Abstract
Background
Coronary artery bypass grafting (CABG) is known to improve heart function and quality of life, while rates of surgery‐related mortality are low. However, delirium and cognitive decline are common complications. We sought to identify preoperative, intraoperative, and postoperative risk or protective factors associated with delirium and cognitive decline (across time) in patients undergoing CABG.
Methods and Results
We conducted a systematic search of Medline, PsycINFO, EMBASE, and Cochrane (March 26, 2019) for peer‐reviewed, English publications reporting post‐CABG delirium or cognitive decline data, for at least one risk factor. Random‐effects meta‐analyses estimated pooled odds ratio for categorical data and mean difference or standardized mean difference for continuous data. Ninety‐seven studies, comprising data from 60 479 patients who underwent CABG, were included. Moderate to large and statistically significant risk factors for delirium were as follows: (1) preoperative cognitive impairment, depression, stroke history, and higher European System for Cardiac Operative Risk Evaluation (EuroSCORE) score, (2) intraoperative increase in intubation time, and (3) postoperative presence of arrythmia and increased days in the intensive care unit; higher preoperative cognitive performance was protective for delirium. Moderate to large and statistically significant risk factors for acute cognitive decline were as follows: (1) preoperative depression and older age, (2) intraoperative increase in intubation time, and (3) postoperative presence of delirium and increased days in the intensive care unit. Presence of depression preoperatively was a moderate risk factor for midterm (1–6 months) post‐CABG cognitive decline.
Conclusions
This meta‐analysis identified several key risk factors for delirium and cognitive decline following CABG, most of which are nonmodifiable. Future research should target preoperative risk factors, such as depression or cognitive impairment, which are potentially modifiable.
Registration
URL: https://www.crd.york.ac.uk/prospero/; Unique identifier: CRD42020149276.
Keywords: cognitive decline, coronary artery bypass grafting, delirium, meta‐analysis
Subject Categories: Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- ACC
aortic cross‐clamp
- CPB
cardiopulmonary bypass
- SMD
standardized mean difference
Clinical Perspective
What Is New?
This meta‐analysis is the first to comprehensively identify risk and protective factors for postoperative delirium and cognitive decline in patients who underwent coronary artery bypass grafting (CABG).
Findings demonstrate that there are many risk and protective factors for delirium and cognitive decline post‐CABG, some of which are modifiable, such as depression, diabetes mellitus, hypertension, and cognitive impairment.
The presence of preoperative depression was a common risk factor across outcomes, which at least doubled the risk of post‐CABG delirium in hospital and cognitive decline acutely and up to 6 months following surgery.
What Are the Clinical Implications?
Risk and protective factors identified in this meta‐analysis could be used to improve delirium and cognitive decline risk prediction tools, leading to more accurate identification of at‐risk patients undergoing CABG, improving care and prognosis.
Findings can inform the design of future intervention trials aimed at reducing the incidence of delirium and cognitive decline post‐CABG, by targeting identified modifiable risk factors.
Coronary artery bypass grafting (CABG) surgery is the main treatment for multivessel coronary disease and remains one of the most common cardiac procedures worldwide. 1 , 2 CABG has low mortality rates, and improves coronary vascularization and cardiac function. 3 However, CABG is associated with high rates of postoperative cognitive impairments, including delirium. 4 , 5 , 6
A recent meta‐analysis investigating post‐CABG cognitive outcomes (cross‐sectional approach by percentage at specific time points) 4 revealed postoperative cognitive impairment or decline was prevalent in 43% of patients up to 4 days, and remains high (39%) up to 1 month post‐CABG. This reduces in the midterm (6–12 months) following CABG to ≈25% and increases up to nearly 40% in the long‐term (1–5 years). The presence of delirium (an acute and fluctuating syndrome of deficits in attention and arousal) was apparent in 24% of patients, up to 1 week post‐CABG, when a standardized tool was used alongside clinical criteria. 4
The presence of cognitive decline following CABG is associated with increased depression risk and decreased quality of life, functional capacity, and the ability to perform activities of daily living. 7 Delirium presence in older adults is associated with increased mortality, length of stay (LOS), hospital readmissions, as well as cognitive decline and dementia, along with reduced quality of life. 8 , 9 , 10 , 11 Research attempting to prevent these post‐CABG cognitive outcomes has been largely unsuccessful, including pharmacological, anesthetic intervention, and surgical techniques. 12 , 13 , 14 , 15 , 16 There has been some evidence of therapeutic effect for advanced surgical methods, such as hypothermia and increasing systemic perfusion intraoperatively. 17 However, the expertise and technology needed are not routinely available.
Understanding risk and protective factors for delirium and cognitive decline post‐CABG has critical clinical implications, including more precise targeting of preoperative and perioperative interventions and the development of a sensitive risk screening tool for these outcomes. The use of a prediction tool for delirium and cognitive decline in a post‐CABG setting could lead to earlier intervention opportunities, greater prognosis, and, in turn, better patient management.
Previous meta‐analyses of all surgical type cardiac patients have provided greater depth of knowledge surrounding the effects of surgery method on cognitive decline (on versus off pump) 15 , 16 and the effect of pharmacological and anesthetic interventions on postoperative delirium. 18 , 19 Specific risk or protective factors for cognitive outcomes (delirium and cognitive decline) have not been comprehensively investigated through meta‐analysis in patients undergoing CABG. In addition, no meta‐analysis has investigated the time course of effects for risk factors in relation to cognitive decline following CABG, especially in the long‐term (>12 months). This systematic review and meta‐analysis aims to investigate risk and protective factors for the following: (1) post‐CABG delirium (1–7 days) and (2) post‐CABG cognitive decline across multiple time points: short‐term (immediately postoperatively up to 1 month), midterm (1–6 months postoperatively), and long‐term (12–15 months postoperatively).
Methods
The protocol for this systematic review and meta‐analysis was registered and published with the international prospective register of systematic reviews (PROSPERO) (registration number: CRD42020149276). This article is reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines. 20 The data that support the findings of this study are available from the corresponding author on reasonable request.
Search Strategy
We updated a search from a published meta‐analysis. 4 We searched Medline, PsycINFO, EMBASE, and the Cochrane databases using the Ovid platform when possible. Searches of all databases were last performed on March 26, 2019. Search terms and medical subject headings used were as follows: (Coronary Artery Bypass/ OR “coronary artery bypass” OR CABG) AND (Cognition/ OR Delirium/ OR Dementia/ OR Alzheimer Disease/ OR Neuropsychological Tests/ OR Cognit* OR Deliri* OR Dementia* OR Alzheimer* MCI or “mild cognitive impairment*” OR “mild‐cognitive impairment*” OR neuropsycholo* OR POCD OR “postoperative cognitive” OR “post‐operative cognitive” OR MMSE OR "mini‐mental state examination” OR “cerebral function” OR neurocognit* OR encephalopath*). Article selection and data extraction, of the updated search, were undertaken by at least 2 reviewers (between D.G., E.S.G., and T.J.R.), with disagreements resolved by consensus.
Study Eligibility
Inclusion criteria were as follows: peer‐reviewed, full‐text, English‐language studies that reported usable risk or protective factor data of those who had undergone CABG surgery (including CABG plus concomitant surgeries). Studies needed to report a cognitive outcome (using a standardized test result, neuropsychological battery, or a clinical diagnosis) for presence of delirium versus no delirium or cognitive decline versus no cognitive decline, and include usable data for at least one risk factor.
Exclusion criteria included the following: case series (n<5), dissertations, book chapters, protocol articles, reviews, news articles, conference abstracts, letters to the editor, editorials, and comment publications; and studies with no description of their operationalization (or definition used for categorizing participants with cognitive decline/delirium) or incomplete reporting in respect to risk factor data.
All possible risk/protective factors were tallied for presence across eligible studies (eg, data reported within text or within a table split by cognitive outcome or results of measures of association, such as odds ratios [ORs]). Unique risk factors that were reported in >10 studies (across delirium and cognitive decline) were included in this review. A list of these factors was circulated to academic clinicians (coauthors P.J.P. and D.H.J.D.) to ensure that no clinically relevant factors had been missed. This led to the additional extraction of delirium as a risk factor for cognitive decline (although only present in 3 studies). Following this, factors were categorized as follows: preoperative, intraoperative, or postoperative. Studies that did not report information pertaining to the target risk factors analyzed within the study (eg, studies reporting data related to hematocrit, height, or sepsis) were subsequently excluded (categorized as inappropriate data). In addition, if multiple studies investigated the same cohort, duplicate samples were excluded.
Quality Assessment
Study design and reporting quality were assessed by at least 2 reviewers (between D.G., E.S.G., and T.J.R.), with disagreements resolved by consensus. An adapted tool was used, on the basis of 2 existing assessment checklists, 21 , 22 where higher scores indicated greater overall quality (0–12) (Data S1).
