Abstract
Background
Human mesenchymal cells are culprit factors in vascular (patho)physiology and are hallmarked by phenotypic and functional heterogeneity. At present, they are subdivided by classic umbrella terms, such as “fibroblasts,” “myofibroblasts,” “smooth muscle cells,” “fibrocytes,” “mesangial cells,” and “pericytes.” However, a discriminative marker‐based subclassification has to date not been established.
Methods and Results
As a first effort toward a classification scheme, a systematic literature search was performed to identify the most commonly used phenotypical and functional protein markers for characterizing and classifying vascular mesenchymal cell subpopulation(s). We next applied immunohistochemistry and immunofluorescence to inventory the expression pattern of identified markers on human aorta specimens representing early, intermediate, and end stages of human atherosclerotic disease. Included markers comprise markers for mesenchymal lineage (vimentin, FSP‐1 [fibroblast‐specific protein‐1]/S100A4, cluster of differentiation (CD) 90/thymocyte differentiation antigen 1, and FAP [fibroblast activation protein]), contractile/non‐contractile phenotype (α‐smooth muscle actin, smooth muscle myosin heavy chain, and nonmuscle myosin heavy chain), and auxiliary contractile markers (h1‐Calponin, h‐Caldesmon, Desmin, SM22α [smooth muscle protein 22α], non‐muscle myosin heavy chain, smooth muscle myosin heavy chain, Smoothelin‐B, α‐Tropomyosin, and Telokin) or adhesion proteins (Paxillin and Vinculin). Vimentin classified as the most inclusive lineage marker. Subset markers did not separate along classic lines of smooth muscle cell, myofibroblast, or fibroblast, but showed clear temporal and spatial diversity. Strong indications were found for presence of stem cells/Endothelial‐to‐Mesenchymal cell Transition and fibrocytes in specific aspects of the human atherosclerotic process.
Conclusions
This systematic evaluation shows a highly diverse and dynamic landscape for the human vascular mesenchymal cell population that is not captured by the classic nomenclature. Our observations stress the need for a consensus multiparameter subclass designation along the lines of the cluster of differentiation classification for leucocytes.
Keywords: atherosclerosis, fibroblasts, myofibroblasts, vascular smooth muscle cells
Subject Categories: Atherosclerosis, Aneurysm, Pathophysiology, Smooth Muscle Proliferation and Differentiation, Vascular Biology
Nonstandard Abbreviations and Acronyms
- AIT
adaptive intimal thickening
- FAP
fibroblast activation protein
- FCP
fibrocalcific plaque
- FSP‐1
fibroblast‐specific protein‐1
- HR
healed rupture
- LFA
late fibroatheroma
- P4HB
prolyl 4‐hydroxylase β
- SM22α
smooth muscle protein 22α
- SMC
smooth muscle cell
- Smemb
nonmuscle myosin heavy chain
- SM‐MHC
smooth muscle myosin heavy chain
- Thy‐1
thymocyte differentiation antigen 1
- αSMA
α‐smooth muscle actin
Clinical Perspective
What Is New?
A classification scheme for the vascular mesenchymal cell population is missing.
This study provides a first framework for a systematic marker‐based classification of human vascular mesenchymal cells, and implies an underappreciated, extremely diverse spectrum of human mesenchymal cells within the aortic wall.
What Are the Clinical Implications?
Mesenchymal cells play a central role in vascular pathological conditions, such as atherosclerosis and abdominal aortic aneurysms.
This systematic evaluation indicates an extreme diverse and dynamic mesenchymal cell landscape, but also implies an unappreciated cellular flexibility with indications for both Endothelial‐to‐Mesenchymal cell Transition as well as Leucocyte‐to‐Mesenchymal cell Transition (fibrocytes) as common events.
This study provides a first step in a better understanding of the role of vascular mesenchymal cells in human disease.
Vascular mesenchymal cells are critically involved in blood vessel development and homeostasis and are progressively acknowledged as key effector cells in vascular pathological conditions, such as atherosclerosis, aneurysmal disease, and neointima formation. 1 , 2 , 3
In the context of vascular pathology, mesenchymal cells are generally subclassified by classic umbrella terms, such as “fibroblasts,” “myofibroblasts,” “smooth muscle cells” (SMCs), 4 “fibrocytes,” 5 “mesangial cells,” 6 and “pericytes.” 7 This generic nomenclature is based on the process under investigation, their presumed function or specific anatomical location, and/or their in vitro behavior. 8 , 9 At this point, a discriminative consensus (sub)classification for vascular mesenchymal cells, let alone classifying marker sets required for mechanistic understanding, is needingly missing. In this light, and in the context of the emerging key roles for mesenchymal cells in human vascular disease, we considered a systematic exploration of potential relevant class‐specific marker sets.
To address this point, we performed a systematic literature search to identify candidate mesenchymal cell‐specific markers, and evaluated the expression pattern and the expression dynamics of the identified markers in different stages of the human atherosclerotic process.
Methods
This study is based on a 2‐step approach. First, we conducted a systematic literature search to map the reported markers for vascular mesenchymal cell subpopulation characterization and classification. Subsequently, we applied immunohistochemistry and immunofluorescence to evaluate the specificity and expression pattern of the identified markers in a representative sample of early, intermediate, and (stabilized) end stages of the human aortic atherosclerotic process (Virmani classification, 10 respectively: adaptive intimal thickening [AIT], late fibroatheroma [LFA], and fibrocalcific plaque [FCP]) (Figure 1).
Figure 1. Histologic overview (Movat pentachrome staining) of selected representative sections of adaptive intimal thickening (AIT), late fibroatheroma (LFA), and fibrotic calcified plaque (FCP).
