For hyperkalemia studies with multiple doses, statistical analysis was performed using either 1‐way ANOVA (passed normality test) or Kruskal‐Wallis nonparametric test (failed normality test), with a false discovery rate (0.1) to correct for multiple comparisons. Significance was defined by *P<0.05.
Results
Storage Age Affects the Biochemical Composition of sRBC
The attributes of a stored blood product shifts as RBC quality declines, which can result in an accumulation of potassium in the supernatant. 29 , 30 , 36 To measure the effect of storage time on the electrolyte composition of blood units, sRBC samples were collected from RBC units on day 7 to 50 postdonor collection, samples were diluted to 10% volume using pH‐buffered KH media, and then electrolyte‐gas measurements were performed on the diluted end product (Table 1). Extracellular potassium levels were elevated in “old” units as compared with “fresh” units (day 7: 5.8±0.2 versus day 40: 9.3±0.3 mM, P<0.0001); but there was variability between age‐matched units near expiration ranging from 7.5 to 11.9 mM [K+] in the 10% diluted end product (day 30–50). Lactate levels were elevated in older blood units (day 7: 0.8±1 versus day 40: 2.4±0.2 mM, P <0.0001), and sodium concentration declined slightly with storage age (day 7: 149±0.3 versus day 40: 145±0.8 mM, P <0.0001). pH and osmolarity values were similar between preparations.
Storage Age Is Associated With Heart Rate Slowing and Sinus Node Dysfunction
Cardiac complications from RBC transfusion include an increased risk of bradycardia and cardiac arrest. 14 , 15 , 18 , 21 These adverse outcomes may be precipitated by elevated extracellular potassium, which diminishes the myocardial resting membrane potential. 53 , 54 Accordingly, we assessed the impact of sRBC exposure on spontaneous heart rate and sinus node function in Langendorff‐perfused hearts. Heart rate remained stable throughout the study when perfused with control media containing 4.5 mM K+ (baseline: 297±10 versus 45 minutes: 288±15 beats per minute), and also remained stable when the perfusate was supplemented with 10% sRBC collected from RBC units aged 7 to 30 days (Figure 2). Similarly, sinus node function remained stable with control media perfusion (SNRT baseline: 223±14 versus 45 minutes: 238±9) and following perfusion with 10% sRBC collected from units aged 7 to 30 days (Figure 2). However, as RBC units neared expiration, sRBC exposure slowed the heart rate by 23% (baseline: 279±5 versus day 40: 216±18 beats per minute, P<0.005). Additionally, sRBC from day 40 units had a significant effect on sinus node function, delaying the recovery time by 46% (SNRT baseline: 243±8 ms versus day 40: 354±23 ms, P<0.005). In the latter, the perfusate media had a mean potassium concentration >9 mM (Table 1). To measure the direct effect of hyperkalemia on automaticity and sinus function, a dose–response study was performed. As the potassium concentration increased from 4.5 to 12 mM, heart rate slowed (linear regression R 2 = 0.92, P=0.01) and SNRT was prolonged (R 2 = 0.86, P=0.02).
Figure 2. RBC storage age is associated with heart rate slowing and sinus node dysfunction.
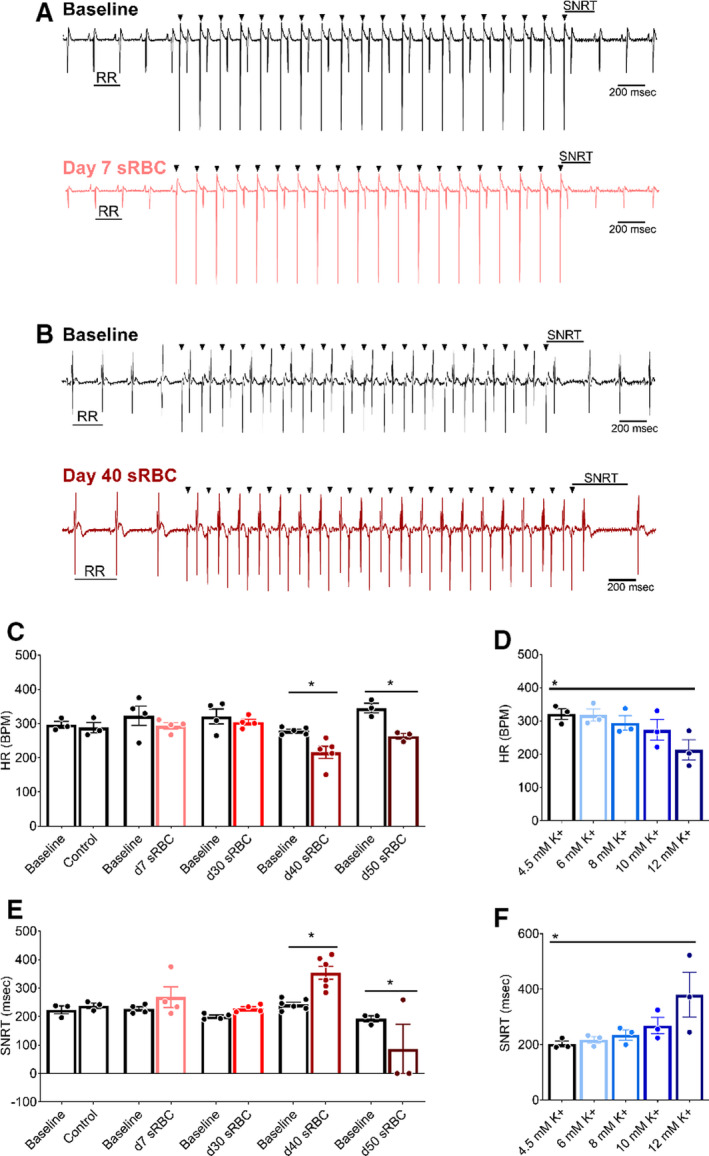
A, Biosignals recorded from isolated hearts perfused with media supplemented with 10% sRBC collected from a day 7 unit, or (B) day 40 unit. ECGs were recorded during sinus rhythm (RR interval highlighted), followed by train of atrial paces (black arrowheads denote pacing spikes). Each atrial pace results in a ventricular response. SNRT was measured from the last pacing spike to resumption of sinus rhythm. C, Stable heart rate following exposure to RBC units aged 7–30 d, but bradycardia observed with sRBC collected from units aged ≥ 40 d. D, HR slowing observed at highest potassium concentration tested (12 mM K+). E, Exposure to day 40 sRBC resulted in slowed sinus node recovery, or complete cessation of sinus function with day 50 sRBC. F, Increased SNRT also observed at highest potassium concentration tested (12 mM K+). Mean±SEM, *P<0.05, n=3–6. BPM indicates beats per minute; HR, heart rate; RBC, red blood cell; SNRT, sinus node recovery time; and sRBC, supernatant from red blood cell units.
