Abstract
Background
Conventional right ventricular pacing (RVP) has been associated with an increased incidence of atrial fibrillation (AF). We sought to compare the occurrence of new‐onset AF and assessed AF disease progression during long‐term follow‐up between His bundle pacing (HBP) and RVP.
Methods and Results
We included patients undergoing initial dual‐chamber pacemaker implants at Rush University Medical Center between January 1, 2016, and June 30, 2019. A total of 360 patients were evaluated, and 225 patients (HBP, n=105; RVP, n=120) were included in the study. Among the 148 patients (HBP, n=72; RVP, n=76) with no history of AF, HBP demonstrated a lower risk of new‐onset AF (adjusted hazard ratio [HR], 0.53; 95% CI, 0.28–0.99; P=0.046) compared with traditional RVP. This benefit was observed with His or RVP burden exceeding 20% (HR, 0.29; 95% CI, 0.13–0.64; P=0.002), ≥40% (HR, 0.31; P=0.007), ≥60% (HR, 0.35; P=0.015), and ≥80% (HR, 0.40; P=0.038). There was no difference with His or RV pacing burden <20% (HR, 0.613; 95% CI, 0.213–1.864; P=0.404). In patients with a prior history of AF, there was no difference in AF progression (P=0.715); however, in a subgroup of patients with a pacing burden ≥40%, HBP demonstrated a trend toward a lower risk of AF progression (HR, 0.19; 95% CI, 0.03–1.16; P=0.072).
Conclusions
HBP demonstrated a lower risk of new‐onset AF compared with RVP, which was primarily observed at a higher pacing burden.
Keywords: Atrial fibrillation, atrial fibrillation progression, his bundle pacing, new‐onset atrial fibrillation, right ventricular pacing
Subject Categories: Atrial Fibrillation, Pacemaker
Nonstandard Abbreviations and Acronyms
- AAD
anti‐arrhythmic drug
- AHRE
atrial high rate episode
- HBP
His bundle pacing
- RVP
right ventricular pacing
Clinical Perspective
What Is New?
In patients without a prior diagnosis of atrial fibrillation, His bundle pacing is associated with a lower incidence of a new‐onset atrial fibrillation when compared with traditional right ventricular pacing.
The lower risk of new‐onset atrial fibrillation with His bundle pacing compared with right ventricular pacing was primarily driven by patients with a higher pacing burden.
What Are the Clinical Implications
Patients with dual‐chamber pacemaker implantations might benefit from His bundle pacing compared with traditional right ventricular pacing, through a reduction in the incidence of atrial fibrillation.
Patients with a higher burden of pacing are more likely to benefit from His bundle pacing over right ventricular pacing.
It is well recognized that conventional right ventricular (RV) apical pacing causes ventricular desynchrony and is associated with an increased risk of atrial fibrillation (AF) and development of cardiomyopathy. 1 , 2 Permanent His bundle pacing (HBP) provides a more physiological form of ventricular activation and has been shown to decrease or reverse some of the adverse clinical outcomes associated with RV pacing (RVP). 3 , 4 There might be some benefit of HBP in reducing onset and progression to persistent AF when compared with RVP. A study that compared HBP with RV septal pacing and RV apical pacing demonstrated that HBP showed a lower risk of progression to persistent or permanent AF. 5 However, the study included patients with and without prior history of AF and did not evaluate the specific end point of a new diagnosis of AF. Whether HBP impacts the development of new‐onset AF or affects the progression of AF has not been systematically evaluated in a large cohort of patients. It is unclear if a higher pacing burden with HBP is associated with a similar increase in the risk of new‐onset AF and progression of AF when compared with RVP. This study was designed to assess the risk of new‐onset AF or AF disease progression among patients with HBP as compared with conventional RVP. We also planned to perform a subgroup analysis based on the ventricular pacing burden to compare the incidence and progression of AF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We performed a retrospective cohort analysis of successful dual‐chamber permanent pacemaker implantations at Rush University Medical Center during the study period from January 1, 2016, through June 30, 2019, in patients with at least a 6‐month duration of follow‐up.
Subject Selection
Patients aged ≥18 years who had an indication for permanent pacemaker implantation based on current guidelines 6 and underwent successful initial dual‐chamber permanent pacemaker implantation qualified for enrollment. Successful HBP implantation was defined as selective or nonselective HB pacing with paced QRS duration ≤130 milliseconds. 3 , 4
Patients with valvular heart disease involving the mitral or aortic valve (moderate or severe stenosis or regurgitation), a history of open‐heart surgery within the past 6 months, a known history of persistent or permanent AF at initial implant, a history of an atrioventricular node ablation or AF ablation, and those patients receiving a single‐chamber pacemaker or cardiac resynchronization therapy device implant were excluded. The study was approved by the institutional review committee. Informed consent was waived, as this was a retrospective study.
Definitions
Paroxysmal AF: AF terminating spontaneously or with intervention within 7 days of onset 7 , 8 , 9 .
Persistent AF: Continuous AF lasting ≥7 days.
Long‐standing persistent AF: Continuous AF lasting ≥12 months.
Permanent AF: AF for which patients and clinicians chose not to employ a rhythm control strategy.
New‐onset AF episode 7 : Device detection of a true AF episode (lasting ≥30 seconds) on intracardiac electrogram. Atrial high‐rate episodes (AHREs) from device recordings were manually reviewed to confirm true AF and rule out other causes of AHREs. AHRE episodes were defined as episodes with an atrial intracardiac electrogram rate ≥190 bpm. AHRE episodes ≥6 minutes were also evaluated.