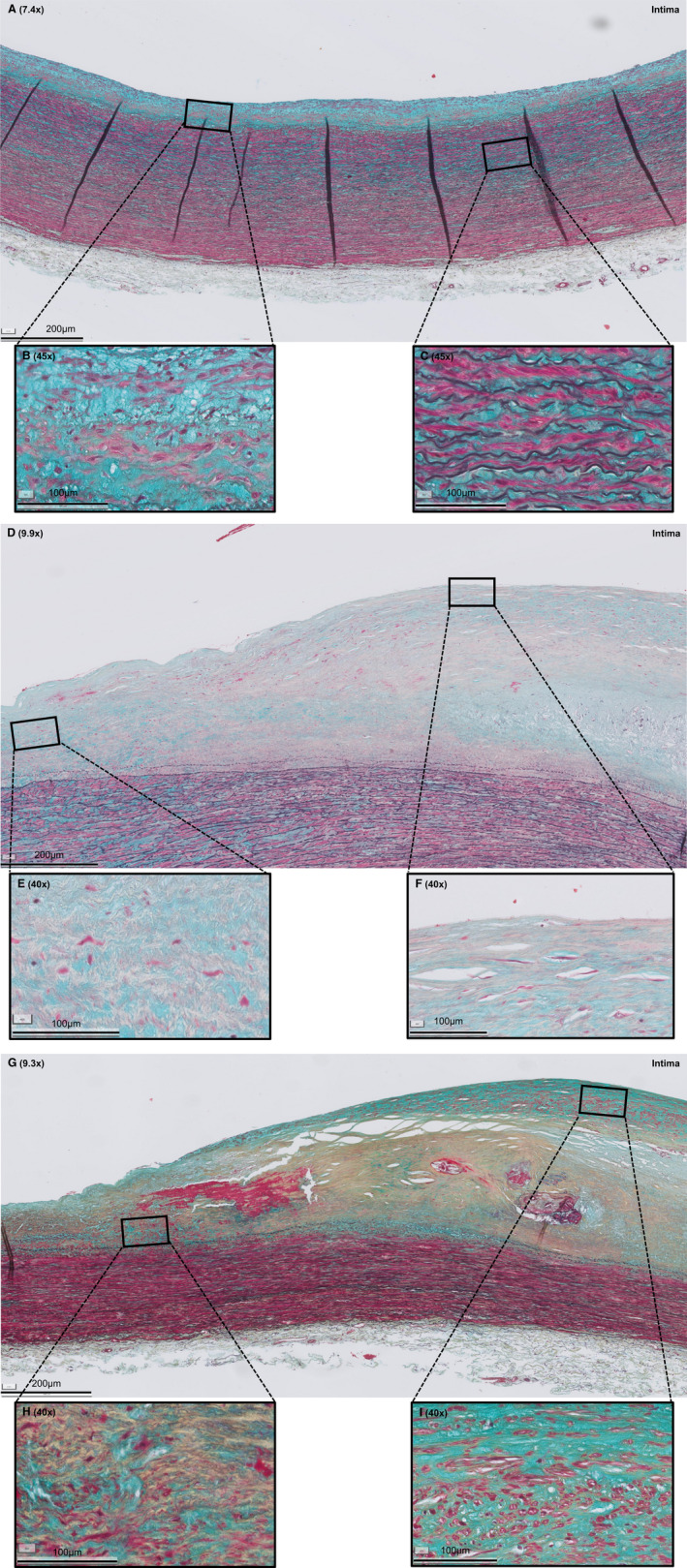
A, AIT is characterized by a thickening intima, consisting of smooth muscle cells (SMCs) in a proteoglycan‐rich matrix (B) and a normal media and adventitia (C). D, LFA is characterized by a necrotic core of cellular debris and cholesterol crystals that is covered by a multilayered fibrous cap, consisting of SMCs in a collagenous proteoglycan‐rich matrix with infiltration of inflammatory cells (F). E, Shoulder regions. G, FCP is characterized by extensive fibrosis, a condensated (former) necrotic core, and ample calcification (H) and neointimal formation (I). Legend to the Movat staining: red, SMCs/fibrin; violet, leukocytes; black, elastin; blue, proteoglycans/mucins; yellow, collagen. Various shades of green reflect colocalization of collagen (yellow) and proteoglycans (blue).
The authors declare that all supporting data are available within the article (and its online supplementary files).
Systematic Literature Review of Phenotypical Immunohistochemical Markers
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. Studies were identified by searching PubMed and Embase. The search strategy (outlined in Data S1 and S2 [Systematic Review Protocol]) was based on 3 search themes, combined in the search by AND. The first theme was created for vascular remodeling and phenotypic heterogeneity. The second theme included descriptions of fibroblasts, myofibroblasts, and SMCs. The final, third theme consisted of terms for atherosclerosis, aortic aneurysmal disease, and fibrosis. Because the focus of the study was on the classic supportive mesenchymal vascular cell type, we considered aspects of osteogenic, adipogenic, and pericyte differentiation beyond the scope of the literature review.
The search was most recently updated in December 2019. First, 2 authors (J.L. and L.B.) independently reviewed the titles and abstracts for eligibility. Thereafter, full‐text articles were assessed.
In parallel to the above phenotypic markers, we mapped reported markers of a synthetic and proinflammatory phenotype for functional subclassification, as these functions are considered independent of the cell phenotype (ie, SMCs, myofibroblasts, and fibroblasts can be synthetic and/or inflammatory).
Human Atherosclerotic Tissue Sampling
Formalin‐fixed, paraffin‐embedded aortic wall samples were selected from the Vascular Tissue Repository at the Department of Vascular Surgery, Leiden, the Netherlands. These human perirenal aortic patches were obtained during clinical organ transplantation with grafts derived from cadaveric donors. Histologic sections were prepared for each tissue block, sections were Movat pentachrome stained (for protocol, see Data S3), and the extent of atherosclerosis was classified (modified American Heart Association classification, according to Virmani et al 10 ) The tissue block showing the highest degree of atherosclerosis was used as the reference block. For this evaluation, we randomly selected preclassified tissue blocks representative for AIT, LFA, and FCP (Figure 1). All stainings were performed on sequential tissue sections from the selected tissue blocks.
To evaluate mesenchymal cell presence in respectively progressive and stabilizing atherosclerotic lesions, representative sections of the unstable lesion thin cap fibroatheroma 10 in addition to the stable lesion LFA and healed rupture (HR) 10 were selected. HR was selected as well because of a suspected enrichment of the mesenchymal cell subtype fibrocytes. 11
Immunohistochemical Staining on Atherosclerotic Lesions
Single‐Labeling Immunohistochemistry
Consecutive (4‐μm) sections were immunostained for the 28 immunohistochemistry markers (Table 1) identified in the literature review. All single stainings were performed by immunohistochemistry, because immunohistochemistry allows for direct clear overview, provides superior contextual information, and is not interfered by background staining (mainly caused by elastin) when assessed by immunofluorescence. Heat‐induced (Tris/EDTA, pH 9.2/citrate, pH 6) or enzyme‐induced antigen retrieval was performed if required (Table 1).
Table 1.