Storage Age Is Associated With Atrioventricular Conduction Slowing
Electrochemical gradients across the cardiomyocyte membrane are essential for cardiac excitation and electrical propagation. Atrial cardiomyocytes are particularly sensitive to deviations in these electrochemical gradients, and an increase in extracellular potassium can slow atrioventricular conduction. 32 , 53 , 70 Atrioventricular conduction remained constant in hearts perfused with control KH media throughout the study (Figure 3), as determined by ECG parameters during sinus rhythm (PR time at baseline: 33±4 versus 45 minutes: 36±2). Similar results were observed before and after exposure to 10% sRBC samples collected from units aged 7 to 30 days, but significant slowing was observed after exposure to sRBC near or after expiration (PR time at baseline: 33±1 versus day 40: 41±3 ms, P<0.05; PR time at baseline: 37±1 versus day 50: 53±8 ms, P<0.005). Atrioventricular node refractoriness was further interrogated by implementing an atrial pacing protocol to measure WBCL (S1‐S1 pacing) and AVNERP (S1‐S2 pacing). These parameters remained unchanged in hearts perfused with control media (WBCL baseline: 79±2 versus 45 min: 83±2; AVNERP baseline: 64±5 versus 45 minutes: 67±4) and hearts exposed to sRBC from “fresh” 7‐day units (Figures 4 and 5). Exposure to day 30 sRBC resulted in a modest increase in AV node refractoriness, increasing WBCL by 9%. Effects on the AV node were more pronounced after exposure to day 40 sRBC, which increased AVNERP by 21% (baseline: 77±2 versus day 40: 93±7 ms, P=0.01) and WBCL by 19% (baseline: 90±1 versus day 40: 107±3 ms, P<0.001). A 66% increase in AVNERP was observed in units stored past expiration (baseline versus day 50 sRBC; Figure 5).
Figure 3. RBC storage age is associated with slowed atrioventricular conduction.
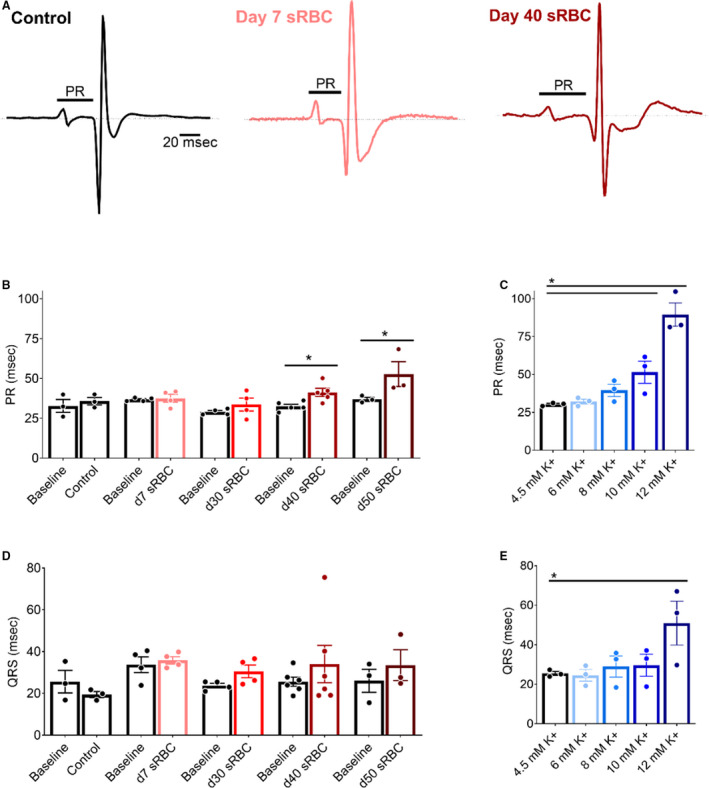
A, Pseudo‐ECGs recorded during sinus rhythm from isolated hearts perfused with control media (left), media supplemented with 10% sRBC collected from a day 7 unit (middle) or day 40 unit (right). PR interval time is denoted. (B,C) Atrioventricular conduction slows in the presence of day 40 and day 50 sRBC, or 10–12 mM K+. (D,E) Exposure to sRBC units had no measurable effect on ventricular depolarization time (QRS) during sinus rhythm. Mean±SEM, *P<0.05, n=3–6. RBC indicates red blood cell; and sRBC, supernatant from red blood cell units.
Figure 4. RBC storage age is associated with increased refractoriness of the AV node.
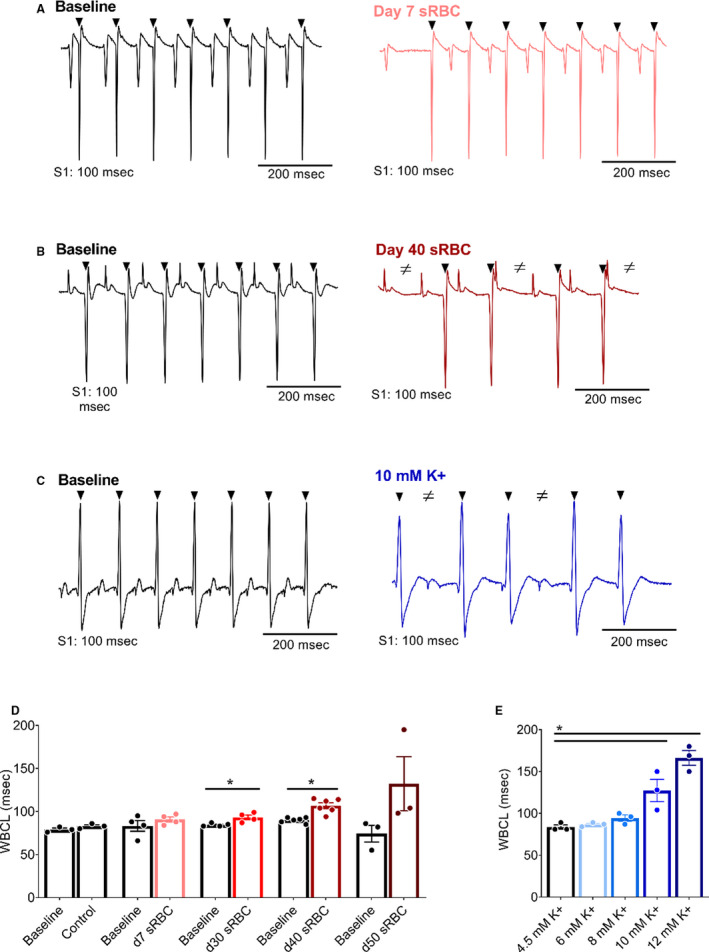
A, Biosignals recorded with atrial pacing (S1‐S1) to measure WBCL in isolated hearts in the presence of day 7 sRBC, (B) day 40 sRBC, or (C) 10 mM K+. D, Slowed AV node conduction following exposure to sRBC from older units, but not “fresh” day 7 units. E, Slowed AV conduction following exposure to 10–12 mM K+. Arrowheads denote ventricular response to atrial pacing at S1 (black) pacing cycle length. ≠ denotes failed conduction. Mean±SEM, *P<0.05, n≥3. AV indicates atrioventricular; sRBC, supernatant from red blood cell units; and WBCL, Wenckebach cycle length.
