Abstract
Depression in patients with cardiovascular disease is independently associated with progression of heart disease, major adverse cardiac events, and mortality. A wide variety of depression treatment strategies have been studied in randomized controlled trials as the field works to identify optimal depression treatments in this population. A contemporary scoping review of the literature can help to consolidate and synthesize the growing and disparate literature on depression treatment trials in people with cardiovascular disease. We conducted a scoping review utilizing a systematic search of the literature via 4 databases (PubMed, PsycINFO, EMBASE, and Google Scholar) from database inception to March 2020. We identified 42 relevant randomized controlled trials of depression treatment interventions in patients with cardiac disease (n=9181 patients with coronary artery disease, n=1981 patients with heart failure). Selective serotonin reuptake inhibitors appear to be safe in patients with cardiac disease and to have beneficial effects on depression (and some suggestion of cardiac benefit) in patients with coronary artery disease, with less evidence of their efficacy in heart failure. In contrast, psychotherapy appears to be effective for depression in coronary artery disease and heart failure, but with less evidence of cardiac benefit. Newer multimodal depression care management approaches that utilize flexible approaches to patients' care have been less studied but appear promising across cardiac patient groups. Selective serotonin reuptake inhibitors may be preferred in the treatment of patients with coronary artery disease, psychotherapy may be preferred in heart failure, and more flexible depression care management approaches have shown promise by potentially using both approaches based on patient needs.
Keywords: antidepressants, collaborative care, coronary artery disease, depression, heart failure, psychotherapy
Subject Categories: Heart Failure, Coronary Artery Disease, Acute Coronary Syndromes, Cardiovascular Disease, Mental Health
Nonstandard Abbreviation and Acronym
- MDD
major depressive disorder
Heart disease is responsible for >30% of deaths worldwide each year. 1 , 2 Among patients with coronary artery disease (CAD), depression is common. Approximately 30% of patients with CAD have elevated depressive symptoms, and 15% to 20% meet criteria for major depressive disorder (MDD), 3 a rate that is 2 to 3 times higher than in the general population. Depression prevalence is comparably elevated in patients with heart failure (HF). 4
Depression is prospectively associated with adverse cardiac outcomes, independent of other cardiovascular risk factors. Among initially healthy people, depression is associated with a higher incidence of new‐onset heart disease than those without depression. 5 , 6 Among those with existing cardiac disease, including CAD, 7 recent acute coronary syndrome (ACS), 8 and coronary artery bypass graft surgery, 9 depression is independently associated with higher rates of recurrent events and death. Based on the large body of evidence linking depression and adverse outcomes in those with an ACS, the American Heart Association has declared depression to be an independent risk factor for poor prognosis following ACS. 8 Likewise, patients with HF who are depressed have increased healthcare utilization, more frequent hospitalizations, and greater mortality, independent of HF severity, 4 and depression is linked with greater recurrence of atrial fibrillation. 10
Moreover, depressive symptoms are often chronic and recurrent in patients with heart disease, 3 and those with untreated or undertreated depression are more likely to have adverse outcomes, including mortality. 11 , 12 , 13 , 14 Reciprocally, some observational studies have found that depression treatment is linked to better outcomes and lower rates of cardiovascular mortality. 15 , 16 , 17 The mechanism by which depression is associated with adverse cardiac outcomes is likely multifactorial, including both physiologic and behavioral factors. 13 , 18
The prevalence of depression, and its impact on prognosis, among people with cardiovascular disease (CVD) highlight an opportunity to optimally treat depression to improve quality of life and potentially prevent adverse cardiac outcomes. Though the relationship between depression and heart disease is by now well established, there remain important practical questions about safe and effective management of depression in this population. There have been a wide variety of approaches to address depression in those with heart disease, with conflicting evidence on the efficacy and clinical impact of such approaches on both psychiatric symptoms and cardiac‐related morbidity.
In this contemporary review, we aim to provide an up‐to‐date overview of the latest evidence regarding interventions to treat depression among patients with cardiac disease. We have limited our focus to randomized clinical trials (RCTs) using antidepressants, psychotherapy, and depression care management programs in people with heart disease.
Methods
We chose to conduct a scoping review to take a more systematic, rigorous approach than a narrative review, while examining a more heterogeneous topic (eg, numerous different depression treatments across patients with different CVDs) than one typically examined in a more precise systematic review, which often is more narrowly focused on a very specific topic. 19 We chose to examine only RCTs in this review given that there have been numerous RCTs testing depression treatments within each main category (eg, antidepressants, psychotherapy) in patients with heart disease, allowing greater consistency within the review and optimizing synthesis across studies, and given that RCTs are considered the criterion standard for efficacy assessment. Regarding the AHA Transparency and Openness Promotion (TOP) Guidelines related to sharing of data and study materials, no original data were generated as part of this scoping review.
We utilized published guidelines for the conduct of a scoping review. 20 , 21 Specifically, to identify RCTs focused on the management of depression in people with heart disease, we conducted systematic literature searches in the following databases: PubMed, PsycINFO, and EMBASE; we also searched Google Scholar using the top 200 references by relevance. 22 We utilized a broad search of cardiac conditions to ensure identification of all trials in heart disease. Specifically, we included a combination of terms such as: (“psychotherapy” OR “antidepressants” OR "stress management" OR “care management”) AND depression AND ("coronary artery disease" OR "acute coronary syndrome" OR "myocardial infarction" OR "heart failure" OR "arrhythmia" OR "heart disease" OR "cardiovascular disease") using database tools to limit identification to intervention trials. We then used a “snowball” technique to include relevant citations within articles if deemed relevant to the review. 23
Articles were included in the review if they (1) were RCTs; (2) examined psychological or psychiatric interventions—defined as those delivered by healthcare professionals, aimed at reducing depressive symptoms (this excludes interventions such as yoga, tai chi, meditation, and related interventions, which promote well‐being but are not typically aimed at the management of depression and are beyond the scope of this review); (3) reported outcome measures that included depressive symptom burden and/or cardiac or medical outcomes; and (4) were written in English. Databases were searched from the time of inception to March 2020.
After screening and selecting articles that met the study criteria, the following data were extracted: (1) reference or study name; (2) population; (3) study design, intervention, and primary aim; (4) psychiatric outcomes; (5) cardiac outcomes; and (6) findings.
The search was conducted by the lead author (J.Z.). After the initial search, the lead author excluded duplicate and irrelevant articles based on the title, then reviewed the abstracts of relevant ones. Full texts of all potentially eligible articles were obtained and reviewed independently by both the lead and senior authors (J.Z. and J.H.), and any disagreements about eligibility were resolved through discussion by these authors. Once the list of articles was finalized, the first author led data extraction, and the senior author reviewed the abstracted data and identified areas for additional clarification/extraction as needed. At this stage, study tables were generated, followed by another round of data confirmation and feedback between these 2 authors. Additional study authors (C.C. and J.J.) provided feedback and clarification at this stage when data from the articles were unclear.
Results
A systematic search of the databases yielded 646 matches (Figure). There were 565 unique articles identified; after review for relevance and match with review criteria, 144 abstracts and 63 full articles were reviewed. Of those, 27 articles were excluded because they did not meet inclusion criteria. Using the snowball technique, 6 additional articles were identified and included. The final review included 42 articles examining a total of 9181 patients with CAD and 1981 with HF. We classified the interventions as antidepressant trials (n=20), psychotherapy interventions (n=14), and care‐management and stepped‐care programs (n=8). We opted to divide interventions into those aimed at treating depression in patients with CAD (eg, those with stable CAD or recent ACS; Table 1) 24 and those for patients with HF (Table 2), because of differences in populations and overall intervention effectiveness. No relevant trials were identified that examined solely patients with arrhythmias.
Figure 1. Search results and articles selection procedures.
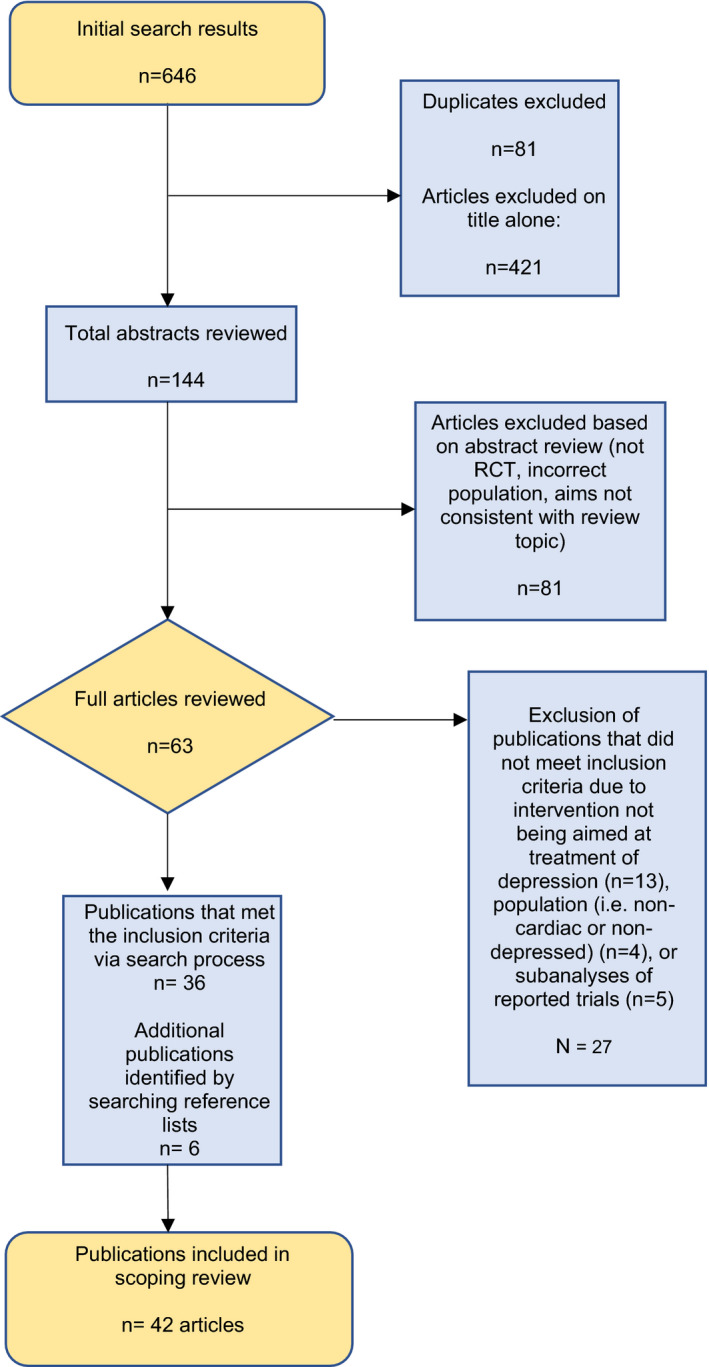
RCT indicates randomized clinical trial.
