Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) utilizes the angiotensin‐converting enzyme‐2 (ACE‐2) receptor to enter human cells. Angiotensin‐converting enzyme inhibitors (ACEI) and angiotensin II receptor antagonists (ARB) are associated with ACE‐2 upregulation. We hypothesized that antecedent use of ACEI/ARB may be associated with mortality in coronavirus disease 2019 (COVID‐19).
Methods and Results
We used the Coracle registry, which contains data of patients hospitalized with COVID‐19 in 4 regions of Italy, and restricted analyses to those ≥50 years of age. The primary outcome was in‐hospital mortality. Among these 781 patients, 133 (17.0%) used an ARB and 171 (21.9%) used an ACEI. While neither sex nor smoking status differed by user groups, patients on ACEI/ARB were older and more likely to have hypertension, diabetes mellitus, and congestive heart failure. The overall mortality rate was 15.1% (118/781) and increased with age (P Trend<0.0001). The crude odds ratios (ORs) for death for ACEI users and ARB users were 0.98, 95% CI, 0.60–1.60, P=0.9333, and 1.13, 95% CI, 0.67–1.91, P=0.6385, respectively. After adjusting for age, hypertension, diabetes mellitus, and congestive heart failure, antecedent ACEI administration was associated with reduced mortality (OR, 0.55; 95% CI, 0.31–0.98, P=0.0436); a similar, but weaker trend was observed for ARB administration (OR, 0.58; 95% CI, 0.32–1.07, P=0.0796).
Conclusions
In those aged ≥50 years hospitalized with COVID‐19, antecedent use of ACEI was independently associated with reduced risk of inpatient death. Our findings suggest a protective role of renin‐angiotensin‐aldosterone system inhibition in patients with high cardiovascular risk affected by COVID‐19.
Keywords: angiotensin‐converting enzyme inhibitor, angiotensin‐converting enzyme‐2, COVID‐19, hospitalization, mortality, renin‐angiotensin converting enzyme inhibitor, SARS‐CoV‐2
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System
Nonstandard Abbreviations and Acronyms
- ACE‐2
angiotensin‐converting enzyme‐2
- ARB
angiotensin II receptor antagonists
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease 2019
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Clinical Perspective
What Is New?
In patients hospitalized with coronavirus disease 2019 (COVID‐19), antecedent use of angiotensin‐converting enzyme inhibitors was associated with decreased odds of inpatient mortality, after adjusting for comorbidities.
What Are the Clinical Implications?
Current users of renin‐angiotensin‐aldosterone inhibitors should continue their use during the COVID‐19 pandemic.
In the midst of the coronavirus disease 2019 (COVID‐19) pandemic, there has been considerable interest and speculation about the effects of prior administration of renin‐angiotensin‐aldosterone system (RAAS) inhibitors, given the understanding that the coronavirus family utilizes the angiotensin‐converting enzyme 2 (ACE‐2) receptor for entry into human somatic cells. 1 , 2 One line of reasoning has been that more ACE‐2 receptors being available for viral entry would lead to more severe, and possibly fatal, infections. An alternative viewpoint is that the ACE‐2 receptor could be functioning in a protective manner despite facilitating viral entry, and that COVID‐19 target mediated destruction and reduction of receptor density could be part of the pathogenesis of the acute respiratory distress syndrome (ARDS) associated with acute infection. By piecing together observations from preclinical models, it has been postulated that children have a relative upregulation of ACE‐2 and a potentially greater pulmonary endothelial density of ACE‐2 expression. 3 It is notable that the rates of symptomatic and fatal cases of COVID‐19 are progressively lower at younger ages. 4 Conversely, the elderly and those who are smokers are believed to have lesser densities of ACE‐2 receptors in the lungs and lower inferred levels of circulating ACE‐2. 5 , 6 Experimental data in ARDS models suggest that ACE‐2 is downregulated and some of its peptide products (Ang 1–7) are consequently diminished, and thus, vascular endothelial protection is lost in the development of ARDS. 7 , 8 , 9 Soluble ACE‐2 has been administered experimentally and appears to lessen pathophysiological correlates of ARDS in vitro and in animal models. 10 , 11 , 12
In humans, long‐standing administration of angiotensin‐converting enzyme inhibitors (ACEI) and or angiotensin II receptor antagonists/blockers (ARB) leads to an upregulation of ACE‐2 on the surface of vascular endothelial cells, and possibly other somatic cell lines. This may be more pronounced with ACEI since angiotensin II, the substrate for ACE‐2, is diminished. By these lines of thinking, ACEI/ARB may be a “double‐edged sword” with either protective or harmful effects on outcomes in older individuals and those with multiple comorbidities when hospitalized for COVID‐19. 13 , 14 In particular, AlGhatrif and colleagues hypothesized that the increase in ACE‐2 levels with RAAS inhibition is likely to be beneficial for vulnerable individuals with COVID‐19 who have reduced ACE‐2 expression (eg older individuals, hypertensives, and/or diabetics). 15 Several professional societies have recommended continuing ACEI/ARB in patients with COVID‐19, but acknowledge a lack of data. 16 , 17 Hence, we sought to determine the association between ACEI/ARB use and inpatient mortality among patients with COVID‐19 ≥50 years using a registry of patients with acute COVID‐19 illness resulting in hospitalization in Italy.
