Abstract
Background: The dissemination of the uropathogenic O25b-ST131 Escherichia coli clone constitutes a threat to public health. We aimed to determine the circulation of E. coli strains belonging to O25b:H4-B2-ST131 and the H30-Rx epidemic subclone causing hospital and community-acquired urinary tract infections (UTI) in Colombia. Methods: Twenty-six nonduplicate, CTX-M group-1-producing isolates causing UTI in the hospital and community were selected for this study. Results: Twenty-two E. coli isolates harboring CTX-M-15, one CTX-M-3, and three CTX-M-55 were identified. Multilocus Sequence Typing (MLST) showed a variety of sequence types (STs), among which, ST131, ST405, and ST648 were reported as epidemic clones. All the E. coli ST131 sequences carried CTX-M-15, from which 80% belonged to the O25b:H4-B2 and H30-Rx pandemic subclones and were associated with virulence factors iss, iha, and sat. E. coli isolates (23/26) were resistant to ciprofloxacin and associated with amino acid substitutions in quinolone resistance-determining regions (QRDR). We detected two carbapenem-resistant E. coli isolates, one coproducing CTX-M-15, KPC-2, and NDM-1 while the other presented mutations in ompC. Additionally, one isolate harbored the gene mcr-1. Conclusions: Our study revealed the circulation of the E. coli ST131, O25b:H4-B2-H30-Rx subclone, harboring CTX-M-15, QRDR mutations, and other resistant genes. The association of the H30-Rx subclone with sepsis and rapid dissemination warrants attention from the public health and infections control.
Keywords: E. coli ST131, O25b:H4, H30-RX subclone, CTX-M, urinary tract infections (UTI), clonal dissemination
1. Introduction
The spread of antimicrobial-resistant microorganisms has become a major threat to global public health, as infections caused by such bacteria are often responsible for the increase in patient mortality and morbidity due to inappropriate therapy [1]. Extended spectrum β-lactamases (ESBLs) are enzymes that provide resistance to penicillins; oxyimino-cephalosporins (like ceftazidime, ceftriaxone, and cefotaxime); and monobactams but not to cephamycins (cefoxitin) or carbapenems [2]. The acquisition of ESBLs is the main mechanism of resistance to oxyimino-cephalosporins in Escherichia coli, which are a major cause of hospital- and community-acquired urinary tract infections (UTI) [3]. The overall prevalence of ESBLs has increased globally, mainly due to the spread of CTX-M enzymes. To date, more than 220 different CTX-M types have been recorded, CTX-M-15 being the most widely distributed [4]. Global surveillance studies have shown high rates of ESBL-producing E. coli in certain areas of Asia, Africa, and Latin America, which has led to an increased use of last-resort antimicrobial drugs such as carbapenems, leading to the emergence of carbapenem-resistant bacteria [5].
The population structure of ESBL-producing E. coli is dominated globally by a high-risk clone named sequence type (ST) 131, which is associated with fluoroquinolone and extended-spectrum cephalosporin resistance and has played a critical role in the current pandemic spread [6]. ST131 has been grouped into three clades, which are associated with specific fimH alleles: A clade (fimH41-O16), B clade (fimH22-O25b), and C clade (fimH30-O25b, including fimH30-Rx). Whole-genome sequencing (WGS) studies identified two subclades within clade C (H30-R and H30-Rx). The most prevalent lineage within ST131 is fimH30, a H30 variant of the type 1 fimbrial adhesin gene, and its subclones H30-R and H30-Rx [7]. Of special interest, isolates belonging to the H30-Rx subclone of ST131 have been associated with upper urinary tract infections (UTIs) and primary sepsis [7].
Other important epidemic clones detected among ESBL producers include ST405, ST38, ST648, ST410, and ST1193 [8]. In 2011, the presence of CTX-M-15-producing ST131 and ST405 E. coli isolates was reported for the first time in Colombia [9]. However, the circulation of the E. coli ST131 clone, B2, O25b:H4, and the H30-Rx subclone of extraintestinal pathogenic E. coli (ExPEC) in the country remains unexplored. Herein, we determined the circulation of CTX-M-15, O25b:H4, and the H30-Rx subclone in ExPEC from hospital or community origins, causing UTI and blood stream infections (BSIs) in Colombia.
