Abstract
Native egg albumin (NEA) was isolated from hen eggs and hydrolyzed by pepsin to produce hydrolyzed egg albumin (HEA). HEA was chemically characterized and screened for its antibacterial activity against 10 pathogenic bacteria (6 Gram (+) and 4 Gram (−)). The SDS-PAGE pattern of NEA showed molecular weights of hen egg albumin subunits ranging from 30 to 180 kDa. The highest intensive bands appeared at a molecular mass of about 50 and 97 kDa. Ultra-performance liquid chromatography (UPLC) of the peptic HEA revealed 44 peptides, 17 of them were dipeptides, and the other 27 fractions corresponded to bigger peptides (3–9 amino acids). The dipeptides and big peptides represented 26% and 74% of the total hydrolysate, respectively. The MIC of HEA was about 100 μg/L for Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Salmonella typhimurium, Streptococcus pyogenes, and Klebsiella oxytoca and 150 μg/L for Pseudomonas aeruginosa, Bacillus subtilis, and Listeria ivanovii and 200 μg/L for Escherichia coli. L. monocytogenes was the most sensitive organism to HEA. Mixtures of HEA with antibiotics showed more significant antibacterial activity than individually using them. Transmission electron microscopy (TEM) revealed various signs of cellular deformation in the protein-treated bacteria. HEA may electrostatically and hydrophobically interact with the cell wall and cell membrane of the susceptible bacteria, engendering large pores and pore channels leading to cell wall and cell membrane disintegration. Higher cell permeability may, thus, occur, leading to cell emptiness, lysis, and finally death. Alternatively, no toxicity signs appeared when HEA was administrated to Wistar Albino rats as one single dose (2000, 5000 mg/kg body weight) or repeated daily dose (500 and 2500 mg/kg body weight/day) for 28 days to disclose the possible toxicity hazards. HEA did not produce any death.
Keywords: egg albumin, pepsin, antibacterial activity, pathogenic bacteria, hydrolysis, acute toxicity
1. Introduction
Natural antimicrobials from food sources are welcomed as food preservatives as they are not hazardous and have no side effects like those caused by synthetic ones [1,2,3,4,5,6]. These natural antimicrobials are quite promising for food bio-preservation to reduce the need for antibiotics, control of microbial spoilage process, and could kill the resistant variants of bacteria that can survive in foods and limit the occurrence of new food-borne disease outbreaks caused by pathogenic bacteria [7,8,9,10,11,12]. Natural extracts are compounds with a wide structural diversity, representing an important source of new chemical compounds with a possibly significant antibacterial action against food-borne pathogens with powerful action against multidrug-resistant bacteria [13,14,15,16,17,18,19,20,21]. The possible negative impact of such chemicals on the environment and human health may preclude synthetic agents’ use [19]. Therefore, novel antimicrobial agents from natural sources are highly required [4,15,17,20]. Many strategies have been suggested to improve proteins’ antimicrobial activities, including chemical modification, e.g., such as esterification [22,23,24,25,26]. Food proteins have long been recognized as important source of bioactive peptides. In particular, peptides derived from protein-based foods have been shown to possess significant antimicrobial capacities. These peptides from either plant or animal sources can be generated through enzymatic hydrolysis, acid or alkali treatment, or fermentation [26]. Bioactive peptides can be obtained from different protein sources, including milk [27] and legume proteins [28,29]. Peptides from various protein resources have been reported to have multiple functions, including antioxidative ability, angiotensin I converting enzyme (ACE) inhibitor properties [30], hepatoprotective activity [31], and antibacterial activity [6,19,20,32,33,34,35,36].
Eggs contain various proteins exhibiting antimicrobial activities, including lysozyme and ovotransferrin, which are commonly used as natural food antimicrobial agents against a wide spectrum of bacteria [37]. Natural antimicrobial peptides exist in different compartments (egg shell, albumen, and vitelline membranes) of eggs. Most of these peptides are cationic or amphipathic, but there are also hydrophobic α-helical peptides that possess antimicrobial activity [38]. Although a wide variety of peptides with different chemical structures and peptide conformations have been reported to exhibit antimicrobial activity, these antimicrobial activities’ mechanism is less well understood in most cases. In fact, several observations suggest that natural peptides can alter cytoplasmic membrane permeability, inhibit cell-wall synthesis, inhibit nucleic-acid synthesis, inhibit protein synthesis and in turn, cause bacterial cell death [39]. Therefore, antimicrobial peptides (AMPs) constitute a promising alternative as therapeutic agents against various pathogenic microbes [40]. More than 60 peptide drugs have reached the market for patients’ benefit, and approximately 140 peptide therapeutics are currently being evaluated in clinical trials [41]. In a previous study [37], the methylated egg white protein inhibited many bacterial pathogens, simulating antibiotics’ inhibitory activity. In an endeavor to develop such work, the present study aimed to determine the antibacterial activity and mode of action of protein hydrolysates produced from egg albumin by enzymatic digestion using pepsin. The toxicity of both native egg albumin (NEA) and hydrolyzed egg albumin (HEA) was studied using Wistar albino rats.
2. Results
2.1. Chemical Characterization of HEA
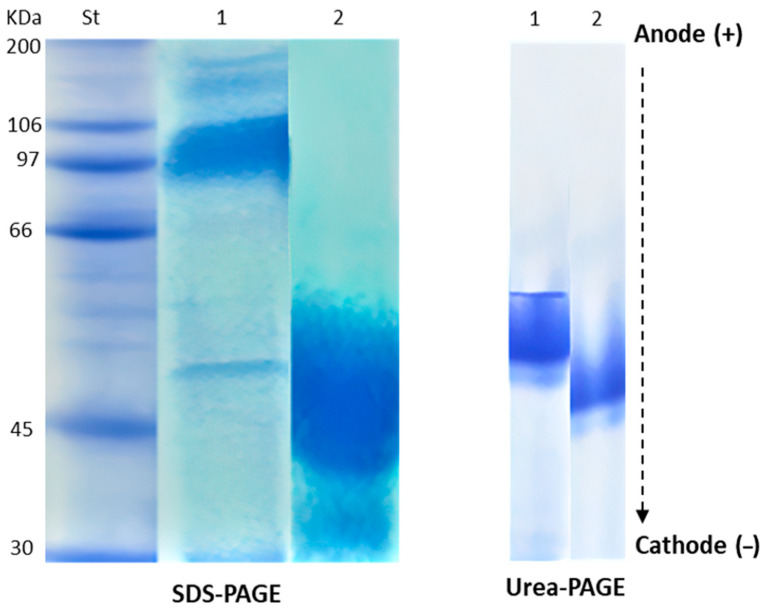
The SDS-PAGE patterns of HEA are shown in Figure 1 and Table 1. The numbers 1–5 refer to the protein fractions of our tested protein (egg albumin) from up to down successively. In Figure 1, the MW200, 106, 97, 66, 45, 30 refer to molecular weight standards in the first lane (St) in Figure 1. In Table 1 the MW 180, 144, 97, 50, 30, refer to the subunits of egg albumin as also shown in the second lane of Figure 1 (1). These molecular weights were deduced using the known standard MW in lane (1) It is evident that the molecular weight of NEA subunits ranged between 30 and 180 kDa. The more intensive bands were presented at molecular masses of about 50 and 97 kDa. The hydrolysate’s electrophoretic pattern indicated that the peptides formed due to hydrolysis were less than 50 kDa. Urea-PAGE patterns of HEA compared to NEA are presented in Figure 1. HEA (lane 2) migrated faster than NEA (lane 1) from anode to cathode due to increased positive charge on the hydrolyzed proteins. Native-PAGE and SDS-PAGE of native and modified proteins represented exchanges in molecular masses between native and modified proteins.
Figure 1.
Electrophoretic patterns of native (1) and hydrolyzed (2) egg albumin (NEA and HEA, respectively), either through SDS-PAGE or UREA_PAGE. St is the standard molecular weight proteins. The arrow refers to the direction of protein migration from anode to cathode. The bands in lane (1) representing NEA correspond to 180, 144, 97, 50, and 30 kDa.
Table 1.
Molecular masses and band intensity of hydrolyzed egg albumin (HEA) compared to native form (NEA).
| Band Number | NEA | HEA | ||
|---|---|---|---|---|
| MW (kDa) | Band Intensity | MW (kDa) | Band Intensity | |
| 1 | 180 | 1.2 | 51 | 10 |
| 2 | 144 | 1.7 | - | - |
| 3 | 97 | 6.9 | - | - |
| 4 | 50 | 1.9 | - | - |
| 5 | 30 | 2.4 | - | - |
MW—molecular weight. The numbers 1–5 refer to protein subunits of NEA as shown in the second column. HEA has only one band.
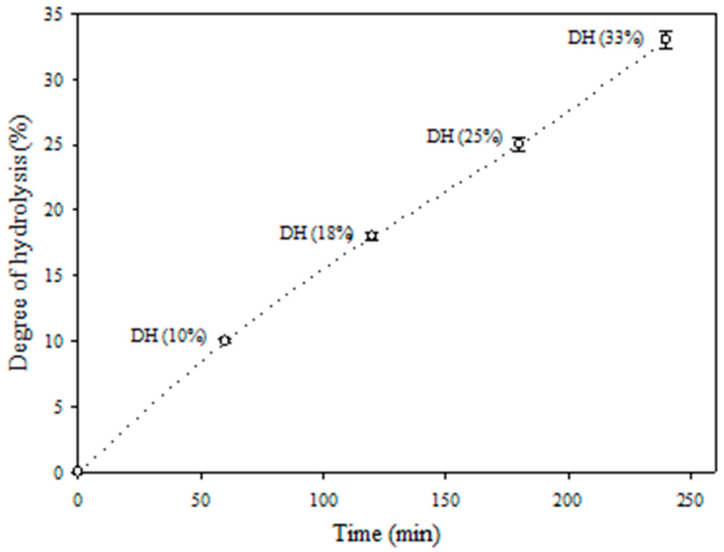
Since using chemical agents in the food and pharmaceutical industries can leave residual organic solvents or toxic chemicals in the products, enzymatic hydrolysis is widely preferred. The hydrolysis of egg albumin with pepsin was monitored for 240 min as recorded in Figure 2. The hydrolysates obtained after 60, 120, 180, and 240 min, exhibited about10%, 18%, 25%, and 3% degree of hydrolysis, respectively.
