The mcr-1 gene is widely reported around the world and has been identified on various plasmids with different replicon types. Resistance to the last-line antibiotic colistin mediated by mcr-1 still represents a threat to global public health.
KEYWORDS: Escherichia coli, IncI2, Père David's deer, blaCTX-M-132, colistin resistance, mcr-1, nanopore sequencing
ABSTRACT
Père David’s deer (Elaphurus davidianus or milu) is an endangered species, and the prevalence of antimicrobial resistance (AMR) such as mcr-1-positive strains among them has been unknown. In this study, we aimed to investigate the genomic characterizations of mcr-1-positive strains and provide insight into the dissemination of AMR in nature reserve settings. Sixty-seven mcr-1-positive Escherichia coli isolates from 97 fecal samples were identified by PCR and found resistant to colistin. The prevalence of β-lactam resistance was very high, and there were 64 mcr-1-positive isolates containing β-lactamase genes. Transconjugants of 66 mcr-1-positive isolates were acquired through conjugation experiments. PCR-based replicon typing (PBRT) showed that 44 strains harbored IncI2 mcr-1-bearing plasmids, eight strains harbored IncX4 mcr-1-carrying plasmids, and 14 strains harbored IncHI2 mcr-1-positive plasmids. Notably, mcr-1 was located in the chromosome of LD27-1. Clonal dissemination and horizontal dissemination of mcr-1 by plasmids coexist. We first report the prevalence of plasmid-mediated mcr-1 in E. coli from Père David’s deer in China. mcr-1-bearing IncI2 plasmid was the most frequent plasmid type, and the first IncI2 plasmid harboring both blaCTX-M-132 and mcr-1 is characterized here. Our results support the implication of Père David’s deer as a potential reservoir for MCR-1-producing E. coli.
IMPORTANCE The mcr-1 gene is widely reported around the world and has been identified on various plasmids with different replicon types. Resistance to the last-line antibiotic colistin mediated by mcr-1 still represents a threat to global public health. Père David’s deer is a highly endangered species originating in China, and many deer are currently being raised in captivity for gradual reintroduction to the wild. If this species carries AMR bacteria, it will pose a potential threat to the environment. Therefore, research on the dissemination of mcr-1-positive E. coli from Père David’s deer is of great significance. This is the first study to investigate the microbiological and genomic surveillance of MCR-1-producing bacteria colonized among Père David’s deer in China, and we uncovered a high prevalence of MCR-1-producing E. coli. The importance of constant surveillance for AMR bacteria in nature reserve settings is emphasized.
OBSERVATION
Plasmid-mediated mobilized colistin resistance (mcr) genes (mcr-1 to mcr-10) have been reported all over the world since the first report of mcr-1 in 2016 (1, 2). However, the mcr-1 gene is still the most widespread of the mcr genes and has been identified on both broad-host-range and narrow-host-range plasmids of different replicons, including IncX3, IncX4, IncH1, IncHI1, IncHI2, IncP, IncI2, IncF, IncFII, and IncY (3–6). Resistance to the last-line antibiotic colistin mediated by mcr-1 represents a threat to global public health. Strains positive for mcr-1 in various sources have been reported. Père David’s deer is a highly endangered species originating in China, and many deer are currently being raised in nature reserve settings. Dissemination of antimicrobial resistance (AMR) bacteria among animals poses a potential threat to the environment. Therefore, research on the dissemination of mcr-1-positive Escherichia coli from Père David’s deer is of great significance. To our knowledge, the distribution of mcr-1 in Père David’s Deer National Nature Reserve remained to be investigated up to now. To further learn the transmission characteristics of mcr-1 among nature reserve sites, we studied the prevalence of mcr-1 in E. coli from Père David’s deer and proved that mcr-1 has been widespread in the nature reserve.
