Abstract
Background
Bleeding is frequent in patients with atrial fibrillation (AF) treated with oral anticoagulant therapy, and may be the first manifestation of underlying cancer. We sought to investigate to what extent bleeding represents the unmasking of an occult cancer in patients with AF treated with oral anticoagulants.
Methods and Results
Using data from CardioCHUVI‐AF (Retrospective Observational Registry of Patients With Atrial Fibrillation From Vigo's Health Area), 8753 patients with AF aged ≥75 years with a diagnosis of AF between 2014 and 2017 were analyzed. Of them, 2171 (24.8%) experienced any clinically relevant bleeding, and 479 (5.5%) were diagnosed with cancer during a follow‐up of 3 years. Among 2171 patients who experienced bleeding, 198 (9.1%) were subsequently diagnosed with cancer. Patients with bleeding have a 3‐fold higher hazard of being subsequently diagnosed with new cancer compared with those without bleeding (4.7 versus 1.4 per 100 patient‐years; adjusted hazard ratio [HR], 3.2 [95% CI, 2.6–3.9]). Gastrointestinal bleeding was associated with a 13‐fold higher hazard of new gastrointestinal cancer diagnosis (HR, 13.4; 95% CI, 9.1–19.8); genitourinary bleeding was associated with an 18‐fold higher hazard of new genitourinary cancer diagnosis (HR, 18.1; 95% CI, 12.5–26.2); and bronchopulmonary bleeding was associated with a 15‐fold higher hazard of new bronchopulmonary cancer diagnosis (HR, 15.8; 95% CI, 6.0–41.3). For other bleeding (nongastrointestinal, nongenitourinary, nonbronchopulmonary), the HR for cancer was 2.3 (95% CI, 1.5–3.6).
Conclusions
In patients with AF treated with oral anticoagulant therapy, any gastrointestinal, genitourinary, or bronchopulmonary bleeding was associated with higher rates of new cancer diagnosis. These bleeding events should prompt investigation for cancers at those sites.
Keywords: atrial fibrillation, bleeding, cancer, oral anticoagulation
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- CardioCHUVI‐AF
Retrospective Observational Registry of Patients With Atrial Fibrillation From Vigo’s Health Area
- COMPASS
Cardiovascular Outcomes for People Using Anticoagulation Strategies
- ICPC‐2
International Classification of Primary Care, Second Edition
- ISTH
International Society on Thrombosis and Haemostasis
- OAC
oral anticoagulant
Clinical Perspective
What Is New?
Among patients with atrial fibrillation treated with oral anticoagulation who experienced bleeding in this study, 1 in 11 was subsequently diagnosed with cancer, and 41.3% of all new cancer diagnoses were in patients with prior bleeding.
Sites of bleeding most commonly associated with new cancer diagnosis were gastrointestinal, genitourinary, and bronchopulmonary, associated with a more than 10‐fold higher hazard for subsequent cancer diagnosis in the respective organ systems.
What Are the Clinical Implications?
Gastrointestinal, genitourinary, and bronchopulmonary bleeding in patients with atrial fibrillation treated with oral anticoagulation should prompt careful investigation for possible underlying cancer in the respective organ systems, even if the bleeding is minor.
Atrial fibrillation (AF) is the most common arrhythmia worldwide, with a lifetime risk of 1 in 3 based on recent data. 1 Recent registry data suggest that an AF diagnosis is associated with higher‐than‐expected cancer incidence rates. 2 , 3 An important number of new‐onset cancers in patients with AF are unmasked following a bleeding episode. 4 In fact, bleeding has been considered an alerting sign to reveal preexisting cancers. 5 , 6 Oral anticoagulants (OACs) could be considered as a “bleeding stress test,” which therefore could potentially unveil an occult cancer and improve the chance of early detection. However, bleeding is not an uncommon complication for patients with AF receiving OACs. 7 , 8 In this sense, a provocative question is emerging: should a bleeding event prompt a search for occult cancer in patients with AF treated with OAC therapy?
