Abstract
Background
Whether circulating growth differentiation factor 15 (GDF‐15) levels differ according to smoking status and whether smoking modifies the relationship between GDF‐15 and mortality in patients with coronary artery disease are unclear.
Methods and Results
Using data from a multicenter, prospective cohort of 2418 patients with suspected or known coronary artery disease, we assessed the association between smoking status and GDF‐15 and the impact of smoking status on the association between GDF‐15 and all‐cause death. GDF‐15 was measured in 955 never smokers, 1035 former smokers, and 428 current smokers enrolled in the ANOX Study (Development of Novel Biomarkers Related to Angiogenesis or Oxidative Stress to Predict Cardiovascular Events). Patients were followed up during 3 years. The age of the patients ranged from 19 to 94 years; 67.2% were men. Never smokers exhibited significantly lower levels of GDF‐15 compared with former smokers and current smokers. Stepwise multiple linear regression analysis revealed that the log‐transformed GDF‐15 level was independently associated with both current smoking and former smoking. In the entire patient cohort, the GDF‐15 level was significantly associated with all‐cause death after adjusting for potential clinical confounders. This association was still significant in never smokers, former smokers, and current smokers. However, GDF‐15 provided incremental prognostic information to the model with potential clinical confounders and the established cardiovascular biomarkers in never smokers, but not in current smokers or in former smokers.
Conclusions
Not only current, but also former smoking was independently associated with higher levels of GDF‐15. The prognostic value of GDF‐15 on mortality was most pronounced in never smokers among patients with suspected or known coronary artery disease.
Keywords: all‐cause death, biomarker, coronary artery disease, prospective cohort study, smoking
Subject Categories: Biomarkers, Clinical Studies, Coronary Artery Disease, Growth Factors/Cytokines, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ANOX
Development of Novel Biomarkers Related to Angiogenesis or Oxidative Stress to Predict Cardiovascular Events
- cTnI
contemporary sensitive cardiac troponin‐I
- GDF‐15
growth differentiation factor 15
- IDI
integrated discrimination improvement
- MACE
major adverse cardiovascular events
- NRI
net reclassification improvement
Clinical Perspective
What Is New?
This is the first, dedicated and large‐scale prospective cohort study to demonstrate that higher levels of growth differentiation factor 15 are independently associated not only with current smoking, but also with former smoking, and that the prognostic value of growth differentiation factor 15 on mortality is attenuated in current and former smokers with suspected or known coronary artery disease.
What Are the Clinical Implications?
An elevated growth differentiation factor 15 level in sera from an arterial catheter sheath at the beginning of elective coronary angiography is independently associated with all‐cause mortality in patients with suspected or known coronary artery disease beyond the potential clinical confounders, N‐terminal pro‐B‐type natriuretic peptide, contemporary sensitive cardiac troponin‐I, and high‐sensitivity C‐reactive protein, and this association is most pronounced in never smokers.
Further studies will be needed to clarify the relationships among smoking status, duration and intensity of smoking, body mass index, growth differentiation factor 15, and mortality/cardiovascular events.
Smoking is a major risk factor for coronary artery disease (CAD), a leading cause of death in the world. Smoking cessation reduces the mortality risk in the general population and in patients with CAD. 1 , 2
Growth differentiation factor 15 (GDF‐15) is a stress‐responsive member of the transforming growth factor ꞵ cytokine superfamily. 3 , 4 GDF‐15 is weakly expressed in human tissues under good health conditions (except for the placenta), but under pathological conditions GDF‐15 can be produced by many cardiovascular and noncardiovascular cell types. 5 , 6 , 7 , 8 Although little is known about the tissues that produce GDF‐15 in patients with cardiovascular disease, GDF‐15 can be measured in serum and plasma by immunoassay. 8 , 9 Circulating levels of GDF‐15 are elevated in various conditions including higher age, current smoking, diabetes mellitus (metabolic syndrome), genetic factors, acute and chronic inflammation, chronic kidney disease, stable CAD, acute coronary syndrome, heart failure, atrial fibrillation, anemia, solid cancers, and terminal illness (cachexia). 8 , 9 , 10
GDF‐15 is an independent predictor of cardiovascular and cancer mortality and morbidity in community‐dwelling individuals. 11 , 12 GDF‐15 is also an independent marker of all‐cause mortality and cardiovascular events in patients with CAD. 13 However, whether GDF‐15 levels differ according to smoking status (never, former, and current smokers) and whether smoking status modifies the relationship between GDF‐15 and mortality in patients with suspected or known CAD are unclear.
In the present study, we first examined the association between smoking status and serum levels of GDF‐15 in a large‐scale, multicenter prospective cohort of patients who have suspected or known CAD and who were undergoing elective coronary angiography. Second, we investigated possible differences in the prognostic impact of GDF‐15 on mortality in never, former, and current smokers.
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The present study was a subanalysis of the ANOX (Development of Novel Biomarkers Related to Angiogenesis or Oxidative Stress to Predict Cardiovascular Events) study. The main analysis results of the ANOX study have been described. 14 In brief, patients with suspected or known CAD (1624 men [67.2%] and 794 women [32.8%]; mean age [range], 70.6 [19–94] years old) undergoing elective coronary angiography were recruited to determine the predictive value of possible novel biomarkers for mortality and major adverse cardiovascular events (MACE) (see details of the entire cohort of the ANOX study in Table 1 and Table S1 in the report by Wada et al). 14 The ANOX study group consists of 15 National Hospital Organization institutions across Japan, and the present study was conducted by nationally certified cardiologists.
Table 1.
