Abstract
Background
Serum levels of vascular cell adhesion molecule‐1 (VCAM‐1) are reflective of endothelial activation. Although VCAM‐1 has been implicated in the pathogenesis of heart failure with preserved ejection fraction (HFpEF), the prospective association of VCAM‐1 with development of clinically overt heart failure (HF) across ejection fraction categories is unclear.
Methods and Results
In MESA (the Multi‐Ethnic Study of Atherosclerosis), we evaluated the association of VCAM‐1 at examination 2 (2002–2004) with incident HF (HFpEF and HF with reduced ejection fraction) after adjustment for cardiovascular risk factors. Incident HF was independently adjudicated as first hospitalization for symptomatic HF. Among 2297 participants (mean age, 63 years; women, 53%), those with higher VCAM‐1 were more likely to be White race, had higher blood pressure, and had lower kidney function. Over a median of 14.4 years, there were 102 HF events (HFpEF=65; HF with reduced ejection fraction=37). After covariate adjustment, each doubling of VCAM‐1 was associated with incident HF (hazard ratio [HR], 1.94; 95% CI, 1.17–3.23; P=0.01). This association appeared stronger among current/former smokers compared with never smokers. On evaluation of HF subtypes, VCAM‐1 was associated with incident HFpEF (HR, 1.97; 95% CI, 1.04–3.72; P=0.04) but not with incident HF with reduced ejection fraction, although risk estimates were consistent (HR, 1.82; 95% CI, 0.79–4.21; P=0.16).
Conclusions
In a multiethnic cohort, VCAM‐1 was significantly associated with incident HF over long‐term follow‐up. These findings suggest a potential role for endothelial activation in driving clinical HF, and specifically HFpEF. Therapies that decrease endothelial activation may prevent the progression from cardiovascular risk factors to clinical HF.
Keywords: cellular adhesion molecule, endothelial activation, heart failure, heart failure with preserved ejection fraction, vascular cell adhesion molecule‐1
Subject Categories: Epidemiology, Primary Prevention, Biomarkers
Nonstandard Abbreviations and Acronyms
- CAM
cellular adhesion molecule
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- MESA
Multi‐Ethnic Study of Atherosclerosis
- VCAM‐1
vascular cell adhesion molecule‐1
Clinical Perspective
What Is New?
Although endothelial activation, as measured by vascular cell adhesion molecule‐1, has been implicated in the pathogenesis of heart failure (HF) with preserved ejection fraction, the prospective association of elevation in vascular cell adhesion molecule‐1 with the development of clinically overt HF with preserved ejection fraction is unclear.
In this analysis of MESA (the Multi‐Ethnic Study of Atherosclerosis), higher vascular cell adhesion molecule‐1 was significantly associated with incident HF over long‐term follow‐up (>14 years).
What Are the Clinical Implications?
These findings substantiate the potential pathogenic roles of endothelial activation, cellular adhesion molecules, and microvascular coronary inflammation in driving symptomatic HF with preserved ejection fraction and HF hospitalization.
Therapies aimed to reduce endothelial activation and specifically vascular cell adhesion molecule‐1 may prevent incident HF among patients at risk.
Heart failure (HF) is a heterogeneous clinical syndrome that carries substantial morbidity and is driven by a multifaceted pathogenesis. The specific mechanisms that lead to HF are unclear, 1 and the elucidation of such mechanisms may provide insights into therapies to halt the progression from cardiovascular risk factors to clinical HF.
Endothelial cell activation may play a particularly important role in the development of HF with preserved ejection fraction (HFpEF) compared with HF with reduced ejection fraction (HFrEF). 2 Chronic systemic inflammation secondary to cardiovascular risk factors results in upregulation of cellular adhesion molecules (CAMs), including vascular cell adhesion molecule‐1 (VCAM‐1), on the surface of endothelial cells. 3 , 4 , 5 Independent of systemic inflammation, it has also been suggested that regional inflammation in the setting of pericardial adipose tissue specifically prompts CAM expression. 6 CAMs facilitate leukocyte transmigration along the endothelium, which may lead to coronary microvascular dysfunction and myocardial interstitial fibrosis. 7 , 8 Indeed, VCAM‐1 expression is upregulated in human myocardial tissue of HFpEF, 8 CAMs are more highly expressed in myocardial tissue of HFpEF compared with HFrEF, 9 and we have previously demonstrated associations of serum CAM levels with left ventricular dysfunction consistent with preclinical HFpEF. 10 In aggregate, these findings suggest a potential pathogenic role for endothelial cell activation, and specifically VCAM‐1, in HFpEF.
Despite current understanding, the prospective associations of VCAM‐1 with development of clinical HFpEF are unknown. Furthermore, the specific role of pericardial adipose tissue in driving VCAM‐1 elevation is not well defined, and may provide understanding of the role of regional, as opposed to systemic, adipose tissue in endothelial activation. Therefore, we evaluated (1) the association of pericardial fat with endothelial activation, as measured by VCAM‐1; and (2) the associations of VCAM‐1 with incident HF, including clinical subtypes, in MESA (the Multi‐Ethnic Study of Atherosclerosis). We hypothesized that (1) pericardial adipose tissue is associated with VCAM‐1 and (2) VCAM‐1 is specifically associated with incident HFpEF.
