Abstract
Background
Cardiogenic shock (CS) is a complex syndrome associated with high morbidity and mortality. In recent years, many US hospitals have formed multidisciplinary shock teams capable of rapid diagnosis and triage. Because of preexisting collaborative systems of care, hospitals with left ventricular assist device (LVAD) programs may also represent “centers of excellence” for CS care. However, the outcomes of patients with CS at LVAD centers have not been previously evaluated.
Methods and Results
Patients with CS were identified in the 2012 to 2014 National Inpatient Sample. Clinical characteristics, revascularization rates, and use of mechanical circulatory support were analyzed in LVAD versus non‐LVAD centers. The association between hospital type and in‐hospital mortality was examined using multivariable logistic regression models. Of 272 075 hospitalizations, 26.0% were in LVAD centers. CS attributable to causes other than acute myocardial infarction represented most cases. In‐hospital mortality was lower in LVAD centers (38.9% versus 43.3%; P<0.001). In multivariable analysis, the odds of mortality remained significantly lower for hospitalizations in LVAD centers (odds ratio, 0.89; P<0.001). In patients with CS secondary to acute myocardial infarction, revascularization rates were similar between LVAD and non‐LVAD centers. The use of intra‐aortic balloon pump (18.7% versus 18.8%) and Impella/TandemHeart (2.6% versus 1.9%) was similar between hospital types, whereas extracorporeal membrane oxygenation was used more frequently in LVAD centers (4.3% versus 0.2%; P<0.001).
Conclusions
Risk‐adjusted mortality was lower in patients with CS who were hospitalized at LVAD centers. These centers likely represent specialized, shock team capable institutions across the country that may be best suited to manage patients with CS.
Keywords: cardiogenic shock, left ventricular assist device, mechanical circulatory support
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- AMI‐CS
cardiogenic shock after acute myocardial infarction
- CS
cardiogenic shock
- IABP
intra‐aortic balloon pump
- MCS
mechanical circulatory support
- NIS
National Inpatient Sample
- Non–AMI‐CS
cardiogenic shock related to causes other than acute myocardial infarction
Clinical Perspective
What Is New?
There has been increased interest in the identification of cardiogenic shock “centers of excellence” that can proficiently implement a “shock team” model.
The present study found that risk‐adjusted mortality was lower in patients with cardiogenic shock hospitalized at left ventricular assist device centers compared with non–left ventricular assist device centers.
What Are the Clinical Implications?
Left ventricular assist device centers may represent cardiogenic shock “centers of excellence” that can leverage collaborative systems of care that are already in place.
This study provides a potential framework to identify “shock team” capable institutions across the United States that can actively participate in larger studies of cardiogenic shock.
Despite the changing landscape of current treatments, cardiogenic shock (CS) remains a devastating condition with high rates of morbidity and mortality. On the basis of data from older studies, 1 , 2 , 3 timely revascularization is still the cornerstone of management for CS after acute myocardial infarction (AMI‐CS), although the impact on survival in the modern era has been less clear. 4 In recent years, percutaneous mechanical circulatory support (MCS) devices have been used with increased frequency and earlier in the course of the illness for a broader population of all comers with CS. However, discordant findings in the literature with respect to mortality have been difficult to reconcile. 5 , 6
In recognition of the hemodynamically complex and heterogeneous nature of this syndrome, the most recent paradigm shift in CS care has focused on “centers of excellence” that can proficiently implement a “shock team” model and serve as tertiary referral centers. Consisting of specialists in advanced heart failure, interventional cardiology, cardiovascular surgery, and critical care medicine, the CS team aims to systematically enhance early recognition of the shock state, identify patients in need of reperfusion, obtain and interpret serial hemodynamic data, consider MCS, and ultimately evaluate patients for weaning, escalation, or withdrawal of support. 7 Evidence for these models has been favorable but comes from a small sample of single‐center experiences, and larger studies are needed to confirm a true benefit. 8 , 9
Hospitals that perform left ventricular assist device (LVAD) implantation frequently have the necessary infrastructure to evaluate and treat patients with advanced heart failure and CS, and the processes already in place at these specialized institutions can be repurposed for the creation of a “shock team.” Therefore, LVAD‐capable institutions may represent potential “centers of excellence” for the management of CS. However, the outcomes of patients with CS at LVAD centers have not been previously reported.
Using the National Inpatient Sample (NIS), we examined CS outcomes in LVAD centers. We hypothesized that LVAD centers would have lower in‐hospital mortality compared with hospitals that did not perform LVAD implantation.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
The NIS is the largest database of inpatient hospital stays in the United States. Data originated from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. In 2012, the NIS was redesigned to approximate a 20% stratified sample of discharges from all nonfederal US hospitals. 10 For each hospitalization, diagnoses and procedures are provided using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9-CM) codes. Because the study cohort was derived from a deidentified, publicly available database, the study was exempt from formal review by the Weill Cornell Medicine (New York, NY) institutional review board.