Methods
Data
To ensure patient confidentiality, the data used for these analyses will not be made available. We used the Coracle (epidemiology, clinical characteristics, and therapy in real life patients affected by severe acute respiratory syndrome coronavirus 2 [Sars‐CoV‐2]) multi‐center registry, which contains data of patients hospitalized with COVID‐19 from participating referral centers in the Piedmont, Lombardy, Tuscany, and Lazio regions of Italy, to perform this analysis. Patients were included in the registry if they were at least 18 years old, hospitalized with COVID‐19 (confirmed using nose or throat swab testing with real‐time reverse transcription polymerase chain reaction) on or after February 22, 2020, and had a known mortality or discharge status as of April 1, 2020. We chose to restrict this analysis to patients ≥50 years because of the significant increase in mortality observed at this age cutoff in previous COVID‐19 publications, as well as for clinical indications of ACEI/ARB. 4 At hospital admission, patients gave written consent to data collection on the condition that the data be anonymized. This work was approved by the ethical committee of Turin (Coracle registry: epidemiology clinical characteristics and therapy in real life patients affected by SARS‐CoV‐2).
Statistical Analysis
The only continuous variable, age, was skewed and is presented as median [quartile 1, quartile 3]. To evaluate risk more clearly, we categorized age based on decade of life as follows: 50 to 59, 60 to 69, 70 to 79, and 80+. Categorical variables were reported as counts and proportions. Differences in characteristics between patients treated with ACEI, ARB, and those not taking ACEI/ARB were assessed via the Kruskal–Wallis Test and Chi‐Square test, respectively. We utilized the Cochran‐Armitage Trend Test to assess for a trend between age group and mortality rate. We examined crude odds ratios (ORs) for death based on ACEI/ARB use, as well as adjusted ORs according to the pre‐specified adjustment for age decade, history of hypertension (defined as blood pressure >140/90 mm Hg), diabetes mellitus, and congestive heart failure via multivariable logistic regression. Analyses were performed using SAS version 9.4 (Cary, NC).
Results
There were 956 patient records reviewed and 175 (18.3%) were excluded because of age <50 years. Hence, 781 patients were included in this analysis. Upon admission, 477 (61.1%) patients were not using ACEI/ARB, 133 (17.0%) were using an ARB, and 171 (21.9%) were using an ACEI. While neither sex nor smoking status differed significantly by ACEI/ARB user groups, patients using ACEI/ARB tended to be older and more likely to have hypertension, diabetes mellitus, and congestive heart failure than those not using ACEI/ARB (Table). Additionally, a total of 225 (28.8%) and 107 (13.8%) patients were treated in the intensive care unit and received invasive ventilation, respectively; neither was associated with antecedent ACEI/ARB use (P=0.2463, P=0.6089).
Table 1.