Figure 2.
Changes in degree of hydrolysis during enzymatic hydrolysis of egg albumin by pepsin for 240 min.
2.2. Ultra-Performance Liquid Chromatography (UPLC) of the Peptic Hydrolysate of Egg Albumin
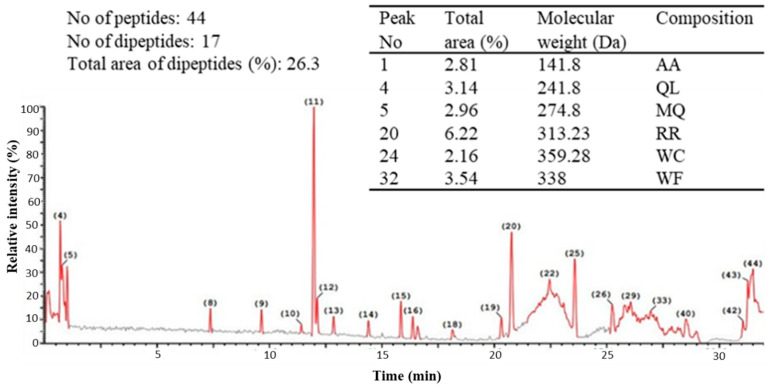
Peptide profile of the peptic hydrolysates of egg albumin was obtained from Ultra performance liquid chromatography (UPLC) analysis (Acquity H Class, Waters) combined with quadrupole-time-of-flight mass spectrometry (Synap G2, Waters), revealed the presence of 44 peptides (Figure 3). The molecular weight of 17 fractions was in the range of dipeptides, representing about 26% of the total peptide fractions based on the corresponding fractions’ total areas. The other 27 peptides’ molecular weights corresponded to peptides ranging from tri-peptides to octa- or nona-peptides, forming about 74% of the total peptides, as concluded from the total areas of the corresponding peaks. The higher relative quantity of the bigger sized peptides (3–9 amino acids) may indicate their higher participation in the hydrolysates’ biological activity. The selected peptides summarized in Table included in Figure 3, were chosen as the major constituting peptides that are expected to produce the highest biological impact.
Figure 3.
Chromatogram of peptides formation from egg albumin hydrolyzed with pepsin and the mass spectrum of major peaks dipeptides.
2.3. Antibacterial Activity of HEA and NEA against Gram (+) and Gram (−) Bacteria
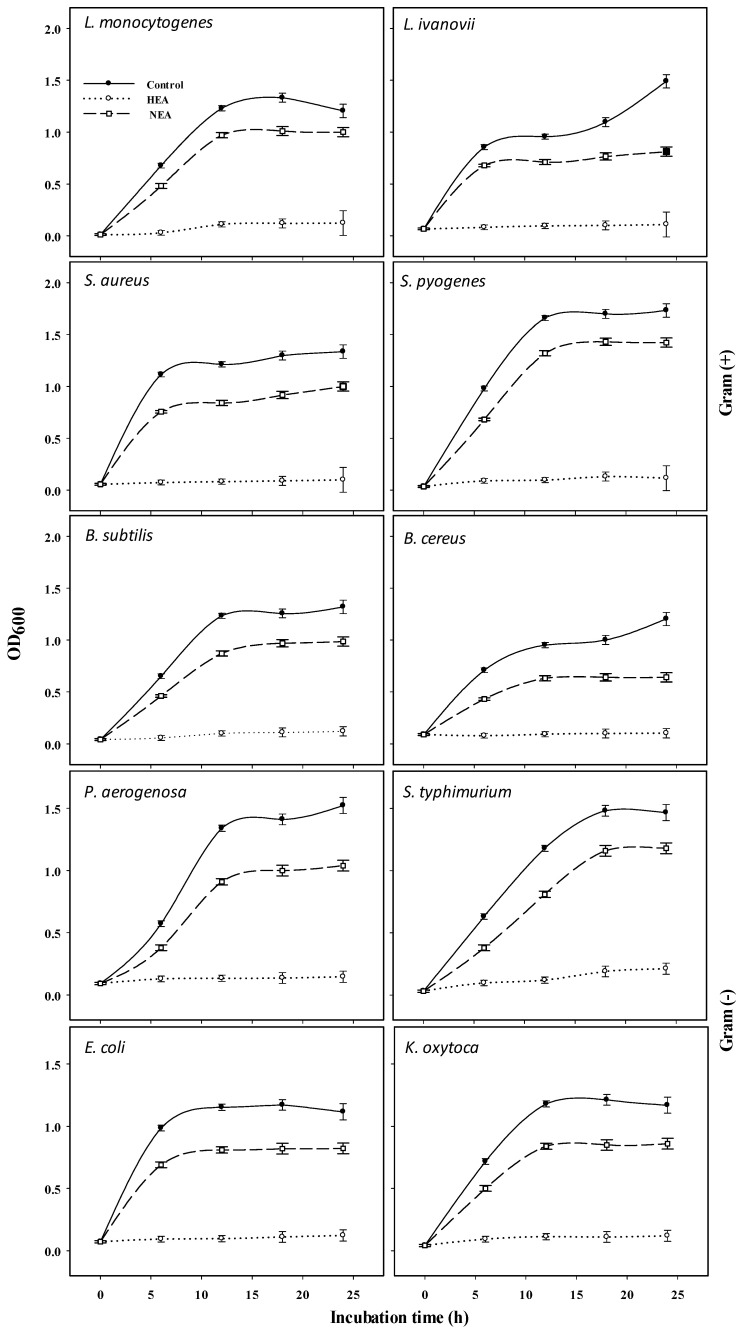
HEA was prepared via enzymatic hydrolysis of egg albumin using pepsin at optimal conditions (pH 2 at 37 °C) to potentially produce cationic antimicrobial peptides with antibacterial effect. Using different HEA and NEA concentrations, Table 2 revealed that 250 μg/mL of HEA was the most inhibitory concentration against all the tested bacteria. Disc diffusion assay indicated concentration-dependent inhibition zone diameters for HEA. The MIC of HEA was 100 μg/mL against L. monocytogenes, B. cereus, S. aureus, S. typhimurium, S. pyogenes, and K. oxytoca, 150 μg/mL against P. aeruginosa, B. subtilis, and L. ivanovii, and 200 μg/mL against E. coli. The data in Figure 4 represent the 24 h growth curves of 10 pathogenic bacteria subjected to one value of MIC of HEA showing general growth inhibition. Among the Gram (+) bacteria, B. subtilis and B. cereus were the most inhibited organisms by HEA. While in the case of Gram (−) bacteria, K. oxytoca showed nearly complete growth inhibition during the 24 h period of incubation. However, there is a moderate effect of NEA on the 10 tested bacteria compared with the control. Therefore, HEA was the most inhibitor compared to NEA and control bacteria (Table 2 and Figure 4).
Table 2.
Antibacterial activity of pepsin hydrolyzed egg albumin (HEA) using disc diffusion assay.
| Inhibition Zone Diameter (IZD) (mm/HEA µg mL−1) | IZD of NEA (mm/NEA µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | 200 | 250 | 250 | |
| L. monocytogens | 0 | 0 | 34 ± 1.16 a | 36 ± 2.31 a | 39 ± 1.73 a | 42 ± 2.31 a | 7 ± 0.58 c |
| K. oxytoca | 0 | 0 | 9 ± 0.58 d | 28 ± 1.73 c | 34 ± 1.73 b | 38 ± 1.73 abc | 5 ± 0.44 d |
| S. pyogenes | 0 | 0 | 24 ± 1.73 b | 32 ± 1.73 b | 35 ± 2.89 ab | 39 ± 2.89 ab | 3 ± 0.38 e |
| L. ivanovii | 0 | 0 | 0 f | 17 ± 0.58 d | 33 ± 1.73 b | 34 ± 1.16 bc | 10 ± 0.58 b |
| S. aureus | 0 | 0 | 20 ± 1.16 c | 28 ± 1.16 c | 30 ± 1.16 bc | 33 ± 1.73 c | 11 ± 1.05 a |
| B. subtilis | 0 | 0 | 0 f | 9 ± 0.58 e | 12 ± 0.58 e | 14 ± 0.58 e | 12 ± 1.28 a |
| P. aerugenosa | 0 | 0 | 0 f | 9 ± 0.58 e | 28 ± 1.73 c | 33 ± 1.73 c | 3 ± 0.11 e |
| S. typhimurium | 0 | 0 | 7 ± 0.57 de | 13 ± 1.16 e | 18 ± 1.16 d | 21 ± 1.15 d | 1 ± 0.08 ef |
| E. coli | 0 | 0 | 0 f | 0 f | 10 ± 0.58 e | 13 ± 1.16 e | 2 ± 0.09 e |
| B. cereus | 0 | 0 | 6 ± 0.58 e | 11 ± 1.16 e | 12 ± 1.16 e | 14 ± 1.16 e | 2 ± 0.22 e |
Every value is the average of three replicates ± SE. Different letters (a–f) in the same column refer to significantly different values (p < 0.05).
Figure 4.
Twenty-four hour growth curves of six pathogenic Gram (+) and four pathogenic Gram (−) in the presence of the value minimum inhibitory concentration (MIC) of HEA.
