Abstract
Background
A population‐scale evidence for the association between moderate‐to‐vigorous physical activity (MV‐PA) and risks of major adverse cardiovascular event (MACE) or all‐cause mortality in people with various metabolic syndrome (MetS) status is warranted.
Methods and Results
We performed a nationwide retrospective cohort study based on the claims database of South Korea. We included people who received ≥3 national health screenings from 2009 to 2013 without a previous MACE history. We determined the MetS status of 6 108 077 people: MetS‐chronic (N=864 063), MetS‐developed (N=348 163), MetS‐recovery (N=348 313), and MetS‐free (N=4 547 538). The exposure was self‐reported MV‐PA frequencies. The outcome was incident MACEs or all‐cause mortality. The incidence rate ratios (IRR) were calculated with adjustments for clinical/demographic characteristics. During the median follow‐up of 4.28 years, 78 770 and 51 840 people experienced MACEs or died, respectively. Those who engaged in MV‐PA had a significantly lower risk of MACEs or all‐cause mortality than those not engaged in MV‐PA in every spectrum of MetS. Even among those who were free from MetS (for MACEs, IRR 0.94 [0.92–0.97], for all‐cause mortality, IRR 0.85 [0.82–0.87]) or who had already recovered from MetS (for MACEs, IRR 0.89 [0.84–0.95], for all‐cause mortality, IRR 0.74 [0.68–0.81]), 1 to 2 days per week of MV‐PA were significantly associated with lower risk of the adverse outcomes when compared with not being engaged in MV‐PA. Those who were engaged in MV‐PA more frequently also had significantly lower risks of MACEs or all‐cause mortality.
Conclusions
This nationwide study suggests that MV‐PA may be recommended to the general population regardless of recent MetS status.
Keywords: cardiovascular outcomes, epidemiology, metabolic syndrome, mortality, physical exercise
Subject Categories: Cardiovascular Disease, Coronary Artery Disease, Exercise
Nonstandard Abbreviations and Acronyms
- MetS
metabolic syndrome
- MV‐PA
moderate‐to‐vigorous physical activity
Clinical Perspective
What Is New?
Regardless of the recent status of metabolic syndrome, individuals engaged in moderate‐to‐vigorous physical activity had lower risk for major adverse cardiovascular events and all‐cause mortality in a large nationwide cohort in South Korea.
Even those who were free from metabolic syndrome or who did not improve in their metabolic profiles showed lower risk for major adverse cardiovascular events and all‐cause mortality when individuals were engaged in any degree of moderate‐to‐vigorous physical activity.
What Are the Clinical Implications?
Healthcare providers should emphasize the importance of moderate‐to‐vigorous physical activity for people even if they are free from metabolic syndrome or have already recovered from metabolic syndrome.
In those with worsening metabolic health or no obvious improvements in metabolic health, moderate‐to‐vigorous physical activity may still be recommended, as certain benefits of moderate‐to‐vigorous physical activity may be independent of the status of measurable metabolic parameters.
Physical activity reduces the risk of major adverse cardiovascular events (MACEs) and is associated with a lower risk of death from all causes. 1 , 2 , 3 , 4 On the other hand, the presence of metabolic risk factors, including central obesity, elevated blood pressure, dyslipidemia, and impaired glucose tolerance, is related to higher MACE risks, and the cluster of such components has been known as metabolic syndrome (MetS). 5 , 6 Considering the significant impact of MetS on cardiovascular health, a substantial amount of medical resources have been invested in promoting physical activity and controlling metabolic risk factors.
Moderate‐to‐vigorous physical activity (MV‐PA), commonly referred to as activities with an intensity equivalent or stronger than that of light bicycling or brisk walking, is considered the main type of activity that leads to a lower MACE or mortality risk. 7 , 8 The role of MV‐PA in improving MetS profiles has been understood to be the major mechanism underlying its beneficial effect on cardiovascular health. 9 However, there were relatively rare large‐scale evidences regarding whether MV‐PA should be promoted to those free from MetS or those lacking improvement in MetS parameters. 2 , 10 In addition, although a dynamic MetS status is prominently associated with an altered MACE risk, 11 a population‐based study showing whether the benefits of MV‐PA are present along the spectrum of MetS has yet to be reported. Such evidence is essential because if the clinical benefits of MV‐PA are present regardless of the presence or severity of MetS, the necessity of encouraging MV‐PA among people with diverse MetS status could be elucidated. 2 , 12 Furthermore, given that mechanisms beyond controlling the traditional risk factors have been emphasized with regard to the benefits of physical activity, 13 , 14 clinical evidence showing whether the benefits of MV‐PA exist independent from MetS status is needed.
In this nationwide cohort study, we investigated the association between self‐reported MV‐PA and the risk of MACEs and all‐cause mortality; the study included ≈6 million people who had different MetS states during a serial course of national health screenings in South Korea. We hypothesized that the benefits of MV‐PA with regard to lower risk of MACEs or all‐cause mortality would be present independent from recent status of MetS or baseline MetS severity.
Methods
Ethical Approval
The institutional review board of Seoul National University Hospital (no. E‐1806‐112‐951) approved the study. The institutional review board waived the need for informed consent as we obtained anonymous data from the National Health Insurance Service of Korea after obtaining approval (no. NHIS‐2018‐1‐247). The study was conducted in accordance with the Declaration of Helsinki.
Data Sources
The data sets analyzed during the current study are available in the National Health Insurance Sharing Service of Korea (URL: https://nhiss.nhis.or.kr/bd/ab/bdaya001iv.do, last accessed January 14, 2020) after acquiring approval from the organization.
Study Design and Setting
The study was a retrospective nationwide cohort study. The study environment has been described in our previous studies. 11 , 15 South Korea provides a nationwide health insurance service for all Korean people. 16 A free‐of‐charge national health screening is provided for insurance subscribers in Korea, including an evaluation for MetS, a cancer screening, a lifestyle assessment, and other clinical investigations. From the National Health Insurance Database, 17 we used the health screening data and claims information to identify the study populations’ dynamic MetS status and the questionnaire results to determine the frequency of MV‐PA. The MACE outcome was identifiable within the claims database through diagnostic codes, history of admission, and intervention histories. The death outcome was collected from the death registries.
Study Population
A graphical description of the time windows in which the study population was identified, covariates or exposure variables were collected, and outcomes during follow‐up were identified is shown in Figure S1. 18 The study population criteria and definitions of the study groups were similar as those implemented in our previous study. 11 We identified adults (≥20 years old) who received ≥3 national health screenings from 2009 to 2013 in Korea. The first 3 health screenings (S1, S2, and S3) were the inclusion period determining the study population.