Baseline Characteristics and Incidence of Events According to Smoking Status
| Baseline Characteristics and Incidence of Events |
Never Smokers (N=955) |
Former Smokers (N=1035) |
Current Smokers (N=428) |
P Value* |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, mean (SD), y | 71.5 (10.8) | 71.8 (9.0) | 65.6 (11.4) | <0.001 |
| Male | 371 (38.9) | 890 (86.0) | 363 (84.8) | <0.001 |
| Body mass index, mean (SD) | 24.1 (4.1) | 24.3 (3.6) | 24.4 (4.1) | 0.365 |
| Obesity† | 365 (38.2) | 395 (38.2) | 176 (41.1) | 0.528 |
| Hypertension | 704 (73.7) | 816 (78.8) | 323 (75.5) | 0.025 |
| Dyslipidemia | 563 (59.0) | 655 (63.3) | 248 (57.9) | 0.065 |
| Diabetes mellitus | 389 (40.7) | 492 (47.5) | 206 (48.1) | 0.003 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 63 (22) | 62 (22) | 67 (23) | <0.001 |
| Chronic kidney disease‡ | 397 (41.6) | 449 (43.4) | 153 (35.8) | 0.026 |
| Gensini score, median (IQR)§ | 8.5 (1.0–29.0) | 14.5 (4.0–41.5) | 14.5 (2.0–37.9) | <0.001 |
| Obstructive coronary artery disease | 490 (51.3) | 656 (63.4) | 246 (57.5) | <0.001 |
| Previous myocardial infarction | 98 (10.3) | 195 (18.8) | 61 (14.3) | <0.001 |
| Previous stroke | 116 (12.2) | 173 (16.7) | 64 (15.0) | 0.015 |
| Previous heart failure hospitalization | 113 (11.8) | 104 (10.1) | 36 (8.4) | 0.134 |
| Atrial fibrillation | 117 (12.3) | 104 (10.1) | 40 (9.4) | 0.162 |
| Malignancies | 68 (7.1) | 122 (11.8) | 36 (8.4) | 0.001 |
| Anemia ‖ | 365 (38.2) | 378 (36.5) | 139 (32.5) | 0.122 |
| Antihypertensive drug use | 779 (81.6) | 838 (81.0) | 350 (81.8) | 0.913 |
| Statin use | 480 (50.3) | 531 (51.3) | 211 (49.3) | 0.765 |
| Aspirin use | 488 (51.1) | 624 (60.3) | 228 (53.3) | <0.001 |
| NT‐proBNP, median (IQR), pg/mL | 194 (79–776) | 190 (67–691) | 228 (73–891) | 0.177 |
| cTnI, median (IQR), pg/mL | 0.0 (0.0–9.0) | 0.0 (0.0–11.0) | 0.0 (0.0–15.8) | 0.001 |
| hs‐CRP, median (IQR), mg/L | 0.8 (0.3–2.6) | 0.9 (0.3–2.8) | 1.5 (0.4–4.8) | <0.001 |
| GDF‐15, median (IQR), pg/mL | 1231 (838–1928) | 1373 (983–1981) | 1370 (927–2096) | <0.001 |
| Incidence of events, no. (/1000 person‐y) | ||||
| All‐cause death | 86 (31.4) | 120 (41.1) | 48 (39.5) | … |
| Cardiovascular death | 24 (8.8) | 47 (16.1) | 17 (14.0) | … |
| Myocardial infarction | 5 (1.8) | 10 (3.4) | 6 (5.0) | … |
| Stroke | 24 (8.8) | 31 (10.8) | 14 (11.7) | … |
| First MACE¶ | 50 (18.5) | 80 (27.9) | 35 (29.4) | … |
Values are expressed as number (percentage) unless otherwise indicated. cTnI indicates contemporary sensitive cardiac troponin‐I; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; MACE, major adverse cardiovascular events; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The P value represents a comparison of the differences between groups, and is based on the χ2 test of independence for categorical variables, and the analysis of variance or Kruskal–Wallis for continuous variables.
Obesity is defined as a body mass index of ≥25.
Chronic kidney disease is defined as an estimated glomerular filtration rate of <60 mL/min per 1.73 m2.
The Gensini score represents the angiographic severity of coronary artery disease using a nonlinear points system for degree of luminal narrowing.
Anemia is defined as a hemoglobin level of <13 g/dL in men and <12 g/dL in women.
MACE is defined as a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Between January 2010 and November 2013, a total of 2513 patients were consecutively enrolled. After the exclusion of 26 patients who did not provide blood samples and 69 patients who withdrew consent, a total of 2418 patients were eligible. All patients were classified according to self‐reported smoking status as never, former, or current smokers. Data on the patients' demographic characteristics, smoking status, medical history, and medication use were collected from medical records. The presence of obstructive CAD was assessed using a modified American Heart Association/American College of Cardiology classification. 15 The severity of CAD was quantified using the Gensini score. 16 The submitted data were examined for completeness and accuracy by the coordinating center (Clinical Research Institute, Kyoto Medical Center, Kyoto, Japan), and data queries were sent to the study sites. The study was approved by the central ethics committee of the National Hospital Organization headquarters and each institution's ethical committee. All of the patients provided written informed consent to participate in the study.
Outcomes and Follow‐Up
The primary outcome was all‐cause death. The secondary outcomes were cardiovascular death and MACE defined as a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The patients were monitored during 3 years (1080 days) for the occurrence of all‐cause death, cardiovascular death, and/or MACE. The follow‐up was performed by personnel blinded to the biomarker data through medical record/chart reviews, a survey letter, and/or telephone interviews.
Sudden death resulting from an unknown but presumed cardiovascular cause in high‐risk patients was included in cardiovascular death. All deaths and MACE were recorded in the official medical chart of the hospitals where the patients received care. The reported deaths, myocardial infarctions, and strokes were reviewed and adjudicated by the expert committee (3 independent and blinded cardiologists). The follow‐up continued even after nonfatal myocardial infarction and/or nonfatal stroke had occurred. At the end of the follow‐up (day 1080), the survival status and detailed information about MACE were available in 2400 patients (99.3%), and 18 patients (0.7%) were lost to follow‐up.