2. Results
2.1. Antibiotic Susceptibility
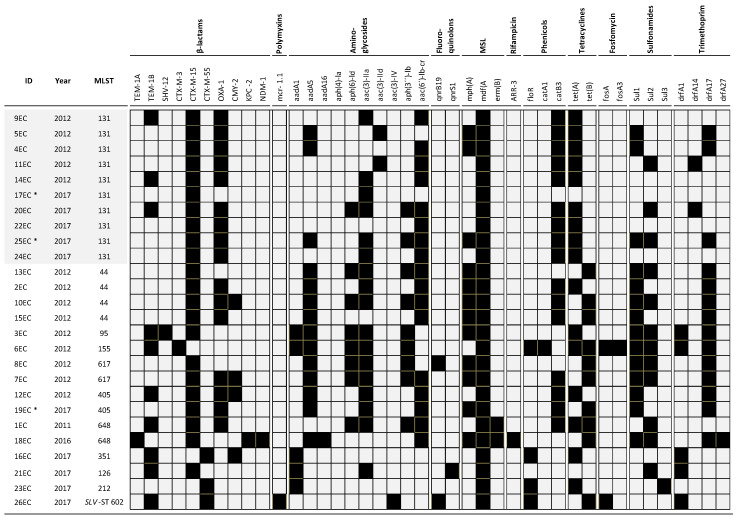
Antimicrobial susceptibilities of all strains evaluated are shown in Table 1. As expected, all isolates were resistant to cefotaxime and ceftriaxone; a resistance to ceftazidime was found in 21/26 (81%) of the isolates, to cefepime in 18/26 (69%), to piperacillin-tazobactam in 2/26 (8%), and to fosfomycin in 1/26 (4%) of the isolates. In addition, 92% susceptibility to ertapenem was found. The complete resistome of the isolates is presented in Figure 1.
Table 1.
Demographic and microbiological data of CTX-M-harboring Escherichia coli isolates.
| ID | City | Collection Date | Source | Ward | MLST | MIC (ug/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRO | CTX | CAZ | FEP | TZP | ETP | CIP | FOS | ||||||
| 1EC | Bogotá | 2011 | Urine | ER | 648 | >4 | >4 | 16 | 32 | 16/4 | ≤0.25 | >4 | ≤32 |
| 2EC | Cali | 2012 | Urine | ER | 44 | >4 | >4 | 32 | 16 | 16/4 | ≤0.25 | >4 | ≤32 |
| 3EC | Cali | 2012 | Urine | ER | 95 | >4 | >4 | 16 | 8 | 8/4 | ≤0.25 | >4 | ≤32 |
| 4EC | Cali | 2012 | Urine | ER | 131 | >4 | >4 | 16 | 8 | 4/4 | ≤0.25 | 4 | ≤32 |
| 5EC | Cali | 2012 | Urine | ER | 131 | >4 | >4 | 8 | 16 | ≤2/4 | ≤0.25 | >4 | ≤32 |
| 6EC | Cali | 2012 | Urine | ER | 155 | >4 | >4 | 1 | 4 | ≤2/4 | ≤0.25 | >4 | >128 |
| 7EC | Cali | 2012 | Urine | ER | 617 | >4 | >4 | 64 | 32 | 8/4 | ≤0.25 | >4 | ≤32 |
| 8EC | Cali | 2012 | Urine | ER | 617 | >4 | >4 | 8 | 16 | 8/4 | ≤0.25 | >4 | ≤32 |
| 9EC | Cali | 2012 | Urine | ER | 131 | >4 | >4 | 16 | 16 | 16/4 | ≤0.25 | >4 | 128 |
| 10EC | Cali | 2012 | Urine | ER | 44 | >4 | >4 | 64 | 32 | 16/4 | ≤0.25 | >4 | ≤32 |
| 11EC | Cali | 2012 | Urine | ER | 131 | >4 | >4 | 4 | 4 | 4/4 | ≤0.25 | >4 | ≤32 |
| 12EC | Cali | 2012 | Urine | ER | 405 | >4 | >4 | 32 | 16 | 8/4 | ≤0.25 | >4 | 64 |
| 13EC | Cali | 2012 | Urine | ER | 44 | >4 | >4 | 16 | 16 | 8/4 | ≤0.25 | >4 | ≤32 |
| 14EC | Bogotá | 2012 | Urine | ER | 131 | >4 | >4 | 16 | 32 | 8/4 | ≤0.25 | >4 | ≤32 |
| 15EC | Bogotá | 2012 | Urine | ER | 44 | >4 | >4 | 16 | 8 | 8/4 | ≤0.25 | >4 | ≤32 |
| 16EC | Ibague | 2017 | Urine | ER | 351 | >4 | >4 | >32 | 64 | 32/4 | ≤0.25 | 0.5 | ≤32 |
| 17EC * | Cucuta | 2017 | Blood | ER | 131 | >4 | >4 | 16 | 16 | ≤2/4 | ≤0.25 | >4 | ≤32 |