We first excluded those who had missing information in variables needed to determine their MetS status, baseline laboratory results used as covariates, and baseline questionnaire results. After these individuals were excluded, people with an identifiable MetS status in ≥3 health examinations without missing information for the covariates or exposures remained. Next, we excluded people according to clinical eligibility; those with undetermined MetS status—those who had transient changes in their MetS status, because we aimed to include people with stable changes in or maintenance of their MetS status, or who had changes at S3, as the maintenance of the change for at least 1 health screening during the inclusion period was needed, were excluded. As we intended to study incident MACE risks in people without significant baseline kidney function impairment, we excluded those with prevalent MACEs before the initiation of follow‐up (a day after S3) and those with indicators of kidney diseases (baseline estimated glomerular filtration rate <60 mL/min per 1.73 m2 calculated using the Modification of Diet in Renal Disease equation, a chronic kidney disease diagnostic code, or a history of renal replacement therapy).
Study Groups
MetS status were determined using the harmonizing criteria in each of the first 3 health screening (S1, S2, and S3), and people fulfilling ≥3 of the following 5 MetS components were considered to have MetS in the according health screening 6 : central obesity (waist circumference ≥90 cm for males, ≥80 cm for females among Asians); high triglycerides (≥150 mg/dL) or the use of a relevant drug; low high‐density lipoprotein cholesterol levels (<40 mg/dL for males, 50 mg/dL for females) or the use of a relevant drug; high blood pressure (systolic ≥130 and/or diastolic ≥80 mm Hg) or the use of an antihypertensive drug; and impaired glucose tolerance (fasting glucose ≥100 mg/dL) or the use of an antidiabetic medication.
Based on the identified MetS status in the first 3 health screenings (S1, S2, and S3), 4 study groups were defined (Figure 1): the MetS‐free group, composed of those who were consistently free from MetS during the inclusion period; the MetS‐chronic group, composed of those who had MetS consistently throughout the inclusion period; the MetS‐recovery group, composed of those who stably recovered from preexisting MetS (presence of MetS at S1 but not at S2 and S3); and the MetS‐developed group, composed of those who newly developed MetS that was consistently identified in subsequent health screenings (absence of MetS at S1 but MetS presence at S2 and S3).
Figure 1. Study population.
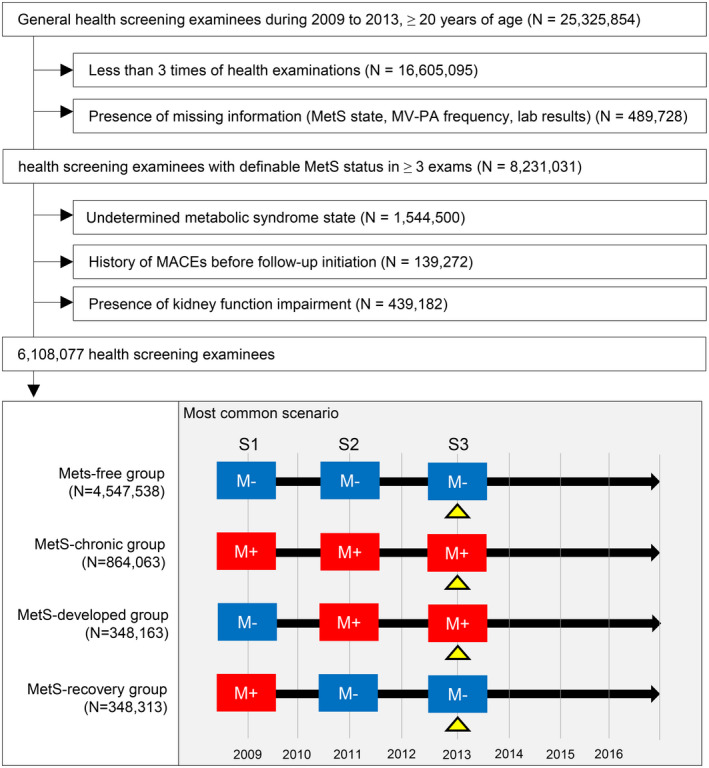
The flow diagram for the study population and the common scenario of the study groups. A blue square with “M−” indicates a health screening without the identification of metabolic syndrome. A red square with “M+” indicates a health screening with the identification of metabolic syndrome. The yellow triangles at S3 indicate the baseline time‐point at which the baseline clinicodemographic characteristics were collected and follow‐up was initiated. MACE indicates major adverse cardiovascular event; MetS, metabolic syndrome; and MV‐PA, moderate‐to‐vigorous physical activity.
Physical Activity Variables and Other Variables
The third health screening (S3) was the time point when baseline characteristics were collected and follow‐up was initiated. The self‐reported frequency of MV‐PA was collected through a questionnaire asking how many days during a week the individual engaged in physical activity, which was stratified into 3 intensities: vigorous activity was defined as more than 20 minutes of activity that renders one almost out of breath, moderate activity was defined as more than 30 minutes of activity requiring fast breathing, and light activity was more than 30 minutes of walking or light MV‐PA per day. The frequency of MV‐PA was stratified as none, 1 to 2 days per week, 3 to 4 days per week, and ≥5 days per week. The representative examples of the MV‐PAs were provided for the responders and the complete questionnaire used during the general health screening can be found in Figure S2. 19 The questionnaire has been developed and distributed by the National Health Insurance Service of Korea with advisory of expert panels. The details of other collected baseline clinical and demographic data are described in Data S1.
Study Outcomes
The first study outcome was the occurrence of MACEs, which consisted of acute myocardial infarction, coronary revascularization, and acute ischemic stroke. Acute myocardial infarction was defined as an admission with an International Classification of Diseases Tenth, Revision (ICD‐10) diagnostic code of I21 or I22. Coronary revascularization was defined by a cardiovascular revascularization procedure history identified in the claims database. Acute ischemic stroke was defined as an admission with an ICD‐10 diagnostic code of I63.
The second study outcome was all‐cause mortality. The death registry information, identifying death dates of all Korean people through death certificates, is available in the claims database and we used the information to define all‐cause mortality. Primary causes of death were not available.
The follow‐up duration started 1 day after the inclusion period and exposure assessment (1 day after S3), and right‐censoring occurred on December 31, 2016, or in death events.