Table 1.
Psychiatric and Psychological Interventions for Depression in Patients With CAD
| Reference or Study Name | Population | Study Design, Intervention, and Primary Aim | Main Psychiatric Outcomes | Main Cardiac and Medical Outcomes | Findings |
|---|---|---|---|---|---|
| Antidepressants | |||||
| SSRIs | |||||
| SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) 25 | 369 post‐ACS patients with MDD | 24‐wk RCT comparing sertraline (50–200 mg) to placebo, aimed at evaluating safety and efficacy of sertraline for MDD in patients with ACS | 17‐item HAM‐D*, CGI‐I* | LVEF†, heart rate, blood pressure, ECG, ventricular premature complexes, heart rate variability, severe cardiovascular events |
No effect on LVEF (primary outcome) or surrogate cardiac markers. Intervention was associated with greater improvements in CGI‐I score (P=0.049) and CGI‐I response (67% sertraline vs 53%; OR, 3.11; 95% CI [2.08–4.63]; P=0.01). No difference in HAM‐D scores in overall sample. CGI‐I response was higher in the group with at least 1 prior episode of depression (72% vs 51%; P=0.003) and in the more severe MDD group (78% vs 45%; P=0.001), and HAM‐D scores were significantly better in both of these subgroups (P<0.01). No differences in rates of severe cardiovascular events (14.5% sertraline vs 22.4%; RR, 0.77, 95% CI [0.51–1.16]) |
| Pizzi et al 26 | 100 patients with CAD and depression | 20‐wk RCT of sertraline (50–200 mg) vs placebo to assess the effects of sertraline on endothelial function and inflammatory markers | BDI*, † | C‐reactive protein, interleukin‐6, endothelial function (flow‐mediated dilation) |
Intervention significantly reduced the BDI score (MD [SD] 8 [7] vs 2 [6] P<0.001), levels of C‐reactive protein (MD 0.4 vs 0.0 mg/dL; P<0.001), interleukin‐6 (MD 0.6 vs 0.0 pg/mL; P<0.001), and fibrinogen (MD 37 vs 7 mg/dL; P<0.001). There was a significant improvement in flow‐dependent endothelium‐mediated dilation (−1.9 vs 0.0; P<0.001) |
| EsDEPACS (Escitalopram for Depression in ACS) 27 , 28 | 300 patients with recent ACS and MDD or minor depression | 24‐wk placebo‐controlled trial of escitalopram (5–20 mg) evaluating efficacy and safety. 1‐y follow‐up and 8‐y follow‐up were conducted | HAM‐D*, † | MACE |
Escitalopram was superior to placebo in reducing HAM‐D scores (MD=2.3; 95% CI [0.7–4.0]; P=0.016, effect size=0.38) after 1 y. MACE was significantly less in intervention group after median 8.1 y of follow‐up (40.9% vs 53.6%; HR, 0.69; 95% CI [0.49–0.96]; P=0.03) |
| Understanding Prognostic benefits of Exercise and Antidepressant Therapy for Persons with Depression and Heart Disease (UPBEAT) 29 | 101 patients with CAD and elevated depressive symptoms | 4‐mo, 3‐arm RCT comparing aerobic exercise (3 times per wk), sertraline, and placebo in a 2:2:1 ratio–assess efficacy in reducing depressive symptoms | 17 item HAM‐D*, † | Heart rate variability, aerobic capacity, flow‐mediated vasodilation, blood pressure, Platelet factor 4, betathromboglobulin |
The exercise (MD −7.5; 95% CI [9.8 to −5.0]) and sertraline (MD −6.1; 95% CI [−8.4 to −3.9]) groups achieved larger reductions in depressive symptoms compared with placebo (MD −4.5; 95% CI [−7.6 to −1.5]); P=0.034, with no significant differences between exercise and sertraline. Exercise and sertraline had nearly significant improvements in heart rate variability compared with placebo (P=0.052); exercise resulted in somewhat greater improvements in heart rate variability compared with sertraline (P=0.09). There were no group differences in other markers |
| CREATE (Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy) 24 , 30 | 284 patients with CAD and MDD |
12‐wk RCT of a 2×2 factorial trial comparing 12 wk of citalopram (20–40 mg) with placebo, and weekly in person or phone therapist‐administered IPT (mean 48 min)+clinical management to clinical management alone; assessing efficacy in reducing depressive symptoms. Also assessed the effect on cardiac and inflammatory markers |
24‐item HAM‐D*, † |
Blood pressure, ECG, MACE P‐selectin, beta‐thromboglobulin, soluble intercellular cell adhesion molecule‐1, and total nitric oxide |
Citalopram was superior to placebo in reducing 12‐wk HAM‐D scores (MD −3.3; 96.7% CI [0.80–5.85]; P=0.005). Mean HAM‐D difference favored clinical management over IPT (−2.26 points; 96.7% CI [−4.78 to 0.27]; P=0.06). No between‐group differences in MACE, blood pressure, or electrocardiographic measures, including QTc intervals. Citalopram was associated with greater increase in total nitric oxide (P=0.005). There were no group differences on the other biomarkers |
| MIND‐IT (Myocardial Infarction and Depression‐Intervention Trial) 31 , 32 | 331 patients with recent MI and depression |
RCT comparing a flexible 6‐mo depression management intervention (mirtazapine, citalopram, or psychiatrist‐recommended management) with usual care in the evaluation of antidepressants in treating depression. 8‐y follow‐up |
BDI*, †, ICD‐10 depression diagnoses | MACE†, mortality |
There were no between‐group differences in BDI depression scores (P=0.45) or ICD‐10 diagnoses (P=0.68) at 18‐mo follow‐up. There was no difference in cardiac events at 18 mo (OR=1.07; 95% CI [0.57–2.00]). At 8 y, the intervention was not associated with lower risk of cardiovascular events or mortality (HR, 0.97; 95% CI [0.67–1.40]) or all‐cause mortality (HR, 0.74 (95% CI [0.41–1.33]) Regardless of randomization status, patients who received depression treatment had reduced all‐cause mortality compared with those who did not receive treatment (HR, 0.52; 95% CI [0.28–0.97]; P=0.041) |
| Other antidepressants | |||||
| Honig et al 33 (Nested RCT within MIND‐IT) | 91 post‐MI patients with MDD | 24‐wk RCT comparing 24 wk of mirtazapine (15–45 mg) with placebo to assess efficacy in this population | HAM‐D*, †, BDI*, dSCL‐90*, CGI* | Intervention was superior to placebo on change in HAM‐D scores (MD 0.20; P=0.003), BDI (MD 0.58; P=0.05), and CGI (MD 0.55; P=0.007), but not dSCL‐90 (MD 0.35; P=0.11) when using a mixed‐effect model at 24 wk | |
| Nelson et al 34 | 81 patients with CAD and MDD | 6‐wk RCT of paroxetine (20–30 mg) or nortriptyline (enough to reach plasma concentration of 50–150 ng/mL) to assess safety and efficacy of paroxetine in this population | 17‐item HAM‐D*, † | Adverse events leading to discontinuation |
Both groups had nonsignificant improvements in HAM‐D scores, with no significant differences between groups. Significantly more patients in the nortriptyline group discontinued treatment prematurely (35% vs 10%, χ2=6.08; P<0.02), and more patients taking nortriptyline had adverse events resulting in termination (25% vs 5%, χ2=5.00; P<0.05) |
| Carney et al 35 | 122 patients with CAD and MDD | 10‐wk RCT comparing omega‐3 fatty acids (EPA/DNA) or placebo added to 50 mg sertraline to determine effectiveness of omega‐3 in improving sertraline's effect on depressive symptoms | BDI*, †, HAM‐D*, † | There were no between‐group differences in change in depression scores at 10 wk BDI (P=0.44) and HAM‐D (P=0.9) | |
| Psychotherapy | |||||
| ENRICHD (Enhancing Recovery in Coronary Heart Disease) 16 , 36 | 2481 post‐MI patients with MDD or low social support | 6‐mo CBT RCT of individual and group, in‐person, therapist‐delivered CBT (mean of 11 sessions), supplemented by sertraline for severe or persistent symptoms, compared with usual care to determine effect on mortality and recurrent MI | 17‐item HAM‐D*, (ENRICHD) BDI* | Composite primary end point of death or recurrent MI† |
Intervention had no significant effect on event‐free survival (75.8% intervention vs 75.9% usual care, HR 1.01; 95% CI [0.86–1.18]) over a mean of 29 mo. Intervention group led to greater improvement in HAM‐D scores (MD‐1.5, 95% CI [−2.3 to −0.1]) and BDI scores (−2.8 95% CI [−3.7 to −2.0]), after 6 mo, both P<0.001 Risk of death or recurrent MI (adjusted HR 0.57; 95% CI [0.38–0.84]) and all‐cause mortality at mean follow‐up of 29 mo (adjusted HR, 0.59; 95% CI [0.37–0.96]) was significantly lower in patients taking SSRIs compared with patients who did not in either condition |
| Lv et al 37 | 75 young and middle‐aged patients (mean age, 52.2±6.2 y) with CAD and anxiety or depression. | 8‐wk CBT intervention consisting of weekly, hour‐long, in‐person, therapist‐delivered CBT vs usual care to investigate effect on mental status and quality of life | 17‐item HAM‐D*, † | None | Intervention significantly reduced HAM‐D 17 scores (MD 3.4, 95% CI [1.46–5.34]; P=0.001) |
| Freedland et al 38 | 123 patients undergoing coronary artery bypass graft (CABG) surgery with depression | 12‐wk, 3‐arm RCT of CBT, stress reduction, and treatment as usual. CBT consisted of a weekly, individual, in‐person, therapist‐delivered intervention. They investigated efficacy in treatment of depression | 17‐item HAM‐D*, †, BDI* | None |
Remission on HAM‐D was higher at 3 mo in CBT group (71%) and supportive stress‐management (57%) vs usual care group (33%) (χ2 12.22; P=0.002) and at 9 mo (73%, 57%, and 35%, respectively; χ2 12.02; P=0.003). They did not differ at 6 mo. CBT was superior to usual care in BDI (P=0.005) |
| Glozier et al 39 | 562 patients with CVD or high CVD risk and mild‐to‐moderate depression | 12‐wk RCT of internet‐delivered CBT (weekly, 30–60‐min modules) vs an attention‐matched health information package to test the effectiveness of CBT on depressive symptoms and adherence to medical advice | PHQ‐9*, † | Medical Outcomes Study (Measures of Patient Adherence Scale), IPAQ (physical activity) |
Intervention group had greater improvement in PHQ‐9 scores (MD 1.06 95% CI [0.23–1.89]; P=0.01), with increasing differences over the intervention period (P=0.012) Intervention led to greater adherence to health behaviors (MD 2.6; 95% CI [0.33–3.99], P=0.02) There was a greater proportion engaging in activity sufficient to confer a health benefit (OR, 1.91; 95% CI [1.01–3.61]) |
| Johansson et al 40 | 144 patients with CVD and depression | 9‐wk program of weekly nurse‐guided internet‐delivered CBT (iCBT) or an active control participating in a Web‐based discussion forum, to evaluate effectiveness in reducing depressive symptoms | PHQ‐9*, †, MADRS‐S* | None | Intervention group had a significant improvement in PHQ‐9 scores (MD=−2.34; 95% CI [−3.58 to −1.10], P<0.001) and MADRS‐S (MD −5.58, 95% CI [−7.72 to −3.44]; P<0.001) |