Antibodies Used for Immunohistochemistry
| Antibody, Clone or Catalog No. | Abbreviation Used in Study | Host Isotype; Subclass | Purification | Cellular Localization | Pretreatment | Protein Block (Dako) | Dilution | Secondary Antibody | Source |
|---|---|---|---|---|---|---|---|---|---|
| Vimentin, 3B4 | Vim |
Mouse IgG2a |
Purified from cell culture supernatant | Cytoskeleton (intermediate filament) | Tris‐EDTA (pH 9.2) | No | 1:2000 |
|
Dako |
| Fibroblast‐specific protein‐1/S100A4, D9F9D | FSP‐1 | Rabbit IgG | Not specified | Nucleus, cytoplasm, and extracellular space | Tris‐EDTA (pH 9.2) | No | 1:6000 | DAKO EnVision+ System, anti‐rabbit | Cell Signaling Technology |
| CD90/thymocyte differentiation antigen 1, ERP3133 | Thy‐1 | Rabbit IgG | Purified from tissue culture supernatant | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:200 | DAKO EnVision+ System, anti‐rabbit | Abcam |
| Fibroblast activation protein, AF3715 | FAP | Sheep IgG | Antigen affinity purified | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:40 | Donkey anti‐sheep IgG‐HRP, A16047 (ThermoFisher) | R&D Systems |
| α‐Smooth muscle actin, 1A4 | αSMA | Mouse IgG2a | Purified from cell culture supernatant | Cytoskeleton (actin filaments) | Tris‐EDTA (pH 9.2) | No | 1:4000 |
|
Dako |
| Nonmuscle myosin heavy chain, NBP2‐38566 | Smemb | Rabbit IgG | Immunogen affinity purified | Cytoskeleton (myosin binding) | Tris‐EDTA (pH 9.2) | No | 1:600 | DAKO EnVision+ System, anti‐rabbit | Novusbio |
| Smooth muscle myosin heavy chain I (SM1), 3F8 | SM‐MHC (SM1) |
Mouse IgG1 |
Ascites | Cytoskeleton (myosin) | Tris‐EDTA (pH 9.2) | No | 1:5000 | DAKO EnVision+ System, anti‐mouse | Abcam |
| Smooth muscle myosin heavy chain 11 (SM2), ab53219 | SM‐MHC (SM2) | Rabbit IgG | Ion exchange chromatography | Cytoskeleton (myosin) | Tris‐EDTA (pH 9.2) | No | 1:500 | DAKO EnVision+ System, anti‐rabbit | Abcam |
| Calponin, hCO | Calp | Mouse IgG1 | Ascites | Cytoskeleton (thin filament‐associated protein) | Tris‐EDTA (pH 9.2) | Yes | 1:3 000 000 |
|
Merck |
| Caldesmon, h‐CALD | h‐Caldes |
Mouse IgG1 |
Protein A or G | Cytoskeleton (microfilaments) and ECM | Citrate (pH 6) | No | 1:2000 | DAKO EnVision+ System, anti‐mouse | Novusbio |
| Desmin, AF3844 | Des | Goat IgG | Antigen affinity purified | Cytoskeleton (intermediate filament) | Tris‐EDTA (pH 9.2) | No | 1:50 | Donkey anti‐goat IgG‐HRP, ab97120 (Abcam) | R&D Systems |
| Smooth muscle protein 22α/transgelin, 8C8 | SM22α | Mouse IgG1 | Antigen affinity purified | Cytoskeleton (actin binding) | No retrieval | No | 1:25000 | DAKO EnVision+ System, anti‐mouse | Novusbio |
| Smoothelin, R4A | Smooth |
Mouse IgG1 |
Not specified | Cytoskeleton (intermediate filament) | Tris‐EDTA (pH 9.2) | No | 1:300 | DAKO EnVision+ System, anti‐mouse | Thermo Fisher Scientific |
| Tropomyosin, F‐6 | Tropo | Mouse IgG2b | Not specified | Cytoskeleton (actin binding) | Tris‐EDTA (pH 9.2) | No | 1:8000 |
|
Santa Cruz Biotechnology |
| Myosin light chain kinase/Telokin A01697‐2 | Telokin | Rabbit IgG | Not specified | Cytoskeleton (myosin binding) | No retrieval | No | 1:1000 | DAKO EnVision+ System, anti‐rabbit | Bosterbio |
| Paxillin, 5H11 | Pax |
Mouse IgG1 |
Protein G |
Cytoplasm > cytoskeleton (focal adhesion protein) |
Tris‐EDTA (pH 9.2) | No | 1:500 | DAKO EnVision+ System, anti‐mouse | Invitrogen |
| Vinculin, hVIN‐1 | Vinc |
Mouse IgG1 |
Unpurified | Cytoskeleton (actin binding) | Citrate (pH 6) | No | 1:200 000 | DAKO EnVision+ System, anti‐mouse | Novusbio |
| Collagen‐I, MBS502155 | Col‐I | Rabbit IgG | Affinity chromatography | ECM | Citrate (pH 6), Tris‐EDTA (pH 9.2), and pepsin | No | No specific signal | DAKO EnVision+ System, anti‐rabbit | MyBioScource |
| Collagen‐I, C7510‐17K | Col‐I | Goat IgG | Affinity chromatography | ECM | Citrate (pH 6), Tris‐EDTA (pH 9.2), and pepsin | No | No specific signal | Donkey anti‐goat IgG‐HRP, AB97120 (Abcam) | USBIO |
| Procollagen‐I, PC8‐7 | Procol‐I | Mouse IgG1 | Not specified | Secreted | Citrate (pH 6) and Tris/EDTA (pH 9.2) | No | No specific signal | DAKO EnVision+ System, anti‐mouse | Abnova |
| Procollagen‐I, M58 | Procol‐I | Rat IgG1 | Affinity chromatography | Secreted | Citrate (pH 6) and Tris‐EDTA (pH 9.2) | No | No specific signal | Goat anti‐rat IgG‐Biotine, BA‐9401 (Vector) | Chemicon International |
| Prolyl 4‐hydroxylase β, 3‐2B12 | P4HB | Mouse IgG1 | Affinity chromatography | Endoplasmic reticulum lumen, cell membrane | Citrate (pH 6) | No | 1:4000 | DAKO EnVision+ System, anti‐mouse | Acris Antibodies |
| Osteopontin, AF1433 | OPN | Polyclonal goat IgG | Antigen affinity purified | ECM | Tris‐EDTA (pH 9.2) | No | 1:100 | Donkey anti‐goat IgG‐HRP, AB97120 (Abcam) | R&D Systems |
| Fibronectin, FBN11 | FBN | Mouse IgG1 | Protein G | ECM | Tris‐EDTA (pH 9.2) | No | 1:900 | DAKO EnVision+ System, anti‐mouse | Thermofisher |
| Laminin, ab11575 | Lam | Polyclonal rabbit IgG | Immunogen affinity purified | ECM | Tris‐EDTA (pH 9.2) | No | 1:200 | DAKO EnVision+ System, anti‐rabbit | Abcam |
| Cellular retinoid‐binding protein‐I, B8 | CRBP‐I | Mouse IgG1 | Not specified | Cytoplasm | Citrate (pH 6) and Tris‐EDTA (pH 9.2) | No | No signal | DAKO EnVision+ System, anti‐mouse | Santa Cruz Biotechnology |