Among 97 samples, 55 (56.70%) yielded 67 mcr-1-positive strains, and no other mcr variants were found. All the mcr-1-positive strains were identified as E. coli. This indicated that mcr-1-positive E. coli strains existed in nature reserve environments at a high prevalence. All 67 mcr-1-positive isolates conferred resistance to colistin (MICs ranging from 4 to 8 mg/liter) (see Table S1 in the supplemental material). The antibiotic resistance rates of β-lactams amoxicillin, aztreonam, and ceftiofur were very high. We further identified genes encoding β-lactamases through multiplex PCR methods, as previously reported (7). There were 65 (97.01%) mcr-1-positive isolates containing β-lactamase genes (Tables S2 and S3), with blaCTX-M (57 strains) and blaTEM (19 strains) most widely distributed in these mcr-1-positive isolates (Tables S2 and S3). blaCTX-M and blaTEM were prevalent extended-spectrum-β-lactamase (ESBL) genes conferring resistance to most β-lactam antibiotics (8). In addition, genes encoding OXA-1-like broad-spectrum β-lactamases were detected in 7 strains harboring IncX4 type plasmids (Table S2). The mcr-1 gene from 66 of 67 isolates and the resistance phenotype were successfully transferred to E. coli J53 (Table S1), suggesting that the mcr-1 gene was located in conjugative plasmids or other mobilizable genetic elements (MGEs) in the 66 isolates. PCR-based replicon typing (PBRT) was performed for all 67 mcr-1-positive isolates and 66 transconjugants. Results showed that 44 transconjugants harbored only an IncI2 plasmid, but corresponding parental strains contained 1 to 4 replicon types, including IncI2. Eight transconjugants harbored only an IncX4 plasmid, and corresponding parental strains also had only an IncX4 plasmid (Table S2). It was concluded that mcr-1 was located in IncI2 or IncX4 plasmids in these strains. Two replicon types of IncHI2 and IncN from 13 transconjugants were identified, and 2 to 4 replicon types, including IncHI2 and IncN, were detected from their corresponding parental strains. It has been reported that the mcr-1-positive IncHI2 plasmid pMCR1_1943 (265,538 bp) also had an IncN replicon (9). S1 nuclease-based pulsed-field gel electrophoresis (S1-PFGE) of 8 transconjugants showed that only one plasmid was visible with a size similar to pMCR1_1943. Therefore, we considered that mcr-1 was located in the IncHI2 plasmid in the 13 strains. However, there were no replicon types detected in strain LD27-1, which implied a possible chromosomal location of mcr-1. In addition, replicon types of one transconjugant were exactly the same as those of its parental strain LD91-1, with four replicon types, IncHI2, IncN, IncFIB, and IncF, detected simultaneously. According to the plasmid size in the S1-PFGE fingerprint of TLD91-1 (transconjugant of LD91-1), we speculated that the plasmid of TLD91-1 was a fused plasmid formed during conjugation whose molecular mechanism was reported previously (10).
Antibiotic susceptibility profiles (mg/liter) of 67 E. coli strains that harbor the mcr-1 gene. Download Table S1, DOCX file, 0.02 MB (26.2KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Replicon types and β-lactamase genes of 67 mcr-1-positive E. coli strains and their transconjugants. Download Table S2, DOCX file, 0.02 MB (18.2KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Positive rate of β-lactamase genes in 67 mcr-1-positive E. coli strains. Download Table S3, DOCX file, 0.01 MB (12.7KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PFGE patterns with a cutoff at 90% similarity were considered to belong to the same phylogenetic cluster and were indicated as groups A to W, implying diverse strain clones. Two different mcr-1-positive strains with diverse PFGE types were detected in the same sample among 12 samples according to their colony difference in morphology and color (Table 1; Fig. S1). A single sample containing multiple mcr-1-positive isolates suggested that mcr-1 had spread in the same microbiota. We also found that the mcr-1-harboring plasmids carried by these strains were diverse. IncX4 and IncI2 type mcr-1-bearing plasmids appeared in two strains, respectively, in samples LD4 and LD9, indicating that coexistence of different mcr-1-bearing plasmids in diverse bacteria of the same microbiota sample would exacerbate the transmission of resistance genes. In samples LD26, LD39, LD54, LD75, and LD91, mcr-1-positive plasmids with IncHI2 and IncI2 types were found simultaneously in two different strains. Ten strains from samples LD24, LD36, LD38, LD70, and LD94 carried mcr-1-positive IncI2 plasmids (Table 1). In these samples, strains with the extension “-2” were distributed mainly in two PFGE types (11 strains belonging to group A and 1 strain belonging to group F), while the strains with names ending in “-1” were classified into 8 PFGE types (B, C, E, G, H, K, L, and S). This result indicates that the samples are highly diverse. Partial mcr-1-positive strains isolated from Père David’s deer were shown to exhibit genetically similar PFGE types, implying that clonal spread occurred in the nature reserve. mcr-1-positive plasmids with the same replicon type were found in different PFGE clusters, indicating that horizontal dissemination of the mcr-1 gene by plasmids also existed (Fig. S1).
TABLE 1.