Study Population
From 2012 to 2014, hospitalizations for patients aged ≥18 years with a diagnosis of CS were identified using ICD‐9-CM code 785.51. Discharges with missing data for in‐hospital death and hospitalizations in which a patient was admitted and discharged alive on the same day were excluded. For hospitalizations that involved interhospital transfer, discharges in which the patient was transferred out to another short‐term care hospital were excluded. However, because transfers in from another hospital had all relevant outcome data, they were included in the analysis. This would avoid the possibility of duplicating a hospitalization for the same patient if it involved interhospital transfer. We excluded discharges for patients who underwent heart transplantation (ICD‐9-CM procedure code 33.6 or 37.51) or LVAD implantation (ICD‐9-CM procedure code 37.66) during the hospitalization to maintain a fair comparison between study groups. Elective hospitalizations were also excluded. Relevant procedures performed during the hospitalization (percutaneous coronary intervention [PCI], coronary artery bypass grafting [CABG], intra‐aortic balloon pump [IABP], Impella/TandemHeart, extracorporeal membrane oxygenation [ECMO], pulmonary artery catheter, mechanical ventilation, and cardiopulmonary resuscitation) were identified using ICD‐9-CM codes (Table S1).
Primary Exposure and Outcome
The primary exposure was whether the hospital type, for each admission, was an LVAD center. To determine this, we separately looked at hospitalizations for patients aged ≥18 years who underwent LVAD implantation from 2012 to 2014 using ICD‐9-CM procedure code 37.66. For our study population, if the admission took place at a hospital that performed at least one LVAD implantation in the same year, a designation of LVAD center was assigned. Non‐LVAD centers were further stratified into nonpeer or peer institutions, with the latter defined as large hospitals providing CABG surgery, similar to a previous study. 11
The primary outcome was in‐hospital, all‐cause mortality. We also examined the association between hospital type and in‐hospital mortality in the following prespecified subgroups: aged ≥75 versus <75 years, female versus male sex, AMI‐CS versus CS related to causes other than AMI (non–AMI‐CS), revascularized versus nonrevascularized patients, and interhospital transfers versus nontransfers.
Statistical Analysis
We compared baseline characteristics between hospital type (LVAD and non‐LVAD centers) using Rao‐Scott χ2 tests for categorical variables and survey‐specific t‐tests for continuous variables. Given the large sample size, standardized differences were calculated similar to previous studies of the NIS, with a difference >10% considered clinically meaningful. 12 To determine whether hospitalization in an LVAD center was independently associated with in‐hospital mortality, we constructed a multivariable logistic regression model incorporating the following variables: age, sex, primary expected payer status, median household income, weekday versus weekend admission, hospital characteristics (region, bed size, location, and teaching status), all Elixhauser comorbidities, cardiac comorbidities (prior myocardial infarction, prior PCI, prior CABG, coronary artery disease, and family history of coronary artery disease), presentation (AMI or non‐AMI), and procedures (cardiac arrest or mechanical ventilation within 24 hours of presentation, PCI, CABG, IABP use, and other MCS use [ECMO or Impella/TandemHeart]). To ensure the robustness of our findings, we repeated a separate analysis in which LVAD centers were compared with only non‐LVAD centers that were considered peer institutions.
For all analyses, we accounted for the complex survey design by using stratification and cluster variables. We weighted the data accordingly to produce nationally representative estimates. Statistical analysis was conducted using SAS software, version 9.4 (SAS Institute Inc, Cary, NC) and IBM SPSS Statistics for Windows, version 26.0 (IBM Corp, Armonk, NY). All P values were 2 sided, with a significance threshold of P<0.05. Categorical variables are expressed as percentages, and continuous variables are expressed as mean±SD.
Results
Patient and Hospital Characteristics
There were 272,075 hospitalizations for CS after applying specific inclusion and exclusion criteria to the database (Figure 1). Of those, 70,685 hospitalizations (26.0%) were in LVAD centers and 201,390 hospitalizations (74.0%) were in non‐LVAD centers. The clinical and hospital characteristics for the study population are presented in Table 1 (additional characteristics in Table S2).
Figure 1. Study population.