2.4. Antibacterial Activity of Antibiotics and HEA-Antibiotic Combinations by Disc Diffusion Assay
An antibiotic sensitivity test was carried out for all the tested pathogenic bacteria using five antibiotics listed in Table 3 and Figure 4. There was variability in the sensitivity of the tested pathogenic bacteria. The results given in Table 3 and Table 4 showed the MICs of antibiotics, HEA, and combinations of them. MICs values (μg/mL) were 7.5, 6, 4, 4, 2, 2, 1, 1, 1, 0.016 for the antibiotics chloramphenicol, gentamycin, ciprofloxacin, vancomycin, chloramphenicol, ciprofloxacin, vancomycin, ciprofloxacin, gentamycin, penicillin as determined against L. ivanovii, K. oxytoca, S. typhimurium, B. cereus, B. subtilis, L. monocytogenes, S. aureus, P. aeruginosa, E. coli, S. pyogenes, respectively. MICs of HEA were in the range 100–200 μg/mL depending on the bacteria. Mixtures of modified proteins at equal MIC of HEA and antibiotics showed greater antibacterial activity than any single treatment of either of them, referring to synergistic action. This effect was most evidently seen in the case of L. monocytogenes with the biggest inhibition zone (36 ± 2.31 mm) followed by S. aureus and S. pyogenes with the second biggest inhibition zones 34 ± 1.16 and 34 ± 0.58 μg/mL, respectively.
Table 3.
Antibacterial activity of mixed combinations of hydrolyzed egg albumin (HEA) and antibiotic.
| Microorganisms | Antibiotic | Antibiotic MIC (µg/mL) | Inhibition Zone (mm) | ||
|---|---|---|---|---|---|
| Antibiotic | HEA | Antibiotic: HEA | |||
| L. monocytogenes | Ciprofloxacin | 2 | 18 ± 1.73 c | 34 ± 1.73 a | 36 ± 2.31 a |
| K. oxytoca | Gentamycin | 6 | 14 ± 1.16 b | 9 ± 0.58 c | 16 ± 0.58 a |
| S. pyogenes | Penicillin G | 0.016 | 29 ± 1.16 b | 24 ± 1.16 c | 34 ± 1.16 a |
| L. ivanovii | Chloramphenicol | 7.5 | 19 ± 1.73 b | 17 ± 1.16 b | 29 ± 1.16 a |
| S. aureus | Vancomycin | 1 | 14 ± 0.58 c | 20 ± 1.16 b | 34 ± 0.58 a |
| B. subtilis | Chloramphenicol | 2 | 18 ± 1.16 b | 9 ± 1.16 c | 20 ± 1.16 a |
| P. aeruginosa | Ciprofloxacin | 1 | 28 ± 1.73 b | 9 ± 0.58 c | 30 ± 1.16 a |
| S. typhimurium | Ciprofloxacin | 4 | 18 ± 1.16 a | 7 ± 0.58 b | 19 ± 1.16 a |
| E. coli | Gentamycin | 1 | 19 ± 1.16 a | 10 ± 1.16 c | 15 ± 1.16 b |
| B. cereus | Vancomycin | 4 | 5 ± 0.29 b | 6 ± 0.29 b | 15 ± 0.58 a |
Every value is the average of three replicates ± SE. Different letters (a–c) in the same rowrefer to significantly different values (p < 0.05).
Table 4.
Body weight gain (g) of male and female albino rats treated orally with daily hydrolyzed egg albumin (HEA) for 28 days.
| Treatment | Control | Tested Proteins Doses (mg/kg b. wt *./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| Initial body wt (g) | 165 ± 2.9 | 163 ± 2.9 | 160 ± 2.9 |
| Final body wt (g) | 245 ± 5.8 | 249 ± 3.5 | 250 ± 2.9 |
| Body wt gain (g) | 80 ± 2.9 | 86 ± 2.3 | 90 ± 5.8 |
| Female | |||
| Initial body wt (g) | 135 ± 2.9 | 140 ± 5.1 | 135 ± 2.9 |
| Final body wt (g) | 187 ± 5.8 | 196 ± 2.3 | 196 ± 5.8 |
| Body wt gain (g) | 52 ± 1.7 b | 56 ± 2.3 ab | 61 ± 2.9 a |
* b. wt; body weight. Every value is the average of 10 replicates ± SE. Different letters (a,b) in the same row refer to significantly different values (p < 0.05).
2.5. TEM Analysis
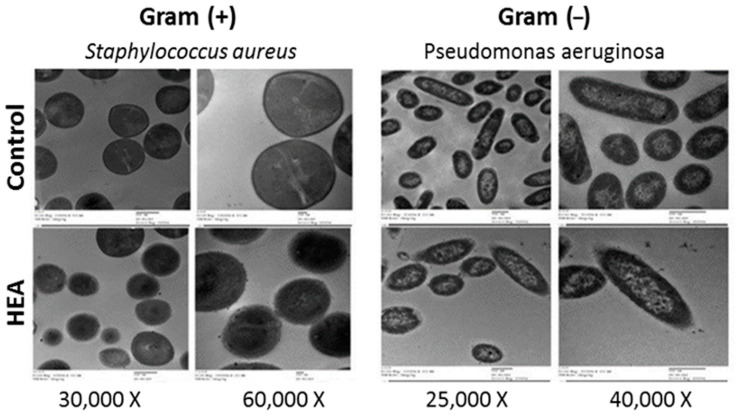
The presence of HEA (100 μg/mL) in BHI broth media containing S. aureus (Gram (+) bacterium), OD600 = 0.5 at the time of application produced TEM images showing reduced relative content of intact cells after 4 h of incubation at 37 °C (Figure 5). TEM images clarified that bacterial cells, escaping death, were subjected to different manifestations of deformation. Similar signs of effects on P. aeruginosa (Gram (−) bacterium), including cell shrinkage, cell membrane wrinkles, pore formation, and emptiness of live cellular material were observed in the HEA (150 μg/mL) treated cells. TEM results indicated that the cationic proteins’ action is rather general and more targeting the cell wall and cell membrane.
Figure 5.
Transmission electron microscopic graphs Staphylococcus aureus and Pseudomonas aeruginosa as subjected to 100 and 150 μg/mL of hydrolyzed egg albumin, respectively.
2.6. Investigation of Toxicity of HEA in Wistar Albino Rats
2.6.1. Acute Oral Toxicity
No mortalities were recorded after oral administration of the native egg albumin (NEA) or its hydrolyzed forms (HEA), administered at different doses in the range of 2000–5000 mg protein/kg body weight/day. LD50 of HEA was higher than 5000 mg/kg body weight/day. Substances with an LD50 between 5000 and 15,000 mg/kg body weight/day are regarded non toxic.
No signs of toxicity, e.g., abnormal breathing and impaired movements, were observed in any rat group. Over the next 14 days, no adverse effects of these treatments were produced. The changes in body weight did not differ significantly between rat groups. They were in the same range 16–18g/rat in each rat group. The non observed adverse effect level is very high, being probably greater than 5000 mg/kg body weight/day Table 4.
2.6.2. Subacute Toxicity
The data presented in Table 4 show relatively insignificant (p < 0.05) changes in the body weights of Wistar Albino rats receiving 5 days per week of low and high doses (500 and 2500 mg/kg body weight/day) of HEA-treated rats. Weight gain was in the same range 80–90 and 52–61g/rat for male and female, respectively. At the end of the experimental period, the organ weight of all rats was nearly in the same range without significant differences (p < 0.05, Table 5).
Table 5.
Effect of hydrolyzed egg albumin (HEA) on organ weight (g) of male and female albino rats treated orally daily for 28 days.
| Organ Weight (g) |
Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| Liver | 6.3 ± 0.35 | 6.23 ± 0.14 | 6.16 ± 0.26 |
| Kidney | 1.45 ± 0.13 | 1.37 ± 0.13 | 1.28 ± 0.06 |
| Brain | 1.37 ± 0.09 | 1.36 ± 0.14 | 1.32 ± 0.09 |
| Spleen | 0.65 ± 0.07 | 0.65 ± 0.07 | 0.63 ± 0.05 |
| Lung | 1.27 ± 0.08 | 1.27 ± 0.08 | 1.25 ± 0.06 |
| Heart | 0.98 ± 0.11 | 0.94 ± 0.08 | 0.95 ± 0.07 |
| Testis | 1.28 ± 0.13 | 1.26 ± 0.12 | 1.24 ± 0.13 |
| Female | |||
| Liver | 5.8 ± 0.23 | 5.78 ± 0.21 | 5.68 ± 0.15 |
| Kidney | 1.29 ± 0.11 | 1.26 ± 0.08 | 1.24 ± 0.09 |
| Brain | 1.27 ± 0.07 | 1.26 ± 0.08 | 1.24 ± 0.15 |
| Spleen | 0.61 ± 0.06 | 0.6 ± 0.04 | 0.58 ± 0.09 |
| Lung | 1.15 ± 0.12 | 1.16 ± 0.08 | 1.14 ± 0.06 |
| Heart | 0.85 ± 0.05 | 0.84 ± 0.13 | 0.83 ± 0.07 |
| Ovary | 0.067 ± 0.02 | 0.064 ± 0.03 | 0.062 ± 0.02 |
2.6.3. Hematological Parameters
The changes in hematological parameters (WBS, RBC, hemoglobin, hematocrit, MCV, MCH, MCHC, platelets, and lymphocytes) were either within the normal range, insignificant and not-dose dependent in rats receiving two levels of HEA for 28 days (Table 6).
Table 6.