Methods
Study Participants
MESA is a prospective cohort of community‐dwelling adults that was designed to understand the risk factors and prevalence of subclinical cardiovascular disease. The recruitment and study design of MESA have been previously described. 11 In brief, between 2000 and 2002, 6814 participants, aged 45 to 84 years, who identified themselves as White, Black, Hispanic, or Chinese were recruited across 6 study sites in the United States (Baltimore, MD; Chicago, IL; St Paul, MN; Forsyth County, North Carolina; New York, NY; and Los Angeles, CA). At baseline, participants had no history of cardiovascular disease, defined as myocardial infarction, angina, stroke, transient ischemic attack, HF, atrial fibrillation, nitroglycerin use, angioplasty, pacemaker or defibrillator, or cardiac surgery. Following recruitment and a baseline in‐person examination (examination 1), 5 additional follow‐up in‐person examinations have been completed at ≈2‐ to 5‐year intervals. Examinations included standardized questionnaires that collected information on demographics, medical history, and medication use. Resting blood pressure, blood sampling, and spot urine specimens were also obtained during examinations. Diabetes mellitus was defined as self‐reported diagnosis, fasting glucose ≥126 mg/dL, or use of antidiabetic medication. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation using examination 1 serum creatinine. 12 Urine albumin was measured using nephelometry, and urine creatine was measured through the Jaffe method. 13 For this analysis, we included participants who had serum VCAM‐1 levels obtained at examination 2 (conducted between 2002 and 2004) and had available data on baseline covariates and follow‐up. Of the 6814 MESA participants, 4517 were initially excluded (Figure S1): 4373 did not have VCAM‐1 levels drawn as part of the MESA Adhesion Ancillary Study, 128 were missing covariate data at examination 2, 10 had HF before examination 2, and 6 terminated follow‐up by examination 2. Thus, the final analytic cohort for this analysis was 2297 participants. The study protocol was approved by the institutional review board of each study site; all participants provided informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request.
Pericardial Fat Assessment
Cardiac computed tomography imaging was performed at initial examination among the MESA cohort. The protocols for computed tomography scan and pericardial fat volume assessment have been previously described. 14 Briefly, computed tomography image slices within 15 mm above and 30 mm below the left main coronary artery were included because it includes fat surrounding the proximal coronary arteries. The anterior and posterior borders of volume were defined by the chest wall and aorta or bronchus, respectively. Fat was discerned from other tissue using a threshold of −190 to −30 Hounsfield units using volume analysis software (GE Health Care, Waukesha, WI), and volume was the sum of all fat‐containing voxels. 14
VCAM‐1 Measurement
At examination 2 (2002–2004), blood samples were obtained from fasting participants and were stored at −70°C. 15 As part of the MESA Adhesion Ancillary Study, serum VCAM‐1 was measured in a subset (n=2372) of the MESA cohort. 16 Serum VCAM‐1 was measured using the Quantikine ELISA Kit (R&D Systems); the minimum detectable level was 0.6 ng/mL, and the interassay coefficient of variation was 3.6% at a mean concentration of 564 ng/mL.
HF Assessment
As part of the MESA, participants were screened for clinical events through regular telephone contact and in‐person examinations. All identified records from hospitalizations for cardiovascular events were abstracted, and MESA personnel transmitted records of symptoms, medical history, biomarkers, ECGs, echocardiograms, cardiac catheterization reports, other imaging studies, and outpatient records (if available) to the MESA coordinating center. Incident HF events were independently adjudicated by 2 study physicians who were blinded to study data outside of hospitalization records. HF events were defined as definite or probable. Both definite and probable HF events required symptoms of HF, including shortness of breath or edema. Definite HF was further defined on the basis of ≥1 of the following criteria: pulmonary edema on chest radiography, left ventricular dilation or decreased systolic function, or evidence of diastolic dysfunction. If criteria for definite HF were not available, probable HF was defined as a physician diagnosis of HF in the clinical record and documentation of medical treatment for HF. The primary outcome of interest in this analysis was incident HF, defined as any probable or definite HF event. We additionally evaluated HF subtypes (incident HFpEF and HFrEF) as secondary outcomes. Incident HFpEF was defined as a HF event with documentation of left ventricular ejection fraction (EF) ≥45% on echocardiogram or radionucleotide study at time of hospitalization, and incident HFrEF was defined as EF <45% at hospitalization. In this analysis, there were no HF events without documented EF on imaging study at time of hospitalization. Event ascertainment of HF in MESA has been fully updated and completed through 2017.
Statistical Analysis
Demographic and clinical characteristics at examination 2 were compared by quartile of VCAM‐1 using χ2 tests for categorical variables and univariate general linear models for continuous variables. A generalized additive model was used to depict the association of urine albumin/creatinine ratio, a biomarker of global endothelial dysfunction, with VCAM‐1.
