Abstract
Background
There is increased risk of hypertension, early cardiovascular disease, and premature mortality in women who have had preeclampsia. This study was undertaken to determine the upper limit of normal blood pressure (BP) 6 months postpartum and the frequency of women with prior preeclampsia who had BP above these limits, as part of the P4 (Post‐Partum Physiology, Psychology and Pediatric) follow‐up study.
Methods and Results
BP was measured by sphygmomanometer, 24‐hour ambulatory BP monitoring, and non‐invasive central BP at 6 months postpartum in 302 women who had normotensive pregnancy and 90 who had preeclampsia. The upper limit of normal BP (mean+2 SD) for women with normotensive pregnancy was 122/79 mm Hg for routine BP, 115/81 mm Hg for central BP, and 121/78 mm Hg for 24‐hour ambulatory BP monitoring. Traditional normal values detected only 3% of women who had preeclampsia as having high BP 6 months postpartum whereas these new values detected between 13% and 19%. Women with preeclampsia had greater body mass index (27.8 versus 25.0, P<0.001) and left ventricular wall thickness but similar augmentation index. They also had lower high‐density lipoprotein (59±15 versus 65±16 mg/dL, P=0.002), higher triglycerides (77±51 versus 61±35 mg/dL, P=0.005), and higher homeostatic model assessment score (2.1±1.8 versus 1.3±1.9, P<0.001).
Conclusions
Clinicians wishing to detect high BP in these women should be aware of the lower than usual upper limit of normal for this young cohort and where possible should use 24‐hour ambulatory BP monitoring to detect these changes. This may define a subgroup of women who had preeclampsia for whom targeted BP lowering therapy would be successful.
Registration
URL: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365295&isReview=true; Unique identifier: ACTRN12613001260718.
Keywords: blood pressure monitoring, cardiovascular risk, high blood pressure, preeclampsia, pregnancy hypertension
Subject Categories: High Blood Pressure, Preeclampsia, Hypertension
Nonstandard Abbreviations and Acronyms
- ABPM
ambulatory blood pressure monitoring
- EVA
early vascular aging
- HOMA
homeostatic model assessment
Clinical Perspective
What Is New?
The upper limit of normal blood pressure (BP) 6 months postpartum for women with normotensive pregnancy was lower than values traditionally considered upper normal.
Using traditional upper limits of normal BP detected high BP in only 3% of women who had preeclampsia 6 months postpartum, whereas using these new values detected between 13% and 19%.
Our data suggest that women who have had preeclampsia have a high frequency of features consistent with early vascular aging.
What Are the Clinical Implications?
All these findings have direct relevance to the postpartum investigation and management of women who have had preeclampsia and should help shape efforts to prevent their longer‐term cardiovascular risk.
Applying new upper normal limits of BP for young women may define a subgroup of women who have had preeclampsia for whom targeted BP lowering therapy would be appropriate.
It is well recognized that women who have had preeclampsia have increased risk for cardiovascular disease, diabetes mellitus, renal disease, and premature death compared with women who have had normotensive pregnancies. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Although this association has been recognized for many years, only recently has preeclampsia become listed as an independent risk factor for cardiac disease. 12 The rate at which women are hospitalized with myocardial infarction is rising, not easily predicted by traditional cardiovascular risk factors, 13 and preeclampsia is probably a contributor to this risk. Indeed, women with preexisting essential hypertension, diabetes mellitus, or obesity are recognized as being at increased risk both for preeclampsia 14 and later life cardiovascular disease.
Women are at least as likely as men to develop cardiovascular disease but healthcare providers are not always aware of preeclampsia being a significant risk factor. 15 Even when women are assessed for their cardiovascular risk after a preeclamptic pregnancy clinicians (understandably) use traditional cut‐off values for risk factors such as blood pressure (BP) when assessing these women. Problems arise firstly in that learned groups differ in their criteria (either 130/80 or 140/90 mm Hg) for defining hypertension, 16 , 17 and secondly that these normal values, and the benefits of anti‐hypertensive treatment, have not been derived from cohorts of young parous women, but more typically from older cohorts, and predominantly men. 18
We therefore undertook this prospective study 6 months after pregnancy to (1) define what constitutes “normal” for BP in young women, and (2) determine the proportion of women who had preeclampsia with BP and other cardiovascular risk factors that lay outside both traditional and these new normal ranges, so as to heighten clinicians' attention to potential risk for such women.
Methods
Study Design, Population, and Recruitment
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed study methodology of the P4 (Postpartum Physiology, Psychology, and Pediatric) follow‐up study, has been published previously. 19 Women who had delivered following a normotensive pregnancy and women who had a preeclamptic pregnancy were invited to participate. Preeclampsia was diagnosed according to the criteria of the International Society for the Study of Hypertension in Pregnancy. 20
Women attended ≈6 months post‐partum to have their BP assessed peripherally with a liquid crystal sphygmomanometer 21 and 24‐hour ambulatory blood pressure monitoring (ABPM), and centrally using non‐invasive applanation tonometry at the radial artery. ABPM was chosen over home BP monitoring as it is considered the best method of assessing BP. 22 Additional testing included echocardiography (with blinded reporting by a cardiologist); liver and renal function; lipids, renin, and aldosterone and urinary albumin; fasting glucose and insulin (with derivation of the homeostatic model assessment (HOMA) score to assess insulin resistance 23 ); and urinalysis. Body mass index (BMI), BP, HOMA score, triglycerides, and high‐density lipoprotein cholesterol were used to assess metabolic syndrome and central BP, and HOMA scores were used as markers of early vascular aging (EVA).