Figure 5. RBC storage age is associated with an increased AV node effective refractory period.
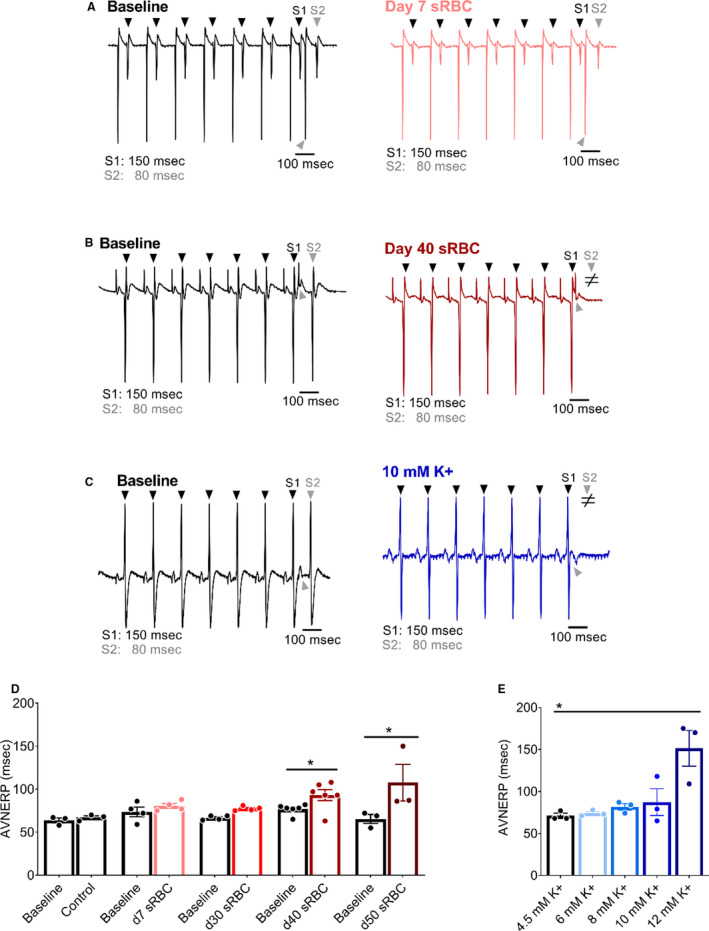
A, Biosignals recorded with atrial pacing (S1–S2) to pinpoint AVNERP in the presence of day 7 sRBC, (B) day 40 sRBC, or (C) 10 mM K+. D, AVNERP did not change after exposure to day 7–30 sRBC, but increased with day 40 and day 50 sRBC exposure. E, AVNERP increased with severe hyperkalemia. Arrowheads denote ventricular response to atrial pacing at S1 (black) or S2 (gray) pacing cycle length. ≠ denotes failed conduction. Mean±SEM, *P<0.05, n=3–6. AVNERP indicates atrioventricular node effective refractory period; RBC, red blood cell; sRBC, supernatant from red blood cell units.
As anticipated, a dose–response relationship was observed when the potassium concentration was increased in the perfusate media, resulting in prolonged atrioventricular conduction time and increased atrioventricular node refractoriness. As the potassium concentration increased from 4.5 to 12 mM, a progressive increase in PR duration (R 2 = 0.85, P<0.05) was observed (Figure 3). At 10 mM K+ (a concentration comparable to day 40 sRBC‐supplemented media), a 51% increase in WBCL was observed (4.5 mM: 84±3 to 10 mM: 127±13 ms, P<0.005), but changes in AVNERP were only observed at 12 mM K+ (4.5 mM: 71±3 to 12 mM: 151±21 ms, P<0.005; Figures 4 and 5). The latter suggests that other factors or substances in the RBC supernatant may also contribute to conduction slowing.
Storage Age Increases Ventricular Refractoriness
Severe hyperkalemia is associated with decreased sodium channel availability and slowed conduction velocity, which results in QRS widening and may precipitate ventricular tachyarrhythmias. 32 , 53 , 70 In our model, exposure to sRBC‐supplemented media did not significantly alter the QRS duration (baseline: 26±2 ms versus day 40: 34±9 ms; Figure 3), QTc duration (baseline: 169±9 versus day 40: 172±11 ms), or increase the incidence of ventricular tachyarrhythmias (data not shown). Furthermore, we were not able to establish a linear trend toward QRS prolongation with increasing potassium concentration (R 2 = 0.72, P=0.07), QTc duration (R 2 = 0.67, P=0.67) or an increased incidence of ventricular tachyarrhythmias, which may be attributed to limitations in our model system. Indeed, ventricular activation and early repolarization can occur simultaneously in the rodent heart, which can influence the QRS complex and result in indistinct T‐waves. 71 Moreover, the rodent myocardium is less than ideal for assessing arrhythmia incidence because of its small size and resilience to fibrillation. 71 As another indicator of ventricular repolarization time, we implemented a pacing protocol to pinpoint ventricular refractoriness. A marginal increase in extracellular potassium can hasten repolarization and shorten action potential duration time, but severe hyperkalemia increases postrepolarization refractoriness. 54 , 72 , 73 As expected, control media perfusion resulted in stable VERP measurements throughout the study (VERP baseline: 45±5 versus 45 minutes: 46±2 ms). VERP measurements were unchanged in heart preparations exposed to sRBC from day 7 to 30 RBC units (Figure 6), but VERP increased by 96% following exposure to day 40 sRBC (baseline: 50±3 versus day 40: 98±10 ms, P<0.0001) and 145% after exposure to expired units (baseline: 51±8 versus day 50: 126±25 ms, P<0.0001). This increase in ventricular refractoriness may be explained, at least partly, by the increase in extracellular potassium levels. In dose–response studies, increasing potassium concentration (4.5–12 mM) also resulted in a progressive increase in VERP (linear regression, R 2 = 0.91, P=0.01).