The association of pericardial fat volume at examination 1 with VCAM‐1 levels at examination 2 was evaluated using linear regression models. Model 1 was unadjusted. Model 2 was adjusted for the following examination 1 covariates: age, sex, race, body mass index (BMI), systolic blood pressure, antihypertensive medication, diabetes mellitus, smoking status, total cholesterol, and eGFR. A generalized additive model was used to visually depict the unadjusted association of pericardial fat volume with VCAM‐1.
Multivariable Cox proportional hazards regression models were used to evaluate the associations of VCAM‐1 with incident HF and its subtypes. We evaluated VCAM‐1 as a continuous variable after log base 2 transformation, which can be interpreted as “per doubling.” Examination 2, the time of VCAM‐1 measurement, was defined as the time origin for this analysis. The proportionality of hazards assumption was confirmed using the time interaction test. We first assessed the potential nonlinear associations of VCAM‐1 and hazard of incident HF using restricted cubic splines in Cox proportional hazards regression. The log relative hazard of incident HF and HFpEF was computed from the fully adjusted model for observed values of log‐VCAM‐1 while holding the values of covariates at their average values. We assessed the associations of VCAM‐1 as a continuous variable (per doubling) with incident HF and its subtypes (HFpEF and HFrEF). We evaluated the time to first HFpEF and HFrEF event using separate Cox proportion hazards models with censoring on the opposing type. For all Cox regression models, covariates were obtained at examination 2, except for eGFR, which was obtained at examination 1. Model 1 adjusted for age, race, and sex. Model 2 further adjusted for BMI, systolic blood pressure, antihypertensive medication treatment, diabetes mellitus, coronary artery disease, smoking, total cholesterol, and eGFR.
In exploratory analyses, we assessed for effect modification of age, sex, BMI, smoking status, and pericardial fat volume on the association of VCAM‐1 and incident HF using interaction terms for age (above versus below median), sex, BMI (above versus below median), smoking status (current/former smokers versus never smokers), and pericardial fat volume (above versus below median). These interaction terms were chosen on the basis of known associations with risk of HF. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two‐tailed P<0.05 was considered statistically significant.
Results
Participant Characteristics
Baseline characteristics of the final analytic cohort, stratified by quartile of VCAM‐1, are shown in Table 1. Participants within the highest quartile of VCAM‐1 were older and more likely men, White race, and former smokers compared with participants in lower VCAM‐1 quartiles. In addition, participants with higher VCAM‐1 tended to have higher systolic blood pressure and lower levels of eGFR and low‐density lipoprotein cholesterol. Participants with higher VCAM‐1 levels also had higher urine albumin/creatinine ratio (Figure 1), consistent with endothelial dysfunction. Compared with participants in the final analytic cohort, MESA participants who were excluded from this analysis were older, more likely White or Black race, and more likely former smokers at examination 1. In addition, excluded participants had higher systolic blood pressure and BMI and lower eGFR at baseline compared with participants included in this analysis (Table S1).
