Abstract
Background
Despite advances in pediatric health care over recent decades, it is not clear whether survival in children with congenital heart disease (CHD) is still increasing.
Methods and Results
We identified all patients with CHD using nationwide Swedish health registries for 1980 to 2017. We examined the survival trends in children with CHD; we investigated the mortality risk in patients with CHD compared with matched controls without CHD from the general population using Cox proportional regression models and Kaplan–Meier survival analysis. Among 64 396 patients with CHD and 639 012 matched controls without CHD, 3845 (6.0%) and 2235 (0.3%) died, respectively. The mean study follow‐up (SD) was 11.4 (6.3) years in patients with CHD. The mortality risk was 17.7 (95% CI, 16.8–18.6) times higher in children with CHD compared with controls. The highest mortality risk was found during the first 4 years of life in patients with CHD (hazard ratio [HR], 19.6; 95% CI, 18.5–20.7). When stratified by lesion group, patients with non‐conotruncal defects had the highest risk (HR, 97.2; 95% CI, 80.4–117.4). Survival increased substantially according to birth decades, but with no improvement after the turn of the century where survivorship reached 97% in children with CHD born in 2010 to 2017.
Conclusions
Survival in children with CHD has increased substantially since the 1980s; however, no significant improvement has been observed this century. Currently, >97% of children with CHD can be expected to reach adulthood highlighting the need of life‐time management.
Keywords: congenital heart disease, nationwide, pediatric, registry study, survivorship
Subject Categories: Congenital Heart Disease, Epidemiology, Cardiovascular Surgery, Primary Prevention
Clinical Perspective
What Is New?
We report for the first time in a large, national cohort study the survival in children with congenital heart disease born until the late 2010s.
The overall mortality risk was 18 times higher in children with congenital heart disease compared with matched controls without congenital heart disease during a period of almost 40 years follow‐up.
Survival increased substantially according to birth decades, but no further overall improvement was noticed after the turn of the century.
What Are the Clinical Implications?
Children with the most complex congenital malformations, such as non‐conotruncal defects, had the highest risk of mortality and could be considered a risk group.
The mortality was still high during the first 4 years of life in children with congenital heart disease which indicates that continuous monitoring and early intervention may be beneficial.
Over 97% of children with congenital heart disease expected to reach adulthood and the need of lifetime management is mandatory.
Congenital heart disease (CHD) is the most common major congenital malformation, having a prevalence of ≈9 per 1000 live births. 1 , 2 Thanks to the development of pediatric health care over the past 70 years, survival among patients with CHD has effectively increased: >90% of such children born in the early 1990s reached adulthood. 3 , 4 , 5 The improvement has been based on developments in diagnostic techniques, catheter interventions, 6 and several surgical innovations, such as the following: surgical treatment of aortic coartation 7 ; repair of atrioventricular septal defects 8 ; Mustard and Senning atrial corrections 9 , 10 ; Rastelli procedure 11 ; the arterial switch 12 ; and the creation of single‐ventricle Fontan circulation. 13 Despite this improvement, the mortality during the first 4 years of life among patients with CHD remains comparatively high 3 , 14 , 15 , 16 , 17 ; the need for further improvement in pediatric care persists.
Advances in pediatric cardiovascular surgery and cardiac interventional catheterization since the new millennium have shown improved outcomes in selected groups of patients with CHD. 18 , 19 , 20 , 21 , 22 , 23 In addition, the antenatal diagnosis of congenital heart malformations has been introduced; currently 37% of all CHD is diagnosed prenatally. 24 However, it is unclear whether recent developments have had an effect on the survival of pediatric patients with CHD over the past decade. Accordingly, we examined the survival trends and risk of mortality in children with CHD compared with controls without CHD from the general population within a nationwide, registry‐based cohort in Sweden from 1980 to 2017.