Table. Characteristics of Patients in Coracle Registry Aged ≥50 Years Hospitalized for COVID‐19
| Characteristic | Renin‐Angiotensin‐Aldosterone System Inhibition | P Value | |||
|---|---|---|---|---|---|
|
Overall (n=781) |
None (n=477) |
ARB (n=133) |
ACEI (n=171) |
||
| Age, y | 69 [60, 78.5] | 66 [58, 78] | 73 [65, 80] | 72 [63.7, 77] | <0.0001 |
| Age category, y | <0.0001 | ||||
| 50–59 | 194 (24.8%) | 150 (31.5%) | 17 (12.8%) | 27 (15.8%) | |
| 60–69 | 205 (26.2%) | 122 (25.6%) | 35 (26.3%) | 48 (28.1%) | |
| 70–79 | 204 (26.1%) | 99 (20.8%) | 46 (34.6%) | 59 (34.5%) | |
| 80+ | 178 (22.8%) | 106 (22.2%) | 35 (26.3%) | 37 (21.6%) | |
| Sex (male) | 498 (63.8%) | 305 (63.9%) | 83 (62.4%) | 110 (64.3%) | 0.9342 |
| History of hypertension2 | 451 (57.9%) | 155 (32.6%) | 130 (97.7%) | 166 (97.1%) | <0.0001 |
| Obstructive lung disease1 | 84 (10.8%) | 52 (10.9%) | 10 (7.5%) | 22 (12.9%) | 0.3236 |
| Diabetes mellitus1 | 143 (18.3%) | 66 (13.8%) | 31 (23.3%) | 46 (27.1%) | 0.0002 |
| Smoking status2 | 0.2524 | ||||
| Yes | 69 (8.9%) | 39 (8.2%) | 11 (8.3%) | 19 (11.1%) | |
| No | 655 (84.1%) | 410 (86.1%) | 108 (81.8%) | 137 (80.1%) | |
| Former | 55 (7.1%) | 27 (5.7%) | 13 (9.8%) | 15 (8.8%) | |
| Congestive heart failure2 | 67 (8.6%) | 31 (6.5%) | 16 (12%) | 20 (11.7%) | 0.0355 |
| Coronary artery disease (ischemia) | 104 (13.3%) | 46 (9.6%) | 28 (21.1%) | 30 (17.5%) | 0.0005 |
| Beta blocker1 | 174 (22.3%) | 82 (17.2%) | 42 (31.6%) | 50 (29.2%) | <0.0001 |
| Calcium channel antagonist1 | 154 (19.7%) | 67 (14.1%) | 40 (30.1%) | 47 (27.5%) | <0.0001 |
| Thiazide diuretic43 | 107 (14.5%) | 42 (9.3%) | 31 (24.6%) | 34 (21.3%) | <0.0001 |
| Loop diuretic43 | 99 (13.4%) | 48 (10.6%) | 26 (20.6%) | 25 (15.6%) | 0.0092 |
| Intensive care unit | 225 (28.8%) | 130 (27.3%) | 37 (27.8%) | 58 (33.9%) | 0.2463 |
| Invasive ventilation6 | 107 (13.8%) | 64 (13.5%) | 16 (12.2%) | 27 (16%) | 0.6089 |
| High flow ventilation without intubation9 | 298 (38.6%) | 169 (35.9%) | 54 (40.9%) | 75 (44.4%) | 0.1257 |
| Oxygen low flow9 | 333 (43.1%) | 214 (45.4%) | 59 (44.7%) | 60 (35.5%) | 0.0758 |
Age is presented as median [quartile 1, quartile 3] and categorical variables are presented as frequency (%). The P value for age was calculated from the Kruskal–Wallis Test. All other P values were calculated using the Chi‐Square test. Superscripts indicate missing data. ACEI indicates angiotensin II‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist/blocker; and COVID‐19, coronavirus disease 2019.
The overall mortality rate was 15.1% (118/781) and an increasing trend with age was detected (P Trend<0.0001, Figure 1). Death by specific subgroups is shown in Figure 2. Overall, the crude ORs for death for those who used ACEI and those who used ARB at admission compared to no ACEI/ARB use were 0.98 (95% CI, 0.60–1.60; P=0.9333) and 1.13 (95% CI, 0.67–1.91; P=0.6385), respectively. Among patients with diabetes mellitus, the crude ORs for death for those who used ACEI and those who used ARB at admission compared to no ACEI/ARB use were 2.05 (95% CI, 0.70–5.98; P=0.1884) and 2.02 (95% CI, 0.62–6.63; P=0.2445), respectively. Among patients with hypertension, the crude ORs for death for those who used ACEI and those who used ARB at admission compared to no ACEI/ARB use were 0.49 (95% CI, 0.28–0.86; P=0.0125) and 0.57 (95% CI, 0.32–1.01; P=0.0552), respectively. Among patients with congestive heart failure, the crude OR for death for those who used ACEI at admission compared to no ACE/ARB use was 0.61 (95% CI, 0.16–2.3; P=0.4721); there were 0 deaths out of the 16 patients with congestive heart failure treated with ARB.