Table 1.
Characteristics at Baseline (Examination 2) by Quartile of VCAM‐1
| Characteristic | Quartile of VCAM‐1 | P Value | |||
|---|---|---|---|---|---|
| 1 (n=574) | 2 (n=574) | 3 (n=575) | 4 (n=574) | ||
| Age, mean±SD, y | 58.3±8.6 | 61.1±9.1 | 64.7±9.5 | 68.1±9.9 | <0.001 |
| Women, n (%) | 305 (53.3) | 331 (57.7) | 301 (52.4) | 282 (49.1) | 0.03 |
| Race/Ethnicity, n (%) | <0.001 | ||||
| Black | 233 (40.6) | 118 (20.6) | 117 (20.4) | 85 (14.8) | |
| White | 73 (12.7) | 150 (26.1) | 161 (28.0) | 183 (31.9) | |
| Hispanic | 124 (21.6) | 160 (27.9) | 142 (24.7) | 157 (27.4) | |
| Chinese | 144 (25.1) | 146 (25.4) | 155 (27.0) | 149 (26.0) | |
| Body mass index, mean±SD, kg/m2 | 28.4±5.5 | 27.9±5.8 | 27.6±5.1 | 27.7±5.5 | 0.05 |
| Systolic blood pressure, mean±SD, mm Hg | 120.0±19.0 | 121.8±20.0 | 125.9±21.8 | 128.4±20.9 | <0.001 |
| Diastolic blood pressure, mean±SD, mm Hg | 71.2±9.8 | 70.7±10.0 | 70.6±10.1 | 69.7±9.6 | 0.07 |
| Antihypertensive medication, n (%) | 192 (33.5) | 214 (37.3) | 224 (39.0) | 289 (50.4) | <0.001 |
| Coronary artery disease/coronary heart disease, n (%) | 4 (0.7) | 6 (1.0) | 3 (0.5) | 7 (1.2) | 0.57 |
| Diabetes mellitus, n (%) | 84 (14.6) | 80 (13.9) | 93 (16.2) | 105 (18.3) | 0.18 |
| Smoking status, n (%) | <0.001 | ||||
| Current smoker | 98 (17.1) | 77 (13.4) | 46 (8.0) | 50 (8.7) | |
| Former smoker | 185 (32.2) | 203 (35.4) | 232 (40.4) | 236 (41.1) | |
| Never smoker | 291 (50.7) | 294 (51.2) | 298 (51.8) | 288 (50.2) | |
| Total cholesterol, mean±SD, mg/dL | 195.9±35.1 | 195.9±36.5 | 191.3±32.1 | 185.5±35.7 | <0.001 |
| LDL cholesterol, mean±SD, mg/dL | 117.7±30.9 | 117.0±33.5 | 113.3±30.1 | 108.8±30.9 | <0.001 |
| HDL cholesterol, mean±SD, mg/dL | 51.9±14.6 | 51.1±14.2 | 52.0±15.5 | 50.0±14.4 | 0.07 |
| eGFR, mean±SD, mL/min per 1.73 m2 | 84.2±14.5 | 81.1±14.0 | 76.8±14.6 | 72.6±17.8 | <0.001 |
| VCAM‐1, mean±SD, ng/mL | 505.1±62.4 | 643.8±31.4 | 766.7±41.9 | 1031.6±218.4 | <0.001 |
eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and VCAM‐1, vascular cell adhesion molecule‐1.
Figure 1. Association of urine albumin/creatinine ratio (UACR) with vascular cell adhesion molecule‐1 (VCAM‐1).
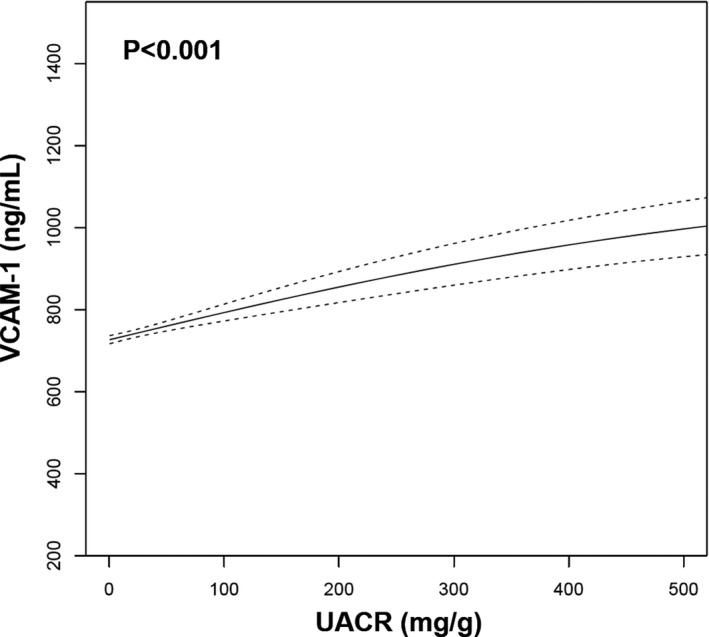
An unadjusted generalized additive model demonstrates the association of UACR, a biomarker of endothelial dysfunction, with VCAM‐1. Dashed lines represent 95% CI.
Association of Pericardial Fat Volume With VCAM‐1
In unadjusted analyses, higher pericardial fat volume at examination 1 was associated with higher VCAM‐1 levels at examination 2 (β coefficient per 1‐SD higher pericardial fat volume, 32.1; SE, 4.7; P<0.001; Figure 2). After full covariate adjustment, there was no significant association of pericardial fat with VCAM‐1 (β coefficient per 1‐SD higher pericardial fat volume, 4.5; SE, 5.8; P=0.44).
Figure 2. Association of pericardial fat volume with vascular cell adhesion molecule‐1 (VCAM‐1).
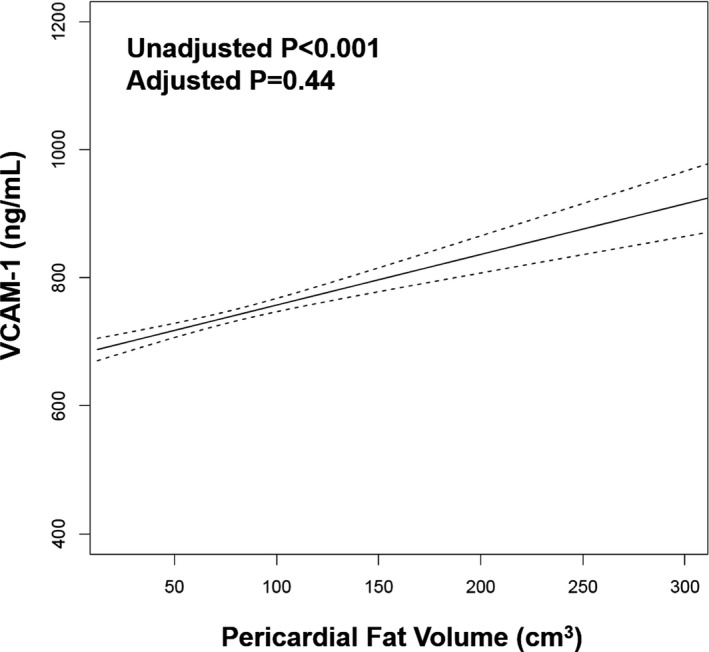
An unadjusted generalized additive model demonstrates the association of pericardial fat with VCAM‐1. Dashed lines represent 95% CI.