Statistical Analysis
We calculated the incidence rate ratios for MACEs and 95% CIs with Poisson regression. First, we compared all the study subgroups divided according to dynamic MetS status and the frequency of MV‐PA, with the MetS‐free group with 0 days per week of self‐reported MV‐PA as the reference group. Second, we assessed the association between the frequency of MV‐PA and the risk of MACE or all‐cause mortalty in each MetS subgroup, further adjusting for the baseline (S3) severity of MetS within a MetS subgroup. The analysis was repeated in several subgroups, divided according to age (20–39, 40–64, ≥65 years old) or sex (male or female), and those who were initially excluded in the main study population due to their undetermined MetS status. Third, the association of the frequency of MV‐PA and MACE risks was assessed in additional subgroups with a consistently absent or present state of individual MetS component. A Kaplan–Meier survival curve was generated with the cumulative incidences. There were no missing values in the data set. Other details regarding the statistical analysis are described in Data S2. All statistical analyses were performed using SAS, and 2‐sided P<0.05 were considered significant.
Results
Study Population
We screened 25 325 854 adults and identified 8 720 759 people who received ≥3 health screenings during the inclusion period (Figure 1). After exclusion, 8 231 031 people had ≥3 health screenings with identifiable MetS status and self‐reported data on MV‐PA. After applying the eligibility criteria, we included 6 108 077 people in the final study population. The median follow‐up duration was 4.28 (interquartile range 3.50–5.25) years. Among them, 4 547 538 (74.5%) subjects were in the MetS‐free group, and 864 063 (14.1%) people were included in the MetS‐chronic group. In addition, 348 163 (5.7%) and 348 313 (5.7%) people were included in the MetS‐developed and MetS‐recovery groups, respectively.
Baseline Characteristics
The MetS‐chronic group had the highest median age, worst metabolic parameters, and highest proportion belonging to the low‐income population (Table 1). The proportion of those who did not engage in MV‐PA was also the highest in the MetS‐chronic group and the lowest in the MetS‐free group, although the absolute difference between the proportions was small. On the other hand, those who engaged in MV‐PA ≥5 days per week were more commonly in the MetS‐chronic or MetS‐developed group than in those free from MetS at baseline. Regarding the number of MetS components, the MetS‐recovery group more frequently had 1 or 2 components of MetS than the MetS‐free group. The MetS‐chronic group had the largest number of people fulfilling all 5 MetS criteria, and over 60% of the MetS‐developed group had 3 MetS components.
Table 1.
Baseline Characteristics of the Study Patients
| Variables | MetS‐Free | MetS‐Recovery | MetS‐Developed | MetS‐Chronic |
|---|---|---|---|---|
| Number of people in each subgroup | 4 547 538 | 348 313 | 348 163 | 864 063 |
| Clinical and demographic characteristics at S3 | ||||
| Age, y | 43.8±12.5 | 50.4±12.7 | 53.2±12.7 | 56.7±12.2 |
| Sex (male) | 2 608 761 (57.4) | 229 853 (66.0) | 209 605 (60.2) | 480 780 (55.6) |
| Height, cm | 165.4±8.8 | 165.2±9.3 | 164.0±9.8 | 162.9±9.9 |
| Weight, kg | 62.3±10.6 | 67.3±11.5 | 69.8±12.9 | 70.7±13.3 |
| Body mass index, kg/m2 | 22.7±2.7 | 24.6±2.8 | 25.8±3.1 | 26.5±3.2 |
| Low‐income status* | 768 815 (16.9) | 66 560 (19.1) | 69 771 (20.0) | 185 068 (21.4) |
| Place of residence | ||||
| Urban | 2 026 920 (44.6) | 149 289 (42.9) | 149 187 (42.9) | 376 087 (43.5) |
| Rural | 2 520 618 (55.4) | 199 024 (57.1) | 198 976 (57.2) | 487 976 (56.5) |
| Charlson Comorbidity Index (score) | 0.5±0.9 | 0.8±1.1 | 1.1±1.4 | 1.6±1.7 |
| Hemoglobin, g/dL | 14.1±1.6 | 14.4±1.6 | 14.4±1.6 | 14.2±1.6 |
| Aspartate aminotransferase, IU/L | 23.7±16.5 | 26.1±20.9 | 28.9±21.4 | 29.4±21.6 |
| Alanine aminotransferase, IU/L | 22.1±21.6 | 26.9±25.2 | 32.6±27.9 | 32.9±27.5 |
| Creatinine, mg/dL | 0.88±0.19 | 0.90±0.19 | 0.89±0.19 | 0.87±0.19 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 93.3±34.5 | 90.8±33.9 | 89.2±30.5 | 88.2±30.0 |
| Self‐reported lifestyle, n (%) | ||||
| Moderate‐to‐vigorous activity | ||||
| None | 2 076 194 (45.7) | 162 282 (46.6) | 163 527 (47.0) | 422 381 (48.9) |
| 1–2 d/wk | 1 444 483 (31.8) | 105 839 (30.4) | 96 746 (27.8) | 225 061 (26.1) |
| 3–4 d/wk | 650 718 (14.3) | 49 482 (14.2) | 51 433 (14.8) | 125 193 (14.5) |
| ≥5 d/wk | 376 143 (8.3) | 30 710 (8.8) | 36 457 (10.5) | 91 428 (10.6) |
| Smoking | ||||
| Nonsmoker | 2 704 258 (59.5) | 181 422 (52.1) | 191 439 (55.0) | 502 946 (58.2) |
| Ex‐smoker | 604 365 (13.3) | 61 512 (17.7) | 54 796 (15.7) | 143 169 (16.6) |
| Current light‐to‐moderate smoker | 750 681 (16.5) | 54 750 (15.7) | 48 995 (14.1) | 96 752 (11.2) |
| Current heavy smoker | 488 234 (10.7) | 50 629 (14.5) | 52 933 (15.2) | 121 196 (14.0) |
| Alcohol | ||||
| No alcohol intake | 2 098 607 (46.2) | 162 816 (46.7) | 176 539 (50.7) | 479 122 (55.5) |
| Moderate consumption | 347 238 (7.6) | 22 231 (6.4) | 19 292 (5.5) | 42 261 (4.9) |
| Heavy consumption | 2 101 693 (46.2) | 163 266 (46.9) | 152 332 (43.8) | 342 680 (39.7) |
| Parameters of MetS | ||||
| Waist circumference, cm | 77.1±8.0 | 82.5±7.5 | 86.4±8.0 | 88.2±8.3 |
| Systolic BP, mm Hg | 117.9±12.9 | 123.8±13.3 | 129.4±13.6 | 130.5±14.1 |
| Diastolic BP, mm Hg | 74.0±9.0 | 77.5±9.2 | 80.5±9.6 | 80.5±9.8 |
| Glucose, mg/dL | 91.6±13.6 | 97.2±20.3 | 106.6±25.3 | 115.3±34.1 |
| Triglycerides, mg/dL | 91.59±13.57 | 97.17±20.26 | 106.55±25.31 | 115.3±34.1 |
| High‐density lipoprotein cholesterol, mg/dL | 105.6±69.3 | 141.4±96.7 | 195.8±139.4 | 205.6±151.7 |
| Baseline N of MetS components, n (%) | ||||
| 0 | 1 843 873 (40.6) | 37 550 (10.8) | 0 (0) | 0 (0) |
| 1 | 1 687 076 (37.1) | 120 133 (34.5) | 0 (0) | 0 (0) |
| 2 | 1 016 589 (22.4) | 190 630 (54.7) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 209 746 (60.2) | 324 261 (37.5) |
| 4 | 0 (0) | 0 (0) | 112 611 (32.3) | 351 379 (40.7) |
| 5 | 0 (0) | 0 (0) | 25 806 (7.4) | 188 423 (21.8) |
There were no missing values in the table. BP indicates blood pressure; MetS, metabolic syndrome; and S3, screening 3.
