Abstract
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia, but the pathogenesis is not completely understood. The application of metabolomics could help in discovering new metabolic pathways involved in the development of the disease.
Methods and Results
We measured 112 baseline fasting metabolites of 3770 participants in the Malmö Diet and Cancer Study; these participants were free of prevalent AF. Incident cases of AF were ascertained through previously validated registers. The associations between baseline levels of metabolites and incident AF were investigated using Cox proportional hazard models. During 23.1 years of follow‐up, 650 cases of AF were identified (incidence rate: 8.6 per 1000 person‐years). In Cox regression models adjusted for AF risk factors, 7 medium‐ and long‐chain acylcarnitines were associated with higher risk of incident AF (hazard ratio [HR] ranging from 1.09; 95% CI, 1.00–1.18 to 1.14, 95% CI, 1.05–1.24 per 1 SD increment of acylcarnitines). Furthermore, caffeine and acisoga were also associated with an increased risk (HR, 1.17; 95% CI, 1.06–1.28 and 1.08; 95% CI, 1.00–1.18, respectively), while beta carotene was associated with a lower risk (HR, 0.90; 95% CI, 0.82–0.99).
Conclusions
For the first time, we show associations between altered acylcarnitine metabolism and incident AF independent of traditional AF risk factors in a general population. These findings highlight metabolic alterations that precede AF diagnosis by many years and could provide insight into the pathogenesis of AF. Future studies are needed to replicate our finding in an external cohort as well as to test whether the relationship between acylcarnitines and AF is causal.
Keywords: acylcarnitines, atrial fibrillation, metabolomics
Subject Categories: Atrial Fibrillation, Metabolism, Epidemiology, Arrhythmias
Nonstandard Abbreviation and Acronym
- MDC
Malmö Diet and Cancer Study
Clinical Perspective
What Is New?
For the first time, we show associations between altered acylcarnitine metabolism and incident atrial fibrillation during a median follow‐up time of >20 years.
The circulating levels of medium‐ and long‐chain acylcarnitines are associated with a higher risk of developing atrial fibrillation, independent of traditional atrial fibrillation risk factors.
What Are the Clinical Implications?
The metabolic disturbances shown to precede atrial fibrillation diagnosis by several years could be future targets for medical or lifestyle interventions.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a worldwide prevalence of ≈33 million as estimated by the 2010 Global Burden of Disease Study. 1 In the European Union, the number of patients with AF is projected to increase from 8.8 million in 2010 to 16.9 million in 2060. 2 AF is associated with increased morbidity in the form of heart failure, stroke, and dementia as well as increased mortality. 1 , 3 The pathogenesis of AF is a complex, not fully understood multifactorial combination of electrical remodeling, structural remodeling, and inflammation. 4 Recently, the application of metabolomics has been suggested as a tool to improve our understanding of AF pathogenesis. 5
In regard to new‐onset AF, metabolomics has so far been underutilized when compared with the research done in cardiometabolic diseases. To our knowledge, 3 cohort studies utilizing metabolomics to investigate the development of new‐onset AF have been published. 6 , 7 , 8 A recent study from the ARIC (Atherosclerosis Risk in Communities) study with a sample size of 3922 and a mean follow‐up time of 20 years found that the 4 metabolites glycochenodeoxycholate, acisoga, pseudouridine, and uridine were associated with incidence of AF in adjusted Cox regression models. 6 In a longitudinal analysis of the Framingham Heart Study with 2458 subjects and 10 years of follow‐up time, no plasma metabolites were found to be associated with the risk of new‐onset AF after adjustment for multiple comparisons. 7 Lastly, in a metabolomics study done on 2023 patients undergoing coronary angiography from the Measurement to Understand Reclassification of Disease of Cabarrus/Kannapolis (MURDOCK) Horizon 1 CV (Horizon 1 Cardiovascular Disease) Study, several metabolite principal component analysis factors composed of medium‐ and long‐chain acylcarnitines were found to associate with new onset of AF after a median follow‐up time of 3.5 years. 8 Together, these studies indicate that metabolic changes can occur several years before AF diagnosis, but that either a large cohort with a long follow‐up time (ARIC), or a cohort with high risk for AF (MURDOCK) is needed in order to gain enough statistical power to find the changes that predispose to AF.
