Abstract
Background
Resting right heart catheterization can assess both left heart filling and pulmonary artery (PA) pressures to identify and classify pulmonary hypertension. Although exercise may further elucidate hemodynamic abnormalities, current pulmonary hypertension classifications do not consider the expected interrelationship between PA and left heart filling pressures. This study explored the utility of this relationship to enhance the classification of exercise hemodynamic phenotypes in pulmonary hypertension.
Methods and Results
Data from 36 healthy individuals (55, 50–60 years, 50% male) and 85 consecutive patients (60, 49–71 years, 48% male) with dyspnea and/or suspected pulmonary hypertension of uncertain etiology were analyzed. Right heart catheterization was performed at rest and during semiupright submaximal cycling. To classify exercise phenotypes in patients, upper 95% CIs were identified from the healthy individuals for the change from rest to exercise in mean PA pressure over cardiac output (ΔmPAP/ΔCO ≤3.2 Wood units [WU]), pulmonary artery wedge pressure over CO (ΔPAWP/ΔCO ≤2 mm Hg/L per minute), and exercise PA pulse pressure over PAWP (PP/PAWP ≤2.5). Among patients with a ΔmPAP/ΔCO ≤3.2 WU, the majority (84%) demonstrated a ΔPAWP/ΔCO ≤2 mm Hg/L per minute, yet 23% demonstrated an exercise PP/PAWP >2.5. Among patients with a ΔmPAP/ΔCO >3.2 WU, 37% had an exercise PP/PAWP >2.5 split between ΔPAWP/ΔCO groups. Patients with normal hemodynamic classification declined from 52% at rest to 36% with exercise.
Conclusions
The addition of PP/PAWP to classify exercise hemodynamics uncovers previously unrecognized abnormal phenotypes within each ΔmPAP/ΔCO group. Our study refines abnormal exercise hemodynamic phenotypes based on an understanding of the interrelationship between PA and left heart filling pressures.
Keywords: exercise, hemodynamics, phenotype, pulmonary artery, right heart catheterization
Subject Categories: Exercise, Hemodynamics, Pulmonary Hypertension
Nonstandard Abbreviations and Acronyms
- BREATH
Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response
- CO
cardiac output
- Cp
pulmonary compliance
- mPAP
mean pulmonary artery pressure
- MRC
Medical Research Council
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- PH‐LHD
pulmonary hypertension due to left heart disease
- PP
pulse pressure
- RHC
right heart catheterization
- Rp
pulmonary resistance
Clinical Perspective
What Is New?
Hemodynamic classifications of pulmonary hypertension at rest or during exercise do not consider the expected interrelationship between pulmonary artery and left heart filling pressures (pulmonary artery pulse pressure/pulmonary artery wedge pressure).
This study examined the normal limits of this relationship and demonstrated the effect of adding pulmonary artery pulse pressure/pulmonary artery wedge pressure to refine the classification of exercise hemodynamic responses in patients presenting with dyspnea and/or suspected pulmonary hypertension of uncertain etiology.
Previously unrecognized abnormal exercise hemodynamic phenotypes were demonstrated.
What Are the Clinical Implications?
Application of a refined understanding of exercise hemodynamics continues to demonstrate previously unrecognized pathophysiology in clinical populations.
Such higher resolution phenotyping provides more precise targets for which to develop therapeutic strategies, which we acknowledge are not yet clearly delineated, based on exercise hemodynamic phenotypes.
Right heart catheterization (RHC) is the gold standard for the diagnosis of pulmonary hypertension (PH) and can identify the presence of either pulmonary arterial hypertension (PAH) or PH due to left heart disease (PH‐LHD). 1 Exercise may be useful to elicit responses consistent with PAH or PH‐LHD when resting PH is either not detectable or if there are overlapping clinical features of PAH or PH‐LHD. 2
The approach to interpretation of exercise hemodynamics is dependent on an understanding of cardiopulmonary vascular physiology in health. The physiologic range of both the increase in pulmonary artery pressure (PAP) or pulmonary artery wedge pressure (PAWP) during exercise relative to the increase in cardiac output (CO; Δmean PAP[mPAP]/ΔCO; ΔPAWP/ΔCO) 3 , 4 are now well described. Abnormal increases in ΔmPAP/ΔCO or ΔPAWP/ΔCO identify hemodynamic responses indicative of PH and PH‐LHD 5 respectively. We, and others, have demonstrated that in healthy individuals during exercise there is a linear relationship between not only the increase in PAWP and changes in PA systolic and diastolic pressures, but also between mean PA and PA systolic and diastolic pressures. 6 , 7 , 8 , 9 , 10 We have further demonstrated that these relationships reflect a predictable exercise associated lowering of pulmonary compliance (Cp) relative to pulmonary resistance (Rp), represented by a decline in the RpCp time product. 9 Based on these observations, we hypothesize that there should be a predictable relationship between the PA pulse pressure and PAWP (PP/PAWP) in health at rest and during exercise. Further, abnormal increases of the PP/PAWP at rest or during exercise may identify patients with pulmonary vascular responses that are out of keeping with the measured values of the PAWP, which would refine exercise phenotyping beyond separate considerations of the ΔmPAP/ΔCO and/or ΔPAWP/ΔCO.
The primary objective of this study was to examine the addition of PP/PAWP to the calculation of ΔmPAP/ΔCO and ΔPAWP/ΔCO to classify exercise hemodynamic phenotypes in a population of patients undergoing exercise RHC for dyspnea of and/or suspected PH of uncertain etiology. In order to achieve this objective, the physiologic range of PP/PAWP in healthy older subjects was first explored.
Methods
Participants
All supporting data are available within the article and online supplementary files. Individual anonymized patient data are available from the corresponding author upon reasonable request. Patients with dyspnea and/or suspected PH of uncertain etiology referred for diagnostic RHC and exercise were consecutively recruited (n=85, 48% male) between November 2016 and February 2019 as part of our diagnostic program known as BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response). Patients also consented to complete dyspnea and quality of life questionnaires. Data from healthy individuals (n=36, 50% male) participating in a previous study of exercise hemodynamics served as a physiological control group. These subjects were recruited from the community by media advertising and had no history of cardiac or systemic disease. Hemodynamics from this group, mainly PAWP responses, were previously reported. 9 , 11 , 12 Institutional research ethics boards approved this study (Mount Sinai Hospital research ethics board; no. 11‐0190‐A and 16‐0217‐E) in accordance with the Declaration of Helsinki. Written informed consent was obtained before study participation.