Currently, systematic screening for cancer in patients taking OACs and having a hemorrhagic episode is not formally conducted. However, the evidence and recommendations regarding the diagnostic workup and management of these bleeding complications remain relatively scarce. 4 , 9 For the diagnosis of hidden cancers after bleeding in patients taking OACs, few data are currently available, most of them focused on gastrointestinal bleeding and gastrointestinal cancers. 4 , 10 Whether bleeding should be considered a sufficient sign to justify an early detection program in anticoagulated patients, similar to the one performed in those without OACs, still remains unclear.
Using data from an unselected real‐word cohort, we sought to estimate the absolute and relative risks of cancer in patients with AF receiving OAC therapy who presented bleeding complications, considering the timing, location, and severity of the hemorrhagic complication.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
A cohort including all patients aged ≥75 years from the health area of Vigo (Galicia, Spain) with a diagnosis of AF between January 1, 2014, and December 31, 2017 (CardioCHUVI‐AF [Retrospective Observational Registry of Patients With Atrial Fibrillation From Vigo's Health Area]), was used for this study. Patients were identified through administrative databases at both the hospital and ambulatory level, using the regional electronic healthcare records system, which also monitored information regarding echocardiographic and laboratory data, use of medication, any hospital or outpatient clinic discharge diagnosis, and all‐cause death. The system has several subcomponents termed Complex Analysis Information Systems, which may be used to retrieve normalized and structured data about primary health care (SIAC‐AP), hospital discharges (SIAC‐HA), pharmacy (SIAC‐PF), and patient characteristics (SIAC‐CID). 11 AF diagnosis was based on codes 427.31 of the International Classification of Diseases, Ninth Revision (ICD‐9), and K78 of the International Classification of Primary Care,Second Edition (ICPC‐2). In all patients, the diagnosis of AF was considered adequate only when an ECG was available to confirm it.
Among a total of 12 083 patients identified, electronic medical records were reviewed, to collect data about baseline clinical variables, therapeutic strategy, and follow‐up events. Patients with preexisting cancer (n=1127), defined as diagnosed before the initiation of OACs, were excluded. We also excluded patients with missing follow‐up data (n=4). Patients were divided into 2 groups: anticoagulated (n=9029) and nonanticoagulated (n=1923). For the purpose of this study, we focused on patients with AF who received OAC therapy (ie, warfarin, phenprocoumon, rivaroxaban, dabigatran, or apixaban); we did not consider patients treated with heparin (n=276). Therefore, the final sample size of our study was composed of 8753 patients with AF treated with OAC therapy and without prior history of cancer (n=8753).
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee (Autonomic Committee of Research Ethics from Galicia, code HAC‐ACO‐2018‐01, registry 2018/258). Informed consent of the patients was waived.
Bleeding Severity and Location
The exposure of interest was bleeding. Medical information was collected for each participant regarding characteristics of bleeding events, location, clinical severity, imaging tests, hemoglobin levels, and blood transfusion.
The severity of bleeding events was classified according to the statement by the International Society on Thrombosis and Haemostasis (ISTH). Major bleeding episodes were defined as fatal bleeding, symptomatic bleeding in a critical area or organ, or bleeding causing a reduced hemoglobin level of ≥2 g/dL, or leading to transfusion of ≥2 units of whole blood or red cells. 12 Clinically relevant nonmajor bleeding was defined as any sign or symptom of hemorrhage that did not fit the criteria for the ISTH definition of major bleeding but that required medical intervention by a healthcare professional, led to hospitalization or increased level of care, or prompted a face‐to‐face evaluation. 13 Given that our aim was to establish the association between bleeding and the subsequent diagnosis of cancer, only bleeding episodes that occurred before—but not after—the clinical diagnosis of cancer were taken into account.
Bleeding outcomes were classified into 4 groups according to their location: gastrointestinal, genitourinary, bronchopulmonary, or bleedings from other sites. We defined gastrointestinal bleeding as hematemesis, melena, or hematochezia. Genitourinary bleeding included hematuria or vaginal bleeding, whereas bronchopulmonary bleeding was defined as any type of hemoptysis.