Data Extraction
Data extracted from each included study consisted of: country, sample size, age, sex, cognitive decline/delirium assessment criteria, and risk factor data relative to time periods and cognitive outcome (delirium versus no delirium): 1 to 7 days postoperatively; postoperative cognitive decline versus no decline: short‐term (immediately postoperatively up to 1 month), midterm (1–6 months postoperatively), and long‐term (12–15 months postoperatively). There may be a small degree of overlap between the outcomes of delirium and acute cognitive decline, yet this overlap is representative of the population at this time point. Many of the studies included in this meta‐analysis did not explicitly aim to assess risk factors for these cognitive outcomes through inferential statistical analyses. Yet, these studies still reported extractable descriptive data related to the cognitive outcome (eg, table presenting counts or mean and SD for preoperative, intraoperative, and postoperative variables, split by cognitive outcome). As fewer articles reported data as a result of an inferential statistical analysis, the extraction of descriptive data was prioritized. For each risk factor, descriptive data (eg, mean and SD/event rates) were extracted when available. In the absence of descriptive data, the results of inferential statistical analyses (eg, ORs) were extracted. To increase the consistency within our analyses, only univariate (or unadjusted) data were extracted, as the number and type of covariates used within risk factor analyses varied greatly across studies. When data were reported and extracted as median and interquartile range values, they were converted to mean and SD values. 23 , 24 Only data pertaining to risk/protective factors could be extracted for each cognitive outcome for the time periods reported in identified studies. There were substantially fewer articles within the literature that investigate midterm and long‐term cognitive decline, compared with delirium and acute cognitive decline. Therefore, fewer risk factors could be investigated for midterm and long‐term cognitive decline. It may be the case that there are important risk factors for these time points that we were unable to identify herein with our approach.
Statistical Analysis
Demographic data were calculated from the reported preoperative samples. The I2 statistic was used to express the proportion of between‐study heterogeneity out of total variance and was classified as low (I2=25%–50%), moderate (I2=50%–75%), or high (I2≥75%), using classification criteria suggested by Higgins et al. 25 Total between‐study variance was quantified using τ2. All analyses were based on random‐effects model. Before data analyses, checks were conducted to detect extreme outliers. Effect size estimates that fell an abnormally large distance from other estimates (mainly because of separation or quasi‐separation for a given outcome) were excluded. This process did not exclude the remaining study data from remaining risk factor analyses.
All analyses were performed in Comprehensive Meta‐Analysis software (version 3). A result was considered statistically significant when P<0.05. Each risk or protective factor was analyzed separately and, therefore, independence from other factors cannot be assumed. Separate random effect meta‐analyses were used to estimate pooled OR for categorical risk factor data and mean difference or standardized mean difference (SMD) for continuous risk factor data, comparing cognitive outcomes (delirium versus no delirium or cognitive decline versus no cognitive decline) post‐CABG. A risk or protective factor was meta‐analyzed when data from ≥2 studies were available for the analysis. All meta‐analyses were conducted on univariate data (no multivariate data were extracted) and therefore should be interpreted as unadjusted pooled estimates. The SMD was also calculated to provide a supplementary common effect size across pooled estimates (Tables S1 through S4). SMD values can be interpreted using the same cutoff as Cohen d, where ≥0.20, ≥0.50, and ≥0.80 are considered as small, moderate, and large, respectively. 26 For cognitive decline post‐CABG, analyses were conducted for each time point: short‐term (immediately postoperatively up to 1 month), midterm (1–6 months postoperatively), and long‐term (12–15 months postoperatively). Some of the extracted predictor variables were presented as both categorical and continuous data across articles (eg, education >12 years [categorical] or total years of education [continuous]). Others provided data that could be sorted into multiple categories (eg, preoperative cognitive test scores): (1) different cognitive tests used between studies (SMD used) or (2) the same test used between studies, such as Mini‐Mental State Examination (mean difference used). In these cases, subanalyses were performed for each data format or category, for each risk factor. For statistically significant results, small study effect was examined by visually inspecting funnel plots of effect size versus SE. 27 When at least 10 studies were available for analyses, small study effect was formally assessed using the Egger test of the intercept. 28 When there was evidence for small study effect (1‐tailed P<0.1), we used the Duvall and Tweedie 29 trim and fill method to quantify the extent of potential bias. When there were <10 studies, we performed sensitivity analyses by removing outliers.
Random‐effects meta‐regressions (using mean age as a covariate within the analysis) were performed to investigate whether age was related to the pooled effect estimates. Only analyses containing both risk factor and age data of ≥10 studies, as stated in recent Cochrane guidelines, 30 were interpreted. We also performed stratified random‐effects subgroup analyses to investigate any possible effects of diagnostic approach for delirium (inclusion of a standardized instrument versus none) for each risk factor. For this, stratified random‐effects meta‐analyses were performed for each risk or protective factor variable relative to (1) studies using a standardized instrument (eg, Confusion Assessment Method or the Delirium Rating Scale) to inform the reference standard and (2) studies not using a specific instrument. Therefore, 2 subgroup meta‐analyses were conducted for each risk factor variable (1 of studies using a diagnostic tool and 1 of studies using no tool), allowing comparison of the pooled estimates. Subgroup analyses investigating differing methods of classifying cognitive decline were not conducted because of the limited numbers of articles across most time points.
Results
The search identified 4260 articles, of which 2647 records were screened by title and abstract, following duplicate removal. Full‐text screening was conducted on 963 articles; of these, 97 were included in this review (Figure 1, see Table S5 for articles excluded and rationale for exclusion, at full‐text review stage).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analysis flow diagram.
CABG indicates coronary artery bypass grafting.
The 97 included studies were published across 4 decades, with 3, 7, 38, and 49 studies published in the 1980s, 1990s, 2000s, and 2010s, respectively. Of the included studies, 17 were conducted in the United States, 13 in Japan, 9 in Canada, 8 in Australia, and 6 each in China and the Netherlands. The remaining 38 studies were conducted across 22 individual countries. The included articles comprised data from 60 479 patients, with individual study sample sizes ranging from 8 to 14 262. The mean age of patients across included studies was 64.54 years, and 68.55% of patients were men (calculated only from studies with available data). The included studies were of good quality on the basis of the critical appraisals, ranging from 4 to 12, with a median study score of 10 (of 12) and interquartile range of 8 to 11.5. No studies were excluded from the analysis on the basis of their quality (see Table S6 for individual study information).
Preanalysis checks for extreme outliers resulted in data from 3 studies being excluded from separate analyses (delirium analyses of: presence of depression, kidney injury, and LOS in intensive care unit [ICU]); however, these studies remained within other analyses and therefore were not excluded from this article.
Delirium
Data from 48 individual studies were used within 33 analyses (including subcategory analyses), investigating 27 separate risk or protective factors for delirium presence post‐CABG. Across the analyses, heterogeneity of statistically significant results spanned from low to high (I2 range, 0–98.40; τ2 range, 0–325.89) (see Table S1 for results of each meta‐analysis and Figure S1 for forest plots). Potential small‐study effect was found in 2 analyses (preoperative age and European System for Cardiac Operative Risk Evaluation (EuroSCORE)), where trim and fill estimation led to decreases in effect size (see Figure S2 for funnel plots and small study effect investigation).
Statistically significant preoperative risk factors of developing delirium post‐CABG, from largest to smallest effect size, were: the presence of cognitive impairment, stroke history, depression, arrhythmia, including atrial fibrillation (AF), peripheral vascular disease, kidney injury/disease, body mass index >30 kg/m2, diabetes mellitus, and hypertension, along with continuous risk factors of higher EuroSCORE and older age. Statistically significant intraoperative risk factors, from largest to smallest effect size, were increased intubation time (hours), duration of surgery (minutes), aortic cross‐clamp (ACC) time (minutes), and cardiopulmonary bypass (CPB) time (minutes). Statistically significant postoperative risk factors, from largest to smallest effect size, were: increased LOS in the ICU (days) and the presence of arrhythmia, including AF. Statistically significant protective factors for developing delirium post‐CABG were higher preoperative cognition test scores and years of education (Table S1 and Figure 2).
Figure 2. Forest plots of pooled estimates for risk or protective factors of post–coronary artery bypass grafting delirium.
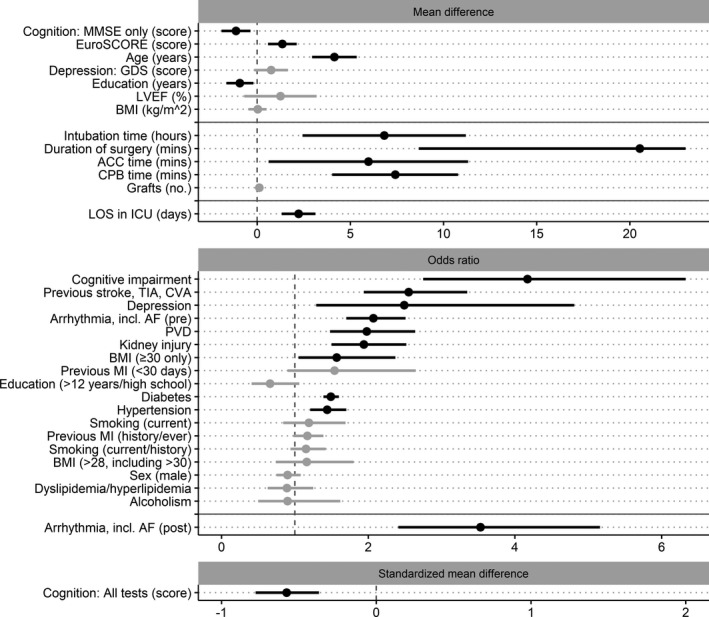
Factors grouped according to the primary pooled estimate of the analysis (mean difference [MD], odds ratio, or standardized MD [SMD]), where solid gray horizontal lines indicate separation of preoperative, intraoperative, and postoperative factors and dashed gray vertical lines divide protective (left side) and risk (right side) factor estimates. The pooled estimates are ordered by the common calculated effect size (SMD) from largest to smallest (largest at the top). Estimates that are black represent statistically significant factors; those that are gray did not reach statistical significance. The scale for all continuous variables (MD and SMD plots) is listed within each factor name. The CIs for duration of surgery extend further than the visible portion of the figure. This was not shown to allow appropriate visibility of all pooled estimates. ACC indicates aortic cross‐clamp; AF, atrial fibrillation; BMI, body mass index; CPB, cardiopulmonary bypass; CVA, cerebrovascular attack; GDS, Geriatric Depression Scale; ICU, intensive care unit; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MMSE, Mini‐Mental State Examination; PVD, peripheral vascular disease; and TIA, transient ischemic attack.