| Platelet‐derived growth factor receptor α, ab61219 | PDGF‐α | Rabbit IgG | Immunogen affinity purified | Cell membrane | Citrate (pH 6) and Tris‐EDTA (pH 9.2) | No | A specific signal (nuclear) | DAKO EnVision+ System, anti‐rabbit | Abcam |
| Phosphorylated nuclear factor‐κB p105, 178F3 | NF‐κB | Rabbit IgG | Not specified | Nucleus | Citrate (pH 6), Tris‐EDTA (pH 9.2), and pepsin | No | No specific signal | DAKO EnVision+ System, anti‐rabbit | Cell Signaling Technology |
| Phosphorylated nuclear factor‐κB p65, MCFA30 | NFκB | Mouse IgG1 | Antigen affinity purified | Nucleus | Citrate (pH 6), Tris‐EDTA (pH 9.2), and pepsin | No | No specific signal | DAKO EnVision+ System, anti‐mouse | eBioscience |
| Interleukin 6, NYRhIL6 | IL‐6 | Mouse IgG2a | Not specified | Secreted |
Citrate (pH 6) |
Yes | 1:300 | DAKO EnVision+ System, anti‐mouse | Santa Cruz Biotechnology |
| Monocyte chemoattractant protein‐I, 23002 | MCP‐I | Mouse IgG2b | Protein A or G | Secreted | Citrate (pH 6), Tris‐EDTA (pH 9.2), and pepsin | No | No signal | DAKO EnVision+ System, anti‐mouse | R&D Systems |
| Interleukin 8, bs0708R | IL‐8 | Rabbit IgG | Protein A | Secreted | Tris‐EDTA (pH 9.2) | No | 1:600 | DAKO EnVision+ System, anti‐rabbit | Bioss |
| CD31, JC70A | CD31 | Mouse IgG1 | Tissue culture supernatant | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:1000 | MACH‐2 Biocare Medical, anti‐mouse | Dako |
| CD45, HI30 | CD45 | Mouse IgG1 | Affinity chromatography | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:2000 | MACH‐2 Biocare Medical, anti‐mouse | Biolegend |
| CD34, M1636 | CD34 | Mouse IgG1 | Affinity chromatography | Cell membrane |
Citrate (pH 6) |
Yes | 1:1 000 000 | MACH‐2 Biocare Medical, anti‐mouse | Sanquin |
| CD4, H‐370 | CD4 | Rabbit IgG | Not specified | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:800 | MACH‐2 Biocare Medical, anti‐rabbit | Santa Cruz Biotechnology |
| CD8, C8/144B | CD8 | Mouse IgG1 | Not specified | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:200 | MACH‐2 Biocare Medical, anti‐mouse | Dako |
|
Rabbit IgG Ref x0903 Lot 20011861 |
Dako | ||||||||
|
Rabbit IgG Ref I5006 Lot SLBK1653V |
Sigma‐Aldrich | ||||||||
|
Mouse IgG Ref x0931 Lot 20023783 |
Dako | ||||||||
|
Rabbit serum Ref x0902 |
Dako | ||||||||
|
Mouse serum Ref x0910 |
Dako |
CD indicates cluster of differentiation; and ECM, extracellular membrane.
All primary antibodies were diluted in 1% BSA/PBS and were incubated overnight at 4°C. Endogenous peroxidase activity was blocked with a 20‐minute incubation of 0.3% hydrogen peroxide. The Envision/3,3'‐diaminobenzidine (Dako, Glostrup, Denmark) system was used for visualization. Nuclei were counterstained by Mayer hematoxylin (Merck Millipore, Amsterdam, the Netherlands). Slides stained for phosphorylated nuclear factor‐κB were washed with Triton X‐100 (Abcam, Cambridge, UK) 0.1% in PBS for 10 minutes. All stainings for a given antibody were processed in a single batch.
Imaging of Immunohistochemistry Slides
Immunohistochemistry images were captured by means of a digital microscope (Philips IntelliSite Pathology Solution Ultra‐Fast Scanner; Philips Eindhoven, the Netherlands).
Evaluation of Marker Expression on Atherosclerotic Tissue
For all markers, expression patterns were inventoried for 6 separate aspects of the aortic wall (see Figure S1 for an outline): intima, inner media, middle media, outer media, adventitia, as well as at the level of the arteriole‐type (thick‐walled) vasa vasorum and venule‐type (thin‐walled) vasa vasorum in the adventitia. In addition, we evaluated the mesenchymal populations in the areas adjacent to shoulders of and covering (multilayered fibrous cap) the necrotic core of the LFA‐type lesion. For the FCP lesion type, the cells in the newly formed intima overlying the fibrous lesion, rather than the remnants of the former fibrous cap, were appreciated.
Scoring was performed by 2 observers using semiquantitative scoring estimates (ie, 0%, <10%, 10%–50%, or >50% positivity) for each region.
Multilabeling Immunohistochemistry
Double‐labeling stainings were primarily performed by immunohistochemistry, for the same reasons single immunohistochemistry stainings were preferred over single immunofluorescence stainings.
Double‐labeling immunohistochemistry stainings were performed by sequential single‐labeling immunohistochemistry. A second heat‐induced antigen retrieval after the first chromogen staining was used to inactivate the previous signal. All epitopes resisted the second heat retrieval. Vulcan red (10 minutes, dilution 1:50) and Ferangi blue (5 minutes, dilution 1:50; both from BioCare Medical, Pacheco, CA; both alkaline phosphatase enzymatic chromogens) were combined in the double staining because these chromogens provide optimal color separation and a clear colocalization signal (purple). Double‐stained slides were not counterstained.
Immunofluorescence Staining
Multilabeling Immunofluorescence
Colocalization of >2 markers was visualized by immunofluorescence, as no triple chromogen panel could be established that provided adequate color differentiation.
All primary (Table 2) and Alexa Fluor secondary antibodies (dilution 1:200; Thermofisher, Waltham, MA) were diluted in 1% PBS/BSA and incubated overnight at 4°C and 60 minutes at room temperature, respectively. Negative controls were created by omitting the primary antibodies, and antigen stability was checked after the first heat retrieval.
Table 2.