| U‐CARE 41 | 239 post‐MI patients with depressive symptoms | 14‐wk therapist‐guided, weekly iCBT vs usual care to evaluate reduction in self‐reported symptoms of depression and anxiety | HADS*, †, MADRS* | None |
HADS scores decreased over time in the total study sample with no difference between the study groups (β=−0.47, 95% CI [−1.95 to 1.00]; P=0.53), and similarly no difference in MADRS P=0.48 Treatment adherence was low (46.2% of intervention group did not complete the introductory module) |
| MoodCare 42 , 43 | 121 patients with ACS and mild‐to‐moderately severe depression | 6‐mo RCT of 10 sessions of telephone‐delivered CBT and risk‐reduction program delivered by master‐level psychologists compared with usual medical care; measuring efficacy and feasibility in reducing depression and improving quality of life | PHQ‐9*, †, Cardiac Depression Scale* | None |
Intervention led to significantly reduced PHQ‐9 scores (MD −1.8; 95% CI [−0.2 to −3.4]; P=0.025) compared with usual care, but no difference on cardiac depression scale (P=0.558) Results were more pronounced for those with a history of depression (PHQ‐9: MD −2.7 [1.32]; P=0.043) at end of intervention and 1‐y follow‐up (P=0.012). At 12‐mo follow‐up, beneficial treatment effects were only observed in those with MDD at baseline (mean score 6.5, 95% CI [4.9–8.0] vs 9.3, 95% CI [7.7–10.9]) |
| Oranta et al 44 | 103 patients with recent MI | 1–6 sessions of nurse‐delivered interpersonal counseling (30 min) in person or by phone to evaluate effect on depressive symptoms and distress | BDI*, † | None | Intervention led to statistically improved depressive symptoms (BDI; OR=0.31; 95% CI [0.16–0.61]; P=0.001) compared with the control group at 18‐mo follow‐up |
| SPIRR‐CAD (Stepwise Psychotherapy Intervention for Reducing Risk in Coronary Artery Disease) 45 | 570 patients with CAD and elevated depression symptoms | Randomized trial of stepwise psychotherapy (3 1:1 supportive‐expressive psychotherapy sessions with 25 group sessions for those still depressed) vs 1 information session in evaluating effectiveness in reducing depressive symptoms | HADS*, †, HAM‐D* | None |
Intervention did not lead to significant group difference in change of depressive symptoms on HADS at 18 mo (MD −0.2 95% CI [−0.8 to 0.4]; P=0.44). There were no significant differences on HAM‐D. There was greater improvement in depressive symptoms (P=0.026) and a trend for greater improvement with the intervention in patients with Type D personality (n=341, P=0.057) |
| Care management intervention trials | |||||
| Bypassing the Blues 46 | 302 post‐coronary artery bypass surgery patients with elevated depressive symptoms | 8‐mo RCT comparing phone‐, nurse‐delivered collaborative care depression management to enhanced usual care (collaborative care for cardiac diagnosis) to evaluate effectiveness in treating depression | SF‐36† (Health‐related quality of life); 17‐item HAM‐D* | DASI (physical function) |
Intervention patients had greater improvements in mental health–related quality of life (MD 3.2; 95% CI [0.5–6.0]; P=0.02). There was a significant improvement in depression scores in the intervention group vs usual care (MD 3.1; 95% CI [1.3–4.9]; P=0.001). There was a significant improvement in physical function in intervention group compared with usual care (MD 4.6; 95% CI [1.9–7.3]; P=0.001) |
| TrueBlue 47 | 400 patients with type 2 diabetes mellitus, coronary heart disease, or both and elevated depression scores | 6‐mo randomized cluster trial comparing a nurse‐delivered collaborative care depression management intervention to enhanced usual care to evaluate effectiveness in reducing depression | PHQ‐9*, † | 10‐y cardiovascular disease risk |
Mean depression scores decreased significantly in intervention group compared with control (MD 5.7±1.3 vs 4.3±1.2; P=0.012). Improvements were sustained after 12 mo. There was a significant (P=0.015) decrease in 10‐y cardiovascular disease risk from 27.4±3.4% to 24.8±3.8% at 12 mo |
| SUCCEED (Screening Utilization and Collaborative Care for More Effective and Efficient Treatment of Depression) 48 | 175 patients with ACS, HF, or arrhythmia and depression | 12‐wk RCT of an inpatient initiated phone‐based, nurse‐delivered collaborative care depression management program compared with enhanced usual care to assess impact on depression, other psychiatric symptoms, and selected medical outcomes | PHQ‐9*, †, SF‐12 mental component score†, HADS‐A (anxiety)†, CPFQ (cognitive symptoms of depression)† | Cardiac symptom scale†, cardiac readmissions |
Collaborative care subjects had significantly greater improvements on all mental health outcomes at 12 wk, including rates of depression response on PHQ‐9 (51.5% vs 34.4%; OR, 2.02; P=0.04 at 12 wk). At 6 mo, intervention subjects had significantly reduced number and intensity of cardiac symptoms. Intervention group did not have significant improvement on SF‐12 MCS (P=0.072), HADS‐A (P=0.099), or CPFQ (P=0.12) compared with placebo at 6 mo. There was no between‐group difference in cardiac rehospitalizations at 6 mo |
| MOSAIC (Management of Sadness and Anxiety in Cardiology) 49 | 183 patients with ACS, HF, or arrhythmia and depression, panic disorder, or generalized anxiety disorder | 24‐wk RCT of an inpatient initiated, phone‐delivered, collaborative care depression and anxiety management program compared with enhanced usual care | SF‐12 mental component score†, PHQ‐9* | DASI (physical function), cardiac readmissions |
Intervention patients had significantly greater improvements in depression (PHQ‐9: MD −2.05, 95% CI [−4.06 to −0.05]; P =0.045), SF‐12 MCS (MD 5.68; 95% CI [2.14–9.22]; P=0.002), and physical function at 24 wk (MD 5.59, 95% CI [1.71–9.46]; P=0.005). There were no significant differences in cardiac readmission rates at 6 mo (32% vs 33%; P=0.83); however, among patients who were readmitted, mean time to readmission was longer in the intervention (92.4 vs 62.5 d; P=0.02 |
| TEAMCare 50 | 214 patients with type 2 diabetes mellitus and/or CAD and depression | 12‐mo RCT of collaborative care management for depression and medical conditions to examine effectiveness in controlling risk factors associated with multiple diseases | SCL‐20* | Hemoglobin A1c, systolic blood pressure, LDL cholesterol level |
Intervention was associated with significant improvements in hemoglobin A1c (MD 0.58%; 95% CI [−0.85 to −0.27]), LDL cholesterol levels (MD 6.9; 95% CI [−17.5 to −0.8] mg/dL), systolic blood pressure (MD 5.1 mm Hg, 95% CI [−6.9 to 0.1]), all P<0.001. Depression scores also significantly improved (MD 0.40 points; 95% CI [−0.56 to −0.26]; P<0.001), compared with usual care |
| COPES (Coronary Psychosocial Evaluation Studies) 51 | 157 post‐ACS patients with elevated depressive symptoms | 6‐mo RCT of a patient preference–based stepped depression care management program, remotely delivered by a clinical nurse specialist, psychologist, social worker, and/or psychiatrist, compared with enhanced usual care to evaluate satisfaction with depression care and improved depressive symptoms | Patient satisfaction with depression care†, BDI* | MACE |
Care management decreased BDI scores significantly more (MD 5.7; 95% CI [−7.6 to −3.8] vs 1.9; 95% CI [−3.8 to −0.1]; P=0.005) and intervention group was more satisfied with treatment (OR, 5.4; 95% CI [2.2–12.9]; P<0.001) when compared with usual care. Intervention was associated with less MACE (4% vs 13%; log‐rank test, χ2 (1)=3.93 [P=0.047]) |
| COADIACS (Comparison of Depression Interventions After Acute Coronary Syndrome) 52 | 150 post‐ACS patients with elevated depression symptoms | 6‐mo RCT of depression stepped‐care management intervention (phone or internet) vs usual care evaluating effectiveness in reducing depressive symptoms | BDI*, † | None | Care management led to significantly reduced depressive symptoms when compared with usual care (BDI; MD −3.5; 95% CI [−6.1 to −0.7]; P=0.01) |
| CODIACS QoL 53 | 1500 patients with ACS |
Three‐arm RCT comparing: (1) systematic depression screening with stepped depression care management for those with positive depression screens, (2) systematic depression screening with PCP notification for those with positive screens, or (3) usual care with no screening. Aim was to evaluate effect of systematic depression screening with and without provision of enhanced depression care on quality‐adjusted life‐years and depressive symptoms |
Quality‐adjusted life‐years in SF‐12†, depression‐free days on CESD‐10*, PHQ‐8* | Adverse effects, mortality |
Intervention led to no group‐related differences in mean (SD) change in quality‐adjusted life‐years (P=0.98) or cumulative mean (SD) depression‐free days (P=0.63) when compared with no screening. Only (7.1%) had elevated 8‐item Patient Health Questionnaire scores indicating depressive symptoms at screening No group differences in mortality |
ACS indicates acute coronary syndrome; BDI, Beck Depression Inventory; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CBT, cognitive behavioral therapy; CESD‐10, Center for Epidemiologic Studies Depression scale; CGI, Clinical Global Impression scale; CGI‐I, Clinical Global Impression Improvement scale; CPFQ, Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire; CVD, cardiovascular disease; DASI, Duke Activity Status Index; dSCL‐90, Symptom Check List 90 items; HADS, Hospital Anxiety and Depression Scale; HAM‐D, Hamilton Depression scale; HF, heart failure; HR, hazard ratio; ICD‐10,International Classification of Diseases, Tenth Revision; IPAQ, International Physical Activity Questionnaire; IPT, interpersonal therapy; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MACE, Major Adverse Cardiac Events; MADRS, Montgomery‐Asberg Depression Rating Scale; MADRS‐S, Montgomery‐Asberg Depression Rating Scale‐Self; MD, mean difference; MDD, major depressive disorder; MI, myocardial infarction; OR, odds ratio; PCP, primary care physician; PHQ‐8, Eight‐item Patient Health Questionnaire; PHQ‐9, Nine‐item Patient Health Questionnaire; RCT, randomized clinical trial; RR, relative risk; SCL‐20, Symptom Checklist–20; SF‐12 MCS, the Mental Component of the Short‐Form 12 Health Survey; SF‐36, 36‐item Short Form Health Survey; SSRI, selective serotonin reuptake inhibitor; and U‐CARE, Uppsala‐CARE.