Exposures, Sample Collection, and Biomarker Measurement
The predictor of this subanalysis was the patients' serum levels of GDF‐15. Fasting blood samples for serum were collected from the arterial catheter sheath at the beginning of each patient's coronary angiography before a heparinized saline flush. The serum was stored at −80°C for a mean of 4 years until it was assayed for GDF‐15 after 1 freeze–thaw cycle. The serum GDF‐15 level was measured with a specific, commercially available, ELISA kit according to the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN). The sensitivity of the assay for GDF‐15 was 2.0 pg/mL. The inter‐/intra‐assay coefficients of variation of the ELISA for GDF‐15 were ≤6%/<3%. The assays were performed by an investigator blinded to the sources of the samples.
The details of the assays for NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), contemporary sensitive cardiac troponin‐I (cTnI), and hs‐CRP (high‐sensitivity C‐reactive protein) are described elsewhere. 14 , 17 Briefly, the serum levels of NT‐proBNP were measured using a validated, sandwich electrochemiluminescence immunoassay (Elecsys; Roche Diagnostics, Indianapolis, IN). Serum cTnI was measured with the ADVIA Centaur Troponin I Ultra assay (Siemens Healthcare Diagnostics, Los Angeles, CA). The serum levels of hs‐CRP were measured with a specific, commercially available ELISA kit according to the manufacturer's instructions (CycLex, MBL, Nagano, Japan). The inter‐/intra‐assay coefficients of variation of ELISA for hs‐CRP were <6%/<4%. The sensitivity of the assay for NT‐proBNP was 5 pg/mL, and the assay coefficient of variation at values of the measuring range (5–35 000 pg/mL) was <10%. The sensitivity of the assay for cTnI was 6 pg/mL, and the assay coefficient of variation at the 99th percentile reference value of 40 pg/mL (potential range, 20–60 pg/mL) was <10%. The sensitivity of the assay for hs‐CRP was 0.0286 mg/L.
Statistical Analysis
We divided the patients into 3 groups according to smoking status. The data were compared among the smoking status groups and significant differences were determined using the Kruskal–Wallis and χ2 tests. The relationships between GDF‐15 and other variables were assessed in simple and stepwise multiple linear regression analyses. Stepwise variable selection was performed in a forward direction with the Bayesian information criterion. Because GDF‐15, the Gensini score, NT‐proBNP, cTnI, and hs‐CRP were normally distributed after logarithmic transformation, the logarithms of these parameters were used in the linear regression analyses. The relationships between the baseline GDF‐15 level and the outcomes were investigated with the use of Cox proportional hazard regression in 3 sets of models: models adjusted for traditional risk factors (age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, and current smoking); models adjusted for potential clinical confounders (the traditional risk factors plus several other potential clinical confounders, ie, the estimated glomerular filtration rate [eGFR], the Gensini score, previous myocardial infarction, previous stroke, previous heart failure hospitalization, malignancies, anemia [defined as a hemoglobin level <13 g/L in men and 12 g/L in women], antihypertensive drug use, statin use, and aspirin use); and models adjusted for potential clinical confounders and established cardiovascular biomarkers (NT‐proBNP, cTnI, and hs‐CRP for 1 SD increase).
We evaluated the incremental predictive performance of biomarkers by calculating changes in the C‐statistic, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) metrics. 18 We assessed the model calibration by comparing predicted probabilities with observed probabilities. A residual analysis was used to assess model fit.
All statistical tests were 2‐sided, and P<0.05 was considered significant. Since all analyses were considered exploratory, the P values were not adjusted for multiple comparisons. The analyses were performed using SPSS ver. 23.0 (IBM Japan, Tokyo), JMP13 (SAS, Cary, NC), and R, ver. 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). Additional details are described elsewhere. 14 , 17
Results
Baseline Characteristics
The median level of GDF‐15 was 1322 pg/mL (interquartile range, 902–1980 pg/mL) for all the patients. The baseline characteristics of the patients divided according to smoking status are shown in Table 1 and Table S1. Never smokers showed the lowest rates of male sex, diabetes mellitus, obstructive CAD, previous myocardial infarction, and previous stroke, and the lowest Gensini score; Former smokers, the highest rates of hypertension, chronic kidney disease, malignancies, and aspirin use; and Current smokers, the lowest age, highest eGFR, and highest levels of cTnI and hs‐CRP. There was no significant difference in the body mass index, the rates of obesity, dyslipidemia, previous heart failure hospitalization, atrial fibrillation, anemia, antihypertensive drug use, statin use, or levels of NT‐proBNP. Importantly, never smokers exhibited significantly lower levels of GDF‐15 compared with former smokers and current smokers (P<0.001).
The correlations of GDF‐15 with other variables are shown in Table 2. Stepwise regression analysis revealed that higher GDF‐15 levels were independently associated with higher age, diabetes mellitus, current smoking, former smoking, lower eGFR, anemia, no use of statins, and higher levels of NT‐proBNP and hs‐CRP.
Table 2.
Simple and Multiple Stepwise Regression Analyses for the GDF‐15 Level* in the Entire Cohort
| Variables | Simple Regression | Independent Determinants | ||||
|---|---|---|---|---|---|---|
| r | SEM | P Value | β | SEM | P Value | |
| Age, y | 0.022 | 0.001 | <0.001 | 0.012 | 0.001 | <0.001 |
| Male | 0.067 | 0.028 | 0.015 | |||
| Body mass index, kg/m2 | −0.017 | 0.003 | <0.001 | |||
| Hypertension | 0.184 | 0.030 | <0.001 | |||
| Dyslipidemia | −0.102 | 0.026 | <0.001 | |||
| Diabetes mellitus | 0.243 | 0.026 | <0.001 | 0.169 | 0.019 | <0.001 |
| Current smoking | 0.026 | 0.034 | 0.445 | 0.166 | 0.027 | <0.001 |
| Former smoking | 0.073 | 0.026 | 0.006 | 0.091 | 0.020 | <0.001 |
| eGFR, mL/min per 1.73 m2 | −0.016 | 0.000 | <0.001 | −0.009 | 0.000 | <0.001 |
| Gensini score* | 0.073 | 0.008 | <0.001 | |||
| Previous myocardial infarction | 0.063 | 0.037 | 0.088 | |||
| Previous stroke | 0.195 | 0.037 | <0.001 | |||
| Previous heart failure hospitalization | 0.449 | 0.041 | <0.001 | |||
| Atrial fibrillation | 0.275 | 0.041 | <0.001 | |||
| Malignancies | 0.193 | 0.044 | <0.001 | |||
| Anemia | 0.535 | 0.025 | <0.001 | 0.202 | 0.020 | <0.001 |
| Antihypertensive drug use | 0.203 | 0.033 | <0.001 | |||
| Statin use | −0.045 | 0.026 | 0.082 | −0.054 | 0.018 | 0.003 |
| Aspirin use | −0.031 | 0.026 | 0.231 | |||
| NT‐proBNP, pg/mL* | 0.208 | 0.006 | <0.001 | 0.101 | 0.006 | <0.001 |
| cTnI, pg/mL* | 0.110 | 0.007 | <0.001 | |||
| hs‐CRP, mg/L* | 0.101 | 0.008 | <0.001 | 0.041 | 0.006 | <0.001 |
cTnI indicates contemporary sensitive cardiac troponin‐I; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; hs‐CRP, high‐sensitivity C‐reactive protein; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Natural log‐transformed to obtain normal distributions.