Effect of hydrolyzed egg albumin (HEA) on hematological parameters of male and female albino rats treated orally daily for 28 days.
| Blood Biochemical Parameters | Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| WBC a (×103/µL) | 7.12 ± 0.13 | 7.09 ± 0.07 | 7.1 ± 0.13 |
| RBC b (×106 µL) | 8.23 ± 0.11 | 8.23 ± 0.09 | 8.22 ± 0.12 |
| HGB c (g/dL) | 12.89 ± 0.23 | 12.89 ± 0.34 | 12.93 ± 0.27 |
| HCT d (%) | 42.7 ± 0.31 | 42.12 ± 0.12 | 42.9 ± 0.17 |
| MCV e (FL) | 51.76 ± 0.17 | 51.56 ± 0.19 | 51.65 ± 0.34 |
| MCH f (pg) | 16.98 ± 0.17 | 16.96 ± 0.13 | 16.94 ± 0.11 |
| MCHC g (g/dL) | 34.45 ± 0.15 | 34.44 ± 0.11 | 34.43 ± 0.09 |
| PLT h (×103/µL) | 820 ± 3.45 | 827 ± 5.91 | 822 ± 4.9 |
| LYM i (%) | 5.27 ± 0.17 | 5.65 ± 0.04 | 5.16 ± 0.06 |
| Female | |||
| WBC a (×103/µL) | 8.02 ± 0.12 | 8.01 ± 0.08 | 8.06 ± 0.12 |
| RBC b (×106 µL) | 8.25 ± 0.11 | 8.22 ± 0.12 | 8.21 ± 0.11 |
| HGB c (g/dL) | 12.99 ± 0.21 | 12.94 ± 0.31 | 12.96 ± 0.22 |
| HCT d (%) | 44.8 ± 0.31 | 43.19 ± 0.15 | 44.9 ± 0.07 |
| MCV e (FL) | 53.70 ± 0.11 | 52.96 ± 0.14 | 53.68 ± 0.14 |
| MCH f (pg) | 17.55 ± 0.12 | 17.86 ± 0.14 | 17.90 ± 0.21 |
| MCHC g (g/dL) | 36.41 ± 0.16 | 36.04 ± 0.09 | 36.33 ± 0.19 |
| PLT h (×103/µL) | 880 ± 62 | 885 ± 5.44 | 882 ± 9.51 |
| LYM i (%) | 6.34 ± 0.11 | 6.25 ± 0.08 | 6.34 ± 0.09 |
a White blood cell. b Red blood cell. c Hemoglobin concentration. d Hematocrit (%). e Mean corpuscular volume. f Mean corpuscular hemoglobin. g Mean corpuscular hemoglobin concentration. h Platelets. i Lymphocyte.
2.6.4. Biochemical Indicators
The treatment with HEA for 28 days (500 and 2500 mg/kg body weight) did not change the biochemical profile of male and female rats, as shown in Table 7.
Table 7.
Effect of hydrolyzed egg albumin (HEA) on blood biochemical parameters of male and female albino rats treated orally daily for 28 days.
| Blood Biochemical Parameters | Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| ALT (U/mL) | 44.82 ± 2.30 a | 37.38 ± 1.71 b | 34.27 ± 1.15 b |
| AST (U/mL) | 113.56 ± 4.05 | 103 ± 4.05 | 98.05 ± 5.84 |
| ALP (U/L) | 145.512 ± 5.8 | 140.05 ± 5.8 | 134.54 ± 5.81 |
| AChE (U/L) | 393.45 ± 5.8 | 376.02 ± 11.60 | 371.36 ± 5.80 |
| T.P. (g/dL) | 6.43 ± 0.58 | 6.18 ± 0.58 | 5.92 ± 0.17 |
| Alb. (g/dL) | 3.28 ± 0.12 | 3.13 ± 0.11 | 3.08 ± 0.91 |
| Urea (mg/dL) | 36.78 ± 3.50 | 32.08 ± 1.15 | 31.23 ± 1.15 |
| Creatinine (mg/dL) | 0.48 ± 0.045 | 0.47 ± 0.024 | 0.42 ± 0.052 |
| Female | |||
| ALT (U/mL) | 38.32 ± 2.91 | 38.02 ± 2.91 | 36.04 ±2.34 |
| AST (U/mL) | 97.19 ± 5.82 | 96.82 ± 3.51 | 93.39 ± 2.94 |
| ALP (U/L) | 114.26 ± 5.81 | 113.53 ± 4.11 | 109.25 ± 5.80 |
| AChE (U/L) | 412.65 ± 11.60 | 408.21 ± 5.80 | 405.03 ± 3.70 |
| T.P. (g/dL) | 6.38 ± 0.58 | 6.37 ± 0.09 | 6.33 ± 0.07 |
| Alb. (g/dL) | 3.31 ± 0.58 | 3.29 ± 0.29 | 3.25 ± 0.14 |
| Urea (mg/dL) | 40.32 ± 2.91 | 39.83 ± 3.51 | 36.83 ± 3.51 |
| Creatinine (mg/dL) | 0.47 ± 0.037 | 0.47 ± 0.04 | 0.45 ± 0.067 |
Every value is the average of ten replicates ± SE. Different letters (a,b) in the same row refer to significantly different values (p < 0.05).
2.6.5. Histo-Pathological Examination
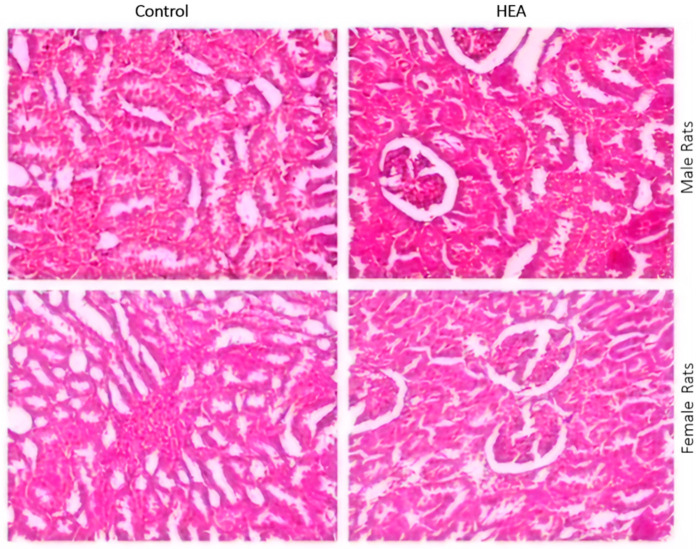
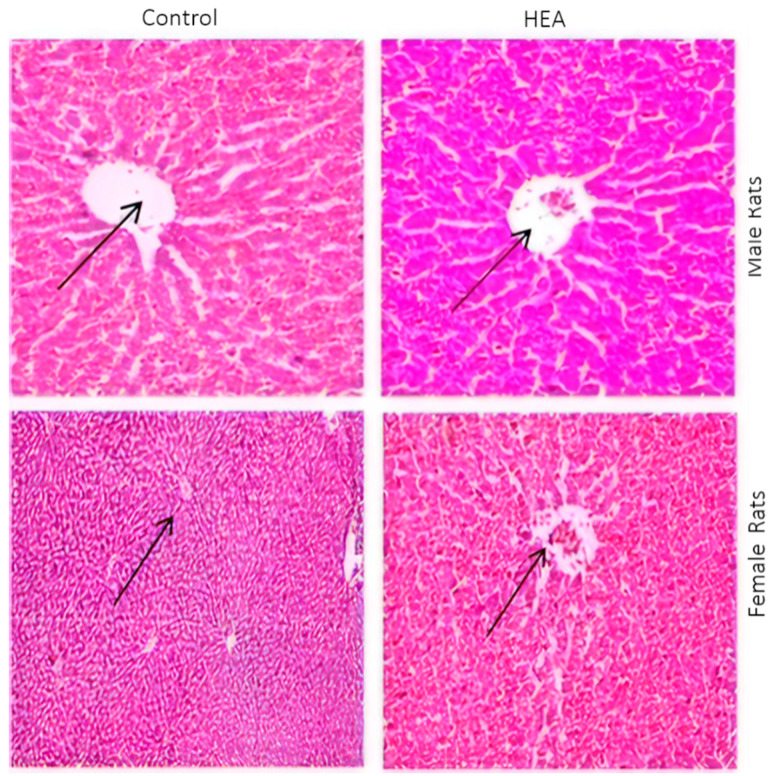
The rat organs were histologically examined at the end of the experimental exposure of rats to the tested substances for any abnormalities. The rat organs were histologically examined at the end of the experimental exposure of rats to the tested substances for any abnormalities. The obtained results are shown in Figure 6 and Figure 7. The macroscopic analysis of the treated animals’ target organs did not show any histological alterations in all organs examined.
Figure 6.
Histopathological examination of H&E stained sections of kidney of male and female albino rats administered 2500 mg/kg body weight of HEA as compared to control (0 mg/kg body weight) using 1200× magnification power.
Figure 7.
Histopathological examination of H&E stained sections of liver of male and female albino rats administered 2500 mg/kg body weight of HEA as compared to control (0 mg/kg body weight) using 1200× magnification power.
3. Discussion
The occurrence and spread of antibiotic resistant bacteria are pressing public health problems worldwide [13,34,42]. Many bacteria have become resistant against many antimicrobial agents. The resistance rates are higher in developing countries [43]. The development of new prophylactic and therapeutic procedures is urgently required to meet the challenges imposed by the emergence of bacterial resistance [44,45,46]. Cationic Antimicrobial Peptides (CAMPs) are promising new antibacterial agents due to their killing mechanism via interaction with bacterial cell walls and membranes [20,47].
Imparting cationic character on native proteins can be achieved through chemical modification, e.g., esterification which blocks free carboxyl groups, elevating the net positive charge on the modified proteins [48,49] or through the enzymatic hydrolysis [50] which potentially affects the molecular size and hydrophobicity, as well as the polar and ionizable groups of protein hydrolysates. This modification produces potent bactericidal peptides that may be classified as effective antimicrobials [51,52].
In line with this trend, HEA was obtained by enzymatic hydrolysis of egg albumin using pepsin at its optimal conditions (pH 2 at 37 °C) to liberate bioactive peptides, a relatively short peptide residue length (e.g., 2–20 amino acids) possessing hydrophobic amino acid residues in addition to proline, lysine, or arginine groups and bioactive peptides. These peptides showed to be an effective antimicrobial [52]. The large number of the peptides (44 fractions) released by peptic hydrolysis are apparently due to the broad specificity of pepsin and the high susceptibility of proteins to this enzyme [53]. The majority of the peptides which had relatively high molecular weight (3–9 amino acids) agrees with the fact that pepsin attacks the protein molecules gradually from the C- and N-terminals [53]. The obtained hydrolysate style, showing increasing and steady rate of hydrolysis with time during 4 h, reflects the nature of pepsin specificity which tends to attack protein molecules gradually from the C- and N-terminals [54,55], targeting preferentially linkages with aromatic or carboxylic L-amino acids [56]. The higher relative quantity of the bigger sized peptides may indicate their higher participation in the biological activity of the hydrolysates; protein hydrolysis may give rise to basic peptides which can directly attack the cell walls and cell membranes of the pathogenic bacteria [57,58].