Association of VCAM‐1 With Incident HF, HFpEF, and HFrEF
Over median follow‐up of 14.4 years (interquartile range, 11.8–14.8 years), there were 102 (4.4%) total HF events. The incidence rate (per 1000 person‐years) for HF was progressively higher for each higher quartile of VCAM‐1 (Figure 3). Of the 102 HF events, 65 (64%) were incident hospitalization for HFpEF and 37 (36%) were incident hospitalization for HFrEF. On evaluation by incident HF by EF categories, the incidence rates for HFpEF and HFpEF were also higher across higher VCAM‐1 quartiles (Figure 3). On spline analysis, higher serum VCAM‐1 was associated with progressively greater risks of HF and HFpEF, with plateauing of risk at the highest levels of VCAM‐1 (Figure 4).
Figure 3. Incidence rates of heart failure (HF) by quartile of vascular cell adhesion molecule‐1 (VCAM‐1).
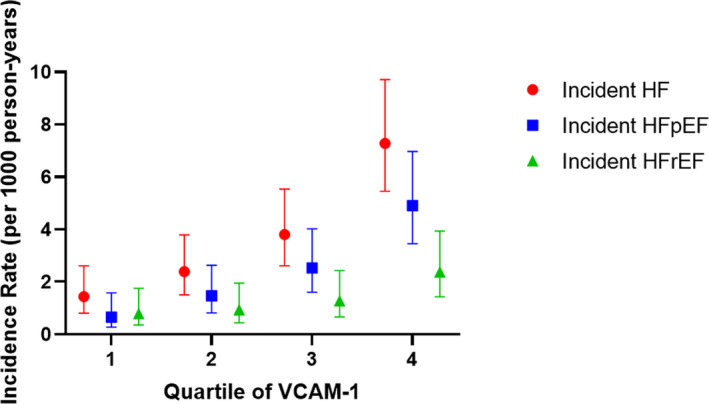
Shown are unadjusted incidence rates. Error bars represent 95% CI. HFpEF indicates HF with preserved ejection fraction; and HFrEF, HF with reduced ejection fraction.
Figure 4. Association of vascular cell adhesion molecule‐1 (VCAM‐1) with incident heart failure (HF).
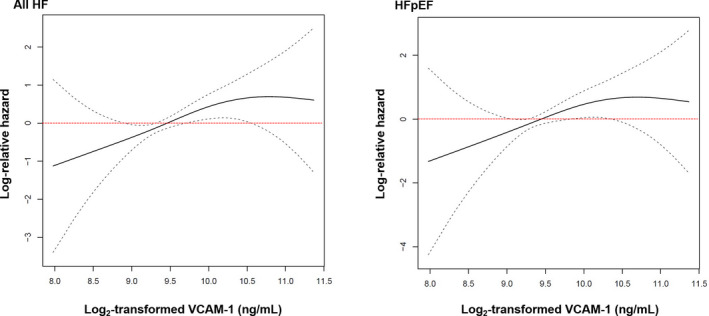
Fully adjusted restricted cubic splines demonstrate the continuous associations of VCAM‐1 with incident HF (left panel) and HF with preserved ejection fraction (HFpEF) (right panel). Log base 2 transformation of VCAM‐1 can be interpreted as “per doubling.” Dashed lines represent 95% CI.
The associations of VCAM‐1 with risk of HF, HFpEF, and HFrEF are shown in Table 2. In unadjusted analyses, higher VCAM‐1 at baseline was associated with increased risk of incident HF hospitalizations. After full covariate adjustment, each doubling of VCAM‐1 was associated with 94% increased risk of HF (hazard ratio [HR], 1.94; 95% CI, 1.17–3.23; P=0.01). On evaluation of HF subtypes, VCAM‐1 remained significantly associated with incident HFpEF after full covariate adjustment, with similar overall magnitude of risk as all HF. Although there was no significant association of VCAM‐1 with incident HFrEF in unadjusted and adjusted models, similar effect estimates as for HFpEF were noted, with wider CIs.
Table 2.