| 18EC | Cali | 2016 | Urine | HOSP | 648 | >4 | >4 | >32 | 32 | >128/4 | >32 | >4 | ≤32 |
| 19EC * | Barranquilla | 2017 | Blood | HOSP | 405 | >4 | >4 | 16 | 32 | 8/4 | ≤0.25 | >4 | ≤32 |
| 20EC | Pereira | 2017 | Urine | HOSP | 131 | >4 | >4 | 32 | 32 | 8/4 | ≤0.25 | >4 | ≤32 |
| 21EC | Pasto | 2017 | Urine | ER | 126 | >4 | >4 | 16 | 8 | ≤2/4 | ≤0.25 | 4 | ≤32 |
| 22EC | Ibague | 2017 | Urine | ER | 131 | >4 | >4 | 32 | 32 | 32/4 | ≤0.25 | >4 | ≤32 |
| 23EC | Bucaramanga | 2017 | Urine | ER | 212 | >4 | >4 | >32 | >64 | ≤2/4 | 8 | ≤0.25 | 64 |
| 24EC | Cucuta | 2017 | Urine | ICU | 131 | >4 | >4 | >32 | 32 | >128/4 | ≤0.25 | >4 | ≤32 |
| 25EC* | Pasto | 2017 | Blood | HOSP | 131 | >4 | >4 | 16 | 8 | 4/4 | ≤0.25 | >4 | ≤32 |
| 26EC | Ibague | 2017 | Urine | HOSP | SLV-ST 602 | >4 | 4 | 8 | 8 | ≤2/4 | ≤0.25 | ≤0.25 | 64 |
CRO: ceftriaxone, CTX: cefotaxime, CAZ: ceftazidime, ER: emergency room, FEP: cefepime, HOSP: hospitalization, ICU: intensive care unit, MIC: minimum inhibitory concentration, MLST: Multilocus Sequence Typing, TZP: piperacillin/tazobactam, ETP: ertapenem, CIP: ciprofloxacin, and FOS: fosfomycin. (*) Isolates recovered from blood samples.
Figure 1.
Antibiotic resistance genes found in the CTX-M Escherichia coli strains. Black squares represent the presence of the respective antibiotic resistance gene, and grey represents its absence. (*) Isolates recovered from blood samples. MLST: Multilocus Sequence Typing.
2.2. Molecular Characterization of β-Lactamase Genes
As shown, the occurrence of ESBL genes remained as follows: blaCTX-M-15 in 22/26 (85%) isolates, blaCTX-M-55 in three (12%) isolates, blaCTX-M-3, and blaSHV-12 with one isolate each (4%) (Figure 1). The prevalence of other β-lactamase-encoding genes, such as blaTEM-1, blaOXA-1, and blaCMY-2 were found in 38%, 54%, and 11% of the evaluated isolates, respectively. Notably, the acquired resistance genes identified among ST131 isolates included blaOXA-1, aac-(6’)lb-cr, mdfA, tetA, and catB3. We detected two carbapenem-resistant isolates (18EC and 23EC isolates), one of them coproducing CTX-M-15, KPC-2, and NDM-1 and the other with point mutations, insertions, and deletions in ompC. Additionally, one isolate harbored the resistance gene mcr-1. None of these three isolates belonged to ST131.