In the present study, we measured fasting plasma levels of 112 metabolites from the baseline examination of a Swedish population‐based prospective cohort study, the MDC (Malmö Diet and Cancer) Study, comprising 3770 individuals without AF at study entry. The associations of metabolite levels and the development of AF were assessed during a median follow‐up time of 23 years. Our aim was to identify metabolites associated with AF risk in order to highlight metabolic changes that predispose to the development of AF, with the opportunity to discover new pathways involved in the complex pathogenesis of AF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The MDC is a population‐based prospective cohort study of individuals who attended baseline examinations between 1991 and 1996 in Malmö, Sweden. The methodology and population have been previously described. 9 , 10 A random sample of 3833 participants in the cardiovascular cohort (MDC‐CC) 11 was included for metabolite measurement. Participants with prevalent AF (n=35) or unknown vital status at follow‐up (n=28) were excluded from all analyses. The remaining 3770 participants constituted our study sample in this post hoc analysis of incident AF. All participants provided written informed consent and the study was approved by the Ethics Committee of Lund University, Lund, Sweden (LU 51–90).
Data on covariates were collected at baseline, and have previously been described. 12 The consumption of alcohol was defined by a 4‐category variable by combining a 7‐day menu book and a food frequency questionnaire as previously described. 13 NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) was measured using the automated Dimension Vista (R) Intelligent Lab System method (Siemens Healthcare Diagnostics Inc., Deerfield, IL). Because of nonnormality, NT‐proBNP underwent logarithmic transformation. 14
Cases of new‐onset AF were ascertained until December 31, 2016 by linkage of Swedish personal identification numbers to the Swedish Hospital Discharge Register and the Swedish Cause of Death Register. Given the similarity of the 2 diseases, AF was defined as persistent or recurring AF or flutter using diagnosis codes 427.92 (International Classification of Diseases, Eighth Revision [ICD‐8]), 427D (ICD‐9), and I48 (ICD‐10). This end point has previously been validated. 15 , 16 Prevalent heart failure was defined as codes 427.00, 427.10, and 428.99 (ICD‐8), 428 (ICD‐9), and I50 and I11.0 (ICD‐10). 16 Prevalent diabetes mellitus was defined as a fasting whole blood glucose ≥6.1 mmol/L (corresponding to a plasma glucose level of ≥7.0 mmol/L) or a history of physician diagnosis of diabetes mellitus or being on antidiabetic medication or having been registered in any of the 6 different national and regional diabetes mellitus registers. 12 Ischemic stroke was coded according to the ICD‐9 code 434 (cerebral infarction) and verified by computed tomography scan or autopsy. To further enrich the end point, only ischemic stroke events that were preceded by or coincided (within 1 month) with a diagnosis of AF were included in order to find plausible cardioembolic stroke cases.
Profiling of plasma metabolites was performed using liquid chromatography–mass spectrometry, and has been previously described in detail. 17 Measured metabolites are listed in an in‐house metabolite library and categorized according to normalization method (Table S1). Thirty‐three of the metabolites were adjusted with an internal standard and 79 were normalized with standard curves calculated from the quality control samples as previously described. 12 The mass spectrometry method was initially created to measure 35 polar metabolites and amino acids. 18 A subsequent study expanded the method to investigate the relationship between metabolites and development of cardiovascular disease and type 2 diabetes mellitus 12 and managed to identify 77 additional metabolites, thus measuring 112 metabolites with suspected relationship to cardiometabolic disease.
R (V.3.6.0) was used for all statistical analysis. Because of nonnormality, metabolite data were log transformed and scaled to multiples of 1 SD and centered on zero before statistical analyses. Outliers that differed >4 SD from the mean after normalization were excluded from the analysis. Percentages of removed samples are reported in the in‐house metabolite library (Table S1).
To assess the associations between baseline fasting levels of metabolite levels and incident AF, Cox proportional hazard models were used. First, hypothesis‐generating analyses were performed with models adjusted for sex and age, and corrected for multiple comparison using false discovery rate. False discovery rate was used instead of a more stringent multiple testing method such as Bonferroni because these methods assume that the statistical tests being performed are independent. In this case, the tests are not independent because the levels of acylcarnitines are closely correlated and using Bonferroni correction would increase the risk of false negatives. Metabolites with significant associations were further analyzed in Cox proportional hazard models adjusted for sex, age, body mass index, baseline smoking status, systolic blood pressure, alcohol intake, use of antihypertensive medicine, NT‐proBNP, prevalent diabetes mellitus, prevalent heart failure, and prevalent ischemic heart disease. Schoenfeld residuals test was used to check the proportional hazard assumptions.