Outcome
The primary outcome was a new diagnosis of cancer, defined as any solid malignancy other than nonmelanoma skin cancer diagnosed after the initiation of OACs. All cancer diagnoses were assessed and validated by medical record review by a physician panel of 4 doctors. Cases where the initial diagnoses were not unanimous were reexamined together until consensus was reached. The priority to confirm the diagnosis of cancer was a definitive pathologic diagnosis from a pathology or cytology report. If tissue sample was not available, cases of cancer were otherwise confirmed based on strong clinical and radiological or laboratory marker evidence. Cancer diagnosis date was assigned based on the date of pathologic diagnosis, or on the clinical diagnosis date (eg, by date of suggestive imaging testing) when the pathology or cytology report was not available. Cancer types were classified by anatomic and system primary involvement. In this study, we specifically classified cancers into 4 groups: gastrointestinal, genitourinary, bronchopulmonary, and cancer from other sites. We defined gastrointestinal cancer as cancer involving the esophagus, stomach, duodenum, jejunum, ileum, colon, or rectum. For genitourinary cancers, we considered those involving the prostate, spermatic cord, uterus, cervix, vagina, kidney, ureter, bladder, or urethra. Bronchopulmonary cancers included those involving the lung and bronchus. All cancers that did not meet these definitions were defined as cancer from other sites.
Statistical Analysis
Baseline characteristics in the overall study population, and by cancer status during follow‐up, were described using frequencies and percentages for categorical data, and mean±SD for continuous data, respectively. Differences in characteristics were assessed using chi‐square tests and unpaired t tests, respectively.
We examined the absolute number and proportion of new cancers diagnosed with and without prior bleeding. We evaluated the association between bleeding and new cancer diagnosis using unadjusted Cox proportional hazards models with the bleeding event modeled as a time‐dependent covariate. The proportional hazards assumption was checked using plots of the log of the negative log of the survival function against the log of time. Multivariate analysis was performed after adjusting for those variables associated with new diagnosis of cancer during follow‐up in the univariate analysis (Table S1), including age, female sex, smoking, prior stroke, prior admission by bleeding, hemoglobin, CHA2DS2‐VASC score, HAS‐BLED score, and digoxin therapy. The interval from the time point of bleeding event to the diagnosis of new‐onset cancer was also explored.
All P values were 2‐sided and values <0.05 were considered significant. All statistical analyses were performed using STATA software version 15 (Stata Corp LLC).
Results
A total of 8753 anticoagulated patients with AF were evaluated. The majority were women (61.7%), with a mean age of 82.7±4.5 years. Vitamin K antagonists were prescribed in 6091 patients (69.6%) and direct OACs in 2662 patients (30.4%). Further baseline characteristics of the study population are described in Table S2.
Over a mean follow‐up of 3.0±1.8 years, we identified bleeding events in 2171 patients (among them, 28.7% were major bleeding), and new diagnosis of cancer in 479 patients. Gastrointestinal and genitourinary bleeding were the most frequent (21.1% and 19.8% of total bleeding, respectively [Figure S1]). Table S3 shows baseline characteristics of patients who experienced bleeding compared with those who did not experience bleeding. Among 2171 patients with bleeding, 198 (9.1%) were diagnosed with new‐onset cancer. For those 623 patients with major bleeding, 78 (12.5%) presented with a new diagnosis of cancer after the bleeding event (Table 1). The rate of new diagnosis of cancer after bleeding events was similar in patients treated with vitamin K antagonists and direct OACs (Table S4).
Table 1.