Incidence of Outcomes
As described in the ANOX study, 14 , 17 254 patients died of any cause (88 cardiovascular and 166 noncardiovascular deaths), and 165 developed first MACE (21 myocardial infarction, 69 stroke, and 75 cardiovascular deaths) during the 3‐year follow‐up. Figure 1 shows the cumulative incidence of all‐cause death according to the quartiles of GDF‐15 levels in the entire cohort, never smokers, former smokers, and current smokers. The highest quartile of GDF‐15 had the greatest risk of all‐cause death irrespective of smoking status. Figures S1 and S2 show the cumulative incidence of cardiovascular death and MACE according to the quartiles of GDF‐15 levels in the entire cohort, never smokers, former smokers, and current smokers, respectively. The highest quartile of GDF‐15 also had the greatest risks of cardiovascular death and MACE irrespective of smoking status. The baseline characteristics of the patients and the incidence of outcomes according to the quartiles of GDF‐15 levels in the entire cohort, never smokers, former smokers, and current smokers are shown in Tables S2 through S5, respectively.
Figure 1. Cumulative incidence of all‐cause death in the entire cohort (A), never smokers (B), former smokers (C), and current smokers (D) according to the serum GDF‐15 level at baseline.
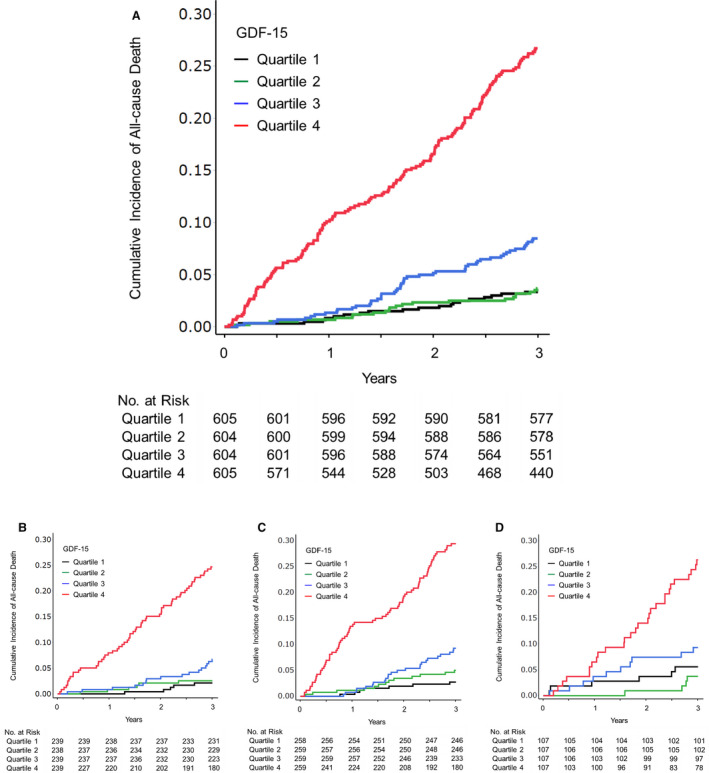
Follow‐up results are truncated after 3 years. GDF‐15 indicates growth differentiation factor 15.
Multivariate Cox Regression Analyses
After adjusting for traditional risk factors (ie, age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, and current smoking), the GDF‐15 level was significantly associated with all‐cause death in the entire cohort, never smokers, former smokers, and current smokers (model 1, Figure 2). After the additional adjustment for other potential clinical confounders (ie, eGFR, the Gensini score, previous myocardial infarction, previous stroke, previous heart failure hospitalization, malignancies, anemia, antihypertensive drug use, statin use, and aspirin use), the GDF‐15 level was significantly associated with all‐cause death in the entire cohort, and this association was independent of smoking status (model 2, Figure 2). Even after additional adjustment for established cardiovascular biomarkers (NT‐proBNP, cTnI, and hs‐CRP), the GDF‐15 level was still significantly associated with all‐cause death in the entire cohort, never smokers, and former smokers, but not in current smokers (model 3, Figure 2).
Figure 2. Hazard ratios for all‐cause death in the entire cohort, never smokers, former smokers, and current smokers according to the serum GDF‐15 level at baseline.