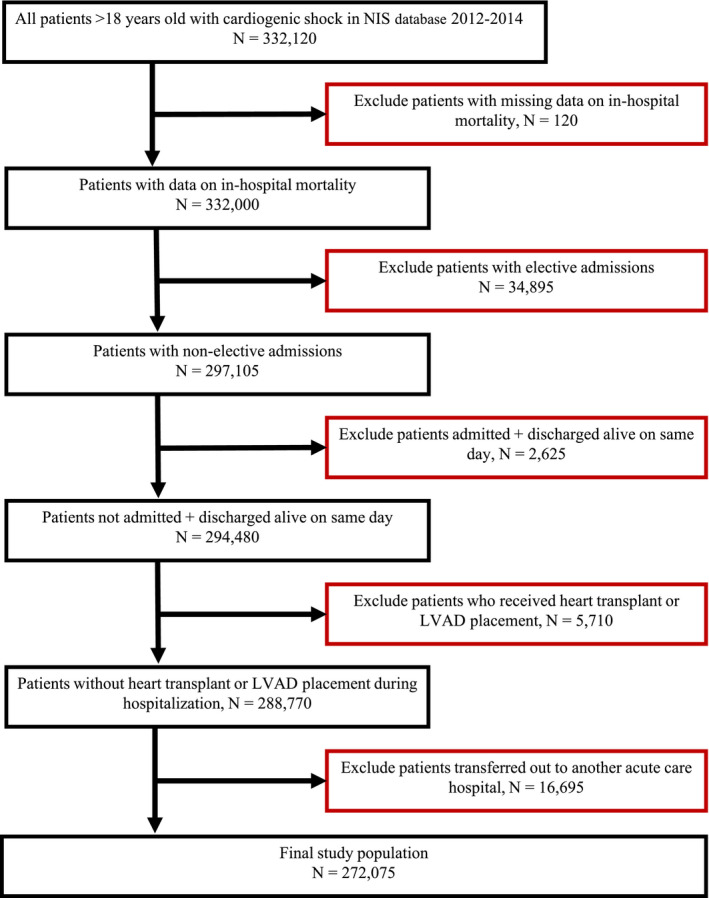
Flowchart of study patients. LVAD indicates left ventricular assist device; and NIS, National Inpatient Sample.
Table 1.
Baseline Characteristics
| Characteristic |
Overall (n=272 075) |
LVAD Hospital (n=70 685) |
Non‐LVAD Hospital (n=201 390) |
P Value | Standardized Difference, % |
|---|---|---|---|---|---|
| Age, y | 67.5±14.5 | 64.1±15.1 | 68.7±14.1 | <0.001 | 13.9 |
| Women | 39.6 | 38.0 | 40.2 | <0.001 | 4.4 |
| Black race | 13.0 | 17.1 | 11.5 | <0.001 | 15.9 |
| Weekend admission | 26.1 | 23.3 | 26.3 | <0.001 | 6.9 |
| Bed size | <0.001 | ||||
| Small | 9.3 | 0.8 | 12.3 | 47.7 | |
| Medium | 23.6 | 8.0 | 29.1 | 56.3 | |
| Large | 67.1 | 91.2 | 58.6 | 81.0 | |
| Urban location | 95.4 | 99.9 | 93.8 | <0.001 | 35.8 |
| Teaching hospital | 66.9 | 98.1 | 55.9 | <0.001 | 115.9 |
| Region | <0.001 | ||||
| Northeast | 18.0 | 23.9 | 15.9 | 20.3 | |
| Midwest | 21.3 | 22.9 | 20.7 | 5.4 | |
| South | 39.6 | 39.7 | 39.6 | 0.2 | |
| West | 21.1 | 13.5 | 23.8 | 26.9 | |
| Cardiac comorbidities | |||||
|
Known coronary artery disease |
53.9 | 50.7 | 55.0 | <0.001 | 8.6 |
|
Family history of coronary artery disease |
3.1 | 3.1 | 3.1 | 0.829 | 0.3 |
| Prior myocardial infarction | 9.9 | 10.3 | 9.8 | 0.208 | 1.6 |
|
Prior percutaneous coronary intervention |
8.8 | 9.5 | 8.5 | 0.005 | 3.6 |
|
Prior coronary artery bypass surgery |
7.5 | 7.8 | 7.4 | 0.215 | 1.4 |
| Elixhauser comorbidities | |||||
| Congestive heart failure | 24.2 | 24.5 | 24.1 | 0.414 | 1.0 |
| Chronic pulmonary disease | 25.2 | 22.0 | 26.3 | <0.001 | 10.1 |
| Coagulopathy | 20.9 | 25.2 | 19.4 | <0.001 | 13.9 |
| Deficiency anemias | 24.1 | 21.8 | 24.9 | <0.001 | 7.3 |
| Diabetes mellitus (uncomplicated) | 27.4 | 26.3 | 27.8 | 0.011 | 3.3 |
| Diabetes mellitus with complications | 7.9 | 6.7 | 8.3 | <0.001 | 6.1 |
| Hypertension | 57.9 | 55.1 | 58.8 | <0.001 | 7.5 |
| Liver disease | 4.4 | 5.1 | 4.1 | <0.001 | 4.5 |
| Fluid and electrolyte disorders | 62.0 | 63.5 | 61.5 | 0.021 | 4.0 |
| Obesity | 14.1 | 14.3 | 14.1 | 0.665 | 0.6 |
| Peripheral vascular disease | 13.8 | 13.8 | 13.8 | 0.916 | 0.1 |
|
Pulmonary circulation disorders |
6.6 | 7.6 | 6.2 | <0.001 | 5.5 |
| Chronic renal failure | 31.4 | 32.3 | 31.0 | 0.054 | 2.8 |
| Valvular disease | 7.0 | 7.3 | 6.9 | 0.171 | 1.6 |
| Weight loss | 12.7 | 14.9 | 12.0 | <0.001 | 8.7 |
| ≥3 Elixhauser comorbidities | 74.5 | 75.3 | 74.3 | 0.279 | 2.2 |
Data are given as mean±SD for age; otherwise, percentages are given. LVAD indicates left ventricular assist device.