Association of VCAM‐1 With Incident HF
| Model | Incident HF | Incident HFpEF | Incident HFrEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events/No. of Participants | HR per Doubling of VCAM‐1 (95% CI) | P Value | No. of Events/No. of Participants | HR per Doubling of VCAM‐1 (95% CI) | P Value | No. of Events/No. of Participants | HR per Doubling of VCAM‐1 (95% CI) | P Value | |
| Model 1* | 102/2297 | 2.36 (1.45–3.85) | <0.001 | 65/2297 | 2.43 (1.33–4.47) | 0.004 | 37/2297 | 2.17 (0.96–4.92) | 0.06 |
| Model 2† | 102/2297 | 1.94 (1.17–3.23) | 0.01 | 65/2297 | 1.97 (1.04–3.72) | 0.036 | 37/2297 | 1.82 (0.79–4.21) | 0.16 |
HF indicates heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; and VCAM‐1, vascular cell adhesion molecule‐1.
Adjusted for age, sex, and race.
Adjusted for model 1 variables plus body mass index, systolic blood pressure, antihypertensive medication treatment, diabetes mellitus, coronary artery disease, smoking, total cholesterol, and estimated glomerular filtration rate.
Association of VCAM‐1 With Incident HF in Subgroups
There were no significant differences in the associations of VCAM‐1 with incident HF when evaluating subgroups based on age (higher than median: HR, 2.59 [95% CI, 1.46–4.57] versus lower than median: HR, 1.56 [95% CI, 0.61–3.99]; P‐interaction=0.36), sex (male: HR, 1.74 [95% CI, 0.93–3.26] versus female: HR, 2.30 [95% CI, 1.09–4.85]; P‐interaction=0.55), BMI (higher than median: HR, 2.05 [95% CI, 1.09–3.83] versus lower than median: HR, 1.77 [95% CI, 0.82–3.79]; P‐interaction=0.76), or pericardial fat volume (higher than median: HR, 2.38 [95% CI, 1.37–4.16] versus lower than median: HR, 0.96 [95% CI, 0.35–2.58]; P‐interaction=0.10). However, the association of VCAM‐1 with incident HF was stronger in current/former smokers compared with never smokers (current/former smokers: HR, 3.19 [95% CI, 1.66–6.14] versus never smokers: HR, 1.12 [95% CI, 0.54–2.33]; P‐interaction=0.03).
Discussion
In this multiethnic cohort of community‐dwelling adults, we evaluated associations of pericardial fat with serum VCAM‐1, and subsequently investigated associations of serum VCAM‐1 with incident HF and its subtypes. Pericardial fat was not associated with serum VCAM‐1 after adjustment for traditional cardiovascular risk factors, suggesting that systemic, as opposed to regional, inflammation may be more integrally involved in endothelial activation. Baseline serum VCAM‐1 was associated with higher risk of incident HF hospitalization after adjustment for traditional cardiovascular risk factors. The risk of incident HF was higher with progressively higher VCAM‐1 levels, and this risk appeared to plateau at the highest levels of VCAM‐1. VCAM‐1 was significantly associated with incident HFpEF. Although the associations of VCAM‐1 and incident HF were consistent across the spectrum of age, sex, and BMI, there was a stronger association among current/former smokers compared with never smokers. In aggregate, these data are consistent with the hypothesis that endothelial activation may play a pathogenic role in the development of clinically manifest HF.
Endothelial activation, VCAM‐1, and coronary microvascular dysfunction have been previously implicated in the pathogenesis of HF, and particularly HFpEF. 17 , 18 Specifically, certain cardiovascular risk factors lead to systemic inflammation and cytokine release, including elevation of tumor necrosis factor‐α. 19 , 20 A known posttranscriptional regulator of VCAM‐1 expression, tumor necrosis factor‐α induces endothelial cell activation and expression of VCAM‐1 on cell surfaces. 4 , 21 VCAM‐1 participates in the rolling, adhesion, and, ultimately, transmigration of leukocytes across the endothelial cell barrier. 22 At the cardiac level, transmigration of leukocytes into cardiac tissue leads to reactive oxidative species production, decreased NO bioavailability, and increased interstitial collagen deposition. 2 , 23 These intersecting pathways result in reduced coronary vasodilation and coronary microvascular dysfunction, and thus suggest a potentially important role of VCAM‐1 in HFpEF pathogenesis. 24 Indeed, there is growing evidence for endothelial activation and VCAM‐1 in HFpEF across experimental and epidemiologic studies. Compared with normal myocardial tissue, VCAM‐1 expression is higher in myocardial tissue of HFpEF 8 and patients with HFpEF have lower coronary microvascular density. 25 In addition, higher serum levels of CAMs in young adults are associated with lower left ventricular global longitudinal strain in later life, 10 suggesting that activation of endothelial cells and its downstream consequences lead to left ventricular dysfunction, a preclinical stage of HF. Our study findings further substantiate the potential mechanistic role of VCAM‐1 in HF through demonstration of the longitudinal association of VCAM‐1 with incident HF hospitalization.