Antibodies Used for Immunofluorescence
| Antibody, Clone | Host Isotype; Subclass | Purification | Cellular Localization | Pretreatment | Protein Block (Dako) | Dilution | Secondary Antibody (+Chromogen If Used) | Reference/Source |
|---|---|---|---|---|---|---|---|---|
| CD45, HI30 | Mouse IgG1 | Affinity chromatography | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:500 |
|
Biolegend |
| CD68, KP1 | Mouse IgG1 | Affinity chromatography | Lysosomes, endosomes, and cell surface | Tris‐EDTA (pH 9.2) | No | 1:6000 |
|
Dako |
| CD31, JC70A | Mouse IgG1 | Tissue culture supernatant | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:500 | Alexa Fluor 546 goat–anti‐mouse IgG1 (ThermoFisher No. 21123) | Dako |
| Vimentin, 3B4 |
Mouse IgG2a |
Purified from cell culture supernatant | Cytoskeleton (intermediate filament) | Tris‐EDTA (pH 9.2) | No | 1:2000 |
|
Dako |
| Vimentin, VI‐10 | Mouse IgM | Precipitation and chromatography | Cytoplasm | Tris‐EDTA (pH 9.2) | No | 1:6000 | Alexa Fluor 488 goat–anti‐mouse IgG (Life Technologies No. 21042) | Abcam |
| α‐Smooth muscle actin, 1A4 | αSMA | Mouse IgG2a | Purified from cell culture supernatant | Cytoskeleton (actin filaments) | Tris/EDTA (pH 9.2) | No | Alexa Fluor 647 goat–anti‐mouse IgG2a (Life Technologies No. 21241) | Dako |
|
FSP‐1/S100A4, D9F9D |
Rabbit IgG | Unknown | Nucleus, cytoplasm, and extracellular space | Tris‐EDTA (pH 9.2) | No | 1:4000 | Alexa Fluor 647 goat–anti‐rabbit IgG (Invitrogen, Thermo Fisher No. 31573) | Cell Signaling Technology |
| CD90/Thy‐1, ERP3133 | Rabbit IgG | Tissue culture supernatant | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:200 | Alexa Fluor 647 goat–anti‐rabbit (Invitrogen, Thermo Fisher No. 31573) |
Abcam |
|
FAP, AF3715 |
Sheep IgG | Antigen affinity purified | Cell membrane | Tris‐EDTA (pH 9.2) | No | 1:40 | Alexa Fluor 546 donkey–anti‐sheep IgG (ThermoFisher No. 16047) | R&D Systems |
| Paxillin, 5H11 | Mouse IgG1 | Protein G | Cytoplasm > cytoskeleton (focal adhesion protein) | Tris‐EDTA (pH 9.2) | No | 1:500 | Alexa Fluor 647 goat–anti‐mouse IgG1 (Life Technologies No. 21240) | Invitrogen |
| Nonmuscle myosin heavy chain, NBP2‐38566 | Rabbit IgG | Immunogen affinity purified | Cytoskeleton (myosin) | Tris‐EDTA (pH 9.2) | No | 1:600 | Alexa Fluor 647 donkey–anti‐rabbit IgG (Life Technologies No. 31573) | Novusbio |
CD indicates cluster of differentiation; FAP, fibroblast activation protein; FSP‐1, fibroblast‐specific protein‐1; and Thy‐1, thymocyte differentiation antigen 1.
In the triple‐labeling immunofluorescence stainings, cluster of differentiation (CD) 45 staining was first performed as a single staining: the CD45 antibody was incubated overnight and visualized using goat anti‐mouse (MACH2 AP‐Polymer; Biocare Medical) as a secondary antibody (30 minutes incubation at room temperature) and visualized using Vulcan Fast Red (10 minutes, dilution 1:50; Biocare Medical) fluorescence. After a second heat retrieval, the other 2 antibodies of different isotypes were incubated overnight and corresponding fluorescent‐labelled seondary antibodies were applied.
Slides were mounted using ProLong Gold with 4′,6‐diamidino‐2‐phenylindole antifade reagent (Thermofisher) and stored at 4°C until analysis. Vulcan Red fluorescence was visualized using a Texas Red Filter (542–582 nm).
Imaging of Immunofluorescence Slides
Digital images were acquired using the Panoramic MIDI Digital Slide Scanner (3D HISTECH Ltd, Budapest, Hungary) and analyzed with CaseViewer software (3D HISTECH Ltd). Minor linear adjustments (brightness and contrast) were performed. Nonlinear adjustments were not performed.
Because (partial) overlapping cells may result in pseudocolocalization in widefield optical microscopy, the anticipated pseudocolocalization of CD31 and respectively FSP‐1 (fibroblast‐specific protein‐1)/thymocyte differentiation antigen 1 (Thy‐1)/FAP (fibroblast activation protein), as well as the anticipated genuine colocalization of CD45 and vimentin, was validated by confocal microscopy (Zeiss LSM 800 CLSM, Oberkochen, Germany). Image analysis was performed with ZEN Lite (Zeiss).
Results
Literature Review
Identification of Phenotypical Immunohistochemistry Markers
The search strategy identified 3246 articles after removal of duplicates, 655 of which were considered of potential relevance (Figure 2 [Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram]). Potentially relevant articles mainly addressed SMC differentiation (n=559 articles), and to a lesser extent aspects of (myo)fibroblastic or mesenchymal differentiation (96 articles). The abstracts of these latter 96 articles were all assessed for potential relevance. Given the large number of publications on SMC differentiation (n=559), it was decided for an alternative approach by focusing on all review articles for assessment of the abstracts (n=69). Because the most recent review was published in March 2018, we additionally screened abstracts of articles published after January 2018 (n=44). On exclusion of articles deemed not relevant for this study, the abstract screening resulted in 190 potentially relevant articles, of which 180 full‐text articles were included for the qualitative synthesis. Motivation for noneligibility of full‐text articles is provided in Table S1.
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram for selection of articles on phenotypical mesenchymal markers.
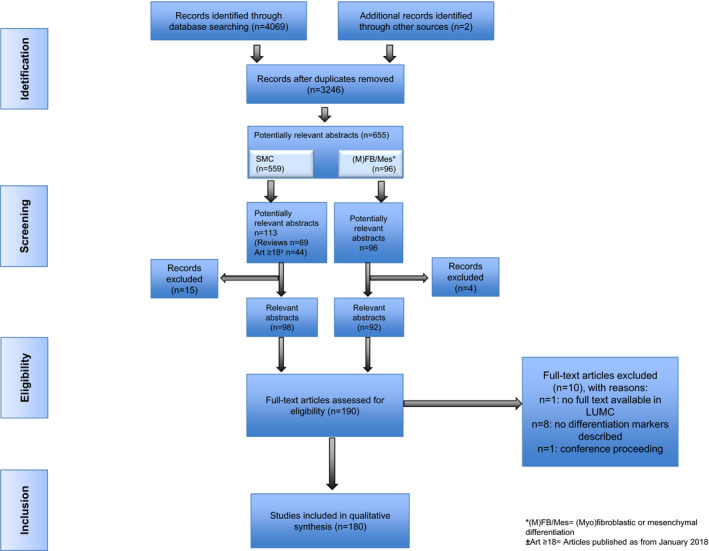
SMC indicates smooth muscle cell.
The identified markers were included for further evaluation (Table 3) 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 if at least 3 independent studies referenced them. Markers excluded in this evaluation are summarized in Table S2. All in all, this strategy yielded 16 candidate markers (either lineage or differentiation specific), which are summarized in Table 3.
Table 3.