Progression of AF: Defined as an absolute increase in pacemaker reported average daily AF burden, by ≥10% from the initial device follow up evaluation at 1 to 2 months to subsequent device interrogations at 6‐month intervals. Additionally, an increase in AF burden by ≥5% and ≥25% were also recorded.
Data Collection and Study Follow‐Up
All patients had scheduled follow‐up at 2 months after implant and every 6 months thereafter until the final follow‐up or death. Data were collected on baseline patient characteristics such as age, sex, race, comorbidities, and other potential risk factors predisposing to AF, which could result in confounding. The percentage of His or ventricular pacingand average daily AF burden at the initial 1‐ to 2‐month follow‐up visit and from each subsequent device interrogation including remote device interrogation were collected. For the end point of new‐onset AF, the subjects were censored at the time of last available follow‐up or death, in the absence of new‐onset AF. For the end point of progression of AF among those patients with a known history of AF, data collection was censored once patients underwent an AF or atrioventricular nodal ablation or were initiated on antiarrhythmic drug (AAD) therapy or at the time of last available follow‐up or death. Reversible causes of AF, such as critical illness AF was not considered to meet the end point for new‐onset AF. Baseline and 12‐month follow‐up transthoracic echocardiogram variables 10 when available were also collected. For the primary analysis of patients with no prior history of AF, data regarding AF episodes were not collected during a lead stabilization period of 4 weeks after device implantation.
Study End Points and Hypothesis
The primary end points were (1) new‐onset AF among patients without a known history of AF and (2) progression of AF defined as an absolute increase in average daily AF burden by ≥10% from the AF burden at initial device follow‐up. The secondary end point was outcomes of new‐onset AF and progression of AF among patients with ≥20% RVP or HBP burden, stratified based on pacing percentage. We hypothesized that in patients with dual‐chamber pacemakers, HBP is associated with a lower risk of new‐onset AF and lower risk of AF progression than conventional RV pacing.
Statistical Analysis
The χ2 test was used to assess the association between categorical variables, whereas an independent samples t‐test was performed to compare continuous variables between pacing sites. The Mann‐Whitney test was used for comparing variables with nonnormal distribution. To determine significant predictors, univariate predictors with a P value <0.10 were entered in a multivariate Cox’s proportional hazard model. Cox’s proportional hazard model was used to estimate the hazard ratio of the first occurrence of new‐onset AF and progression of AF according to different pacing site (HBP or RVP), adjusted for various potential confounders identified between left ventricular ejection fraction (LVEF), left atrial indexed volume, percentage of atrial and ventricular pacing, age, sex, diabetes mellitus, hypertension, coronary disease, QRS morphology, bundle branch block, use of antiarrhythmic drugs (propafenone, flecainide, dofetilide, sotalol, dronedarone, and amiodarone). 4 , 11 A 2‐tailed P value of <0.05 was considered statistically significant. SPSS software, version 21 (IBM Corp., Armonk, NY) was used in all statistical analyses.
Results
There were 360 permanent pacemaker implantations performed at our institution between January 1, 2016, and June 30, 2019. After exclusion criteria were applied, 225 patients were included in the analysis (Figure 1). There were 120 patients in the RVP group and 105 patients in the HBP group. Baseline characteristics are shown in Table 1. Age was significantly lower in the HBP group compared with the RVP group by about 4 years (P=0.006). Hypertension was the most common comorbidity seen in 84% of the patients included. LVEF was predominantly preserved, with a mean LVEF of 60% in the HBP group and 61% in the RVP group (P=0.262). There was no significant difference in the 2 groups in the rest of the baseline characteristics (Table 1). Among the 105 patients in the HBP group, 5 patients (4.8%) required HBP lead revision during follow‐up. Among them, 3 were performed for high His bundle capture threshold, and 2 were performed for loss of His bundle capture.
Figure 1. Flowchart demonstrating the patients included in the study as well as the inclusion and the exclusion criteria.

AF indicates atrial fibrillation; CRT, cardiac resynchronization therapy; and RV, right ventricular.
Table 1.
Baseline Characteristics of All Patients Included in the Study
| Characteristic | HBP Group (n=105) | RVP Group (n=120) | P Value |
|---|---|---|---|
| Age, y | 72.65±11.04 | 76.54±9.87 | P=0.006 |
| Sex, n (%) | P=0.422 | ||
| Female | 53 (50.5) | 67 (55.8) | |
| Male | 52 (49.5) | 53 (44.2) | |
| Race/Ethnicity, n (%) | P=0.407 | ||
| Black | 32 (31.4 | 50 (42) | |
| White | 53 (52) | 54 (45.4) | |
| Hispanic | 11 (10.8) | 12 (10.1) | |
| Asian | 3 (2.9) | 1 (0.8) | |
| Other (including Native American or if race/ethnicity was unknown) | 3 (2.9) | 2 (1.7) | |
| Body mass index, kg/m2 | 30.30±6.99 | 29.1±6.46 | P=0.184 |
| Hypertension, n (%) | 87 (82.9) | 105 (87.5) | P=0.326 |
| Diabetes mellitus, n (%) | 31 (29.5) | 40 (33.3) | P=0.540 |
| Coronary artery disease, n (%) | 31 (29.5) | 30 (25) | P=0.446 |
| Heart failure, n (%) | 15 (14.3) | 21 (17.5) | P=0.512 |
| History of AF at implant, n (%) | 33 (31.4) | 44 (36.7) | P=0.409 |
| Indication for implant, n (%) | P=0.244 | ||
| Sinus node dysfunction, n (%) | 54 (51.4) | 71 (59.2) | |
| Atrioventricular block, n (%) | 51 (48.6) | 49 (40.8) | |
| Native QRS morphology | P=0.159 | ||
| Narrow, % | 62.4 | 74.8 | |
| LBBB, % | 13.9 | 6.7 | |
| RBBB, % | 19.8 | 15.1 | |
| IVCD, % | 4 | 3.4 | |
| Native QRS duration, ms | 113.7±32.2 | 105.5±25.3 | P=0.299 |
| ACEi or ARB use, n (%) | 50 (47.6) | 54 (45) | P=0.694 |
| AAD at implant, n (%) | 14 (13.3) | 11 (9.2) | P=0.332 |
| LVEF baseline, % | 59.84±8.06 | 61.00±7.19 | P=0.262 |
| LVEF at follow‐up, % | 56.67±9.29 | 57.85±7.80 | P=0.466 |
AAD indicates antiarrhythmic drug; ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; HBP, His bundle pacing; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; and RVP, right ventricular pacing.