Methods
Data Source and Study Population
Sweden is a northern European country of almost 10 million inhabitants with a through taxation publicly financed healthcare system. There are 70 acute care hospitals, all publicly financed and all but a handful also run by the regional authorities. Two complete university affiliated congenital heart care units exist where all congenital heart surgery, pediatric and adult, is performed. We linked data from Swedish health registers to identify patients who were born from January 1, 1980 to December 31, 2017 and who were recorded at any time with a diagnosis of CHD with at least 1 of the following registers: National Hospital Inpatient (complete since 1987, but with coverage of all hospitals performing thoracic surgery since 1970); National Hospital Outpatient (complete since 2001); and National Cause of Death Registers in Sweden (complete since 1968). All diagnoses were coded according to the International Statistical Classification of Diseases, Eighth, Ninth, and Tenth Revisions (ICD‐8, ICD‐9, ICD‐10). Follow‐up and comorbidity data were collected until December 31, 2017 or death.
Each patient with CHD was matched by birth year and sex with 10 control individuals without diagnosis of CHD from the Total Population Register in Sweden. 25 We used hierarchical CHD categorization to classify patients with CHD into different groups according to CHD lesions. The study design has been described previously. 3 , 26 , 27
The study was conducted according to the ethical guidelines of the Declaration of Helsinki and it was approved by the Gothenburg Regional Research Ethics Board (Gpg 912‐16, T 616‐18). All national registration numbers were replaced with a unique code for every individual in the final data set by the Swedish National Board of Health and Welfare and under collaboration with the Statistics Sweden. The requirement for informed consent was waived. The data, methods used in the analysis, and study materials used to conduct the present study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Definitions
We defined patients with CHD as having at least 1 hospital discharge, an outpatient visit, or a death certificate with a registered ICD‐8, ICD‐9, and ICD‐10 diagnosis of CHD (Table S1). To categorize CHD into different lesion groups according to severity, we used the hierarchic classification initially suggested by Botto et al and subsequently used in observational studies, 27 , 28 , 29 , 30 (Table S2). Lesion group 1 was defined as patients with conotruncal defects (such as common arterial trunk, transposition of the great vessels, double‐outlet right ventricle, double‐outlet left ventricle, discordant atrioventricular connection, tetralogy of Fallot, and aortopulmonary septal defect). Lesion group 2 was defined as patients with non‐conotruncal defects (such as endocardial cushion defects, common ventricle, and hypoplastic left heart syndrome). We defined lesion group 3 as patients with coarctation of the aorta. Lesion group 4 was defined as patients with ventricular septal defect. Lesion group 5 was defined as patients with atrial septal defect. Lesion group 6 included all other heart and circulatory system anomalies and all other CHD diagnoses not included in lesion groups 1 to 5.
Cardiac intervention was defined as patients with CHD having undergone at least 1 cardiovascular surgery or cardiac interventional catheterization related to CHD, according to the classification of operations (Sixth edition, Swedish version) 31 or following the classification of surgical procedures (1.9 edition, Swedish version). 32
Statistical Analysis
Baseline characteristics were reported as proportions and percentages of sex, birth period, and number of deaths for patients with CHD and controls separately. For continuous variables, mean and median with SD and interquartile range were reported. We used survival analysis techniques to compare patients with CHD and matched controls in terms of mortality outcomes. Incidence rate was estimated as the number of deaths divided by total follow‐up time and reported as per 10 000 person‐years. We estimated survival by means of the Kaplan–Meier estimator; we compared patients with CHD with controls (both overall and within groups) from birth until the age of 18 years. We compared the survival curves using non‐parametric log‐rank tests. We did not adjust for any confounders such as comorbidities because the follow‐up of the study started at birth and there were no recorded comorbidities at that time. Patients with CHD and matched controls that died shortly after birth were accounted in the present study; however, those who died the same date as their date of birth, were given 1 day of follow‐up.
Hazard ratios (HRs) and the associated 95% CIs were calculated by means of the Cox proportional hazards model. We used 2‐sided P values and considered a P value of <0.05 as statistically significant. Statistical analyses were conducted with SAS software (version 9.4; SAS institute, Cary, NC, USA) or R software (version 3.6.1; Free Software Foundation for Statistical Computing, Vienna, Austria).
Results
We identified 64 396 patients with CHD and 639 012 matched controls; the characteristics of the study population appear in Table 1. The majority of the patients with CHD and matched controls were born in Sweden; 94.8% and 83.2% respectively. From birth and with a mean (SD) follow‐up of 11.4 (6.3) years in patients with CHD and 12.2 (6.0) years in matched controls, 3845 (6.0%) and 2235 (0.3%) respectively, died. The characteristics were similar for male and females in the study population (Table S3).