Figure 6. RBC storage age is associated with increased ventricular refractoriness.
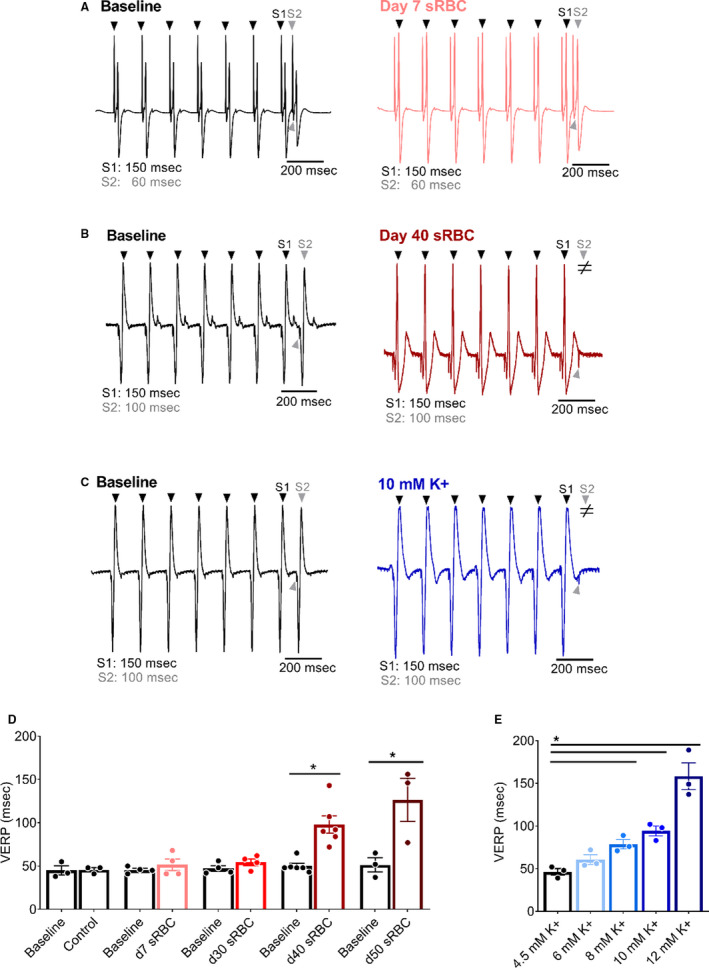
A, Biosignals recorded with ventricular pacing (S1–S2) to pinpoint the VERP in isolated hearts perfused with media supplemented with 10% sRBC collected from a day 7 unit, (B) day 40 unit, or (C) 10 mM K+. D, Ventricular refractoriness was unchanged after exposure to day 7–30, but increased with day 40–50 sRBC and (E) media supplemented with 8–12 mM K+. Arrowheads denote ventricular response to pacing at S1 (black) or S2 (gray) pacing cycle length. ≠ denotes failed conduction. Mean±SD, *P<0.05, n=3–6. RBC indicates red blood cell; sRBC, supernatant from red blood cell units; and VERP, ventricular effective refractory period.
hiPSC‐CM Are Susceptible to Electrical Disturbances
Rodent models are frequently employed in cardiovascular research studies, although species‐specific differences in ion channel expression are established. 74 , 75 Accordingly, we performed a follow‐up study using hiPSC‐CM to validate the effects of sRBC exposure. Using a MEA system, we noted an increase in the beating rate of hiPSC‐CM over time when treated with day 7 sRBC (5 minutes: 12±6% rate increase P=0.09 versus 60 minutes: 33±5% P<0.005, Figure 7). Conversely, cardiomyocytes demonstrated bradycardia after exposure to “older” sRBC products, which was more severe than reported in the whole heart experiments. The spontaneous beating rate of hiPSC‐CM decreased by 47±7% in day 35 samples and 75±9% in day 40 samples relative to baseline measurements (P<0.0001). Significant slowing in the spontaneous beating rate was also observed with increasing potassium concentrations (4.5–12 mM K+; R 2 = 0.999, P=0.01). Notably, treatment did not appear to have a lasting effect on cardiomyocyte viability, as the beating rate quickly returned to normal after washing out the sRBC or hyperkalemic media (Figure 7).
Figure 7. Reduced automaticity in hiPSC‐CM.
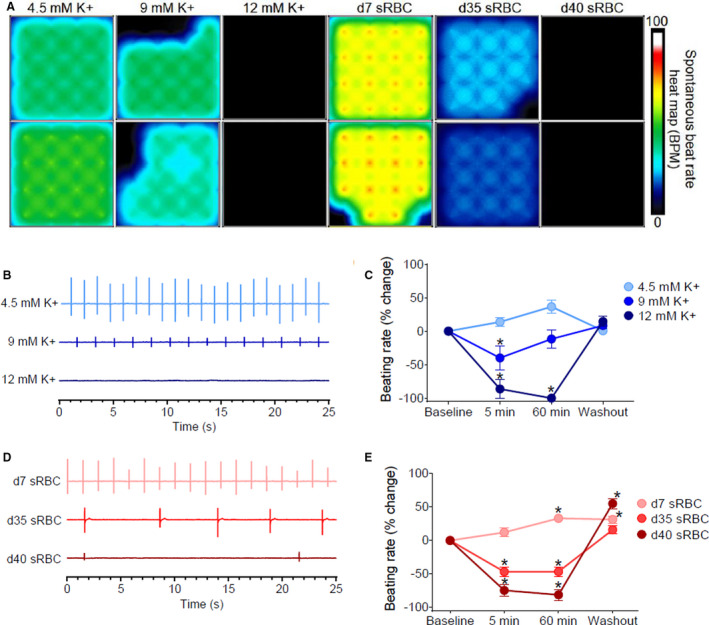
A, Microelectrode array heat map shows 16‐electrode recordings from hiPSC‐CM treated with control media (4.5 mM K+), media with increasing potassium concentrations (9–12 mM K+), or 10% sRBC collected from RBC units aged 7–40 days. The heat map corresponds to the spontaneous beating rate. B, Biosignals recorded from hiPSC‐CM show a decline in beating rate with elevated potassium concentrations. C, Percent change in beating rate following treatment with elevated potassium concentrations, compared with baseline. D, Biosignals show a decline in the beating rate with “older” sRBC samples (day 35–40) but not “fresh” sRBC samples (day 7). E, Percent change in beating rate following sRBC treatment, compared with baseline. Mean±SEM, n=12–24, *Significantly different from baseline, P<0.05. BPM indicates beats per minute; hiPSC‐CM, human induced pluripotent stem cell–derived cardiomyocytes; RBC, red blood cell; and sRBC, supernatant from red blood cell units.