Because of the high amount of samples with miniscule caffeine levels (16%) (Table S1), additional Cox regression models were made using quintiles of caffeine levels including all samples. The lowest quintile was set as reference quintile.
The relationships between metabolites were investigated using Spearman correlations, displayed in a heat map with metabolites ordered by their first component.
To test the associations between metabolite levels and risk factors, partial Spearman correlations adjusted for sex and age were used. The associations between metabolite levels and sex were only adjusted for age, and the associations between metabolite levels and age were only adjusted for sex.
The associations between AF‐associated metabolites and likely cardioembolic stroke were tested using Cox proportional hazard models adjusted for sex and age. Before analyses, patients with prevalent stroke, incident subarachnoid hemorrhage, or incident intracerebral hemorrhage were excluded. Associations were further tested in Cox proportional hazard models adjusted for sex, age, body mass index, baseline smoking status, systolic blood pressure, alcohol intake, use of antihypertensive medicine, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes mellitus, prevalent heart failure, and prevalent ischemic heart disease. Additional adjustments were made with prevalent chronic obstructive pulmonary disease, prevalent cancer, and estimated glomerular filtration rate.
Results
The average baseline age was 57.7 years, and 59% were female. General characteristics of the study population can be found in Table. Among the 3770 participants free from prevalent AF, we identified 650 incident cases of AF during a median follow‐up time of 23.1 years (incidence rate: 8.6 per 1000 person‐years).
Table 1.
General Characteristics of Study Participants
|
Total No.=3770 Mean (SD) or % (No.) |
Non‐Incident No.=3120 Mean (SD) or % |
Incident AF No.=650 Mean (SD) or % |
P Value | |
|---|---|---|---|---|
| N | 3770 | 3120 (83%) | 650 (17%) | |
| Age, y | 58 (6.0) | 57 (6.0) | 60 (5.4) | <0.001 |
| Sex (% female) | 59% (2224) | 61% (1914) | 48% (310) | <0.001 |
| BMI, kg/m2 | 25.7 (3.9) | 25.5 (3.8) | 26.5 (4.3) | <0.001 |
| LDL‐C, mmol/L | 4.16 (1.0) | 4.17 (1.0) | 4.13 (0.9) | 0.3 |
| HDL‐C, mmol/L | 1.40 (0.4) | 1.40 (0.4) | 1.39 (0.4) | 0.4 |
| Glucose, mmol/L | 5.20 (1.4) | 5.15 (1.3) | 5.41 (1.6) | <0.001 |
| NT‐proBNP, ng/L | 96 (151) | 89.1 (139) | 129.1 (152) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 75.7 | 76.2 | 73.4 | <0.001 |
| Systolic blood pressure, mm Hg | 142 (19) | 141 (19) | 148 (19) | <0.001 |
| Diastolic blood pressure, mm Hg | 87 (9.5) | 86.5 (9.5) | 88.7 (9.2) | <0.001 |
| Antihypertensive treatment | 15.9% (599) | 14.1% (439) | 24.6% (160) | <0.001 |
| Smoking status (3% missing) | 27% (995) | 28% (840) | 25% (155) | 0.11 |
| Alcohol intake, g/d (3% missing) | 10.4 (12) | 10.0 (12) | 11.8 (14) | 0.002 |
| Prevalent coronary artery disease | 2.1% (79) | 1.4% (45) | 5.2% (34) | <0.001 |
| Prevalent diabetes mellitus | 9.8% (368) | 9.3% (289) | 12.2% (79) | 0.03 |
| Prevalent heart failure | 0.1% (5) | 0.1% (3) | 0.31% (2) | 0.4 |
| Prevalent COPD | 0.6% (24) | 0.6% (20) | 0.6% (4) | 1 |
| Prevalent cancer | 5.8% (219) | 5.6% (172) | 7.2% (47) | 0.1 |
Values are displayed as mean (SD) or percentages. BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Using sex‐ and age‐adjusted Cox proportional hazard models, out of 112 metabolites measured, 15 were associated with an increase or decrease in risk for developing AF (false discovery rate <0.05). In fully adjusted models, 11 metabolites remained significantly associated with AF incidence, most of which were acylcarnitines (Figure 1 and Table S2). Caffeine was associated with the highest increase in risk for AF (hazard ratio [HR] per 1 SD increment of caffeine, HR, 1.17; 95% CI, 1.06–1.28, P=0.001) in the fully adjusted model followed by acylcarnitine 16:1 (HR per 1 SD increment of acylcarnitine 16:1, HR, 1.13; 95% CI, 1.04–1.23, P=0.004). The proportional hazard assumptions were met for all statistically significant models shown in Figure 1. Further adjustments for prevalent chronic obstructive pulmonary disease, prevalent cancer, and estimated glomerular filtration rate did not change the results (Table S3).