Basic information about 12 samples detected with two mcr-1-positive strains
| Sample name | Strain designation |
Replicon type(s) |
||
|---|---|---|---|---|
| Parental strain | Transconjugant | Parental strain | Transconjugant | |
| LD4 | LD4-1 | TLD4-1 | IncX4 | IncX4 |
| LD4-2 | TLD4-2 | IncI2 | IncI2 | |
| LD9 | LD9-1 | TLD9-1 | IncX4 | IncX4 |
| LD9-2 | TLD9-2 | IncI2 | IncI2 | |
| LD24 | LD24-1 | TLD24-1 | IncHI2/IncF/IncFIB/IncI2 | IncI2 |
| LD24-2 | TLD24-2 | IncI2 | IncI2 | |
| LD26 | LD26-1 | TLD26-1 | IncHI2/IncN/IncFIB/IncF | IncHI2/IncN |
| LD26-2 | TLD26-2 | IncI2 | IncI2 | |
| LD36 | LD36-1 | TLD36-1 | IncHI2/IncF/IncFIB/IncI2 | IncI2 |
| LD36-2 | TLD36-2 | IncI2 | IncI2 | |
| LD38 | LD38-1 | TLD38-1 | IncHI1/IncI2/IncFIB | IncI2 |
| LD38-2 | TLD38-2 | IncI2 | IncI2 | |
| LD39 | LD39-1 | TLD39-1 | IncHI2/IncN/IncFIA/IncFIB | IncHI2/IncN |
| LD39-2 | TLD39-2 | IncI2 | IncI2 | |
| LD54 | LD54-1 | TLD54-1 | IncHI2/IncN | IncHI2/IncN |
| LD54-2 | TLD54-2 | IncI2 | IncI2 | |
| LD70 | LD70-1 | TLD70-1 | IncI2 | IncI2 |
| LD70-2 | TLD70-2 | IncI2 | IncI2 | |
| LD75 | LD75-1 | TLD75-1 | IncHI2/IncN/IncFIB/IncF | IncHI2/IncN |
| LD75-2 | TLD75-2 | IncI2 | IncI2 | |
| LD91 | LD91-1 | TLD91-1 | IncHI2/IncN/IncFIB/IncF | IncHI2/IncN |
| LD91-2 | TLD91-2 | IncI2/IncFIB | IncI2 | |
| LD94 | LD94-1 | TLD94-1 | IncI2 | IncI2 |
| LD94-2 | TLD94-2 | IncI2 | IncI2 | |
PFGE-XbaI dendrogram of mcr-1-positive E. coli isolates. The PFGE assay was conducted according to the standard protocol. PFGE patterns with a cutoff at 90% similarity (indicated by a dashed line) were considered to belong to the same PFGE cluster and are indicated as groups A to W. The last column indicates replicon types of mcr-1-bearing plasmids. Download FIG S1, JPG file, 0.2 MB (205.9KB, jpg) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To obtain a comprehensive view of the genetic features of mcr-1-bearing MGEs in these isolates, five representative mcr-1-positive E. coli strains were selected for further genome characterization. Whole-genome sequencing (WGS) and bioinformatics analyses showed that isolate LD27-1 harbored a chromosome (sequence type 10 [ST10]) 4,694,065 bp in length (Table 2), with mcr-1 located in the chromosome. A 9,974-bp chromosomal segment containing mcr-1 was extracted, and BLASTn analysis was performed, showing that the segment had 99.98% identity (100% coverage) to the sequence of the E. coli L73 chromosome (CP033378) isolated from goose and 99.99% identity (100% coverage) to the sequence of the E. coli PE15 chromosome (CP041628) of pig origin. A hypothetical protein was disrupted by insertion of ISApl1-mcr-1-orf, which may derive from Tn6330 (ISApl1-mcr-1-orf-ISApl1) that L73 and PE15 chromosomes contained (3) (Fig. S2).
TABLE 2.
Detailed information about plasmids of five sequenced mcr-1-positive strains
| Strains | Sequence type | Plasmid | Accession no. | Size (bp) | Replicon type(s) (accession no.) | Resistance gene(s) |
|---|---|---|---|---|---|---|