Discussion
Clinical case reports have documented transfusion‐associated hyperkalemia, which can lead to conduction disturbances, ventricular tachycardias, and/or cardiac arrest. 14 , 15 , 18 , 19 , 22 , 32 , 52 Furthermore, studies suggest that transfusion‐associated adverse events may be associated with the storage age of blood products, as RBCs undergo a cascade of morphological, biochemical, and metabolic changes over time that are collectively termed the “RBC storage lesion” or “metabolic aging.” 14 , 18 , 19 , 76 This study is the first to demonstrate that “older” blood products may directly impact myocardial automaticity and electrical conduction, using experimental cardiac models. Importantly, we show that supernatant collected from “fresh” RBC units (7 days postdonor collection) had no effect on heart rate, sinus node function, atrial or atrioventricular conduction, or myocardial refractoriness in an isolated, whole heart model. A follow‐up study in hiPSC‐CM revealed that supplementation with 10% sRBC from “fresh” units (day 7) caused a modest increase in the spontaneous beating rate over time, which may be attributed to mild hyperkalemia (5.8±0.2 mM K+). In comparison, whole heart preparations exposed to supernatant from aged RBC units (>30 days postcollection) displayed bradycardia, slowed atrial and atrioventricular conduction, and an increase in the refractoriness of the ventricle and AV node. Notably, other groups have suggested that the maximal allowable RBC storage duration be reduced from 42 to 35 days, because of increased hemolysis and a sharp increase in nontransferrin‐bound iron after 5 weeks in refrigerated storage. 24 Although we did not measure either free iron or nontransferrin‐bound iron levels in this study, our results closely align with this conclusion, as electrophysiological disturbances were predominately observed in units stored 30+ days postdonor collection.
Mechanistic Links Between RBC Transfusion and Adverse Cardiac Outcomes
Blood transfusion complications include an increased risk of bradycardia and cardiac arrest, which may be precipitated by an elevated potassium level in the supernatant of RBC units. 14 , 15 , 21 , 22 , 52 As extracellular potassium increases, electrochemical gradients are diminished and the cardiomyocyte resting membrane potential becomes less negative. 54 , 70 , 72 Accordingly, mild hyperkalemia can enhance cardiomyocyte excitability, similar to our observation with day 7 sRBC treatment in hiPSC‐CM. However, with more severe hyperkalemia, the change in resting membrane potential decreases the availability of voltage‐gated sodium channels that are critical to depolarization and myocardial excitability. 54 Accordingly, severe hyperkalemia is marked by sinus node dysfunction and sinus arrest. 53 Similar observations were observed in our study when cardiac preparations were exposed to increasing potassium concentrations, a prominent biomarker of RBC storage lesion that can, at least in part, contribute to the electrical disturbances observed in this study.
As described above, hyperkalemia shifts the resting membrane potential and reduces the availability of voltage‐gated sodium channels. As the action potential upstroke slows, electrical conduction slows, which manifests as a prolongation of P‐waves, PR interval, and QRS interval time. 54 , 70 , 72 Atrial cardiomyocytes are the most sensitive to elevated potassium concentrations, followed by the ventricular myocardium and then specialized conductive tissue, including the sinoatrial node and bundle of His. 54 , 70 , 72 Accordingly, electrical disturbances attributed to high [K+] are initially observed as widened p‐waves with shorter amplitudes, followed by atrioventricular and ventricular conduction delays as extracellular [K+] continues to increase. Instead of a gradual change in cardiac parameters, we observed a global depression in electrical conduction that was largely limited to sRBC samples near expiration and/or 10 to 12 mM K+ perfusion. The latter may be attributed to the sensitivity of our model system, 71 species‐specific differences in ion channel expression and electrophysiology, 74 , 75 and/or other attributes of the RBC storage lesion (eg, lactate, free‐iron, and plasticizer leaching) that may have additional effects on cardiac electrophysiology. 24 , 25 , 27 , 63 , 77
Although not investigated in the present study, phthalate chemical exposure is another potential contributor to heart rate slowing and sinus node dysfunction. Phthalate chemicals are frequently used as plasticizers in blood bags, and studies have shown that storage age is associated with an accumulation of harmful phthalate chemicals in the supernatant of stored RBC products (18‐fold increase, day 5 versus 42 postdonor collection). 77 Phthalate chemical exposure has been associated with bradycardia in vivo, 78 in vitro, 79 and using an isolated heart model. 80 Moreover, our laboratory previously reported that phthalate plasticizers can lead to sinus node dysfunction in an isolated heart model, delaying SNRT by 54% compared with control. 63 Additional studies are needed to investigate the additive effects that may result from hyperkalemia and phthalate chemical exposure.
Clinical Implications
In the current study, we focused our attention on hyperkalemia as 1 potential mechanism for the electrophysiology disturbances observed in our model system after exposure to “old” RBC samples. Hyperkalemia has been reported in >70% of adult trauma patients following transfusion, 18 and observed in 18% to 23% of pediatric trauma patients following transfusion. 33 Moreover, Smith et al. reported that an increase in serum potassium levels (5.9–9.2 mEq/L) was associated with a higher risk of cardiac arrest, 15 which is more likely to occur following rapid transfusion, large‐volume transfusion, or in cases of low cardiac output that impairs the redistribution of potassium. 14 , 19 Potential solutions to help mitigate the risk of hyperkalemia include prebypass filtering, 81 washing RBCs, 21 or limiting RBC storage duration. 14 , 15 , 18 , 20 , 24 Notably, longer blood storage duration has been associated with suboptimal outcomes in high‐risk pediatric surgery cases 12 and cardiac operations. 17 , 20 Recent randomized controlled trials have indicated that transfusion of “fresh” blood (eg, 1–10 days) does not decrease the risk of mortality when compared with standard of care (eg, 2–3 weeks). 44 , 45 , 46 , 47 , 48 However, much less is known about the safety of prolonged RBC storage (eg, 30–42 days) or the impact of “old” blood products on secondary cardiac end points. 35 , 49 Accordingly, expert panels have highlighted the lack of evidence‐based data to reach consensus on the safety of RBC storage age in relation to critically ill children, including those undergoing surgical repair for congenital heart defects or those undergoing extracorporeal membrane oxygenation. 82 , 83 The presented study highlights the importance of studying the direct impact of RBC storage lesion on end‐organ function, with an emphasis on cardiac electrophysiology given the sensitivity of the heart to electrolyte disturbances.