Figure 1. Frequency of survival and inpatient death of patients with coronavirus disease 2019 (COVID‐19) by age group.
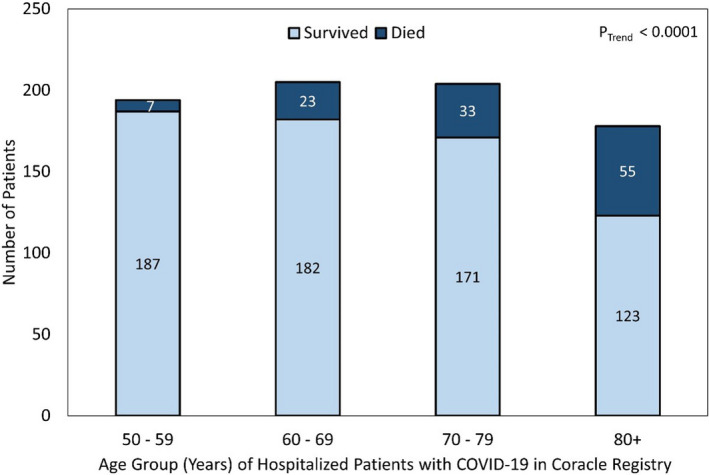
Figure 2. Inpatient mortality rates by renin‐angiotensin‐aldosterone system inhibitor use before admission for coronavirus disease 2019 (COVID‐19).
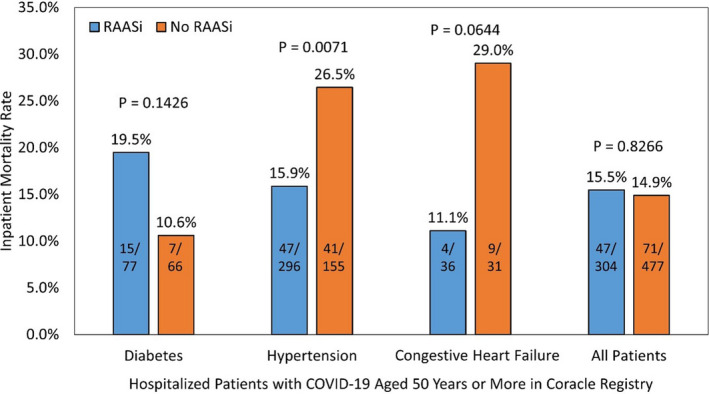
RAASi indicates renin‐angiotensin‐aldosterone system inhibitor.
In unadjusted analyses, neither ACEI nor ARB significantly changed the odds of death compared to no ACEI/ARB use (Figure 3). However, after adjusting for age decade, hypertension, diabetes mellitus, and congestive heart failure, ACEI decreased the odds of death by ≈45% (OR, 0.553; 95% CI, 0.311–0.983; P=0.0436). The large shift in ORs is primarily attributed to the adjustment for hypertension. Specifically, although only 57.9% of the patients in this analysis were hypertensive, they accounted for 74.6% of the deaths. Further, the mortality rates for patients with hypertension were 15.1%, 16.9%, and 26.5% for ACEI users, ARB users, and no RAASi users, respectively. ARB use did not significantly change the odds of death compared to patients using neither ACEI nor ARB, under the same adjustment (OR, 0.586; 95% CI, 0.322–1.065; P=0.0796). The Hosmer‐Lemeshow test yielded a P value of 0.8093, indicating the model fit the data well. Additionally, the area under the receiver operating characteristic curve was 0.737, indicating that the model had good ability to discriminate inpatient mortality from survival.
Figure 3. Forest plot of odds ratios for death pertaining to renin‐angiotensin‐aldosterone system inhibitor use.

ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist/blocker; COVID‐19, coronavirus disease 2019; and RAASi, renin‐angiotensin‐aldosterone system inhibitor.