Patient Characteristics and Self‐Reported Symptom Status
Standardized case report forms were populated to capture medical history. All patients completed the Medical Research Council (MRC) Breathlessness Scale 13 and the Medical Outcomes Study 36‐Item Short‐Form Health Survey (SF‐36). 14
Cardiac Catheterization Procedures
Right Heart Catheterization
RHC was performed with the patient in the supine position from peripheral venous access. 9 , 11 , 12 In brief, a 7Fr multilumen balloon flotation PA catheter (Swan‐Ganz Thermodilution PacePort Catheter; Edwards Lifesciences) was advanced under fluoroscopic guidance to a main branch of the PA. Following catheterization, patients were transferred to a purpose‐built electronically braked cycle ergometer (Ergoselect 1200E or Ergoline Ergoselect 12, Bitz, Germany) and inclined to a semiupright position for rest and exercise. Pressure transducers were zeroed at the midaxillary line.
Submaximal Exercise and Data Acquisition
The exercise protocol was previously employed in our study of healthy individuals. 9 , 11 , 12 Based on this experience, we developed the submaximal BREATH exercise protocol. Following 5 minutes of rest in the exercise position, up to 2 sequential 6‐minute stages of constant‐load submaximal exercise was performed. Patients with a MRC breathlessness scale score of 4 or more underwent an exercise protocol of 15/25 W. Patients with scores of 3 or less completed exercise at 25/40 W for women and 40/70 W for men. Pressure within the right atrium and PA were recorded continuously while PAWP was intermittently sampled and stored for offline analysis (MacLab version 6.5, 300 Hz; GE Healthcare, Little Chalfont, UK). CO was determined in triplicate via thermodilution with <10% variation between measurements. Hemodynamic variables were assessed 2 minutes into the pre‐exercise rest period and then again 5 minutes and 30 seconds into each submaximal exercise stage. Thermodilution CO was measured 2 minutes and 30 seconds into rest and each exercise stage.
Hemodynamic Analysis and Classification of Exercise Phenotypes
Hemodynamic analysis was performed on digital recordings as previously described. 12 Derived indices including systemic vascular resistance ([mean arterial blood pressure−right atrial pressure]/CO×80), pulse pressures (systolic−diastolic pressure), transpulmonary pressure gradient (mPAP−PAWP), diastolic pressure gradient (pulmonary artery diastolic pressure−PAWP), and RpCp time product (Rp=transpulmonary pressure gradient/(CO×1000/60); Cp=stroke volume/PA PP). Exercise was reported as the greatest single work rate completed by an individual. PAWP was reported at end‐expiration 15 as we have previously described. 12 During exercise, ΔmPAP/ΔCO, ΔPAWP/ΔCO, and PP/PAWP were calculated.
Evaluating Pressure‐Flow Relationships and the Association Between PAWP and PA Pressures in Healthy Controls: Determination of 95% CIs
Hemodynamic data from the healthy control cohort were used to calculate 95% CIs, the upper limit of “normal” (Figure 1A). The 95% upper confidence limit was 3.2 for ΔmPAP/ΔCO, 2.0 for ΔPAWP/ΔCO and 2.5 for exercise PP/PAWP.
Figure 1. Phenotype classification approach.
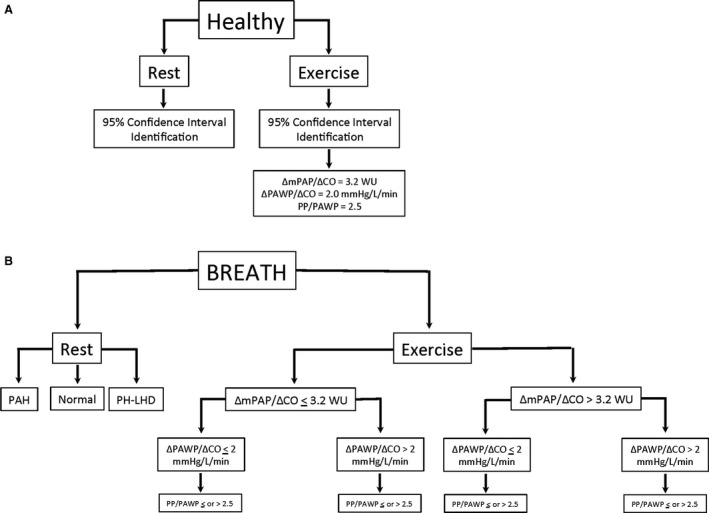
A, Healthy control cohort 95% confidence limit upper threshold identification. B, Algorithmic classification approach in BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort. CO indicates cardiac output; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PH‐LHD, pulmonary hypertension due to left heart disease; and PP/PAWP, pulse pressure/pulmonary artery wedge pressure.
Resting Hemodynamic Phenotype Classification
Resting hemodynamics were classified as Normal (mPAP ≤20 mm Hg, PAWP ≤15 mm Hg) PAH (mPAP >20 mm Hg, PAWP ≤15 mm Hg), and PH‐LHD (mPAP >20 mm Hg, PAWP >15 mm Hg).
Exercise Hemodynamic Phenotype Classification
The BREATH cohort was iteratively classified either above or below the upper limit of normal from healthy controls for ΔmPAP/ΔCO, ΔPAWP/ΔCO, and PP/PAWP (Figure 1B). To fully explore the association between the PAWP and the PA pressures, we evaluated the relationships between the PAWP and the systolic/diastolic/mean PA pressure as well as the RpCp time product.
Statistical Analysis
Normality was assessed visually with Q‐Q plots and quantitatively with a Shapiro–Wilk test. Physiologic parameters at semiupright rest (rest) and during exercise were analyzed using a mixed model, repeated measure analysis of variance, and a 1‐way analysis of variance. For the repeated measures analysis of variance the assumption of sphericity was met. Clinical parameters were analyzed with chi‐square, 1‐way analysis of variance, Mann–Whitney U test, and non‐parametric test of medians. Only significant F‐statistics were followed up with Bonferroni corrected post hoc t tests. Associations between continuous hemodynamic variables were explored with linear regressions. All physiologic data are presented as mean±SD. Demographic data are presented as mean±SD or median, interquartile range (Q1–Q3). Questionnaire data are reported as median, interquartile range (Q1–Q3). Statistics were completed using SPSS 20 (IBM Corp, Armonk, NY). Statistical significance was set at P<0.05.
Results
Participant Characteristics and Semiupright Resting Hemodynamics
Demographic information, patient comorbidities, medications, and self‐reported symptom status results are presented in Table 1. Semiupright resting hemodynamics are presented in Table 2. Additional results can be found in Data S1.
Table 1.