2.3. Multilocus Sequence Typing (MLST) and Phylogenetic Analysis
The molecular typing information of the isolates is presented in Table 2. According to the MLST protocol applied, the 26 E. coli isolates were distributed into 11 different STs, including a new ST, a single locus variant of ST602. The most common ST was ST131 (10/26 isolates; 38%), followed by ST44 (4/26 isolates; 15%) and ST405, ST617, and ST648 (2/26 isolates; 8% each). Lastly, ST95, ST155, ST351, ST126, ST212, and SLV-ST602, with one isolate each. Accordingly, the most common phylogenetic group was B2 (13/26, 50%), followed by phylogroup A (6/26, 23%), B1 (3/26, 11%), and C and D, with two isolates each. The epidemic strain ST131 O25b:H4-B2-H30-Rx represented 80% (8/10) of all ST131 studied during the periods 2011 and 2012 and 2016 and 2017 in Colombia (Table 2).
Table 2.
Molecular data of CTX-M-harboring E. coli isolates.
| ID | MLST | CTX-M | Phylogroup | Serogroup | fimH | Subclone | Allele gyrA/parC | CIP |
|---|---|---|---|---|---|---|---|---|
| 4EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | 4 |
| 5EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 9EC | 131 | CTX-M-15 | B2 | O25b:H4 | 35 | No-H30 | gyrA1AB/parC1aAB | >4 |
| 11EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 14EC | 131 | CTX-M-15 | B2 | O25b:H4 | 35 | No-H30 | gyrA1AB/parC1aAB | >4 |
| 17EC * | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 20EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 22EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 24EC | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 25EC * | 131 | CTX-M-15 | B2 | O25b:H4 | 30 | H30-Rx | gyrA1AB/parC1aAB | >4 |
| 16EC | 351 | CTX-M-55 | B1 | O18a:H7 | 31 | - | 0.5 | |
| 2EC | 44 | CTX-M-15 | A | O89:H4 | 54 | gyrA1AB | >4 | |
| 10EC | 44 | CTX-M-15 | A | O89:H4 | 54 | gyrA1AB | >4 | |
| 13EC | 44 | CTX-M-15 | A | O89:H4 | 54 | gyrA1AB | >4 | |
| 15EC | 44 | CTX-M-15 | A | O89:H4 | 54 | gyrA1AB | >4 | |
| 3EC | 95 | CTX-M-15 | B2 | O50:H4 | 27 | gyrA1AB | >4 | |
| 21EC a | 126 | CTX-M-15 | B2 | H5 | 26 | - | 4 | |
| 6EC | 155 | CTX-M-3 | B1 | O162:H19 | 32 | gyrA1AB | >4 | |
| 23EC | 212 | CTX-M-55 | B2 | O18ac:H49 | 38 | - | ≤0.25 | |
| 12EC | 405 | CTX-M-15 | D | O102:H6 | 27 | gyrA1AB | >4 | |
| 19EC * | 405 | CTX-M-15 | D | O102:H6 | 27 | gyrA1AB | >4 | |
| 7EC | 617 | CTX-M-15 | A | O89:H10 | - | gyrA1AB | >4 | |
| 8EC | 617 | CTX-M-15 | A | O89:H10 | - | gyrA1AB | >4 | |
| 1EC | 648 | CTX-M-15 | F | O1:H6 | 27 | gyrA1AB | >4 | |
| 18EC | 648 | CTX-M-15 | F | H6 | 27 | gyrA1AB | >4 | |
| 26EC | SLV-ST 602 | CTX-M-15 | B1 | H21 | 86 | - | ≤0.25 |
CIP: ciprofloxacin MIC, MLST: Multilocus Sequence Typing. (*) Blood stream isolates. a This isolate only harbors the known Asp87Tyr substitution in GyrA.
2.4. O25b-ST131 E. coli Clone Detection
In regards of the serotypes, all of the ST131 were 025:H4; ST44 were serotype 089:H4, and ST617 were serotype 089:H10. Of the two ST648, one had serotype O1:H6, and the other had an unknown O-group but belonged to H6.