Limitations
The scope of our study was limited to the effects of acute cardiac exposure to supernatant collected from RBC units. Whole heart and cardiomyocyte models were used to investigate the direct effects of sRBC‐mediated biochemical disturbances on electrical activity. Although hiPSC‐CM are frequently employed in preclinical testing, it is important to note that these cells can display a less mature phenotype compared with adult cardiomyocytes. 84 Moreover, in vitro and ex vivo results may differ from those observed in vivo, with an intact vascular and autonomic nervous system. To mimic patient exposure following a large transfusion, we estimated 10% supernatant volume exposure from reconstituted blood, based on volumes reported in trauma cases, cardiac surgery, and/or extracorporeal membrane oxygenation studies. Additional studies are warranted to assess additional effects that may result from reconstituted blood containing aged RBCs, or the risk to sensitive populations including those with low cardiac output. Further studies are warranted to investigate alternative mechanisms that may contribute to cardiovascular dysfunction, including additional biochemical and metabolic alterations in stored RBC units. 26 , 36 , 77
Sources of Funding
This work was supported by the National Institutes of Health (R01HL139472 to NGP and UL1TR001876), Sheikh Zayed Institute for Pediatric Surgical Innovation, and the Children’s National Heart Institute. This publication was also supported by the Gloria and Steven Seelig family.
Disclosures
None.
Acknowledgments
The authors gratefully acknowledge Dr. Luther Swift for experimental technical assistance, Drs. Nobuyuki Ishibashi and Takuya Maeda for assistance with electrolyte measurements, and Drs. Pranava Sinha and Charles Berul for helpful discussions. We also acknowledge Dr. Meghan Delaney and Antoine Tavares Da Souza in the Children’s National Blood Bank for their assistance with procuring, storing, and collecting RBC supernatant samples for this study.
Author contributions: Marissa R, NC, TP, CB, DG, Manelle R, and RN performed experiments; Marissa R, NC, TP, DG, and NGP analyzed data; Marissa R, NC, TP, DG, and NGP prepared figures; Marissa R, TP, NC, CB, NL, and NGP drafted the manuscript; NL and NGP conceived and designed experiments; Marissa R, CB, NC, TP, DG, Manelle R, RN, NL, and NGP approved the manuscript.
(J Am Heart Assoc. 2020;9:017748 DOI: 10.1161/JAHA.120.017748.)
For Sources of Funding and Disclosures, see page 13.
Preprint posted on BioRxiv May 25, 2020. https://doi.org/10.1101/2020.05.22.111302.
References
- 1. Jonas RA. Comprehensive Surgical Management of Congenital Heart Disease. London: Hodder Education Group; 2004. [Google Scholar]
- 2. Punjabi PP, Taylor KM. The science and practice of cardiopulmonary bypass: From cross circulation to ECMO and SIRS. Glob Cardiol Sci Pract. 2013;2013:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Speiss BD. Transfusion and outcome in heart surgery. Ann Thorac Surg. 2002;74:986–987. [DOI] [PubMed] [Google Scholar]
- 4. Chung K-W, Basavaraju SV, Mu Y, van Santen KL, Haass KA, Henry R, Berger J, Kuehnert MJ. Declining blood collection and utilization in the United States. Transfusion. 2016;56:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones JM, Sapiano MRP, Savinkina AA, Haass KA, Baker ML, Henry RA, Berger JJ, Basavaraju SV. Slowing decline in blood collection and transfusion in the United States - 2017. Transfusion. 2020;60(Suppl 2):S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, Jacobs B, Markovitz B, Goldstein B, Hanson JH, et al. Anemia, blood Loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. [DOI] [PubMed] [Google Scholar]
- 7. Department of Health and Human Services . The 2011 National Blood Collection and Utilization Survey Report. Available at: http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf.
- 8. Keung C, Smith K, Savoia H, Davidson A. An audit of transfusion of red blood cell units in pediatric anesthesia. Paediatr Anaesth. 2009;19:320–328 [DOI] [PubMed] [Google Scholar]
- 9. Savinkina AA, Haass KA, Sapiano MRP, Henry RA, Berger JJ, Basavaraju SV, Jones JM. Transfusion-associated adverse events and implementation of blood safety measures - findings from the 2017 National Blood Collection and Utilization Survey. Transfusion. 2020;60:S10–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. [DOI] [PubMed] [Google Scholar]
- 11. Iyengar A, Scipione CN, Sheth P, Ohye RG, Riegger L, Bove EL, Devaney EJ, Hirsch-Romano JC. Association of complications with blood transfusions in pediatric cardiac surgery patients. Ann Thorac Surg. 2013;96:910–916. [DOI] [PubMed] [Google Scholar]
- 12. Manlhiot C, McCrindle BW, Menjak IB, Yoon H, Holtby HM, Brandão LR, Chan AK, Schwartz SM, Ben Sivarajan V, Crawford-Lean L, et al. Longer blood storage is associated with suboptimal outcomes in high-risk pediatric cardiac surgery. Ann Thorac Surg. 2012;93:1563–1569. [DOI] [PubMed] [Google Scholar]
- 13. Josephson CD, Heath Mondoro T, Ambruso DR, Sanchez R, Sloan SR, Luban NLC, Widness JA. One size will never fit all: the future of research in pediatric transfusion medicine. Pediatr Res. 2014;76:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee AC, Reduque LL, Luban NLC, Ness PM, Anton B, Heitmiller ES. Transfusion-associated hyperkalemic cardiac arrest in pediatric patients receiving massive transfusion. Transfusion. 2014;54:244–254. [DOI] [PubMed] [Google Scholar]
- 15. Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: A case series. Anesth Analg. 2008;106:1062–1069. [DOI] [PubMed] [Google Scholar]
- 16. Karkouti K, Callum JL, Acker JP, Yip P, Rao V. Red cell transfusion-associated hemolysis in cardiac surgery. Anesth Analg. 2017;124:1986–1991. [DOI] [PubMed] [Google Scholar]
- 17. Ranucci M, Carlucci C, Isgrò G, Boncilli A, De Benedetti D, De la Torre T, Brozzi S, Frigiola A. Duration of red blood cell storage and outcomes in pediatric cardiac surgery: an association found for pump prime blood. Crit Care. 2009;13:R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raza S, Ali Baig M, Chang C, Dabas R, Akhtar M, Khan A, Nemani K, Alani R, Majumder O, Gazizova N, et al. A prospective study on red blood cell transfusion related hyperkalemia in critically Ill patients. J Clin Med Res. 2015;7:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown KA, Bissonnette B, McIntyre B. Hyperkalaemia during rapid blood transfusion and hypovolaemic cardiac arrest in children. Can J Anaesth. 1990;37:747–754. [DOI] [PubMed] [Google Scholar]