Patients at LVAD centers were younger, more likely to be Black, and more likely to have private insurance compared with patients at non‐LVAD centers (P<0.001). There were similar rates of cardiovascular risk factors, including hypertension, diabetes mellitus, obesity, coronary artery disease, chronic heart failure, valvular disease, and peripheral vascular disease (all standardized differences <10%). LVAD centers were more likely to be large hospitals (91.2% versus 58.6%), urban centers (99.9% versus 95.4%), and teaching institutions (98.1% versus 55.9%) compared with non‐LVAD centers (P<0.001).
In the overall population, non–AMI‐CS cases were more common compared with AMI‐CS cases (52.5% versus 47.5%), with a higher prevalence in LVAD centers (62.3% versus 37.7%; P<0.001; Table 2). Accordingly, revascularization (PCI or CABG) was performed in a higher percentage of patients with CS in non‐LVAD centers. In the subpopulation of patients with AMI‐CS (129,330 cases), there were similar rates of PCI or CABG in LVAD and non‐LVAD centers (53.6% versus 52.6%), but CABG rates were higher in LVAD centers (subgroup characteristics and procedures in Tables S3‐S6). There was a higher percentage of AMI‐CS cases treated with early PCI (≤24 hours) in non‐LVAD centers (32.1% versus 24.6%; P<0.001). In the subgroup of AMI‐CS cases treated with early PCI, in‐hospital mortality was the same in both LVAD and non‐LVAD centers (31.7% versus 31.8%).
Table 2.
Case Presentations and Procedures
| Variable |
Overall (n=272 075), % |
LVAD Hospital (n=70 685), % |
Non‐LVAD Hospital (n=201 390), % |
P Value | Standardized Difference, % |
|---|---|---|---|---|---|
| Presentation | |||||
| Non–AMI‐CS | 52.5 | 62.3 | 49.0 | <0.001 | 27.0 |
| AMI‐CS | 47.5 | 37.7 | 51.0 | <0.001 | 27.0 |
| Procedures | |||||
|
CPR or intubated <24 h of admission |
34.8 | 31.1 | 36.1 | <0.001 | 10.4 |
| IABP | 18.8 | 18.7 | 18.8 | 0.847 | 0.3 |
| Percutaneous support (Impella/TandemHeart) | 2.1 | 2.6 | 1.9 | <0.001 | 5.2 |
| ECMO | 1.3 | 4.3 | 0.2 | <0.001 | 27.4 |
| PCI or CABG | 28.7 | 25.2 | 29.9 | <0.001 | 10.3 |
| CABG | 8.7 | 10.7 | 8.0 | <0.001 | 9.4 |
| PCI | 21.2 | 15.5 | 23.1 | <0.001 | 19.4 |
| Mechanical ventilation | 53.6 | 51.5 | 54.3 | <0.001 | 5.6 |
| Pulmonary artery catheter | 7.0 | 14.6 | 4.3 | <0.001 | 35.4 |
AMI‐CS indicates cardiogenic shock after acute myocardial infarction; CABG, coronary artery bypass grafting; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; LVAD, left ventricular assist device; Non–AMI‐CS, cardiogenic shock related to causes other than acute myocardial infarction; and PCI, percutaneous coronary intervention.
Differences in the use of temporary MCS for the study population are illustrated in Figure 2. Over the study period, IABP was the predominant type of circulatory support device, and was used far more frequently in AMI‐CS compared with non–AMI‐CS cases. IABP use was similar in both LVAD and non‐LVAD centers. Other devices, such as Impella/TandemHeart and ECMO, were used less frequently overall. ECMO was used in 4.3% of hospitalizations in LVAD centers compared with 0.2% of hospitalizations in non‐LVAD centers, with a similar rate in AMI‐CS and non–AMI‐CS cases (4.4% versus 4.2%). Impella/TandemHeart devices were used in 2.6% of hospitalizations in LVAD centers compared with 1.9% in non‐LVAD centers (standardized difference <10%). In addition, the rate of pulmonary artery catheter placement was higher in LVAD centers compared with non‐LVAD centers (Table 2).