New‐Onset AF
There were 72 patients in the HBP group and 76 patients in the RVP group without prior history of AF (Table 2). The mean follow‐up duration was 1.95±0.9 years. A new diagnosis of AF was noted in 15 (20.8%) patients in the HBP group and 31 (40.8%) patients in the RVP group (P=0.009) (Table 3). A new diagnosis of AHRE ≥6 minutes was seen in 14 (19.4%) of patients in the HBP group and 28 (36.8%) of the patients in the RVP group (P=0.019). The type of device (ie, HBP/RVP) was the only statistically significant univariate predictor of new‐onset AF (Table 4). Cox regression analysis demonstrated that there was a lower risk of a new diagnosis of AF in the HBP group when compared with RVP (hazard ratio [HR], 0.50; 95% CI, 0.27–0.94; P=0.029). When adjusted for the confounder (Table S1) of age, the risk of new‐onset AF was still lower in HBP (HR, 0.53; 95% CI, 0.28–0.99; P=0.046) (Figure 2). A significantly lower burden of new‐onset AF was observed in HBP across all pacing burden groups (Figure 3). Results of stratified Cox regression analysis for secondary outcomes based on His or RV pacing burden is shown in Figure 4. The benefit of HBP was primarily driven by His or ventricular pacing burden ≥20%. Adjusted risk of new diagnosis of AF was significantly lower in patients with HBP compared with RVP in the subgroups with His or RV pacing burdens ≥20 % (HR, 0.29; 95% CI, 0.13–0.64; P=0.002), ≥40% (HR, 0.31; 95% CI, 0.13–0.72; P=0.007), ≥60% (HR, 0.35; 95% CI, 0.15–0.81; P=0.015) and ≥80% (HR, 0.40; 95% CI, 0.17–0.95; P=0.038). In the patients with His or RV pacing burden <20%, there was no difference between the 2 groups (P=0.404). HBP also demonstrated a lower risk of a new diagnosis of AHRE ≥6 minutes in patients with His or RV pacing burden ≥20% (HR, 0.36; 95% CI, 0.16–0.83; P=0.016).
Table 2.
Baseline Characteristics of Patients With No Prior History of Atrial Fibrillation
| Characteristic | HBP Group (n=72) | RVP Group (n=76) | P Value |
|---|---|---|---|
| Age, y | 72.33±11.58 | 75.71±10.19 | P=0.061 |
| Sex, n (%) | P=0.612 | ||
| Female | 33 (45.8) | 38 (50) | |
| Male | 39 (54.2) | 38 (50) | |
| Race/Ethnicity, n (%) | P=0.955 | ||
| Black | 25 (35.7) | 31 (41.3) | |
| White | 32 (45.7) | 32 ((42.7) | |
| Hispanic | 9 (12.9) | 9 (12) | |
| Asian | 1 (1.4) | 1 (1.3) | |
| Other (including Native American or if race/ethnicity was unknown) | 3 (4.3) | 2 (2.7) | |
| Body mass index, kg/m2 | 30.44±7.26 | 28.79±6.39 | P=0.144 |
| Hypertension, n (%) | 60 (83.3) | 62 (81.6) | P=0.779 |
| Diabetes mellitus, n (%) | 25 (34.7) | 25 (32.9) | P=0.814 |
| Coronary artery disease, n (%) | 24 (33.3) | 21 (27.6) | P=0.451 |
| Heart failure, n (%) | 9 (12.5) | 12 (15.8) | P=0.566 |
| Indication for implant, n (%) | P=0.298 | ||
| Sinus node dysfunction | 28 (38.9) | 36 (47.4) | |
| Atrioventricular block | 44 (61.1) | 40 (52.6) | |
| Native QRS morphology, % | P=0.458 | ||
| Narrow | 61.8 | 64 | |
| LBBB | 7.4 | 9.3 | |
| RBBB | 23.5 | 22.7 | |
| IVCD | 2.9% | 4 | |
| Native QRS duration, ms | 114.24±31.85 | 113.07±26.50 | P=0.884 |
| ACEi or ARB use, n (%) | 35 (48.6) | 33 (43.4) | P=0.527 |
| LVEF baseline, % | 60.2±8.3 | 61.24±7.58 | P=0.439 |
| LVEF at follow‐up, % | 57.03±9.6 | 56.34±8.9 | P=0.890 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blockers; HBP, His bundle pacing; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; and RVP, right ventricular pacing.
Table 3.