2.5. Genes Related with Ciprofloxacin Resistance
A resistance to ciprofloxacin (CIP), found in 23/26 (88%) isolates, was attributed to mutations in the gyrA and parC (Table 2). Mutations at the quinolone resistance-determining regions (QRDR) of gyrA and parC (yielding to substitutions at Ser83 and Asp87 and Ser80 and Glu84 of the DNA gyrase and topoisomerase IV, respectively) were the most frequent. Of note, one ciprofloxacin-resistant isolate carried only the Asp87Tyr substitution in GyrA, lacking the Ser83Leu change that typically co-occurs. Likewise, only one isolate belonging to ST131 presented the Glu84 substitution. Lastly, a low prevalence of qnr genes (3/26, 11.5%) was found among these isolates.
2.6. Virulence Factor-Encoding Genes and Incompatibility Groups
Regarding the virulome, the most frequent virulence factor (VF)-encoding genes detected were gad (50%), hlyD (92%), iutA (88%), K1 and K2 (96% each), and traT (88%) (Figure S1). Other VF-encoding genes, such as iha, sat, and pap genes, were detected frequently among ST131 E. coli isolates, whereas the pap(ACGH) genes were associated with the H30-Rx subclone. Lastly, the most prevalent plasmid types found were of incompatibility groups IncFIA (20/26, 77%), IncFIB (24/26, 88%), and IncFII (23/26, 92%).
3. Discussion
ExPEC is one of the most important causes of BSIs and UTIs. Particular attention has been on the E. coli ST131 clone, which is considered a high-risk clone due to its epidemic potential, in both community and hospital settings, and its virulence and high level of antimicrobial resistance [10]. As expected, ST131 accounted for the majority (38%) of the STs detected in this study. Other ExPEC epidemic clones that have been detected among ESBL producers include ST405, ST38, ST648, ST410, and ST1193 [8], among which, ST405 and ST648 were also detected among the strains included.
According to the susceptibility testing, carbapenems were found to be the most active against the isolates included in this study, followed by fosfomycin. This result confirms that carbapenem resistance is still low among E. coli isolates in Colombia, given that only two strains, which were isolated in 2016, were resistant to these β-lactams. In accordance to the increasing reports of pathogenic isolates co-expressing two or more carbapenemases [11], one of the carbapenem-resistant ExPEC harbored both blaKPC-2 and blaNDM-1 genes. This gene combination has been reported worldwide and possesses a great challenge for therapeutic options [12].
Currently, the ESBL most widely distributed globally is CTX-M-15 [1]. In the ExPEC isolates included in our study, the acquisition of blaCTX-M-15 and blaOXA-1 were the most common β-lactam resistance-conferring mechanisms. We also noted that some of the isolates harboring the intact and wild type versions of blaCTX-M-15 were susceptible to ceftazidime, an uncommon phenotype that has been reported previously [13]. Based on what has been described for other antimicrobial resistance determinants [14], we asserted that the blaCTX_M copy number and changes in the upstream genetic environment (e.g., promoter region) affect the expression levels of CTX-M. This, combined with changes in the outer membrane, could give rise to different phenotypes [15]. Additionally, 65% of E. coli strains carried the aac(6′)Ib-cr gene, which encodes for an aminoglycoside-modifying enzyme that provides resistance to amikacin and other aminoglycosides but is also known to confer low-level resistance to ciprofloxacin [16].
In general, we did not observe differences in the resistance to β-lactams, ciprofloxacin, and fosfomycin in ST131 compared to non-ST131 isolates. However, we found that the non-ST131 isolates carried more resistance genes to aminoglycosides, macrolides, trimethoprim, and sulfonamides (Table 1). An increased resistance to antibiotics in non-ST131 was already previously reported by Hojabri et al. [17].