Table 1.
Study Population Characteristics
| Characteristics |
Patients With Congenital Heart Disease (n=64 396) |
Controls (n=639 012) |
|---|---|---|
| Male, n (%) | 32 334 (50.2) | 323 340 (50.6) |
| Mean follow‐up, y (SD) | 11.4 (6.3) | 12.2 (6.0) |
| Median follow‐up, y (IQR) | 12.5 (5.6–18.0) | 13.7 (6.8–18.0) |
| Born in Sweden, n (%) | 61 054 (94.8) | 531 866 (83.2) |
| Deaths, n (%) | 3845 (6.0) | 2235 (0.3) |
| Birth period | ||
| Born 1980–1989, n (%) | 9814 (15.2) | 98 140 (15.4) |
| Born 1990–1999, n (%) | 13 997 (21.7) | 139 970 (21.9) |
| Born 2000–2009, n (%) | 21 459 (33.3) | 212 177 (33.2) |
| Born 2010–2017, n (%) | 19 126 (29.7) | 188 725 (29.5) |
IQR indicates interquartile range.
Overall, the risk of mortality was 17.7 times higher for patients with CHD (95% CI, 16.8–18.6; P<0.001) than in matched controls (Table 2). All the lesion groups in patients with CHD had increased mortality risk compared with matched controls. The highest relative risk of mortality was found in patients with non‐conotruncal defects (such as endocardial cushion defects, common ventricle, and hypoplastic left heart syndrome) with an HR of 97.2 (95% CI, 80.4–117.4; P<0.001).
Table 2.
Mortality Risk in Patients With Congenital Heart Disease Compared With Matched Controls According to Lesion Group
| Categorical Hierarchy Group | Deaths in Patients With CHD/All Patients With CHD, n (%) | Deaths in Controls/All Controls, n (%) | HR (95%, CI)* |
|---|---|---|---|
| Lesion group 1 | 764/4593 (16.63) | 171/45 710 (0.37) | 48.8 (41.3–57.6) |
| Lesion group 2 | 972/3081 (31.55) | 121/30 700 (0.39) | 97.2 (80.4–117.4) |
| Lesion group 3 | 199/2773 (7.18) | 112/27 610 (0.41) | 18.4 (14.6–23.2) |
| Lesion group 4 | 525/21 649 (2.43) | 690/214 296 (0.32) | 7.6 (6.8–8.6) |
| Lesion group 5 | 271/13 376 (2.03) | 398/132 561 (0.29) | 6.8 (5.8–8.0) |
| Lesion group 6 | 1114/18 924 (5.87) | 743/188 135 (0.39) | 15.4 (14.0–16.9) |
| All groups | 3845/64 396 (5.97) | 2235/639 012 (0.35) | 17.7 (16.8–18.6) |
CHD indicates congenital heart disease; and HR, hazard ratio.
All P<0.001
Overall survival probability in patients with CHD and matched controls appear in Figure S1. The survival curve in patients with CHD diverged within from the survival curve for controls, mostly within the first 4 years of life when mortality was higher for patients with CHD. However, the survival curves continued to separate more modestly until the age of 18 years (P<0.001).
The survival trends for patients with CHD and matched controls according to birth period are shown in Figure 1. Survival increased markedly in patients with CHD that were born in the 1980s, 1990s, and 2000s. However, we did not observe any change in survival in patients with CHD born in the 2010s compared with such patients born in the 2000s: there was practically identical survival (almost 97%) over the first years in the 2 birth cohorts. We did not find any significant difference in the survival trends between male and female patients with CHD (Figure S2).
Figure 1. Kaplan–Meier survival curves of patients with congenital heart disease and matched controls according to birth period.