Statistical Analysis
Data were analyzed using SPSS version 25. Comparisons between groups were tested using Student independent t‐test for normally distributed continuous variables and Chi‐square testing for categorical variables. The primary outcome was the prevalence of women who formerly had preeclampsia with mean 24‐hour systolic or diastolic BP >2 SD greater than the mean BP derived from women who had normotensive pregnancies. The study was powered on the proportion of 24‐hour mean diastolic BP readings ≥2 SD above the mean for women who were normotensive in pregnancy, with preliminary data that suggested 56 women after preeclampsia and normotensive pregnancy would be required to assess this with 85% power (α=0.05). For appropriate power to construct a normal range (95% reference range) for BP 6 months postpartum, 292 women post‐normotensive pregnancy were required 19 and to accommodate potential dropouts we recruited 90 women with preeclampsia. Because of multiple comparisons made between groups, apart from the primary outcomes (considered significant at P=0.05), after using a Bonferroni correction only a P≤0.005 was considered statistically significant.
The study was approved by the South Eastern Sydney Local Health District Ethics committee. All women gave written informed consent.
Results
We studied 302 women after normotensive pregnancy, who represented 11% of those invited (302 of 2754), and 90 women who had preeclampsia, 62% of those invited (90 of 145). Studies were undertaken at an average of 27 (SD, 1.3) weeks postpartum.
Baseline Characteristics of Participants and Non‐Participants
Women with normotensive pregnancies who consented to participate in this study (n=302) were slightly older (average, 33 versus 31 years; P<0.001), had slightly higher BP (average, 108/67 versus 101/62 mm Hg; P<0.001) in their first trimester, and were more likely to have had a normal vaginal birth than women who did not consent (n=2452) (P<0.001), but did not differ in gestation at birth (39 weeks). Women with preeclampsia who consented to the study (n=90) were similar in age, BMI, first trimester BP, and gestation at birth as those who did not participate (n=55) but were more likely to have received intravenous magnesium sulfate (24% versus 4%, P=0.001) (Table S1).
Baseline Characteristics of Women With Normotensive and Preeclamptic Pregnancies
Women with preeclampsia were more likely primiparous, had greater BMI, and had slightly higher BP at the start of their pregnancy (10 weeks of gestation) upon referral from their primary care physician (P=0.002) than women who had a normotensive pregnancy (Table 1). This BP difference was also present at their first visit to the hospital, on average at 16 weeks of gestation (P<0.001). There was no difference in frequency of smokers between groups. Women withpreeclampsia gave birth earlier (37 versus 39 weeks, P<0.001) with more babies born preterm (33% versus 6%), and more babies born small for gestational age (24% versus 7%, P<0.001).
Table 1.
Baseline Characteristics of Women Who Had Normotensive Pregnancy and Those Who Had Preeclampsia
| Normotensive | Preeclampsia | P Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| BMI at booking | 302 | 24 | 5 | 90 | 26 | 5 | 0.003 |
| Gestation at booking BP (wk) with GP | 281 | 10 | 5 | 73 | 10 | 5 | 0.237 |
| Booking GP SBP, mm Hg | 281 | 108 | 11 | 74 | 113 | 12 | 0.002 |
| Booking GP DBP, mm Hg | 281 | 67 | 8 | 74 | 71 | 8 | <0.001 |
| Gestation at hospital booking BP, wk | 276 | 15 | 4 | 81 | 16 | 4 | 0.243 |
| Hospital booking SBP, mm Hg | 276 | 103 | 10 | 82 | 109 | 10 | <0.001 |
| Hospital booking DBP, mm Hg | 276 | 63 | 7 | 82 | 67 | 9 | <0.001 |
| Gestation at birth, wk | 302 | 39 | 2 | 90 | 37 | 3 | <0.001 |
| Birth weight (baby), g | 302 | 3367 | 511 | 90 | 2753 | 733 | <0.001 |
| Maternal age, y | 302 | 33 | 5 | 90 | 32 | 5 | 0.016 |
| Apgar, 1 min | 300 | 9 | 1 | 90 | 8 | 2 | <0.001 |
| Apgar, 5 min | 300 | 9 | 1 | 90 | 9 | 1 | 0.003 |
|
Normotensive (%) n=302 |
Preeclampsia (%) n=90 |
P Value | |
|---|---|---|---|
| Primigravidas | 50 | 73 | <0.001 |
| Episodes of severe hypertension (≥160/110) | 0 | 38 | <0.001 |
| Preterm birth (<37 wk) | 6 | 33 | <0.001 |
| SGA (<10th percentile) | 7 | 24 | <0.001 |
| Vaginal birth | 81 | 51 | <0.001 |
| Magnesium Sulfate given | 0 | 24 | <0.001 |
| Education | |||
| Secondary | 9 | 4 | 0.044 |
| Diploma | 23 | 36 | |
| University degree | 68 | 60 | |
| Smoker | |||
| Never | 67 | 61 | 0.193 |
| Former | 28 | 29 | |
| Current | 5 | 10 | |
| Labor onset | |||
| Spontaneous | 58 | 9 | <0.001 |
| Induced | 32 | 66 | |
| No labor | 10 | 26 | |
| Background/Ethnicity/Race | |||
| White | 54 | 52 | 0.152 |
| Asian | 22 | 20 | |
| ATSI | 0.7 | 2 | |
| Polynesian | 0.3 | 3 | |
| European | 14 | 12 | |
| Other | 9 | 10 | |
| Breastfeeding on discharge | |||
| Yes | 94 | 86 | 0.012 |
| No | 3 | 3 | |
| Both breast and bottle | 3 | 11 | |
“Booking” is the gestation at first presentation to the primary care physician or hospital clinic. ATSI indicates Aboriginal and Torres Strait Islander; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; GP, general practitioner/primary care physician; SBP, systolic blood pressure; and SGA, small for gestational age.