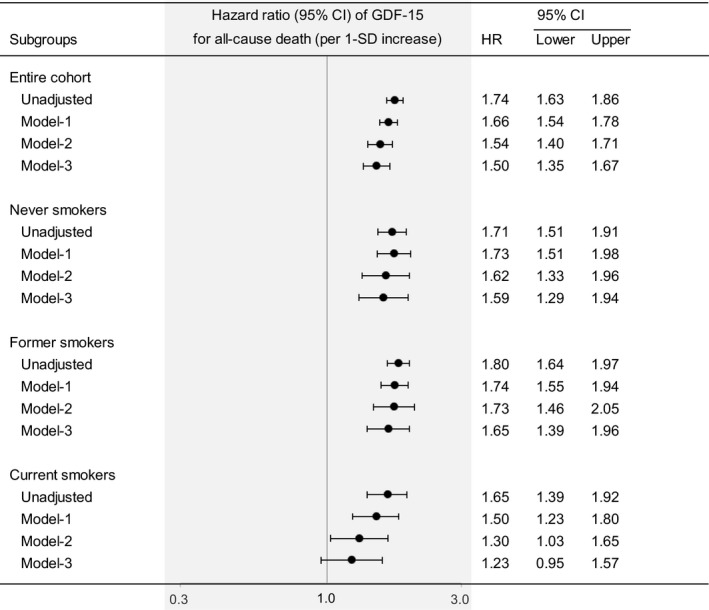
Values are for 1 SD increase. The data were adjusted for the following variables: model‐1, age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, and current smoking; model‐2, the variables in model‐1 plus estimated glomerular filtration rate, the Gensini score, previous myocardial infarction, previous stroke, previous heart failure hospitalization, malignancies, anemia, antihypertensive drug use, statin use, and aspirin use; model‐3, the variables in model‐2 plus N‐terminal pro‐B‐type natriuretic peptide, contemporary sensitive cardiac troponin‐I, and high‐sensitivity C‐reactive protein. The biomarkers were modeled as continuous variables. GDF‐15 indicates growth differentiation factor 15; and HR, hazard ratio.
We also performed quartile analyses. After full adjustment, the quartiles of GDF‐15 levels were significantly associated with all‐cause death in the entire cohort, never smokers, and former smokers, but not in current smokers (Figure S3).
Discrimination, Reclassification, and Calibration
The C statistics for all‐cause death by the model with potential clinical confounders (base model) were 0.789 in the entire cohort, 0.815 in never smokers, 0.793 in former smokers, and 0.796 in current smokers (Table 3). The C statistics of GDF‐15 alone were 0.776 in the entire cohort, 0.788 in never smokers, 0.782 in former smokers, and 0.735 in current smokers. The C statistics of other single cardiovascular biomarkers (NT‐proBNP, cTnI, and hs‐CRP) in the entire cohort are shown in Table 2 of the report by Wada et al, 17 and those in never, former, and current smokers are shown in Table 3. The combination of established cardiovascular biomarkers significantly improved the prediction of all‐cause death in the entire cohort and in former smokers, but not in never smokers or in current smokers (Table 3). Notably, the addition of GDF‐15 to the model with potential clinical confounders and established cardiovascular biomarkers significantly improved the prediction of all‐cause death in the entire cohort (P<0.001 for NRI, P<0.001 for IDI) and in never smokers (P<0.001 for NRI, P=0.010 for IDI), but not in former smokers (P=0.236 for NRI, P=0.013 for IDI) or in current smokers (P=0.126 for NRI, P=0.090 for IDI) (Table 3). Calibration of the models with or without GDF‐15 showed no evidence of lack of fit.
Table 3.
Model Performance Measures for All‐Cause Death in the Entire Cohort, Never Smokers, Former Smokers, and Current Smokers
| Risk Factors and Biomarkers | C Statistics | ∆C Statistics | Continuous NRI, 95% CI | P Value | IDI, 95% CI | P Value |
|---|---|---|---|---|---|---|
| Entire cohort | ||||||
| Base model* | 0.789 | … | … | … | ||
| GDF‐15 | 0.776 | … | … | … | ||
| Base model+NT‐proBNP+cTnI+hs‐CRP† | 0.803 | 0.015 | 0.386 (0.257 to 0.514) | <0.001 | 0.017 (0.006 to 0.028) | 0.003 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+GDF‐15‡ | 0.826 | 0.022 | 0.276 (0.147 to 0.405) | <0.001 | 0.036 (0.021 to 0.051) | <0.001 |
| Never smokers | ||||||
| Base model* | 0.815 | … | … | … | ||
| NT‐proBNP | 0.734 | … | … | … | ||
| cTnI | 0.673 | … | … | … | ||
| hs‐CRP | 0.646 | … | … | … | ||
| GDF‐15 | 0.788 | … | … | … | ||
| Base model+NT‐proBNP+cTnI+hs‐CRP† | 0.827 | 0.011 | 0.349 (0.129 to 0.569) | 0.002 | 0.008 (−0.006 to 0.021) | 0.246 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+GDF‐15‡ | 0.847 | 0.020 | 0.465 (0.245 to 0.684) | <0.001 | 0.028 (0.006 to 0.049) | 0.010 |
| Former smokers | ||||||
| Base model* | 0.793 | … | … | … | ||
| NT‐proBNP | 0.723 | … | … | … | ||
| cTnI | 0.673 | … | … | … | ||
| hs‐CRP | 0.640 | … | … | … | ||
| GDF‐15 | 0.782 | … | … | … | ||
| Base model+NT‐proBNP+cTnI+hs‐CRP† | 0.815 | 0.022 | 0.275 (0.085 to 0.464) | 0.004 | 0.059 (0.027 to 0.090) | <0.001 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+GDF‐15‡ | 0.838 | 0.023 | 0.114 (−0.075 to 0.303) | 0.236 | 0.025 (0.005 to 0.046) | 0.013 |
| Current smokers | ||||||
| Base model* | 0.796 | … | … | … | ||
| NT‐proBNP | 0.701 | … | … | … | ||
| cTnI | 0.577 | … | … | … | ||
| hs‐CRP | 0.629 | … | … | … | ||
| GDF‐15 | 0.735 | … | … | … | ||
| Base model+NT‐proBNP+cTnI+hs‐CRP† | 0.832 | 0.036 | 0.380 (0.084 to 0.676) | 0.012 | 0.023 (−0.004 to 0.05) | 0.096 |
| Base model+NT‐proBNP+cTnI+hs‐CRP+GDF‐15‡ | 0.842 | 0.010 | 0.233 (−0.066 to 0.532) | 0.126 | 0.022 (−0.003 to 0.05) | 0.090 |
Follow‐up results are truncated after 3 years. The biomarkers were modeled as continuous variables (for 1 SD increase). The ΔC statistic, continuous NRI, and IDI show the change in model performance from “Base model” or “Base model+NT‐proBNP+cTnI+hs‐CRP.” cTnI indicates contemporary sensitive cardiac troponin‐I; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; hs‐CRP, high‐sensitivity C‐reactive protein; IDI, integrated discrimination improvement; NRI, net reclassification improvement; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The base model is based on age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, current smoking, eGFR, the Gensini score, previous myocardial infarction, previous stroke, previous heart failure hospitalization, atrial fibrillation, malignancies, anemia, antihypertensive drug use, statin use, and aspirin use.