Outcomes of the Patients Included in the Study Based on Prior History of AF
| (A) Patients With No Prior History of AF | |||
|---|---|---|---|
| Characteristic | HBP Group (n=72), n (%) | RVP Group (n=76), n (%) | P Value |
| His or RV pacing burden ≥20% | 53 (73.6) | 45 (59.2) | 0.064 |
| New diagnosis of AF | 15 (20.8) | 31 (40.8) | 0.009 |
| New diagnosis of AHRE ≥6 min | 14 (19.4) | 28 (36.8) | 0.019 |
| (B) Patients With Prior History of AF | |||
|---|---|---|---|
| Characteristic | HBP Group (n=33), n (%) | RVP Group (n=44), n (%) | P Value |
| His or RV pacing burden ≥20% | 21 (63.6) | 22 (50) | 0.233 |
| AAD at implant | 13 (39.4) | 10 (23.3) | 0.129 |
| Intervention performed* | 9 (27.3) | 8 (18.2) | 0.341 |
| AF burden increase by ≥25% | 6 (18.2) | 10 (22.7) | 0.627 |
| AF burden increase by ≥10% | 7 (21.2) | 12 (27.3) | 0.542 |
| AF burden increase by ≥5% | 9 (27.3) | 13 (29.5) | 0.827 |
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; AHRE, atrial high‐rate episode; HBP, His bundle pacing; and RVP, right ventricular pacing
Interventions included AF ablation, atrioventricular nodal ablation, and initiation or up‐titration of antiarrhythmic medication.
Table 4.
Univariate Predictors of New‐Onset AF in Patients With No Prior History of AF
| Characteristic | New Diagnosis of AF (46) | No New Diagnosis of AF (n=102) | P Value |
|---|---|---|---|
| Age, y | 76.22±11.55 | 73.10±10.63 | 0.110 |
| Sex, n (%) | 0.740 | ||
| Male | 23 (50) | 54 (52.9) | |
| Female | 23 (50) | 48 (47.1) | |
| Body mass index, kg/m2 | 30.03±6.43 | 29.39±7.05 | 0.599 |
| Diabetes mellitus, n (%) | 14 (31.8) | 35(35.4) | 0.681 |
| Hypertension, n (%) | 36 (81.8) | 84 (84.8) | 0.649 |
| Heart failure | 4 (8.7) | 17 (16.7) | 0.151 |
| Indication for implant | 0.969 | ||
| Sinus node dysfunction, n (%) | 20 (43.5) | 44 (43.1) | |
| Atrioventricular block, n (%) | 26 (56.5) | 58 (56.9) | |
| Type of device | 0.009* | ||
| HBP, n (%) | 15 (32.6) | 57 (55.9) | |
| RVP, n (%) | 31 (67.4) | 45 (44.1) | |
| LVEF, % | 58.59±6.17 | 56.98±8.34 | 0.282 |
| Pacing % burden ≥20%, n (%) | 31 (67.4) | 67 (65.7) | 0.839 |
| Atrial pacing burden | 47.46±30.01 | 41.00±32.80 | 0.247 |
| Native QRS duration, ms | 116±26 | 113±30 | 0.502 |
AF indicates atrial fibrillation; HBP, His bundle pacing; LVEF, left ventricular ejection fraction; and RVP, right ventricular pacing.
Statistically significant univariate predictor.
Figure 2. Cumulative proportion of patients free of a new diagnosis of AF based on the type of device (HBP vs RVP).
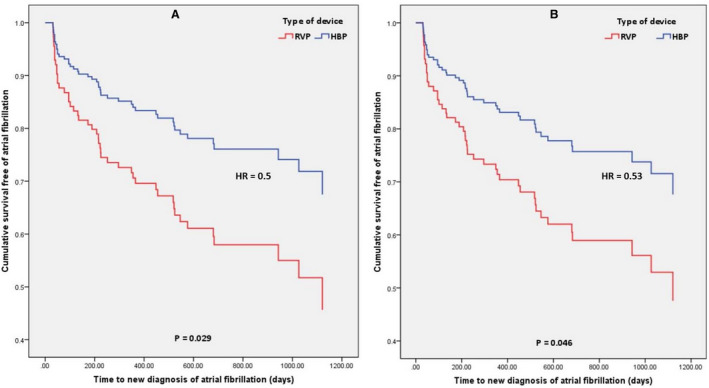
A, Unadjusted for confounders; (B) adjusted for confounders. The representations are derived from Cox model survival function. The P values are from Cox proportional hazards model. HBP indicates His bundle pacing; HR, hazard ratio;
and RVP, right ventricular pacing.
Figure 3. Comparison of new‐onset AF by percentage between HBP and RVP in all patients and the subgroups based on ventricular pacing burden.
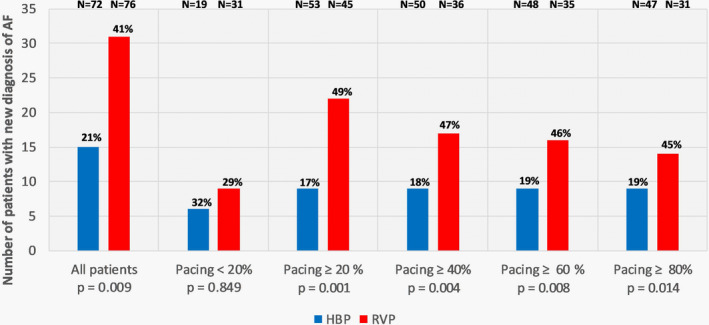
AF
indicates atrial fibrillation; HBP, His bundle pacing; and RVP, right ventricular pacing.
Figure 4. Cumulative proportion of patients free of a new diagnosis of AF based on the type of device (HBP vs RVP) and percentage of ventricular pacing adjusted for confounders.