Demographic Information, Patient Comorbidities, Medications, and Self‐Reported Symptom Status
| Variable | Healthy Control (n=36) |
BREATH ΔmPAP/ΔCO ≤3.2 WU (n=44) |
BREATH ΔmPAP/ΔCO >3.2 WU (n=41) |
BREATH Cohort (n=85) |
|---|---|---|---|---|
| Demographic Information | ||||
| Sex (% male) | 50 | 50 | 46 | 48 |
| Age, y | 55, 50–60 | 53, 44–63 | 66, 57–75*, † | 60, 49–71 |
| Height, cm | 170±9 | 172±9 | 167±11 | 170±10 |
| Weight, kg | 74, 62–85 | 79, 72–100 | 82, 64–95 | 82, 68–98 |
| Body mass index, kg/m2 | 25.3±3.0 | 28.5±5.2† | 28.5±5.6† | 28.5±5.4 |
| Comorbidities | ||||
| Diabetes mellitus (% yes) | … | 11 | 37* | 24 |
| Hypertension (% yes) | … | 41 | 71* | 55 |
| Dyslipidemia (% yes) | … | 20 | 51* | 35 |
| Heart failure (% yes) | … | 9 | 34* | 21 |
| Creatinine, mmol/L | … | 79, 69–92 | 97, 75–129* | 84, 73–111 |
| Estimated glomerular filtration rate, mL/min per 1.73m2 | … | 83±22 | 62±24* | 72±25 |
| History of CAD (% yes) | … | 9 | 27* | 18 |
| Non CAD surgery (% yes) | … | 2 | 10 | 6 |
| Asthma or chronic obstructive pulmonary disease (% yes) | … | 11 | 34* | 22 |
| Smoking (% never/current/previous) | … | 77/3/20 | 49/2/49* | 64/2/34 |
| Connective tissue disorder (% yes) | … | 11 | 5 | 8 |
| Documented previous pulmonary embolism (% yes) | … | 45 | 10* | 28 |
| Medications | ||||
| Angiotensin‐converting enzyme inhibitor (% yes) | … | 18 | 27 | 22 |
| Angiotensin blocker (% yes) | … | 7 | 27* | 17 |
| Beta blockers (% yes) | … | 20 | 49* | 34 |
| Calcium channel blocker (% yes) | … | 20 | 24 | 22 |
| Acetylsalicylic acid (% yes) | … | 25 | 41 | 33 |
| Anticoagulant (% yes) | … | 52 | 46 | 49 |
| Insulin (% yes) | … | 7 | 17 | 12 |
| Other antidiabetic | … | 11 | 27 | 19 |
| Diuretic (% yes) | … | 32 | 61* | 46 |
| Statin (% yes) | … | 25 | 56* | 40 |
| Short Form Health Survey Questionnaire and MRC Breathlessness Scale | ||||
| Physical functioning | … | 45, 25–65 | 30, 15–58 | 35, 20–65 |
| Physical role functioning | … | 0, 0–50 | 0, 0–63 | 0, 0–50 |
| Emotional role functioning | … | 100, 33–100 | 66, 0–100 | 100, 0–100 |
| Energy/fatigue | … | 35, 15–50 | 40, 20–50 | 40, 20–50 |
| Emotional well‐being | … | 72, 56–84 | 68, 52–80 | 70, 52–84 |
| Social functioning | … | 63, 38–88 | 50, 50–88 | 50, 38–88 |
| Pain | … | 78, 45–90 | 58, 28–76 | 68, 38–86 |
| General health | … | 45, 25–65 | 35, 20–60 | 40, 25–60 |
| Health change | … | 50, 25–50 | 25, 25–67 | 38, 25–50 |
| MRC Breathlessness Score | … | 3, 2–4 | 4, 3–5 | 3, 2–4 |
Data are mean±SD or median, interquartile range (Q1–Q3). BREATH indicates Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response; CAD, coronary artery disease; CO, cardiac output; mPAP, mean pulmonary artery pressure; MRC, medical research council; and WU, Wood unit.
Statistically significant difference between BREATH ΔmPAP/ΔCO ≤3.2 WU and BREATH ΔmPAP/ΔCO >3.2 WU (P<0.05).
Statistically significant difference from Healthy Control and BREATH ΔmPAP/ΔCO ≤3.2 WU or BREATH ΔmPAP/ΔCO >3.2 WU respectively (P<0.05).
Table 2.
Semiupright Rest and Exercise Hemodynamic Data
| Variable | Healthy Control (n=36) |
BREATH ΔmPAP/ΔCO ≤3.2 WU (n=44) |
BREATH ΔmPAP/ΔCO >3.2 WU (n=41) |
|||
|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| WR, W | … | 67±26 | … | 51±27* | … | 30±19*, † |
| Heart rate, bpm | 64±8 | 121±3‡ | 68±11 | 110±20*, ‡ | 70±10* | 103±20*, ‡ |
| Stroke volume, mL | 76±16 | 93±23‡ | 78±20 | 104±25‡ | 65±17*, † | 69±23*, †, ‡ |
| CO, L/min | 4.8±0.8 | 11.2±2.7‡ | 5.2±1.3 | 11.3±3.0‡ | 4.5±1.3† | 7.1±2.6* |
| Systolic blood pressure, mm Hg | 128±13 | 169±15‡ | 130±17 | 155±21‡ | 132±25 | 152±34*, ‡ |
| Diastolic blood pressure, mm Hg | 79±8 | 80±9 | 82±10 | 81±16 | 76±11 | 81±19 |
| Mean arterial blood pressure, mm Hg | 96±8 | 109±8 | 97±10 | 105±14 | 94±14 | 104±21 |
| Right atrial pressure, mm Hg | 6±2 | 6±3 | 3±3* | 5±4‡ | 6±5 | 15±7*, †, ‡ |
| Pulmonary artery systolic pressure, mm Hg | 25±4 | 36±7‡ | 25±8 | 41±12‡ | 54±26*, † | 81±28*, †, ‡ |
| Pulmonary artery diastolic pressure, mm Hg | 11±3 | 15±4‡ | 10±4 | 17±5‡ | 19±9*, † | 31±9*, †, ‡ |
| mPAP, mm Hg | 17±3 | 25±5‡ | 17±5 | 28±7‡ | 33±15*, † | 53±16*, †, ‡ |
| mPAP ≤20 mm Hg (%) | 31 (86) | … | 31 (86) | … | 9 (22) | … |