Discussion
We found that among individuals aged ≥50 years hospitalized with COVID‐19 infection, that antecedent ACEI administration was associated with a considerably reduced case fatality rate after adjusting for age, hypertension, diabetes mellitus, and heart failure. Because of the predisposition of these comorbidities to mortality, only after adjustment did the protective effect of ACEI usage become apparent. Because it is common that hypotension and likely withdrawal of ACEI (or ARB) occurred in the sequence of events before death in COVID‐19 infections, we would infer it is the antecedent chronic use and not the acute administration of these agents that had an effect.
Our findings are in line with Lui, who reported on 46 patients over age 65 with hypertension hospitalized with COVID‐19, that both ACEI and ARB were associated with unadjusted reduced odds for mortality, but the point estimates were unstable and only ARB use had an adjusted P value of 0.046. 18 The finding is reassuring in that in older individuals with common and compelling indications for ACEI/ARB use, including chronic kidney disease and heart failure, there was no excessive risk of mortality with previous chronic administration and this supports most professional societies that have advised that patients on ACEI/ARB be continued on therapy in the setting of COVID‐19. 19
Our study raises many questions for the current COVID‐19 pandemic. Could chronic ACEI/ARB use be protective against initial infection or reduce the severity of COVID‐19 pneumonia? Large population studies including the large fraction of patients with COVID‐19 who shelter at home and are not hospitalized will need to be performed. Would administration of ACEI/ARB in hospital be protective against the development of ARDS? For this question, daily ACEI/ARB administration is needed or, preferably, a randomized trial recruiting hospitalized subjects. Finally, do these data suggest, along with the body of information on ACE‐2 and COVID‐19, that therapeutic upregulation of ACE‐2 or soluble ACE‐2 be a therapeutic strategy suitable for other agents or possibly recombinant ACE‐2?
Our study has all the limitations of retrospective observational cohort studies performed with limited information in the setting of a critical pandemic. We lacked clarity on the primary indication of ACEI/ARB prescription, dose, duration, and tolerability with respect blood pressure and azotemia. We did not have information on the choice of ACEI versus ARB and whether ARB treated patients were more likely to be ACEI intolerant. Additionally, other variables of interest, such as body mass index, or measures of ACE‐2 activity or its genetic determinants, were not available for study. Further, the relatively small sample size could have hindered the ability to detect an association between antecedent ARB administration and inpatient mortality, as its point estimate was similar to that of ACEI's; an adjusted pooled analysis comparing patients taking either ACEI or ARB against those taking neither ACEI nor ARB revealed significance (OR, 0.568; 95% CI, 0.347–0.928; P=0.0239). Lastly, our data may have limited generalizability attributable to the relatively homogeneous nature of the Italian population studied. Despite these limitations, the Coracle registry provides a representation of patients from regions of Italy that experienced high levels of viral spread, as well as those that were less affected, likely attributable to lockdown.
In summary, antecedent use of ACEI in patients aged 50 and older who were hospitalized with COVID‐19 was associated with a reduced risk of mortality after adjustment for common indications for ACEI/ARB administration. Future research is urgently needed for a better understanding on how the renin angiotensin system and its related pharmacological therapies influence the frequency, severity, and outcomes related to COVID‐19 infection.
Sources of Funding
This work was partially funded by the Baylor Health Care System Foundation.
Disclosures
None.