Figure 2. Temporary mechanical support in left ventricular assist device (LVAD) vs non‐LVAD centers, subdivided into the cardiogenic shock after acute myocardial infarction (AMI‐CS) and cardiogenic shock related to causes other than acute myocardial infarction (non–AMI‐CS) subpopulations.
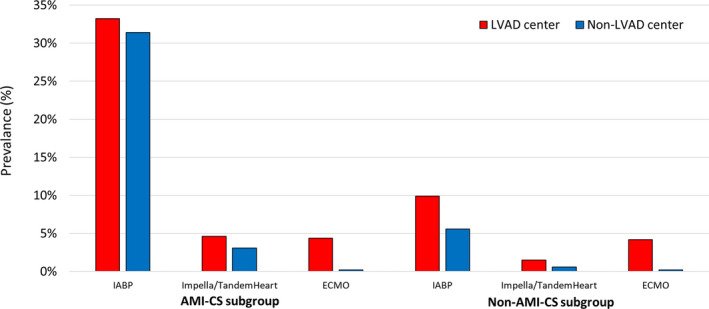
ECMO indicates extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; and LVAD, left ventricular assist device.
In‐Hospital Mortality
The in‐hospital mortality rates were 38.9% and 43.3% in patients hospitalized at LVAD centers and non‐LVAD centers, respectively (unadjusted odds ratio [OR], 0.84; 95% CI, 0.79–0.88; P<0.001). After multivariable adjustment, the odds of mortality remained lower for hospitalization in an LVAD center (adjusted OR, 0.89; 95% CI, 0.83–0.94; P<0.001). Revascularization was associated with lower in‐hospital morality (adjusted OR for PCI, 0.40; 95% CI, 0.34–0.43; P<0.001). Percutaneous support was associated with increased odds of mortality (adjusted OR for Impella/TandemHeart, 2.5; 95% CI, 2.1–2.8; P<0.001; and adjusted OR for ECMO, 4.4; 95% CI, 3.6–5.4; P<0.001). There was a significant interaction between hospital type and age ≥75 years for in‐hospital mortality (P interaction=0.004; Figure 3). The odds of in‐hospital mortality were lower in patients hospitalized at LVAD centers for those aged <75 years, whereas there was no difference in mortality for older patients. Also, in the subgroup of patients who were not transferred between hospitals, mortality rates were still lower in LVAD centers (Figure 3).
Figure 3. Association between left ventricular assist device (LVAD) centers vs non‐LVAD centers and in‐hospital mortality in cardiogenic shock.
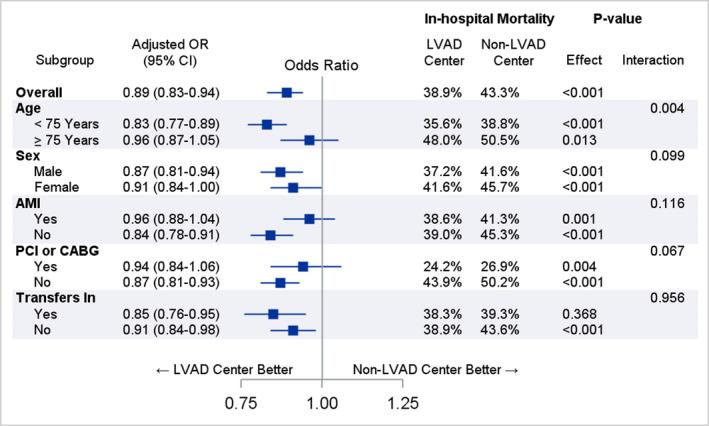
AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; OR, odds ratio; and PCI, percutaneous coronary intervention.
When LVAD centers were compared with peer institutions (defined as CABG‐capable centers with a large bed size; characteristics and procedures in Tables S7 and S8) in a sensitivity analysis, in‐hospital mortality was lower in LVAD centers (adjusted OR, 0.89; 95% CI, 0.84–0.95; P<0.001). The rate of CABG in both groups was similar (10.7% versus 10.3%; P=0.44), and the rate of PCI was higher (15.5% versus 25.8%) in the peer institutions. The use of Impella/TandemHeart in both groups was similar (2.6% versus 2.5%), but ECMO remained more frequent in LVAD centers (4.3% versus 0.3%; P<0.001).
Last, the odds of in‐hospital mortality were even lower in LVAD centers when the study population criteria were amended to include patient subgroups that were initially excluded. For example, when the 5710 CS hospitalizations in which the patient went on to receive heart transplant or LVAD implantation on the same admission were included as part of the study population, the in‐hospital mortality rates were 37% and 43.3% for LVAD centers and non‐LVAD centers, respectively (adjusted OR, 0.83; 95% CI, 0.78–0.88; P<0.001). When elective admissions (N=34 895) were included as part of the original study population, the adjusted OR was 0.86 (95% CI, 0.81–0.91; P<0.001).