BP at 6 Months
The average routine sphygmomanometry BP 6 months postpartum for women who had a normotensive pregnancy was 104/66 mm Hg with an upper limit of normal 122/79 mm Hg (Figure 1). Women who had preeclampsia had average BP significantly higher than that of women who had normotensive pregnancies whether measured routinely (113/72 mm Hg) or as central BP, awake, sleep or 24‐hour mean BP (all P<0.001).
Figure 1. Routine sphygmomanometry and ambulatory awake, sleep, and central systolic and diastolic blood pressures and pulse pressures 6 months postpartum.
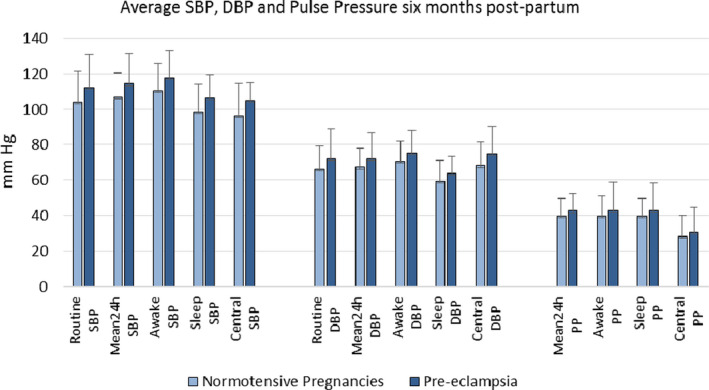
All measurements were significantly higher in women who had preeclampsia, P<0.001. DBP indicates diastolic blood pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Awake, sleep, and 24‐hour pulse pressures (all P<0.001) and central pulse pressure (P=0.021) were significantly higher 6 months postpartum in women who had preeclampsia. Twenty‐four‐hour (73 versus 76 bpm, P=0.002), awake (77 versus 80 bpm, P=0.002), and sleep average heart rates (63 versus 67 bpm, P<0.001) were also significantly higher 6 months postpartum in women who had preeclampsia.
Routine systolic BP fell by 4 mm Hg between 10 weeks of gestation and 6 months postpartum in women who had normotensive pregnancies (P<0.001) but diastolic BP was unchanged. Women who had preeclampsia had no change in routine BP between 10 weeks of gestation and 6 months postpartum (Table 2).
Table 2.