Association Between Bleeding and New Cancer Diagnosis According to Bleeding Severity
| Population | Patients, n | New Cancers Diagnosed |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| No bleeding | 6582 | 281 | 4.3 | Reference | Reference | Reference |
| Any bleeding | 2171 | 198 | 9.1 | 3.7 (3.0–4.5) | 3.2 (2.6–3.9) | <0.001 |
| Nonmajor bleeding | 1548 | 120 | 7.8 | 2.9 (2.3–3.7) | 2.5 (1.9–3.1) | <0.001 |
| Major bleeding | 623 | 78 | 12.5 | 6.2 (4.6–8.2) | 4.2 (3.1–5.7) | <0.001 |
Among patients with a new diagnosis of cancer (n=479), 198 (41.3%) were diagnosed in patients with prior bleeding, and 78 (16.3%) in patients with prior major bleeding (Table 2). Gastrointestinal cancer was the most frequent (n=162; 33.8%), followed by genitourinary cancer (n=139; 29.0%) (Figure S2). Bronchopulmonary cancer represented 7.7% of total cancers (n=37). Figure 1 presents data on the rate of new cancers—according to location—preceded by bleeding in the respective organ systems. Among 162 patients with a new diagnosis of gastrointestinal cancer, 42 (25.9%) were diagnosed in those with prior gastrointestinal bleeding. Among 139 patients with a new diagnosis of genitourinary cancer, 44 (31.7%) were diagnosed in those with prior genitourinary bleeding. Among 37 patients with a new diagnosis of bronchopulmonary cancer, 5 (13.5%) were diagnosed in those with prior bronchopulmonary bleeding. Of the remaining 141 new cancer diagnoses, 26 were diagnosed in patient with other bleeding that was not gastrointestinal, genitourinary, or bronchopulmonary. Diagnostic statistics including sensitivity and specificity and positive and negative predictive values for cancer diagnosis according to severity and location of bleeding are shown in Table S5.
Table 2.
Number of Patients With Bleeding or New Cancer Diagnosis and the Percentage of New Cancers Diagnosed in Patients With Bleeding According to Bleeding Site
| Patients With Bleeding, n | Patients With Cancer, n (%) | |||||
|---|---|---|---|---|---|---|
| Organ System | Any Bleeding | Major Bleeding | CIF Cancer (Per 100 Patient‐y) | Total Patients | In Patients With Bleeding | In Patients With Major Bleeding |
| Any | 2171 | 623 | 4.7 (4.1–5.4) | 479 | 198* (41.3) | 78* (16.3) |
| Gastrointestinal* | 458 | 197 | 6.2 (4.5–8.7) | 162 | 42* (25.9) | 28* (17.3) |
| Genitourinary* | 429 | 79 | 6.0 (4.5–8.0) | 139 | 44* (31.7) | 18* (12.9) |
| Bronchopulmonary* | 111 | 21 | 2.1 (0.9–4.9) | 37 | 5* (13.5) | 1* (2.7) |
| Other sites* | 1173 | 326 | 1.1 (0.8–1.7) | 141 | 26* (18.4) | 3* (2.1) |
CIF indicates cumulative incidence function.
The number of specific cancers is reported based on the location of the bleeding.
Figure 1. Proportion of bleeding in patients with cancer according to cancer site.

BP indicates bronchopulmonary; GI, gastrointestinal; GU, genitourinary; and other cancer, nongastrointestinal, nongenitourinary, and nonbronchopulmonary.
The cumulative incidence of cancer after a bleeding episode is illustrated in Figure 2. Patients with any bleeding have a 3‐fold higher hazard of being subsequently diagnosed with new cancer compared with those without prior bleeding (4.7 per 100 patient‐years versus 1.4 per 100 patient‐years; hazard ratio [HR], 3.7 [95% CI, 3.0–4.5]). Figure 3 shows data on the association between bleeding site and new diagnoses of cancer. Gastrointestinal bleeding (n=458) was associated with a 13‐fold higher hazard of new gastrointestinal cancer diagnosis (HR for any gastrointestinal bleeding, 13.4; 95% CI, 9.1–19.8). Genitourinary bleeding (n=429) was associated with an 18‐fold higher hazard of new genitourinary cancer diagnosis (HR for any genitourinary bleeding, 18.1; 95% CI, 12.5–26.2). Bronchopulmonary bleeding (n=111) was associated with a 15‐fold higher hazard of new bronchopulmonary cancer diagnosis (HR for any bronchopulmonary bleeding, 15.8; 95% CI, 6.0–41.3). For other bleeding that was not gastrointestinal, genitourinary, or bronchopulmonary (n=1173), the HR for cancer from sites other than the gastrointestinal, genitourinary, and bronchopulmonary tracts was 2.3 (95% CI, 1.5–3.6). For further information, see Figures S3 through S6.
Figure 2. Cumulative frequency of new cancer diagnosis in relation to bleeding.

The bars represent percentages of patients with a new diagnosis of cancer after bleeding (according to severity) or without prior bleeding. HR indicates hazard ratio.