The higher relative quantity of the bigger sized peptides (3–9 amino acids) may indicate their higher participation in the biological activity of the hydrolysates, i.e., peptides of more than three amino acids may be essentially more responsible for the antibacterial activity since they represent more than 74% of the peptide mixture. However, the data available from the analysis cannot give information about the specific peptide that was most responsible for this activity.
The observed molecular mass range of NEA subunits between 50 and 180 kDa agrees with its ability to dissociate into more active subunits under different ionic strength and pH [59,60,61,62]. The electrophoretic patterns of HEA confirmed the transformation of NEA into lower molecular weight peptides. The faster migration from anode to cathode, in case of the hydrolyzed protein, than the native one refers to bigger positive charges. This may imply the liberation of some cationic peptides in the course of hydrolysis in accordance with previous studies [63,64]. HEA exhibited a distinctive inhibition against the pathogenic bacteria, while NEA showed only scant inhibition corroborating previous results [65,66,67,68].
The higher antimicrobial activity of HEA than NEA could be due to the more hydrophobicity and cationic nature of HEA [26,37,69,70,71,72]; initiating the electrostatic interaction between the positive charges of the antimicrobial agent and the negatives charges on the bacterial cell walls or membranes. The hydrophobic aggregation between the similar regions of the two reactants can further stabilize this complex. Oscillating random Brownian motion of these aggregations [71] may produce large-sized pores channels and disintegrate the cell wall and cell membranes, engendering higher cell permeability, cell emptiness, and death. The mechanism of antimicrobial peptides is based on their amino acid composition, amphipathicity, and cationic charges allowing their attachment to cell membrane bilayers to form pores by ‘barrel-stave’, ‘carpet’, or ‘toroidal-pore’ mechanisms [37,39].
Since HEA shows broad-spectrum antimicrobial activity, it can promote the activity of antibiotics against all tested bacteria. This was realized in the tested HEA-antibiotic combinations which showed greater antibacterial action than the single antibiotics, especially against L. monocytogenes, B. cereus, and P. aeruginosa. This synergism between antibiotics and HEA may be due to accentuating the hydrogen bonding and hydrophobic–hydrophobic interactions with targeted microbes [73] or through using different mechanisms of bacteria inhibition.
This Gram (−) bacteria (especially P. aeruoginosa) is known for being notorious for its ability to survive in the environment, particularly in moist conditions. It may contaminate medicines, surgical equipment, clothing, and dressing with the ability to cause serious infections in immune compromised patients [74]. Therefore, it was used in this study for TEM studies. Additionally, S. aureus bacterium was selected from the Gram (+) group as it was more sensitive and because of its public health concerns. S. aureus is a pathogen of skin abscesses, pharyngitis, sinusitis, meningitis, pneumonia, endocarditis, osteomyelitis, toxic shock syndrome, sepsis, and wound infections following surgery. Moreover, it responsible for food poisoning illness as it is capable of producing several virulence factors such as enterotoxins, adhesins, hemolysins, invasins, superantigens, and surface factors that inhibit its phagocytic engulfment [73,75,76].
The signs of the irregular wrinkled outer surface, fragmentation, adhesion, and aggregation of damaged cells or cellular debris of HEA-treated bacterial cells, manifested by TEM examination confirm the antimicrobial action and follow previously published works [23,52,77].
The potential toxicity of NEA and HEA was assessed by determining acute oral toxicity when administrated to Wistar Albino rats. Mortality absence after a single administration of up to 5000 mg/kg body weight/day of the two tested substances indicates their safety and their possible biological utilization. The absence of significant body weight changes or any abnormality signs during 14 days after the single high dose administration of HEA (5000 mg/kg body weight/day) indicates its harmlessness. Since no deaths emerged in response to the used range of doses, the LD50 of HEA could not be calculated, but it may be assumedly more than 5000 mg/kg body weight/day. Therefore, HEA may be regarded nontoxic since its LD50lies in the range 5000–15,000 mg/kg body weight/day according to [78].
Extending the administration of two HEA doses (500 and 2500 mg/kg body weight/day) for 28 days did not either produce evident signs of toxicity or pathogenicity on the body or organ weights, indicating the absence of any specific organ toxicity. Changes in body or organ weights are taken as indicators of toxicity [79] while normal body weight changes are indicators of food safety and lack of toxicity [80]. The rats conserved normal healthy status in spite of repeated force-feeding with high doses. The obtained results revealed slight reductions in these two parameters and showed absence of any negative effects of the two studied substances on the renal function during repeated dose administration for 28 days. The rats conserved normal healthy status despite repeated force-feeding with high drug doses. The obtained results revealed the absence of any negative effects on the renal or hepatic function as well as the histological status during repeated-dose administration for 28 days in agreement with a previous study [81]. Therefore, our data demonstrate the importance of hydrolyzed egg albumin in controlling resistant bacteria. Further work will be necessary to study pathogenic bacteria’s inhibition by HEA in processed foods based on egg albumin.
4. Materials and Methods
4.1. Collection of Pathogenic Bacteria
L. monocytogenes LMG10470, L. ivanovii LMG11388, B. subtilis LMG7135, B. cereus ATCC14579, S. aureus DSM1104, S. pyogenes ATCC12384, K. oxytoca LMG3055, P. aeruginosa LMG8029, E. coli LMG8223, and S. typhimurium LMG10395 were used. All the bacteria were stored as stock cultures in glass beads at −20 °C and subcultured propagated in brain heart infusion broth (BHIB, Oxoid), before use. Slope cultures were grown on (BHIB, Oxoid, Columbia, MD, USA) as described previously by Abdel-Shafi S. et al., 2019a [34] and preserved stored at 4 °C during experimentation.
4.2. Egg Albumin Preparation
Fresh hen eggs were obtained from the Farm of Faculty of Agriculture, Zagazig University, Zagazig, Egypt. They were hand broken and the whites were separated from the whole eggs. The egg white was gently subjected to a magnetic stirrer for 30 min to reduce the viscosity. They were then lyophilized (Thermo-electron Corporation—Heto power dry LL 300 Freeze dryer). The lyophilized protein product was designated native egg albumin (NEA) according to Abdel-Shafi S. et al., 2016 [37].
4.3. Enzymatic Hydrolysis of Egg Albumin
Enzymatic hydrolysis of egg albumin was performed using pepsin at optimal conditions (pH 2 at 37 °C). The protein substrate was dissolved in the relevant buffer (0.1 M Glycine–HCl pH 2) at 10 mg/mL and the enzyme was added at 1:2 (w/w) enzyme-to-substrate ratio. After 4 h of reaction, the sample was heated in a boiling water bath for 10 min to inactivate the enzyme in accordance to Otte J. et al., 2015 [82]. The hydrolysates were centrifuged at 4000× g for 15 min and the supernatant was lyophilized and stored at −20 °C.
Characterization of Egg Albumin Hydrolysates
The degree of hydrolysis (DH) (the percentage of peptide bonds cleaved), was calculated at different periods (0, 1, 2, 3, and 4 h) by determining free amino groups through reaction with L-Leucine, 2,4,6-trinitrobenzenesulphonic acid (TNBS) following Adler-Nissen J., 1986 [83].
SDS–PAGE of both NEA and HEA was carried out as described previously by Sitohy M. and Osman A. 2010 [84] in 3% and 17% acrylamide for the stacking and resolving gels, respectively, as described previously by Evans R.W and Williams J., 1980 [85].
Native egg albumin hydrolyzed with pepsin for 4 h was analyzed by Urea-PAGE in 3% stacking and 10% resolving gels according to CLSI, 2008 and Standards N.C.F.C.L. et al., 1999 [86,87].
Ultra performance liquid chromatography (UPLC) was conducted in the Center of Drug Discovery Research and Development, Faculty of Pharmacy, Ain-Shams University, Egypt, using (Acquity H Class, Waters) coupled with quadrupole-time-of-flight mass spectrometry (Synap G2, Waters). An aliquot of 10 µL of the final peptide solution was injected into the chromatograph and peptides were separated on an ACQUITY UPLC—BEH C18 1.7 µm—2.1 × 50 mm. The mobile phase combined deionized water with 0.1% formic acid as solvent A, while acetonitrile with 0.1% formic acid represented solvent B. The initial conditions were 95% A and 5% B to establish a 95% (v/v) linear gradient of B at 15 min. The running specifications were 25 min total run time, 5 min post-delay time, and 0.2 mL min−1 flow rate. A high-resolution mass spectrometric analysis was carried out in the positive electro spray ionization (ESI+). High-purity nitrogen was used for dissolution (800 L h−1) and cone (25 L h−1). Spectra were recorded over the mass/charge (m/z) range of 100–1000.
4.4. Antibacterial Activity Estimation
4.4.1. Disc Diffusion Assay
The antibacterial activity of both NEA and HEA were tested against the experimental pathogenic bacteria by the Kirby-Bauer disc diffusion method as described previously by Schafer E.W. et al., 1972 [88]. The bacterial suspensions were spread over the surface of nutrient agar plates, then sterilized paper discs of 6mm in diameter were soaked in either diluted NEA solutions (0, 10, 25, 50, 100, and 250 μg/mL) or HEA solutions (0, 50,100,150, 200, and 250 μg/mL). They were then laid onto the surface of nutrient agar media seeded with the pathogenic bacteria at appropriate distances, separating them from each other. The nutrient agar plates were incubated at 37 °C for 24 h before recording the results in accordance to OECD, 2001 [89].
4.4.2. Minimum Inhibitory Concentration (MIC)
MIC of HEA and five antibiotics, ciprofloxacin (10 μg), gentamycin (10 μg), penicillin G (10 μg), chloramphenicol (30 μg), andvancomycin (30 μg) was evaluated using standard inoculums of about 2 × 105 CFU/mL following Abdel-Shafi S. et al., 2019b [35]. Serial dilutions of the test compounds, previously dissolved in sterilized distilled water, were prepared to final concentrations to each tube (containing 10 mL BHI broth), 100 μL of the inoculum was added and incubated for 24 h at 37 °C. At the end of incubation time, MIC was visually identified as the lowest concentration of the test compound, inhibiting the visible growth as confirmed by measuring the optical density at 600 nm. Sterilized distilled water was used as a negative control.