| mPAP 21–24 mm Hg (%) | 5 (14) | … | 5 (14) | 7 (17) | … | |
| mPAP ≥25 mm Hg (%) | 0 (0) | … | 0 (0) | … | 25 (61) | … |
| PAWP, mm Hg | 11±2 | 15±5‡ | 9±4 | 14±6‡ | 13±7† | 23±10*, †, ‡ |
| PAWP ≤15 [C] 25 [E] mm Hg (%) | 34 (94) | 34 (97) | 34 (94) | 40 (91) | 28 (68) | 25 (61) |
| PAWP >15 [C] 25 [E] mm Hg (%) | 2 (6) | 1 (3) (missing n=1) | 2 (6) | 4 (9) | 13 (32) | 16 (39) |
| Systemic PP, mm Hg | 49±12 | 89±16‡ | 48±16 | 74±24*, ‡ | 56±22 | 71±33*, ‡ |
| Systemic vascular resistance, dyn/s per cm5 | 1538±287 | 778±199‡ | 1524±445 | 758±237‡ | 1666±525 | 1108±438*, †, ‡ |
| Pulmonary PP, mm Hg | 14±3 | 21±5‡ | 16±5 | 24±9‡ | 35±19*, † | 49±22*, †, ‡ |
| Transpulmonary pressure gradient, mm Hg | 6±2 | 10±3‡ | 8±4 | 13±6‡ | 20±15*, † | 30±21*, †, ‡ |
| Diastolic pressure gradient, mm Hg | −1±1 | −0.1±3 | 1±3 | 2±5 | 6±8*, † | 8±13*, †, ‡ |
| Rp, mm Hg/s per mL | 0.07±0.22 | 0.06±0.02‡ | 0.10±0.05 | 0.07±0.04‡ | 0.29±0.24*, † | 0.30±0.25*, † |
| Cp, mL/mm Hg | 5.6±1.6 | 4.6±1.7 | 5.3±1.8 | 4.8±2.1 | 2.4±1.2 | 1.7±0.9 |
| RpCp‐time, s | 0.39±0.10 | 0.23±0.007 | 0.44±0.11 | 0.31±0.10 | 0.45±0.15 | 0.33±0.11 |
| PP/PAWP | 1.3±0.4 | 1.5±0.5 | 1.0±0.3* | 2.0±1.0 | 1.1±0.2* | 3.9±6.1*, †, ‡ |
| ΔPAWP/ΔCO | 0.7±0.8 | 1.1±1.0* | 6.3±7.8*, † | |||
| PAWP/[WR/weight], mm Hg/W per kg | … | 18±9 | … | 33±32 | … | 83±73*, † |
Data are mean±SD. BREATH, Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response; CO indicates cardiac output; Cp, pulmonary compliance; mPAP, mean pulmonary artery pressure; ; PAWP, end‐expiratory pulmonary artery wedge pressure; PP, pulse pressure; Rp, pulmonary vascular resistance; WR, work rate; WU, Wood unit; and Δ difference between exercise and semiupright rest.
Statistically significant difference between healthy controls within condition (P<0.05).
Statistically significant difference between BREATH ΔmPAP/ΔCO ≤3.2 WU within condition (P<0.05).
Statistically significant difference between exercise and semiupright rest within a group (P<0.05).
Healthy Control Cohort
Participants had a median age of 55, 50 to 60 with a body mass index of 25.3±3.0 kg/m2. Among this healthy, older, control cohort, the mPAP was ≤20 mm Hg in 86%. PAWP was ≤15 mm Hg in 94% of individuals (Table 2).
BREATH Cohort
Patients had a median age of 60, 49 to 71 with a body mass index of 28.5±5.4 kg/m2. Identifying risk factors for PAH, 8% had a history of connective tissue disorders and 28% had a previous pulmonary embolus. Over 50% had a history of systemic hypertension (>140/90 mm Hg), and 69% had at least 1 of the following: diabetes mellitus, systemic hypertension, elevated body mass index (>30 kg/m2), or a history of coronary artery disease. Breathlessness assessment revealed a median MRC score of 3, 2 to 4 and the SF‐36 demonstrated impairments in all quality of life outcomes. PAWP was ≤15 mm Hg in 79% of patients. Forty‐eight percent demonstrated PH. Of patients with PH, 56% were classified as PAH, 44% were classified as PH‐LHD (Table 2).
Exercise Hemodynamic Phenotyping by ΔmPAP/ΔCO Slope
Exercise hemodynamic data are presented in Table 2 for healthy controls and BREATH patients. In the BREATH cohort, 44 patients (54%) demonstrated a ΔmPAP/ΔCO slope ≤3.2 WU and 41 patients (46%) demonstrated a ΔmPAP/ΔCO slope >3.2 WU (Figure 2).
Figure 2. Individual total pulmonary resistance (TPR) slope from semiupright rest to steady state submaximal exercise.
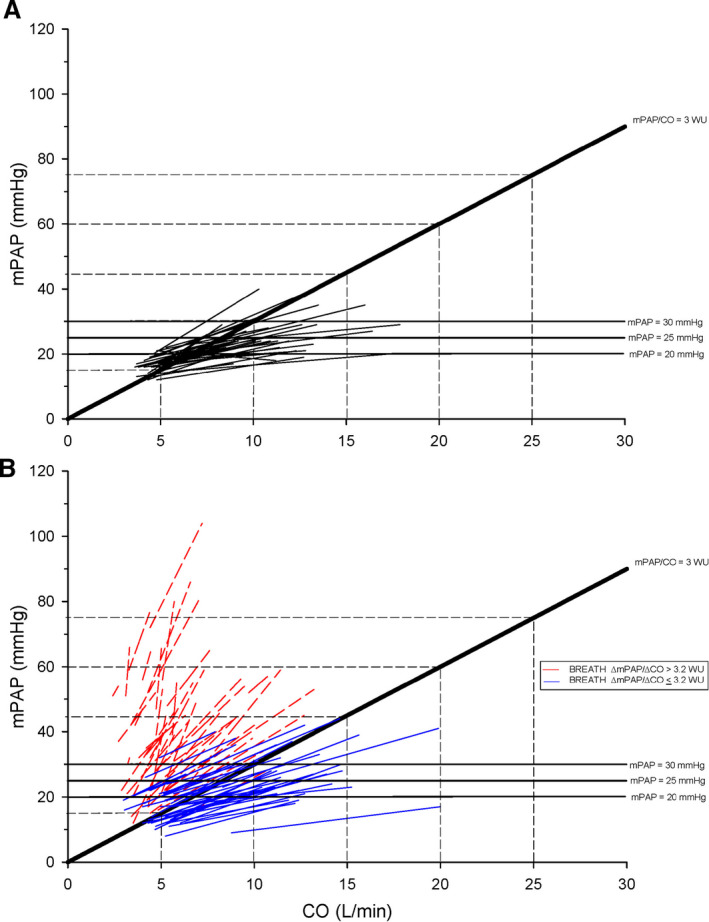
A, Healthy control cohort. B, BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort. TPR slope of 3 WU is denoted within each panel. CO indicates cardiac output; and mPAP, mean pulmonary artery pressure.
Clinical Characteristics
BREATH cohort—ΔmPAP/ΔCO ≤3.2 WU
The median age was 53, 44 to 63, 50% were male, and 57% had at least 1 of the following: diabetes mellitus, systemic hypertension, or coronary artery disease. The median MRC Breathlessness Score was 3, 2 to 4 and the SF‐36 results demonstrated impairments in all facets of quality of life (Table 1). At rest, 20% demonstrated PH and PAWP was ≤15 mm Hg in 89% of patients (Figure 2B). Of patients with PH, 56% were classified as PAH and 44% were classified as PH‐LHD.