- 20. Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. [DOI] [PubMed] [Google Scholar]
- 21. Swindell CG, Barker TA, McGuirk SP, Jones TJ, Barron DJ, Brawn WJ, Horsburgh A, Willetts RG. Washing of irradiated red blood cells prevents hyperkalaemia during cardiopulmonary bypass in neonates and infants undergoing surgery for complex congenital heart disease. Eur J Cardio-Thoracic Surg. 2007;31:659–664. [DOI] [PubMed] [Google Scholar]
- 22. Carvalho B, Quiney NF. ‘Near-miss’ hyperkalaemic cardiac arrest associated with rapid blood transfusion. Anaesthesia. 1999;54:1094–1096. [DOI] [PubMed] [Google Scholar]
- 23. Gruenwald CE, McCrindle BW, Crawford-Lean L, Holtby H, Parshuram C, Massicotte P, Van Arsdell G. Reconstituted fresh whole blood improves clinical outcomes compared with stored component blood therapy for neonates undergoing cardiopulmonary bypass for cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2008;136:1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rapido F, Brittenham GM, Bandyopadhyay S, La Carpia F, L’Acqua C, McMahon DJ, Rebbaa A, Wojczyk BS, Netterwald J, Wang H, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2016;127:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D’Alessandro A, Zimring JC, Busch M. Chronological storage age and metabolic age of stored red blood cells: are they the same? Transfusion. 2019;59:1620–1623. [DOI] [PubMed] [Google Scholar]
- 26. Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia. 2015;70:29-e12. [DOI] [PubMed] [Google Scholar]
- 29. Wallas CH. Sodium and potassium changes in blood bank stored human erythrocytes. Transfusion. 1979;19:210–215. [DOI] [PubMed] [Google Scholar]
- 30. Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci. 2007;104:17063–17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–59. [DOI] [PubMed] [Google Scholar]
- 32. Friedensohn A, Faibel HE, Bairey O, Goldbourt U, Schlesinger Z. Malignant arrhythmias in relation to values of serum potassium in patients with acute myocardial infarction. Int J Cardiol. 1991;32:331–338. [DOI] [PubMed] [Google Scholar]
- 33. Livingston MH, Singh S, Merritt NH. Massive transfusion in paediatric and adolescent trauma patients: incidence, patient profile, and outcomes prior to a massive transfusion protocol. Injury. 2014;45:1301–1306. [DOI] [PubMed] [Google Scholar]
- 34. Davey RJ, McCoy NC, Yu M, Sullivan JA, Spiegel DM, Leitman SF. The effect of prestorage irradiation on posttransfusion red cell survival. Transfusion. 1992;32:525–528. [DOI] [PubMed] [Google Scholar]
- 35. Belpulsi D, Spitalnik S, Hod E. The controversy over the age of blood: What do the clinical trials really teach us? Blood Transfus. 2017;15:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, Zolla L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–219. [DOI] [PubMed] [Google Scholar]
- 37. Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion. 2016;56:1462–1468. [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinberg JA, McGwin G, Griffin RL, Huynh VQ, Cherry SA, Marques MB, Reiff DA, Kerby JD, Rue LW. Age of transfused blood: An independent predictor of mortality despite universal leukoreduction. J Trauma Inj Infect Crit Care. 2008;65:279–284. [DOI] [PubMed] [Google Scholar]
- 40. Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pettilä V, Westbrook AJ, Nichol AD, Bailey MJ, Wood EM, Syres G, Phillips LE, Street A, French C, Murray L, et al. Blood observational study investigators for ANZICS clinical trials group. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15(2):R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keidan I, Amir G, Mandel M, Mishali D. The metabolic effects of fresh versus old stored blood in the priming of cardiopulmonary bypass solution for pediatric patients. J Thorac Cardiovasc Surg. 2004;127:949–952. [DOI] [PubMed] [Google Scholar]
- 43. Zubair AC. Clinical impact of blood storage lesions. Am J Hematol. 2010;85:117–122. [DOI] [PubMed] [Google Scholar]
- 44. Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, Very LOW-birth-weight infants. JAMA. 2012;308:1443. [DOI] [PubMed] [Google Scholar]
- 45. Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lacroix J, Hébert P, Fergusson D, Tinmouth A, Cook D, Marshall J, Clayton L, McIntyre L, Callum J., Turgeon AF, Blajchman MA, et al. Age of transfused blood in critically Ill adults. N Engl J Med. 2015;372:1410–1418. [DOI] [PubMed] [Google Scholar]
- 47. Heddle N, Cook R, Arnold D, Liu Y, Barty R, Crowther M, Devereaux P, Hirsh J., Warkentin TE, Webert KE, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937–1945. [DOI] [PubMed] [Google Scholar]
- 48. Spinella PC, Tucci M, Fergusson DA, Lacroix J, Hébert PC, Leteurtre S, Schechtman KB, Doctor A, Berg RA, Bockelmann T, et al. Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically Ill pediatric patients. JAMA. 2019;322:2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Remy KE, Spinella PC. Red blood cell storage age – what we know from clinical trials. Expert Rev Hematol. 2016;9:1011–1013. [DOI] [PubMed] [Google Scholar]
- 50. Klein HG, Cortés-Puch I, Natanson C. More on the age of transfused red cells. N Engl J Med. 2015;373:283–284. [DOI] [PubMed] [Google Scholar]
- 51. McQuilten ZK, Cooper DJ. Age of red blood cells for transfusion in critically Ill pediatric patients. JAMA. 2019;322:2175. [DOI] [PubMed] [Google Scholar]
- 52. Baz EMK, Kanazi GE, Mahfouz RAR, Obeid MY. An unusual case of hyperkalaemia-induced cardiac arrest in a paediatric patient during transfusion of a “fresh” 6-day-old blood unit. Transfus Med. 2002;12:383–386. [DOI] [PubMed] [Google Scholar]
- 53. El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18:233–245. [PubMed] [Google Scholar]
- 54. Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10:e004667 DOI: 10.1161/CIRCEP.116.004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Food and Drug Administration . Additional standards for human blood and blood products. 2020. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=640&showFR=1.