Figure 1. Cox proportional hazard models comparing circulating metabolite levels with risk for atrial fibrillation during the median follow‐up time of 23.1 years.
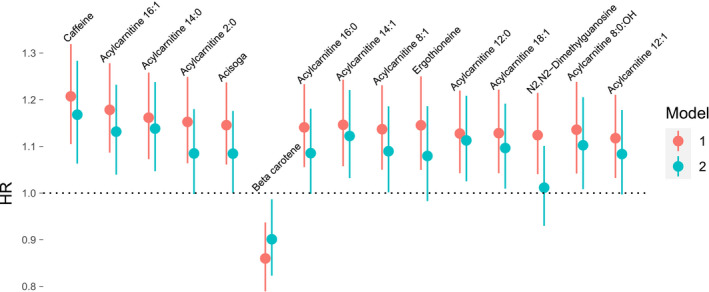
Model 1 was adjusted for sex and age. Model 2 was adjusted for sex, age, smoking, body mass index, systolic blood pressure, alcohol intake, use of hypertensive medicine, N‐terminal pro‐B‐type natriuretic peptide, and prevalent diabetes mellitus, heart failure, and coronary artery disease. The HR is calculated as the increase or decrease in risk per 1 SD increment of metabolite levels with 95% CI. HR indicates hazard ratio.
Since there were many samples with caffeine levels below limit of detection (16%) (Table S1), additional analysis of caffeine split into quintiles was made including all samples. In these Cox regression models with quintile 1 as reference, quintile 5 had a significantly higher risk for developing AF in the sex‐ and age‐adjusted model (Figure 2). The fully adjusted models showed no significant associations between quintiles of caffeine and AF risk.
Figure 2. Cox proportional hazard models comparing caffeine levels with risk for atrial fibrillation.
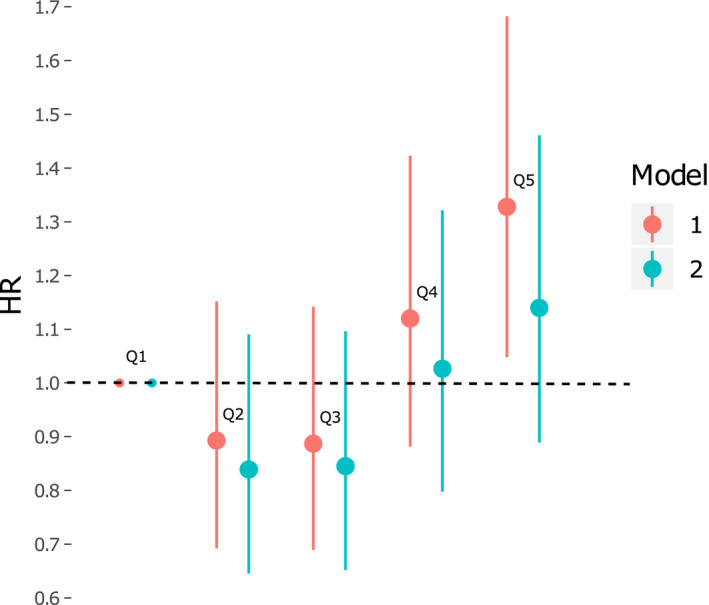
Model 1 was adjusted for sex and age. Model 2 was adjusted for sex, age, smoking, body mass index, systolic blood pressure, alcohol intake, use of hypertensive medicine, N‐terminal pro‐B‐type natriuretic peptide, and prevalent diabetes mellitus, heart failure, and coronary artery disease. The HR is calculated as the increase or decrease in risk per 1 SD increment of metabolite levels with 95% CI. HR indicates hazard ratio.
Since many of the metabolites significantly associated with AF were in the same class of metabolites, namely, acylcarnitines, the correlations between AF‐associated metabolites levels were examined. The heat map with Spearman correlations show that acylcarnitines correlate strongly with each other, except for acylcarnitine 8:1, which had a weaker correlation with other acylcarnitines (Figure 3).