Figure 3. New diagnosis of gastrointestinal (GI) (A), genitourinary (GU) (B), bronchopulmonary (BP) (C), and other (nongastrointestinal, nongenitourinary, nonbronchopulmonary; D) cancer after any gastrointestinal, genitourinary, bronchopulmonary, and other‐site bleeding, respectively.

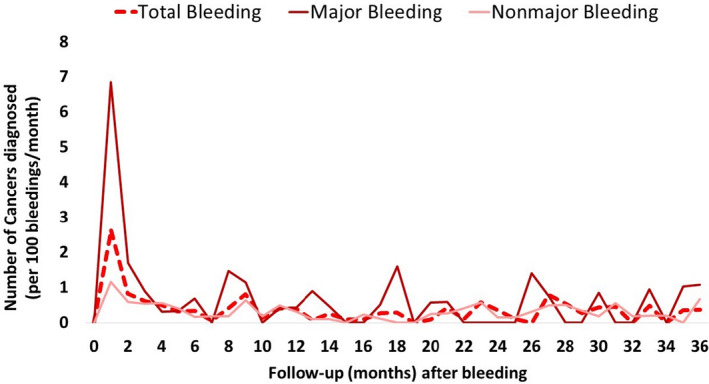
Figure 4 shows the percentage of new diagnosed cancers in relation to the time after the bleeding event. Of new cancers diagnosed after bleeding, 36 of 198 (18.2%) were diagnosed in the first month after the bleeding episode (29.5% after a major bleeding) and 71 (35.9%) within 6 months after bleeding (61.5% after a major bleeding).
Figure 4. Timing of new cancer diagnosis in relation to bleeding.
In the group of nonanticoagulated patients with AF (n=1923), antiplatelet therapy was prescribed in 55.7% (n=1072). Over a mean follow‐up of 2.6±1.8 years, we identified bleeding events in 245 nonanticoagulated patients (12.7%) and new diagnosis of cancer in 130 nonanticoagulated patients (6.8%). The cumulative incidence of cancer was similar in anticoagulated and nonanticoagulated patients (2.0 [95% CI, 1.8–2.2] versus 2.1 [95% CI, 1.7–2.5] per 100 patient‐years, respectively; P=0.803). However, the percentage of cancers that were diagnosed after a bleeding episode was significantly higher in anticoagulated patients (41.3% versus 17.7%, P<0.001). Like in anticoagulated patients, bleeding was associated with an increased risk of subsequent diagnosis of cancer in nonanticoagulated patients with AF (adjusted HR, 1.8; 95% CI, 1.1–2.9 [P=0.012]) (Table S6).
Discussion
In this large observational study of anticoagulated patients with AF, we report that gastrointestinal, genitourinary, and bronchopulmonary bleeding may identify a high‐risk group for new cancer diagnosis. Among 2171 patients who experienced bleeding, 1 in 11 were subsequently diagnosed with new cancer. When we restricted our analysis to major bleeding form the gastrointestinal, genitourinary, or bronchopulmonary tracts, 1 in 7 patients with prior major gastrointestinal bleeding were diagnosed with gastrointestinal cancer, 1 in 4 patients with prior major genitourinary bleeding were diagnosed with genitourinary cancer, and 1 in 21 patients with prior major bronchopulmonary bleeding were diagnosed with bronchopulmonary cancer. For bleeding from other sites, the association with new cancer other than gastrointestinal, genitourinary, and bronchopulmonary cancers was much weaker (only 1 in 45 bleedings [2.2%]).