Low income status was determined as the lowest quartile of the nation according to the medical insurance fee.
MACE and All‐Cause Mortality Risks
The numbers of cases and incidence rates for MACEs and all‐cause mortality in each subgroup according to MetS status and the frequencies of self‐reported MV‐PA are shown in Tables 2 and 3. The unadjusted risks showed that the overall risks of MACEs and all‐cause mortality were higher in the MetS‐developed and MetS‐chronic groups than in the MetS‐recovery and MetS‐free groups. However, within the subgroups, those who did not MV‐PA had a higher risk of MACEs or all‐cause mortality than those who did (Figure 2, Tables 2 and 3). MV‐PA, even when engaged in only 1 to 2 days per week, was associated with a lower risk of MACEs or all‐cause mortality when compared with those who were not engaging in MV‐PA. Similar trends were identified with regard to the individual MACE outcomes, including acute myocardial infarction, revascularization, and acute ischemic stroke (Tables S1 through S3), but the trend was relatively weak with the revascularization outcome.
Table 2.
Risks of Major Adverse Cardiovascular Events According to the Frequency of Moderate‐to‐Vigorous Physical Activity and MetS Status
| MetS status | Frequency of MV‐PA | Number of People | Number of MACEs | Follow‐Up (Person‐Years) | Incidence Rate (/1000 Person‐Years) | Unadjusted Model | Multivariable Model | ||
|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | ||||||
| MetS‐free | 0 d/wk | 1 840 026 | 17 237 | 7 981 115 | 2.16 | Reference | Reference | ||
| 1–2 d/wk | 1 531 152 | 8953 | 6 878 662 | 1.30 | 0.60 (0.59–0.62) | <0.001 | 0.84 (0.82–0.87) | <0.001 | |
| 3–4 d/wk | 756 270 | 5226 | 3 367 586 | 1.55 | 0.72 (0.70–0.74) | <0.001 | 0.80 (0.77–0.82) | <0.001 | |
| ≥5 d/wk | 420 090 | 4027 | 1 825 140 | 2.21 | 1.02 (0.99–1.06) | 0.222 | 0.84 (0.81–0.86) | <0.001 | |
| MetS‐recovery | 0 d/wk | 145 047 | 3222 | 610 909 | 5.27 | 2.44 (2.35–2.54) | <0.001 | 1.40 (1.35–1.46) | <0.001 |
| 1–2 d/wk | 104 911 | 1423 | 468 051 | 3.04 | 1.41 (1.33–1.49) | <0.001 | 1.24 (1.17–1.31) | <0.001 | |
| 3–4 d/wk | 59 716 | 910 | 262 636 | 3.46 | 1.60 (1.50–1.71) | <0.001 | 1.17 (1.09–1.25) | <0.001 | |
| ≥5 d/wk | 38 639 | 772 | 163 687 | 4.72 | 2.18 (2.03–2.35) | <0.001 | 1.18 (1.09–1.26) | <0.001 | |
| MetS‐developed | 0 d/wk | 156 540 | 4347 | 634 581 | 6.85 | 3.17 (3.07–3.28) | <0.001 | 1.47 (1.43–1.53) | <0.001 |
| 1–2 d/wk | 101 511 | 1840 | 436 850 | 4.21 | 1.95 (1.86–2.05) | <0.001 | 1.41 (1.34–1.48) | <0.001 | |
| 3–4 d/wk | 54 830 | 1084 | 231 489 | 4.68 | 2.17 (2.04–2.31) | <0.001 | 1.22 (1.14–1.3) | <0.001 | |
| ≥5 d/wk | 35 282 | 922 | 143 535 | 6.42 | 2.97 (2.78–3.18) | <0.001 | 1.24 (1.16–1.33) | <0.001 | |
| MetS‐chronic | 0 d/wk | 406 137 | 15 625 | 1 624 863 | 9.62 | 4.45 (4.36–4.55) | <0.001 | 1.60 (1.56–1.64) | <0.001 |
| 1–2 d/wk | 225 524 | 6096 | 965 660 | 6.31 | 2.92 (2.84–3.01) | <0.001 | 1.60 (1.55–1.65) | <0.001 | |
| 3–4 d/wk | 136 733 | 3867 | 572 284 | 6.76 | 3.13 (3.02–3.24) | <0.001 | 1.37 (1.32–1.42) | <0.001 | |
| ≥5 d/wk | 95 669 | 3219 | 384 603 | 8.37 | 3.88 (3.73–4.02) | <0.001 | 1.30 (1.25–1.35) | <0.001 | |
Multivariable model was adjusted for baseline age, sex, estimated glomerular filtration rate (continuous, mL/min per 1.73 m2), alanine aminotransferase (continuous, IU/mL), aspartate aminotransferase (continuous, IU/mL), hemoglobin (continuous, g/dL), low‐income status (the lowest quartile in the nation), body mass index (continuous, kg/m2), Charlson Comorbidity Index score, and place of residence (urban or rural). IRR indicates incidence rate ratio; MACE, major adverse cardiovascular events; MetS, metabolic syndrome; and MV‐PA, moderate‐to‐vigorous physical activity.
Table 3.