Depression scale.
Main outcome.
Table 2.
Psychiatric and Psychological Interventions for Depression in Patients With HF
| Reference or Study Name | Population | Study Design, Intervention, and Primary Aim | Main Psychiatric Outcomes | Main Cardiac and Medical Outcomes | Findings |
|---|---|---|---|---|---|
| Antidepressants | |||||
| SSRIs | |||||
| SADHART‐CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) 54 | 469 patients with HF and MDD | 12‐wk RCT comparing 12 wk of sertraline (50–200 mg) with placebo evaluating safety and efficacy | 17‐item HAM‐D*, † | Composite cardiovascular score*, MACE |
Sertraline did not provide greater reduction in depression (MD −0.4; 95% CI [−1.7 to 0.92] P=0.89) at 12 wk. There were no differences in composite cardiovascular score (P=0.78) or MACE |
| MOOD‐HF (The Effects of Selective Serotonin Re‐Uptake Inhibition on Morbidity, Mortality, and Mood in Depressed Heart Failure Patients) 55 | 372 patients with HF and reduced LVEF (<45%) and MDD | 24‐mo RCT of escitalopram (10–20 mg) or placebo to assess safety and efficacy | 10‐item MADRS† | Time to all‐cause death or hospitalization* | Intervention did not significantly reduce MADRS scores (MD, −0.9; 95% CI [−2.6 to 0.7]; P=0.26) or all‐cause mortality or hospitalization (HR, 0.99; 95% CI [ 0.76–1.27]; P=0.92) |
| Fraguas et al 56 | 37 elderly (>65 y) patients with HF and depression | 8‐wk RCT of citalopram vs placebo with weekly psychiatric follow‐up evaluating efficacy | 17‐item HAM‐D*, †, MADRS† | None |
There was nonsignificant superiority of intervention over placebo in HAM‐D depression response rate (68% vs 54%; P=0.46) or MADRS (P=0.089). RCT was interrupted because of high placebo rate |
| Other antidepressants | |||||
| OCEAN (Omega‐3 Supplementation for Co‐Morbid Depression and Heart Failure Treatment) 57 | 108 patients with HF and MDD | 12‐wk 3‐arm RCT comparing a 400/200 eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) fish oil (2 g), an almost pure EPA (2 g), and placebo to assess effect on depressive symptoms, omega‐3 fatty acid levels, and other psychosocial factors | HAM‐D*, †, BDI‐II† | Red blood cell (RBC) levels of EPA*, DHA, docosapentaenoic acid (DPA), and omega‐3‐index (% of EPA/DHA in RBCs) |
There were no between‐group differences in change in depression scores at 12 wk (HAMD; P=0.74; BDI‐II; P=0.60). All omega‐3 variables were significantly elevated in the omega‐3 groups (P<0.0001) |
| Psychotherapy | |||||
| Freedland et al 58 | 158 patients with HF and MDD | 6‐mo RCT of CBT (weekly, 1‐h sessions delivered by masters or doctoral‐level therapists)+usual care vs enhanced usual care (educational materials) to determine the efficacy of intervention for depression and HF self‐care | BDI‐II*, †, Self‐Care Maintenance and Confidence subscale of the Self‐Care of Heart Failure Index*, HAM‐D† | Hospitalizations, mortality |
Intervention was associated with lower depression scores on BDI‐II (12.8 [10.6] vs 17.3 [10.7]; P=0.008) at 6 mo. Intervention also had greater remission rates on BDI‐II (46% vs 19%; number needed to treat=3.76; 95% CI [3.62–3.90]; P<0.001) and on HAM‐D (51% vs 20%; Number needed to treat=3.29; 95% CI, 3.15–3.43; P<0.001). The groups did not differ on the Self‐Care Maintenance or Confidence subscales. CBT was associated with lower rates of hospitalization at 1 y, compared with usual care (incidence rate ratio 0.47; 95% CI [0.30–0.76]; P=0.002). There was no difference in mortality. (33% of the patients were taking an antidepressant at baseline) |
| Gary et al 59 | 74 patients with HF and depression | 12‐wk 4‐arm RCT comparing a nurse‐delivered, home‐based exercise+CBT (1‐h/wk) program, with CBT alone, exercise alone, and usual care comparing effectiveness | 17‐item HAM‐D*, † | 6‐min walk test |
Intervention did not significantly reduce HAM‐D scores but was most reduced in the exercise+CBT group (MD −10.4 [3.9]) over time compared with CBT (−9.6), exercise (−7.3), and usual care (−6.2). The combined group had a significant increase in 6‐min walk distance at 24 wk compared with other groups (P=0.001). Among those with moderate‐to‐major depression, only those in combined group had sustained lower HAM‐D scores at 12 (P=0.001) and 24 wk (P=0.014), and 6‐min walk test distances were significantly greater at 12 (P=0.018) and 24 (P=0.013) wks |
| Dekker et al 60 | 41 patients with HF and depressive symptoms | RCT of a 1‐session nurse‐delivered cognitive therapy intervention, and 1 booster phone call after 1 wk to evaluate effectiveness on depressive symptoms and health outcomes after 3 mo | BDI‐II*, † | Cardiac event‐free survival |
Depression scores improve in both groups, with no significant between‐group differences (P=0.24). Intervention led to longer cardiac event‐free survival compared with control (80% vs 40%; P=0.048) |
| Care management intervention trials | |||||
| See CAD section for SUCCEED 48 and MOSAIC 49 RCTs that also included patients with HF | |||||
| Hopeful Hearts 61 | 625 patients with HF and depression and 125 nondepressed HF patients | 12‐mo RCT comparing telephone‐delivered "blended" care (collaborative care management for HF and depression) vs “enhanced usual care” (collaborative care for HF alone) to evaluate effectiveness | SF‐12 MCS* (health‐related quality of life), 17‐item HAM‐D† | Readmissions, mortality |
Intervention led to greater depression response rates at 12 mo when compared with usual care (OR 0.47, P<0.0001). Blended care also led to improvement in health‐related quality of life at 12 mo (OR 0.34, P=0.002). There was no difference in readmissions (P=0.49) or mortality (P=0.79) between groups at 12 mo |
BDI indicates Beck Depression inventory; CBT, cognitive behavioral therapy; HAM‐D, Hamilton Depression scale; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MADRS, Montgomery‐Asberg Depression Rating Scale; MD, mean difference; MDD, major depressive disorder; OR, odds ratio; RCT, randomized clinical trial; SF‐12 MCS; the Mental Component of the Short‐Form 12 Health Survey; and SSRIs, selective serotonin reuptake inhibitors.
Main outcome.
Depression scale.
Antidepressants
Across antidepressant classes, selective serotonin reuptake inhibitors (SSRIs) have been the most studied in patients with heart disease.
SSRIs in Patients With CAD
Details of included studies are outlined in Table 1. Initial placebo‐controlled trials of SSRIs aimed at evaluating depressive symptoms found these agents to be safe and largely effective in the treatment of MDD in patients with recent ACS 25 , 27 , 62 , 63 and stable CAD. 30 Glassman and colleagues evaluated sertraline in the treatment of MDD in 369 patients post‐ACS in the SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) RCT. They found sertraline to have higher clinical global improvement scores and depression response rates, but there were no between‐group differences on the Hamilton Depression Scale (HAM‐D). They did, however, find greater response rates and significant improvement on HAM‐D in patients with previous depressive episodes and more severe MDD. 25 Pizzi and associates also compared sertraline and placebo among 100 patients with CAD and depressive symptoms, and found significant improvement in depression scores compared with placebo after 20 weeks. 26 Escitalopram likewise was also found superior to placebo in reducing depressive symptoms in 300 recent patients with ACS with depression at 24 weeks and 1‐year follow‐up in the EsDEPACS (Escitalopram for Depression in ACS) trial. 27 Finally, in a smaller trial, fluoxetine was found to have greater depression response rates but no significant differences in depression symptoms in 54 patients with depression with recent myocardial infarction. 62
Along with these placebo‐controlled trials, SSRIs have also been compared with other interventions for the treatment of depression in patients with stable and unstable CAD. In the UPBEAT (Understanding Prognostic Benefits of Exercise and Antidepressant Therapy for Persons with Depression and Heart Disease) study, sertraline was compared with aerobic exercise and placebo in a 3‐arm trial in 101 patients with CAD and elevated depressive symptoms. The sertraline and exercise groups achieved larger reductions in depressive symptoms compared with placebo, with no significant differences between exercise and sertraline. 29 The CREATE (Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy) trial used a factorial design to compare citalopram with placebo, interpersonal therapy, and clinical management in 284 patients with CAD and MDD. 30 They found citalopram to be superior to placebo in reducing depressive symptoms.