We demonstrate for the first time, to our knowledge, the prospective associations of baseline VCAM‐1 with symptomatic HF, as determined by incident HF hospitalization, across the spectrum of EF. Consistent with multiple epidemiologic studies, the definition of incident HF hinges primarily on hospitalization for symptoms in the MESA cohort. Indeed, such definitions are highly specific for HF, as individuals with severe symptoms are most likely to require hospitalization. In addition, the requirement of evidence for imaging, treatment, or physician diagnosis of HF within the medical record further enhances the specificity of adjudicated HF events within the MESA cohort. It is possible that such rigorous adjudication may not detect early or mild HF, in which symptoms are not sufficiently severe to warrant hospitalization. However, the exclusion of such cases would tend to bias our results toward the null. In addition, in light of our previous findings, which demonstrated associations of CAMs with preclinical HFpEF, our current findings further substantiate the association of CAMs, and specifically VCAM‐1, with development of HF across the clinical spectrum, from stage B HF (ie, cardiac functional derangement but lack of symptoms) to severe stage C HF (ie, symptoms requiring hospitalization).
There was no association of pericardial fat with VCAM‐1 levels, providing insight into the relationship between regional adipose tissue and endothelial activation. Pericardial fat is associated with adverse cardiovascular structure and function and increased risk of several cardiovascular diseases, including HF. 26 Furthermore, individuals with HFpEF and pericardial fat had a higher prevalence of atrial fibrillation and diabetes mellitus along with more severe derangements in biomarkers of myocardial injury. 27 Given these findings and the known proinflammatory effect of adipose tissue, it has thus been suggested that pericardial adipose tissue specifically may be involved in HFpEF pathogenesis through regional, cardiac‐specific inflammatory signaling. 6 It is possible that such regional inflammation may drive endothelial activation and subsequent VCAM‐1 expression at the level of the heart. Although pericardial fat was associated with serum VCAM‐1 in unadjusted analyses, there was no significant association after cardiovascular risk factor adjustment. Although these findings suggest that systemic inflammation attributable to cardiovascular risk factors, and not uniquely pericardial fat, may drive VCAM‐1 elevation, pericardial fat volume does not reflect the nature of pericardial fat composition or the metabolic activity of pericardial fat. The associations of pericardial fat with adverse cardiovascular outcomes in previous investigations may be explained by mechanisms independent of endothelial activation, including activation of the renin‐angiotensin‐aldosterone axis. 28
On categorization of HF events by prespecified EF categories, VCAM‐1 was significantly associated with incident HFpEF hospitalization; the effect estimate for HFrEF was similar, although nonsignificant. However, given the low incidence rate of HFrEF events in our study, these results should be interpreted with caution. Such low rates of HFrEF events may limit the power to detect true associations of VCAM‐1 and HFrEF (type II error). Further investigations in populations with higher HFrEF event rates may be useful to confirm these findings. Despite this, the distinct association of VCAM‐1 with incident HFpEF in our study is consistent with our primary hypothesis and is in line with proposed mechanistic paradigms of HFpEF pathogenesis. Although chronic, systemic risk factors drive both HF syndromes, they may play a more influential role in HFpEF development because HFrEF may be additionally influenced by acute, direct myocardial injury (eg, acute myocardial infarction). 29 Such acute events do not appear as influential in driving HFpEF, and, therefore, the downstream effects of chronic comorbidity burden appear to be particularly important. Endothelial activation is a consequence of chronic exposure to inflammatory cardiovascular risk factors, 10 including obesity, and thus may be particularly involved in HFpEF development. Indeed, CAMs are more highly expressed in myocardial tissue of HFpEF compared with HFrEF specimens. 9 Our findings, in light of previous investigations, suggest that expression of VCAM‐1 may play a more influential role in microvascular endothelial‐cardiomyocyte interaction (ie, substrate for HFpEF) than for macrovascular plaque stability (ie, substrate for HFrEF). Taken together, our long‐term, observational study substantiates previous tissue‐based molecular findings about the specific pathogenic role of endothelial activation in HFpEF.
Our study has strengths and limitations. MESA participants represent a large, ethnically diverse, community‐based cohort and have been well characterized through longitudinal in‐person examinations, accompanied by rigorous, blinded event adjudication. Despite this, several participants were excluded from the current analysis because of lack of VCAM‐1 measurement. Participants excluded from this analysis represented a somewhat higher‐risk group with more cardiovascular risk factors, and their exclusion would likely bias our results toward the null. Further investigation is required to understand if serum VCAM‐1 levels are reflective of VCAM‐1 activity and expression at the level of the coronary microvasculature. Although we adjusted for various demographic, clinical, and laboratory covariates, our findings remain subject to potential residual confounding. Incident HF hospitalizations (and their subtypes) were relatively low in this multiethnic cohort, and the requirement of hospitalization for HF adjudication prevents detection of mild, outpatient cases of HF, which may have limited power to detect interactions by various subgroups (type II error). However, such events were carefully adjudicated by an independent coordinating center and are thus highly specific for true, clinically severe HF. Further investigations among populations with higher HF event rates may be required to verify our findings of specific associations of VCAM‐1 with incident HFpEF, to identify potential high‐risk subgroups, and to confirm our finding of a stronger association between VCAM‐1 and incident HF among current/former smokers. Finally, further investigations are required to understand drivers of cellular adhesion molecule expression, the role of cumulative VCAM‐1 exposure (as defined by serial measurements) in HF risk, and clinically meaningful changes in VCAM‐1 over one's lifetime.