BREATH cohort—ΔmPAP/ΔCO >3.2 WU
The median age was 66, 57 to 75 and 46% were male. Compared with the ΔmPAP/ΔCO ≤3.2 WU group, these patients were older (P=0.01), more likely to have diabetes mellitus (P=0.006), a history of coronary artery disease (P=0.032), and treated with cardiovascular medications. The median MRC Breathlessness Score was 4, 3 to 5 and the SF‐36 results demonstrated impairments in all facets of quality of life (Table 1). At rest, 78% demonstrated PH and PAWP was ≤15 mm Hg in 68% (Figure 2B). Of patients with PH, 56% were classified as PAH and 44% were classified as PH‐LHD.
Semiupright Rest and Exercise Hemodynamics
Healthy control cohort
Hemodynamics are presented in Table 2.
BREATH cohort—ΔmPAP/ΔCO ≤3.2 WU
Mean resting values for PA pressures, PAWP, CO, transpulmonary pressure gradient, diastolic pressure gradient, Rp, and Cp were not different from the healthy control cohort. Compared with healthy controls, exercise work rate was lower (P=0.01), but exercise hemodynamics were not different.
BREATH cohort—ΔmPAP/ΔCO >3.2 WU
Compared with healthy controls and ΔmPAP/ΔCO ≤3.2 WU, this group had abnormal mean resting PA pressures, transpulmonary pressure gradient, diastolic pressure gradient, Rp, and Cp. Exercise work rate was lower compared to healthy controls and BREATH cohort ΔmPAP/ΔCO ≤3.2 WU (P<0.001). With exercise, stroke volume and CO responses were lower and both right atrial pressure and PAWP were increased compared with healthy controls and ΔmPAP/ΔCO ≤3.2 WU (all P<0.05, Table 2).
In contrast to both healthy controls and BREATH ΔmPAP/ΔCO ≤3.2 WU, the linear relationship between PAWP and pulmonary artery systolic pressure was lost and the diastolic pressure gradient was elevated. Similar to both healthy controls and BREATH ΔmPAP/ΔCO ≤3.2 WU the relationship between the decline in RpCp time and PAWP was preserved, yet this cohort presented on the horizontal, as opposed to the vertical, limb of the RpCp time relationship (Figure S1).
Exercise Hemodynamic Phenotyping ΔPAWP/ΔCO Slope
Healthy Control Cohort
The ΔPAWP/ΔCO slope was ≤2 mm Hg/L per minute in 97% of healthy controls. Exercise PAWP ≤25 mm Hg was also observed in 97% of healthy controls.
BREATH Cohort—ΔmPAP/ΔCO ≤ or >3.2 WU
Among patients demonstrating a ΔmPAP/ΔCO ≤3.2 WU, ΔPAWP/ΔCO slope was ≤2 mm Hg/L per minute in 84%. Among patients demonstrating a ΔmPAP/ΔCO >3.2 WU, ΔPAWP/ΔCO was ≤2 mm Hg/L per minute in 27% of patients and exercise PAWP did not exceed 25 mm Hg. Among patients demonstrating a ΔmPAP/ΔCO >3.2 WU, ΔPAWP/ΔCO slope was >2 mm Hg/L per minute in 73% of patients and exercise PAWP exceeded 25 mm Hg in 57% of these patients (Figure 3).
Figure 3. Coupling of the change in end‐expiratory pulmonary artery wedge pressure (PAWP) to cardiac output (CO) from semiupright rest to steady state submaximal exercise in the BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort.
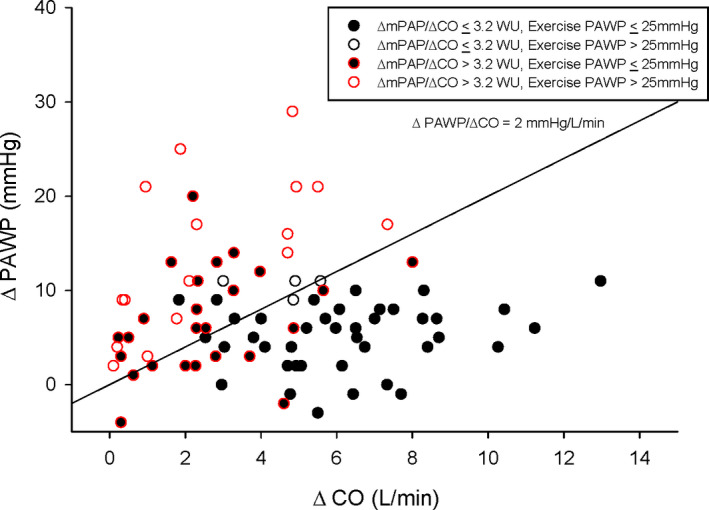
ΔPAWP/ΔCO of 2 mm Hg/L per minute is denoted. CO indicates cardiac output; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; and WU, Wood unit.
Exercise Hemodynamic Phenotyping by Association of PAWP and Pulmonary Pressures: the PP/PAWP Ratio
Healthy Control Cohort
The PP/PAWP ratio was 1.3±0.3 at rest, 1.5±0.5 during exercise. A frequency histogram demonstrates a narrow range in the PP/PAWP ratio at rest and during exercise (Figure 4A), with an upper 95% CI ratio of 2.5 during exercise.
Figure 4. Frequency distribution of pulmonary artery pulse pressure (PP) to end‐expiratory pulmonary artery wedge pressure (PAWP; PP/PAWP) during semiupright rest and during steady state submaximal exercise.