Discussion
Using a nationally representative sample of patients hospitalized with CS, we found that LVAD centers had lower in‐hospital mortality compared with non‐LVAD centers. This difference remained significant after multivariable adjustment for patient and hospital characteristics, as well as relevant in‐hospital procedures. Most CS hospitalizations took place in non‐LVAD centers, and there were more hospitalizations for non–AMI‐CS. In addition, younger patients derived the most survival benefit from LVAD centers compared with non‐LVAD centers. Finally, although revascularization was associated with improved survival, the difference in mortality between LVAD and non‐LVAD centers was more apparent in patients with non–AMI‐CS and patients who did not receive revascularization.
Although patients admitted to non‐LVAD centers had a similar burden of Elixhauser and cardiovascular comorbidities compared with patients hospitalized in LVAD centers, they were older on average, and age was independently associated with worse survival. Patients aged ≥75 years had higher case fatality rates, consistent with previous studies of CS that have reported a worse prognosis and a variable response to interventions in this population. 1 , 13 , 14 , 15 More important, it was younger patients with CS (aged <75 years) who benefited most from hospitalization in an LVAD center compared with a non‐LVAD center. This is not surprising as younger patients with CS are less likely to have end‐organ failure, neurological injury, comorbid conditions, including frailty, and bleeding disorders. 16 Therefore, they are more likely to be candidates for aggressive treatment measures, including early revascularization, mechanical ventilation, therapeutic hypothermia, hemodialysis, and MCS.
Consistent with previous studies of CS, there was a clear survival benefit in patients who received revascularization. 1 , 2 , 3 However, even when controlling for revascularization, hospital type (LVAD center versus non‐LVAD center) remained independently associated with in‐hospital mortality. Furthermore, in the AMI‐CS subpopulation, revascularization rates (CABG or PCI) were similar between LVAD and non‐LVAD centers. Most hospitalizations in our study were actually for non–AMI‐CS, and they represented 62% of hospitalizations in LVAD centers. This finding is consistent with other observational studies from centers considered referral hubs for CS. In 2 contemporary single‐center studies of CS, decompensated heart failure was the cause in 60% and 63% of cases. 8 , 17 Recently, in a study of 3049 admissions from 16 hospitals in North America, more than two thirds of all CS cases were for non–AMI‐CS. 18 These findings are not unexpected. Patients with advanced cardiomyopathy, both ischemic and nonischemic, are often referred to heart failure specialists in LVAD centers and are therefore more likely to be admitted to the same center if they decompensate. Also, there are rare causes of acute heart failure (giant cell myocarditis and checkpoint inhibitor myocarditis) that may require right ventricular biopsy, immunosuppressive medications, and urgent MCS necessitating earlier transfer to tertiary centers.
The difference in survival between LVAD and non‐LVAD centers was more impressive for the non–AMI‐CS subpopulation. This may be partially explained by variations in the pattern of MCS use in LVAD centers versus non‐LVAD centers. The use of Impella/TandemHeart and IABP devices was similar between hospital types and, as expected, was more frequent in AMI‐CS cases compared with non–AMI‐CS. However, ECMO use was significantly more prevalent in LVAD centers, and it was deployed with similar rates in AMI‐CS and non–AMI‐CS hospitalizations. Although there is still no compelling evidence that any MCS device improves outcomes in AMI‐CS, their use in non–AMI‐CS remains largely understudied. Venoarterial ECMO has not been the subject of randomized trials but, nonetheless, is being used with increased frequency in many centers. 19 This trend, particularly over the past year, may reflect recent changes in the heart transplant organ allocation policy, which now prioritizes transplant for patients on venoarterial ECMO. 20 , 21 Despite a paucity of data overall and some controversy about this policy change, 22 there is a physiologic rationale for ECMO with or without left ventricular unloading in certain phenotypes of non–AMI‐CS, such as acute on chronic biventricular failure, right ventricular failure with hypoxia, and pulmonary hypertensive crisis. 23 , 24 Still, in our study as in others, ECMO use was associated with increased odds of in‐hospital death, most likely related to increased illness severity but also MCS‐associated complications. 9 Further studies are needed to elucidate the full impact of ECMO and other MCS devices on CS outcomes.