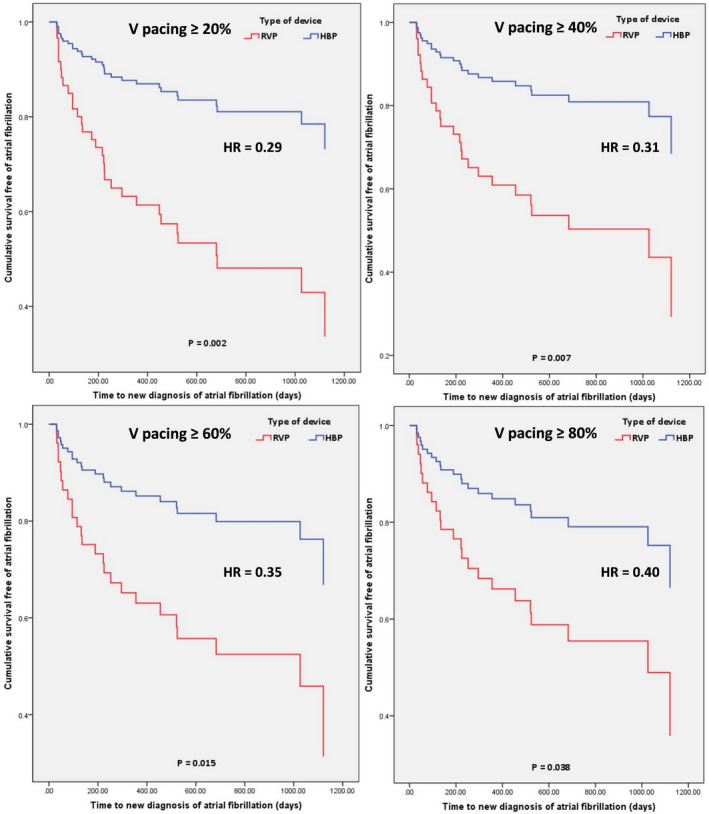
(A) Ventricular pacing (VP) ≥ 20%; (B) VP ≥40%; (C) VP ≥60%; (D) ≥80%. The representations are derived from stratified Cox model survival function. The P values are from Cox proportional hazards model. HBP indicates His bundle pacing; HR, hazard ratio; and RVP, right ventricular pacing.
AF Disease Progression
There were 33 patients in the HBP group and 44 patients in the RVP group who had a prior history of AF (Table 5). The mean follow‐up duration was 1.97±0.8 years. AAD use at implant was not different between the 2 groups (P=0.129). There was no difference between the 2 groups in the history of AF ablation, atrioventricular nodal ablation, or AAD initiation (P=0.341). Progression of AF as defined by an increase in AF burden by ≥10% (AF10%) was seen in 7 (21.2%) patients in the HBP group and 12 (27.3%) patients in the RVP group with no statistically significant difference between the 2 pacing sites (P=0.542) (Table 3). There were no univariate predictors for AF disease progression (Table 6). There was no difference in diagnosis of AF 10% between HBP and RVP (HR, 0.84; 95% CI, 0.33–2.13; P=0.715) (Figure 5). There was no difference between the 2 groups in AF burden increase of ≥25% (P=0.627) and ≥5% (P=0.827). On adjusting for confounders (Table S2) of age, hypertension, and native QRS duration, there was no difference between the 2 groups (HR, 0.94; 95% 0.32–2.79; P=0.910) (Figure 5). Results of further analysis of secondary outcomes based on His or RV pacing percentage are shown in Figure 6. There was no statistically significant difference in adjusted risk of incidence of AF 10% between HBP and RVP, in patients with His or RV pacing ≥20% (HR, 0.44; 95% CI, 0.11–1.74; P=0.243), ≥40% (HR, 0.19; 95% CI, 0.03–1.16; P=0.072), ≥60% (HR, 0.30; 95% CI, 0.051.79] P=0.188), and ≥80% (HR, 0.32; 95% CI, 0.02–4.79; P=0.410). However, separation of the curves between HBP and RVP suggested a potential clinical difference between the 2 groups in patients with His or RV pacing burden ≥40%, which did not reach statistical significance (Figure 6B).
Table 5.
Baseline Characteristics of Patients With History of Atrial Fibrillation
| Characteristic | HBP Group (n=33) | RVP Group (n=44) | P Value |
|---|---|---|---|
| Age, y | 73.33±9.9 | 77.98±9.23 | P=0.037 |
| Sex, n (%) | P=0.632 | ||
| Female | 20 (60.6) | 29 (65.9) | |
| Male | 3 (39.4) | 15 (34.1) | |
| Race/Ethnicity, n (%) | P=0.111 | ||
| Black | 7 (21.9) | 19 (43.2) | |
| White | 21 (65.6) | 22 (50) | |
| Hispanic | 2 (6.3) | 3 (6.8) | |
| Asian | 2 (6.3) | 0 | |
| Body mass index, kg/m2 | 30±6.45 | 29.65±6.62 | P=0.818 |
| Hypertension, n (%) | 27 (81.8) | 43 (97.7) | P=0.016 |
| Diabetes mellitus, n (%) | 6 (18.2) | 15 (34.1) | P=0.121 |
| Coronary artery disease, n (%) | 7 (21.2) | 9 (20.5) | P=0.935 |
| Heart failure, n (%) | 6 (18.2) | 9 (20.5) | P=0.803 |
| Indication for implant, n (%) | P=0.935 | ||
| Sinus node dysfunction | 26 (78.8) | 35 (79.5) | |
| Atrioventricular block | 7 (21.2) | 9 (20.5) | |
| Native QRS morphology, n (%) | P=0.458 | ||
| Narrow | 63.6 | 93.2 | |
| LBBB | 18.2 | 2.3 | |
| RBBB | 12.1 | 2.4 | |
| IVCD | 6.1 | 2.3 | |
| Native QRS duration, ms | 112.64±33.53 | 92.59±16.81 | P=0.003 |
| ACEi or ARB use, n (%) | 15 (45.5) | 21 (47.7) | P=0.843 |
| AAD at implant, n (%) | 13 (39.4) | 10 (22.7) | P= 0.114 |
| LVEF baseline, % | 59.06±7.58 | 60.61±6.60 | P=0.341 |
| LVEF at follow up, % | 56.06±8.93 | 58.56±5.94 | P=0.265 |
AAD indicates antiarrhythmic drug; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HBP, His bundle pacing; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; and RVP, right ventricular pacing.