Renal and Liver Function, Blood Count, Lipids, Insulin Resistance, Vitamin D, and Change in Systolic and Diastolic Blood Pressure From the First Trimester to 6 Months Postpartum
| Normotensive | Preeclampsia | P Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| BMI | 302 | 25.0 | 5.1 | 90 | 27.8 | 5.9 | <0.001 |
| Creatinine, mg/dL | 299 | 0.7 | 0.1 | 89 | 0.7 | 0.1 | 0.225 |
| eGFR, mL/min | 299 | 95.0 | 8.9 | 89 | 96.7 | 7.7 | 0.075 |
| ALP, IU | 299 | 77.5 | 21.3 | 87 | 83.3 | 20.6 | 0.024 |
| GGT, IU | 299 | 14.5 | 14.5 | 87 | 20.0 | 24.4 | 0.044 |
| ALT, U/L | 299 | 19.7 | 9.2 | 87 | 21.1 | 11.4 | 0.314 |
| AST, U/L | 298 | 19.8 | 5.2 | 85 | 20.9 | 5.7 | 0.118 |
| Glucose, mg/dL | 299 | 83.7 | 7.6 | 89 | 85.8 | 7.0 | 0.014 |
| Urate, mg/dL | 299 | 4.6 | 1.0 | 88 | 4.9 | 1.1 | 0.020 |
| White cell count, 109/L | 297 | 5.3 | 1.2 | 89 | 5.8 | 1.8 | 0.022 |
| Hemoglobin, g/dL | 297 | 13.2 | 0.8 | 89 | 13.0 | 1.0 | 0.042 |
| Platelets, 109/L | 296 | 247.3 | 53.3 | 88 | 260.0 | 59.1 | 0.073 |
| Cholesterol, mg/dL | 299 | 177.6 | 27.6 | 88 | 180.5 | 34.1 | 0.469 |
| LDL, mg/dL | 299 | 100.4 | 26.3 | 88 | 105.6 | 30.6 | 0.149 |
| HDL, mg/dL | 299 | 65.2 | 15.7 | 89 | 59.4 | 14.7 | 0.002 |
| Triglyceride, mg/dL | 299 | 60.8 | 34.6 | 88 | 77.4 | 50.9 | 0.005 |
| Aldosterone, ng/dL | 298 | 9.0 | 6.4 | 87 | 10.8 | 9.4 | 0.093 |
| Renin, mU/L | 296 | 17.0 | 13.3 | 84 | 16.2 | 11.0 | 0.553 |
| Aldosterone:renin ratio | 296 | 21.6 | 25.6 | 84 | 33.2 | 75.6 | 0.173 |
| Insulin, mU/L | 298 | 6.1 | 7.7 | 88 | 9.6 | 8.2 | 0.001 |
| HbA1c, % | 298 | 5.2 | 0.3 | 89 | 5.2 | 0.3 | 0.946 |
| Vitamin D, ng/mL | 299 | 27.8 | 8.5 | 88 | 27.3 | 7.4 | 0.561 |
| HOMA score | 298 | 1.3 | 1.9 | 88 | 2.1 | 1.8 | 0.001 |
| Urine albumin/creatinine, mg/g | 297 | 10.5 | 31.4 | 88 | 19.6 | 27.9 | 0.010 |
| Difference between 6‐m routine SBP and first trimester SBP | 279 | −3.9 | 11.8 | 73 | −0.9 | 10.1 | 0.033 |
| Difference between 6‐m routine DBP and first trimester DBP | 279 | −0.3 | 8.7 | 73 | 0.8 | 7.4 | 0.291 |
BMI indicates body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; eGFR indicates estimated glomerular filtration rate; HOMA, homeostatic model assessment; LDL, low‐density lipoprotein; SBP, systolic blood pressure. ALP, Alkaline Phosphatase; GGT, Gamma glutamyl transpeptidase; ALT, Alanine transaminase; and AST, aspartate aminotransferase.
Cardiovascular Risk Factors 6 Months Postpartum
Women with preeclampsia still had slightly greater BMI than those who had normotensive pregnancy (BMI, 27.8 versus 25.0, P<0.001), having begun their pregnancies that way (Table 2). High‐density lipoprotein was slightly lower and triglycerides higher along with higher insulin and HOMA score (P<0.001) but hemoglobin A1c was similar. Plasma renin, aldosterone, and aldosterone/renin ratio were similar between groups as was serum creatinine and estimated glomerular filtration rate. Although serum uric acid and urinary albumin/creatinine were slightly higher in those with preeclampsia this was not statistically significant when accounting for multiple comparisons. Augmentation index was similar between groups (mean 23% in both groups).
Echocardiographic Findings at 6 Months
Echocardiography was obtained in 74 women who had normotensive pregnancies and 44 who had preeclampsia (Table 3). Women with previous preeclampsia had significantly greater septal and posterior wall thickness and left ventricular mass as well as lower mitral E/A ratio and higher E/E′ ratios, consistent with more diastolic dysfunction 6 months postpartum, even though these measurements lie within traditionally “normal” ranges.
Table 3.
Echocardiographic Findings 6 Months Postpartum in Women Who Had Preeclampsia or Normotensive Pregnancies
| Normotensive | Preeclampsia | P Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Age, y | 74 | 33 | 4.0 | 44 | 31 | 4.7 | 0.010 |
| Height, cm | 74 | 166 | 7.2 | 44 | 164 | 6.2 | 0.204 |
| Weight, kg | 74 | 69 | 14.2 | 44 | 79 | 18.8 | 0.003 |
| BSA, m2 | 74 | 1.7 | 0.2 | 44 | 1.9 | 0.2 | 0.004 |
| LVIDD, mm | 74 | 46.0 | 3.1 | 44 | 46.3 | 4.1 | 0.693 |
| IVS, mm | 74 | 7.9 | 1.0 | 44 | 8.7 | 1.2 | <0.001 |
| PW, mm | 74 | 7.5 | 1.0 | 44 | 8.4 | 1.1 | <0.001 |
| Relative wall thickness | 74 | 0.3 | 0.0 | 44 | 0.4 | 0.1 | 0.001 |
| LV mass, g | 74 | 88.4 | 24.6 | 44 | 109.0 | 29.0 | <0.001 |
| LV mass indexed, g/m2 | 74 | 50.6 | 12.3 | 44 | 58.8 | 12.6 | 0.001 |
| Mitral E/A ratio | 74 | 1.6 | 0.4 | 44 | 1.4 | 0.3 | 0.005 |
| E/E′ ratio septal | 74 | 7.51 | 1.4 | 44 | 8.94 | 2.3 | <0.001 |
| E/E′ ratio lateral | 74 | 5.44 | 1.0 | 44 | 6.33 | 1.6 | 0.001 |
| RV free‐wall annulus, S'm/s | 74 | 0.12 | 0.0 | 44 | 0.13 | 0.0 | 0.028 |
| TAPSE | 74 | 21.6 | 2.3 | 44 | 22.2 | 3.0 | 0.235 |
| EF, % | 74 | 64.6 | 4.0 | 44 | 63.7 | 3.9 | 0.244 |
| GLS | 74 | −21.6 | 2.0 | 44 | −20.7 | 1.9 | 0.022 |
BSA indicates body surface area; EF, ejection fraction; GLS, global longitudinal strain; IVS, interventricular septum; LA, left atrial; LV, left ventricular; LVIDD, left ventricular internal dimension diastole; PW, posterior wall; RV, right ventricular; TAPSE, tricuspid annular planar systolic excursion; and TDI, tissue Doppler imaging.