Figure 3. A heat map of Spearman correlation coefficients between metabolites that were associated with incident atrial fibrillation.
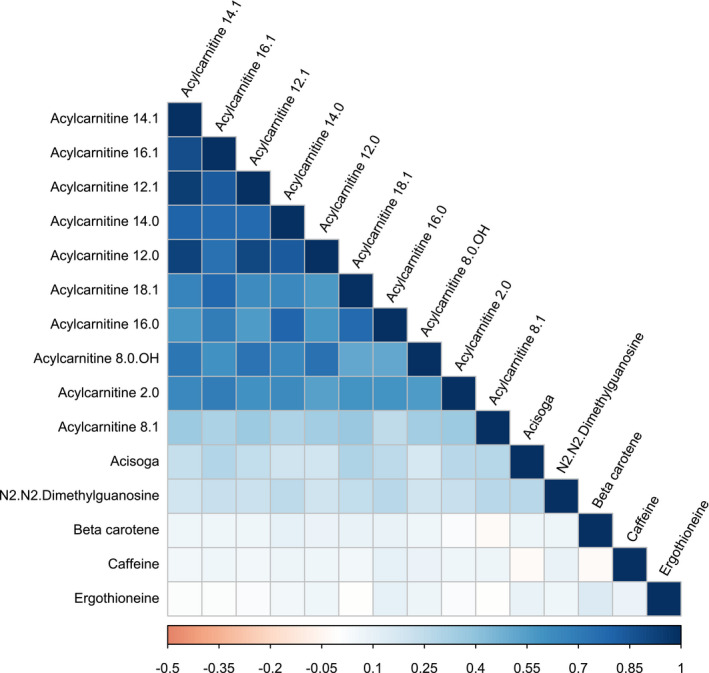
Metabolites are ordered by their first component.
In partial Spearman correlations comparing metabolite levels to risk factors, acylcarnitines were associated with higher age (Figure 4). The strongest correlation was between ergothioneine and alcohol intake. The correlations with the other risk factors, systolic blood pressure, use of antihypertensive treatment, smoking status, body mass index, and NT‐proBNP were small overall.
Figure 4. Partial Spearman correlation coefficients adjusted for sex and age between risk factors and metabolite levels.
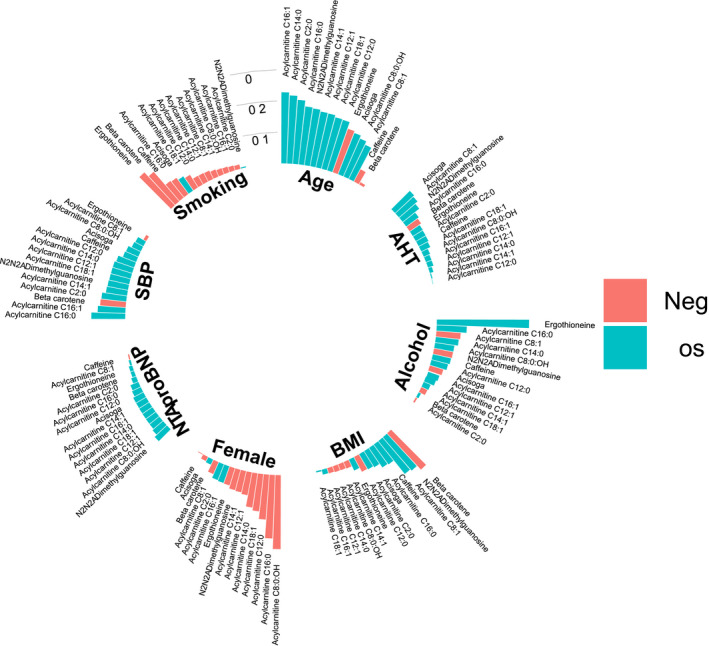
The directions of the associations are color coded. AHT indicates antihypertensive treatment; BMI, body mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and SBP, systolic blood pressure.
In a median follow‐up time of 23.2 years, 329 cases of ischemic stroke were identified after exclusion of patients with prevalent stroke (n=29), incident subarachnoid hemorrhage (n=7), incident intracerebral hemorrhage (n=48), or unspecified incident stroke subtype (n=13) (n after exclusion 3681). Out of these 329 cases, 83 were preceded by or coincided (within a month) with AF diagnosis and were therefore deemed likely to be cardioembolic in nature.