Evaluated the change of model performance from the “Base model.”
Evaluated the change of model performance from the “Base model+NT‐proBNP+cTnI+hs‐CRP.”
Discussion
In this prospective cohort of patients with suspected or known CAD, we first showed that higher levels of GDF‐15 were independently associated not only with current smoking, but also with former smoking. In addition, we demonstrated that the GDF‐15 level independently predicted mortality in the entire cohort and in never smokers, and that this prognostic impact was attenuated in current smokers and in former smokers. To the best of our knowledge, this is the first large‐scale, multicenter study to examine the independent prognostic value of GDF‐15 on mortality over potential clinical confounders and established cardiovascular biomarkers in Asian high‐risk patients. The strengths of our investigation include the large sample size, multicenter prospective design, and high follow‐up rate (99.3%).
At baseline, independent determinants of the GDF‐15 level were age, diabetes mellitus, current smoking, former smoking, eGFR, anemia, no use of statins, NT‐proBNP, and hs‐CRP. These findings are mostly consistent with previous reports, except with respect to former smoking. 8 , 9 , 10 There was no significant association between GDF‐15 and former smoking in the previous study involving the 2 community‐based cohorts. 12 This discrepancy merits consideration.
The ages of participants at enrollment in the 2 cohort studies were 70 years old (PIVUS [Prospective Study of the Vasculature in Uppsala Seniors ULSAM, Uppsala Longitudinal Study of Adult Men]: n=969; male, 50%) and 77 years old (ULSAM [Uppsala Longitudinal Study of Adult Men]: n=717; male, 100%), respectively. Thus, the mean age of participants in the present study at enrollment (70.6 years old) was similar to that of PIVUS and younger than that of ULSAM, and ranged from 19 to 94 years old. In general, the prevalence of current smokers was lower in those aged 65 or more than in those aged 18 to 64. 19 Accordingly, the prevalence of former smokers who had stopped smoking <5 years ago could have been lower in the 2 community‐based cohorts compared with the present study. In other words, the present study could have included “relatively new” former smokers who had shorter duration since smoking cessation compared with the former smokers in the 2 community‐based cohort studies. A previous meta‐analysis demonstrated that compared with never smokers, the excess risk in former smokers decreased continuously with time since smoking cessation. 20 Thus, the relatively higher levels of GDF‐15 in former smokers in the present study may reflect higher excess risk compared with those in the 2 community‐based cohort studies.
The median GDF‐15 level was higher in former smokers than in current smokers (Table 1). However, the GDF‐15 level was affected not only by smoking status, but also by age, diabetes mellitus, low eGFR, anemia, and so on. In the present study, former smokers exhibited higher age, lower eGFR, and higher rate of anemia than current smokers (Table 1). In addition, although the independent determinants of the GDF‐15 level included both current smoking and former smoking, the standardized partial regression coefficient of current smoking was higher than that of former smoking (0.166 versus 0.091, Table 2). Thus, the impact of current smoking on GDF‐15 was stronger than that of former smoking.
After smoking cessation, considerable weight gain may occur, 21 and this is associated with an increased short‐term risk of type 2 diabetes mellitus. 22 Since the GDF‐15 level is elevated in patients with diabetes mellitus or metabolic syndrome, 9 the GDF‐15 level may—at least in part—depend on the weight gain after cessation.
Paradoxically, previous studies showed that active smokers who smoke more intensively tend to weigh more than light smokers. 23 , 24 , 25 In addition, higher levels of general and abdominal adiposity influence smoking status and smoking intensity (number of cigarettes smoked per day). 26 Unfortunately, we did not collect data on the smoking–pack years in this cohort. Therefore, further studies will be needed to clarify the relationships among smoking status, duration and intensity of smoking, body mass index, GDF‐15, and mortality/cardiovascular events.
Previous experimental studies have shown that GDF‐15 expression can increase in response to diverse cellular stress signals, such as oxidative stress, hypoxia/anoxia, inflammation, acute tissue injuries, and the tumor process. 5 , 6 , 7 , 8 GDF‐15 thus appears to be a general marker of diseases, and a wide range of (cardiovascular or noncardiovascular) cell types could be its sources. Nonetheless, the direct impact of cigarette smoking exposure on the excessive production of GDF‐15 should be considered.
Recent studies reported that human airway epithelial cells produce excessive GDF‐15 in response to cigarette smoking exposure, which subsequently activates the activin receptor–like kinase 1/Smad1 pathway to promote airway epithelial senescence. Senescent airway epithelial cells can produce high‐mobility group box 1 and proinflammatory cytokines (eg, IL‐ 6) to augment cigarette smoking–induced airway inflammation, which has been implicated in chronic obstructive pulmonary disease pathogenesis. 27 , 28 Interestingly, GDF‐15 deficiency in mice attenuated the cigarette smoking–induced pulmonary inflammation, suggesting that increased GDF‐15—as observed in the lungs of smokers and patients with chronic obstructive pulmonary disease—contributes to cigarette smoking–induced pulmonary inflammation. 29 Thus, human airway epithelial cells could be one of the sources of excessive GDF‐15 in current and former smokers. Further studies are needed to elucidate the sources and significance of excessive GDF‐15 in ever smokers.