Normal BP 6 Months Postpartum
The upper limits of normal for young parous women 6 months postpartum were 122/79 mm Hg for routine BP, 115/81 mm Hg for central BP, 121/78 mm Hg for 24‐hour ambulatory BP, 126/82 mm Hg for awake ABPM and 114/71 mm Hg for sleep BP.
Women Who Had Preeclampsia With High BP at 6 Months
BP in women who had preeclampsia was compared against both a “Traditional” upper normal threshold value for routine BP of 139 mm Hg systolic and 89 mm Hg diastolic 16 and also against upper threshold values of 129 mm Hg systolic and 79 mm Hg diastolic to account for more recent recommendations of the American College of Cardiology/American Heart Association 17 (Figure 2). Central systolic BP of 119 mm Hg was considered the upper normal and mean 24‐hour ABPM blood pressure <130/80 mm Hg was considered traditionally normal. 24
Figure 2. Percentage of women who have had preeclampsia with blood pressures above the normal ranges according to our new data (blue), traditional cut‐offs (red), and American College of Cardiology/American Heart Association definitions (green).
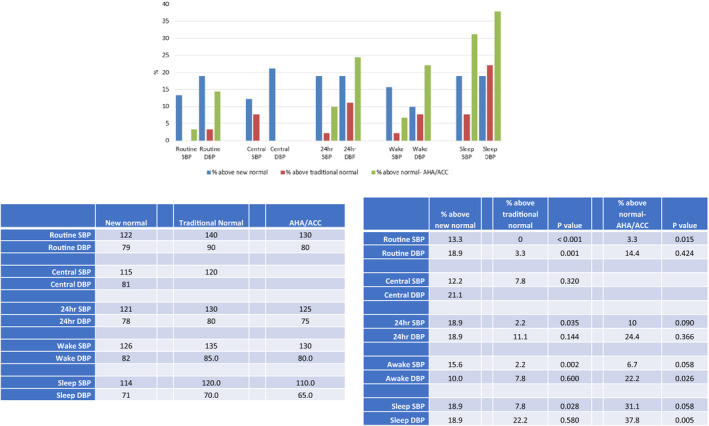
Significant difference in frequencies are indicated by P values of comparisons with percentage above new normal. All blood pressures are expressed as mm Hg. ACC/AHA indicates American College of Cardiology/American Heart Association; DBP, diastolic blood pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Using a traditional cut‐off defining hypertension by routine sphygmomanometry BP as ≥140/90 mm Hg detected only 3% of women with preeclampsia as having high BP whereas 13% to 19% were considered abnormal using the new normal range defined within this study for young women 6‐months postpartum.
Central BP measurement offered only a small change in frequency of detection, detecting 8% with traditional levels and 12% using the new cut‐off level. Twenty‐four‐‐hour ABPM increased detection of high BP from 2% to 19% for systolic BP and 11% to 19% for diastolic BP using the newly defined range for normal women in this study rather than traditional ranges and ABPM diagnosed high BP more often than routine sphygmomanometry measurement. Seventeen of the 90 women who had preeclampsia had 24‐hour ABPM systolic BP above the new normal range determined in this study but only 6 of these women had routine sphygmomanometer systolic BP above the new normal range. Only 2 women who had preeclampsia had 24‐hour ABPM systolic BP above traditionally normal ABPM values and neither of these had routine systolic BP above traditional normal values. Nine of the 90 women with preeclampsia had 24‐hour ABPM systolic BP above normal values as more recently defined by American College of Cardiology/American Heart Association but none of these women had routine systolic BP above normal as defined by American College of Cardiology/American Heart Association. Similar results were found when hypertension was defined solely by awake ABPM rather than by 24‐hour ABPM (Table S2).
Women who had preeclampsia with elevated sphygmomanometry BP at 6 months had similar values for all other cardiovascular risk factors as women who had preeclampsia with ongoing normal BP at 6 months (Table S3).