Preoperative factors that did not reach statistical significance were: the presence of alcoholism, body mass index >28 kg/m2, dyslipidemia/hyperlipidemia, >12 years of education, male sex, previous myocardial infarction, and previous/current smoking; and continuous factors of higher body mass index, depression score, and left ventricular ejection fraction. With respect to intraoperative factors, number of grafts did not reach statistical significance (Table S1 and Figure 2).
Subgroup analyses investigating the effect of diagnostic criteria for delirium (studies using standardized measurement tool along with diagnostic criteria versus studies using no tool) revealed no meaningful differences for any risk factors, with CIs overlapping for all analyses (Table S7). Meta‐regressions with mean age as a model factor (covariate) revealed statistically significant results for risk factors of ACC time (age: β=−1.33, Z=−2.49, P=0.013, R 2=0.50) and LOS in ICU (age: β=−0.22, Z=−1.99, P=0.046, R 2=0.10). These results suggest that as the mean age of the study sample increases, the delirium risk associated with ACC time and LOS in ICU decreases. The results also suggest that 50% (for ACC time) and 10% (for LOS in ICU) of the variance in delirium presence relating to these risk factors can be attributed to age.
Acute Cognitive Decline (Immediately to 1‐Month Post‐CABG)
Data from 35 individual studies were used within 30 analyses (including subcategory analyses), investigating 25 separate risk or protective factors for the presence of cognitive decline acutely (immediately up to 1 month) post‐CABG. Across the analyses, heterogeneity of statistically significant results spanned from low to high (I2 range, 0–92.85; τ2 range, 0–32.28) (see Table S2 for results of each meta‐analysis and Figure S3 for forest plots). Potential small study effect was found in 2 analyses. Trim and fill estimation for preoperative age led to a decrease in effect size (see Figure S4 for funnel plots and small study effect investigation). A sensitivity analysis was performed for postoperative delirium (removal of outlier), which resulted in a decrease in effect size (Table S2 and Figure 3).
Figure 3. Forest plots of pooled estimates for risk or protective factors of post–coronary artery bypass grafting acute cognitive decline.
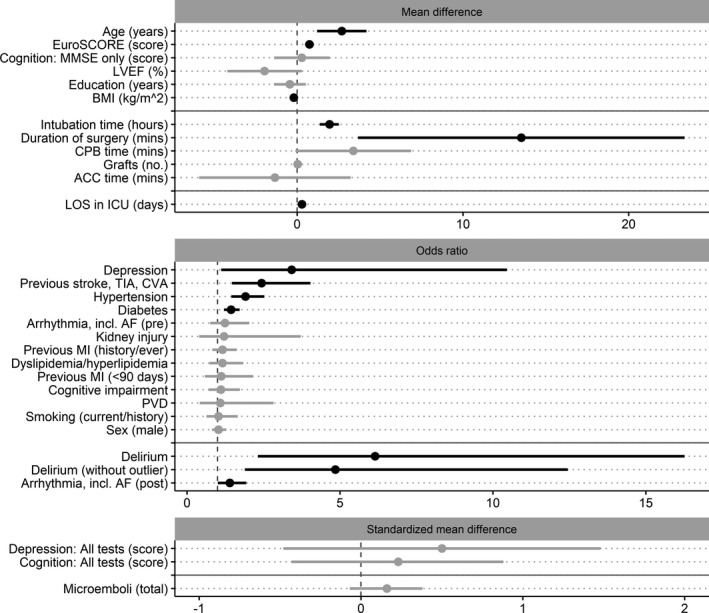
Factors grouped according to the primary pooled estimate of the analysis (mean difference [MD], odds ratio, or standardized MD [SMD]), where solid gray horizontal lines indicate separation of preoperative, intraoperative, and postoperative factors and dashed gray vertical lines divide protective (left side) and risk (right side) factor estimates. The pooled estimates are ordered by the common calculated effect size (SMD) from largest to smallest (largest at the top). Estimates that are black represent statistically significant factors; those that are gray did not reach statistical significance. The scale for all continuous variables (MD and SMD plots) is listed within each factor name. ACC indicates aortic cross‐clamp; AF, atrial fibrillation; BMI, body mass index; CPB, cardiopulmonary bypass; CVA, cerebrovascular attack; ICU, intensive care unit; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MMSE, Mini‐Mental State Examination; PVD, peripheral vascular disease; and TIA, transient ischemic attack.
Statistically significant preoperative risk factors for acute post‐CABG cognitive decline, from largest to smallest effect size, were: the presence of depression, stroke history, hypertension, and diabetes mellitus, along with continuous risk factors of older age and higher EuroSCORE. Statistically significant intraoperative continuous risk factors, from largest to smallest effect size, were increased intubation time (hours) and duration of surgery (minutes). Statistically significant postoperative risk factors, from largest to smallest effect size, were: the presence of delirium and arrhythmia, including AF, and the continuous risk factor of increased LOS in the ICU (days). Higher body mass index was a statistically significant protective factor for acute post‐CABG cognitive decline (Table S2 and Figure 3).
Preoperative factors that did not reach statistical significance were the presence of arrhythmia, including AF, cognitive impairment, dyslipidemia/hyperlipidemia, male sex, kidney injury/disease, previous myocardial infarction, peripheral vascular disease, and previous/current smoking; and continuous factors of higher cognitive test score, depression score, years of education, and lower left ventricular ejection fraction. Intraoperative factors that did not reach statistical significance were increase in ACC time (minutes), CPB time (minutes), number of grafts, and total microemboli count (Table S2 and Figure 3).
Meta‐regressions revealed that 49% of the variance in acute cognitive decline for the risk factor of increased CPB time (age: β=−0.88, Z=−2.24, P=0.025, R 2=0.49) can be attributed to age. These results suggest that as the mean age of the study sample increases, the risk of cognitive decline associated with CPB time decreases.
Midterm Cognitive Decline (1–6 Months Post‐CABG)
Data from 24 individual studies were used within 19 analyses (including subcategory analyses), investigating 17 separate risk or protective factors for the presence of cognitive decline in the midterm (1–6 months) post‐CABG. Across the analyses, heterogeneity of statistically significant results spanned from low to moderate (I2 range, 0–68.84; τ2 range, 0–0.04) (see Table S3 for results of each meta‐analysis and Figure S5 for forest plots). Two analyses revealed statistically significant results, with no indication of small study effect (Figure S6). Preoperative depression and higher cognitive test scores (across all tests) were risk factors for midterm post‐CABG cognitive decline (Table S3 and Figure 4).
Figure 4. Forest plots of pooled estimates for risk or protective factors of post–coronary artery bypass grafting midterm cognitive decline.
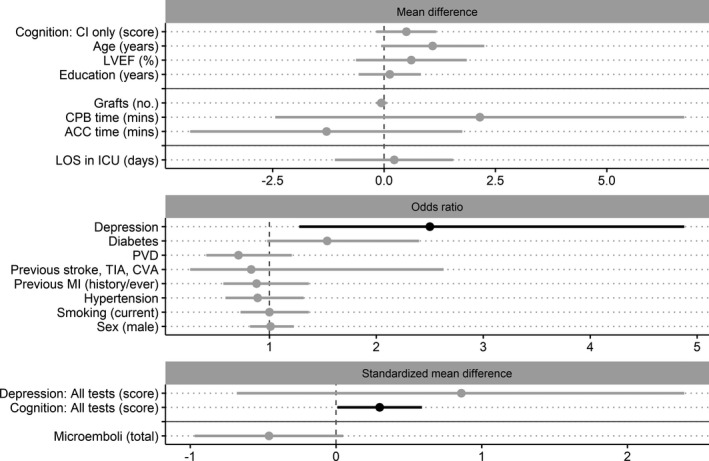
Factors grouped according to the primary pooled estimate of the analysis (mean difference [MD], odds ratio, or standardized MD [SMD]), where solid gray horizontal lines indicate separation of preoperative, intraoperative, and postoperative factors and dashed gray vertical lines divide protective (left side) and risk (right side) factor estimates. The pooled estimates are ordered by the common calculated effect size (SMD) from largest to smallest (largest at the top). Estimates that are black represent statistically significant factors; those that are gray did not reach statistical significance. The scale for all continuous variables (MD and SMD plots) is listed within each factor name. ACC indicates aortic cross‐clamp; CI, cognitive index score; CPB, cardiopulmonary bypass; CVA, cerebrovascular attack; ICU, intensive care unit; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PVD, peripheral vascular disease; and TIA, transient ischemic attack.
Preoperative factors that did not reach statistical significance were the presence of diabetes mellitus, male sex, hypertension, previous myocardial infarction, stroke history, peripheral vascular disease, and current smoking; and continuous factors of higher age, cognitive test score (when using cognitive index), depression score, years of education, and left ventricular ejection fraction. No intraoperative or postoperative factors reached statistical significance, including increase in ACC time (minutes), CPB time (minutes), number of grafts, total microemboli count, and LOS in ICU (days) (Table S3 and Figure 4). No meta‐regressions investigating the influence of age were significant for this time point.