Risks of All‐Cause Mortality According to the Frequency of Moderate‐to‐Vigorous Physical Activity and MetS Status
| MetS status | Frequency of MV‐PA | Number of People | Number of Death | Follow‐Up (Person‐Years) | Incidence Rate (/1000 Person‐Years) | Unadjusted Model | Multivariable Model | ||
|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | ||||||
| MetS‐free | 0 d/wk | 1 840 026 | 15 621 | 8 012 877 | 1.95 | Reference | Reference | ||
| 1–2 d/wk | 1 531 152 | 6733 | 6 896 304 | 0.98 | 0.50 (0.49–0.52) | <0.001 | 0.79 (0.77–0.82) | <0.001 | |
| 3–4 d/wk | 756 270 | 4145 | 3 377 828 | 1.23 | 0.63 (0.61–0.65) | <0.001 | 0.77 (0.74–0.80) | <0.001 | |
| ≥5 d/wk | 420 090 | 3559 | 1 832 813 | 1.94 | 1.00 (0.96–1.03) | 0.832 | 0.82 (0.79–0.85) | <0.001 | |
| MetS‐recovery | 0 d/wk | 145 047 | 2376 | 616 827 | 3.85 | 1.98 (1.89–2.06) | <0.001 | 1.24 (1.19–1.29) | <0.001 |
| 1–2 d/wk | 104 911 | 760 | 470 783 | 1.61 | 0.83 (0.77–0.89) | <0.001 | 0.89 (0.82–0.95) | 0.001 | |
| 3–4 d/wk | 59 716 | 526 | 264 399 | 1.99 | 1.02 (0.94–1.11) | 0.647 | 0.88 (0.80–0.96) | 0.003 | |
| ≥5 d/wk | 38 639 | 489 | 165 185 | 2.96 | 1.52 (1.39–1.66) | <0.001 | 0.90 (0.82–0.99) | 0.025 | |
| MetS‐developed | 0 d/wk | 156 540 | 2467 | 642 750 | 3.84 | 1.97 (1.89–2.05) | <0.001 | 1.10 (1.06–1.15) | <0.001 |
| 1–2 d/wk | 101 511 | 933 | 440 524 | 2.12 | 1.09 (1.02–1.16) | 0.014 | 1.03 (0.97–1.10) | 0.342 | |
| 3–4 d/wk | 54 830 | 550 | 233 594 | 2.35 | 1.21 (1.11–1.31) | <0.001 | 0.85 (0.78–0.92) | <0.001 | |
| ≥5 d/wk | 35 282 | 472 | 145 281 | 3.25 | 1.67 (1.52–1.83) | <0.001 | 0.79 (0.72–0.87) | <0.001 | |
| MetS‐chronic | 0 d/wk | 406 137 | 7601 | 1 654 198 | 4.59 | 2.36 (2.29–2.42) | <0.001 | 1.09 (1.06–1.13) | <0.001 |
| 1–2 d/wk | 225 524 | 2368 | 977 978 | 2.42 | 1.24 (1.19–1.30) | <0.001 | 0.92 (0.88–0.96) | <0.001 | |
| 3–4 d/wk | 136 733 | 1663 | 579 996 | 2.87 | 1.470 (1.4–1.55) | <0.001 | 0.83 (0.79–0.87) | <0.001 | |
| ≥5 d/wk | 95 669 | 1577 | 390 821 | 4.04 | 2.07 (1.97–2.18) | <0.001 | 0.84 (0.80–0.89) | <0.001 | |
Multivariable model was adjusted for baseline age, sex, estimated glomerular filtration rate (continuous, mL/min per 1.73 m2), alanine aminotransferase (continuous, IU/mL), aspartate aminotransferase (continuous, IU/mL), hemoglobin (continuous, g/dL), low‐income status (the lowest quartile in the nation), body mass index (continuous, kg/m2), Charlson Comorbidity Index score, and place of residence (urban or rural). IRR indicates incidence rate ratio; MACE, major adverse cardiovascular events; MetS, metabolic syndrome; and MV‐PA, moderate‐to‐vigorous physical activity.
Figure 2. Kaplan–Meier survival curve plotting the incidence probability of major adverse cardiovascular events and all‐cause mortality according to the frequency of self‐reported moderate‐to‐vigorous physical activity.
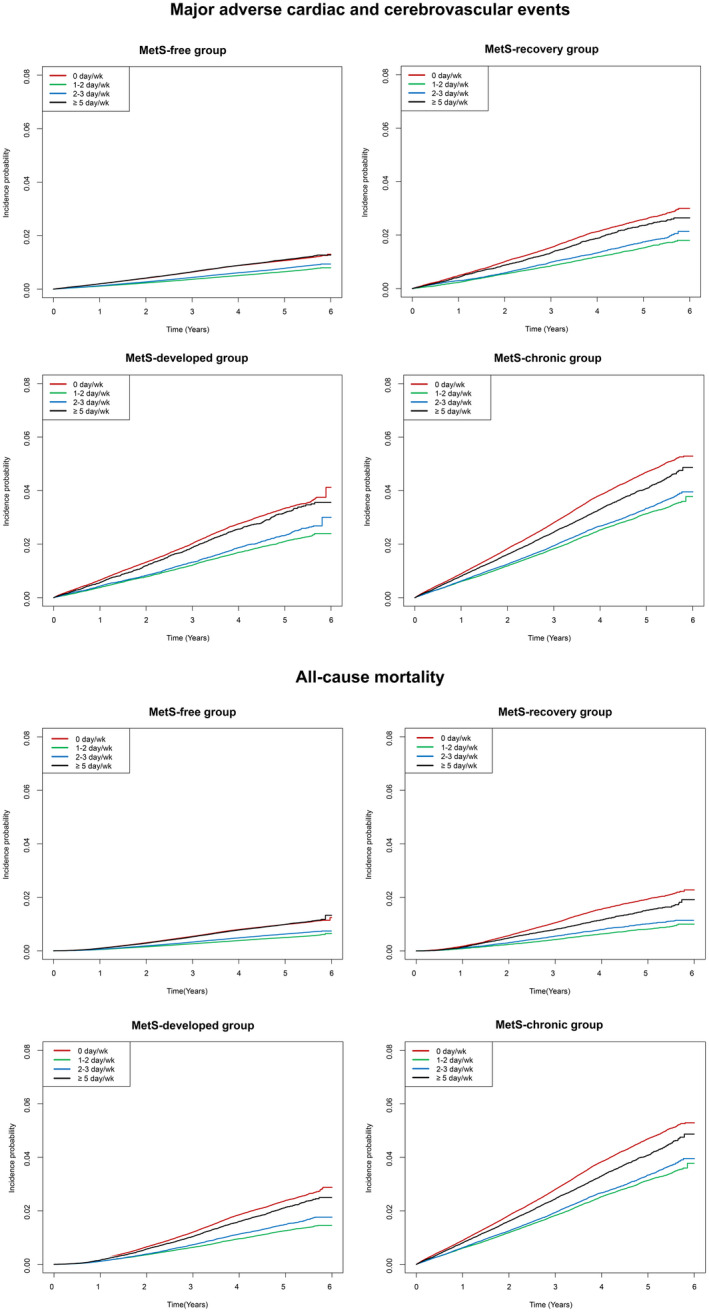
The survival curves were plotted in each metabolic syndrome subgroup. The y‐axes indicate the incidence probability of major adverse cardiovascular events and all‐cause mortality. The x‐axes indicate the time (years). The tables below the survival curves show the numbers of people at risk. MetS indicates metabolic syndrome; and MV‐PA, moderate‐to‐vigorous physical activity.