Discussion
This study highlights 4 key findings: (1) young women who have had a normotensive pregnancy 6 months ago have upper limits of BP that are lower than that usually recommended to define hypertension. Accordingly, high BP is found more often postpartum in women who have had preeclampsia if it is defined by these new limits rather than those traditionally recommended; (2) Use of 24‐hour ABPM, the gold standard for diagnosing hypertension, detects hypertension more often in women who have had preeclampsia than accurate sphygmomanometric BP measurement; (3) Six months after pregnancy women who have had preeclampsia have more insulin resistance, higher BP, and more features of metabolic syndrome than women who had normotensive pregnancies; as they had greater BMI and higher BP at the start of their pregnancy it is likely that these observations 6 months postpartum reflect abnormalities brought into their pregnancy rather than having developed as a consequence of their pregnancy; (4) Our data suggest that women who have had preeclampsia have a high frequency of features consistent with EVA. All these findings have direct relevance to the postpartum investigation and management of women who have had preeclampsia and should help shape efforts to prevent long‐term cardiovascular risk.
The heightened long‐term cardiovascular and mortality risk of women with preeclampsia has been recognized for some time now 25 and appears to be a universal phenomenon 26 that has been confirmed in more recent studies. 1 , 8 , 9 It is known that the highest cause of death in women is cardiovascular disease and that at all age groups their frequency of hypertension is close to or even greater than that of men. 27 Although women overall are more aware of a diagnosis of hypertension, this appears to often go undetected in postpartum follow‐up. 28 A compounding factor is knowing what upper level of BP a caregiver should ascribe to young women after pregnancy, as this issue has not been studied specifically to date.
In 300 women who had normotensive pregnancies we found an upper limit of normal BP at 122/79 mm Hg for routine BP, similar to that defined by 24‐hour ABPM in this cohort, and closer to recommended levels for defining hypertension by the American College of Cardiology/American Heart Association 17 than by the European Society of Cardiology/European Society of Hypertension committee. 16 When using these values as cut‐off for defining high BP rather than general population norms we detected 13% to 19% of women who previously had preeclampsia as having high BP. The clinical implication is that to detect high BP 6 months after pregnancy this new threshold level of BP should be used. It is unknown whether this subgroup of women who had preeclampsia with postpartum elevated BP are those women who will develop a cardiovascular event but it seems logical that this subgroup carries increased vascular risk and might respond better to encouragement of the need for non‐pharmacological therapies than women who had preeclampsia who have normal BP postpartum.
In addition to BP, BMI, insulin, HOMA score, triglycerides, and high‐density lipoprotein cholesterol all differed significantly between women with normotensive pregnancy and women with preeclampsia at 6 months postpartum, similar to what we had observed previously in a smaller study at variable and later stages postpartum. 29 Controversy continues as to whether the cardiovascular risk of preeclampsia is independent of standard vascular risk factors or preexisting diabetes mellitus 30 or is affected by ongoing hyperlipidemia, chronic hypertension, and type 2 diabetes mellitus. 4 Unfortunately, adding a history of preeclampsia to standard risk assessment tools does not appear to improve sensitivity for cardiovascular risk detection. 31 However, our data confirm that women who have had preeclampsia have a greater propensity for metabolic syndrome 6 months postpartum than women who had a normotensive pregnancy.
It is likely that weight reduction, healthy diet, and exercise will improve long‐term outcomes 32 , 33 , 34 , 35 but anecdotally it can be difficult to engage women in non‐drug therapies postpartum. Indeed, in this study the subgroup with ongoing elevated BP postpartum did not have a higher frequency of other vascular risk factors than those who have had preeclampsia with normal BP 6 months postpartum, making it hard to emphasize the cardiovascular risk. Perhaps by defining a subgroup of women with preeclampsia with demonstrably high BP postpartum we can help at least this subgroup engage more readily in lifestyle changes.
The BP at 10 weeks of gestation is a surrogate marker for pre‐pregnancy BP, which was not available in this study. A new observation in this study was that BP fell slightly by 6 months postpartum from that measured by the primary care physician in early pregnancy within women with normotensive pregnancy (−4/0 mm Hg) but not in women with preeclampsia (−1/1 mm Hg; Table 2). This implies that the higher BP observed postpartum in women with preeclampsia was because of the elevated BP they brought with them into the pregnancy, rather than a specific consequence of the pregnancy.
It is generally agreed that 24‐hour ABPM provides the best way of detecting true hypertension that portends the greatest cardiovascular risk. 36 , 37 We found that high BP was more likely to be detected using ABPM than routine sphygmomanometry (Figure 2) and would therefore recommend that this be incorporated in the assessment of women with preeclampsia 6 months postpartum wherever possible.
Our study confirms the findings of others 38 that women who had preeclampsia have greater left ventricular mass and indices suggestive of mild diastolic dysfunction postpartum but we do not have pre‐pregnancy data to know whether these women entered their pregnancy with these abnormalities, a possibility given that women with preeclampsia women had elevated BP at the start of pregnancy compared with women with normotensive pregnancy.