Associations between likely cardioembolic stroke and AF‐associated metabolites had similar effect sizes as risk for incident AF, but after multiple test corrections, no associations were significant (Table S4).
Discussion
In this metabolomics study of 3770 individuals in a prospective cohort, we found 10 metabolites to associate significantly with new‐onset AF in models adjusted for AF risk factors. Most of the metabolites associated with incident AF were acylcarnitines, suggesting a dysfunction of carnitine metabolism that precedes AF diagnosis by many years and which also might contribute to the pathogenesis of AF.
In a metabolomics study done on 2023 patients from the MURDOCK Study, several metabolite factors composed of medium‐ and long‐chain acylcarnitines were found to associate with new onset of AF. 8 The cohort consisted of patients subjected to coronary angiography with a high risk of new‐onset AF, and 12.3% of study participants developed AF during the 2.8‐year follow‐up. We extend these findings displaying associations between several acylcarnitines and risk for AF in a general population without increased risk for AF. Furthermore, we show the associations between AF and levels of specific acylcarnitines to be independent of several risk factors for AF, including NT‐proBNP. In the ARIC study mentioned earlier, some acylcarnitines were associated with an increased risk of AF, but the associations were not significant after multiple test correction.
Acylcarnitines are mostly derived from mitochondrial fatty acid oxidation, but can be formed from almost any coenzyme A ester. 19 Changed levels of acylcarnitines in circulation have been suggested to provide indirect evidence of altered mitochondrial metabolism, and accumulation of acylcarnitines could be seen as a sign of poor metabolic status. 20 Stressed myocardial cells can change from fatty acid oxidation in the mitochondria to glycolysis, 21 and the subsequent accumulation of long‐chain acylcarnitines in the cytoplasm could contribute to membrane instability by inhibiting the exchange of sodium and calcium ions in the sarcolemma and thus lead to the development of arrhythmia. 22 In a study of patients undergoing coronary artery bypass grafting surgery, adenosine‐diphosphate‐stimulated mitochondrial respiration supported by acylcarnitine 16:0 was significantly lower in patients who developed postoperative AF. 23 Levels of short‐, medium‐, and long‐chain acylcarnitines have all been associated with an increased risk of cardiovascular death and acute myocardial infarction. 24
Furthermore, circulating levels of long‐chain acylcarnitines have been associated with maladaptive left ventricular remodeling. 25 The increased levels of acylcarnitines could therefore be associated with either electrical remodeling or structural remodeling, which together with inflammation form the 3 dominant theories on AF pathogenesis. 4 The structural remodeling seems to be independent of the heart‐failure‐associated remodeling, given that the associations displayed in the fully adjusted models were independent of NT‐proBNP levels. Additional studies are needed to replicate our finding and to further investigate the potentially causal associations between increased acylcarnitines levels and AF.
The finding of caffeine being associated with new‐onset AF was surprising, because caffeine exposure has not been shown to increase the risk of AF in a systematic review of >100 000 individuals. 26 The results from this large meta‐analysis even suggest that low‐dose caffeine may have a protective effect. Given that many of our participants had caffeine levels below the limit of detection, quintile analyses were made with all samples included. This analysis showed that the potential association between plasma caffeine levels and AF is not linear. Caffeine quintile 5 displayed a significantly higher risk for AF compared with quintile 1, but other associations were not significant.
To our knowledge, our study is the first to study caffeine levels in plasma in relation to AF instead of studying caffeine exposure, based on reported intake, in relation to AF risk. Participants of the study were overnight fasting, and data on caffeine exposure preceding days before the analyses are not available. There are numerous factors influencing caffeine intake, absorption, metabolism, and physiologic and functional effects, which all could affect caffeine plasma levels, such as age, sex, hormonal status, diet, smoking, exposure to drugs, and genetic background. 27 Adjustments for genetic polymorphism of CYP1A2 (cytochrome P450 1A2), the main metabolizer of caffeine, has not shown any improvement in AF risk prediction, but the addition of caffeine concentration measurements to such studies could bring more insight into the caffeine–AF interaction. 28
An article published last year studying incident AF in 3922 individuals from the ARIC study found that the 4 metabolites acisoga, glycochenodeoxycholate, pseudouridine, and uridine were associated with AF. 6 The polyamine acisoga is a breakdown product of spermidine, and its precise role is unknown. 29 In the present study, acisoga was found to associate with AF in a sex‐ and age‐adjusted Cox regression model, but the relationship was strongly attenuated after adjustment. The liquid chromatography–mass spectrometry method used in our study did not measure the other 3 metabolites that were significantly associated with AF in the ARIC study.