4.4.3. Antibacterial Activity of Antibiotics and Combinations of Both HEA and Antibiotics
Ready antibiotic discs (μg/disc) of ciprofloxacin (10 μg), gentamycin (10 μg), penicillin G (10 μg), chloramphenicol (30 μg), and vancomycin (30 μg) were laid onto the surface of nutrient agar media, seeded previously with all the tested bacteria, leaving appropriate distances separating them. The nutrient agar plates were incubated at 37 °C for 24 h, and the diameters of the inhibition zones (mm) were measured. Results of antibiotic sensitivity were taken according to OECD, 2001 and Osman A. et al., 2019 [89,90]. In another experiment, a disc of the more effective antibiotic against each bacterium was selected and was soaked in MIC μg/mL of HEA and placed onto surface of nutrient agar seeded with the indicator bacterium. After 48h of incubation at 37 °C, results were taken as mentioned above.
4.4.4. Quantitative Inhibition of Pathogenic Bacteria by HEA
A number of test tubes, containing 10 mL of BHIB (Oxoid, United Kingdom), were inoculated with 100 µL of bacterial suspension (2 × 105 CFU/mL) at the log phase and combined with 100 μg/mL HEA for L. monocytogenes, B. cerueus, S. aureus, S. typhimurium, S. pyogenes, and K. oxytoca and 150 μg/mL HEA for P. aeruginosa, B. subtilis, and L. ivanovii and 200 μg/mL HEA for E. coli. Control test tubes contained BHIB (Oxoid) using uniquely the bacteria without treatment. Samples and controls were incubated at 37 °C before determining the growth after 0.0, 6, 12, 18, and 24 h of incubations by measuring the turbidity at OD600 using a spectrophotometer (ENWAY—England 6405 UV/VIS, England).
4.5. Transmission Electron Microscopy Examination
Morphological and ultrastructural changes of bacterial cells of S. aureus and P. aeruginosa were examined using transmission electron microscopy (TEM) (JEOL-TME-2100F, Japan) as described previously by Standards N.C.F.C.L. et al., 1999 [87]. Prior to TEM imaging, each bacterial strain was grown in tryptone glucose yeast extract broth supplemented with or without 100 μg/mL of HEA and incubated at 35 °C for 24 h.
4.6. Investigation of Toxicity for HEA in Wistar Albino Rats
Zagazig University Animal Care Board approved the design of the toxicological animal experiment (Approval number: ZU-IACUC/1/F/134/2020). Animals used and the design of the experiment were carried out following Osman A. et al., 2019 [90]. Healthy male and female white albino rats (Rattus norvegicus. Bork), Wistar strain (135 ± 10 g, bodyweight for female and 155 ± 15 g, bodyweight for male) were obtained from the Organization of Biological Products and Vaccine (Helwan farm, Cairo, Egypt) and housed in plastic cages in groups of five animals/cage.
The experimental animals were allowed to acclimatize under the laboratory conditions (temperature of 28 ± 2 °C; relative humidity 50–70% and normal light/dark cycle) for 2 weeks prior to the experiment. They were provided with balanced pelleted diet (23% protein) and tap water ad libitum throughout the adaptation and experimental period. The experimental design included two phases; the first phase was destined to assess the acute oral medium lethal doses (LD50) while the second one was to evaluate HEA subacute toxicity.
4.6.1. Acute Oral Toxicity
Fifteen male Wistar Albino rats (160 ± 10 g, body weight) were divided into three groups (five rats each). The first group received 2 mL distilled water free from any external treatment (control). The groups 2 and 3 received one acute dose of HEA of 2000 and 5000 mg/kg body weight, respectively. Each group received a stomach tube force-feeding of different specified doses dissolved in distilled water (2 mL). All rats were kept under observation for 24 h and symptoms of toxicity or mortality were recorded. Animals that were still alive were observed daily for their behavior and body weight changes for 14 days. The acute oral LD50 values were calculated accordance to Osman A. et al., 2019 and Guidelines for Hematoxylin and Eosin Staining, 2001 [90,91].
4.6.2. Subacute Toxicity
Fifteen male Wistar Albino rats (160 ± 10 g, body weight) were divided into three groups (five rats each). The first group received 2 mL distilled water free from any external treatment (control). Groups 2 and 3 received one acute dose of HEA of 2000 and 5000 mg/kg body weight. Each group received a stomach tube force-feeding of different specified doses dissolved in distilled water (2 mL). All rats were kept under observation for 24 h and symptoms of toxicity or mortality were recorded. Animals that were still alive were observed daily for their behavior and body weight changes for 14 days. The acute oral LD50 values were calculated. Fifteen male rats were divided into three groups of five rats each. Each group was intubated orally by force-feeding using a stomach tube with different HEA doses five days/week for 28 days. The groups received the following doses: Group 1: the same amount of distilled water without any treatment and served as control. Groups 2 and 3: HEA at doses of 500 and 2500 mg/kg body (weight/day), respectively. The same experiment was applied to 15female rats. All animals, including control, were supplied with a balanced pelleted diet (23% protein) and tap water ad libitum during the experimental period. Daily body weight of rats was recorded, and the weight gains were calculated during the testing period as follows;
At the end of the administration period on day 28, the rats were sacrificed and blood samples were collected into clean, dry, and labeled Eppendorf tubes containing heparin as an anticoagulant (7.5 I.U). The collected blood samples were centrifuged at 3600× g for 15 min at 4 °C to separate serum from plasma. Serum samples were divided into aliquots and kept in deep freezer at −20 °C for the biochemical and hematological assays of the as described previously by Guidelines for Hematoxylin and Eosin Staining, 2001 [91]. The animal organs were removed, weighed, and used for histopathological examination.
4.6.3. Biochemical Analyses
The serum was assayed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), acetyl choline esterase (AchE), total proteins, albumin, urea, and creatinine. Hematological analysis using the SYSMEX Hematology Auto Analyzer (Japan) was conducted to estimate the white blood cell (WBC), platelet counts (PLT), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and lymphocyte (LYM).The organs of randomly selected three rats from each group were excised, examined grossly, and subsequently fixed in 15% formalin saline then used for histopathological examination. The fixed tissues were processed by dehydration in a series of graded ethanol concentrations, cleared with xylol, and embedded in paraffin blocks. Fourµ thick sections were stained by Hematoxylin–Eosin stain (H & E) for the histopathological analysis under alight microscope (model OLYMPUS CX 41) at 1200× magnification following Osman A. et al., 2019 and Guidelines for Hematoxylin and Eosin Staining, 2001 [90,91].
5. Conclusions
Food microbiological control is one of the most important approaches for extending its shelf life, ensuring its safety. Based on obtained results, hydrolyzed egg albumin (HEA) effectively inhibits the pathogenic bacteria, where L. monocytogenes was the most sensitive organism. Ultra-performance liquid chromatography (UPLC) of the peptic HEA revealed 44 peptides, 17 of them were dipeptides and the other 27 fractions corresponded to bigger peptides (3–9 amino acids). The dipeptides and big peptides represented 26% and 74% of the total hydrolysate, respectively. Mixtures of HEA (MICs) with antibiotics showed more significant antibacterial activity than either of them individually. The enzymatically modified proteins’ ability to act synergistically with antibiotics may be a new approach, overcoming partially the bacterial antibiotic-resistance. HEA has a broad-spectrum antimicrobial activity [6 G (+) and 4 G (−)], enabling it to partially replace the synthetic antibiotic to significant extents. HEA has abroad-spectrum antimicrobial activity (6 G (+)) enabling its recommendation as a food-safe antibacterial treatment since administering it to Wistar albino rats a high ratio of 2500 mg/kg bodyweight for 28 days did not negatively affect liver or kidney functions or trigger any toxicity signs in the animals.