It is known that cardiovascular disease occurs fairly soon after preeclamptic pregnancies, more commonly in the first decade or so than later, 1 , 30 which may represent EVA in these women, but this has been little studied to date. A key feature of EVA is increased arterial stiffness due predominantly to changes in medial wall function and structure, identified amongst other factors by elevated pulse pressure and increased central systolic and pulse pressure 39 and commonly associated with elevated HOMA score, triglycerides, and lower high‐density lipoprotein cholesterol. 40 We observed all of these features in our cohort of women with preeclampsia 6 months postpartum, supporting the notion that this group represents a cohort with EVA, either genetically or environmentally determined.
Strengths and Limitations
Strengths include the concise and consistent criteria for diagnosing preeclampsia, the prospective nature of the study with detailed analyses of BP by several methods including the gold standard of ABPM, the development of new limits of normal for these parameters based upon an appropriately aged and parous cohort, and the capacity to analyze individual changes in BP from 10 weeks of gestation to 6 months postpartum. Weaknesses include the likelihood that “sleep” BPs were overestimated as, although we recorded sleep BP from a patient diary, it is still likely that sleep was disturbed often in a cohort of women with young babies. Secondly, we have defined the upper limit of normal BPs according to the 95% confidence limits; ideally a discriminant level would be that which defined a higher versus lower risk of actual clinical cardiovascular outcomes but this will not be known in this group for many years. Thirdly, we do not yet know whether these findings will be persistent later after pregnancy, though we are studying this. 19 Finally, although we are following up with these women 19 we do not know whether the subgroup of women with preeclampsia we have identified as having elevated BP using newly defined cut‐off levels or those exhibiting features of EVA at 6 months postpartum will be those women who develop the cardiovascular events, as this too will require at least a 10‐year follow‐up.
Conclusions
The main new clinical finding in this prospective study is the definition of new upper levels of sphygmomanometry, ambulatory, and central BPs in young women who have had a pregnancy 6 months ago. Using these levels diagnoses up to 1 in 5 women who have had preeclampsia as having ongoing elevated BP, a much greater frequency than if using traditional values to define hypertension. Only time will tell whether this subgroup are those who develop a vascular event and this will require ongoing research. In the meantime it seems prudent to advise these women in particular that they may be at higher cardiovascular risk than other women with preeclampsia and should focus even more diligently upon modifiable vascular risk factors. 34 At a minimum this might help engage such women in cardiovascular disease prevention.
Perspectives
Women with preeclampsia exhibit features of metabolic syndrome and possibly EVA 6 months postpartum. Clinicians wishing to detect high BP in these women should be aware of the lower than usual upper limit of normal for this young cohort and where possible should use 24‐hour ABPM to detect these changes. Although we did not incorporate home BP in this study, it is likely that this would be a useful alternative when ABPM is not available. Future research will elucidate whether targeted BP lowering therapy is successful in preventing cardiovascular disease in this subgroup of women who have had preeclampsia.
Sources of Funding
This study was supported by The St George and Sutherland Medical Research Foundation and by a philanthropic donation from Emeritus Professor Richard Henry.
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The authors wish to thank Prof Caroline Homer and the following students for their assistance with this study: Michelle Bai, Gemma Bylos, Rose Kennedy, Annie Lu, Sarah McLennan, Melissa Ojurovic, Sai Sankare Siritharan, Sathia Sushil, Jaime Worboys, and Amanda Yao.
(J Am Heart Assoc. 2020;9:e018604 DOI: 10.1161/JAHA.120.018604.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018604
For Sources of Funding and Disclosures, see page 9.