Ergothioneine and N2N2‐dimethylguanosine were both associated with an increased risk for AF in sex‐ and age‐adjusted models, but the associations were not significant after full adjustment. We have previously found that ergothioneine was associated with a decreased risk of cardiovascular disease, cardiovascular mortality, and all‐cause mortality 12 and N2N2‐dimethylguanosine with incident type 2 diabetes mellitus. 18 The attenuated association between ergothioneine and AF could further be explained by the strong association between ergothioneine and alcohol intake, a known risk factor for AF. 30
Beta carotene was associated with a decreased risk of AF in sex‐ and age‐adjusted models, an association that was attenuated after full adjustment. Beta carotene has previously been associated with higher risk for AF, which contradicts our findings. 31 The association found in the present study between beta carotene and AF could be explained by an association with heart failure, a disease with closely aligned risk factors and pathogenesis with AF. 32 , 33
When testing the association between metabolite levels and likely cardioembolic stroke, associations showed the same directionality and effect size as risk for incident AF. However, the results were nonsignificant, which could partly have been explained by a lack of statistical power because of the low incidence rate and because we did not have sufficient information about stroke subtypes to validate the end point cardioembolic stroke.
In a short perspective, our results must be validated before they are integrated into clinical care. If the results are generalizable, test for acylcarnitines might be a part of AF risk stratification as we move towards personalized medicine. If further research shows a causative link between altered acylcarnitine metabolism and AF, acylcarnitine metabolism might become a target for drug development in order to lower the risk of AF.
The main strengths of our study are the large cohort with follow‐up data of good quality combined with a large number of AF cases found by a previously validated method. Since AF cases were diagnosed via registers and not through ECGs at follow‐up visits, there is the potential that undiagnosed cases are excluded. There were no data available on electrophysiological parameters or cardiac imaging, which might have provided additional information about what parameters the acylcarnitines affect. Moreover, with the prospective but observational study design of the MDC study, the causal link between metabolites and AF cannot be tested.
Conclusions
For the first time, we show associations between altered acylcarnitine metabolism and incident AF independent of traditional risk factors in a general population. These findings highlight metabolic alterations that precede AF diagnosis by many years. Future studies are needed to replicate our finding in an external cohort as well as to test whether the relationship between acylcarnitines and AF is causal.
Sources of Funding
Smith was supported by the Hulda and Conrad Mossfelt Foundation. Melander was supported by research grants from the Knut and Alice Wallenberg Foundation, Göran Gustafsson Foundation, the Swedish Heart‐ and Lung Foundation, the Swedish Research Council, the Novo Nordisk Foundation, Region Skåne, Skåne University Hospital and Lund University. Fernandez was supported by the Albert Påhlsson Research Foundation, the Crafoord Research Foundation, the Ernhold Lundström Research Foundation, the Royal Physiographic Society of Lund, and the Åke Wiberg Foundation. Ottosson was supported by Ernhold Lundströms Research Foundation.
Disclosures
None.
Supporting information
Tables S1–S4
Acknowledgments
We thank all the participants and staff in the Malmö Diet and Cancer—Cardiovascular Cohort.