Author Contributions
M.S., G.E., A.O. and S.A. suggested the work protocol, interpreted the results, and revised the manuscript. S.A.-S., M.E.-N., A.O. and M.A.T. performed the laboratory experiments regarding microbiological and chemical investigations and prepared the manuscript. A.-R.A.-M. revised and interpreted the results and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Zagazig University, Zagazig. Egypt supported the experimental work. A. R. Al- Mohammadi from Department of Science, King Khalid Military Academy, Riyadh 11495, P.O. Box 22140, Saudi Arabia, was responsible for paying the publication fees.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sitohy M.Z., Osman A.O. Enhancing milk preservation with esterified legume proteins. Probiotics Antimicrob. Proteins. 2011;3:48–56. doi: 10.1007/s12602-010-9060-5. [DOI] [PubMed] [Google Scholar]
- 2.Enan G., Addallah F.M., Sobhy H. Effect of acyclovir on bovine herpesvirus type 1 infection in vitro cultured cells. Int. J. Virol. 2012;8:307–312. doi: 10.3923/ijv.2012.307.312. [DOI] [Google Scholar]
- 3.Enan G., Abdel-Shafi S., Ouda S., Negm S. Novel antibacterial activity of Lactococcus lactis subspecies lactis Z11 isolated from Zabady. Int. J. Biomed. Sci. 2013;9:144–180. [PMC free article] [PubMed] [Google Scholar]
- 4.Enan G., Abdel-Shafi S., Abdel-Halem M.F., Negm S. Characterization of probiotic lactic acid bacteria to be used as starter and protective cultures for dairy fermentations. Int. J. Probiotics Prebiotics. 2013;8:157–163. [Google Scholar]
- 5.Enan G., Osman M.E., Abdel-Haliem M.E.F., Abdel-Ghany S.E. Advances in Microbial and Nucleic Acids Biotechnology. BioMed Res. Int. 2018:1–2. doi: 10.1155/2018/3102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Shafi S., Osman A., Al-Mohammadi A.-R., Enan G., Kamal N., Sitohy M. Biochemical, biological characteristics and antibacterial activity of glycoprotein extracted from the epidermal mucus of African catfish (Clarias gariepinus) Int. J. Biol. Macromol. 2019;138:773–780. doi: 10.1016/j.ijbiomac.2019.07.150. [DOI] [PubMed] [Google Scholar]
- 7.Ismaiel A.A., Ali A.-E.S., Enan G. Incidence of Listeria in Egyptian meat and dairy samples. Food Sci. Biotechnol. 2014;23:179–185. doi: 10.1007/s10068-014-0024-5. [DOI] [Google Scholar]
- 8.Mahgoub S.A.M., Osman A., Ramadan M.F. Inhibitory effect of Nigella sativa oil against Listeria monocytogenes and Salmonella enteritidis inoculated in minced beef meat. J. Food Meas. Charact. 2017;11:2043–2051. doi: 10.1007/s11694-017-9587-1. [DOI] [Google Scholar]
- 9.Osman A., El-Didamony G., Sitohy M., Khalifa M., Enan G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. Appl. Res. Nat. Prod. 2016;9:17–26. [Google Scholar]
- 10.Osman A., Goda H.A., Sitohy M. Storage stability of minced beef supplemented with chickpea legumin at 4 °C as a potential substitute for nisin. Food Sci. Technol. 2018;93:434–441. doi: 10.1016/j.lwt.2018.03.071. [DOI] [Google Scholar]
- 11.Osman A.O., Mahgoub S.A., Sitohy M.Z. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 °C. J. Dairy Res. 2013;80:174–183. doi: 10.1017/S0022029913000095. [DOI] [PubMed] [Google Scholar]
- 12.Sitohy M., Osman A. Bioactive Compounds in Soybean Proteins and Its Applications in Food Systems, Handbook of Environmental Chemistry. Springer; Berlin/Heidelberg, Germany: 2019. pp. 147–160. [Google Scholar]
- 13.Enan G., Abdul Aziz A.-A. Novel plantaricin UG1 production by Lactobacillus plantarum UG1 in enriched whey permeate in batch fermentation processes. J. Food Agric. Environ. 2006;4:84–88. [Google Scholar]
- 14.Abdel-Shafi S., Ouda S.M., Elbalat I., Enan G. Characterization and identification of multidrug resistant bacteria from some Egyptian patients. Biotechnology. 2013;12:65–73. doi: 10.3923/biotech.2013.65.73. [DOI] [Google Scholar]
- 15.Enan G., Al-Mohammadi A.-R., El-Didamony G., Abdel-Haliem M.E.F. Antimicrobial activity of Enterococcus faecium NM2 isolated from urine: Purification, characterization and bactericidal action of enterocin NM2. Asian J. Appl. Sci. 2014;7:721–734. doi: 10.3923/ajaps.2014.621.634. [DOI] [Google Scholar]
- 16.Abdel-Shafi S., Al-Mohammadi A.-R., Negm S., Enan G. Antibacterial activity of Lactobacillus delbreukii subspecies bulgaricus isolated from zabady. Life Sci. J. 2014;11:264–270. [Google Scholar]
- 17.Enan G., El-Didamony G., Mohammed M.E.F., Zakaria A. Novel antibacterial activity of Enterococcus faecium NM2 isolated from urine of healthy people. Asian J. Appl. Sci. 2014;7:66–78. doi: 10.3923/ajaps.2014.66.78. [DOI] [Google Scholar]
- 18.Osman A., Abbas E., Mahgoub S., Sitohy M. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016;82:293–301. doi: 10.1007/s10327-016-0686-3. [DOI] [Google Scholar]
- 19.Akyil D., Ozkara A., Konuk M. Environmental Health Risk-Hazardous Factors to Living Species. Intech Open; London, UK: 2016. Pesticides, Environmental Pollution, and Health. [DOI] [Google Scholar]
- 20.Osman A., Abdel-Shafi S., Al-Mohammadi A.R., Kamal N., Enan G., Sitohy M. Catfish glycoprotein, a highly powerful safe preservative of minced beef stored at 4 °C for 15 Days. Foods. 2020;9:1115. doi: 10.3390/foods9081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas E., Osman A., Sitohy M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020;100:2889–2897. doi: 10.1002/jsfa.10314. [DOI] [PubMed] [Google Scholar]
- 22.Mahgoub S., Osman A., Sitohy M. Inhibition of growth of pathogenic bacteria in raw milk by legume protein esters. J. Food Prot. 2011;74:1475–1481. doi: 10.4315/0362-028X.JFP-11-065. [DOI] [PubMed] [Google Scholar]
- 23.Mahgoub S.A., Sitohy M.Z., Osman A.O. Counteracting recontamination of pasteurized milk by methylated soybean protein. Food Bioproc. Tech. 2013;6:101–109. doi: 10.1007/s11947-011-0653-0. [DOI] [Google Scholar]
- 24.Osman A., Mahgoub S., El-Masry R., Al-Gaby A., Sitohy M. Extending the technological validity of raw buffalo milk at room temperature by esterified legume proteins. J. Food Process. Preserv. 2014;38:223–231. doi: 10.1111/j.1745-4549.2012.00768.x. [DOI] [Google Scholar]
- 25.Osman A., Mahgoub S., Sitohy M. Hindering milk quality storage deterioration by mild thermization combined with methylated chickpea protein. Int. Food Res. J. 2014;21:693–701. [Google Scholar]
- 26.Sitohy M., Mahgoub S., Osman A. Controlling psychrotrophic bacteria in raw buffalo milk preserved at 4 °C with esterified legume proteins. Food Sci. Technol. 2011;44:1697–1702. doi: 10.1016/j.lwt.2011.03.008. [DOI] [Google Scholar]
- 27.Abdel-Hamid M., Romeih E., Saporito P., Osman A., Mateiu R.V., Mojsoska B., Jenssen H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control. 2020;111:107056. doi: 10.1016/j.foodcont.2019.107056. [DOI] [Google Scholar]
- 28.Osman A., El-Araby G.M., Taha H. Potential use as a bio-preservative from lupin protein hydrolysate generated by alcalase in food system. J. Appl. Biol. Biotechnol. 2016;4:076–081. [Google Scholar]
- 29.Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: Characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26:567–577. doi: 10.1007/s10989-019-09863-x. [DOI] [Google Scholar]
- 30.Abdel-Hamid M., Otte J., De Gobba C., Osman A., Hamad E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017;66:91–98. doi: 10.1016/j.idairyj.2016.11.006. [DOI] [Google Scholar]
- 31.Abdel-Hamid M., Osman A., El-Hadary A., Romeih E., Sitohy M., Li L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020;103:1884–1893. doi: 10.3168/jds.2019-17355. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Hamid M., Goda H.A., De Gobba C., Jenssen H., Osman A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016;61:91–98. doi: 10.1016/j.idairyj.2016.04.004. [DOI] [Google Scholar]
- 33.Osman A., Goda H.A., Abdel-Hamid M., Badran S.M., Otte J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. Food Sci. Technol. 2016;65:480–486. doi: 10.1016/j.lwt.2015.08.043. [DOI] [Google Scholar]
- 34.Abdel-Shafi S., Al-Mohammadi A.-R., Osman A., Enan G., Abdel-Hameid S., Sitohy M. Characterization and antibacterial activity of 7S and 11S Globulins isolated from Cowpea seed protein. Molecules. 2019;24:1082. doi: 10.3390/molecules24061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdel-Shafi S., Al-Mohammadi A.R., Sitohy M., Mosa B., Ismaiel A., Enan G., Osman A. Antimicrobial activity and chemical constitution of the crude, phenolic-rich extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules. 2019;24:4280. doi: 10.3390/molecules24234280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Shafi S., Al-Mohammadi A.R., Almanaa T.N., Moustafa A.H., Saad T.M.M., Ghonemy A., Anacarso I., Enan G., El-Gazzar N. Identification and testing antidermatophyticoxaborole-6-benzene sulphonoamide derivative (OXBS) from Streptomyces atrovirens KM192347 isolated from soil. Antibiotics. 2020;9:176. doi: 10.3390/antibiotics9040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Shafi S., Osman A., Enan G., El-Nemer M., Sitohy M. Antibacterial activity of methylated egg white proteins against pathogenic G+ and G− bacteria matching antibiotics. SpringerPlus. 2016;5:983. doi: 10.1186/s40064-016-2625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Z., Yin Y., Zhao W., Chen F., Liu J. Application and bioactive properties of proteins and peptides derived from hen eggs: Opportunities and challenges. J. Sci. Food Agric. 2014;94:2839–2845. doi: 10.1002/jsfa.6670. [DOI] [PubMed] [Google Scholar]
- 39.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 40.Cruz J., Ortiz C., Guzman F., Fernandez-Lafuente R., Torres R. Antimicrobial peptides: Promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014;21:2299–2321. doi: 10.2174/0929867321666140217110155. [DOI] [PubMed] [Google Scholar]
- 41.Fosgerau K., Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Enan G., Abdel-Shafi S., Ouda S.M., Elbalat I. Genetic linkage of the antibiotic resistance ability in the Escherichia coli UR4 strain isolated from urine. J. Med. Sci. 2013;13:261–268. doi: 10.3923/jms.2013.261.268. [DOI] [Google Scholar]