Table 6.
Univariate Predictors of Progression of AF in Patients With Prior History of AF
| Characteristic | Progression of AF 10% (n=19) | No Progression of AF (n=58) | P Value |
|---|---|---|---|
| Age, y | 76.63±9.59 | 75.78±9.86 | 0.742 |
| Sex, n (%) | |||
| Male | 6 (31.6) | 22 (37.9) | 0.617 |
| Female | 13 (68.4) | 36 (62.1) | |
| Body mass index, kg/m2 | 30.28±7.79 | 29.64±6.10 | 0.710 |
| Diabetes mellitus, n (%) | 5 (26.3) | 16 (27.6) | 0.914 |
| Hypertension, n (%) | 18 (94.7) | 52 (89.7) | 0.504 |
| Heart failure, n (%) | 3 (15.8) | 12 (20.7) | 0.461 |
| Indication for implant, n (%) | 0.204 | ||
| Sinus node dysfunction | 17 (89.5) | 44 (75.9) | |
| Atrioventricular block, n (%) | 2 (10.5%) | 14 (24.1%) | |
| Type of device | 0.542 | ||
| HBP, n (%) | 7 (36.8 | 26 (44.8) | |
| RVP, n (%) | 12 (63.2) | 32 (55.2 | |
| LVEF, % | 56.94±6.22 | 57.02±7.43 | 0.970 |
| Pacing burden ≥20%, n (%) | 13 (68.4) | 30 (51.7) | 0.203 |
| Native QRS duration, ms | 107.33±29.51 | 106.31±31.77 | 0.931 |
| AAD at implant, n (%) | 5 (26.3) | 18 (31) | 0.697 |
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; HBP, His bundle pacing; LVEF, left ventricular ejection fraction; and RVP, right ventricular pacing.
Figure 5. Cumulative proportion of patients free of progression of AF burden by 10%, based on the type of device (HBP vs RVP).
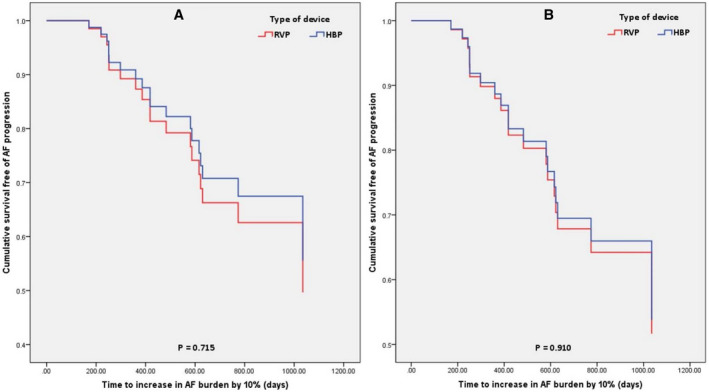
A, Unadjusted for confounders; (B) adjusted for confounders. The representations are derived from Cox model survival function. The P values are from Cox proportional hazards model. AF indicates atrial fibrillation; HBP, His bundle pacing; and RVP, right ventricular pacing.
Figure 6. Cumulative proportion of patients free of progression of AF burden by 10%, based on the type of device (HBP vs RVP) and His or ventricular pacing percentage adjusted for confounders.
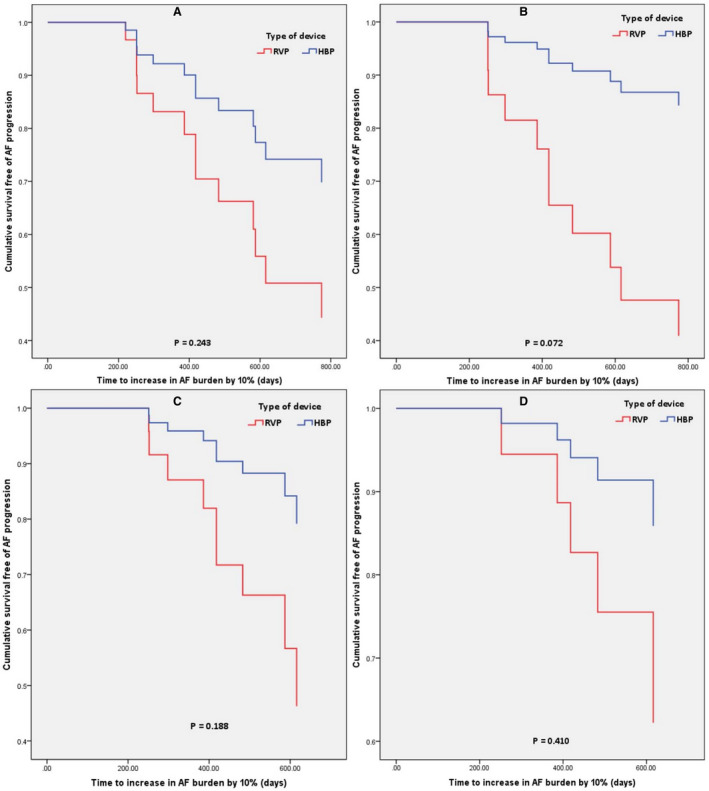
A, Ventricular pacing (VP) ≥20%; (B) VP ≥40%; (C) VP ≥60%; (D) ≥80%. The representations are derived from stratified Cox model survival function. The P values are from Cox proportional hazards model. AF indicates atrial fibrillation; HBP, His bundle pacing and RVP, right ventricular pacing.