(J. Am. Heart Assoc. 2020;9:e016737 DOI: 10.1161/JAHA.120.016737.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016737
For Sources of Funding and Disclosures, see page 8.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;1288–1294. [DOI] [PubMed] [Google Scholar]
- 4. Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;243–252. [DOI] [PubMed] [Google Scholar]
- 5. Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol. 2016;2850–2870. [DOI] [PubMed] [Google Scholar]
- 6. Alonso A, Yu B, Sun YV, Chen LY, Loehr LR, O'Neal WT, Soliman EZ, Boerwinkle E. Serum metabolomics and incidence of atrial fibrillation (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2019;1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ko D, Riles EM, Marcos EG, Magnani JW, Lubitz SA, Lin H, Long MT, Schnabel RB, McManus DD, Ellinor PT, et al. Metabolomic profiling in relation to new‐onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2016;1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harskamp RE, Granger TM, Clare RM, White KR, Lopes RD, Pieper KS, Granger CB, Newgard CB, Shah SH, Newby LK. Peripheral blood metabolite profiles associated with new onset atrial fibrillation. Am Heart J. 2019;54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;45–51. [DOI] [PubMed] [Google Scholar]
- 10. Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden. N Engl J Med. 2007;1009–1019. [DOI] [PubMed] [Google Scholar]
- 11. Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated Lp‐PLA2 levels add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle‐aged nondiabetic subjects. Arterioscler Thromb Vasc Biol. 2007;1411–1416. [DOI] [PubMed] [Google Scholar]
- 12. Smith E, Ottosson F, Hellstrand S, Ericson U, Orho‐Melander M, Fernandez C, Melander O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart. 2020;691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ericson U, Brunkwall L, Alves Dias J, Drake I, Hellstrand S, Gullberg B, Sonestedt E, Nilsson PM, Wirfalt E, Orho‐Melander M. Food patterns in relation to weight change and incidence of type 2 diabetes, coronary events and stroke in the Malmo Diet and Cancer cohort. Eur J Nutr. 2019;1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith JG, Newton‐Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engstrom G, Wang TJ, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;95–102. [DOI] [PubMed] [Google Scholar]
- 16. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;787–791. [DOI] [PubMed] [Google Scholar]
- 17. Ottosson F, Ericson U, Almgren P, Nilsson J, Magnusson M, Fernandez C, Melander O. Postprandial levels of branch chained and aromatic amino acids associate with fasting glycaemia. J Amino Acids. 2016;8576730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ottosson F, Smith E, Gallo W, Fernandez C, Melander O. Purine metabolites and carnitine biosynthesis intermediates are biomarkers for incident type 2 diabetes. J Clin Endocrinol Metab. 2019;4921–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;553–572. [DOI] [PubMed] [Google Scholar]
- 21. Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation–a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1333–1350. [DOI] [PubMed] [Google Scholar]
- 22. Wu J, Corr PB. Influence of long‐chain acylcarnitines on voltage‐dependent calcium current in adult ventricular myocytes. Am J Physiol. 1992;H410–H417. [DOI] [PubMed] [Google Scholar]
- 23. Montaigne D, Marechal X, Lefebvre P, Modine T, Fayad G, Dehondt H, Hurt C, Coisne A, Koussa M, Remy‐Jouet I, et al. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof‐of-concept study in patients with metabolic syndrome in whom post‐operative atrial fibrillation develops. J Am Coll Cardiol. 2013;1466–1473. [DOI] [PubMed] [Google Scholar]
- 24. Strand E, Pedersen ER, Svingen GF, Olsen T, Bjorndal B, Karlsson T, Dierkes J, Njolstad PR, Mellgren G, Tell GS, et al. Serum acylcarnitines and risk of cardiovascular death and acute myocardial infarction in patients with stable angina pectoris. J Am Heart Assoc. 2017;9:e003620 DOI: 10.1161/JAHA.116.003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elmariah S, Farrell LA, Furman D, Lindman BR, Shi X, Morningstar JE, Rhee EP, Gerszten RE. Association of acylcarnitines with left ventricular remodeling in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JAMA Cardiol. 2018;242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta‐analysis of observational studies. Heart. 2013;1383–1389. [DOI] [PubMed] [Google Scholar]
- 27. Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. 2018;384–411. [DOI] [PubMed] [Google Scholar]
- 28. Casiglia E, Tikhonoff V, Albertini F, Gasparotti F, Mazza A, Montagnana M, Danese E, Benati M, Spinella P, Palatini P. Caffeine intake reduces incident atrial fibrillation at a population level. Eur J Prev Cardiol. 2018;1055–1062. [DOI] [PubMed] [Google Scholar]
- 29. van den Berg GA, Kingma AW, Elzinga H, Muskiet FA. Determination of N‐(3-acetamidopropyl)pyrrolidin‐2-one, a metabolite of spermidine, in urine by isotope dilution mass fragmentography. J Chromatogr. 1986;251–258. [DOI] [PubMed] [Google Scholar]
- 30. Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;2567–2576. [DOI] [PubMed] [Google Scholar]
- 31. Karppi J, Kurl S, Makikallio TH, Ronkainen K, Laukkanen JA. Low levels of plasma carotenoids are associated with an increased risk of atrial fibrillation. Eur J Epidemiol. 2013;45–53. [DOI] [PubMed] [Google Scholar]
- 32. Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol. 2005;2054–2060. [DOI] [PubMed] [Google Scholar]
- 33. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