The observational nature of this study limits our ability to know with certainty the reasons why LVAD centers had lower mortality rates. Although there may be several more traditional hospital‐level factors that are more commonly associated with tertiary referral centers in general and less specific to LVAD centers only, we feel that that this was unlikely to account for the differences in outcome. First, although LVAD centers were more likely to be large, teaching hospitals in urban locations, neither hospital size nor teaching status had an impact on mortality in our logistic regression model. Also, when we compared LVAD centers with peer institutions defined as large hospitals with cardiac surgery capabilities, LVAD centers still performed better. Because the 2012 to 2014 NIS sampling scheme precluded accurate determination of hospital case volume, it was not included in our logistic regression model. In one NIS study of CS hospitalizations between 2004 and 2011, there was a correlation between case‐volume and in‐hospital mortality, but notably, LVAD centers were part of every volume quartile, suggesting that there are benefits that extend beyond increased caseload. 25 And finally, hospitalizations in specialized centers do not universally equate to better outcomes. In a study of Medicare patients with heart failure, 30‐day risk‐adjusted mortality was similar between heart transplant centers and nontransplant institutions in the same geographic area. 11
It is more likely that LVAD centers have better CS outcomes because they can leverage collaborative systems of care that are already in place. In a recently published report from the Cardiac Safety Research Consortium ThinkTank on CS, potential centers of excellence were described as hospitals with multidisciplinary shock teams capable of accurate diagnosis, rapid stratification of cause, and appropriate triage. 26 They would also be institutions with a track record for consistently collecting high‐quality data that could be used in registries and trials of drugs and devices. LVAD centers have established multidisciplinary teams with processes in place to optimize unstable patients with heart failure, bridge them to surgery, recover them in postoperative units, and manage complications, including right heart failure, respiratory failure, ventricular arrhythmias, and vasoplegia. These programs are also required to analyze LVAD related clinical data and contribute to an audited registry. 27 Last, LVAD centers have the necessary expertise to identify patients with CS who will not recover and evaluate them for durable devices and transplant. To our knowledge, this is the first study to show, on a national level, that specialized LVAD centers have better CS outcomes. These data provide a potential framework to identify “shock team” capable institutions across the United States that can actively participate in larger studies of CS.
A major strength of this study was that we used a nationally representative all‐payer cohort to capture a real‐world case mix of CS with a large number of hospitalizations. However, there are also notable limitations. First, our definitions of both CS and AMI were based on ICD‐9-CM codes. Although these definitions are imperfect, they have nonetheless been used in multiple previous studies of the NIS and validated in other studies as well. 12 , 28 , 29 , 30 Second, starting in 2012, the NIS was redesigned to represent a 20% national patient‐level sample of all US hospitals. Because of the stratified sampling technique, it is possible that our definition of hospital type (LVAD versus non‐LVAD) may have missed some hospitals with a low procedural volume (≤5 LVADs annually). However, our data corresponded with data from the Centers for Medicare and Medicaid Services. There were 137 LVAD hospitals identified in our study for the year 2014, which is the same number as the 137 Centers for Medicare and Medicaid Services–approved ventricular assist device institutions by the end of 2014. 31 Third, the NIS lacks information on medications, CS severity, hemodynamics, cardiac biomarkers, left ventricular ejection fraction, coronary anatomical features, lactate levels, bilirubin levels, renal function, and frailty, all of which could have impacted our findings. Fourth, the NIS contains only inpatient data and does not include outcomes on long‐term survival. Finally, miscoded and missing data can occur in large administrative databases; however, quality control procedures are routinely performed to confirm that data in the NIS are valid and reliable.
Conclusions
In this large national study of CS hospitalizations, LVAD centers had lower in‐hospital mortality compared with non‐LVAD centers, even after accounting for various patient and hospital characteristics, revascularization, and use of mechanical support devices. LVAD centers may represent centers of excellence for the management of CS and could serve as research sites for future investigations.
Sources of Funding
This work was supported by grants from the Michael Wolk Heart Foundation and the New York Cardiac Center, Inc. The Michael Wolk Heart Foundation and the New York Cardiac Center, Inc, had no role in the design and conduct of the study.
Disclosures
Dr Goyal is supported by the National Institute on Aging grant R03AG056446 and American Heart Association grant 18IPA34170185 and is a recipient of a National Institute on Aging Loan Repayment Plan. The remaining authors have no disclosures to report.
Supporting information
Table S1–S8
Acknowledgements
Author contributions: All authors had access to the data and a role in writing the manuscript.