Discussion
Some important observations can be made from this study. First, in patients without a prior diagnosis of AF, HBP is associated with a lower incidence of a new‐onset AF when compared with RVP. Second, the lower risk of new‐onset AF with HBP compared with RVP was primarily driven by patients with a higher pacing burden, providing further evidence of a true relationship. In patients with a prior diagnosis of AF, HBP and RV pacing did not demonstrate a statistically significant difference in AF disease progression; however, there was a trend toward lower risk of progression of AF with HBP in the patients with His or RV pacing burden ≥40%.
We demonstrated that HBP was associated with a 50% relative risk reduction and 20% absolute risk reduction of new‐onset AF, compared with RVP. These results are consistent with the study by Pastore et al, 5 which showed the risk of progression to persistent or permanent AF in those without a history of AF was lower with HBP. The mechanism by which ventricular pacing induces AF is unclear, but the left atrial dysfunction induced by left ventricular dyssynchrony is a probable culprit. 5 , 12 , 13 HBP generates physiological electromechanical activation of the left ventricle without dyssynchrony when compared with RVP, potentially leading to the clinical benefit of the lower incidence of AF. 5 , 13 Prior studies on RVP have demonstrated that a higher ventricular pacing burden is associated with a higher risk of AF. 14 In our study, HBP was associated with a 71% relative risk reduction and 30% absolute risk reduction when compared with RVP in patients with His or ventricular pacing burdens ≥20%. This benefit was primarily driven by patients with a higher ventricular pacing burden. Our results add to the previously reported benefits of physiological pacing with HBP when compared with RVP in improving LVEF, quality of life, and New York Heart Association functional class. 15 Current guidelines recommend the use of physiological conduction system pacing implants like HBP in patients with LVEF <50% who are anticipated to require frequent pacing (>40%), to reduce the risk of pacing cardiomyopathy. 6 Our observations suggest that patients with normal LVEF who may require frequent pacing might also benefit from HBP by decreasing the risk of incident AF. This must be weighed against the concern for a higher pacing threshold with HBP compared with RVP, potentially resulting in reduced battery longevity and the requirement for HBP lead revision. 4 Further randomized studies are required to validate these observations.
We were unable to demonstrate a statistically significant difference in the progression of AF between the 2 groups. These results are consistent with the study by Pastore et al, 5 which demonstrated that in a subgroup of patients with prior history of AF, HBP was not associated with a statistically significant difference in progression to persistent or permanent AF compared with RVP. This could be attributable to our sample size and follow‐up duration, which might have underpowered our study to detect any difference in the progression of AF (type II error). Although we did not demonstrate a statistically significant difference, there was a trend toward a reduced risk of AF progression among patients with HBP, especially with pacing burden ≥40% as evident by a separation of the survival curves from about 6 months after device implantation in favor of HBP (Figure 6B). A sufficiently sized study may identify a significant difference in AF progression.
In the study by Pastore et al, 5 HBP was associated with a lower risk of progression to persistent or permanent AF in the subgroup analysis of patients with no prior history of AF, and in the subgroup of patients with a prior history of AF, there was no difference between the HBP and RVP groups. However, our definition for the end point of a new diagnosis of AF and progression of AF is different from that of Pastore et al. In addition, Pastore et al did not evaluate the impact of pacing modality on the occurrence of new‐onset paroxysmal AF as demonstrated in our study, which makes our results unique. The mean left ventricular systolic function was preserved in both studies. Further studies in patients with reduced ejection fraction are needed to evaluate the potential beneficial effects of HBP compared with traditional biventricular pacing in terms of AF occurrence and progression.
The incidence of a new diagnosis of AF in our study was 21% in the HBP group and 41% in the RVP group. In the analysis of patients from the study by Sweeney et al, the incidence of a new diagnosis of AF based on ECG confirmation only ranged from 21% to 24 % based on the pacing mode, during a follow‐up duration of 6 years. 1 In the same study, the risk of AF increased by 1% for each 1% increase in ventricular pacing burden in patients with DDDR mode. 1 Although the incidence of a new diagnosis of AF in our study was higher when compared with older data using only ECG diagnosis, it is consistent with the recent studies that used implantable device detection of AF to establish the diagnosis. In the study by Reiffel et al evaluating patients with risk factors (CHADS2 ≥2) who underwent implantable loop recorders the incidence of a new diagnosis of AF was 29.3% at 18 months and 40% at 30 months. 16 A pooled analysis of 3 studies involving 6850 patients with cardiac implanted electronic devices showed the incidence of new AF ≥5 minutes was 34% during a follow‐up period of 2.4 years. 17
Limitations
There are several limitations to our study. This was a retrospective, observational study that has inherent limitations. We used the Cox proportional hazards model to help minimize the effect of confounders as much as possible. Our study may have been underpowered to detect the outcomes of the progression of AF. In patients with a prior history of AF, a minority of patients were on AAD therapy, which may be a potential bias; however, the subgroup analysis excluding patients on AAD yielded similar results to the primary analysis. Also, variation in the time between device checks may have affected the AF burden calculation, which may, in turn, bias our results. Our end point for AF progression based on the device detected change in AF burden by 10% was chosen to demonstrate the effect of pacing modality and identify patients at risk of persistent or permanent AF earlier, but the clinical significance of this end point is unclear. Hence, our results regarding the progression of AF must be interpreted with caution. Multiplicity adjustment was not performed. The CI of the hazard ratio for the new diagnosis of AF is broad, especially when stratified on the basis of pacing percentage, which needs to be noted when interpreting these results. The difference in manufacturers of the pacemakers used in the HBP and RVP groups might have confounded the results. The variation in follow‐up duration between the HBP and RVP groups might have also confounded the results.