Long‐Term Cognitive Decline (12–15 Months Post‐CABG)
Data from 5 individual studies were used within 6 separate risk factor analyses for cognitive decline in the long‐term (12–15 months) post‐CABG. No analyses revealed statistically significant results, including presence of preoperative cognitive impairment, diabetes mellitus, male sex, and hypertension, nor older age or higher number of intraoperative grafts (see Table S4 for results of each meta‐analysis, Figure S7 for forest plots, and Figure 5). No meta‐regressions were performed for this time point.
Figure 5. Forest plots of pooled estimates for risk or protective factors of post–coronary artery bypass grafting long‐term cognitive decline.
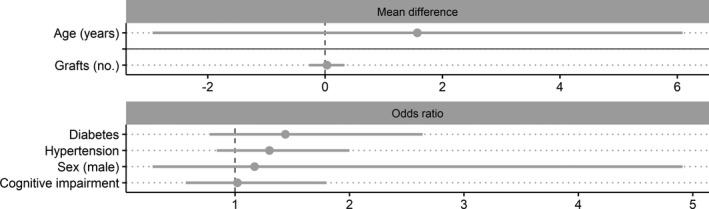
Factors grouped according to the primary pooled estimate of the analysis (mean difference [MD], odds ratio, or standardized MD [SMD]), where solid gray horizontal lines indicate separation of preoperative, intraoperative, and postoperative factors and dashed gray vertical lines divide protective (left side) and risk (right side) factor estimates. The pooled estimates are ordered by the common calculated effect size (SMD) from largest to smallest (largest at the top). Estimates that are black represent statistically significant factors; those that are gray did not reach statistical significance. The scale for all continuous variables (MD and SMD plots) is listed within each factor name.
Discussion
This meta‐analysis quantifies data from >60 000 patients to identify risk and protective factors for the development of cognitive decline, including delirium, immediately following CABG and in the midterm and long‐term. Findings highlight that there are many risk factors for both delirium and cognitive decline following CABG. These factors could be integrated into existing delirium tools or shortlisted in the development of prediction tools for postoperative cognitive decline. 31 , 32 Further development of these clinical risk screening tools for both delirium and cognitive decline post‐CABG could lead to more accurate identification of at‐risk patients, improved prognosis, targeting of interventions, and patient management.
Risk prediction for delirium has been discussed at length for nonsurgical patients, with current models generally thought to have inadequate accuracy. 32 Most published delirium prediction tools are based on individual clinical studies with low statistical power, decreasing their generalizability. 33 , 34 , 35 , 36 To our knowledge, no tools have been developed for predicting postoperative cognitive decline, nor have they been developed for delirium specifically following CABG. The results of this meta‐analysis can provide a shortlist of risk and protective factors that should be considered in future research for the modeling of prediction tools. Specifically, results should be considered when modifying or developing tools related to post‐CABG cognitive outcomes, as the operative process differs from other surgeries (eg, the use of CPB). Similar risk and protective factors may be applicable to other surgery types (cardiac and noncardiac); however, these factors cannot be ascertained from the current meta‐analysis. The development of CABG‐specific tools (delirium and cognitive decline) may lead to better prognosis, because of earlier identification and risk reduction strategies.
Delirium has been said to be preventable in up to 40% of cases. 9 Recent editorials 37 , 38 have highlighted the importance of decreasing the incidence of delirium and cognitive decline to decrease patient and economic burden. In this meta‐analysis, modifiable risk factors, such as the presence of preoperative depression, diabetes mellitus, and hypertension, were found to increase the risk (ORs, 1.44—3.42) for both delirium and cognitive decline acutely post‐CABG. Future research should investigate the effectiveness of implementing preoperative management strategies of these factors on cognitive outcomes (delirium and cognitive decline) post‐CABG. The presence of cognitive impairment resulted in over a 4‐fold increase in risk of developing post‐CABG delirium. Cognition is known to be modifiable through cognitive training in older populations, including those presenting with heart failure, 39 , 40 , 41 and therefore may be a viable preoperative target of intervention. 42
In this meta‐analysis, preoperative depression moderately (moderate effect sizes) increased the risk of delirium (OR, 2.49), acute cognitive decline (OR, 3.42), and midterm cognitive decline (OR, 2.50) post‐CABG. In addition, a higher preoperative depression score revealed moderate to large (SMD, 0.50–0.86) increases in the risk of developing acute and midterm cognitive decline post‐CABG, yet these analyses were not statistically significant, possibly because of high heterogeneity (I2, 93.32–96.08; τ2, 0.92–1.75). Depression in late life is known to occur concurrently with cognitive impairment and can hasten the onset of dementia. 43 The presence of vascular disease (indicative of undergoing CABG) is considered to have a strong link to the development of depression and dementia. 44 Therefore, the effects seen across the meta‐analyses in relation to depression may not be independent from other factors. We endeavored to investigate the influence of these factors through meta‐regression, yet it was not possible because of limited studies concurrently reporting data relating to depression, cognitive impairment, and vascular disease (eg, peripheral vascular disease, hypertension, and dyslipidemia).
The presence of delirium following CABG resulted in a near 5‐fold increase (OR, 4.85, following sensitivity analysis) in risk of acute post‐CABG cognitive decline (up to 1 month). This pooled effect size was not adjusted for any preoperative or intraoperative risk factors and, therefore, its independence cannot be assumed and should be interpreted with this in mind. It may be argued that in a short‐term setting, this risk can be inflated because of the cognitive deficits of the delirium episode itself. However, the presence of delirium at this time (acute cognitive decline) is unlikely, as the assessment period for the 3 included studies was between days 7 and 9, whereas we know delirium typically resolves by day 5. 45 , 46 , 47 No studies reported data related to associations between post‐CABG delirium and cognitive decline in the midterm and long‐term. Delirium in late life (not specifically surgery related) is associated with doubling the rate of cognitive decline 37 and greatly increases the risk of incident dementia. 48 It should therefore be a priority for surgery‐related research to investigate if post‐CABG delirium has similar impact on long‐term cognitive decline and even dementia incidence.
Only 5 studies assessed cognitive decline in the long‐term (>12 months post‐CABG), restricting risk or protective factors that could be extracted. These analyses revealed no significant results, likely because of smaller sample sizes and study variability. Cognitive decline is seen in nearly 40% of patients 1 to 5 years post‐CABG. 4 The presence of cognitive decline is associated with decreased quality of life, functional capacity, and increased rates of depression. 7 In addition, longer‐term cognitive decline can lead to a loss of support networks, such as friends and neighbors, and can strain familial relationships. 49 Yet, from this meta‐analysis, because of the lack of data at this time point, no possible risk reduction strategies can be suggested.
Meta‐regressions generally found that age was not related to the pooled effect estimates. The 3 significant meta‐regressions (delirium: ACC time and LOS in ICU; acute cognitive decline: CPB time) revealed a negative relationship with age, meaning as mean age of the study sample increased, the effect of the risk factor decreased. For example, as age increased, there was a smaller difference in ACC time between those who developed delirium and those who did not. These results could be influenced by older age increasing the risk of post‐CABG complications (eg, AF, dialysis, reintubation, and stroke). 50 These complications are likely to increase LOS in the ICU, regardless of the presence of delirium or cognitive decline. In addition, because of increased complications, greater surgical precautions may be taken with older adults (eg, prioritizing dangerously stenosed arteries over complete revascularization of coronary arteries), which may decrease overall ACC and CPB time, minimizing group differences. Although these meta‐regressions reached significance, most of the variance (≥50%) was not explained by age. Therefore, these risk factors should still be considered clinically meaningful.
This meta‐analysis revealed multiple risk factors for post‐CABG delirium and cognitive decline based on group‐level data from included studies. Future research could identify clusters of risk factors by accessing patient‐level data. This investigation could be guided by common risk factors identified in this meta‐analysis, specifically depression, cognitive impairment, stroke history, diabetes mellitus, and vascular factors (hypertension and AF).
This is the only meta‐analysis to investigate risk and protective factors for multiple outcomes (delirium and cognitive decline) across multiple time points in patients undergoing CABG. Although this study is not without limitations, the pooled sample size is >60 000 patients, allowing for greater generalizability of the results. The pooled results of this meta‐analysis cannot be directly compared across time (for cognitive decline), as the same individuals are not represented at all time points. As only studies published in English were included, there may be a geographical bias. All extracted data within this meta‐analysis were unadjusted for covariates, which does not permit investigation of independence. In addition, no temporal adjustments were conducted (eg, adjusting for preoperative depression within the intraoperative and postoperative factor meta‐analyses). Therefore, caution should be used in interpreting study results, especially on the utility of identified intraoperative and postoperative risk factors in risk prediction tools. Within the literature, substantially fewer articles investigated midterm and long‐term cognitive decline (than acute cognitive decline), which means that there may be important risk factors for these time periods that our approach could not identify. Many analyses conducted herein resulted in medium to high heterogeneity. Investigation into small study effect (publication bias) generally did not change the conclusions of this study (Figures S2, S4, and S6). The heterogeneity may be partially driven by the wide range of tests, screening tools, and methods of classifying delirium and cognitive decline within the included studies (Tables S8 and S9), although, notably, our subgroup analyses for delirium diagnosis (when using a diagnostic tool versus no tool) revealed no meaningful differences (Table S7).