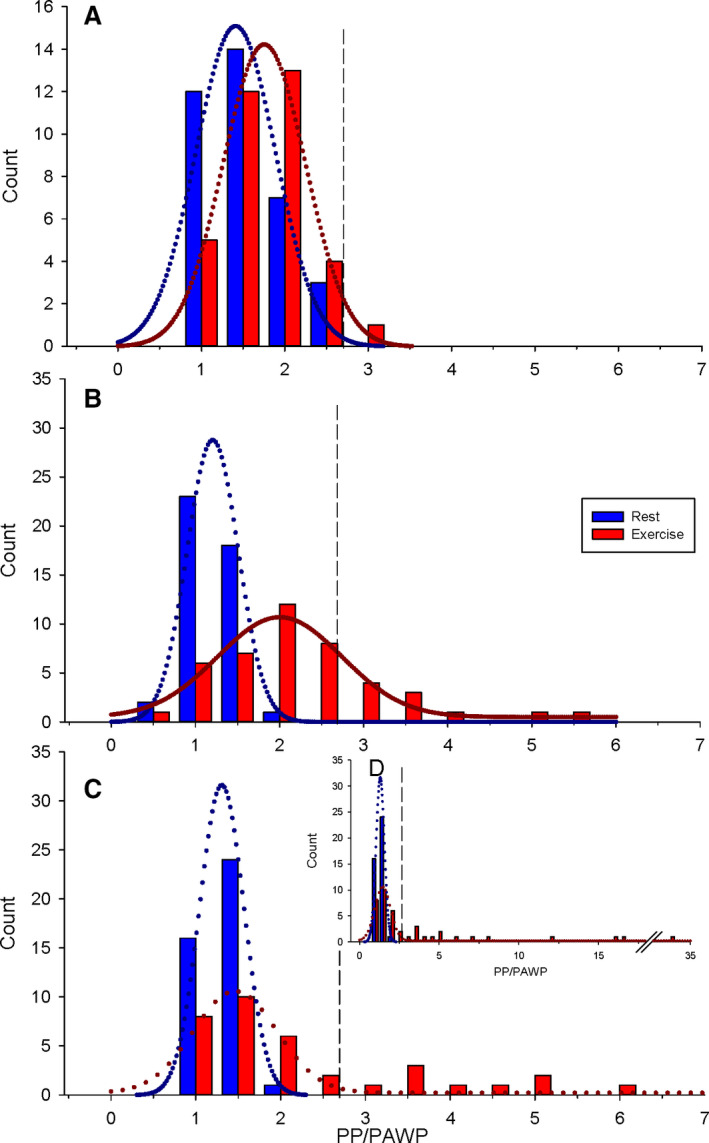
A, Healthy control cohort. B, BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort ΔmPAP/ΔCO ≤3.2 WU. C, BREATH cohort ΔmPAP/ΔCO >3.2 WU. D, BREATH cohort ΔmPAP/ΔCO >3.2 WU expanded PP/PAWP axis. Vertical dashed lines represent the upper limit of normal (2.5) in each panel. CO indicates cardiac output; mPAP, mean pulmonary artery pressure; and WU, Wood unit
BREATH Cohort—ΔmPAP/ΔCO ≤3.2 WU
The PP/PAWP ratio was 1.0±0.3 at rest, 2.0±1.0 during exercise. In contrast to healthy controls, the frequency histogram of the PP/PAWP ratio with exercise revealed a subpopulation (23% of patients) demonstrating an abnormal PP/PAWP ratio extending beyond the upper 95% CI of 2.5 (Figure 4B).
BREATH Cohort—ΔmPAP/ΔCO >3.2 WU
The PP/PAWP ratio was 1.1±0.2 at rest, 3.9±6.1 during exercise. The frequency histogram of the PP/PAWP ratio with exercise in this group revealed a subpopulation (37% of patients) demonstrating a markedly abnormal PP/PAWP ratio extending well beyond the upper 95% CI of 2.5 (Figure 4C and 4D).
Partitioning ΔmPAP/ΔCO ≤3.2 WU and ΔmPAP/ΔCO >3.2 WU by exercise PP/PAWP ≤ or >2.5, all groups demonstrated a linear relationship between PAWP and PA pressures, except PAWP and pulmonary artery systolic pressure in the ΔmPAP/ΔCO >3.2 WU, exercise PP/PAWP >2.5 group. Regardless of ΔmPAP/ΔCO slope, exercise PP/PAWP >2.5 groups presented with PAWP responses not exceeding 25 mm Hg and a shift of the relative position on the RpCp time relationship downwards. All groups continued to demonstrate a linear relationship between PAWP and the decline in RpCP time (Figure S2).
Approach to Exercise Hemodynamic Phenotyping Based on Pressure‐Flow Relationships and the Pulmonary PP/PAWP Ratio
Exercise hemodynamic phenotypes can be illustrated by plotting ΔPAWP/ΔCO on the x‐axis and PP/PAWP on the y‐axis while denoting the upper limits of normal (dashed horizontal and vertical lines). Patients in the left lower quadrant exhibit both ΔPAWP/ΔCO and exercise PP/PAWP that are within normal limits, which would include our healthy control population, as illustrated in the inset panel (Figure 5B). Patients demonstrating an elevated exercise PP/PAWP yet a normal ΔPAWP/ΔCO and are observed in the left upper quadrant. Patients with an elevated ΔPAWP/ΔCO yet a normal exercise PP/PAWP and are found in the right lower quadrant. Patients with both abnormal elevations in both exercise PP/PAWP and ΔPAWP/ΔCO response are present in the right upper quadrant. Within each quadrant patients with either a ΔmPAP/ΔCO ≤3.2 WU or ΔmPAP/ΔCO >3.2 WU are noted.
Figure 5. Coupling of the exercise pulmonary artery pulse pressure (PP) over end‐expiratory pulmonary artery wedge pressure (PAWP; PP/PAWP) to the change in PAWP over cardiac output (CO; ΔPAWP/ΔCO) from semiupright rest to steady state submaximal exercise.
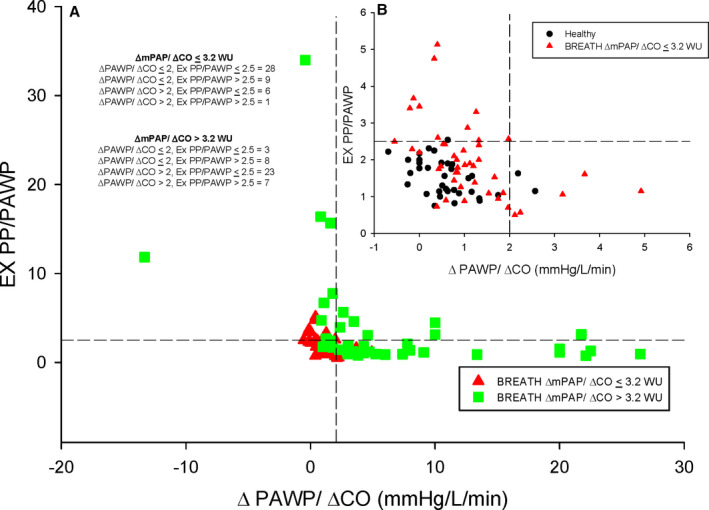
A, BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort. B, Healthy control cohort and BREATH cohort ΔmPAP/ΔCO ≤3.2 WU. Horizontal dashed line represents the upper limit of normal for Ex PP/PAWP (2.5). Vertical dashed line represents the upper limit of normal for ΔPAWP/ΔCO (2 mm Hg/L per minute). CO indicates cardiac output; mPAP, mean pulmonary artery pressure; and WU, Wood unit
Resting to Exercise Phenotypes—Higher Resolution Classification Based on Pressure‐Flow Relationships and the Pulmonary PP/PAWP Ratio
At rest, hemodynamics were within normal limits in 52% of patients. After exercise reclassification, the proportion of normal (ΔmPAP/ΔCO ≤3.2 WU, ΔPAWP/ΔCO ≤2 mm Hg/L per minute, and PP/PAWP ≤2.5) declined to 36%. Independent of a normal or abnormal ΔmPAP/ΔCO, abnormal hemodynamic phenotypes were identified. If ΔmPAP/ΔCO ≤3.2 WU was observed during exercise, the following abnormal phenotypes were still observed: 16% exhibited an abnormal ΔPAWP/ΔCO >2 mm Hg/L per minute; 23% demonstrated an abnormal exercise PP/PAWP ratio >2.5, and a single patient had abnormal elevations in both ΔPAWP/ΔCO and exercise PP/PAWP (Figure 6A). If ΔmPAP/ΔCO >3.2 WU was observed during exercise, the following abnormal phenotypes were observed: 56% exhibited an abnormal ΔPAWP/ΔCO >2 mm Hg/L per minute; 20% demonstrated an abnormal exercise PP/PAWP ratio >2.5, and 17% had abnormal elevations in both ΔPAWP/ΔCO and exercise PP/PAWP (Figure 6B).