Physicians have long been aware of the potential for bleeding to unmask underlying cancer, but most reports of the association between bleeding and cancer diagnosis are based on retrospective small case series, or analyses of databases designed to address other questions and do not provide reliable measures of the strength of association. Recently, a nationwide Danish retrospective study of anticoagulated patients with AF demonstrated high relative risks of colorectal cancer in patients with lower gastrointestinal bleeding. 4 The authors showed a 15‐fold higher hazard of new colorectal cancer diagnosis with lower gastrointestinal bleeding in patients aged ≥75 years (1‐year risk of colorectal cancer, 8.1% versus 0.5% in patients with and without bleeding). In our study, gastrointestinal bleeding was associated with a 13‐fold higher hazard of new gastrointestinal cancer diagnosis, similar to the Danish study. 4 However, the novelty of our findings relies in extending these results to other bleeding and cancers, such as those that come from the genitourinary or bronchopulmonary tract. The significance of hematuria in patients receiving OACs has been addressed only by several underpowered studies, which were conducted in relatively small samples of <100 patients. 14 Only one study by Yu et al, 15 in 3750 patients with AF with hematuria, showed a higher prevalence and early detection of genitourinary cancer in patients with AF who had hematuria. For bronchopulmonary bleeding, it is known that 15% to 25% of cases of hemoptysis are caused by primary lung cancer. 16 Hirshberg and colleagues, 17 after reviewing 208 patients without a history of malignancy who presented with hemoptysis, found that 19% had lung cancer. In our study, patients with genitourinary and bronchopulmonary bleeding were associated with an 18‐ and 15‐fold higher hazard of new genitourinary and bronchopulmonary cancer, respectively. This strong association between bleeding and cancer, specifically for gastrointestinal, genitourinary, and bronchopulmonary with the corresponding cancers, highlight the importance of searching for occult cancer at the site of bleeding in patients who experience those bleeding episodes. Therefore, gastrointestinal, genitourinary, and bronchopulmonary bleedings during OAC treatments should not be dismissed as diffuse mucosal bleeding attributable to OACs, and these patients should be examined for an underlying malignant cause. OACs could led to identifying previously unknown lesions (malignant or premalignant) in more than one third of cases (41.3% in our study), resulting in earlier treatment and better prognosis.
Although the greater the bleeding the greater the association with cancer, a relevant finding of our study was that 60.6% of cancers in patients with prior bleeding were diagnosed after minor bleeding. We found a 3‐fold higher hazard of new cancer diagnosis with nonmajor bleeding in our study population, which is even more relevant in patients with gastrointestinal (7‐fold higher hazard), genitourinary (14‐fold higher hazard), and bronchopulmonary (14‐fold higher hazard) bleeding. This was consistent with the recent study by Eikelboom et al 6 using data from the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial. In a population of patients with established atherosclerosis treated with aspirin±low‐dose rivaroxaban, authors found that 69% of all new cancer diagnoses after bleeding were in patients with minor bleeding. This finding highlights the potential for minor bleeding to unmask new cancers.
We also reported a temporal relationship between bleeding and subsequent cancer. Most of the cancers were diagnosed in the first 6 months after hemorrhagic complication. This was consistent with prior studies, which reported a critical period of 6 months after bleeding in which there is a higher risk for occurrence of cancer. 10 , 18 , 19 Many cancers are asymptomatic until advanced stages of disease when treatment has a minimal effect on survival. Paradoxically, postdischarge bleeding can be interpreted positively as an opportunity for timely diagnosis of hidden cancers. A quick proactive screening of cancer after bleeding could potentially provide early detection of cancers and could result in earlier treatment, better prognosis, and possibly increased long‐term survival.
Our provocative results raise both clinical and research questions. Clinically, should a bleeding episode in anticoagulated patients prompt an early screening for occult cancer? Several factors argue against routine screening, including the low absolute risk of cancer and the potential cost and burden of cancer screening. 20 However, the important association we have found between gastrointestinal, genitourinary, and bronchopulmonary bleedings with their respective cancers makes it necessary to reflect about it. Although bleeding is undesirable, it may help physicians make an earlier diagnosis of cancer, and this could lead to improved outcomes. Gaining insight into the relationship between atrial fibrillation and cancer is of great public health relevance. Our results, together with the results of the Danish and COMPASS trials, 4 , 9 support that gastrointestinal, genitourinary, and bronchopulmonary bleeding in patients receiving OACs should prompt a careful search for undiagnosed cancer, even when the bleeding is minor.
Despite the interest of our findings based on a large contemporary patient cohort with real‐word patients with AF, several limitations should be considered. This is a retrospective observational single‐center study with unselected real‐life patients; hence, no causal inferences can be made. A potential limitation is that the protocol of our study did not mandate investigation of patients with bleeding for cancer but left this to the discretion of the physician. Unfortunately, our study also has no data to analyze the stage of cancer and the events after cancer. Last, information regarding some risk factors for cancer was lacking, such as dietary habits.