Overview of Selected Potential Phenotypic Markers
| Antibody* | Function† | Specificity in Vasculature† | Fibroblastic Cells | Myofibroblastic Cells | C‐SMCs | |
|---|---|---|---|---|---|---|
| Mesenchymal lineage | 1. Vimentin 12 , 13 , 14 | Stabilization of cytoskeletal interactions and maintenance of cell shape and integrity of the cytoplasm 48 , 49 | Expressed in leukocytes and endothelial cells as well 50 , 51 | |||
| 2. S100A4/FSP‐1 15 , 16 , 17 | Involved in proteolytic activity during ECM remodeling and cell migration, proliferation, differentiation, and contractility 52 , 53 , 54 | Expressed in leukocytes as well 52 , 54 , 55 | ||||
| 3. CD90/Thy‐1 15 , 18 , 19 | Involved in cell adhesion and cell communication 56 | Expressed in endothelial cells as well 57 | ||||
| 4. FAP 20 , 21 , 22 | Thought to promote cellular invasion through the ECM 58 | Expressed in leukocytes as well 59 | ||||
| Contractile apparatus | 5. αSMA 23 , 24 , 25 | Force generation 60 , 61 , 62 | Expressed in macrophage‐like phenotype (foam cells) as well 63 , 64 | |||
| 6. Smemb 25 , 26 , 27 | Thought to be involved in diverse processes, including cytokinesis, cell shape, and secretion 65 | Restricted to mesenchymal cells 66 | ||||
| 7. SM‐MHC 28 , 29 , 30 | Force generation 17 , 67 | Restricted to mesenchymal cells 68 | ||||
| Accessory contractile | 8. h1‐Calponin 28 , 31 , 32 | Regulation and modulation of smooth muscle contraction 69 | Restricted to mesenchymal cells 70 | |||
| 9. h‐Caldesmon 13 , 33 , 34 | Regulation of smooth muscle contraction 71 | Restricted to mesenchymal cells 72 | ||||
| 10. Desmin 13 , 34 , 35 | Maintenance of myofibril, myofiber, and whole muscle tissue structural and functional integrity 73 , 74 | Restricted to mesenchymal cells (in vasculature) 75 | ||||
| 11. SM22α 22 , 28 , 29 | Involved in the reorganization of the actin cytoskeleton and cell contractility 76 , 77 | Restricted to mesenchymal cells 78 | ||||
| 12. Smoothelin‐B 28 , 36 , 37 | Associates with stress fibers and constitutes part of the cytoskeleton 79 | Restricted to mesenchymal cells 80 | ||||
| 13. a‐Tropomyosin 27 , 38 , 39 | Regulation of smooth muscle contraction 81 | Restricted to mesenchymal cells 82 | ||||
| 14. Telokin 40 , 41 , 42 | Involved in initiation or maintenance of smooth muscle relaxation as well as in contraction 83 , 84 | Restricted to mesenchymal cells 85 | ||||
| Focal adhesion | 15. Paxillin 43 , 44 , 45 | Focal adhesion protein involved in cell‐matrix adhesion and presumably coordination of transmission of downstream signals in cell movement and migration 86 | Expressed in leukocytes, endothelial cells, and epithelial cells as well 87 | |||
| 16. Vinculin 38 , 46 , 47 | F‐actin binding protein involved in cell‐matrix adhesion and cell‐cell interactions 88 | Expressed in leukocytes, endothelial cells, and epithelial cells as well 89 |
The selected phenotypical markers are divided in 3 main groups, of which the contractile markers are subdivided on the basis of whether they are part of the contractile apparatus or they are accessory proteins that regulate actin‐myosin interaction. Fibroblastic cells, myofibroblastic cells, and C‐SMCs were evaluated on their reported discriminative power: dark gray represents controversy over discriminating ability of the marker, and light gray represents consensus that the marker is nondiscriminative. CD indicates cluster of differentiation; C‐SMC, contractile smooth muscle cell; ECM, extracellular membrane; FAP, fibroblast activation protein; FSP‐1, fibroblast‐specific protein‐1; αSMA, α‐smooth muscle actin; SM22α, smooth muscle protein 22α; Smemb, nonmuscle myosin heavy chain; SM‐MHC, smooth muscle myosin heavy chain; and Thy‐1, thymocyte differentiation antigen 1.
References from systematic review.
Supplementary references (not from systematic review).
Cell Identity Markers
On the basis of the literature, identified markers were classified as (mesenchymal) lineage or (sub)class specific (ie, potentially discriminating between fibroblasts, myofibroblasts, or SMCs). The literature synopsis did not indicate a discriminatory marker(set) for myofibroblasts versus SMCs (Table 3), nor a single fibroblast‐specific marker.
In this context, it was decided to categorize the identified markers along the following lines: we first defined a group of 4 markers that are reported as lineage specific (eg, vimentin) and a second group consisting of 3 markers associated with the principal force generating machinery (α‐smooth muscle actin [αSMA], the 2 smooth muscle myosin heavy chain [SM‐MHC] isoforms [SM1 and SM2], and nonmuscle myosin heavy chain [Smemb]). This cluster may allow differentiation between contractile (expressed in both SMC‐like and myofibroblastic classes) and noncontractile mesenchymal cells.
A third group constituted of 7 molecules that are accessory to the contractile machinery (eg, tropomyosin). The final 2 markers (final fourth subset) are associated with cell‐cell and/or cell‐matrix interactions (eg, vinculin).
Markers of Functional Status
The literature review for candidate functional markers (ie, proinflammatory and synthetic markers) in the context of vascular biology research identified 222 full‐text articles (Figure 3). Motivation for noneligibility of the reviewed full‐text articles is provided in Table S3. Again, a threshold of at least 3 independent references for each functional marker was adopted to include the marker for immunohistochemistry evaluation (Tables S4 and S5).
Figure 3. Diagram for selection of articles on synthetic/proinflammatory markers.
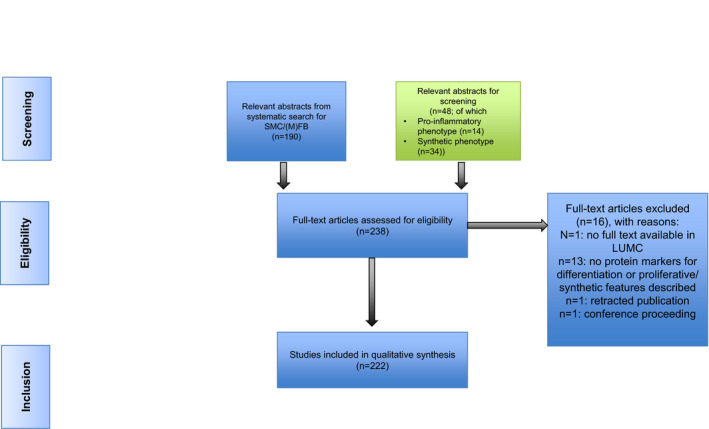
SMC indicates smooth muscle cell; and LUMC, Leiden University Medical Center.
On the basis of this search strategy, 8 synthetic and 4 proinflammatory markers were selected for further evaluation (Table 4). 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128
Table 4.