Conclusions
HBP was associated with a lower risk of new‐onset AF compared with conventional RVP. Patients with a higher burden of ventricular pacing are more likely to benefit from HBP over RVP. HBP was associated with a trend toward reduced risk of AF progression. These findings should be further evaluated in randomized studies with a larger sample size.
Sources of Funding
This study was funded by Medtronic.
Disclosures
Dr Trohman reports serving as an advisor to Boston Scientific/Guidant; receiving research grants from Boston Scientific/Guidant, Medtronic Inc., and St. Jude Medical (Abbott); serving as a consultant for St. Jude Medical (Abbott); and receiving speaker’s fees or honoraria from Boston Scientific/Guidant CRM, Medtronic Inc., and St. Jude Medical (Abbott). Dr Huang reports serving as a consultant for Cardiofocus and receiving research grants from Medtronic. Dr Krishnan serves as a consultant to Abbott/St. Jude Medical, Cardiva, and Zoll and research funding from Abbott/St. Jude Medical. Dr Sharma has been a speaker for Medtronic and has been a consultant for Abbott, Boston Scientific, and Biotronik. Dr Vijayaraman has been a consultant to Abbott, Biotronik, Boston Scientific, and Medtronic; he also has a patent pending for a His delivery tool. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
Author contributions: Drs Sharma, Vijayaraman, and Ravi contributed to the conception and design of the work. Drs Ravi, Pietrasik, Ooms, Hanifin, and Ayub contributed to the acquisition, analysis, and interpretation of data for the work. Drs Ravi, Beer, Sharma, and Vijayaraman drafted the manuscript. Drs Ravi, Beer, Larsen, Huang, Krishnan, Sharma, Vijayaraman, and Trohman critically reviewed and revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
(J Am Heart Assoc. 2020;9:e018478 DOI: 10.1161/JAHA.120.018478.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;2932–2937. [DOI] [PubMed] [Google Scholar]
- 2. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, Frankel DS. Incidence and predictors of right ventricular pacing‐induced cardiomyopathy. Heart Rhythm. 2014;1619–1625. [DOI] [PubMed] [Google Scholar]
- 3. Sharma P.S., Dandamudi G., Naperkowski A., Oren J.W., Storm R.H., Ellenbogen K.A., Vijayaraman P.. Permanent His‐bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;305–312. [DOI] [PubMed] [Google Scholar]
- 4. Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, Oren JW, Dandamudi G, Vijayaraman P. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;2319–2330. [DOI] [PubMed] [Google Scholar]
- 5. Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, Boaretto G, Roncon L, Noventa F, Barold SS. The risk of atrial fibrillation during right ventricular pacing. Europace. 2016;353–358. [DOI] [PubMed] [Google Scholar]
- 6. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, et al 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Circulation. 2019;e382–e482. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, ACC, AHA Task Force Members, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;1–55. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Interv Card Electrophysiol. 2017;50:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;178–183. [DOI] [PubMed] [Google Scholar]
- 10. Kronborg MB, Mortensen PT, Poulsen SH, Gerdes JC, Jensen HK, Nielsen JC. His or para‐His pacing preserves left ventricular function in atrioventricular block: a double‐blind, randomized, crossover study. Europace. 2014;1189–1196. [DOI] [PubMed] [Google Scholar]
- 11. Stambler BS, Ellenbogen KA, Orav EJ, Sgarbossa EB, Estes NA, Rizo‐Patron C, Kirchhoffer JB, Hadjis TA, Goldman L, Lamas GA; Pacemaker Selection in the Elderly Trial Investigators. Predictors and clinical impact of atrial fibrillation after pacemaker implantation in elderly patients treated with dual chamber versus ventricular pacing. Pacing Clin Electrophysiol. 2003;2000–2007. [DOI] [PubMed] [Google Scholar]
- 12. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;427–436. [DOI] [PubMed] [Google Scholar]
- 13. Xie JM, Fang F, Zhang Q, Chan JYS, Yip GWK, Sanderson JE, Lam Y‐Y, Yan BP, Yu C‐M. Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int J Cardiol. 2012;364–369. [DOI] [PubMed] [Google Scholar]
- 14. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus‐node disease. N Engl J Med. 2007;1000–1008. [DOI] [PubMed] [Google Scholar]
- 15. Slotwiner DJ, Raitt MH, Del‐Carpio Munoz F, Mulpuru SK, Nasser N, Peterson PN. Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: a systematic review for the 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;e483–e503. [DOI] [PubMed] [Google Scholar]
- 16. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD; REVEAL AF Investigators . Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high‐risk population: the REVEAL AF study. JAMA Cardiol. 2017;1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di S Stefano L, Sepsi M, Landolina M, Lunati M, Lewalter T, Camm AJ. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device‐detected atrial fibrillation burden. Heart Rhythm. 2018;376–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