| LD26-1 | ST3714 | pLD26-1-MCR1 | CP047666 | 251,000 | IncHI2, IncHI2A, IncN | mph(A), aac(3)-IV, aadA1, aadA2, aph(3')-Ia, aph(4)-Ia, fosA3, dfrA12, mcr-1, sul1, sul2, sul3, blaCTX-M-14, cmlA1, floR |
| pLD26-1-135kb | CP047667 | 135,123 | IncFIB (AP001918), IncFII | dfrA14, tet(A), floR, blaTEM-135, qnrS1 | ||
| LD22-1 | ST3714 | pLD22-1- MCR1 | CP047877 | 251,000 | IncHI2, IncHI2A, IncN | blaCTX-M-14, mph(A), mcr-1, aac(3)-IV, aadA1, aadA2, aph(3‘)-Ia, aph(4)-Ia, cmlA1, floR, fosA3, sul1, sul2, sul3, dfrA12 |
| pLD22-1-135kb | CP047878 | 135,123 | IncFIB (AP001918), IncFII | dfrA14, tet(A), floR, blaTEM-135, qnrS1 | ||
| pLD22-1-6kb | CP047879 | 6,430 | None | None | ||
| LD39-1 | ST2325 | pLD39-1- MCR1 | CP047659 | 251,000 | IncHI2, IncHI2A, IncN | blaCTX-M-14, fosA3, mph(A), cmlA1, floR, mcr-1, aac(3)-IV, aadA1, aadA2, aph(3')-Ia, aph(4)-Ia, dfrA12, sul1, sul2, sul3 |
| pLD39-1-134kb | CP047660 | 134,831 | IncFIB (pB171), IncFIA | None | ||
| pLD39-1-6kb | CP047661 | 6,938 | None | None | ||
| LD67-1 | ST1485 | pLD67-1-MCR1 | CP061186 | 66,568 | IncI2 | mcr-1, blaCTX-M-132 |
| pLD67-1-157kb | CP061187 | 157,028 | IncHI2, IncHI2A | aadA1, dfrA14, tet(A), qnrS1, ARR-2, blaOXA-10, cmlA1, floR | ||
| pLD67-1-165kb | CP061188 | 165,427 | IncFIA, IncFIB (AP001918) | dfrA14, blaTEM-1B, aph(3'')-Ib, aph(6)-Id, sul2 | ||
| LD93-1 | ST7511 | pLD93-1-90kb | CP047663 | 90,674 | None | None |
| pLD93-1-MCR1 | CP047664 | 33,309 | IncX4 | mcr-1 |
Linear sequence alignment between the selected fragment of E. coli LD27-1 chromosome and E. coli L73 and PE15 chromosome fragments. Download FIG S2, JPG file, 0.1 MB (142.7KB, jpg) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
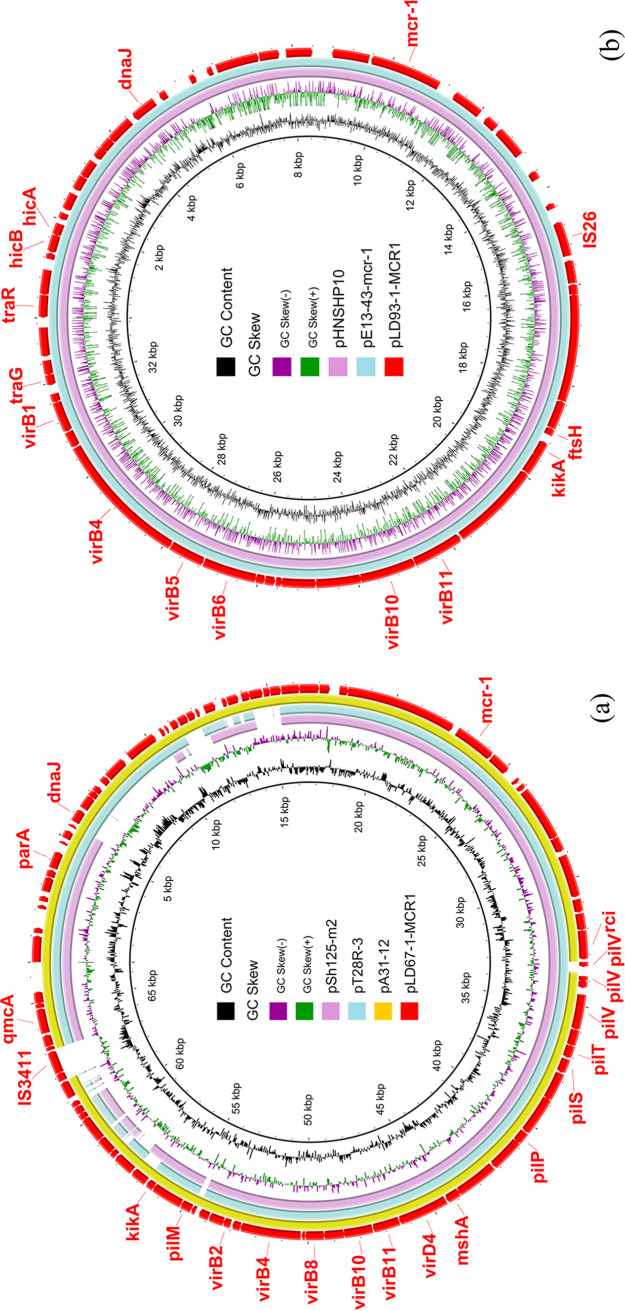
LD67-1 harbored a chromosome (ST1485) and three plasmids consisting of pLD67-1-MCR1 (66,568 bp), pLD67-1-157kb (157,028 bp), and pLD67-1-165kb (165,427 bp). pLD67-1-MCR1 was an mcr-1-bearing IncI2 plasmid carrying the ESBL gene blaCTX-M-132 and with an overall 99.14% nucleotide identity and 100% query coverage to the sequence of E. coli A31-12 plasmid pA31-12 (GenBank accession no. KX034083) (Fig. 1a), which was an mcr-1-harboring plasmid with blaCTX-M-55. pA31-12 was the first IncI2 plasmid coharboring blaCTX-M-55 and mcr-1 (11). pLD67-1-MCR1 showed 99.61% identity (90% coverage) to E. coli T28R plasmid pT28R-3 (CP049356) (Fig. 1a), which was an mcr-1- and blaCTX-M-64-carrying plasmid. No other CTX-M genes were found in mcr-1-carrying IncI2 plasmid in the nr/nt database (as of 1 September 2020). We first reported the IncI2 plasmid harboring both blaCTX-M-132 and mcr-1 in this study. pLD67-1-MCR1 showed 99.79% identity (82% coverage) with Shigella sonnei SH11Sh125 mcr-1-carrying plasmid pSh125-m2 (KY363998) (Fig. 1a) without CTX-M genes. Cotransfer of mcr-1 with blaCTX-M-132 by a single conjugative plasmid constitutes a serious threat. pLD67-1-157kb (157,028 bp) was an IncHI2 plasmid with resistance genes aadA1, dfrA14, tet(A), qnrS1, ARR-2, blaOXA-10, cmlA1, and floR (Table 2). pLD67-1-165kb (165,427 bp) was an IncFIA and IncFIB multidrug resistance plasmid carrying dfrA14, blaTEM-1B, aph(3′')-Ib, aph(6)-Id, and sul2 (Table 2).
FIG 1.