Overview of Selected Potential Functional Markers
| Subdivision | Antibody, Reference | Function | Tested Immunohistochemistry Suitability |
|---|---|---|---|
| Synthetic | 1. Collagen type I 28 , 90 , 91 |
Provides tensile strength of the arterial wall 110 In pathologic conditions, collagen contributes to plaque growth and serves as a depot for atherogenic molecules 111 |
Nonspecific staining |
| 2. Procollagen type 1 25 , 92 , 93 | Precursor of collagen I, the most abundant collagen in ECM 112 | Weak to no staining; in higher concentrations, nonspecific staining | |
| 3. Prolyl 4‐hydroxylase β 16 , 33 , 94 | Involved in hydroxylation of prolyl residues in preprocollagen 113 , 114 | Works well | |
| 4. Osteopontin 40 , 95 , 96 | Mediates cell migration, adhesion, and survival of SMCs and endothelial cells and functions as Th1 cytokine 115 , 116 | Works well, but extracellular presence hampers cell phenotyping | |
| 5. Fibronectin 25 , 48 , 97 | ECM constituent with great diversity of cellular functions, including adhesion, cytoskeletal organization, migration, growth, and differentiation 117 | Works well, but extracellular presence hampers cell phenotyping | |
| 6. Laminin 40 , 97 , 98 | ECM constituent (base membrane) with great diversity of cellular functions, including adhesion, migration, differentiation, and proliferation 118 , 119 | Works well, but extracellular presence hampers cell phenotyping | |
| 7. CRBP‐1 27 , 47 , 99 | Regulation of uptake, intracellular transport, and metabolism of retinol 120 | No protein expression detectable in AAA and atherosclerotic tissue | |
| 8. PDGFR‐α 43 , 100 , 101 | PDGF‐α is a mitogen for mesenchymal cells and regulates proliferation, migration, and differentiation during embryonic development 121 , 122 | Nonspecific staining | |
| Proinflammatory | 9. NF‐ĸB 29 , 102 , 103 | Pivotal mediator of inflammatory responses by inducing proinflammatory genes and regulating the survival, activation, and differentiation of innate immune cells and inflammatory T cells 123 | Low protein expression in vessel wall |
| 10. Interleukin 6 103 , 104 , 105 | Proangiogenic and proinflammatory/anti‐inflammatory cytokine, predominantly associated with plasma cells and macrophages 124 , 125 | Works well | |
| 11. MCP‐1 105 , 106 , 107 | Regulates migration and infiltration of monocytes/macrophages 126 | No protein expression detectable in AAA and atherosclerotic tissue | |
| 12. Interleukin 8 104 , 108 , 109 | Proangiogenic and proinflammatory cytokine, predominantly associated with lymphocytes and neutrophils; promotes neutrophil infiltration and activation, and can exert strong proangiogenic activities 127 , 128 | Works well |
AAA, abdominal aortic aneurysm; CRBP‐1 indicates cellular retinol‐binding protein 1; ECM, extracellular membrane; MCP‐1, monocyte chemoattractant protein 1; NF‐κB, nuclear factor‐κB; PDGF‐α, platelet‐derived growth factor α; PDGFR‐α, PDGF‐α receptor; SMC, smooth muscle cell; and Th1, T‐helper type 1 T‐cell.
Histological Validation
Interference by Rabbit Polyclonal Antibodies
A particular point of concern that emerged from the histological evaluation was an apparent interference when using rabbit polyclonal antibodies on formalin‐fixed, paraffin‐embedded vessel wall samples. Interference was consistent for different sources and batches of isotype controls, and found for rabbit serum (Figure S2). In fact, all rabbit immunoglobulins in concentrations beyond 1 μg/mL produced a characteristic staining pattern on the arterial wall samples. This phenomenon was rabbit IgG/serum specific. Consequently, we avoided the use of rabbit polyclonal antibodies requiring working dilutions of ≥1 μg/mL in this evaluation.
Vascular Distribution and Specificity
We validated the expression patterns and staining specificity of the phenotypical and functional cell markers identified in the literature review on the vessel wall samples from the biobank. All markers selected in the review process were stained (single staining) on consecutive slides of the reference tissue block. Results from the evaluation (summarized as semiquantitative scores for the different aspects of the arterial wall) are summarized in Figure 4.
Figure 4. Semiquantitative evaluation of single immunohistochemistry (IHC) stainings.

The presence of immunohistochemical markers is appreciated in 6 zones of the vessel wall: intima (I), inner media underlying lesion (M1), middle media (M2), outer media (M3), adventitia (Ad), thin‐walled, venous‐type vasa vasorum (VV thin), and thick‐walled artery‐type vasa vasorum (VV thick). In late fibroatheroma (LFA), the intima is divided in a central cap region (Cap) and shoulder regions (Sh); and in fibrotic calcified plaque (FCP), the neointima (Neo) overlaying the fibrous cap is considered. AIT indicates adaptive intimal thickening; FAP, fibroblast activation protein; FSP, fibroblast‐specific protein; α‐SMA, α‐smooth muscle actin; SM22α, smooth muscle protein 22α; Smemb, nonmuscle myosin heavy chain; SM‐MHC, smooth muscle myosin heavy chain; and Thy1, thymocyte differentiation antigen 1.
Lineage (mesenchymal) markers
Vimentin, FSP‐1/S100A4, Thy‐1/CD90, and FAP were identified as mesenchymal lineage‐specific markers (Figure 5A).
Figure 5. Histological validation of the selected immunohistochemistry (IHC) markers.
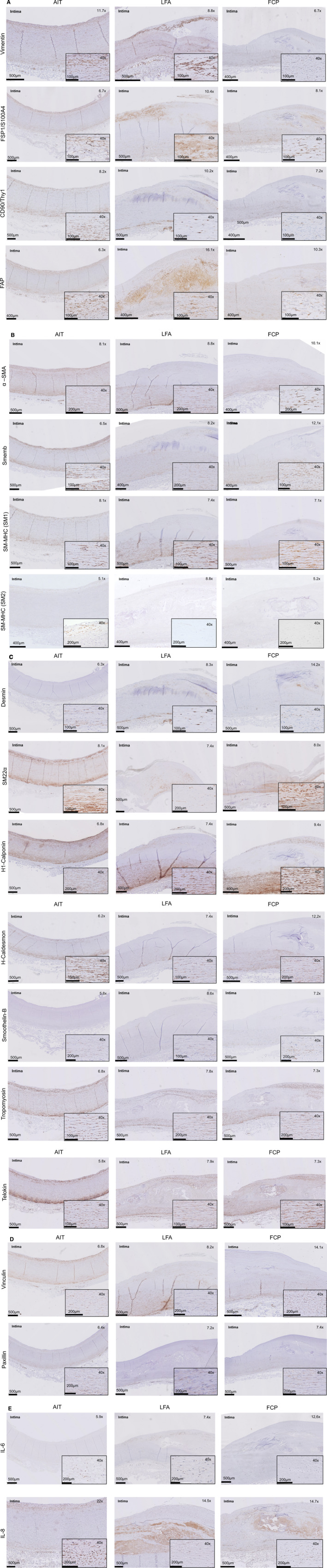
A, Mesenchymal lineage IHC markers. Overview of staining patterns in the selected representative atherosclerotic sections of adaptive intimal thickening (AIT) (early), late fibroatheroma (LFA) (progressive), and fibrotic calcified plaque (FCP) (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. B, Generic contractile IHC markers. Overview of staining patterns in the selected representative atherosclerotic sections of AIT (early), LFA (progressive), and FCP (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. C, Accessory contractile IHC markers. Overview of staining patterns in the selected representative atherosclerotic sections of AIT (early), LFA (progressive), and FCP (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. D, Focal adhesion IHC markers. Overview of staining patterns in the selected representative atherosclerotic sections of AIT (early), LFA (progressive), and FCP (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. E, Interleukin 6 (IL‐6) and interleukin 8 (IL‐8) staining (activation markers). Overview of staining patterns in the selected representative atherosclerotic sections of AIT (early), LFA (progressive), and FCP (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. F, Prolyl 4‐hydroxylase β (P4HB) staining (synthetic marker). Overview of staining patterns in the selected representative atherosclerotic sections of AIT (early), LFA (progressive), and FCP (end stage). Close ups in LFA and FCP represent cap regions and neointima, respectively. CD indicates cluster of differentiation; FAP, fibroblast activation protein; FSP, fibroblast‐specific protein; α‐SMA, α‐smooth muscle actin; SM22α, smooth muscle protein 22α; Smemb, nonmuscle myosin heavy chain; SM‐MHC, smooth muscle myosin heavy chain; and Thy1, thymocyte differentiation antigen 1.