- 56. Muszynski JA, Reeder RW, Hall MW, Berg RA, Shanley TP, Newth CJL, Pollack MM, Wessel D, Carcillo J, Harrison R, et al. Eunice kennedy shriver national institute of child health and human development collaborative pediatric critical care research network (CPCCRN). RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018;46:e552–e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Durandy Y. Blood transfusion in pediatric cardiac surgery. Artif Organs. 2010;34:1057–1061. [DOI] [PubMed] [Google Scholar]
- 58. Jennings LK, Watson S. Massive Transfusion. StatPearls [Internet], Treasure Island (FL): StatPearls Publishing; 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499929/. [PubMed] [Google Scholar]
- 59. Murthi SB, Dutton RP, Edelman BB, Scalea TM, Hess JR. Transfusion medicine in trauma patients. Expert Rev Hematol. 2008;1:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chambers LA, Cohen DM, Davis JT. Transfusion patterns in pediatric open heart surgery. Transfusion. 1996;36:150–154. [DOI] [PubMed] [Google Scholar]
- 61. Jaimes R, Kuzmiak-Glancy S, Brooks DM, Swift LM, Posnack NG, Kay MW. Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate. Pflügers Arch - Eur J Physiol. 2016;468:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641:187–192. [DOI] [PubMed] [Google Scholar]
- 63. Jaimes R 3rd, McCullough D, Siegel B, Swift L, McInerney D, Hiebert J, Perez-Alday EA, Trenor B, Sheng J, Saiz J, et al. Plasticizer interaction with the heart: Chemicals used in plastic medical devices can interfere with cardiac electrophysiology. Circ Arrhythmia Electrophysiol. 2019;12:e007294 DOI: 10.1161/CIRCEP.119.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swift L, Jaimes R, McCullough D, Burke M, Reilly M, Maeda T, Zhang H, Ishibashi N, Rogers J, Posnack NG. Optocardiography and electrophysiology studies of ex vivo langendorff-perfused hearts. J Vis Exp. 2019; 153 DOI: 10.3791/60472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Swift LM, Burke M, Guerrelli D, Reilly M, Ramadan M, McCullough D, Prudencio T, Mulvany C, Chaluvadi A, Jaimes R, et al. Age-dependent changes in electrophysiology and calcium handling: implications for pediatric cardiac research. Am J Physiol Circ Physiol. 2020;318:H354–H365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, Zamora V, Smith G, Crumb WJ, Pang L, et al. Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol Sci. 2017;155:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colatsky T, Fermini B, Gintant G, Pierson JB, Sager P, Sekino Y, Strauss DG, Stockbridge N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative - Update on progress. J Pharmacol Toxicol Methods. 2016;81:15–20. [DOI] [PubMed] [Google Scholar]
- 68. Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clements M. Multielectrode Array (MEA) Assay for Profiling Electrophysiological Drug Effects in Human Stem Cell-Derived Cardiomyocytes. Current Protocols in Toxicology. Hoboken, NJ: John Wiley & Sons, Inc.; 2016:22.4.1-22.4.32. DOI: 10.1002/cptx.2. [DOI] [PubMed] [Google Scholar]
- 70. Dittrich KL, Walls RM. Hyperkalemia: ECG manifestations and clinical considerations. J Emerg Med. 1986;4:449–455. [DOI] [PubMed] [Google Scholar]
- 71. Boukens BJ, Rivaud MR, Rentschler S, Coronel R. Misinterpretation of the mouse ECG: “musing the waves of Mus musculus”. J Physiol. 2014;592:4613–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Texas Hear Inst J. 2006;33:40–47. [PMC free article] [PubMed] [Google Scholar]
- 73. Coronel R, Janse MJ, Opthof T, Wilde AA, Taggart P. Postrepolarization refractoriness in acute ischemia and after antiarrhythmic drug administration: action potential duration is not always an index of the refractory period. Heart Rhythm. 2012;9:977–982. [DOI] [PubMed] [Google Scholar]
- 74. Zimmer T, Haufe V, Blechschmidt S. Voltage-gated sodium channels in the mammalian heart. Glob Cardiol Sci Pract. 2014;2014:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Edwards AG, Louch WE. Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin Med Insights Cardiol. 2017;11 DOI: 10.1177/1179546816686061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smits-Wintjens VEHJ, Rath MEA, van Zwet EW, Oepkes D, Brand A, Walther FJ, Lopriore E. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology. 2013;103:141–147. [DOI] [PubMed] [Google Scholar]
- 77. D’alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016;14:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rock G., Labow RS, Franklin C, Burnett R, Tocchi M. Hypotension and cardiac arrest in rats after infusion of mono(2-ethylhexyl) phthalate (MEHP), a contaminant of stored blood. N Engl J Med. 1987;316:1218–1219. [DOI] [PubMed] [Google Scholar]
- 79. Rubin RJ, Jaeger RJ. Some pharmacologic and toxicologic effects of Di-2-ethylhexyl phthalate (DEHP) and other plasticizers. Environ Health Perspect. 1973;3:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aronson CE, Serlick ER, Preti G. Effects of di-2-ethylhexyl phthalate on the isolated perfused rat heart. Toxicol Appl Pharmacol. 1978;44:155–169. [DOI] [PubMed] [Google Scholar]
- 81. Delaney M, Axdorff-Dickey RL, Crockett GI, Falconer AL, Levario MJ, McMullan DM. Risk of extracorporeal life support circuit-related hyperkalemia is reduced by prebypass ultrafiltration. Pediatr Crit Care Med. 2013;14:e263–e267. [DOI] [PubMed] [Google Scholar]
- 82. Valentine SL, Bembea MM, Muszynski JA, Cholette JM, Doctor A, Spinella PC, Steiner ME, Tucci M, Hassan NE, Parker RI, et al. Pediatric critical care transfusion and anemia expertise initiative (TAXI), pediatric critical care blood research network (BloodNet), and the pediatric acute lung injury and sepsis investigators (PALISI) network. Consensus recommendations for RBC transfusion practice in critically Ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018;19:884–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cholette JM, Willems A, Valentine SL, Bateman ST, Schwartz SM. Pediatric critical care transfusion and anemia expertise initiative (TAXI), pediatric critical care blood research network (BloodNet), and the pediatric acute lung injury and sepsis investigators (PALISI) network. Recommendations on RBC transfusion in infants and children with acquired and congenital heart disease from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018;19:S137–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