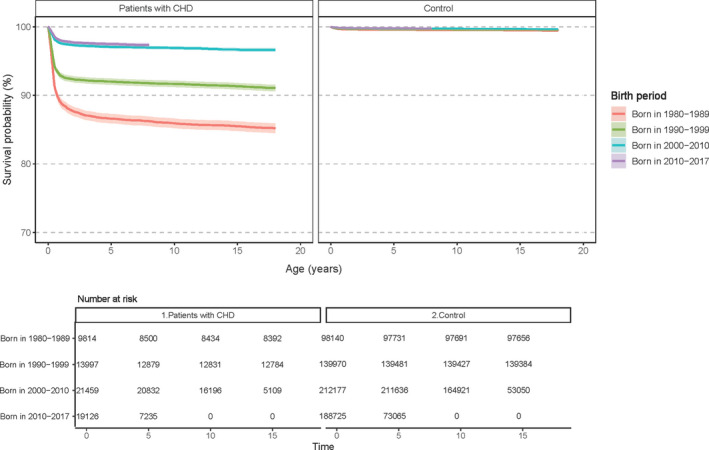
CHD indicates congenital heart disease.
The risk of mortality according to birth period in patients with CHD relative to that of controls appears in Table 3. In all birth periods, patients with CHD had higher risk of mortality than matched controls; that difference decreased over the birth period. Patients with CHD born in the 1980s had the highest relative mortality: HR, 29.0; 95% CI, 26.2 to 31.9; P<0.001. However, the risk was similar in patients with CHD born during the 2000s and 2010s: HR, 10.7 (95% CI, 9.6–11.9; P<0.001) and HR, 11.4 (95% CI, 10.0–13.0; P<0.001) respectively.
Table 3.
Risk of All‐Cause Mortality in Patients With Congenital Heart Disease Compared With Matched Controls According to Birth Period and Sex
| Birth Period | Deaths in Patients With CHD, No./All Patients With CHD, n (%) | Deaths in Controls, No./All Controls, n (%) | HR (95%, CI)* |
|---|---|---|---|
| Birth period | |||
| Born 1980–1989 | 1452/9814 (14.80) | 542/98 140 (0.55) | 29.0 (26.2–32.0) |
| Born 1990–1999 | 1248/13 997 (8.92) | 647/139 970 (0.46) | 20.2 (18.4–22.2) |
| Born 2000–2009 | 688/21 459 (3.21) | 646/212 177 (0.30) | 10.7 (9.6–11.9) |
| Born 2010–2017 | 457/19 126 (2.39) | 400/188 725 (0.22) | 11.4 (10.0–13.0) |
| Sex | |||
| Male | 2026/32 334 (6.27) | 1276/323 340 (0.39) | 16.4 (15.3–17.6) |
| Female | 1819/32 062 (5.67) | 958/315 672 (0.30) | 19.3 (17.9–20.9) |
CHD indicates congenital heart disease; and HR, hazard ratio.
All P<0.001
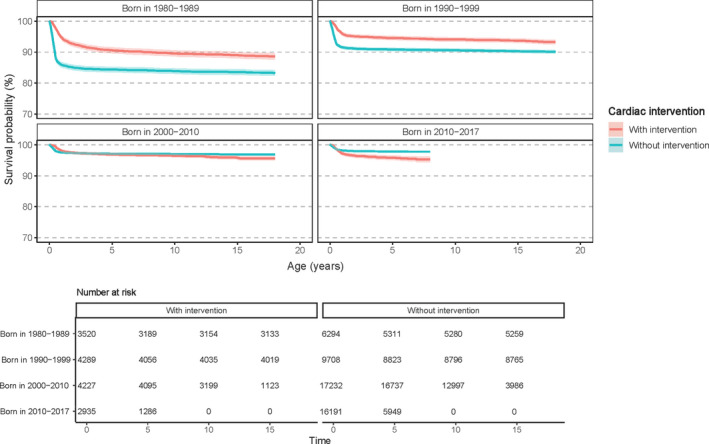
Altogether, 23.2% (n=14 971) of patients with CHD underwent a cardiac intervention related to their CHD between birth and the age of 18 years. Survival increased in patients with CHD with and without cardiac intervention until the 2000s (Figure 2). However, in the past decade, no further improvement in survival appeared in patients with CHD who had undergone at least 1 cardiac intervention: the mortality was up to 4.5% at the age of 7 years.
Figure 2. Kaplan–Meier survival curves of patients with congenital heart disease with or without a cardiac intervention according to birth period.
Survival showed a significant improvement in patients with CHD who were born between the 1980s and 2010s, particularly in those with complex congenital malformations (Figure S3). Among complex lesion groups, survival improved from about 70% and 50%, respectively, at the age of 18 years to >90% in lesion group 1 and >80% in lesion group 2; the latter included highly complex conditions such as hypoplastic left heart syndrome. However, survival was stable and similar in all lesion groups after the millennium: no further improvement was evident.