E. coli ST131 strains are mostly of serotype O25:H4, with a specific O25b type. However, ST131 E. coli isolates of serotype O16:H5 have recently been identified in Europe, Japan, Australia, and Pakistan [18]. Additionally, the H30 subclone within E. coli ST131 has emerged as the dominant E. coli lineage among clinical isolates [19]. In our study, all of the ST131 clones had the fimH adhesin gene, and most of them were H30-Rx (8/10, 80%), as reported in previous studies [20]. The other two ST131 isolates belonged to the H35 subclone. All ST131 isolates carried both mutations described in gyrA and gyrB. Our results suggest that CIP resistance in E. coli ST131 in Colombia is associated with the lineages H30 and H35, which harbor a distinct gyrA and parC allele combination (gyrA1AB/parC1aAB). However, resistance to CIP was found in both ST131 and non-ST131 isolates (Table 2).
On the other hand, E. coli ST131 has an expansive range of VFs, which permits its survival in extraintestinal niches, including certain adhesins (afa/dra and iha); siderophore receptors (iutA and fyuA); toxins (sat and hylA); capsule variants (kpsMT II and K2); protection factors (ompT, traT, and iss); and others (usp and malX) [17]. In our study, the VFs that were almost exclusively associated with the E. coli O25:H4-B2-ST131 clone were iha and pap(ACGH), which code for adherence factors, senB, which promotes toxin production, and sat, which encodes for a protease [19]. In addition, 66% of E. coli isolates, including all the ST131, have the iss gene, a virulence factor that promotes immune evasion by increasing serum survival [21]. Banerjee et al. [19] identified three virulence genes (iha, sat, and iutA), which are more prevalent among H30 than in non-H30 ST131 isolates [19]. We found the iha and sat genes in both H30 and H35 ST131 E. coli subclones and differences in the pap(ACGH) genes, which were exclusively associated with the H30-Rx subclone. Interestingly, it has been reported that the presence of this VF is associated with the production of pyelonephritis and sepsis, because these isolates are easier to colonize and invade the upper urinary tract [22]. Of the three blood isolates, two belonged to the ST131 subclone H30 and had the same virulence genes as the urinary isolates of subclone H30 (Supplemental Figure S1).
CTX-M-15 has been frequently reported on plasmids of the IncF incompatibility group, which have the ability to acquire resistance genes and disseminate rapidly [23]. These plasmids might carry important VFs implicated in the spread of epidemic clone O25b:H4-ST131 [24].
Our study faced some limitations, such as the fact that the number of sequenced E. coli isolates was too small for a significant evaluation and that most isolates were from urine and community onset infections.
4. Materials and Methods
4.1. Bacterial Isolates
Two sets of isolates were included in this study. The first set was composed by 15 E. coli CTX-M group 1-positive isolates collected between 2011 and 2012 from community-acquired UTIs, as defined by the Center for Disease Control and Prevention (CDC). The second set included 8 oxyimino-cephalosporins-resistant E. coli isolates recovered from UTIs and 3 E. coli isolates recovered from bloodstream infections and collected from 2016 to 2017. Isolates from the second set were randomly selected and could be from community and hospital-acquired infections.
4.2. Susceptibility Testing
Minimum inhibitory concentrations (MICs) were determined by the broth microdilution method using customized Sensititer plates (Trek Diagnostic Systems, East Grinstead, West Sussex, UK) following the manufacturer’s recommendations. MIC values of ciprofloxacin (CIP) were determined with E-test™ (BioMerieux, Marcy L’Etoile, France). E. coli ATCC 25922 was used as the quality control strain, as per Clinical and Laboratory Standards Institute (CLSI) recommendations.
4.3. Whole-Genome Sequencing (WGS) and Analysis
Genomic DNA was extracted using the DNA Blood & Tissue Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany), followed by library preparation using the Nextera XT library (Illumina, San Diego, CA, USA), and sequenced on an Illumina MiSeq platform using the MiSeq v3 reagent kit (Illumina, USA). Phylogenetic groups (A, B1, B2, C, D, E, and F) were determined following the protocol published by Clermont et al. [25]. Sequence type (ST; according to the Achtman scheme [26]), O:H serotypes, serogroup, plasmid replicon types, virulence genes, clonotype determined by fumC and fimH (CH), and resistome were determined using the tools freely available at the Center for Genomic Epidemiology (http://www.genomicepidemiology.org/). The H30-Rx subclone was identified by detecting a specific single-nucleotide polymorphism (SNP) (G723A) from the allantoin permease (ybbW) gene [19]. The O25b subtype was determined by targeting 347 bp of the para(p)-aminobenzoate synthase (pabB) gene fragment. The E. coli K-12 strain was used as the reference genome for the WGS analysis (GenBank accession number NC000913).