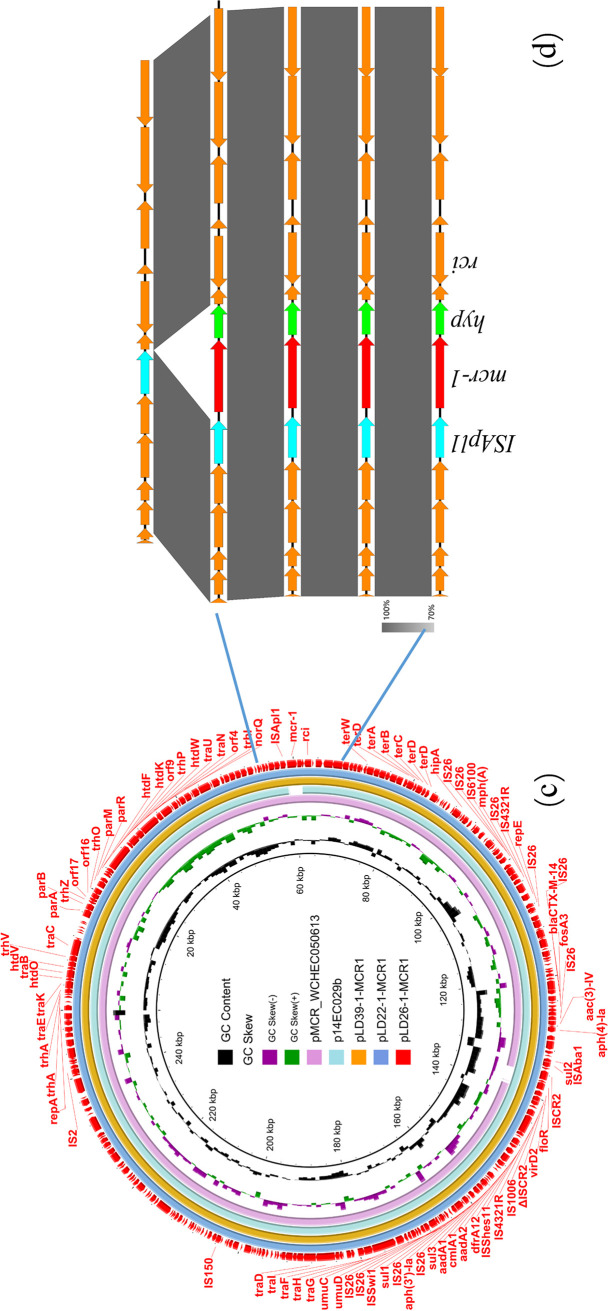
Sequence comparison of plasmids harboring the mcr-1 gene. (a) Circular comparison between mcr-1-bearing IncI2 plasmid pLD67-1-MCR1 in strain LD67-1 in this study and three similar IncI2 plasmids in the NCBI nr database. pLD67-1-MCR1 was used as the reference in the outermost ring. (b) Circular comparison between mcr-1-carrying IncX4 plasmid pLD93-1-MCR1 in strain LD93-1 in this study and two similar IncX4 plasmids in the NCBI nr database. The outer circle with red arrows denotes annotation of reference plasmid pLD93-1-MCR1. (c) Circular comparison between three mcr-1-bearing IncHI2 plasmids and two similar IncHI2 plasmids in the NCBI nr database. (d) Linear comparison of the partial sequence containing mcr-1 of IncHI2 type plasmids (pLD22-1, pLD26-1, and pLD39-1) with two similar structures of plasmids pMCR_WCHEC050613 and p14EC029b in the NCBI nr database.
One chromosome and two plasmids, pLD93-1-90kb (90,674 bp) and pLD93-1-MCR1 (33,309 bp), were found in strain LD93-1, which belonged to ST7511. The mcr-1-positive plasmid pLD93-1-MCR1 was an IncX4 plasmid sharing 100% identity (100% coverage) to pE13-43-mcr-1 (GenBank accession no. MG747473) (Fig. 1b) from human urine. pLD93-1-MCR1 also showed 100% identity and 100% coverage to pHNSHP10 (MF774182) (Fig. 1b) of pig origin, indicating that mcr-1-bearing IncX4 plasmids could disseminate in human, food animals, and Pere David’s deer. There were no plasmid replicons and resistance genes found in pLD93-1-90kb (Table 2).
Three mcr-1-bearing IncHI2-positive strains, LD22-1, LD26-1, and LD39-1, were randomly selected to perform genomic analysis. LD22-1 and LD26-1 belonged to ST3714, and LD39-1 belonged to ST2325. They possessed the typical IncHI2 mcr-1-bearing plasmids pLD22-1-MCR1, pLD26-1-MCR1, and pLD39-1-MCR1, sharing 100% identity with each other (251,000 bp in length) and carried the same resistance genes, namely, blaCTX-M-14, fosA3, mph(A), cmlA1, floR, aac(3)-IV, aadA1, aadA2, aph(3′)-Ia, aph(4)-Ia, dfrA12, sul1, sul2, and sul3 (Table 2). BLASTn analysis showed that pLD26-1-MCR1 exhibited 99.98% identity (98% coverage) to plasmid pMCR_WCHEC050613 (GenBank accession no. CP019214) and 99.99% identity (98% coverage) to plasmid p14EC029b (CP024143) (Fig. 1c). ISApl1 upstream of mcr-1 played a crucial part in the presence of mcr-1 (Fig. 1d). IncFIB-IncFII plasmid pLD22-1-135kb (135,123 bp) showed 100% identity to pLD26-1-135kb (135,123 bp), carrying resistance genes dfrA14, tet(A), floR, blaTEM-135, and qnrS1. pLD39-1-134kb (134,831 bp) was an IncFIA-IncFIB plasmid and had no resistance genes. Small plasmids pLD22-1-6kb and pLD39-1-6kb were found in strain LD22-1 and LD39-1 (Table 2).