Finally, the MIND‐IT (Myocardial Infarction and Depression‐Intervention Trial) was a flexible depression management trial offering pharmacological (mirtazapine or citalopram) and nonpharmacological therapy (at the discretion of the study psychiatrist) for 331 patients with depression with myocardial infarction (MI); patients in the usual‐care group were allowed to seek treatment for depression outside the study. There were no between‐group differences in depression scores at the end of the intervention. 31
Regarding the effects of SSRIs on cardiac outcomes, some, 17 , 64 but not all, 65 , 66 observational studies have observed associations between SSRI prescriptions and cardiovascular benefits. None of the above RCTs were designed or powered to measure effects on cardiac outcomes/markers at initial follow‐up time points. However, some of them had co‐primary cardiac outcomes or extended follow‐up assessments to further explore the effects of SSRIs on cardiac‐related outcomes. The SADHART trial, for example, found no adverse effect of sertraline on left ventricular ejection fraction or other surrogate cardiac markers including electrocardiographic changes, and no differences in rates of cardiovascular events. 25 Pizzi and associates observed mixed but largely negative effects of SSRIs on cardiac markers in the UPBEAT study, with nearly significant increases in heart rate variability with sertraline compared with exercise or placebo in the 3‐arm trial, but no change in other markers of inflammation, endothelial and platelet function, or baroreflex sensitivity. 29
Three studies have examined longer‐term effects on major cardiac events. Follow‐up from the EsDEPACS trial found those assigned to the escitalopram group to have lower rates of major adverse cardiac events at 8 years compared with those who had received placebo, 28 but the MIND‐IT trial found no effects on cardiac events at the end of the intervention, 31 or at 8‐year follow‐up, and no effect on all‐cause mortality. However, patients in that study who received any depression treatment (pharmacotherapy or psychotherapy/counseling) had reduced all‐cause mortality compared with those who did not, regardless of randomization status. 32
Finally, the ENRICHD (Enhancing Recovery in Coronary Heart Disease) study was a psychotherapy intervention trial (cognitive behavioral therapy [CBT] versus usual care) in 2481 post‐MI patients with MDD in which some participants in the CBT arm (those with severe or persistent depression) were offered supplemental sertraline, and those in the treatment as usual group were sometimes provided SSRIs by their primary clinicians. Among the patients with depression in either condition, risk of death or recurrent MI and all‐cause mortality was significantly reduced in patients prescribed SSRIs, compared with patients who were not (effects of the primary intervention on depression is discussed below, under Psychotherapy). 16
SSRIs for HF
Interventions for patients with HF are outlined in Table 2. Sertraline was studied against placebo in 469 patients with HF in the SADHART‐CHF (Sertraline Against Depression and Heart Disease in Congestive Heart Failure) trial. This trial examined sertraline compared with enhanced usual care that included a nurse‐facilitated support intervention. The investigators found no between‐group differences in depression reduction in depression at 12 weeks. 54 Escitalopram was also explored in the MOOD‐HF (Effects of Selective Serotonin Re‐Uptake Inhibition on Morbidity, Mortality, and Mood in Depressed Heart Failure Patients) trial on 372 patients with depression with HF and likewise found no differences in depression scores. 55 In a smaller study, Fraguas and associates studied citalopram in 37 elderly patients with HF with depression, where they found nonsignificant superiority of citalopram over placebo. 56 Regarding cardiac outcomes, the SADHART‐CHF found no effect on cardiovascular events or surrogate markers at 12 weeks 54 and the MOOD‐HF trial found no reduction in all‐cause mortality or hospitalization after 18 months. 55
Other Antidepressants
Other antidepressants have been less studied in patients with heart disease. Mirtazapine, a 5‐hydroxytryptamine‐2 and α‐2 receptor antagonist antidepressant, was found to improve depressive symptoms in a nested RCT of the MIND‐IT trial in post‐MI patients, 33 though this agent has been linked to weight gain and hypercholesterolemia, making it potentially problematic for patients with heart disease. 67 , 68 When the tricyclic antidepressant nortriptyline was compared with paroxetine in 41 patients with depression with CAD, nortriptyline had significantly more side effects, including sinus tachycardia, severe angina associated with ST changes, and increase in ventricular ectopy, which led to premature discontinuation, 34 consistent with a larger literature suggesting that tricyclic antidepressants are suboptimal for this population. 69 There have not been RCTs of serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine or duloxetine), nor have there been RCTs on other antidepressant classes such as dopamine and norepinephrine reuptake inhibitors (eg, bupropion), for depression in patients with cardiac disease, though bupropion has been safely prescribed for post‐ACS patients for smoking cessation. 70
Finally, omega‐3‐fatty acids have been tested as antidepressant monotherapy or as adjuncts to SSRIs in a pair of RCTs. In these trials, these agents were not associated with significant benefits on mood symptoms or cardiac outcomes in patients with CAD 35 or HF. 57
Cost‐Effectiveness
The SADHART trial was the only trial that reported data on costs and found no significant increase in overall medical care costs for sertraline compared with placebo after including the costs of medication 71 (Table 3).
Table 3.
Cost Effectiveness Data of Interventions
| Reference or Study Name | Population | Study Design, Intervention, and Primary Aim | Findings |
|---|---|---|---|
| SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) 71 | 369 post‐ACS patients with MDD | 24‐wk RCT comparing sertraline (50–200 mg) with placebo, aimed at evaluating safety and efficacy of sertraline for MDD in patients with ACS | The mean cost of psychiatric and medical care per patient for the intervention group was 2733 US dollars and 3326 US dollars for placebo, albeit not statistically significant (P=0.32). There was no increase in overall medical care costs for sertraline compared with placebo after including the costs of the sertraline over 24 wk |
| Bypassing the Blues 72 | 302 patients post–coronary artery bypass surgery with elevated depressive symptoms | 8‐mo RCT comparing phone‐, nurse‐delivered collaborative care depression management to enhanced usual care (collaborative care for cardiac diagnosis) to evaluate effectiveness in treating depression | Patients in the intervention group had $2068 lower median costs, although nonsignificant, compared with usual care (P=0.30). There were no significant changes in sensitivity analyses after removing outliers and doubling estimated cost of intervention to account for overlooked costs. The incremental cost‐effectiveness ratio was −$9889 per additional QALY |
| TEAMCare 73 | 214 patients with type 2 diabetes mellitus and/or CAD and depression | 12‐mo RCT of collaborative care management for depression and medical conditions to examine effectiveness in controlling risk factors associated with multiple diseases | The intervention group had lower mean outpatient health costs of $594 per patient (95% CI, −$3241 to $2053) relative to UC patients |
| MOASAIC (Management of Sadness and Anxiety in Cardiology) 74 | 183 patients with ACS, HF, or arrhythmia and depression, panic disorder, or generalized anxiety disorder | 24‐wk RCT of an inpatient initiated, phone‐delivered, collaborative care depression and anxiety management program compared with enhanced usual care | The cost of mental health care was greater in intervention than control group ($209.86 vs $34.59; z=−11.71; P<0.001). The incremental cost‐effectiveness ratio was $3337.06 per QALY saved, $13.36 per depression‐free day, and $13.74 per anxiety‐free day. Compared with enhanced usual care, the intervention was also associated with fewer emergency department visits but no differences in overall costs |
| COADIACS (Comparison of Depression Interventions After Acute Coronary Syndrome) 52 | 150 post‐ACS patients with elevated depression symptoms | 6‐mo RCT of depression stepped‐care management intervention (phone or internet) vs usual care evaluating effectiveness in reducing depressive symptoms | The intervention group had significantly higher mental health cost (adjusted change, $687; 95% CI, $466–$909; P<0.001), while average hospital costs were lower (adjusted change, −$1010; 95% CI, −$3294 to $1274; P=0.39). Total healthcare costs in the study intervention group resulted in nonsignificantly lower costs than the control group (adjusted change, −$325; 95% CI, −$2639 to $1989; P=0.78) |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; HF, heart failure; MDD, major depressive disorder; QALY, quality‐adjusted life‐year; RCT, randomized clinical trial; and UC, usual care.
Psychotherapy
A number of studies have examined the use of psychotherapy to manage depression in patients with heart disease. CBT has been the most studied psychotherapy among those with heart disease. CBT is an umbrella term for therapies that focus on recognizing and restructuring cognitive distortions, modifying problematic behaviors, and regulation of emotions, 75 and it is effective in the treatment of depression in patients without cardiac disease. 76
CBT and Related Interventions for Patients With CAD
CBT has been studied in several trials in CAD (Table 1). The largest psychotherapy intervention trial for depression in this population has been the abovementioned ENRICHD trial. In this trial, individual (mean 11 sessions) and supplemental group therapist–delivered CBT was compared with usual care in 2481 post‐MI patients with MDD or low social support. Patients with depression who received CBT had greater improvement in depressive symptoms compared with those in usual care after 6 months. There was, however, no significant difference in event‐free survival between usual care and CBT over a mean 29 months' follow‐up. 36
Other trials have also explored the effectiveness of CBT in cardiac populations. Lv and colleagues found a significant reduction in depressive symptoms between an 8‐session weekly therapist‐delivered CBT intervention and usual care in 75 patients with CAD undergoing percutaneous intervention who had elevated anxiety or depressive symptoms. 37 Freedland et al also conducted a 3‐arm trial comparing CBT, stress reduction (both were weekly, in‐person, and therapist‐delivered), and usual care among 123 patients undergoing coronary artery bypass graft. They found greater significant reductions of depressive symptoms and rates of depression remission in the CBT and stress management groups compared with usual care at 9 months; cardiovascular outcomes were not examined. 38
MoodCare, a phone‐delivered, 10‐session, CBT risk‐reduction program delivered by psychologists, was compared with usual care in a 6‐month trial of 121 patients with ACS with mild to moderately severe depression. CBT led to significantly greater reductions in depression at 6 months, with effects more pronounced among those with a history of depression. 42 At 12‐month follow‐up, however, only those who met MDD criteria at enrollment experienced significantly greater improvements. 43
There have also been studies of remotely delivered CBT in patients with heart disease. Glozier and associates randomized 562 patients with depression and CVD or high CVD risk to a 12‐week internet‐delivered CBT. The internet‐delivered CBT group had significantly greater reduction in depression and increased adherence to medical and health behaviors. 39 A similar trial by Johansson et al explored a 9‐week nurse‐guided, weekly internet‐delivered CBT intervention compared with an active control in 144 patients with CVD and depression, and also showed significant reductions in depression. 40 However, the Uppsala‐CARE (U‐CARE) study compared 14 weeks of therapist‐guided internet‐delivered CBT to treatment as usual in 239 post‐MI patients with depressive symptoms and found no statistically significant reduction in depression scores. 41 No cardiovascular outcomes were explored in these 3 studies.