In a multiethnic, community‐based cohort, baseline serum VCAM‐1 was significantly associated with incident HF hospitalization. The risk of HF increased progressively with higher levels of VCAM‐1, with plateauing of risk at the highest levels of this biomarker. Our findings suggest that higher VCAM‐1 may be particularly associated with incident HFpEF. The association of VCAM‐1 with incident HF appears stronger among individuals with a history of smoking. Taken together, our findings substantiate the potential pathogenic roles of endothelial activation, cellular adhesion molecules, and microvascular coronary inflammation in driving symptomatic HFpEF and HF hospitalization. Therapies aimed to reduce endothelial activation and specifically VCAM‐1 may prevent incident HF among patients at risk.
Sources of Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 and grant R01 HL98077 from the National Heart, Lung, and Blood Institute, and by grants KL2TR001424, UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences.
Disclosures
Dr Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis, and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Cardiora, Eisai, Ironwood, Merck, MyoKardia, Novartis, Sanofi, and United Therapeutics. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA (Multi‐Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2020;9:e019390 DOI: 10.1161/JAHA.120.019390.)
This work was presented as an abstract at the American Heart Association's Scientific Sessions, November 13 to 17, 2020.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;591–602. [DOI] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;263–271. [DOI] [PubMed] [Google Scholar]
- 3. Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi‐Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine‐induced endothelial protein that binds to lymphocytes. Cell. 1989;1203–1211. [DOI] [PubMed] [Google Scholar]
- 4. Croft D, McIntyre P, Wibulswas A, Kramer I. Sustained elevated levels of VCAM‐1 in cultured fibroblast‐like synoviocytes can be achieved by TNF‐alpha in combination with either IL‐4 or IL‐13 through increased mRNA stability. Am J Pathol. 1999;1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh RJ, Mason JC, Lidington EA, Edwards DR, Nuttall RK, Khokha R, Knauper V, Murphy G, Gavrilovic J. Cytokine stimulated vascular cell adhesion molecule‐1 (VCAM‐1) ectodomain release is regulated by TIMP‐3. Cardiovasc Res. 2005;39–49. [DOI] [PubMed] [Google Scholar]
- 6. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;2360–2372. [DOI] [PubMed] [Google Scholar]
- 7. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;44–52. [DOI] [PubMed] [Google Scholar]
- 9. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite‐Moreira AF, Musters R, Niessen HW, Linke WA, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;312–324. [DOI] [PubMed] [Google Scholar]
- 10. Patel RB, Colangelo LA, Reiner AP, Gross MD, Jacobs DR Jr, Launer LJ, Lima JAC, Lloyd‐Jones DM, Shah SJ. Cellular adhesion molecules in young adulthood and cardiac function in later life. J Am Coll Cardiol. 2020;2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;871–881. [DOI] [PubMed] [Google Scholar]
- 12. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wattanakit K, Folsom AR, Criqui MH, Kramer HJ, Cushman M, Shea S, Hirsch AT. Albuminuria and peripheral arterial disease: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2008;212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, et al. The association of pericardial fat with incident coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis MR, Callas PW, Jenny NS, Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2001;1495–1500. [PubMed] [Google Scholar]
- 16. Christoph MJ, Allison MA, Pankow JS, Decker PA, Kirsch PS, Tsai MY, Sale MM, de Andrade M, Sicotte H, Tang W, et al. Impact of adiposity on cellular adhesion: the Multi‐Ethnic Study of Atherosclerosis (MESA). Obesity (Silver Spring). 2016;223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juni RP, Kuster DWD, Goebel M, Helmes M, Musters RJP, van der Velden J, Koolwijk P, Paulus WJ, van Hinsbergh VWM. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC Basic Transl Sci. 2019;575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor‐alpha contributes to obesity‐related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hauner H, Bender M, Haastert B, Hube F. Plasma concentrations of soluble TNF‐alpha receptors in obese subjects. Int J Obes Relat Metab Disord. 1998;1239–1243. [DOI] [PubMed] [Google Scholar]
- 21. Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA‐126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;85–97. [DOI] [PubMed] [Google Scholar]
- 23. Duca F, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2016;e005277. [DOI] [PubMed] [Google Scholar]
- 24. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink‐Nelson L, Ljung Faxen U, Fermer ML, Broberg MA, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J. 2018;3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE, Jensen MK, Koch M, Allison M, Kawel‐Boehm N, et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the Multi‐Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2017;1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail. 2018;1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huby AC, Antonova G, Groenendyk J, Gomez‐Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemele EJ. Adipocyte‐derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;2134–2145. [DOI] [PubMed] [Google Scholar]
- 29. Sanders‐van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner‐La Rocca HP; TIME‐CHF Investigators . Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;1006–1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