Acknowledgments
We are indebted to the following individuals for their contribution to this work: Francesco Mojoli, MD (Intensive Care University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy), Federico Franchi (AOUS Le Scotte Hospital, University of Siena Italy), Alex Di Nizio (AOUS Le Scotte Hospital, University of Siena Italy), Marco Schiavone (Azienda Ospedaliera ‐ Polo Universitario ‐ "Luigi Sacco" Milano Italy), Gianfranco Mitacchione (Azienda Ospedaliera ‐ Polo Universitario ‐ "Luigi Sacco" Milano Italy), Gabriella D'Ettorre (Department of public health and infectious diseases, La Sapienza, University of Rome, Italy), Giancarlo Ceccarelli (Department of public health and infectious diseases, La Sapienza, University of Rome, Italy), Claudio Mastroianni (Department of public health and infectious diseases, La Sapienza, University of Rome, Italy), Paolo Severino (Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Sapienza, University of Rome, Italy), Lucia Ilaria Birtolo (Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Sapienza, University of Rome, Italy), Francesco Pugliese (Department of General Surgery, Surgical Specialities "Paride Stefanini" Rome Italy), Franco Ruberto (Department of General Surgery, Surgical Specialities "Paride Stefanini" Rome Italy), Francesco Alessandri (Department of General Surgery, Surgical Specialities "Paride Stefanini" Rome Italy), Maria Chiara Colaiacomo (Department of Radiological, Oncological, and Pathological Anatomy Sciences, Sapienza University of Rome, Italy), Gaia Cartocci (Department of Radiological, Oncological, and Pathological Anatomy Sciences, Sapienza University of Rome, Italy), Alessio Mattei (Division of Pneumology University of Torino, AOU Città della salute e della Scienza, Torino, Italy), and Monica Andriani (Division of Cardiology University of Torino, AOU Città della salute e della Scienza, Torino, Italy).
(J Am Heart Assoc. 2020;9:e017364 DOI: 10.1161/JAHA.120.017364.)
For Sources of Funding and Disclosures, see page 6.
References
- 1. Lin HX, Feng Y, Wong G, Wang L, Li B, Zhao X, Li Y, Smaill F, Zhang C. Identification of residues in the receptor‐binding domain (RBD) of the spike protein of human coronavirus NL63 that are critical for the RBD‐ACE2 receptor interaction. J Gen Virol. 2008;89:1015–1024. [DOI] [PubMed] [Google Scholar]
- 2. Lo KB, McCullough PA, Rangaswami J. Antihypertensive drugs and risk of COVID‐19? Lancet Respir Med. 2020;8:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colafella KM, Hilliard LM, Denton KM. Epochs in the depressor/pressor balance of the renin‐angiotensin system. Clin Sci (Lond). 2016;130:761–771. [DOI] [PubMed] [Google Scholar]
- 4. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323:1775–1776. [DOI] [PubMed] [Google Scholar]
- 5. Fernández‐Atucha A, Izagirre A, Fraile‐Bermúdez AB, Kortajarena M, Larrinaga G, Martinez‐Lage P, Echevarría E, Gil J. Sex differences in the aging pattern of renin‐angiotensin system serum peptidases. Biol Sex Differ. 2017;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan YM, Luo L, Guo Z, Yang M, Ye RS, Luo C. Activation of renin‐angiotensin‐aldosterone system (RAAS) in the lung of smoking‐induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst. 2015;16:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M, Dyer MD, Teal TH, Proll S, van den Brand J, et al. Early upregulation of acute respiratory distress syndrome‐associated cytokines promotes lethal disease in an aged‐mouse model of severe acute respiratory syndrome coronavirus infection. J Virol. 2009;83:7062–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wösten‐van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin‐(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–627. [DOI] [PubMed] [Google Scholar]
- 9. Kuba K, Imai Y, Penninger JM. Angiotensin‐converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batlle D, Wysocki J, Satchell K. Soluble angiotensin‐converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond). 2020;134:543–545. [DOI] [PubMed] [Google Scholar]
- 12. Treml B, Neu N, Kleinsasser A, Gritsch C, Finsterwalder T, Geiger R, Schuster M, Janzek E, Loibner H, Penninger J, et al. Recombinant angiotensin‐converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide‐induced lung injury in piglets. Crit Care Med. 2010;38:596–601. [DOI] [PubMed] [Google Scholar]
- 13. Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double‐edged sword. Circulation. 2020;142:426–428. [DOI] [PubMed] [Google Scholar]
- 14. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9:e016219 DOI: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AlGhatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 2020;5:747–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID‐19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020;5:745–747. [DOI] [PubMed] [Google Scholar]
- 17. Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus disease 2019 (COVID‐19): do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9:e016509 DOI: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lui Y. Anti‐hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID‐19 patients. medRxiv preprint. DOI: 10.1101/2020.03.20.20039586. [DOI]
- 19. Talreja H, Tan J, Dawes M, Supershad S, Rabindranath K, Fisher J, Valappil S, van der Merwe V, Wong L, van der Merwe W, et al. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID‐19 (corona virus disease 2019). N Z Med J. 2020;133:85–87. [PubMed] [Google Scholar]