Other Psychotherapy Interventions for Patients With CAD
Other forms of psychotherapy are less well‐studied in this population. Interpersonal therapy focuses on symptomatic recovery and resolving interpersonal problems. Like CBT, interpersonal therapy is time‐limited, focused, and regimented. 77 In the abovementioned factorial‐design CREATE trial, weekly therapist‐delivered interpersonal therapy plus clinical management showed no benefit over weekly clinical management alone in patients with CAD and MDD. 30 In another trial, however, Oranta and colleagues found greater depressive symptom improvements in 103 post‐MI patients at 18‐month follow‐up among those randomized to nurse‐delivered interpersonal therapy compared with treatment as usual. 44
Finally, the SPIRR‐CAD (Psychotherapy Intervention for Reducing Risk in Coronary Artery Disease) study utilized a stepped‐care psychotherapy approach among 570 patients with CAD with elevated depression or anxiety symptoms. The intervention utilized 3 supportive‐expressive psychotherapy sessions plus 25 group sessions for those still depressed; this was compared with a control condition consisting of usual care plus 1 information session. They found no difference in improvement in depressive symptoms or cardiac outcomes. 45
Psychotherapy Interventions for Patients With HF
CBT has been the only form of therapy formally studied in patients with HF. In a 6‐month trial of therapist‐delivered weekly CBT intervention versus usual care in 158 patients with HF with major depression, CBT was associated with significant improvements in depressive symptoms and remission rates, but not in HF self‐care. 58 CBT was also associated with lower rates of hospitalization at 1 year, compared with usual care. Gary and colleagues conducted a 4‐arm clinical trial in 74 patients with HF and depression comparing 12 weeks of home‐based exercise+nurse‐delivered CBT, versus CBT alone, exercise alone, and usual care. The exercise+CBT group had a greater but nonsignificant improvement in depression compared with CBT, exercise, and usual care. 59 A smaller study by Dekker et al compared a 1‐session, nurse‐delivered cognitive therapy intervention to usual care in 41 hospitalized patients with HF with depressive symptoms and found no significant differences between groups. 60
No trials focused solely on psychotherapy reported data on cost analyses.
Care Management
Depression care management approaches that utilize serial symptom assessment and flexible, stepped care that includes medication or therapy have also been studied in cardiac populations. Collaborative care programs utilize a nonphysician/nonpsychologist care manager to assess and longitudinally monitor symptoms and deliver psychosocial interventions. Medication recommendations are iteratively made by a team psychiatrist, with all medications prescribed by the primary medical provider. Stepped care management is a similar approach, in which cases are systematically discussed by a treatment team and psychotherapy or medication are offered to patients in a stepwise manner depending on patients' level of symptoms or need; in stepped care, the interventions are delivered/prescribed by therapists or clinicians rather than the care manager.
Collaborative Care in CAD
Numerous clinical trials have explored collaborative care depression management programs for patients with CVD 78 (Table 1). In the Bypassing the Blues collaborative care trial, patients (N=302) with recent coronary artery bypass graft and comorbid MDD were randomized to an 8‐month phone‐delivered collaborative care program or usual care. Collaborative care led to significantly greater improvements in depression and physical function at 1 year on the HAM‐D; there were no reductions in cardiac readmissions associated with the intervention. 46 The TrueBlue study (N=400) was a 6‐month collaborative care intervention trial for depression in patients with CAD and/or type 2 diabetes mellitus. They found greater improvements in depression on the Nine‐item Patient Health Questionnaire and greater adherence to depression stepped care and physical exercise in the intervention group; medical/cardiac outcomes were not reported. 47
The SUCCEED (Screening Utilization and Collaborative Care for More Effective and Efficient Treatment of Depression) trial was an inpatient‐initiated 12‐week nurse‐delivered collaborative care trial for patients admitted for ACS, arrhythmia, or HF, and comorbid depression. The intervention was associated with significant improvements in depression and other mental health outcomes at 12 weeks, with fewer cardiac symptoms and improved self‐reported health behavior adherence by 24 weeks, compared with enhanced usual care; there was no effect on 6‐month readmissions. 48 The same group conducted the MOSAIC (Management of Sadness and Anxiety in Cardiology) randomized trial, a 24‐week program for the same cardiac diagnoses that managed depression, panic disorder, and/or generalized anxiety disorder. The intervention was associated with significantly greater improvements in depression and physical function at 24 weeks; there were no group differences in rates of cardiac readmission at 6 months. 49
An expanded version of collaborative care, known as “blended” collaborative care (with nurse care manager intervening on both mental health and medical targets) was studied in the TEAMCare trial of 214 patients with type 2 diabetes mellitus and/or CAD using a blended collaborative care intervention for 12 months; patients in the intervention group had greater overall 12‐month improvement across hemoglobin A1c, low‐density lipoprotein cholesterol, systolic blood pressure, and depression scores, compared with usual care. 50
Stepped Care for Patients With CAD
Davidson and colleagues utilized a stepped‐care approach for patients with ACS with elevated depressive symptoms. The COPES (Coronary Psychosocial Evaluation Studies) trial delivered 6 months of patient preference–based depression stepped care remotely to 157 patients with ACS with elevated depressive symptoms; the intervention led to greater improvements in depression (Beck Depression inventory and lower rates of major adverse cardiac events), compared with usual care. 51 The CODIACS (Comparison of Depression Interventions After Acute Coronary Syndrome) trial was a follow‐up study in 150 patients post‐ACS with persistent depressive symptoms in which the intervention was delivered by phone or internet, and was likewise found to reduce depressive symptoms at 6 months compared with usual care; no cardiac outcomes were reported. 52 Finally, in a broader approach, the CODIACS‐QoL program examined routine depression screening and stepped depression care in 1500 patients post‐ACS. Participants were randomized to screening plus stepped care, screening and primary care practitioner notification, or no screening. The program was associated with no greater improvement in depression‐free days, depressive symptoms, quality‐adjusted life‐years, or mortality than screening and primary care practitioner notification, or treatment as usual. 53 Of note, only 71 of the 1000 screened patients had depressive symptoms at baseline.
Collaborative Care for Patients With HF
The Hopeful Hearts trial was a blended collaborative care trial for patients with HF and comorbid depression (N=629) (Table 2). This was a 3‐arm trial, in which patients were randomized to a “blended” group, where nurse care managers were used to address depression and medical targets over 12 months, an “enhanced care” group where nurse care managers targeted HF targets only, or usual care. They found greater improvements in depression response rates in 1 of 2 scales in those randomized to the collaborative care program compared with either medical care management or treatment as usual, but there were no significant group differences in cardiac admissions or other medical outcomes. 61
Cost‐Effectiveness
Data on cost analysis are reported in Table 3. The Bypassing the Blues trial found lower median of medical care ($2068) in the intervention group compared with usual care, though this difference was nonsignificant (P=0.30), with an incremental cost‐effectiveness ratio of −$9889 per additional quality‐adjusted life‐year. 72 Mean outpatient costs were also lower in the TEAMCare trial intervention group when compared with control ($594 per patient [95% CI, −$3241 to $2053]). 73 The MOSAIC clinical trial found the intervention group to be more costly than the control group. However, there was an incremental cost‐effectiveness ratio of $3337.06 per quality‐adjusted life‐year saved, $13.36 per depression‐free day, and $13.74 per anxiety‐free day, and fewer emergency department visits in the intervention group, resulting in no differences in overall costs. 74 Finally, the CODIACS study also found higher mental health costs in the intervention group, while average hospital costs were lower, resulting in nonsignificantly lower total healthcare costs in the intervention group than the control group (adjusted change, −$325; 95% CI, −$2639 to $1989; P=0.78). 52
Discussion
Our review of randomized intervention trials for depression in patients with cardiac disease has revealed several themes. First, antidepressants, psychotherapy, and care management have all been proven safe and largely—though not universally—effective in treating and improving mood symptoms among patients with elevated depressive symptoms or MDD in CAD or ACS. In HF, however, antidepressant efficacy has not been demonstrated, while psychotherapy and care management generally have been more effective.
Regarding antidepressants, overall, based on existing evidence, SSRIs should be considered a first‐line therapy in patients with cardiac disease who merit treatment based on a diagnosis of MDD, especially those with CAD. SSRIs are the most studied agents in populations with cardiac disease, with efficacy in treating depression in most studies. Sertraline has been the most studied SSRI, and may be preferred because it does not (in contrast to citalopram and escitalopram) prolong the QT interval. 79 Among patients with CAD/outcomes, observational evidence points to possible cardiovascular benefits of SSRIs, 16 and nonresponse of mood symptoms to antidepressants is associated with adverse cardiac outcomes, including an elevated risk of mortality, in patients with cardiac disease. 11 , 80 However, at this stage there have been few definitive prospective trials that have found SSRIs to prevent cardiac events, readmissions, or mortality. The EsDEPACS trial did show reduction in major adverse cardiac events and all‐cause mortality over 8‐year follow‐up when escitalopram was used in patients with ACS, 28 showing potential promise in using SSRIs to improve cardiac outcomes, but substantial further study is needed.
As noted, antidepressants have been less effective in HF, with similar response rates in antidepressant and placebo groups. It is possible that “elevated depressive symptoms” measured by depression scales may simply be measuring somatic symptoms common in this chronic disease rather than true depression, that the mechanism of depression differs in patients with HF, or more powerful or individualized interventions are needed in this chronic disease. Study design may also play a role. For example, the SADHART‐CHF trial provided weekly nursing support, which may have improved mood symptoms sufficiently to mask any sertraline‐specific effects. 54 Fortunately, SSRIs do appear to be safe in patients with HF, which still makes them a potentially reasonable approach for individuals when other treatments are not readily available or as an inexpensive complement to psychotherapeutic or care management supportive interventions.