The risk of mortality in patients with CHD, compared with controls, declined dramatically with increasing age and by birth period (Table S4). The highest mortality was found during the first 4 years of life in patients with CHD born in the 1980s (HR, 34.3, 95% CI, 30.7–38.3, P<0.001); however, the HRs decreased by two thirds in the most recent birth period cohort (2010–2017).
A sensitivity analysis was performed after excluding individuals that were not born in Sweden and the overall risk of mortality in patients with CHD born in Sweden was 15.7 times higher (95% CI, 14.9–16.5) compared with matched controls.
Discussion
In Sweden, survival among pediatric patients with CHD has increased dramatically since the 1980s; currently, >97% can be expected to reach adulthood. However, no further improvement in survivorship was observed in children with CHD over the past decade. Although declining, mortality remains comparatively high in relative as well as absolute terms with the most complex conditions and during the first years of life: the mortality in patients with complex CHD lesions born 1980s and 2000s decreased from almost 50% to <20%, respectively, at the age of 18 years.
We observed an increase in the number of patients with CHD over time which is explained by population growth. This may in part be explained by increased diagnosis of less severe CHD cases. However, also among complex CHD we observed no further improvement in prognosis in recent years (Figure S3). This is also reflected in the comparatively stable level of interventions.
Numerous studies have reported outcomes with particular diagnostic groups for post‐surgical results. 22 , 23 , 33 , 34 , 35 However, our study is the first nationwide report to cover recent trends and examine an unselected, broad, representative population of children with CHD (including matched controls), including patients where no cardiac interventions took place.
The use of antenatal screening for CHD diagnosis have increased over the past decade 36 , 37 ; though feasible, it is not clear whether the use of antenatal CHD diagnosis leads to improved care or survival. There are several reports indicating that in some countries including Sweden, increasing prenatal diagnosis of highly complex malformations such as hypoplastic left heart syndrome will lead to more frequent terminations of pregnancy and fewer live born children with this condition. 38 , 39 Prenatal screening currently detects almost 40% of fetuses with major CHD in Sweden. However, that trend does not appear to translate into increased survival—at least not on a national level. Improved detection rates, particularly with major CHD, lead to increased rates of pregnancy termination, with a subsequent decrease in the incidence of the most severe complex CHD. 38 , 40 , 41 Also in Sweden, data indicate that increased antenatal CHD diagnosis leads to more pregnancy terminations, with little—if any—effect on the overall survivorship in children born with CHD. 39 Live births with the most complex CHD may have become less frequent; however, other moderately complex congenital heart conditions, related to increasing maternal age and obesity, may increase. 42 In the present study, we did not observe any further improvement in survivorship after the new millennium in children with CHD in general–particularly in those with complex CHD. Whether a higher rate of antenatal screening and detection can be translated into a better survival, on a national level, is still unclear. Variations in the rate of antenatal screening have not been tied to variations in outcome for children with CHD and improvements in general in outcome because of antenatal screening remains unproven. 43 , 44
Mortality has declined for patients with CHD who underwent cardiac intervention (surgical or catheter intervention) from the early (1980–1989) to the latest era (2010–2017); despite the introduction and expansion of complicated and high‐risk procedures, such as Fontan palliation for univentricular heart defects and Norwood surgery for hypoplastic left heart syndrome during this period. This strongly implies that improved therapy was an important explanation for the improved survival seen in the entire population. However, we have also observed that mortality among children who did not undergo a cardiac intervention decreased over time. This may reflect improved selection of patients with CHD for cardiovascular surgery or catheter interventions; but it may, also reflect improved diagnostic techniques, especially on echocardiography, for example an increased rate of diagnosis of less complex congenital heart malformations such as mild shunts. 1
During the last study period cohort (2010–2017), we observed a worse outcome among children with CHD who underwent a cardiac intervention, different from results from earlier birth periods. This most likely reflects an increase in detection of mild conditions of CHD where no intervention is needed, and the condition has little if any impact on the health of the child. It may also reflect a further improvement in interventional techniques with CHD children with extremely high risk undergoing reparative or palliative procedures. This is further supported by results from patients with the most complex CHD groups, such as the lesion group 1 and lesion group 2, doing better until the turn of the millennium, after which no further improvement is observed. Sweden's 6 cardiothoracic surgery clinics have registered all hospitalizations and interventions since 1970. Swedish hospital records have been mandatory since 1987, based on each individual in the country having a unique 10‐digit personal identity number, which includes their sex and date of birth. Administrative health databases have become a powerful resource for studying several medical conditions; they are valuable owing to the large sample sizes and possibility of long observation periods. The strength of the present report is that it is a nationwide study based on the Swedish healthcare system, which is mainly government funded, universal, and offers free access to all citizens. The current data are representative for Sweden but may be less applicable to other countries with different access to and organization and financing of the healthcare system. Our data may be considered as support for regionalization and centralization of the care of the complex congenital heart conditions. 45 By using national registers, we were able to achieve almost complete follow‐up, with limited risk because of emigration as a possible cause of the loss to follow‐up.