Sequence data were deposited in the National Center for Biotechnology Information (NCBI) database (Bioproject: PRJNA608731).
4.4. Ethical Approval
The protocol was approved by the ethics committee of Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) and the participating healthcare institutions (6 July 2011). Collection of the microbiological isolates was part of the regular diagnostic process, as established by each of the participating healthcare institutions. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
5. Conclusions
Our study revealed the circulation of E. coli strains belonging to the C clade, O25b:H4-B2 ST131 clone, and H30-Rx subclone from outpatients and inpatients with UTIs and BSIs in Colombia similar to what has been reported on other continents, describing the spread of these endemic clones to this geographical region. To our knowledge, this is the first study that described the presence of the H30-Rx subclone in ST131 ExPEC in Colombia.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/12/899/s1: Figure S1: Virulence genotypes and incompatibility plasmids of the studied isolates.
Author Contributions
Conceptualization, M.F.M., E.D.L.C., A.C., C.J.P., and M.V.V.; methodology, E.D.L.C., N.C., and M.F.M.; validation, M.F.M., A.C., T.M.A., J.C.G.-B., C.J.P., and M.V.V.; formal analysis, E.D.L.C. and M.F.M; investigation, E.D.L.C., M.F.M., N.C., A.C., and T.M.A.; data curation, E.D.L.C., N.C., and M.F.M.; writing—original draft preparation, E.D.L.C.; writing—review and editing, M.F.M., E.D.L.C., T.M.A., J.C.G.-B., and M.V.V.; supervision, E.D.L.C., M.F.M., A.C., J.C.G.-B., and M.V.V.; project administration, E.D.L.C.; and funding acquisition, E.D.L.C. and M.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad El Bosque, grant number PCI-2017-9453.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marchaim D., Gottesman T., Schwartz O., Korem M., Maor Y., Rahav G., Karplus R., Lazarovitch T., Braun E., Sprecher H., et al. National Multicenter Study of Predictors and Outcomes of Bacteremia upon Hospital Admission Caused by Enterobacteriaceae Producing Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2010;54:5099–5104. doi: 10.1128/AAC.00565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson D.L., Bonomo R.A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo T.A., Johnson J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/S1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 4.Naas T., Oueslati S., Bonnin R.A., Dabos M.L., Zavala A., Dortet L., Iorga B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzym. Inhib. Med. Chem. 2017;32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Antimicrobial Resistance: Global Report on Surveillance. [(accessed on 10 February 2020)]; Available online: http://www.ncbi.nlm.nih.gov/pubmed/22247201%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2536104&tool=pmcentrez&rendertype=abstract.