Conclusions
There are many risk factors for delirium and cognitive decline (acutely and in the midterm) following CABG, which could be used in clinical practice, including the development or modification of a clinical prediction tool. Use of a CABG‐specific risk tool could improve prognosis and, in turn, lead to better patient management. This is especially critical for delirium, as it is severely underrecognized and has serious outcomes. 9 To improve prediction ability of these risk tools, future development could also integrate the results of functional neuroimaging (eg, electroencephalography) and biomarker research, related to CABG.
The most clinically meaningful finding from this meta‐analysis was the identification of modifiable preoperative risk factors for delirium and cognitive decline, of depression, diabetes mellitus, hypertension, and cognitive impairment. Improving the management of depression, diabetes mellitus, and hypertension in a preoperative setting may result in reductions in incident delirium and cognitive decline post‐CABG. Targeting cognitive impairment through cognitive training interventions also has potential. Even if these are small reductions in incidence rates, they will have great impact at scale. Future work should investigate if we can target modifiable risk factors to reduce the incidence of delirium and cognitive decline post‐CABG.
Sources of Funding
D. Greaves is supported by the Australian Government Research Training Program Scholarship. Dr Keage is supported by a National Health and Medical Research Council Boosting Dementia Research Leadership Fellowship (GNT1135676) and the National Heart Foundation of Australia Vanguard Grant (101758–VG 2017). Dr Psaltis is supported by a National Heart Foundation of Australia Future Leader Fellowship (FLF100412) and a National Health and Medical Research Council Career Development Fellowship (CDF1161506). Dr Davis is supported by a Wellcome Trust Intermediate Clinical Fellowship (WT107467). Dr Lampit is supported by a National Health and Medical Research Council–Australian Research Council Dementia Research Development Fellowship (GNT1108520). Dr Smith is supported by a National Health and Medical Research Council–Australian Research Council Dementia Research Development Fellowship (GNT1097397). This project was supported by a National Heart Foundation of Australia Vanguard Grant (101758–VG 2017).
Disclosures
None.
Supporting information
Acknowledgments
The authors would like to acknowledge and thank Monique Boord, who was involved in the screening of articles from the original search, before being updated for this meta‐analysis.
(J Am Heart Assoc. 2020;9:e017275 DOI: 10.1161/JAHA.120.017275.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Head S, Milojevic M, Taggart D, Puskas J. Current practice of state‐of-the‐art surgical coronary revascularization. Circulation. 2017;136:1331–1345. [DOI] [PubMed] [Google Scholar]
- 2. Melly L, Torregrossa G, Lee T, Jansens J‐L, Puskas J. Fifty years of coronary artery bypass grafting. J Thorac Dis. 2018;10:1960–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Velazquez E, Lee K, Jones R, Al‐Khalidi H, Hill J, Panza J, Michler R, Bonow R, Doenst T, Petrie M, et al. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greaves D, Psaltis P, Ross T, Davis D, Smith A, Boord M, Keage H. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta‐analysis of 91,829 patients. Int J Cardiol. 2019;289:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman M, Kirchner J, Phillips‐Bute B, Gaver V, Grocott H, Jones R, Mark D, Reves J, Blumenthal J. Longitudinal assessment of neurocognitive function after coronary‐artery bypass surgery. N Engl J Med. 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 6. Santos F, Velasco I, Fráguas R Jr. Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16:175–193. [PubMed] [Google Scholar]
- 7. Phillips‐Bute B, Mathew J, Blumenthal J, Grocott H, Laskowitz D, Jones R, Mark D, Newman M. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–375. [DOI] [PubMed] [Google Scholar]
- 8. Fong T, Davis D, Growdon M, Albuquerque A, Inouye S. The interface of delirium and dementia in older persons. Lancet Neurol. 2015;14:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inouye S, Westendorp R, Saczynski J. Delirium in elderly people. Lancet. 2014;383:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, Elmi‐Sarabi M, Hiebert B, Macdonald K, Giles‐Smith L, et al. Long‐term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. 2016;102:1391–1399. [DOI] [PubMed] [Google Scholar]
- 11. Robinson T, Raeburn C, Tran Z, Angles E, Brenner L, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. [DOI] [PubMed] [Google Scholar]
- 12. Holinski S, Claus B, Alaaraj N, Dohmen P, Kirilova K, Neumann K, Uebelhack R, Konertz W. Cerebroprotective effect of piracetam in patients undergoing coronary bypass surgery. Med Sci Monitor. 2008;14:Pi53–Pi57. [PubMed] [Google Scholar]
- 13. Waegemans T, Wilsher C, Danniau A, Ferris S, Kurz A, Winblad B. Clinical efficacy of piracetam in cognitive impairment: a meta‐analysis. Dement Geriatr Cogn Disord. 2002;13:217–224. [DOI] [PubMed] [Google Scholar]
- 14. Ottens T, Dieleman J, Sauër A‐M, Peelen L, Nierich A, de Groot W, Nathoe H, Buijsrogge M, Kalkman C, van Dijk D. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121:492–500. [DOI] [PubMed] [Google Scholar]
- 15. Sun J, Wu X, Wang W, Jin L. Cognitive dysfunction after off‐pump versus on‐pump coronary artery bypass surgery: a meta‐analysis. J Int Med Res. 2012;40:852–858. [DOI] [PubMed] [Google Scholar]
- 16. Kennedy E, Choy K, Alston R, Chen S, Farhan‐Alanie M, Anderson J, Ang Y, Moore D, MacKenzie S, Sykes R. Cognitive outcome after on‐ and off‐pump coronary artery bypass grafting surgery: a systematic review and meta‐analysis. J Cardiothorac Vasc Anesth. 2013;27:253–265. [DOI] [PubMed] [Google Scholar]
- 17. Bhamidipati D, Goldhammer J, Sperling M, Torjman M, McCarey M, Whellan D. Cognitive outcomes after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31:707–718. [DOI] [PubMed] [Google Scholar]
- 18. Liu X, Xie G, Zhang K, Song S, Song F, Jin Y, Fang X. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta‐analysis with trial sequential analysis of randomized controlled trials. J Crit Care. 2017;38:190–196. [DOI] [PubMed] [Google Scholar]
- 19. Tao R, Wang X‐W, Pang L‐J, Cheng J, Wang Y‐M, Gao G‐Q, Liu Y, Wang C. Pharmacologic prevention of postoperative delirium after on‐pump cardiac surgery: a meta‐analysis of randomized trials. Medicine (Baltimore). 2018;97:e12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, Clarke M, Devereaux P, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, et al. Chapter 7: systematic reviews of etiology and risk In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer's Manual. Adelaide, South Australia, Australia: The Joanna Briggs Institute; 2017. 219–269. [Google Scholar]
- 22. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–153. [DOI] [PubMed] [Google Scholar]
- 23. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2016;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 27. Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, Carpenter J, Rücker G, Harbord R, Schmid C, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 30. Deeks J, Higgins J, Altman D. Chapter 10: analysing data and undertaking meta‐analyses In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2019. 241–284. [Google Scholar]
- 31. van Meenen L, van Meenen D, de Rooij S, ter Riet G. Risk prediction models for postoperative delirium: a systematic review and meta‐analysis. J Am Geriatr Soc. 2014;62:2383–2390. [DOI] [PubMed] [Google Scholar]
- 32. Lindroth H, Bratzke L, Purvis S, Brown R, Coburn M, Mrkobrada M, Chan M, Davis D, Pandharipande P, Carlsson C, et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open. 2018;8:e019223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winterer G, Androsova G, Bender O, Boraschi D, Borchers F, Dschietzig T, Feinkohl I, Fletcher P, Gallinat J, Hadzidiakos D, et al. Personalized risk prediction of postoperative cognitive impairment – rationale for the EU‐funded BioCog project. Eur Psychiatry. 2018;50:34–39. [DOI] [PubMed] [Google Scholar]
- 34. Marcantonio E, Goldman L, Mangione C, Ludwig L, Muraca B, Haslauer C, Donaldson M, Whittemore A, Sugarbaker D, Poss R, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 35. Xing H, Zhou W, Fan Y, Wen T, Wang X, Chang G. Development and validation of a postoperative delirium prediction model for patients admitted to an intensive care unit in China: a prospective study. BMJ Open. 2019;9:e030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mestres Gonzalvo C, de Wit H, van Oijen B, Deben D, Hurkens K, Mulder W, Janknegt R, Schols J, Verhey F, Winkens B, et al. Validation of an automated delirium prediction model (DElirium MOdel (DEMO)): an observational study. BMJ Open. 2017;7:e016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fong T, Inouye S, Jones R. Delirium, dementia, and decline. JAMA Psychiatry. 2017;74:212–213. [DOI] [PubMed] [Google Scholar]