References
- 1. Leon LJ, McCarthy FP, Direk K, Gonzalez‐Izquierdo A, Prieto‐Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records. Circulation. 2019;140:1050–1060. [DOI] [PubMed] [Google Scholar]
- 2. Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre‐eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khashan AS, Evans M, Kublickas M, McCarthy FP, Kenny LC, Stenvinkel P, Fitzgerald T, Kublickiene K. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PloS Med. 2019;16:e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James‐Todd TM, Rich‐Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergen NE, Schalekamp‐Timmermans S, Roos‐Hesselink J, Roeters van Lennep JE, Jaddoe VVW, Steegers EAP. Hypertensive disorders of pregnancy and subsequent maternal cardiovascular health. Eur J Epidemiol. 2018;33:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siepmann T, Boardman H, Bilderbeck A, Griffanti L, Kenworthy Y, Zwager C, McKean D, Francis J, Neubauer S, Yu GZ, et al. Long‐term cerebral white and gray matter changes after preeclampsia. Neurology. 2017;88:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd H. Risk of post‐pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Horvath J, Hennessy A. Hypertension in pregnancy and long‐term cardiovascular mortality: a retrospective cohort study. Am J Obstet Gynecol. 2016;214:722.e1–722.e6. [DOI] [PubMed] [Google Scholar]
- 9. Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, Esplin M. All‐cause and cause‐specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postma IR, Groen H, Easterling TR, Tsigas EZ, Wilson ML, Porcel J, Zeeman GG. The brain study: cognition, quality of life and social functioning following preeclampsia; an observational study. Pregnancy Hypertens. 2013;3:227–234. [DOI] [PubMed] [Google Scholar]
- 11. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]
- 12. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Himmelfarb CD, Goldberger ZD, Hahn EJ, Khera A, Lloyd‐Jones D, McEvoy JW, et al.2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. American College of Cardiology; 2019. Available at: https://www.onlinejacc.org/sites/default/files/additional_assets/guidelines/Prevention‐Guidelines‐Made‐Simple.pdf. Accessed September 2, 2020. [Google Scholar]
- 13. Vaccarino V. Myocardial infarction in young women. Circulation. 2019;139:1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Block‐Abraham DM, Turan OM, Doyle LE, Kopelman JN, Atlas RO, Jenkins CB, Blitzer MG, Baschat AA. First‐trimester risk factors for preeclampsia development in women initiating aspirin by 16 weeks of gestation. Obstet Gynecol. 2014;123:611–617. [DOI] [PubMed] [Google Scholar]
- 15. Wilkins‐Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by women’s health care providers of long‐term cardiovascular disease risk after preeclampsia. Obstet Gynecol. 2015;125:1287–1292. [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 17. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 18. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 19. Davis GK, Roberts L, Mangos G, Henry A, Pettit F, O’Sullivan A, Homer CSE, Craig M, Harvey SB, Brown MA. Postpartum physiology, psychology and paediatric follow up study (P4 Study)—study protocol. Pregnancy Hypertens. 2016;6:374–379. [DOI] [PubMed] [Google Scholar]
- 20. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall D, Warren C, Adoyi G, Ishaku S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- 21. Davis GK, Roberts LM, Mangos GJ, Brown MA. Comparisons of auscultatory hybrid and automated sphygmomanometers with mercury sphygmomanometry in hypertensive and normotensive pregnant women: parallel validation studies. J Hypertens. 2015;33:499–506. [DOI] [PubMed] [Google Scholar]
- 22. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mossmann M, Wainstein MV, Gonçalves SC, Wainstein RV, Gravina GL, Sangalli M, Veadrigo F, Matte R, Reich J, Costa FG, et al. HOMA‐IR is associated with significant angiographic coronary artery disease in non‐diabetic, non‐obese individuals: a cross‐sectional study. Diabetol Metab Syndr. 2015;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Head GA, McGrath BP, Mihailidou AS, Nelson MR, Schlaich MP, Stowasser M, Mangone AA, Cowley D, Brown MA, Ruta L‐A, et al. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J Hypertens. 2012;30:253–266. [DOI] [PubMed] [Google Scholar]
- 25. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lisowska M, Pietrucha T, Sakowicz A. Preeclampsia and related cardiovascular risk: common genetic background. Curr Hypertens Rep. 2018;20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmad A, Oparil S. Hypertension in women. Hypertension. 2017;70:19–26. [DOI] [PubMed] [Google Scholar]
- 28. Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, Margolis KL, O’Connor P, Magid DJ, Bibbins‐Domingo K. Missed opportunities in cardiovascular disease prevention? Low rates of hypertension recognition for women at medicine and obstetrics‐gynecology clinics. Hypertension. 2011;57:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012;30:351–358. [DOI] [PubMed] [Google Scholar]
- 30. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 31. Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, Rexrode KM, Mukamal KJ, Rich‐Edwards JW. Hypertensive disorders of pregnancy and 10‐year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich‐Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ. 2017;358:j3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berks D, Hoedjes M, Raat H, Duvekot JJ, Steegers EAP, Habbema JDF. Risk of cardiovascular disease after pre‐eclampsia and the effect of lifestyle interventions: a literature‐based study. BJOG. 2013;120:924–931. [DOI] [PubMed] [Google Scholar]
- 34. Spaan J, Peeters L, Spaanderman M, Brown M. Cardiovascular risk management after a hypertensive disorder of pregnancy. Hypertension. 2012;60:1368–1373. [DOI] [PubMed] [Google Scholar]
- 35. Scholten RR, Thijssen DJH, Lotgering FK, Hopman MTE, Spaanderman MEA. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol. 2014;211:516.e1–516.e11. [DOI] [PubMed] [Google Scholar]
- 36. O’Brien E, White WB, Parati G, Dolan E. Ambulatory blood pressure monitoring in the 21st century. J Clin Hypertens. 2018;20:1108–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aung K, Htay T. Relationship between outpatient clinic and ambulatory blood pressure measurements and mortality. Curr Cardiol Rep. 2019;21:28. [DOI] [PubMed] [Google Scholar]
- 38. Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–715. [DOI] [PubMed] [Google Scholar]
- 39. Nilsson PM. Hemodynamic aging as the consequence of structural changes associated with early vascular aging (EVA). Aging Dis. 2014;5:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gottsäter M, Östling G, Persson M, Engström G, Melander O, Nilsson PM. Non‐hemodynamic predictors of arterial stiffness after 17 years of follow‐up: the Malmö Diet and Cancer study. J Hypertens. 2015;33:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