One of the study's limitations is that administrative data from Swedish outpatient clinics before 2001 and data for primary care were unavailable. Thus, they were not included in this study: that could have led to an underestimation of the mortality of patients with less severe lesions that were not detected at birth or detected during follow‐up at outpatient clinics or by primary care physicians. Another limitation is that there have been no published formal validations of CHD diagnostic codes in the Swedish registry system; however, several cardiovascular and other medical conditions or interventions have been shown to have high validity. 46 , 47 The limited number of variables available for analysis may also limit the assertation of both cases and causes of death. One should also acknowledge that our results may be less valid in a different healthcare setting with less centralization and different access to care, different rate of antenatal diagnosis as well as differences in public opinion and regulations on termination of pregnancy. The classification and grouping of CHD into larger groups such as the 6 lesion groups used in the present study will by definition group together conditions that may have had different evolution over the last decades. Our data may be an example of Simpson paradox i.e., that while prognosis improves for some of the CHD conditions included in e.g., lesion group 1, other conditions in the same group may have changed in a different direction. Dividing, congenital cardiac malformations into smaller or more distinct groups may provide further insights but at the cost of statistical power.
The significant improvements made in cardiovascular care since the 1970s and 1980s have resulted in a dramatic improvement in survival for children with CHD. Further advances in the past 2 decades have not yet resulted in increased survival for patients with CHD in general nor for specific lesion groups of patients. Clearly, there is still room for improvement in survival and development of care in patients with the most complex lesion groups, where mortality is still high and where 10% to 20% of those born die before becoming adults. Our data may be interpreted as a sign of optimized surgical procedures, interventional techniques, and diagnostic improvements but where no further improvement, given today's technology and knowledge, may be difficult to obtain. Our data may reflect the, as yet, unfulfilled hope for improvement in a case with antenatal diagnostics. Our finding may also indicate a place for improved surgical supporting techniques, such as better ventricular assist devices, improvements in myocardial and cerebral protection to push the dramatic improvement in congenital heart care over the last decades of the 19th century, even further.
Furthermore, our results point to the obvious need for more cardiologists, nurses, physiotherapist to develop skills and knowledge of how to care for adults with CHD, since they are increasing in number and will continue to do so in the future.
In summary, our study shows that survival among patients with CHD has improved dramatically in the past 40 years. Over the past decade, no further improvement has been observed, and survivorship in children with CHD has been at about 97% since the beginning of this century. Despite these trends, the most complex conditions are still characterized by a high early mortality. For patients with less complex conditions, focusing on lifetime management and preventing acquired diseases may be the key to future improvement.
Sources of Funding
This work was funded by the Swedish state under an agreement between the Swedish Government and County Councils (the Avtal om Läkarutbildning och Forskning agreement, Grant Number: 236611), the Swedish Heart‐Lung Foundation (Grant Number: 20090724), and the Swedish Research Council (2019‐00193 SIMSAM).