- 38. Keage H, Smith A, Loetscher T, Psaltis P. Cognitive outcomes of cardiovascular surgical procedures in the old: an important but neglected area. Heart Lung Circ. 2016;25:1148–1153. [DOI] [PubMed] [Google Scholar]
- 39. Hill N, Mowszowski L, Naismith S, Chadwick V, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta‐analysis. Am J Psychiatry. 2016;174:329–340. [DOI] [PubMed] [Google Scholar]
- 40. Kelly M, Loughrey D, Lawlor B, Robertson I, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta‐analysis. Ageing Res Rev. 2014;15:28–43. [DOI] [PubMed] [Google Scholar]
- 41. Ellis M, Edwards J, Peterson L, Roker R, Athilingam P. Effects of cognitive speed of processing training among older adults with heart failure. J Aging Health. 2014;26:600–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greaves D, Psaltis P, Lampit A, Davis D, Smith A, Bourke A, Worthington M, Valenzuela M, Keage H. Computerised cognitive training to improve cognition including delirium following coronary artery bypass grafting surgery: protocol for a blinded randomised controlled trial. BMJ Open. 2020;10:e034551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennett S, Thomas A. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79:184–190. [DOI] [PubMed] [Google Scholar]
- 44. Byers A, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whitlock E, Vannucci A, Avidan M. Postoperative delirium. Minerva Anestesiol. 2011;77:448–456. [PMC free article] [PubMed] [Google Scholar]
- 46. Eide L, Ranhoff A, Fridlund B, Haaverstad R, Hufthammer K, Kuiper K, Nordrehaug J, Norekvål T. Comparison of frequency, risk factors, and time course of postoperative delirium in octogenarians after transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol. 2015;115:802–809. [DOI] [PubMed] [Google Scholar]
- 47. Zhang W, Hu W, Shen M, Ye X, Huang Y, Sun Y. Profiles of delirium and the clinical outcomes of patients who underwent coronary artery bypass grafting: a prospective study from China. J Clin Nurs. 2016;25:631–641. [DOI] [PubMed] [Google Scholar]
- 48. Davis D, Muniz‐Terrera G, Keage H, Rahkonen T, Oinas M, Matthews F, Cunningham C, Polvikoski T, Sulkava R, MacLullich A, et al. Delirium is a strong risk factor for dementia in the oldest‐old: a population‐based cohort study. Brain. 2012;135:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aartsen M, van Tilburg T, Smits C, Knipscheer K. A longitudinal study of the impact of physical and cognitive decline on the personal network in old age. J Soc Pers Relat. 2004;21:249–266. [Google Scholar]
- 50. Mortasawi A, Arnrich B, Walter J, Frerichs I, Rosendahl U, Ennker J. Impact of age on the results of coronary artery bypass grafting. Asian Cardiovasc Thorac Ann. 2004;12:324–329. [DOI] [PubMed] [Google Scholar]
- 51. Al Tmimi L, Van de Velde M, Meyns B, Meuris B, Sergeant P, Milisen K, Pottel H, Poesen K, Rex S. Serum protein S100 as marker of postoperative delirium after off‐pump coronary artery bypass surgery: secondary analysis of two prospective randomized controlled trials. Clin Chem Lab Med. 2016;54:1671–1680. [DOI] [PubMed] [Google Scholar]
- 52. Baba T, Goto T, Maekawa K, Ito A, Yoshitake A, Koshiji T. Early neuropsychological dysfunction in elderly high‐risk patients after on‐pump and off‐pump coronary bypass surgery. J Anesth. 2007;21:452–458. [DOI] [PubMed] [Google Scholar]
- 53. Boodhwani M, Rubens F, Wozny D, Rodriguez R, Alsefaou A, Hendry P, Nathan H. Predictors of early neurocognitive deficits in low‐risk patients undergoing on‐pump coronary artery bypass surgery. Circulation. 2006;114:461–466. [DOI] [PubMed] [Google Scholar]
- 54. Braekken S, Reinvang I, Russell D, Brucher R, Svennevig J. Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J Neurol Neurosurg Psychiatry. 1998;65:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bucerius J, Gummert J, Walther T, Doll N, Barten M, Falk V, Mohr F. Diabetes in patients undergoing coronary artery bypass grafting: impact on perioperative outcome. Z Kardiol. 2005;94:575–582. [DOI] [PubMed] [Google Scholar]
- 56. Caldas J, Panerai R, Bor‐Seng-Shu E, Ferreira G, Camara L, Passos R, de‐Lima-Oliveira M, Galas F, Almeida JP, Nogueira R. Dynamic cerebral autoregulation: a marker of post‐operative delirium? Clin Neurophysiol. 2019;130:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Y, Ding S, Tao X, Feng X, Lu S, Shen Y, Wu Y, An X. The quality of life of patients developed delirium after coronary artery bypass grafting is determined by cognitive function after discharge: a cross‐sectional study. Int J Nurs Pract. 2017;23:e12563. [DOI] [PubMed] [Google Scholar]
- 58. Christiansen C, Berg R, Plovsing R, Ronit A, Holstein‐Rathlou N‐H, Yndgaard S, Møller K. Dynamic cerebral autoregulation after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2016;64:569–574. [DOI] [PubMed] [Google Scholar]
- 59. Coffey C, Massey E, Roberts K, Curtis S, Jones R, Pryor D. Natural history of cerebral complications of coronary artery bypass graft surgery. Neurology. 1983;33:1416–1421. [DOI] [PubMed] [Google Scholar]
- 60. Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric‐Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg. 2015;47:447–454. [DOI] [PubMed] [Google Scholar]
- 61. Cumurcu B, Karlidag R, Unal S, Sezer O, Battaloglu B, Mendil D, But K, Etikan I. Plasma iron, copper, zinc levels in patients experiencing delirium following coronary artery bypass grafting. Neurol Psychiatry Brain Res. 2008;15:167–174. [Google Scholar]
- 62. de Tournay‐Jette E, Dupuis G, Bherer L, Deschamps A, Cartier R, Denault A. The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:95–104. [DOI] [PubMed] [Google Scholar]
- 63. Dieleman J, Sauer A, Klijn C, Nathoe H, Moons K, Kalkman C, Kappelle J, Van Dijk D. Presence of coronary collaterals is associated with a decreased incidence of cognitive decline after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2009;35:48–53. [DOI] [PubMed] [Google Scholar]
- 64. Djaiani G, Phillips‐Bute B, Blumenthal J, Newman M. Chronic exposure to nicotine does not prevent neurocognitive decline after cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17:341–345. [DOI] [PubMed] [Google Scholar]
- 65. Dong S, Li C, Liang W, Chen M, Bi Y, Li X. Postoperative plasma copeptin levels independently predict delirium and cognitive dysfunction after coronary artery bypass graft surgery. Peptides. 2014;59:70–74. [DOI] [PubMed] [Google Scholar]
- 66. Eriksson M, Samuelsson E, Gustafson Y, Aberg T, Engstrom K. Delirium after coronary bypass surgery evaluated by the organic brain syndrome protocol. Scand Cardiovasc J. 2002;36:250–255. [DOI] [PubMed] [Google Scholar]
- 67. Goto T, Baba T, Yoshitake A, Shibata Y, Ura M, Sakata R. Craniocervical and aortic atherosclerosis as neurologic risk factors in coronary surgery. Ann Thorac Surg. 2000;69:834–840. [DOI] [PubMed] [Google Scholar]
- 68. Gottesman R, Grega M, Bailey M, Pham L, Zeger S, Baumgartner W, Selnes O, McKhann G. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hall R, Fordyce D, Lee M, Eisenberg B, Lee R, Holmes J, Campbell W. Brain spect imaging and neuropsychological testing in coronary artery bypass patients: single photon emission computed tomography. Ann Thorac Surg. 1999;68:2082–2088. [DOI] [PubMed] [Google Scholar]
- 70. Harmon D, Ghori K, Eustace N, O'Callaghan S, O'Donnell A, Shorten G. Aprotinin decreases the incidence of cognitive deficit following CABG and cardiopulmonary bypass: a pilot randomized controlled study. Can J Anaesth. 2004;51:1002–1009. [DOI] [PubMed] [Google Scholar]
- 71. Harmon D, Eustace N, Ghori K, Butler M, O'Callaghan S, O'Donnell A, Moore‐Groarke G, Shorten G. Plasma concentrations of nitric oxide products and cognitive dysfunction following coronary artery bypass surgery. Eur J Anaesthesiol. 2005;22:269–276. [DOI] [PubMed] [Google Scholar]
- 72. Humphreys J, Denson L, Baker R, Tully P. The importance of depression and alcohol use in coronary artery bypass graft surgery patients: risk factors for delirium and poorer quality of life. J Geriatr Cardiol. 2016;13:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kadoi Y, Saito S, Goto F, Fujita N. Decrease in jugular venous oxygen saturation during normothermic cardiopulmonary bypass predicts short‐term postoperative neurologic dysfunction in elderly patients. J Am Coll Cardiol. 2001;38:1450–1455. [DOI] [PubMed] [Google Scholar]
- 74. Kadoi Y, Saito S, Goto F, Fujita N. Slow rewarming has no effects on the decrease in jugular venous oxygen hemoglobin saturation and long‐term cognitive outcome in diabetic patients. Anesth Analg. 2002;94:1395–1401. [DOI] [PubMed] [Google Scholar]