Our investigation found that there is a supplementary feeding area in the nature reserve where commercial feed additives such as colistin may have been used in feedstuff before May 2017. Widespread mcr-1 dissemination in Père David’s deer may be attributable to selective pressure exerted by colistin. It has been suggested that mcr-1 spreads to humans from farmed animals (1, 12). As a result, China banned the use of colistin as a feed additive for animals on 1 May 2017 (13). It has been reported that the mcr-1 prevalence decreased significantly in national pig farms after the ban of colistin in animal feed (14). A reduction in the mcr-1-positive E. coli population size following the colistin ban could also be expected in Père David’s deer, which warrants further surveillance.
The global spread of ESBL producers is of great concern to human and animal health, and CTX-Ms are the most predominant ESBLs worldwide (15). CTX-M-producing E. coli isolates were recognized as a major cause of hospital- and community-onset infections (16, 17). The coexistence of ESBL genes and mcr-1 in E. coli with multidrug resistance was first reported in China in 2016 (18). Evidence showed that ESBL-producing E. coli was more likely to recruit the mcr-1 gene than non-ESBL-producing E. coli (19). Given the fact that colistin is one of the last-line antibiotics for managing multidrug-resistant infections, cotransfer of mcr-1 with ESBL genes by a single mobile plasmid might compromise clinical treatment considerably. In our work, a high prevalence of IncI2 and IncHI2 plasmids harboring both blaCTX-M and mcr-1 (Table 2; Table S2) indicates a widespread situation of such notorious MDR plasmids among animals. The emergence of the IncI2 plasmid harboring blaCTX-M-132 and mcr-1 reported here should arouse our attention.
The limitations of this study are that geographical distribution and the number of samples collected are relatively confined. Although we collected fresh fecal samples from areas as scattered as possible to guarantee that fecal samples were derived from different individuals of Pere David's deer, duplicate sampling of the same animal was possible.
To conclude, this research is the first report of the prevalence of colistin resistance gene mcr-1 in E. coli from Père David’s deer in China that was mainly mediated by plasmids. Mobilizable mcr-1-positive plasmids (IncI2, IncX4, and IncHI2) were widespread in Père David’s Deer National Nature Reserve, which may be an important reservoir of mcr-1. The IncI2 mcr-1-bearing plasmid was the most frequent plasmid type (44/67, 65.67%) in the nature reserve, and we are the first to report the IncI2 plasmid harboring both blaCTX-M-132 and mcr-1. Wide spread of mcr-1-harboring IncHI2 plasmids carrying multiple resistance genes in the nature reserve constitutes an easily missed source of multidrug-resistant Gram-negative bacteria. The horizontal dissemination of the mcr-1 gene by plasmids needs to be further investigated, and monitoring of other important emerging resistance genes in nature reserves of animals should be performed.
Bacterial isolates and identification.
On 18 August 2018, a total of 97 fresh fecal samples were collected from Père David’s Deer National Nature Reserve in Dafeng, Jiangsu Province, China. Individual fresh fecal samples were collected using a sterile swab, subsequently diluted in brain heart infusion broth containing 2 mg/liter colistin, and incubated for 6 h at 37°C. The cultures were plated on MacConkey agar and incubated for 12 h at 37°C. One to three colonies with different morphological characteristics from each MacConkey agar plate were purified and subsequently screened for mcr-1 to mcr-5 genes by multiplex PCR method as previously reported (20). 16S rRNA gene sequencing was performed to confirm bacterial species.
Antimicrobial susceptibility testing.
The MICs of colistin (CST), aztreonam (ATM), amoxicillin (AMC), florfenicol (FFC), ceftiofur (CFF), streptomycin (STR), doxycycline (DOX), meropenem (MEM), and enrofloxacin (ENR) for all mcr-1-bearing isolates were determined by the broth microdilution method and interpreted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (21) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (https://www.eucast.org/clinical_breakpoints/). Colistin was interpreted in accordance with the EUCAST (susceptible, ≤2 mg/liter; resistant, >2 mg/liter). Reference strain E. coli ATCC 25922 served as the quality control strain.
Conjugation experiments and plasmid replicon typing.
To investigate the transferability of mcr-carrying plasmids, conjugation assays were performed using MCR-producing strains as donors and E. coli J53 (Azir) as the recipient. Bacterial strains were streaked onto LB agar plates, followed by inoculation into LB broth overnight. Cultures of donors and the recipient were mixed 1:3, and then 100 μl of mixed culture was applied onto LB agar plates, followed by incubation at 37°C for 16 to 20 h. After incubation, we subsequently diluted the mixed culture on LB agar plates in sterile saline. LB agar plates supplemented with colistin (2 mg/liter) and sodium azide (150 mg/liter) were used to recover transconjugants. The presence of mcr genes in transconjugants was confirmed by PCR and antimicrobial susceptibility testing as described above. The plasmid contents of mcr-positive strains and transconjugants were determined by PBRT (22) with the addition of PCR detections of IncX4 and IncI2 plasmids, as previously described (3).