- 6.Stoesser N., Sheppard A.E., Pankhurst L., De Maio N., Moore C.E., Sebra R., Turner P., Anson L.W., Kasarskis A., Batty E.M., et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. mBio. 2016;7:e02162-15. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price L.B., Johnson J.R., Aziz M. The Epidemic of Extended-Spectrum-β-Lactamase-Producing Subclone, H 30-Rx. mBio. 2013;4:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coque T.M., Baquero F., Cantón R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance. 2008;13:1–11. [PubMed] [Google Scholar]
- 9.Ruiz S.J., Montealegre M.C., Ruiz-Garbajosa P., Correa A., Briceño D.F., Martinez E., Rosso F., Muñoz M., Quinn J.P., Cantón R., et al. First Characterization of CTX-M-15-Producing Escherichia coli ST131 and ST405 Clones Causing Community-Onset Infections in South America. J. Clin. Microbiol. 2011;49:1993–1996. doi: 10.1128/JCM.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolas-Chanoine M.H., Bertrand X., Madec J.Y. Escherichia coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meletis G., Chatzidimitriou D., Malisiovas N. Double- and multi-carbapenemase-producers: The excessively armored bacilli of the current decade. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1487–1493. doi: 10.1007/s10096-015-2379-9. [DOI] [PubMed] [Google Scholar]
- 12.Miyakis S., Pefanis A., Tsakris A. The Challenges of Antimicrobial Drug Resistance in Greece. Clin. Infect. Dis. 2011;53:177–184. doi: 10.1093/cid/cir323. [DOI] [PubMed] [Google Scholar]
- 13.Titelman E., Iversen A., Kahlmeter G., Giske C.G. Antimicrobial susceptibility to parenteral and oral agents in a largely polyclonal collection of CTX-M-14 and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. APMIS. 2011;119:853–863. doi: 10.1111/j.1600-0463.2011.02766.x. [DOI] [PubMed] [Google Scholar]
- 14.Kitchel B., Rasheed J.K., Endimiani A., Hujer A.M., Anderson K.F., Bonomo R.A., Patel J.B. Genetic Factors Associated with Elevated Carbapenem Resistance in KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2010;54:4201–4207. doi: 10.1128/AAC.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endimiani A., Perez F., Bajaksouzian S., Windau A.R., Good C.E., Choudhary Y., Hujer A.M., Bethel C.R., Bonomo R.A., Jacobs M.R. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J. Clin. Microbiol. 2010;48:4417–4425. doi: 10.1128/JCM.02458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez M.S., Tolmasky M.E. Aminoglycoside Modifying Enzymes. Drug Resist. Updates. 2010;16:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hojabri Z., Darabi N., Arab M., Saffari F., Pajand O. Clonal diversity, virulence genes content and subclone status of Escherichia coli sequence type 131: Comparative analysis of E. coli ST131 and non-ST131 isolates from Iran. BMC Microbiol. 2019;19:1–10. doi: 10.1186/s12866-019-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanc V., Leflon-Guibout V., Blanco J., Haenni M., Madec J.-Y., Rafignon G., Bruno P., Mora A., Lopez C., Dahbi G., et al. Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising O25b:H4 and O16:H5 ST131 strains. J. Antimicrob. Chemother. 2014;69:1231–1237. doi: 10.1093/jac/dkt519. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee R., Robicsek A., Kuskowski M.A., Porter S., Johnston B.D., Sokurenko E., Tchesnokova V., Price L.B., Johnson J.R. Molecular Epidemiology of Escherichia coli Sequence Type 131 and Its H30 and H30-Rx Subclones among Extended-Spectrum-β-Lactamase-Positive and -Negative E. coli Clinical Isolates from the Chicago Region, 2007 to 2010. Antimicrob. Agents Chemother. 2013;57:6385–6388. doi: 10.1128/AAC.01604-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitout J.D.D., DeVinney R. Escherichia coli ST131: A multidrug-resistant clone primed for global domination. F1000Research. 2017;6:195. doi: 10.12688/f1000research.10609.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson T.J., Wannemuehler Y.M., Nolan L.K. Evolution of the iss Gene in Escherichia coli. Appl. Environ. Microbiol. 2008;74:2360–2369. doi: 10.1128/AEM.02634-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J.R., Porter S.B., Zhanel G., Kuskowski M.A., Denamur E. Virulence of Escherichia coli Clinical Isolates in a Murine Sepsis Model in Relation to Sequence Type ST131 Status, Fluoroquinolone Resistance, and Virulence Genotype. Infect. Immun. 2012;80:1554–1562. doi: 10.1128/IAI.06388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carattoli A. Resistance Plasmid Families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009;53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco M., Alonso M.P., Nicolas-Chanoine M.-H., Dahbi G., Mora A., Blanco J.E., López C., Cortés P., Llagostera M., Leflon-Guibout V., et al. Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases in Lugo (Spain): Dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2009;63:1135–1141. doi: 10.1093/jac/dkp122. [DOI] [PubMed] [Google Scholar]
- 25.Clermont O., Bonacorsi S., Bingen E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000;66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth T., Falush D., Lan R., Colles F., Mensa P., Wieler L.H., Karch H., Reeves P.R., Maiden M.C.J., Ochman H., et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.