- 43.Simon C., Foxman B., Nriagu J. Prevalence of antibiotic resistance bacteria and treatment. Appl. Environ. Microbiol. 2009;75:5714–5718. doi: 10.1128/AEM.00382-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enan G., Sitohy M., Osman A., Khalifa M., El-Didamony G. Inhibition of Methicillin Resistant-Vancomycin Intermediate S. aureus (MRSA-VISA) by Glycin Basic Subunit In Vitro and in Minced Meat. First International Congress on Food Safety and Regulatory Measures; Birmingham, UK: 2015. pp. 37–45. [Google Scholar]
- 45.Chopra I., Hodgson J., Metcalf B., Poste G. New approaches to control of infections caused by antibiotic resistant bacteria. Jama. 1996;275:401–403. doi: 10.1001/jama.1996.03530290071040. [DOI] [PubMed] [Google Scholar]
- 46.Chopra I., Hodgson J., Metcalf B., Poste G. The search for antimicrobial agents effective against bacterial resistant to multiple antibiotics. Antimicorb. Agents Chemother. 1997;41:497–503. doi: 10.1128/AAC.41.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedrich C.L., Moyles D., Beveridge T.J., Hancock R.E.W. Antimicrobial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob. Agents Chemother. 2000;44:2086–2092. doi: 10.1128/AAC.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halpin M.I., Richardson T. Elected functionality changes of Beta-lacto globulin upon esterification of side chain carboxyl groups. J. Dairy Sci. 1985;68:3189–3198. doi: 10.3168/jds.S0022-0302(85)81226-1. [DOI] [PubMed] [Google Scholar]
- 49.Sitohy M., Mahgoub S., Osman A. Bioactive proteins against pathogenic and spoilage bacteria. Funct. Food Health Dis. 2014;4:451–462. doi: 10.31989/ffhd.v4i10.155. [DOI] [Google Scholar]
- 50.Liu Q., Kong B., Xiong Y.L., Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- 51.Klompong V., Benjakul S., Kantachote D., Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influence by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- 52.Sharma S., Singh R., Rana R. Bioactive Peptides: A Review. Int. J. Bioautom. 2011;15:223–250. [Google Scholar]
- 53.El-Zahar K., Sitohy M., Choiset Y., Métro F., Haertle T., Chobert J.-M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int. Dairy J. 2005;15:17–27. doi: 10.1016/j.idairyj.2004.06.002. [DOI] [Google Scholar]
- 54.Sitohy M., Chobert J., Haertlé T. Peptic hydrolysis of methyl-, ethyl-, and propyl-esters of beta-casein and alpha-lactalbumin. Milchwissenshaft Milk Sci. Int. 2001;56:303–307. [Google Scholar]
- 55.Sitohy M., Chobert J.M., Dalgalarrondo M., Haertle T. Factors influencing pepsinolysis of methyl-, ethyl-, and propyl-esters of derivatives of β-lactoglobulin. J. Food Biochem. 2001;25:181–198. doi: 10.1111/j.1745-4514.2001.tb00733.x. [DOI] [Google Scholar]
- 56.Lehninger A.L., Nelson D.L., Cox M.M. Lehninger Principles of Biochemistry. Macmillan; New York, NY, USA: 2005. [Google Scholar]
- 57.El-Zahar K., Chobert J.M., Sitohy M., Dalgalarrondo M., Haertlé T. Proteolytic degradation of ewe milk proteins during fermentation of yoghurts and storage. Nahr. Food. 2003;47:199–206. doi: 10.1002/food.200390046. [DOI] [PubMed] [Google Scholar]
- 58.El-Zahar K., Sitohy M., Choiset Y., Metro F., Haertle T., Chobert J. Antimicrobial activity of ovine whey protein and their peptic hydrolysates. Milchwissenshaft Milk Sci. Int. 2004;59:653–656. [Google Scholar]
- 59.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 60.Sitohy M.Z., Mahgoub S.A., Osman A.O. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012;154:19–29. doi: 10.1016/j.ijfoodmicro.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Enan G., El-Essawy A.A., Uytendaale M., Debevere J. Antibacterial activity of L. plantarum UG1 isolated from dry sausage: Characterization, production and bactericidal action of plantaricinUG1. Int. J. Food Microbiol. 1996;30:189–215. doi: 10.1016/0168-1605(96)00947-6. [DOI] [PubMed] [Google Scholar]
- 62.Utsumi S., Matsumura Y., Mori T. Structure-function relationships of soy proteins. In: Damodaran S., Paraf A., editors. Food Proteins and Their Application. Marcel Dekker; New York, NY, USA: 1997. pp. 257–291. [Google Scholar]
- 63.Sitohy M., Mahgoub S., Osman A., El-Masry R., Al-Gaby A. Extent and mode of action of cationic legume proteins against Listeria monocytogenes and Salmonella enteritidis. Probiotics Antimicrob. Proteins. 2013;5:195–205. doi: 10.1007/s12602-013-9134-2. [DOI] [PubMed] [Google Scholar]
- 64.Kuipers B.J. Gruppen, H. Identification of strong aggregating regions in soy glycinin upon enzymatic hydrolysis. J. Agric. Food Chem. 2008;56:3818–3827. doi: 10.1021/jf703781j. [DOI] [PubMed] [Google Scholar]
- 65.Ibrahim H.R. On the novel catalytically-independent antimicrobial function of hen egg-white lysozyme: A conformation-dependent activity. Nahrung. 1998;42:187–193. doi: 10.1002/(SICI)1521-3803(199808)42:03/04<187::AID-FOOD187>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Hughey V.L., Johnson E.A. Antimicrobial activity of lysozyme against bacteria involved in food spoilage and food-borne disease. Appl. Environ. Microbiol. 1987;53:2165–2170. doi: 10.1128/AEM.53.9.2165-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bera A., Herbert S., Jacob A., Vollmer W., Gotz F. Why are pathogenic Staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferaze OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 2005;55:778–787. doi: 10.1111/j.1365-2958.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 68.Atrih A., Bacher G., Allmaier G., Williamson M.P., Foster S.J. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 1999;181:3956–3966. doi: 10.1128/JB.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szwajkowska M., Wolanciuk A., Barłowska J., Król J., Litwińczuk Z. Bovine milk proteins as the source of bioactive peptides influencing the consumers’ immune system—A review. Anim. Sci. Pap. Rep. 2011;29:269–280. [Google Scholar]
- 70.Jeremy J.M., Sondra C.F., Anthony C.J.L. Longer-duration uses of tetracyclines and penicillins in U.S. food-producing animals: Indications and microbiologic effects. Environ. Int. 2011;37:991–1004. doi: 10.1016/j.envint.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Lavalette D., Te´treau C., Tourbez M., Blouquit Y. Microscopic viscosity and rotational diffusion of proteins in a macromolecular environment. Biophys. J. 1999;76:2744–2751. doi: 10.1016/S0006-3495(99)77427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murzyn K., Rog T., Pasenkiewicz-Gierula M. Phosphatidylethanolamine-phosphatidyl glycerol bilayer as a model of the inner bacterial membrane. Biophys. J. 2005;88:1091–1103. doi: 10.1529/biophysj.104.048835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Enan G., Al-Mohammadi A.-R., Mahgoub S., Abdel-Shafi S., Askar E., Ghaly M.F., Taha M.A., El-Gazzar N. Inhibition of Staphylococcus aureus LC 554891 by Moringa oleifera seed extract either singly or in combination with antibiotics. Molecules. 2020;25:4583. doi: 10.3390/molecules25194583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hugo W., Russel A. Pharmaceutical Microbiology. 5th ed. Black Well Scientific Publications; Oxford, UK: 1990. pp. 369–380; 487–492. [Google Scholar]
- 75.Giuliani A., Pirri G., Rinaldi A.C. Antimicrobial peptides: The LPS connection. Methods Mol. Biol. 2010;618:137–154. doi: 10.1007/978-1-60761-594-1_10. [DOI] [PubMed] [Google Scholar]
- 76.Castro A., Silva J., Teixeira P. Staphylococcus aureus, a Food Pathogen: Virulence Factors and Antibiotic Resistance. Food Borne Dis. 2018:213–238. doi: 10.1016/B978-0-12-811444-5.00008-7. [DOI] [Google Scholar]
- 77.Hoek K.S., Milne J.M., Grieve P.A., Dionysius D.A., Smith R. Antibacterial activity of bovine lactoferrin–derived peptides. Antimicrob. Agents Chemother. 1997;41:54–59. doi: 10.1128/AAC.41.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loomis T.A., Hayes A.W. Loomis’s Essentials of Toxicology. Academic Press; San Diego, CA, USA: 1996. pp. 205–248. [Google Scholar]
- 79.Andersen H., Larsen S., Spliid H., Christensen N.D. Multivariate statistical analysis of organ weights in toxicity studies. Toxicology. 1999;136:67–77. doi: 10.1016/S0300-483X(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 80.Morita C., Nishida T., Ito K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral Biol. 2011;56:359–366. doi: 10.1016/j.archoralbio.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Sitohy M., Osman A., Gharib A., Chobert J.-M., Haertlé T. Preliminary assessment of potential toxicity of methylated soybean protein and methylated β-lactoglobulin in male Wistar rats. Food Chem. Toxicol. 2013;59:618–625. doi: 10.1016/j.fct.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 82.Otte J., Abdel-Hamid M., Osman A. Comparative assessment of peptide concentration in milk protein hydrolysates and fractions. Int. J. Dairy Sci. 2015;10:228–235. doi: 10.3923/ijds.2015.228.235. [DOI] [Google Scholar]
- 83.Adler-Nissen J. Enzymic Hydrolysis of Food Proteins. Elsevier Applied Science Publishers; Amsterdam, The Netherlands: 1986. [Google Scholar]
- 84.Sitohy M., Osman A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010;120:66–73. doi: 10.1016/j.foodchem.2009.09.071. [DOI] [Google Scholar]
- 85.Evans R.W., Williams J. The electrophoresis of transferrins in urea/polyacrylamide gels. Biochem. J. 1980;189:541. doi: 10.1042/bj1890541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement. CLSI; Wayne, PA, USA: 2008. [Google Scholar]
- 87.National Committee for Clinical Laboratory Standards. Barry A.L. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. National Committee for Clinical Laboratory Standards Wayne; Wayne, PA, USA: 1999. [Google Scholar]
- 88.Schafer E.W. The acute oral toxicity of 369 pesticidal, pharmaceutical and other chemicals to wild birds. Toxicol. Appl. Pharmacol. 1972;21:315–330. doi: 10.1016/0041-008X(72)90151-2. [DOI] [PubMed] [Google Scholar]
- 89.OECD . Guidelines for The Testing of Chemicals: Acute Oral Toxicity—Fixed Dose Procedure. OECD Environment Directorate, Environment, Health and Safety Division; Paris, France: 2001. OECD/OCDE 420. [Google Scholar]
- 90.Osman A., Abd-Elaziz S., Salama A., Eita A.A., Sitohy M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. Eur. Asian J. Biosci. 2019;13:105–112. [Google Scholar]
- 91.Guidelines for Hematoxylin and Eosin Staining. National Society for Histotechnology; Ellicott City, MD, USA: 2001. [Google Scholar]