XbaI-PFGE and S1-PFGE.
XbaI pulsed-field gel electrophoresis (PFGE) was performed to assess the genetic relatedness of all mcr-positive isolates. Briefly, the whole-cell DNA of E. coli isolates was digested with the XbaI restriction enzyme for 3 h at 37°C. Electrophoresis was conducted on a CHEF-DR III apparatus (Bio-Rad, Hercules, CA, USA) through a 1% agarose gel in 0.5× Tris-borate-EDTA buffer using an initial pulse time of 4 s and a final pulse time of 45 s at a voltage of 200 V for 20 h at 14°C (23). Genomic DNA of the Salmonella enterica serovar Braenderup strain H9812 restricted with XbaI (TaKaRa, Osaka, Japan) was used as the reference standard. Cluster analysis of XbaI-PFGE fingerprints was typically performed by using BioNumerics 7.6 (Applied Maths, Sint-Martens-Latem, Belgium). Bacterial DNA was prepared in agarose plugs and digested with 1 U of S1 nuclease (New England Biolabs) for S1 nuclease-based PFGE (S1-PFGE).
Plasmid sequencing and bioinformatics analyses.
The genomic DNA of several mcr-1-positive E. coli strains was extracted using a TIANamp bacteria DNA kit (Tiangen, Chain) in accordance with the manufacturer’s recommendation. Whole-genome sequencing was performed via Illumina HiSeq and Oxford Nanopore Technologies (ONT) MinION platforms. De novo assembly was performed by Unicycler, combining short-read and long-read data (24). The Rapid Annotation using Subsystems Technology annotation website server (https://rast.nmpdr.org/rast.cgi) was then used to annotate the genomes (25). Online tools including PlasmidFinder 2.1, ResFinder 3.2, and MLST 2.0 (multilocus sequence typing) were utilized to assemble and characterize the mcr-1-bearing genomes (https://cge.cbs.dtu.dk/services/). Comparisons with highly homologous complete plasmid sequences available in NCBI for the plasmids in the study were performed with BRIG (26). To visualize the genetic comparison features, Easyfig was used to generate linear figures (27).
Data availability.
The complete sequences of LD22-1, LD26-1, LD27-1, LD39-1, LD67-1, and LD93-1 were deposited in the NCBI database under the following accession numbers: LD22-1 chromosome, CP047876; LD26-1 chromosome, CP047665; LD27-1 chromosome, CP047594; LD39-1 chromosome, CP047658; LD67-1 chromosome, CP061185; and LD93-1 chromosome, CP047662. Accession numbers of all plasmids are listed in Table 2.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 31872523 to Ruichao Li), the National Natural Science Foundation of China (grant no. 31872526 to Zhiqiang Wang), Natural Science Foundation of Jiangsu Province (grant no. BK20180900 to Ruichao Li) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
REFERENCES
- 1.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. 2020. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect 9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Xie M, Zhang J, Yang Z, Liu L, Liu X, Zheng Z, Chan EW, Chen S. 2017. Genetic characterization of mcr-1-bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J Antimicrob Chemother 72:393–401. doi: 10.1093/jac/dkw411. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Yang Y, Miao M, Chavda KD, Mediavilla JR, Xie X, Feng P, Tang YW, Kreiswirth BN, Chen L, Du H. 2016. Complete sequences of mcr-1-harboring plasmids from extended-spectrum-beta-lactamase- and carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4351–4354. doi: 10.1128/AAC.00550-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Feng Y, Liu F, Jiang H, Qu Z, Lei M, Wang J, Zhang B, Hu Y, Ding J, Zhu B. 2017. A phage-like IncY plasmid carrying the mcr-1 gene in Escherichia coli from a pig farm in China. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.02035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Sun J, Li J, Ding Y, Li XP, Lin J, Hassan B, Feng Y. 2017. Expanding landscapes of the diversified mcr-1-bearing plasmid reservoirs. Microbiome 5:70. doi: 10.1186/s40168-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, He T, Han J, Wang J, Foley SL, Yang G, Wan S, Shen J, Wu C. 2012. Prevalence of ESBLs and PMQR genes in fecal Escherichia coli isolated from the non-human primates in six zoos in China. Vet Microbiol 159:53–59. doi: 10.1016/j.vetmic.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Ma K, Feng Y, Zong Z. 2018. Fitness cost of a mcr-1-carrying IncHI2 plasmid. PLoS One 13:e0209706. doi: 10.1371/journal.pone.0209706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Lu X, Peng K, Liu Y, Xiao X, Wang Z. 2020. Reorganization of mcr-1-bearing large MDR plasmids resolved by nanopore sequencing. J Antimicrob Chemother 75:1645–1647. doi: 10.1093/jac/dkaa046. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Li XP, Yang RS, Fang LX, Huo W, Li SM, Jiang P, Liao XP, Liu YH. 2016. Complete nucleotide sequence of an IncI2 plasmid coharboring blaCTX-M-55 and mcr-1. Antimicrob Agents Chemother 60:5014–5017. doi: 10.1128/AAC.00774-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Chan EW, Xie M, Ye L, Dong N, Chen S. 2017. Widespread distribution of mcr-1-bearing bacteria in the ecosystem, 2015 to 2016. Euro Surveill 22:17-00206. doi: 10.2807/1560-7917.ES.2017.22.39.17-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh TR, Wu Y. 2016. China bans colistin as a feed additive for animals. Lancet Infect Dis 16:1102–1103. doi: 10.1016/S1473-3099(16)30329-2. [DOI] [PubMed] [Google Scholar]