- 75. Kadoi Y, Saito S, Kunimoto F, Goto F, Fujita N. Comparative effects of propofol versus fentanyl on cerebral oxygenation state during normothermic cardiopulmonary bypass and postoperative cognitive dysfunction. Ann Thorac Surg. 2003;75:840–846. [DOI] [PubMed] [Google Scholar]
- 76. Kadoi Y, Saito S, Fujita N, Goto F. Risk factors for cognitive dysfunction after coronary artery bypass graft surgery in patients with type 2 diabetes. J Thorac Cardiovasc Surg. 2005;129:576–583. [DOI] [PubMed] [Google Scholar]
- 77. Kadoi Y, Goto F. Sevoflurane anesthesia did not affect postoperative cognitive dysfunction in patients undergoing coronary artery bypass graft surgery. J Anesth. 2007;21:330–335. [DOI] [PubMed] [Google Scholar]
- 78. Kadoi Y, Kawauchi C, Kuroda M, Takahashi K, Saito S, Fujita N, Mizutani A. Association between cerebrovascular carbon dioxide reactivity and postoperative short‐term and long‐term cognitive dysfunction in patients with diabetes mellitus. J Anesth. 2011;25:641–647. [DOI] [PubMed] [Google Scholar]
- 79. Kadoi Y, Kawauchi C, Ide M, Kuroda M, Takahashi K, Saito S, Fujita N, Mizutani A. Preoperative depression is a risk factor for postoperative short‐term and long‐term cognitive dysfunction in patients with diabetes mellitus. J Anesth. 2011;25:10–17. [DOI] [PubMed] [Google Scholar]
- 80. Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R, Sobow T, Kloszewska I. Mild cognitive impairment with associated inflammatory and cortisol alterations as independent risk factor for postoperative delirium. Dement Geriatr Cogn Disord. 2014;38:65–78. [DOI] [PubMed] [Google Scholar]
- 81. Kazmierski J, Sieruta M, Banys A, Jaszewski R, Sobow T, Liberski P, Kloszewska I. The assessment of the T102C polymorphism of the 5HT2a receptor gene, 3723G/A polymorphism of the NMDA receptor 3A subunit gene (GRIN3A) and 421C/A polymorphism of the NMDA receptor 2B subunit gene (GRIN2B) among cardiac surgery patients with and without delirium. Gen Hosp Psychiatry. 2014;36:753–756. [DOI] [PubMed] [Google Scholar]
- 82. Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL‐2 and TNF‐alpha concentrations are associated with postoperative delirium in patients undergoing coronary‐artery bypass graft surgery. Int Psychogeriatr. 2014;26:845–855. [DOI] [PubMed] [Google Scholar]
- 83. Khan I, Khan A, Gull S, Kausar S, Iqbal M, Waheed A. Incidence and predictors of delirium in postoperative coronary artery bypass surgery patients in Pakistani population. Pak J Med Health Sci. 2014;8:92–97. [Google Scholar]
- 84. Khatri P, Babyak M, Clancy C, Davis R, Croughwell N, Newman M, Reves J, Mark D, Blumenthal J. Perception of cognitive function in older adults following coronary artery bypass surgery. Health Psychol. 1999;18:301–306. [DOI] [PubMed] [Google Scholar]
- 85. Kok W, Koerts J, Tucha O, Scheeren T, Absalom A. Neuronal damage biomarkers in the identification of patients at risk of long‐term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia. 2017;72:359–369. [DOI] [PubMed] [Google Scholar]
- 86. Kumpaitiene B, Svagzdiene M, Sirvinskas E, Adomaitiene V, Petkus V, Zakelis R, Krakauskaite S, Chomskis R, Ragauskas A, Benetis R. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypass are related to postoperative cognitive deterioration: prospective observational study. Minerva Anestesiol. 2019;85:594–603. [DOI] [PubMed] [Google Scholar]
- 87. Lachmann G, Feinkohl I, Borchers F, Ottens T, Nathoe H, Sauer A‐M, Dieleman J, Radtke F, van Dijk D, Spies C. Diabetes, but not hypertension and obesity, is associated with postoperative cognitive dysfunction. Dement Geriatr Cogn Disord. 2018;46:193–206. [DOI] [PubMed] [Google Scholar]
- 88. Leenders J, Overdevest E, van Straten B, Golab H. The influence of oxygen delivery during cardiopulmonary bypass on the incidence of delirium in CABG patients: a retrospective study. Perfusion. 2018;33:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li H, Chen Y, Chiu M, Fu M, Huang G, Chen C. Delirium, subsyndromal delirium, and cognitive changes in individuals undergoing elective coronary artery bypass graft surgery. J Cardiovasc Nurs. 2015;30:340–345. [DOI] [PubMed] [Google Scholar]
- 90. Liu Y, Wang D, Li L, Wu X, Shan G, Su Y, Li J, Yu Q, Shi C, Huang Y, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass graft surgery. Anesth Analg. 2009;109:1013–1022. [DOI] [PubMed] [Google Scholar]
- 91. Loponen P, Luther M, Wistbacka J‐O, Nissinen J, Sintonen H, Huhtala H, Tarkka M. Postoperative delirium and health related quality of life after coronary artery bypass grafting. Scand Cardiovasc J. 2008;42:337–344. [DOI] [PubMed] [Google Scholar]
- 92. Mardani D, Bigdelian H. Predictors and clinical outcomes of postoperative delirium after administration of dexamethasone in patients undergoing coronary artery bypass surgery. Int J Prev Med. 2012;3:420–427. [PMC free article] [PubMed] [Google Scholar]
- 93. Mariscalco G, Cottini M, Zanobini M, Salis S, Dominici C, Banach M, Onorati F, Piffaretti G, Covaia G, Realini M, et al. Preoperative statin therapy is not associated with a decrease in the incidence of delirium after cardiac operations. Ann Thorac Surg. 2012;93:1439–1447. [DOI] [PubMed] [Google Scholar]
- 94. Martin B, Buth K, Arora R, Baskett R. Delirium as a predictor of sepsis in post‐coronary artery bypass grafting patients: a retrospective cohort study. Crit Care. 2010;14:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Martin B, Buth K, Arora R, Baskett R. Delirium: a cause for concern beyond the immediate postoperative period. Ann Thorac Surg. 2012;93:1114–1120. [DOI] [PubMed] [Google Scholar]
- 96. Mathew J, Rinder H, Smith B, Newman M, Rinder C. Transcerebral platelet activation after aortic cross‐clamp release is linked to neurocognitive decline. Ann Thorac Surg. 2006;81:1644–1649. [DOI] [PubMed] [Google Scholar]
- 97. Mathew J, Podgoreanu M, Grocott H, White W, Morris R, Stafford‐Smith M, Mackensen G, Rinder C, Blumenthal J, Schwinn D, et al. Genetic variants in P‐selectin and C‐reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. [DOI] [PubMed] [Google Scholar]
- 98. Miyazaki S, Yoshitani K, Miura N, Irie T, Inatomi Y, Ohnishi Y, Kobayashi J. Risk factors of stroke and delirium after off‐pump coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. 2011;12:379–383. [DOI] [PubMed] [Google Scholar]
- 99. Mu D, Wang D, Li L, Shan G, Li J, Yu Q, Shi C. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care. 2010;14:R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mu D, Li L, Wang D, Li N, Shan G, Li J, Yu Q, Shi C. High postoperative serum cortisol level is associated with increased risk of cognitive dysfunction early after coronary artery bypass graft surgery: a prospective cohort study. PLoS One. 2013;8:e77637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Newman S, Smith P, Treasure T, Joseph P, Ell P, Harrison M. Acute neuropsychological consequences of coronary artery bypass surgery. Curr Psychol. 1987;6:115–124. [Google Scholar]
- 102. Nikolić B, Putnik S, Lazovic D, Vranes M. Can we identify risk factors for postoperative delirium in cardiac coronary patients? Our experience. Heart Surg Forum. 2012;15:E195–E199. [DOI] [PubMed] [Google Scholar]
- 103. Norkiene I, Ringaitiene D, Misiuriene I, Samalavicius R, Bubulis R, Baublys A, Uzdavinys G. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J. 2007;41:180–185. [DOI] [PubMed] [Google Scholar]
- 104. Norkiene I, Samalavicius R, Ivaskevicius J, Budrys V, Paulauskiene K. Asymptomatic carotid artery stenosis and cognitive outcomes after coronary artery bypass grafting. Scand Cardiovasc J. 2011;45:169–173. [DOI] [PubMed] [Google Scholar]
- 105. Oh Y, Kim J, Shim J, Yoo K, Lee J, Kwak Y. Diabetes mellitus does not affect jugular bulb oxygen saturation in patients undergoing off‐pump coronary artery bypass graft surgery. Circ J. 2008;72:1259–1264. [DOI] [PubMed] [Google Scholar]
- 106. Oh C‐S, Park S, Hong S, Kang W‐S, Yoon T‐G, Kim S‐H. Postoperative delirium in patients undergoing off‐pump coronary artery bypass grafting according to the anesthetic agent: a retrospective study. J Cardiothorac Vasc Anesth. 2017;31:1988–1995. [DOI] [PubMed] [Google Scholar]
- 107. Oldham M, Hawkins K, Yuh D, Dewar M, Darr U, Lysyy T, Lee H. Cognitive and functional status predictors of delirium and delirium severity after coronary artery bypass graft surgery: an interim analysis of the neuropsychiatric outcomes after heart surgery study. Int Psychogeriatr. 2015;27:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Oldham M, Hawkins K, Lin I‐H, Deng Y, Hao Q, Scoutt L, Yuh D, Lee H. Depression predicts delirium after coronary artery bypass graft surgery independent of cognitive impairment and cerebrovascular disease: an analysis of the neuropsychiatric outcomes after heart surgery study. Am J Geriatr Psychiatry. 2019;27:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Omiya H, Yoshitani K, Yamada N, Kubota Y, Takahashi K, Kobayashi J, Ohnishi Y. Preoperative brain magnetic resonance imaging and postoperative delirium after off‐pump coronary artery bypass grafting: a prospective cohort study. Can J Anaesth. 2015;62:595–602. [DOI] [PubMed] [Google Scholar]