In the present study, each of NT‐proBNP, cTnI, or hs‐CRP was independently associated with current smoking but not with former smoking (data not shown). The relationship between elevated CRP levels and smoking is well established, 30 whereas it is controversial whether or not higher levels of NT‐proBNP and cTnI are associated with current smoking. 31 , 32 , 33 , 34 , 35 However, previous studies demonstrated that NT‐proBNP, cTnI, and hs‐CRP levels were not significantly affected by the changes in smoking status. 36 , 37 , 38 , 39 To compare with these results on NT‐proBNP, cTnI, and hs‐CRP, long‐term follow‐up studies will be needed to clarify whether GDF‐15 levels are affected by the changes in smoking status.
Notably, the combination of established cardiovascular biomarkers did not add statistically significant prognostic information to the model with potential clinical confounders in never smokers (Table 3). However, the addition of GDF‐15 markedly improved the prediction of all‐cause mortality, suggesting that GDF‐15 could be superior to established cardiovascular biomarkers to predict mortality in never smokers. These findings are consistent with a previous report. 11 By contrast, the combination of established cardiovascular biomarkers remarkably improved the prediction of mortality over the base model with potential clinical confounders in former smokers, whereas further addition of GDF‐15 hardly improved the prediction of mortality, suggesting the limited additional value of GDF‐15 in former smokers. Neither the established cardiovascular biomarkers nor GDF‐15 added statistically significant prognostic information on mortality in current smokers. This may have been because of the smaller sample size, younger mean age, lower mortality rate, and thus limited statistical power of the current smokers compared with former smokers and never smokers. Otherwise, a potential explanation for these findings is that the harmful effects of cigarette smoking exposure may increase circulating levels of these biomarkers, leading to an elevation of their optimal cut‐off values to discriminate the risk of mortality, and an attenuation of their prognostic impact. Further investigations are required to define the additive prognostic impact of these biomarkers on mortality over potential clinical confounders in current smokers. From a clinical point of view, these findings also indicate the importance of verifying the smoking status in order to more accurately predict each patient's prognosis using cardiovascular biomarkers.
The quartile analyses of GDF‐15 for all‐cause death may indicate the presence of a nonlinear threshold at around the 75th percentile in never and former smokers (Table S3). Future studies including both development and validation cohorts will be needed to determine the optimal cut‐off values of GDF‐15 in never and former smokers.
Limitations
First, we did not collect data on smoking–pack years. Future studies including the data on smoking–pack years would clarify the effect of duration and intensity of smoking on the GDF‐15 level and its prognostic value. Second, the patients' blood samples were drawn from the arterial sheath. It is possible that concentrations of GDF‐15 in the arterial and venous circulation differ. In our preliminary data (n=40), the GDF‐15 levels in sera from the arterial sheath were closely correlated with those from the peripheral vein before cardiac catheterization (β, 0.955; SEM, 0.031; P<0.001). However, to validate the potential of GDF‐15 to be used more widely in risk prediction, other cohort studies using peripheral venous blood samples are necessary, such as our EXCEED‐J (Establishment of the Method to Extract a High‐Risk Population Employing Novel Biomarkers to Predict Cardiovascular Events in Japan) study (UMIN000018807). Third, we used a contemporary sensitive troponin I assay, but not a high‐sensitivity troponin I or T assay. Fourth, we had no collected data of cardiovascular imaging, such as echocardiography, cardiovascular magnetic resonance, computed tomography, intravascular ultrasound/optical coherence tomography, or nuclear imaging. Fifth, we had no collected data of a history of chronic obstructive pulmonary disease. Sixth, this was an observational study, and other unmeasured confounding factors may have existed. Finally, because the ANOX study cohort consists exclusively of Asian individuals with suspected or known CAD, our results may not be generalizable to general Asian populations, or to other ethnic groups.
Conclusions
Nevertheless, our results clearly demonstrate that not only current smoking, but also former smoking was independently associated with higher levels of GDF‐15. An elevated serum GDF‐15 value was independently associated with all‐cause mortality beyond the potential clinical confounders and cardiovascular biomarkers (NT‐proBNP, cTnI, and hs‐CRP) in patients with suspected or known CAD undergoing elective coronary angiography. This association was most pronounced in never smokers.
Sources of Funding
The ANOX study is supported by a Grant‐in‐Aid for Clinical Research from the National Hospital Organization in Japan.
Disclosures
None.
Supporting information
Appendix S1
Tables S1–S5
Figures S1–S3
Acknowledgments
We thank Shuichi Ura, who substantially contributed to this work but passed away before this manuscript was drafted. We also thank the other members, cooperators, and participants of the ANOX study for their valuable contributions. The other members of the ANOX study group are listed in the Appendix in the Supplemental Material.