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S3
(J Am Heart Assoc. 2020;9:e017704 DOI: 10.1161/JAHA.120.017704.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017704
Preprint posted on SSRN June 23, 2020. doi: http://dx.doi.org/10.2139/ssrn.3566151.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. Int J Epidemiol. 2019;48:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. [DOI] [PubMed] [Google Scholar]
- 3. Mandalenakis Z, Rosengren A, Skoglund K, Lappas G, Eriksson P, Dellborg M. Survivorship in children and young adults with congenital heart disease in Sweden. JAMA Intern Med. 2017;177:224–230. [DOI] [PubMed] [Google Scholar]
- 4. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122:2264–2272. [DOI] [PubMed] [Google Scholar]
- 5. Morris CD, Menashe VD. 25‐year mortality after surgical repair of congenital heart defect in childhood. A population‐based cohort study. JAMA. 1991;266:3447–3452. [PubMed] [Google Scholar]
- 6. Waldhausen JA, Boruchow I, Miller WW, Rashkind WJ. Transposition of the great arteries with ventricular septal defect. Palliation by atrial septostomy and pulmonary artery banding. Circulation. 1969;39:I215–I221. [DOI] [PubMed] [Google Scholar]
- 7. Crafoord C, Ejrup B, Gladnikoff H. Coarctation of the aorta. Thorax. 1947;2:121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMullan MH, Wallace RB, Weidman WH, McGoon DC. Surgical treatment of complete atrioventricular canal. Surgery. 1972;72:905–912. [PubMed] [Google Scholar]
- 9. Mustard WT, Keith JD, Trusler GA, Fowler R, Kidd L. The surgical management of transposition of the great vessels. J Thorac Cardiovasc Surg. 1964;48:953–958. [PubMed] [Google Scholar]
- 10. Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966–980. [PubMed] [Google Scholar]
- 11. Rastelli GC, Wallace RB, Ongley PA. Complete repair of transposition of the great arteries with pulmonary stenosis. A review and report of a case corrected by using a new surgical technique. Circulation. 1969;39:83–95. [DOI] [PubMed] [Google Scholar]
- 12. Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–370. [PubMed] [Google Scholar]
- 13. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the united states, 1999 to 2006. Circulation. 2010;122:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khairy P, Ionescu‐Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. [DOI] [PubMed] [Google Scholar]
- 16. Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. [DOI] [PubMed] [Google Scholar]
- 17. Yeh SJ, Chen HC, Lu CW, Wang JK, Huang LM, Huang SC, Huang SK, Wu MH. Prevalence, mortality, and the disease burden of pediatric congenital heart disease in Taiwan. Pediatr Neonatol. 2013;54:113–118. [DOI] [PubMed] [Google Scholar]
- 18. Feltes TF, Bacha E, Beekman RH III, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the american heart association. Circulation. 2011;123:2607–2652. [DOI] [PubMed] [Google Scholar]
- 19. McRobb CM, Mejak BL, Ellis WC, Lawson DS, Twite MD. Recent advances in pediatric cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2014;18:153–160. [DOI] [PubMed] [Google Scholar]
- 20. Canter CE, Shaddy RE, Bernstein D, Hsu DT, Chrisant MR, Kirklin JK, Kanter KR, Higgins RS, Blume ED, Rosenthal DN, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;115:658–676. [DOI] [PubMed] [Google Scholar]
- 21. Jayaram N, Spertus JA, Kennedy KF, Vincent R, Martin GR, Curtis JP, Nykanen D, Moore PM, Bergersen L. Modeling major adverse outcomes of pediatric and adult patients with congenital heart disease undergoing cardiac catheterization: observations from the NCDR impact registry (national cardiovascular data registry improving pediatric and adult congenital treatment). Circulation. 2017;136:2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raissadati A, Nieminen H, Jokinen E, Sairanen H. Progress in late results among pediatric cardiac surgery patients: a population‐based 6‐decade study with 98% follow‐up. Circulation. 2015;131:347–353; discussion 353. [DOI] [PubMed] [Google Scholar]
- 23. Erikssen G, Liestol K, Seem E, Birkeland S, Saatvedt KJ, Hoel TN, Dohlen G, Skulstad H, Svennevig JL, Thaulow E, et al. Achievements in congenital heart defect surgery: a prospective, 40‐year study of 7038 patients. Circulation. 2015;131:337–346; discussion 346. [DOI] [PubMed] [Google Scholar]
- 24. SWEDCON . Swedcon year report. 2018.
- 25. Register SSTP . Sweden s. Total population register. 2019. Available at: https://www.scb.se/contentassets/8f66bcf5abc34d0b98afa4fcbfc0e060/rtb-bar-2016-eng.pdf. Accessed December 30, 2020.