Conclusions
Among patients with AF treated with OAC therapy, gastrointestinal, genitourinary, and bronchopulmonary bleeding are strongly and relatively specifically associated with new diagnosis of cancer within the respective organ systems. The strength of this association increases with the severity of bleeding, with most cancers identified within the first 6 months after bleeding event. A prompt evaluation of bleeding could be useful for enabling early detection of cancer, especially gastrointestinal, genitourinary, and bronchopulmonary cancers.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S6
(J Am Heart Assoc 2020;9:e016836 DOI: 10.1161/JAHA.120.016836.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Weng LC, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation. 2018;1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;e102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of malignant cancer among women with new‐onset atrial fibrillation. JAMA Cardiol. 2016;389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen PV, Dalgaard F, Gislason GH, Brandes A, Johnsen SP, Grove EL, Torp‐Pedersen C, Dybro L, Harboe L, Munster AB, et al. Gastrointestinal bleeding and the risk of colorectal cancer in anticoagulated patients with atrial fibrillation. Eur Heart J. 2020:ehz964. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Norgaard M, Veres K, Ording AG, Djurhuus JC, Jensen JB, Sorensen HT. Evaluation of hospital‐based hematuria diagnosis and subsequent cancer risk among adults in Denmark. JAMA Netw Open. 2018;e184909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eikelboom JW, Connolly SJ, Bosch J, Shestakovska O, Aboyans V, Alings M, Anand SS, Avezum A, Berkowitz SD, Bhatt DL, et al. Bleeding and new cancer diagnosis in patients with atherosclerosis. Circulation. 2019;1451–1459. [DOI] [PubMed] [Google Scholar]
- 7. Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, Becker RC, Singer DE, Halperin JL, Hankey GJ, et al. gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;2271–2281. [DOI] [PubMed] [Google Scholar]
- 8. O'Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, Kowey PR, Mahaffey KW, Chang P, Fonarow GC, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;3258–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay J‐F, Granger CB, Mauri L, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;527–536. [DOI] [PubMed] [Google Scholar]
- 10. Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant‐related gastrointestinal bleeding–could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med. 2014;672–678. [DOI] [PubMed] [Google Scholar]
- 11. Cobas Paz R, Raposeiras Roubín S, Abu Assi E, Barreiro Pardal C, García Comesaña J, González‐Carrero López A, Caneiro Queija B, Cespón Fernández M, Muñoz Pousa I, Domínguez Erquicia P, et al. Impact of anticoagulation in patients with dementia and atrial fibrillation. Results of the CardioCHUVI‐FA registry. Rev Esp Cardiol (Engl Ed). 2020:S1885‐5857(20)30001–3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;692–694. [DOI] [PubMed] [Google Scholar]
- 13. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of A . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;2119–2126. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen M, Qaseem A; High Value Care Task Force of the American College of P . Hematuria as a marker of occult urinary tract cancer: advice for high‐value care from the American College of Physicians. Ann Intern Med. 2016;488–497. [DOI] [PubMed] [Google Scholar]
- 15. Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B. Clinical significance of hematuria in atrial fibrillation with oral anticoagulation therapy. Circ J. 2017;158–164. [DOI] [PubMed] [Google Scholar]
- 16. Mondoni M, Carlucci P, Job S, Parazzini EM, Cipolla G, Pagani M, Tursi F, Negri L, Fois A, Canu S, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur Respir J. 2018;1701813. [DOI] [PubMed] [Google Scholar]
- 17. Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;440–444. [DOI] [PubMed] [Google Scholar]
- 18. Halpern JA, Chughtai B, Ghomrawi H. Cost‐effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Intern Med. 2017;800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke MA, Long BJ, Del Mar MA, Arbyn M, Bakkum‐Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta‐analysis. JAMA Intern Med. 2018;1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahman F, Ko D, Benjamin EJ. Association of atrial fibrillation and cancer. JAMA Cardiol. 2016;384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S6