Psychotherapy interventions have had efficacy comparable to SSRIs in most cases, especially those that have used CBT or related interventions, but there is less compelling data on their potential cardiac benefits. There are also concerns about scalability, especially for individual‐level therapy, but remotely delivered and group therapies that could be more broadly implemented are preferable, because they are easier to deliver and likely more cost‐effective. Care management interventions have likewise shown good efficacy in the management and reduction of depressive symptoms. These programs represent a promising area of healthcare delivery, especially those that integrate depression and cardiac symptom management, and such interventions (which use nurses or social workers to provide front‐line management) have proven cost‐effective. 81 However, as with antidepressants, in existing randomized trials, there have been limited effects on cardiac biomarkers or cardiac events, with many studies not yet powered to measure such effects. Finally, exercise programs have been used as an active comparator in a small number of trials, and as in populations without cardiac disease, they appear to have similar efficacy to other depression management approaches.
Where does this leave clinicians in terms of selecting treatment for depressed patients with cardiac disease? It is important to emphasize that comparative effectiveness trials of depression interventions in heart disease are lacking. Taken together, there are modest data suggesting that antidepressants could be preferred in patients with CAD, given that they were found to be more effective than psychotherapy alone in 1 trial, 30 and that SSRIs, but not CBT, were found to be associated with reduced cardiac mortality and recurrent MI in the ENRICHD trial. 16 In populations with HF, however, there is some evidence for the efficacy of CBT, while SSRIs have not been efficacious.
Combining interventions could be even more effective than a single modality, potentiating the beneficial effects of both, as observed in the UPBEAT trial 29 and recent secondary prevention studies in nondepressed individuals. 82 , 83 Interventions such as collaborative care may be particularly effective in this manner, providing the potential for both pharmacologic and therapeutic treatments via a stepped‐care and patient‐preference‐focused approach, in a generally cost‐effective manner. 84
Finally, there are several important gaps in this literature. It is important to note that there has been minimal study in RCTs of depression treatment approaches among patients with arrhythmias. While a small number of trials have included patients with arrhythmia, 48 , 49 we identified no RCTs that specifically targeted patients with arrhythmia. This is an important area of future research. For example, atrial fibrillation is exceedingly common, occurring in ≈5 million Americans, 85 and depression has been linked with elevated mortality risk in this population. 86 Furthermore, there has been very limited study of commonly used non‐SSRI classes of antidepressants (eg, serotonin–norepinephrine reuptake inhibitors, bupropion) in patients with heart disease. Likewise, there has been minimal study of other modalities such as non‐CBT psychotherapies, exercise programs that can be delivered at scale, or nutritional interventions.
There are some limitations to this review that must be noted. For instance, though we systematically reviewed numerous databases, this was a scoping review and not a full systematic review, and thus some clinical trials may have been missed in this slightly less structured screening of articles. Additionally, we only reviewed publications in English, and did not examine interventions for other mental health conditions such as anxiety or bipolar disorders. There is also the potential for unpublished negative data. Finally, it was not possible to conduct a quantitative analysis because of substantial heterogeneity in research designs and outcome definitions.
In conclusion, antidepressant medications (especially SSRIs), psychotherapy, and depression care management programs all appear to be well‐accepted and tolerable in patients with acute and chronic heart disease, with associated improvements in mental health outcomes and quality of life in many cases. However, this synthesis has revealed that the majority of interventions have not found improvements in cardiac risk factors or cardiac outcomes, with most studies underpowered to detect group differences in such outcomes. Given the potential health consequences and elevated cost of untreated depression in patients with cardiac disease, there is a need for controlled prospective trials to assess the impact of psychiatric and psychologic interventions on cardiac outcomes and mortality. Moreover, cost‐effectiveness of depression interventions and barriers to their broad implementation in clinical care must also continue to be investigated.
Sources of Funding
Time for analysis and article preparation was funded by the National Heart, Lung, and Blood Institute through grants R01HL133149 (Huffman) and K23HL123607 (Celano).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e018686 DOI: 10.1161/JAHA.120.018686.)
For Sources of Funding and Disclosures, see page 17.
References
- 1. Organization WH . Cardiovascular diseases (CVDs). Fact sheet: World Health Organization. 2017. Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds. Accessed July 12, 2020.
- 2. Heron M. Deaths: leading causes for 2017. National Vital Statistics Reports; 2019. Contract No.: 6. [PubMed] [Google Scholar]
- 3. Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev. 2011;130–142. [DOI] [PubMed] [Google Scholar]
- 4. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;1527–1537. [DOI] [PubMed] [Google Scholar]
- 5. Jee YH, Chang H, Jung KJ, Jee SH. Cohort study on the effects of depression on atherosclerotic cardiovascular disease risk in Korea. BMJ Open. 2019;e026913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wium‐Andersen MK, Wium‐Andersen IK, Prescott EIB, Overvad K, Jørgensen MB, Osler M. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta‐analytic approach. Br J Psychiatry. 2020;434–441. [DOI] [PubMed] [Google Scholar]
- 7. May HT, Horne BD, Knight S, Knowlton KU, Bair TL, Lappé DL, Le VT, Muhlestein JB. The association of depression at any time to the risk of death following coronary artery disease diagnosis. Eur Heart J Qual Care Clin Outcomes. 2017;296–302. [DOI] [PubMed] [Google Scholar]
- 8. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure‐Smith N, Freedland KE, Jaffe AS, Leifheit‐Limson EC, Sheps DS, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;1350–1369. [DOI] [PubMed] [Google Scholar]
- 9. Geulayov G, Novikov I, Dankner D, Dankner R. Symptoms of depression and anxiety and 11‐year all‐cause mortality in men and women undergoing coronary artery bypass graft (CABG) surgery. J Psychosom Res. 2018;106–114. [DOI] [PubMed] [Google Scholar]
- 10. Lange HW, Herrmann‐Lingen C. Depressive symptoms predict recurrence of atrial fibrillation after cardioversion. J Psychosom Res. 2007;509–513. [DOI] [PubMed] [Google Scholar]
- 11. Carney RM, Freedland KE. Treatment‐resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seligman F, Nemeroff CB. The interface of depression and cardiovascular disease: therapeutic implications. Ann N Y Acad Sci. 2015;25–35. [DOI] [PubMed] [Google Scholar]
- 13. Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scherrer JF, Chrusciel T, Garfield LD, Freedland KE, Carney RM, Hauptman PJ, Bucholz KK, Owen R, Lustman PJ. Treatment‐resistant and insufficiently treated depression and all‐cause mortality following myocardial infarction. Br J Psychiatry. 2012;137–142. [DOI] [PubMed] [Google Scholar]
- 15. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1‐year mortality after acute myocardial infarction: insights from the TRIUMPH Registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status). Circulation. 2017;1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, Kaufmann PG, Shuster J, Mellman T, Blumenthal JA, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;792–798. [DOI] [PubMed] [Google Scholar]
- 17. Kimmel SE, Schelleman H, Berlin JA, Oslin DW, Weinstein RB, Kinman JL, Sauer WH, Lewis JD. The effect of selective serotonin re‐uptake inhibitors on the risk of myocardial infarction in a cohort of patients with depression. Br J Clin Pharmacol. 2011;514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piña IL, Di Palo KE, Ventura HO. Psychopharmacology and cardiovascular disease. J Am Coll Cardiol. 2018;2346–2359. [DOI] [PubMed] [Google Scholar]
- 19. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;141–146. [DOI] [PubMed] [Google Scholar]
- 20. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sayers A. Tips and tricks in performing a systematic review. Br J Gen Pract. 2007;759. [PMC free article] [PubMed] [Google Scholar]
- 24. van Zyl LT, Lespérance F, Frasure‐Smith N, Malinin AI, Atar D, Laliberté MA, Serebruany VL. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) biomarker sub‐study. J Thromb Thrombolysis. 2009;48–56. [DOI] [PubMed] [Google Scholar]
- 25. Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. J Am Med Assoc. 2002;701–709. [DOI] [PubMed] [Google Scholar]
- 26. Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;527–532. [DOI] [PubMed] [Google Scholar]
- 27. Kim JM, Bae KY, Stewart R, Jung BO, Kang HJ, Kim SW, Shin IS, Hong YJ, Kim JH, Shin HY, et al. Escitalopram treatment for depressive disorder following acute coronary syndrome: a 24‐week double‐blind, placebo‐controlled trial. J Clin Psychiatry. 2015;62–68. [DOI] [PubMed] [Google Scholar]
- 28. Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, Kang HJ, Bae KY, Kim SW, Shin IS, et al. Effect of escitalopram vs placebo treatment for depression on long‐term cardiac outcomes in patients with acute coronary syndrome: a randomized clinical trial. JAMA. 2018;350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, O'Hayer CV, Mabe S, Johnson J, Doraiswamy PM, et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 2012;1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lesperance F, Frasure‐Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. J Am Med Assoc. 2007;367–379. [DOI] [PubMed] [Google Scholar]
- 31. van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;460–466. [DOI] [PubMed] [Google Scholar]
- 32. Zuidersma M, Conradi HJ, van Melle JP, Ormel J, de Jonge P. Depression treatment after myocardial infarction and long‐term risk of subsequent cardiovascular events and mortality: a randomized controlled trial. J Psychosom Res. 2013;25–30. [DOI] [PubMed] [Google Scholar]
- 33. Honig A, Kuyper AM, Schene AH, van Melle JP, de Jonge P, Tulner DM, Schins A, Crijns HJ, Kuijpers PM, Vossen H, et al. Treatment of post‐myocardial infarction depressive disorder: a randomized, placebo‐controlled trial with mirtazapine. Psychosom Med. 2007;606–613. [DOI] [PubMed] [Google Scholar]
- 34. Nelson JC, Kennedy JS, Pollock BG, Laghrissi‐Thode F, Narayan M, Nobler MS, Robin DW, Gergel I, McCafferty J, Roose S. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry. 1999;1024–1028. [DOI] [PubMed] [Google Scholar]