- 14.Shen C, Zhong L-L, Yang Y, Doi Y, Paterson DL, Stoesser N, Ma F, El-Sayed Ahmed MAE-G, Feng S, Huang S, Li H-Y, Huang X, Wen X, Zhao Z, Lin M, Chen G, Liang W, Liang Y, Xia Y, Dai M, Chen D-Q, Zhang L, Liao K, Tian G-B. 2020. Dynamics of mcr-1 prevalence and mcr-1-positive Escherichia coli after the cessation of colistin use as a feed additive for animals in China: a prospective cross-sectional and whole genome sequencing-based molecular epidemiological study. Lancet Microbe 1:e34–e43. doi: 10.1016/S2666-5247(20)30005-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhou K, Zheng B, Zhao L, Shen P, Ji J, Wei Z, Li L, Zhou J, Xiao Y. 2016. High prevalence of ESBL-producing Klebsiella pneumoniae causing community-onset infections in China. Front Microbiol 7:1830. doi: 10.3389/fmicb.2016.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li LM, Wang MY, Yuan XY, Wang HJ, Li Q, Zhu YM. 1969. Characterization of integrons among Escherichia coli in a region at high incidence of ESBL-EC. Pak J Med Sci 30:177–180. doi: 10.12669/pjms.301.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitout JD, Nordmann P, Laupland KB, Poirel L. 2005. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother 56:52–59. doi: 10.1093/jac/dki166. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Seward CH, Wu Z, Ye H, Feng Y. 2016. Genomic insights into the ESBL and MCR-1-producing ST648 Escherichia coli with multi-drug resistance. Sci Bull (Beijing) 61:875–878. doi: 10.1007/s11434-016-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Wang Y, Shi X, Wang S, Ren H, Shen Z, Wang Y, Lin J, Wang S. 2018. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008–2014. Emerg Microbes Infect 7:30. doi: 10.1038/s41426-018-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, Guerra B, Malorny B, Borowiak M, Hammerl JA, Battisti A, Franco A, Alba P, Perrin-Guyomard A, Granier SA, De Frutos Escobar C, Malhotra-Kumar S, Villa L, Carattoli A, Hendriksen RS. 2018. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill 23:17-00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed, CLSI supplement M100. CLSI, Wayne, PA, USA. [Google Scholar]
- 22.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Kim YR, Kim SI, Lee JY, Park YJ, Lee KY, Kang MW. 2005. Nosocomial transmission of CTX-M-15 and OXA-30 beta-lactamase-producing Escherichia coli in a neurosurgical intensive care unit. Ann Clin Lab Sci 35:297–301. [PubMed] [Google Scholar]
- 24.Li R, Xie M, Dong N, Lin D, Yang X, Wong MHY, Chan EW, Chen S. 2018. Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. Gigascience 7:1–9. doi: 10.1093/gigascience/gix132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia FF, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibiotic susceptibility profiles (mg/liter) of 67 E. coli strains that harbor the mcr-1 gene. Download Table S1, DOCX file, 0.02 MB (26.2KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Replicon types and β-lactamase genes of 67 mcr-1-positive E. coli strains and their transconjugants. Download Table S2, DOCX file, 0.02 MB (18.2KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Positive rate of β-lactamase genes in 67 mcr-1-positive E. coli strains. Download Table S3, DOCX file, 0.01 MB (12.7KB, docx) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PFGE-XbaI dendrogram of mcr-1-positive E. coli isolates. The PFGE assay was conducted according to the standard protocol. PFGE patterns with a cutoff at 90% similarity (indicated by a dashed line) were considered to belong to the same PFGE cluster and are indicated as groups A to W. The last column indicates replicon types of mcr-1-bearing plasmids. Download FIG S1, JPG file, 0.2 MB (205.9KB, jpg) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Linear sequence alignment between the selected fragment of E. coli LD27-1 chromosome and E. coli L73 and PE15 chromosome fragments. Download FIG S2, JPG file, 0.1 MB (142.7KB, jpg) .
Copyright © 2020 Lu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The complete sequences of LD22-1, LD26-1, LD27-1, LD39-1, LD67-1, and LD93-1 were deposited in the NCBI database under the following accession numbers: LD22-1 chromosome, CP047876; LD26-1 chromosome, CP047665; LD27-1 chromosome, CP047594; LD39-1 chromosome, CP047658; LD67-1 chromosome, CP061185; and LD93-1 chromosome, CP047662. Accession numbers of all plasmids are listed in Table 2.