Figure 6. Resting and exercise hemodynamic phenotypes within the BREATH (Breathlessness Revealed Using Exercise to Assess the Hemodynamic Response) cohort.
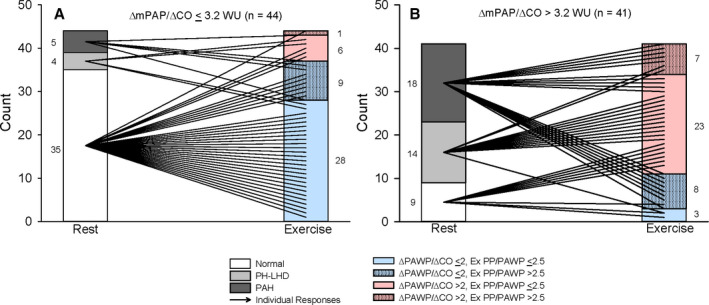
A, BREATH cohort ΔmPAP/ΔCO ≤3.2 WU. B, BREATH cohort ΔmPAP/ΔCO >3.2 WU. Numerical representation of patients within each phenotype is denoted on each panel. Exercise phenotypes have variability introduced into the y‐axis variable to allow for delineation of individual responses from rest to exercise. CO indicates cardiac output; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PH‐LHD, pulmonary hypertension due to left heart disease; and WU, Wood unit
Discussion
In this study, we approached the evaluation of exercise hemodynamic phenotypes with expanded concepts of integrated physiology between the pulmonary vasculature and the left heart observed from a healthy control cohort. Among patients with a ΔmPAP/ΔCO ≤3.2 WU, most, but not all, of this group demonstrated a ΔPAWP/ΔCO slope ≤2 mm Hg/L per minute. However, exercise revealed a clear subpopulation with an abnormal exercise PP/PAWP >2.5; suggestive of an inappropriate or exaggerated pulmonary vascular to PAWP response. Among patients with a ΔmPAP/ΔCO >3.2 WU, demonstration of both a ΔPAWP/ΔCO slope ≤2 mm Hg/L per minute and an exercise PP/PAWP >2.5 clearly indicated abnormal PA pressure responses not driven by left heart filling abnormalities. Further, we identified patients with an elevated ΔmPAP/ΔCO >3.2 WU, for whom responses were driven by a ΔPAWP/ΔCO slope >2 mm Hg/L per minute with or without the addition of an abnormal exercise PP/PAWP ratio. Our study refines quantitative classification of abnormal exercise hemodynamic phenotypes based on a more detailed understanding of the interrelationship between left heart filling pressure and pulmonary vascular behaviour.
Healthy Control Cohort and Identification of 95% CIs
In healthy individuals, we identified an upper 95% confidence limit for the ΔmPAP/ΔCO slope of 3.2 WU during submaximal exercise, which is similar to the work of Naeije et al 4 who reported an upper limit of normal threshold of 3 WU. Although this relationship identifies abnormal increases in PA pressure, it does not discriminate between pulmonary vascular disease or PH‐LHD. As such, ΔPAWP/ΔCO slope was applied to determine the presence of an abnormal increase in PAWP. As others have, we demonstrated in healthy controls that the ΔPAWP/ΔCO slope does not exceed 2 mm Hg/L per minute and that an exercise PAWP >25 mm Hg is rare during sustained submaximal exercise. 3 , 11
We then evaluated the exercise PP/PAWP ratio as the next step in the analysis of exercise hemodynamics. The rationale for this parameter arose from work in our laboratory 9 , 12 and others 6 , 10 that documented predictable interactions between the PAWP and the pulmonary vasculature in health. As PAWP increases, the RpCp time product is reduced. This reduction in the RpCp time product reflects a downward shift in the relationship between Cp at a given Rp, that stems from a greater reduction in Cp than Rp. 9 In the current study we demonstrated that the physiologic range of PP/PAWP has an upper 95% CI of 2.5 during exercise. The PP/PAWP ratio represents a novel, simple, physiologically rational means to discern whether the PA pressure behaves normally or abnormally in the context of the PAWP responses.
Hemodynamic Phenotyping of the Study Cohort
The study cohort as a whole was older, and the prevalence of risk factors for cardiovascular disease was 70%. This patient cohort presenting with dyspnea and/or suspected PH of uncertain etiology, often with clinical risk factors for both PAH or PH‐LHD is a therapeutic challenge and have typically been excluded from clinical trials. 16 Although slightly over half of the population had normal hemodynamics at rest, these patients were clearly symptomatic; suffering impairments in quality of life across the SF‐36 compared with similarly aged Canadians. 17 Exercise as an intervention during RHC may improve the clinician's understanding of the pathophysiologic abnormalities and allow personalization of clinical care. The ΔmPAP/ΔCO slope ≤ or >3.2 WU was employed as the initial screen for exercise hemodynamic abnormalities in the study cohort. This relationship threshold is similar and does provide validation to the proposed threshold of ΔmPAP/ΔCO >3 WU by the European Respiratory Society. 18 Patients with a ΔmPAP/ΔCO ≤3.2 WU were indistinguishable hemodynamically from the healthy control cohort. 6 , 7 , 8 , 9 However, this grouping did not entirely rule out pathophysiologic abnormalities during exercise that may contribute to dyspnea. A small number of individuals presented with a ΔPAWP/ΔCO >2 mm Hg/L per minute with a further subset of these patients demonstrating a PP/PAWP ratio >2.5 during exercise. In health, the limited PP/PAWP ratio response is related to a relatively high Cp, 19 characteristic of the low‐pressure pulmonary circulation. Therefore, an increase in the PP/PAWP ratio may suggest increased stiffness of the PA, without an increase in Rp, as a mechanism for dyspnea experienced by this population.