- 110. Otomo S, Maekawa K, Goto T, Baba T, Yoshitake A. Pre‐existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;17:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Palmbergen W, van Sonderen A, Keyhan‐Falsafi A, Keunen R, Wolterbeek R. Improved perioperative neurological monitoring of coronary artery bypass graft patients reduces the incidence of postoperative delirium: the Haga Brain Care Strategy. Interact Cardiovasc Thorac Surg. 2012;15:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, Karck M, Kopitz J. Early postoperative delirium after open‐heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin‐6. Intensive Care Med. 2010;36:2081–2089. [DOI] [PubMed] [Google Scholar]
- 113. Reents W, Muellges W, Franke D, Babin‐Ebell J, Elert O. Cerebral oxygen saturation assessed by near‐infrared spectroscopy during coronary artery bypass grafting and early postoperative cognitive function. Ann Thorac Surg. 2002;74:109–114. [DOI] [PubMed] [Google Scholar]
- 114. Restrepo L, Wityk R, Grega M, Borowicz L, Barker P, Jacobs M, Beauchamp N, Hillis A, McKhann G. Diffusion‐ and perfusion‐weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002;33:2909–2915. [DOI] [PubMed] [Google Scholar]
- 115. Ringaitiene D, Gineityte D, Vicka V, Zvirblis T, Sipylaite J, Irnius A, Ivaskevicius J, Kacergius T. Impact of malnutrition on postoperative delirium development after on pump coronary artery bypass grafting. J Cardiothorac Surg. 2015;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Robson M, Alston R, Deary I, Andrews P, Souter M, Yates S. Cognition after coronary artery surgery is not related to postoperative jugular bulb oxyhemoglobin desaturation. Anesth Analg. 2000;91:1317–1326. [DOI] [PubMed] [Google Scholar]
- 117. Rodriguez R, Rubens F, Wozny D, Nathan H. Cerebral emboli detected by transcranial doppler during cardiopulmonary bypass are not correlated with postoperative cognitive deficits. Stroke. 2010;41:2229–2235. [DOI] [PubMed] [Google Scholar]
- 118. Rolfson D, McElhaney J, Rockwood K, Finnegan B, Entwistle L, Wong J, Suarez‐Almazor M. Incidence and risk factors for delirium and other adverse outcomes in older adults after coronary artery bypass graft surgery. Can J Cardiol. 1999;15:771–776. [PubMed] [Google Scholar]
- 119. Rolfson D, McElhaney J, Jhangri G, Rockwood K. Validity of the confusion assessment method in detecting postoperative delirium in the elderly. Int Psychogeriatr. 1999;11:431–438. [DOI] [PubMed] [Google Scholar]
- 120. Royse A, Royse C, Ajani A, Symes E, Maruff P, Karagiannis S, Gerraty R, Grigg L, Davies S. Reduced neuropsychological dysfunction using epiaortic echocardiography and the exclusive Y graft. Ann Thorac Surg. 2000;69:1431–1438. [DOI] [PubMed] [Google Scholar]
- 121. Royse C, Andrews D, Newman S, Stygall J, Williams Z, Pang J, Royse A. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66:455–464. [DOI] [PubMed] [Google Scholar]
- 122. Rudolph J, Babikian V, Birjiniuk V, Crittenden M, Treanor P, Pochay V, Khuri S, Marcantonio E. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53:462–466. [DOI] [PubMed] [Google Scholar]
- 123. Rudolph J, Jones R, Grande L, Milberg W, King E, Lipsitz L, Levkoff S, Marcantonio E. Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2006;54:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rudolph J, Babikian V, Treanor P, Pochay V, Wigginton J, Crittenden M, Marcantonio E. Microemboli are not associated with delirium after coronary artery bypass graft surgery. Perfusion. 2009;24:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Şahan C, Sungur Z, Çamcı E, Sivrikoz N, Sayin Ö, Gurvit H, Şentürk M. Effects of cerebral oxygen changes during coronary bypass surgery on postoperative cognitive dysfunction in elderly patients: a pilot study. Braz J Anesthesiol. 2018;68:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Scott D, Silbert B, Doyle T, Blyth C, Borton M, O'Brien JL, de L. Horne DJ. Centrifugal versus roller head pumps for cardiopulmonary bypass: effect on early neuropsychologic outcomes after coronary artery surgery. J Cardiothorac Vasc Anesth. 2002;16:715–722. [DOI] [PubMed] [Google Scholar]
- 127. Sevuk U, Baysal E, Ay N, Altas Y, Altindag R, Yaylak B, Alp V, Demirtas E. Relationship between cobalamin deficiency and delirium in elderly patients undergoing cardiac surgery. Neuropsychiatr Dis Treat. 2015;11:2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C, Beyersdorf F. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011;40:200–207. [DOI] [PubMed] [Google Scholar]
- 129. Silbert B, Scott D, Evered L, Lewis M, Kalpokas M, Maruff P, Myles P, Jamrozik K. A comparison of the effect of high‐ and low‐dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology. 2006;104:1137–1145. [DOI] [PubMed] [Google Scholar]
- 130. Silbert B, Evered L, Scott D, McCutcheon C, Jamrozik K. Homocysteine and C‐reactive protein are not markers of cognitive impairment in patients with major cardiovascular disease. Dement Geriatr Cogn Disord. 2008;25:309–316. [DOI] [PubMed] [Google Scholar]
- 131. Slater J, Guarino T, Stack J, Vinod K, Bustami R, Brown JM III, Rodriguez A, Magovern C, Zaubler T, Freundlich K, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–45. [DOI] [PubMed] [Google Scholar]
- 132. Smith P, Treasure T, Newman S, Joseph P, Ell P, Schneidau A, Harrison M. Cerebral consequences of cardiopulmonary bypass. Lancet. 1986;1:823–825. [DOI] [PubMed] [Google Scholar]
- 133. Smith M, Wagenknecht L, Legault C, Goff D, Stump D, Troost B, Rogers A. Age and other risk factors for neuropsychologic decline in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2000;14:428–432. [DOI] [PubMed] [Google Scholar]
- 134. Stump D, Kon N, Rogers A, Hammon J. Emboli and neuropsychological outcome following cardiopulmonary bypass. Echocardiography. 1996;13:555–558. [DOI] [PubMed] [Google Scholar]
- 135. Subramaniam B, Shankar P, Shaefi S, Mueller A, O’Gara B, Banner‐Goodspeed V, Gallagher J, Gasangwa D, Patxot M, Packiasabapathy S. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Suksompong S, Prakanratrana U, Chumpathong S, Sriyoschati S, Pornvilawan S. Neuropsychological alterations after coronary artery bypass graft surgery. J Med Assoc Thai. 2002;85(suppl 3):S910–S916. [PubMed] [Google Scholar]
- 137. Swaminathan M, McCreath B, Phillips‐Bute B, Newman M, Mathew J, Smith P, Blumenthal J, Stafford‐Smith M; Perioperative Outcomes Research Group. Serum creatinine patterns in coronary bypass surgery patients with and without postoperative cognitive dysfunction. Anesth Analg. 2002;95:1–8. [DOI] [PubMed] [Google Scholar]
- 138. Sylivris S, Levi C, Matalanis G, Rosalion A, Buxton B, Mitchell A, Fitt G, Harberts D, Saling M, Tonkin A. Pattern and significance of cerebral microemboli during coronary artery bypass grafting. Ann Thorac Surg. 1998;66:1674–1678. [DOI] [PubMed] [Google Scholar]
- 139. Tagarakis G, Tsolaki‐Tagaraki F, Tsolaki M, Diegeler A, Tsilimingas N, Papassotiropoulos A. The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen. 2007;22:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tamura K, Maruyama T, Sakurai S. Preventive effect of suvorexant for postoperative delirium after coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2019;25:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Toeg H, Nathan H, Rubens F, Wozny D, Boodhwani M. Clinical impact of neurocognitive deficits after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:1545–1549. [DOI] [PubMed] [Google Scholar]
- 142. Trubnikova O, Mamontova A, Syrova I, Maleva O, Barbarash O. Does preoperative mild cognitive impairment predict postoperative cognitive dysfunction after on‐pump coronary bypass surgery? J Alzheimers Dis. 2014;42:S45–S51. [DOI] [PubMed] [Google Scholar]
- 143. Tully P, Baker R, Eld H, Turnbull D. Depression, anxiety disorders and type d personality as risk factors for delirium after cardiac surgery. Aust N Z J Psychiatry. 2010;44:1005–1011. [DOI] [PubMed] [Google Scholar]
- 144. van Dijk D, Moons K, Keizer A, Jansen E, Hijman R, Diephuis J, Borst C, Jaegere P, Grobbee D, Kalkman C. Association between early and three month cognitive outcome after off‐pump and on‐pump coronary bypass surgery. Heart. 2004;90:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yilmaz S, Aksoy E, Diken A, Yalcinkaya A, Erol M, Cagli K. Dopamine administration is a risk factor for delirium in patients undergoing coronary artery bypass surgery. Heart Lung Circ. 2016;25:493–498. [DOI] [PubMed] [Google Scholar]
- 146. Zhang W, Wu W, Gu J, Sun Y, Ye X, Qiu W, Su C, Zhang S, Ye W. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. 2015;30:606–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