- 35. Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega‐3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. J Am Med Assoc. 2003;3106–3116. [DOI] [PubMed] [Google Scholar]
- 37. Lv J, Zhang X, Ou S, Gu S, Su Z, Tong S, Liu B, Song Z, Chi L. Influence of cognitive behavioral therapy on mood and quality of life after stent implantation in young and middle‐aged patients with coronary heart disease. Int Heart J. 2016;167–172. [DOI] [PubMed] [Google Scholar]
- 38. Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Davila‐Roman VG, Steinmeyer BC, Hogue CW Jr. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. 2009;387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glozier N, Christensen H, Naismith S, Cockayne N, Donkin L, Neal B, Mackinnon A, Hickie I. Internet‐delivered cognitive behavioural therapy for adults with mild to moderate depression and high cardiovascular disease risks: a randomised attention‐controlled trial. PLoS One. 2013;e59139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansson P, Westas M, Andersson G, Alehagen U, Broström A, Jaarsma T, Mourad G, Lundgren J. An internet‐based cognitive behavioral therapy program adapted to patients with cardiovascular disease and depression: randomized controlled trial. JMIR Ment Health. 2019;e14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norlund F, Wallin E, Olsson EMG, Wallert J, Burell G, von Essen L, Held C. Internet‐based cognitive behavioral therapy for symptoms of depression and anxiety among patients with a recent myocardial infarction: the U‐CARE heart randomized controlled trial. J Med Internet Res. 2018;e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Neil A, Taylor B, Sanderson K, Cyril S, Chan B, Hawkes AL, Hare DL, Jelinek M, Venugopal K, Atherton JJ, et al. Efficacy and feasibility of a tele‐health intervention for acute coronary syndrome patients with depression: results of the "MoodCare" randomized controlled trial. Ann Behav Med. 2014;163–174. [DOI] [PubMed] [Google Scholar]
- 43. O'Neil A, Taylor B, Hare DL, Sanderson K, Cyril S, Venugopal K, Chan B, Atherton JJ, Hawkes A, Walters DL, et al. Long‐term efficacy of a tele‐health intervention for acute coronary syndrome patients with depression: 12‐month results of the MoodCare randomized controlled trial. Eur J Prev Cardiol. 2015;1111–1120. [DOI] [PubMed] [Google Scholar]
- 44. Oranta O, Luutonen S, Salokangas RK, Vahlberg T, Leino‐Kilpi H. The outcomes of interpersonal counselling on depressive symptoms and distress after myocardial infarction. Nord J Psychiatry. 2010;78–86. [DOI] [PubMed] [Google Scholar]
- 45. Herrmann‐Lingen C, Beutel ME, Bosbach A, Deter HC, Fritzsche K, Hellmich M, Jordan J, Junger J, Ladwig KH, Michal M, et al. A stepwise psychotherapy intervention for reducing risk in coronary artery disease (SPIRR‐CAD): results of an observer‐blinded, multicenter, randomized trial in depressed patients with coronary artery disease. Psychosom Med. 2016;704–715. [DOI] [PubMed] [Google Scholar]
- 46. Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, Kapoor WN, Schulberg HC, Reynolds CF III. Telephone‐delivered collaborative care for treating post‐CABG depression: a randomized controlled trial. J Am Med Assoc. 2009;2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morgan MA, Coates MJ, Dunbar JA, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open. 2013;e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huffman JC, Mastromauro CA, Sowden G, Fricchione GL, Healy BC, Januzzi JL. Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;198–205. [DOI] [PubMed] [Google Scholar]
- 49. Huffman JC, Mastromauro CA, Beach SR, Celano CM, DuBois CM, Healy BC, Suarez L, Rollman BL, Januzzi JL. Collaborative care for depression and anxiety disorders in patients with recent cardiac events. JAMA Intern Med. 2014;927–935. [DOI] [PubMed] [Google Scholar]
- 50. Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, Albanese G, Kronish I, Hegel M, Burg MM. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, Dornelas E, Duer‐Hefele J, Frasure‐Smith N, Freedland KE, et al. Centralized, stepped, patient preference‐based treatment for patients with post‐acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Intern Med. 2013;997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kronish IM, Moise N, Cheung YK, Clarke GN, Dolor RJ, Duer‐Hefele J, Margolis KL, St Onge T, Parsons F, Retuerto J, et al. Effect of depression screening after acute coronary syndromes on quality of life: the CODIACS‐QoL randomized clinical trial. JAMA Intern Med. 2019;45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART‐CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Angermann CE, Gelbrich G, Störk S, Gunold H, Edelmann F, Wachter R, Schunkert H, Graf T, Kindermann I, Haass M, et al. Effect of escitalopram on all‐cause mortality and hospitalization in patients with heart failure and depression: the MOOD‐HF randomized clinical trial. JAMA. 2016;2683–2693. [DOI] [PubMed] [Google Scholar]
- 56. Fraguas R, da Silva Telles RM, Alves TC, Andrei AM, Rays J, Iosifescu DV, Wajngarten M. A double‐blind, placebo‐controlled treatment trial of citalopram for major depressive disorder in older patients with heart failure: the relevance of the placebo effect and psychological symptoms. Contemp Clin Trials. 2009;205–211. [DOI] [PubMed] [Google Scholar]
- 57. Jiang W, Whellan DJ, Adams KF, Babyak MA, Boyle SH, Wilson JL, Patel CB, Rogers JG, Harris WS, O'Connor CM. Long‐chain omega‐3 fatty acid supplements in depressed heart failure patients: results of the OCEAN Trial. JACC Heart Fail. 2018;833–843. [DOI] [PubMed] [Google Scholar]
- 58. Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self‐care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dekker RL, Moser DK, Peden AR, Lennie TA. Cognitive therapy improves three‐month outcomes in hospitalized patients with heart failure. J Card Fail. 2012;10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rollman B, Anderson A, Abebe K, Jeong K, Herbeck BB, Ramani R, Muldoon M, Karp J. Blended collaborative care for treating systolic heart failure and co‐morbid depression: 12‐month primary outcomes from the Hopeful Heart Trial. J Gen Intern Med. 2019;783–789.30877456 [Google Scholar]
- 62. Strik JJ, Honig A, Lousberg R, Lousberg AH, Cheriex EC, Tuynman-Qua HG, Kuijpers PM, Wellens HJ, Van Praag HM. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62:783–789. [DOI] [PubMed] [Google Scholar]
- 63. Hansen BH, Hanash JA, Rasmussen A, Hansen JF, Andersen NL, Nielsen OW, Birket‐Smith M. Effects of escitalopram in prevention of depression in patients with acute coronary syndrome (DECARD). J Psychosom Res. 2012;11–16. [DOI] [PubMed] [Google Scholar]
- 64. Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;1358–1367. [DOI] [PubMed] [Google Scholar]
- 65. Almuwaqqat Z, Jokhadar M, Norby FL, Lutsey PL, O'Neal WT, Seyerle A, Soliman EZ, Chen LY, Bremner JD, Vaccarino V, et al. Association of antidepressant medication type with the incidence of cardiovascular disease in the ARIC study. J Am Heart Assoc. 2019;e012503 DOI: 10.1161/JAHA.119.012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oh SW, Kim J, Myung SK, Hwang SS, Yoon DH. Antidepressant use and risk of coronary heart disease: meta‐analysis of observational studies. Br J Clin Pharmacol. 2014;727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000;883–889. [PubMed] [Google Scholar]
- 68. Nicholas LM, Ford AL, Esposito SM, Ekstrom RD, Golden RN. The effects of mirtazapine on plasma lipid profiles in healthy subjects. J Clin Psychiatry. 2003;64:883–889. [DOI] [PubMed] [Google Scholar]
- 69. Marshall JB, Forker AD. Cardiovascular effects of tricyclic antidepressant drugs: therapeutic usage, overdose, and management of complications. Am Heart J. 1982;401–414. [DOI] [PubMed] [Google Scholar]
- 70. Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J. 2013;3259–3267. [DOI] [PubMed] [Google Scholar]
- 71. O'Connor CM, Glassman AH, Harrison DJ. Pharmacoeconomic analysis of sertraline treatment of depression in patients with unstable angina or a recent myocardial infarction. J Clin Psychiatry. 2005;346–352. [DOI] [PubMed] [Google Scholar]
- 72. Donohue JM, Belnap BH, Men A, He F, Roberts MS, Schulberg HC, Reynolds CF III, Rollman BL. Twelve‐month cost‐effectiveness of telephone‐delivered collaborative care for treating depression following CABG surgery: a randomized controlled trial. Gen Hosp Psychiatry. 2014;453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, Peterson D, Young B, Von Korff M. Cost‐effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Celano CM, Healy B, Suarez L, Levy DE, Mastromauro C, Januzzi JL, Huffman JC. Cost‐effectiveness of a collaborative care depression and anxiety treatment program in patients with acute cardiac illness. Value Health. 2016;185–191. [DOI] [PubMed] [Google Scholar]
- 75. Rachman S. The evolution of behaviour therapy and cognitive behaviour therapy. Behav Res Ther. 2015;1–8. [DOI] [PubMed] [Google Scholar]
- 76. Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;3–7. [DOI] [PubMed] [Google Scholar]
- 77. Weissman MM. Interpersonal psychotherapy: history and future. Am J Psychother. 2020;73:3–7. [DOI] [PubMed] [Google Scholar]
- 78. Huffman JC, Adams CN, Celano CM. Collaborative care and related interventions in patients with heart disease: an update and new directions. Psychosomatics. 2018;1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beach SR, Celano CM, Sugrue AM, Adams C, Ackerman MJ, Noseworthy PA, Huffman JC. QT prolongation, torsades de pointes, and psychotropic medications: a 5‐year update. Psychosomatics. 2018;105–122. [DOI] [PubMed] [Google Scholar]
- 80. Jiang W, Krishnan R, Kuchibhatla M, Cuffe MS, Martsberger C, Arias RM, O'Connor CM; Investigators S‐C . Characteristics of depression remission and its relation with cardiovascular outcome among patients with chronic heart failure (from the SADHART‐CHF Study). Am J Cardiol. 2011;545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Katon WJ, Seelig M. Population‐based care of depression: team care approaches to improving outcomes. J Occup Environ Med. 2008;459–467. [DOI] [PubMed] [Google Scholar]
- 82. Huffman JC, Feig EH, Millstein RA, Freedman M, Healy BC, Chung WJ, Amonoo HL, Malloy L, Slawsby E, Januzzi JL, et al. Usefulness of a positive psychology‐motivational interviewing intervention to promote positive affect and physical activity after an acute coronary syndrome. Am J Cardiol. 2019;1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jacob V, Chattopadhyay SK, Sipe TA, Thota AB, Byard GJ, Chapman DP; Force CPST . Economics of collaborative care for management of depressive disorders: a community guide systematic review. Am J Prev Med. 2012;539–549. [DOI] [PubMed] [Google Scholar]
- 85. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wändell P, Carlsson AC, Gasevic D, Wahlström L, Sundquist J, Sundquist K. Depression or anxiety and all‐cause mortality in adults with atrial fibrillation—a cohort study in Swedish primary care. Ann Med. 2016;59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