MACE and All‐Cause Mortality Risks According to the Frequency of MV‐PA in Each Dynamic MetS Status Group
In each MetS status subgroup, the association between engaging in MV‐PA and a lower risk of MACEs or all‐cause mortality was similarly identified (Tables 4 and 5). Even after controlling for MetS severity and smoking and alcohol consumption behaviors, those who engaged in MV‐PA had a significantly lower risk of MACEs or all‐cause mortality than those who did not engaged in MV‐PA in every studied spectrum of MetS. In the fully adjusted model, engaging in MV‐PA for 1 to 2 days per week was associated with a 6% to 11% lower risk of MACEs and 9% to 26% for all‐cause mortality, respectively, when compared with those who did not engage in MV‐PA. Among those who engaged in MV‐PA more frequently, the effect sizes were similar or even larger, and engaged in MV‐PA ≥5 days per week was associated with a 12% to 16% lower risk of MACEs 14% to 25% lower risk for all‐cause mortality, respectively, when compared with those who did not engage in MV‐PA.
Table 4.
Risks of Major Adverse Cardiovascular Events According to the Frequency of Moderate‐to‐Vigorous Physical Activity in Each Study Group
| Subgroups and Exposure | Model 1. Adjusted for the Baseline Severity of MetS (Number of MetS Components)* | Model 2. Adjusted for the Baseline Severity of MetS (Laboratory Parameters of MetS) † | Model 3. Adjusted for the Baseline Severity of MetS (Number of MetS Components) and Other Lifestyle Variables (Smoking, Alcohol) ‡ | |||
|---|---|---|---|---|---|---|
| Adjusted IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | |
| MetS‐free | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.92 (0.89–0.94) | <0.001 | 0.91 (0.89–0.94) | <0.001 | 0.94 (0.92–0.97) | <0.001 |
| 3–4 d/wk | 0.83 (0.81–0.86) | <0.001 | 0.84 (0.81–0.87) | <0.001 | 0.87 (0.84–0.90) | <0.001 |
| ≥5 d/wk | 0.82 (0.80–0.85) | <0.001 | 0.84 (0.81–0.87) | <0.001 | 0.86 (0.83–0.89) | <0.001 |
| MetS‐recovery | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.87 (0.81–0.93) | <0.001 | 0.87 (0.82–0.93) | <0.001 | 0.89 (0.84–0.95) | <0.001 |
| 3–4 d/wk | 0.82 (0.77–0.89) | <0.001 | 0.83 (0.77–0.89) | <0.001 | 0.87 (0.80–0.93) | <0.001 |
| ≥5 d/wk | 0.83 (0.77–0.91) | <0.001 | 0.84 (0.78–0.91) | <0.001 | 0.87 (0.81–0.94) | <0.001 |
| MetS‐developed | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.90 (0.85–0.95) | <0.001 | 0.91 (0.86–0.96) | <0.001 | 0.92 (0.87–0.98) | 0.006 |
| 3–4 d/wk | 0.81 (0.75–0.86) | <0.001 | 0.81 (0.76–0.86) | <0.001 | 0.84 (0.78–0.90) | <0.001 |
| ≥5 d/wk | 0.85 (0.79–0.91) | <0.001 | 0.86 (0.80–0.92) | <0.001 | 0.88 (0.82–0.95) | <0.001 |
| MetS‐chronic | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.92 (0.89–0.95) | <0.001 | 0.93 (0.90–0.95) | <0.001 | 0.94 (0.91–0.97) | <0.001 |
| 3–4 d/wk | 0.82 (0.79–0.85) | <0.001 | 0.83 (0.81–0.86) | <0.001 | 0.85 (0.82–0.88) | <0.001 |
| ≥5 d/wk | 0.82 (0.79–0.85) | <0.001 | 0.84 (0.81–0.87) | <0.001 | 0.85 (0.82–0.89) | <0.001 |
IRR indicates incidence rate ratio; and MetS, metabolic syndrome.
Model 1 was adjusted for age, sex, baseline estimated glomerular filtration rate (continuous, mL/min per 1.73 m2), alanine aminotransferase (continuous, IU/mL aspartate aminotransferase (continuous, IU/mL), hemoglobin (continuous, g/dL), low‐income status (the lowest quartile in the nation), body mass index (continuous, kg/m2), Charlson Comorbidity Index score, place of residence (urban or rural), and the number of MetS components at the baseline health screening.
Model 2 was adjusted for the same variables as Model 1, but the exact values of waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, triglycerides, and high‐density lipoprotein cholesterol were included in the multivariable model instead of the number of MetS components.
Model 3 was adjusted for the same variables as Model 1, but other lifestyle variables (smoking: none, previous, and current; alcohol consumption: none, moderate, heavy) were additionally included.
Table 5.