(J Am Heart Assoc. 2020;9:e018217 DOI: 10.1161/JAHA.120.018217.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. GBD 2013 Risk Factors Collaborators , Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all‐cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;924–934. [DOI] [PubMed] [Google Scholar]
- 3. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, et al. MIC‐1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF‐beta superfamily. Proc Natl Acad Sci USA. 1997;11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauskin AR, Zhang HP, Fairlie WD, He XY, Russell PK, Moore AG, Brown DA, Stanley KK, Breit SN. The propeptide of macrophage inhibitory cytokine (MIC‐1), a TGF‐beta superfamily member, acts as a quality control determinant for correctly folded MIC‐1. EMBO J. 2000;2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini‐Vittori M, Korf‐Klingebiel M, Napp LC, Hansen B, et al. GDF‐15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;581–588. [DOI] [PubMed] [Google Scholar]
- 6. de Jager SC, Bermúdez B, Bot I, Koenen RR, Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJ, Weber C, et al. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2‐mediated macrophage chemotaxis. J Exp Med. 2011;217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonaterra GA, Zügel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, Kinscherf R. Growth differentiation factor‐15 deficiency inhibits atherosclerosis progression by regulating interleukin‐6-dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;e002550 DOI: 10.1161/JAHA.112.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corre J, Hébraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;140–151. [DOI] [PubMed] [Google Scholar]
- 10. Ho JE, Mahajan A, Chen MH, Larson MG, McCabe EL, Ghorbani A, Cheng S, Johnson AD, Lindgren CM, Kempf T, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett‐Connor E. Growth‐differentiation factor‐15 is a robust, independent predictor of 11‐year mortality risk in community‐dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang B, Svensson P, Ärnlöv J, Sundström J, Lind L, Ingelsson E. Effects of cigarette smoking on cardiovascular‐related protein profiles in two community‐based cohort studies. Atherosclerosis. 2016;52–58. [DOI] [PubMed] [Google Scholar]
- 13. Hagström E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug‐Gourley S, Mohler ER III. Steg PG, et al.; STABILITY Investigators . Growth differentiation factor 15 predicts all‐cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;325–333. [DOI] [PubMed] [Google Scholar]
- 14. Wada H, Suzuki M, Matsuda M, Ajiro Y, Shinozaki T, Sakagami S, Yonezawa K, Shimizu M, Funada J, Takenaka T, et al.; ANOX Study Investigators . VEGF‐C and mortality in patients with suspected or known coronary artery disease. J Am Heart Assoc. 2018;e010355 DOI: 10.1161/JAHA.118.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;5–40. [DOI] [PubMed] [Google Scholar]
- 16. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;606. [DOI] [PubMed] [Google Scholar]
- 17. Wada H, Suzuki M, Matsuda M, Ajiro Y, Shinozaki T, Sakagami S, Yonezawa K, Shimizu M, Funada J, Takenaka T, et al.; ANOX Study Investigators . Distinct characteristics of VEGF‐D and VEGF‐C to predict mortality in patients with suspected or known coronary artery disease. J Am Heart Assoc. 2020;9:e015761 DOI: 10.1161/JAHA.119.015761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, et al.; CHANCES Consortium . Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta‐analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta‐analysis. BMJ. 2012;e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, Willett WC, Manson JE, Hu FB, Sun Q. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, Khaw KT. Cigarette smoking and fat distribution in 21,828 British men and women: a population‐based study. Obes Res. 2005;1466–1475. [DOI] [PubMed] [Google Scholar]
- 24. Sneve M, Jorde R. Cross‐sectional study on the relationship between body mass index and smoking, and longitudinal changes in body mass index in relation to change in smoking status: the Tromso Study. Scand J Public Health. 2008;397–407. [DOI] [PubMed] [Google Scholar]
- 25. Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross‐sectional study of 499,504 middle‐aged adults in the UK general population. PLoS One. 2015;e0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carreras‐Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, Martin RM. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ. 2018;k1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Q, Jiang D, Chu HW. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over‐expression. Innate Immun. 2012;617–626. [DOI] [PubMed] [Google Scholar]
- 28. Wu Q, Jiang D, Matsuda JL, Ternyak K, Zhang B, Chu HW. Cigarette smoke induces human airway epithelial senescence via growth differentiation factor 15 production. Am J Respir Cell Mol Biol. 2016;429–438. [DOI] [PubMed] [Google Scholar]
- 29. Verhamme FM, Seys LJM, De Smet EG, Provoost S, Janssens W, Elewaut D, Joos GF, Brusselle GG, Bracke KR. Elevated GDF‐15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol. 2017;1400–1411. [DOI] [PubMed] [Google Scholar]
- 30. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;1557–1566. [DOI] [PubMed] [Google Scholar]
- 31. Otsuka T, Kawada T, Seino Y, Ibuki C, Katsumata M, Kodani E. Relation of smoking status to serum levels of N‐terminal pro‐brain natriuretic peptide in middle‐aged men without overt cardiovascular disease. Am J Cardiol. 2010;1456–1460. [DOI] [PubMed] [Google Scholar]
- 32. Nadruz W Jr, Gonçalves A, Claggett B, Querejeta Roca G, Shah AM, Cheng S, Heiss G, Ballantyne CM, Solomon SD. Influence of cigarette smoking on cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Heart Fail. 2016;629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glick D, deFilippi CR, Christenson R, Gottdiener JS, Seliger SL. Long‐term trajectory of two unique cardiac biomarkers and subsequent left ventricular structural pathology and risk of incident heart failure in community‐dwelling older adults at low baseline risk. JACC Heart Fail. 2013;353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lyngbakken MN, Skranes JB, de Lemos JA, Nygård S, Dalen H, Hveem K, Røsjø H, Omland T. Impact of smoking on circulating cardiac troponin I concentrations and cardiovascular events in the general population: the HUNT Study (Nord‐Trøndelag Health Study). Circulation. 2016;1962–1972. [DOI] [PubMed] [Google Scholar]
- 35. Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE, Packard CJ, Mills NL. High‐sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eggers KM, Lindahl B, Venge P, Lind L. Predictors of 10‐year changes in levels of N‐terminal pro B‐type natriuretic peptide and cardiac troponin I in the elderly. Int J Cardiol. 2018;300–305. [DOI] [PubMed] [Google Scholar]
- 37. Wada H, Ura S, Satoh‐Asahara N, Kitaoka S, Mashiba S, Akao M, Abe M, Ono K, Morimoto T, Fujita M, et al. α1‐Antitrypsin low‐density‐lipoprotein serves as a marker of smoking‐specific oxidative stress. J Atheroscler Thromb. 2012;47–58. [DOI] [PubMed] [Google Scholar]
- 38. Komiyama M, Shimada S, Wada H, Yamakage H, Satoh‐Asahara N, Shimatsu A, Akao M, Morimoto T, Takahashi Y, Hasegawa K. Time‐dependent changes of atherosclerotic LDL complexes after smoking cessation. J Atheroscler Thromb. 2016;1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Komiyama M, Wada H, Yamakage H, Satoh‐Asahara N, Sunagawa Y, Morimoto T, Ozaki Y, Shimatsu A, Takahashi Y, Hasegawa K. Analysis of changes on adiponectin levels and abdominal obesity after smoking cessation. PLoS One. 2018;e0201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S5
Figures S1–S3