As a whole, the ΔmPAP/ΔCO >3.2 WU group exhibited significantly abnormal hemodynamics, although ≈20% still had normal resting hemodynamics. The combination of a normal exercise PP/PAWP and a ΔPAWP/ΔCO >2 mm Hg/L per minute provides strong physiological rationale that left heart disease is entirely responsible for the ΔmPAP/ΔCO >3.2 WU. Patients with an exercise PP/PAWP ≤2.5 demonstrated preserved linkages between left heart filling and PA pressures. An elevated exercise PP/PAWP identified patients with the highest PA pressures, limited PAWP responses to exercise, and altered pulmonary vascular to PAWP relationships. In a small group, detection of an elevated exercise PP/PAWP in addition to a ΔPAWP/ΔCO >2 mm Hg/L per minute suggested the pulmonary vascular response was abnormal over and above left heart disease.
Reclassification of Resting Phenotypes
Exercise hemodynamic testing serves to reveal abnormal responses not evident or unclear at rest. The phenotypes identified by our integrative physiological approach revealed abnormalities even among patients with a ΔmPAP/ΔCO slope ≤3.2 WU. Clinically, higher resolution phenotyping provides more precise targets for which to develop therapeutic strategies, which we acknowledge are not yet clearly delineated for exercise hemodynamic phenotypes; perhaps with the exception of an interatrial septal device for an exercise PAWP ≥25 mm Hg. 20 , 21 Finally, our observations underline the notion that dyspnea is a common and disabling symptom related to disparate mechanisms and for 35% of our cohort, we did not identify a hemodynamic abnormality. It remains challenging to fully attribute symptom status to hemodynamic mechanisms.
Limitations
There are limitations to this study that merit discussion. The study cohort was a consecutive sample arising from patients referred for diagnostic RHC and exercise and as such, presented with a range of underlying comorbidities. Although we have attempted to standardize and minimize artefacts that confound interpretation of hemodynamic waveforms, there is still debate as to how intrathoracic pressure swings should be handled. 15 , 18 This is particularly relevant to clinical states of excess adiposity or the presence of respiratory disorders including chronic obstruction pulmonary disease. Underlying pathology may create a bias to group partitioning by hemodynamic responses but does suggest the utility of algorithmic exercise hemodynamic classification across a range of clinical profiles. Future investigations are required to corroborate study findings. Although exercise PP/PAWP represents a novel marker of abnormal exercise hemodynamics, whether there is an association to clinical end points such as functional capacity is currently unknown. The limited sample size may bring into question the generalizability of the upper 95% confidence limits to discern normal versus abnormal exercise phenotypes. However, a larger sample size would only further reduce the SD and thereby confidence limits, thus more easily identifying abnormalities in the BREATH population.
Conclusions
We have demonstrated that in a diverse cohort of patients presenting with dyspnea and/or suspected PH of uncertain etiology, an approach involving ΔmPAP/ΔCO slope, ΔPAWP/ΔCO slope, and exercise PP/PAWP serves to uncover previously unrecognized hemodynamically abnormal phenotypes compared with healthy controls.
Sources of Funding
This research was supported by the Peter Munk Cardiac Centre Innovation Fund and the Ontario Research Fund.
Disclosures
None.
Supporting information
Data S1
Figures S1–S2
Acknowledgments
The authors would like to thank the research staff of the Mecklinger Family and Posluns Family Cardiac Catheterization Clinical Research Laboratory for their support.
(J Am Heart Assoc. 2020;9:e016339 DOI: 10.1161/JAHA.120.016339.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sorajja P, Borlaug BA, Dimas VV, Fang JC, Forfia PR, Givertz MM, Kapur NK, Kern MJ, Naidu SS. SCAI/HFSA clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. Catheter Cardiovasc Interv. 2017;E233–E247. [DOI] [PubMed] [Google Scholar]
- 3. Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;e004750 DOI: 10.1161/CIRCHEARTFAILURE.117.004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery JL, Lewis GD. Exercise‐induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naeije R, Saggar R, Badesch D, Rajagopalan S, Gargani L, Rischard F, Ferrara F, Marra AM, D'Aalto M, Bull TM, et al. Exercise‐induced pulmonary hypertension: translating pathophysiological concepts into clinical practice. Chest. 2018;10–15. [DOI] [PubMed] [Google Scholar]
- 6. Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013;217–223. [DOI] [PubMed] [Google Scholar]
- 7. Kaltman AJ, Herbert WH, Conroy RJ, Kossmann CE. The gradient in pressure across the pulmonary vascular bed during diastole. Circulation. 1966;377–384. [DOI] [PubMed] [Google Scholar]
- 8. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright SP, Granton JT, Esfandiari S, Goodman JM, Mak S. The relationship of pulmonary vascular resistance and compliance to pulmonary artery wedge pressure during submaximal exercise in healthy older adults. J Physiol. 2016;3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kind T, Faes TJ, Vonk‐Noordegraaf A, Westerhof N. Proportional relations between systolic, diastolic and mean pulmonary artery pressure are explained by vascular properties. Cardiovasc Eng Technol. 2011;15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esfandiari S, Wright SP, Goodman JM, Sasson Z, Mak S. Pulmonary artery wedge pressure relative to exercise work rate in older men and women. Med Sci Sports Exerc. 2017;1297–1304. [DOI] [PubMed] [Google Scholar]
- 12. Wright SP, Esfandiari S, Gray T, Fuchs FC, Chelvanathan A, Chan W, Sasson Z, Granton JT, Goodman JM, Mak S. The pulmonary artery wedge pressure response to sustained exercise is time‐variant in healthy adults. Heart. 2016;438–443. [DOI] [PubMed] [Google Scholar]
- 13. Stenton C. The MRC breathlessness scale. Occup Med (Lond). 2008;226–227. [DOI] [PubMed] [Google Scholar]
- 14. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;473–483. [PubMed] [Google Scholar]
- 15. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grunig E, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;834–844. [DOI] [PubMed] [Google Scholar]
- 17. Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, Joseph L, Brown JP, Murray TM, Adachi JD, et al. Canadian normative data for the SF‐36 health survey. Canadian Multicentre Osteoporosis Study Research Group. CMAJ. 2000;265–271. [PMC free article] [PubMed] [Google Scholar]
- 18. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grunig E, Howard L, Humbert M, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;1700578. [DOI] [PubMed] [Google Scholar]
- 19. Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc. 2016;276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldman T, Komtebedde J, Burkhoff D, Massaro J, Maurer MS, Leon MB, Kaye D, Silvestry FE, Cleland JG, Kitzman D, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP‐HF I). Circ Heart Fail. 2016;e003025 DOI: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 21. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1–S2