Risks of All‐Cause Mortality According to the Frequency of Moderate‐to‐Vigorous Physical Activity in Each Study Group
| Subgroups and Exposure | Model 1. Adjusted for the Baseline Severity of MetS (Number of MetS Components)* | Model 2. Adjusted for the Baseline Severity of MetS (Laboratory Parameters of MetS) † | Model 3. Adjusted for the Baseline Severity of MetS (Number of MetS Components) and Other Lifestyle Variables (Smoking, Alcohol) ‡ | |||
|---|---|---|---|---|---|---|
| Adjusted IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | Adjusted IRR (95% CI) | P Value | |
| MetS‐free | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.82 (0.8–0.85) | <0.001 | 0.82 (0.8–0.84) | <0.001 | 0.85 (0.82–0.87) | <0.001 |
| 3–4 d/wk | 0.79 (0.76–0.82) | <0.001 | 0.8 (0.77–0.82) | <0.001 | 0.83 (0.8–0.86) | <0.001 |
| ≥5 d/wk | 0.82 (0.79–0.86) | <0.001 | 0.83 (0.8–0.87) | <0.001 | 0.86 (0.83–0.89) | <0.001 |
| MetS‐recovery | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.72 (0.66–0.79) | <0.001 | 0.72 (0.66–0.78) | <0.001 | 0.74 (0.68–0.81) | <0.001 |
| 3–4 d/wk | 0.72 (0.65–0.79) | <0.001 | 0.71 (0.65–0.79) | <0.001 | 0.75 (0.68–0.83) | <0.001 |
| ≥5 d/wk | 0.74 (0.67–0.81) | <0.001 | 0.73 (0.66–0.81) | <0.001 | 0.77 (0.7–0.85) | <0.001 |
| MetS‐developed | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.89 (0.82–0.96) | 0.002 | 0.88 (0.81–0.95) | 0.001 | 0.91 (0.84–0.99) | 0.024 |
| 3–4 d/wk | 0.73 (0.66–0.8) | <0.001 | 0.76 (0.69–0.83) | <0.001 | 0.77 (0.7–0.84) | <0.001 |
| ≥5 d/wk | 0.71 (0.64–0.79) | <0.001 | 0.75 (0.68–0.83) | <0.001 | 0.75 (0.68–0.83) | <0.001 |
| MetS‐chronic | ||||||
| 0 d/wk | Reference | Reference | Reference | |||
| 1–2 d/wk | 0.81 (0.77–0.85) | <0.001 | 0.82 (0.78–0.86) | <0.001 | 0.83 (0.79–0.87) | <0.001 |
| 3–4 d/wk | 0.73 (0.69–0.77) | <0.001 | 0.77 (0.72–0.81) | <0.001 | 0.76 (0.72–0.81) | <0.001 |
| ≥5 d/wk | 0.77 (0.73–0.82) | <0.001 | 0.81 (0.76–0.85) | <0.001 | 0.81 (0.77–0.85) | <0.001 |
IRR indicates incidence rate ratio; and MetS, metabolic syndrome.
Model 1 was adjusted for age, sex, baseline estimated glomerular filtration rate (continuous, mL/min per 1.73 m2), alanine aminotransferase (continuous, IU/mL aspartate aminotransferase (continuous, IU/mL), hemoglobin (continuous, g/dL), low‐income status (the lowest quartile in the nation), body mass index (continuous, kg/m2), Charlson Comorbidity Index score, place of residence (urban or rural), and the number of MetS components at the baseline health screening.
Model 2 was adjusted for the same variables as Model 1, but the exact values of waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, triglycerides, and high‐density lipoprotein cholesterol were included in the multivariable model instead of the number of MetS components.
Model 3 was adjusted for the same variables as Model 1, but other lifestyle variables (smoking: none, previous, current; and alcohol consumption: none, moderate, heavy) were additionally included.
Subgroup Analysis Results
When we stratified the subjects according to age being engaged on MV‐PA was generally associated with lower risk of MACE (Tables S4 through S6) or all‐cause mortality (Tables S7 through S9) in the middle (40–64 years old) and the old age (≥65 years old) subgroups but not in the young age (20–39 years old) subgroup. Regarding sex, although the overall incidence rate for MACE (Tables S10 and S11) or death rates (Tables S12 and S13) were lower in the females, MV‐PA was associated significantly lower adverse outcome risks in both sexes except for few associations with marginal significance. When we tested the association between the frequency of MV‐PA and MACE or all‐cause mortality risks in 1 297 589 individuals with undetermined MetS status (Table S14), who were initially excluded in the main analysis, the results were similar (Table S15). When we assessed this association in each subgroup with a consistently absent or present state of each individual MetS component, a similar result was identified (Tables S16 and S17), and MV‐PA was associated with lower MACE or all‐cause mortality risks in all subgroups after clinicodemographic characteristics were adjusted.
Discussion
In this nationwide study, we found that the beneficial association of MV‐PA with the risk of MACE or all‐cause mortality was present in individuals with various dynamic status of MetS regardless of MetS severity. Those who engaged in MV‐PA had a better cardiovascular outcome and survival than those who did not MV‐PA among those who remained free from MetS or had already recovered from MetS. Also, in the population who had or developed MetS, a lower risk of MACE or all‐cause mortality was identified in those who MV‐PA, even after controlling for the baseline severity of MetS. Our study provides population‐scale evidence that supports encouraging MV‐PA in all people regardless of their metabolic risk profiles.
Managing metabolic risk factors is the mainstay of the current method of reducing the risk of MACEs or mortality. 9 , 20 , 21 We also reported a population‐scale study that showed that recovery from or the development of MetS is associated with altered risks of MACE, further highlighting the importance of the controlling or prevention of MetS. 11 MV‐PA has been acknowledged as one of the main lifestyle improvements to ameliorate MetS and further reduce the risk of MACEs or deaths. 9 However, there have been remaining questions regarding whether targeting metabolic risk profiles is the sole beneficial effect of MV‐PA on the cardiovascular system, as certain aspects of adverse outcome risks are not explained by the metabolic profiles. 13 , 14 To determine whether MV‐PA has benefits in the general population with various MetS status, we performed this study, and the results suggest that there may be certain benefits of MV‐PA independent of MetS status or severity. Our study has strengths in that (1) we successfully stratified the dynamic MetS status of ≈6 million people by analyzing a unique nationwide cohort who underwent successive health screenings, (2) a self‐reported questionnaire was administered to a large number of people, and (3) we showed that MV‐PA was associated with lower actual MACE or all‐cause mortality risks, not just reduced individual MetS parameters, in the general population. Therefore, according to our study results, healthcare providers may encourage MV‐PA to reduce the risk of MACEs or all‐cause mortality in people regardless of their current or recent metabolic risk status.
Based on our results, MV‐PA may be recommended even for those who are free from or already recovered from MetS, and the MetS‐free population who engages in MV‐PA may have an even lower risk of MACE or deaths than people who are free from MetS but do not participate in MV‐PA. Namely, the absence of metabolic risk factors may not indicate that there is a limited advantage of MV‐PA with regard to cardiovascular health for such people who are MetS free. In our previous report, 11 MetS recovery was associated with an ≈20% lower adjusted risk of MACE or all‐cause mortality when compared with those who remained in the chronic MetS state. The current study suggests that those who engage in MV‐PA may have even lower adverse outcome risks after their recovery than those who do not. The overall MACE or all‐cause mortality risks were higher in those with MetS than in those without MetS; however, MV‐PA was still related to lower MACE or death risks in those with MetS. It is common for people to still have MetS even though they engage in MV‐PA, and prominent improvements in measurable MetS parameters are not easily achieved through lifestyle modifications in the real world. Our study shows that even in people with chronic MetS, MV‐PA should be consistently recommended to at least partially reduce their high risk of MACEs and all‐cause mortality.