- 26. Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. [DOI] [PubMed] [Google Scholar]
- 27. Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson PO, Skoglund K, Fedchenko M, Dellborg M. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928–937. [DOI] [PubMed] [Google Scholar]
- 28. Botto LD, Lin AE, Riehle‐Colarusso T, Malik S, Correa A; National Birth Defects Prevention S . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79:714–727. [DOI] [PubMed] [Google Scholar]
- 29. Oyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu S, Joseph KS, Luo W, Leon JA, Lisonkova S, Van den Hof M, Evans J, Lim K, Little J, Sauve R, et al. Effect of folic acid food fortification in canada on congenital heart disease subtypes. Circulation. 2016;134:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welfare NBoHa . The National Board of Health and Welfare, Classification of Operations, Swedish Version. 6th ed 1993; 2019. Available at: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/dokument-webb/klassifikationer-och-koder/klassifikation-av-operationer-sjatte-upplagan.pdf. Accessed October 30, 2020. [Google Scholar]
- 32. Welfare NBoHa . The national board of health and welfare, classification of surgical procedures, version 1.9, Swedish version 1997. 2019.
- 33. Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L. Trends in long‐term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsen SH, Olsen M, Emmertsen K, Hjortdal VE. Interventional treatment of patients with congenital heart disease: nationwide Danish experience over 39 years. J Am Coll Cardiol. 2017;69:2725–2732. [DOI] [PubMed] [Google Scholar]
- 35. Kempny A, Dimopoulos K, Uebing A, Diller GP, Rosendahl U, Belitsis G, Gatzoulis MA, Wort SJ. Outcome of cardiac surgery in patients with congenital heart disease in England between 1997 and 2015. PLoS One. 2017;12:e0178963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wright LK, Ehrlich A, Stauffer N, Samai C, Kogon B, Oster ME. Relation of prenatal diagnosis with one‐year survival rate for infants with congenital heart disease. Am J Cardiol. 2014;113:1041–1044. [DOI] [PubMed] [Google Scholar]
- 37. Oster ME, Kim CH, Kusano AS, Cragan JD, Dressler P, Hales AR, Mahle WT, Correa A. A population‐based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113:1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, Jorgensen FS, Sondergaard L. Live‐born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. 2018;3:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohman A, El‐Segaier M, Bergman G, Hanseus K, Malm T, Nilsson B, Pivodic A, Rydberg A, Sonesson SE, Mellander M. Changing epidemiology of hypoplastic left heart syndrome: results of a national Swedish cohort study. J Am Heart Assoc. 2019;8:e010893 DOI: 10.1161/JAHA.118.010893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tararbit K, Bui TT, Lelong N, Thieulin AC, Goffinet F, Khoshnood B. Clinical and socioeconomic predictors of pregnancy termination for fetuses with congenital heart defects: a population‐based evaluation. Prenat Diagn. 2013;33:179–186. [DOI] [PubMed] [Google Scholar]
- 41. Vincenti M, Guillaumont S, Clarivet B, Macioce V, Mura T, Boulot P, Cambonie G, Amedro P. Prognosis of severe congenital heart diseases: do we overestimate the impact of prenatal diagnosis? Arch Cardiovasc Dis. 2019;112:261–269. [DOI] [PubMed] [Google Scholar]
- 42. Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population‐based study. Am J Clin Nutr. 2010;91:1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, Jacobs ML, Kim S, Ungerleider RM. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136:e378–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakker MK, Bergman JEH, Krikov S, Amar E, Cocchi G, Cragan J, de Walle HEK, Gatt M, Groisman B, Liu S, et al. Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. BMJ Open. 2019;9:e028139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Backer CL, Pasquali SK, Dearani JA. Improving national outcomes in congenital heart surgery: the time has come for regionalization of care. Circulation. 2020;141:943–945. [DOI] [PubMed] [Google Scholar]
- 46. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Forss A, Myrelid P, Olen O, Everhov AH, Nordenvall C, Halfvarson J, Ludvigsson JF. Validating surgical procedure codes for inflammatory bowel disease in the Swedish National Patient Register. BMC Med Inform Decis Mak. 2019;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