(J Am Heart Assoc. 2020;9:e017326 DOI: 10.1161/JAHA.120.017326.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock: SHOCK Investigators: Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;625–634. [DOI] [PubMed] [Google Scholar]
- 2. Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD. Early revascularization and long‐term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;448–454. [DOI] [PubMed] [Google Scholar]
- 4. Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, Charitakis K, Feldman DN, Dakik HA, Mauri L, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. 2016;341–351. [DOI] [PubMed] [Google Scholar]
- 5. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;1249–1258. [DOI] [PubMed] [Google Scholar]
- 6. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short‐term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;1407–1415. [DOI] [PubMed] [Google Scholar]
- 7. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd, O'Neill W. Cardiac shock care centers: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;1972–1980. [DOI] [PubMed] [Google Scholar]
- 8. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;1659–1669. [DOI] [PubMed] [Google Scholar]
- 9. Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi‐Nicolau J, Alvarez Villela M, Welt F, Stehlik J, Gilbert EM, et al. Shock Team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support: a proof of concept. Circulation. 2019;98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. HCUP Databases . Healthcare Cost and Utilization Project (HCUP). December 2019. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Published December 17, 2019. Accessed March 24, 2020
- 11. Hummel SL, Pauli NP, Krumholz HM, Wang Y, Chen J, Normand SL, Nallamothu BK. Thirty‐day outcomes in Medicare patients with heart failure at heart transplant centers. Circ Heart Fail. 2010;244–252. [DOI] [PubMed] [Google Scholar]
- 12. Echouffo‐Tcheugui JB, Kolte D, Khera S, Aronow HD, Abbott JD, Bhatt DL, Fonarow GC. Diabetes mellitus and cardiogenic shock complicating acute myocardial infarction. Am J Med. 2018;1144–1152. [DOI] [PubMed] [Google Scholar]
- 13. Aissaoui N, Puymirat E, Juilliere Y, Jourdain P, Blanchard D, Schiele F, Guéret P, Popovic B, Ferrieres J, Simon T, et al. Fifteen-year trends in the management of cardiogenic shock and associated 1-year mortality in elderly patients with acute myocardial infarction: the FAST-MI programme. Eur J Heart Fail. 2016;18:1144–1152. [DOI] [PubMed] [Google Scholar]
- 14. Masoumi A, Rosenblum H, Garan A. Cardiogenic shock in older adults. Curr Cardiovasc Risk Rep. 2016;10. [Google Scholar]
- 15. Damluji AA, Bandeen‐Roche K, Berkower C, Boyd CM, Al‐Damluji MS, Cohen MG, Forman DE, Chaudhary R, Gerstenblith G, Walston JD, et al. Percutaneous coronary intervention in older patients with ST‐segment elevation myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2019;1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non‐infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;287–303. [DOI] [PubMed] [Google Scholar]
- 17. Lim HS, Howell N. Cardiogenic shock due to end‐stage heart failure and acute myocardial infarction: characteristics and outcome of temporary mechanical circulatory support. Shock. 2018;167–172. [DOI] [PubMed] [Google Scholar]
- 18. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;9:e005618. DOI: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Batra J, Toyoda N, Goldstone AB, Itagaki S, Egorova NN, Chikwe J. Extracorporeal membrane oxygenation in New York State: trends, outcomes, and implications for patient selection. Circ Heart Fail. 2016;e003179 DOI: 10.1161/CIRCHEARTFAILURE.116.003179. [DOI] [PubMed] [Google Scholar]
- 20. Cogswell R, John R, Estep JD, Duval S, Tedford RJ, Pagani FD, Martin CM, Mehra MR. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant. 2020;1–4. [DOI] [PubMed] [Google Scholar]
- 21. Stevenson LW, Kormos RL, Young JB, Kirklin JK, Hunt SA. Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant. 2016;547–549. [DOI] [PubMed] [Google Scholar]
- 22. Trivedi JR, Slaughter MS. "Unintended" consequences of changes in heart transplant allocation policy: impact on practice patterns. ASAIO J. 2020;125–127. [DOI] [PubMed] [Google Scholar]
- 23. Rosenzweig EB, Gannon WD, Madahar P, Agerstrand C, Abrams D, Liou P, Brodie D, Bacchetta M. Extracorporeal life support bridge for pulmonary hypertension: a high‐volume single‐center experience. J Heart Lung Transplant. 2019;1275–1285. [DOI] [PubMed] [Google Scholar]
- 24. Keebler ME, Haddad EV, Choi CW, McGrane S, Zalawadiya S, Schlendorf KH, Brinkley DM, Danter MR, Wigger M, Menachem JN, et al. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail. 2018;503–516. [DOI] [PubMed] [Google Scholar]
- 25. Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;9:e001462 DOI: 10.1161/JAHA.114.001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samsky M, Krucoff M, Althouse AD, Abraham WT, Adamson P, Aguel F, Bilazarian S, Dangas GD, Gilchrist IC, Henry TD, et al. Clinical and regulatory landscape for cardiogenic shock: a report from the cardiac safety research consortium ThinkTank on cardiogenic shock. Am Heart J. 2020;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, et al. The Society of Thoracic Surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg. 2019;341–353. [DOI] [PubMed] [Google Scholar]
- 28. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;99–104. [DOI] [PubMed] [Google Scholar]
- 29. Metcalfe A, Neudam A, Forde S, Liu M, Drosler S, Quan H, Jetté N. Case definitions for acute myocardial infarction in administrative databases and their impact on in‐hospital mortality rates. Health Serv Res. 2013;290–318. DOI: 10.1111/j.1475-6773.2012.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguere M, Carroll C, Beauchamp C, Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico‐administrative hospital data? Can J Cardiol. 2012;162–168. [DOI] [PubMed] [Google Scholar]
- 31. VAD Destination Therapy Facilities . Centers for Medicare & Medicaid Services, Baltimore, MD. https://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/VAD-Destination-Therapy-Facilities. Published July 5, 2016. Accessed March 25, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S8