Although controlling metabolic risk factors has been considered the main mechanism underlying the benefits of MV‐PA, there are other effects of MV‐PA on the cardiovascular system (eg, improvement in vascular remodeling, 22 , 23 cardiac adaptation, 24 and MV‐PA‐induced adaptations 25 ). As such beneficial effects may not be directly reflected in measurable laboratory parameters, our study results can serve as large‐scale clinical evidence that those nontraditional mechanisms may actually lead to a better cardiovascular prognosis. In addition, a future study investigating the benefits of MV‐PA on cardiovascular disease may further elucidate the various mechanisms through which the benefits of MV‐PA occur. 13 , 14 Particularly, as in our study results, those with middle or old age, rather than those with young age, would be the primary target group of interest for a such trial.
Our study has several limitations. First, a retrospective study based on a claims database innately has limited sensitivity, so missed events or confounders may be present. Second, although the questionnaire was relatively simple, the accuracy of the questionnaire has not been validated, and we were able to study only “self‐reported” frequencies of MV‐PA. Additional studies regarding the types or intensities of MV‐PA in detail may provide specific recommendations for how the risk of MACE is effectively reduced by MV‐PA. Third, although the study included one of the largest nationwide cohorts with reported frequencies of MV‐PA, the follow‐up duration was relatively short. Last, the study examined a population in a single nation with limited ethnic diversity, so whether the results can be generalized to other countries is unknown.
In conclusion, MV‐PA is associated with a lower MACE and all‐cause mortality risk in general people regardless of their previous or current MetS status. Healthcare providers should emphasize the importance of MV‐PA for people even if they are free from MetS or have already recovered from MetS. In those with worsening metabolic health or no obvious improvements in metabolic health, MV‐PA may still be recommended, as certain benefits of MV‐PA may be independent of the status of measurable MetS parameters.
Sources of Funding
This work was supported by the Industrial Strategic Technology Development Program ‐ Development of Bio‐Core Technology (10077474, Development of early diagnosis technology for acute/chronic renal failure) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). The study was performed independently by the authors.
Disclosures
None.
Supporting information
Acknowledgments
This study used the National Health Insurance Database managed by the National Health Insurance Service of Korea, and the approach was approved by the organization (NHIS‐2018‐1‐247).
(J Am Heart Assoc. 2020;9:e016806 DOI: 10.1161/JAHA.120.016806.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;2395–2401. [DOI] [PubMed] [Google Scholar]
- 2. Broekhuizen LN, Boekholdt SM, Arsenault BJ, Despres JP, Stroes ES, Kastelein JJ, Khaw KT, Wareham NJ. Physical activity, metabolic syndrome, and coronary risk: the EPIC‐Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil. 2011;209–217. [DOI] [PubMed] [Google Scholar]
- 3. Bennett DA, Du H, Clarke R, Guo Y, Yang L, Bian Z, Chen Y, Millwood I, Yu C, He P, et al. Association of physical activity with risk of major cardiovascular diseases in Chinese men and women. JAMA Cardiol. 2017;1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekelund U, Tarp J, Steene‐Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, et al. Dose‐response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta‐analysis. BMJ. 2019;l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;2709–2716. [DOI] [PubMed] [Google Scholar]
- 6. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;1640–1645. [DOI] [PubMed] [Google Scholar]
- 7. Lee IM, Paffenbarger RS Jr. Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;293–299. [DOI] [PubMed] [Google Scholar]
- 8. Saint‐Maurice PF, Troiano RP, Berrigan D, Kraus WE, Matthews CE. Volume of light versus moderate‐to‐vigorous physical activity: similar benefits for all‐cause mortality? J Am Heart Assoc. 2018;e008815 DOI: 10.1161/JAHA.118.008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe M, Yokotsuka M, Yamaoka K, Adachi M, Nemoto A, Tango T. Effects of a lifestyle modification programme to reduce the number of risk factors for metabolic syndrome: a randomised controlled trial. Public Health Nutr. 2017;142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all‐cause mortality, and longevity of college alumni. N Engl J Med. 1986;605–613. [DOI] [PubMed] [Google Scholar]
- 11. Park S, Lee S, Kim Y, Lee Y, Kang MW, Han K, Han SS, Lee H, Lee JP, Joo KW, et al. Altered risk for cardiovascular events with changes in the metabolic syndrome status: a nationwide population‐based study of approximately 10 million persons. Ann Intern Med. 2019;875–884. [DOI] [PubMed] [Google Scholar]
- 12. Moholdt T, Lavie CJ, Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. J Am Coll Cardiol. 2018;1094–1101. [DOI] [PubMed] [Google Scholar]
- 13. Fiuza‐Luces C, Santos‐Lozano A, Joyner M, Carrera‐Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;731–743. [DOI] [PubMed] [Google Scholar]
- 14. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park S, Lee S, Kim Y, Lee Y, Kang MW, Han K, Lee H, Lee JP, Joo KW, Lim CS, et al. Reduced risk for chronic kidney disease after recovery from metabolic syndrome: a nationwide population‐based study. Kidney Res Clin Pract. 2020;180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, Kang HJ, Do CH, Song JS, Lee EJ, et al. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schneeweiss S, Rassen JA, Brown JS, Rothman KJ, Happe L, Arlett P, Dal Pan G, Goettsch W, Murk W, Wang SV. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med. 2019;398–406. [DOI] [PubMed] [Google Scholar]
- 19. Jeong HG, Kim DY, Kang DW, Kim BJ, Kim CK, Kim Y, Yang W, Park ES, Lee SH. Physical activity frequency and the risk of stroke: a nationwide cohort study in Korea. J Am Heart Assoc. 2017;e005671 DOI: 10.1161/JAHA.117.005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blair SN, Kampert JB, Kohl HW III, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all‐cause mortality in men and women. JAMA. 1996;205–210. [PubMed] [Google Scholar]
- 21. Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;63–67. [PubMed] [Google Scholar]
- 22. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age‐related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;2896–2901. [DOI] [PubMed] [Google Scholar]
- 23. Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, Phillips SA. Improved arterial flow‐mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens. 2016;1309–1316. [DOI] [PubMed] [Google Scholar]
- 24. Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, et al. Exercise‐induced changes in glucose metabolism promote physiological cardiac growth. Circulation. 2017;2144–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004;1288–1294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