All 4 markers were diffusely expressed throughout the vessel wall and vasa vasorum in the early atherosclerotic (AIT) reference sample. However, a notable inconsistent expression was found for these markers in the media, with subsets of spindle‐shaped cells being vimentin+ and FAP+, but negative for both FSP‐1 and Thy‐1, challenging Thy‐1 and FSP‐1 as generic mesenchymal lineage markers. Indeed, validation of this observation in triple immunofluorescence stainings of (Thy‐1/FSP‐1/FAP)/vimentin/αSMA showed that up to 10% of the spindle‐shaped αSMA+/vimentin+ or αSMA+/FAP+ cells in the media were negative for both Thy‐1 and FSP‐1 (Figure S3).
This dissociation between vimentin and Thy‐1 expression became even more pronounced in the more advanced atherosclerotic stages by an apparent inverse association between Thy‐1 expression and disease progression, with a particularly low expression of Thy‐1 in the neointima. Further discrepancies were observed for vimentin and FAP. Although medial vimentin expression remained stable in advanced‐stage (LFA) atherosclerotic disease samples, FAP expression varied (Figure S4).
For explorative purposes, we also evaluated vimentin and αSMA coexpression in the cap of progressive lesions (ie, LFA and the unstable progressive atherosclerotic lesion [thin cap fibroatheroma]), as well as a stabilized lesion type (HR) (Figure S5). The cap in LFA was rich in mesenchymal cells (elongated vimentin+ cells). Transition to a thin cap fibroatheroma was associated with a clear decrease in cap cell density. In both LFA and thin cap fibroatheroma, ≈80% of the vimentin+ cells were double vimentin+/αSMA+. Similarly, 80% of the vimentin+ cells in the cell‐rich/proteoglycan‐rich luminal granulation tissue associated with healing of a ruptured atherosclerotic lesion (HR) were double vimentin+/αSMA+.
On the basis of these observations, vimentin classified as the most inclusive lineage marker. However, we did observe a small population of spindle‐shaped FAP+/vimentin– cells in the cap of LFA (Figure S4.2), defying vimentin as an all‐inclusive panmesenchymal marker.
Specificity of vimentin as a mesenchymal lineage marker was challenged by the diffuse presence of round, vimentin+ cells in the vicinity of the vasa vasorum in the adventitia. Validation studies that included triple immunofluorescence staining for the panleucocyte marker CD45, the macrophage marker CD68, and vimentin showed subsets of triple‐positive cells in the adventitia (Figure S6.1/2). This colocalization was observed for different vimentin antibodies, and confirms the expression of vimentin in subsets of macrophages. Along similar lines, we identified (small) subsets CD45+/CD68+ and FSP‐1+, Thy‐1+, or FAP+ triple‐positive cells in the adventitia, consistent with subsets of FSP‐1+, Thy‐1+, and FAP+ macrophages (Figure S6.3‐5).
Moreover, indications were found for vimentin expression in subsets of endothelial cells. Endothelial cell‐specific expression was confirmed by CD31/vimentin double staining (Figure S7). Confocal microscopy characterized the apparent spatial associations between CD31 and FSP‐1, Thy‐1, and FAP as pseudocolocalization. Distinct, small populations of solitary vimentin+/CD31+ and vimentin+/CD34+ were observed in the vicinity of the adventitial vasa vasorum (Figure S8).
A third nonclassic population of vimentin+ cells was observed in the granulation tissue of HR. Approximately 10% of these spindle‐shaped cells were double Vim+/CD45+(Figure S9.1). Incidental (<5% of the population) double Vim+/CD45+ cells were also observed in the cap and neointima of LFA and FCP reference sections. Distinct (spindle‐shaped and round) morphological features may imply distinct subpopulations (Figure S9.2/3).
Contractile/noncontractile phenotype markers
αSMA, SM‐MHC (isoforms SM1 and SM2), and Smemb (embryonic form of SM‐MHC) are principle parts of the contractile machinery that is characteristic for SMCs and myofibroblasts (Figure 5B).
αSMA was expressed in virtually all spindle‐shaped cells in the intima, media, and adventitia. In mesenchymal cells covering the vasa vasorum, αSMA was consistently expressed. Expression of the SM‐MHC isoforms and Smemb was more variable: SM‐MHC (SM1) expression was notably less in the middle section of the media than in the inner and outer segments of the media, and expression of the second isoform (SM2) was limited to the outer segment of the media. Smemb expression was more pronounced in the outer medial segment than in other medial segments. A discriminatory expression profile was seen for SM‐MHC/Smemb expression in the vasa vasorum with parallel expression in the thick‐walled arteriole‐like vessels, but Smemb single positivity was found in the thin‐walled venule‐like vessels.
Progressive stages of atherosclerosis showed stable αSMA expression, whereas SM‐MHC and Smemb expression were negatively and positively associated, respectively, with disease progression, potentially disqualifying SM‐MHC and Smemb as all‐encompassing contractile markers. Smemb expression has been linked to a synthetic phenotype. Indeed, most, but not all, of the Smemb+ cells in the cap and shoulder regions expressed the synthetic marker prolyl 4‐hydroxylase β (P4HB) (Figure S10).
Auxiliary contractile markers
The third group of markers identified in the review consisted of a group of auxiliary molecules to the contractile machinery (SM22α [smooth muscle protein 22α], h1‐Calponin, h‐Caldesmon, Telokin, Tropomyosin, Desmin, and Smoothelin) (Figure 5C).
Spatial expression of these markers was variable: subsets of spindle‐shaped cells in the intima, media, and vaso vasora were positive for SM22α and h‐Caldesmon. Desmin and Smoothelin expression were both selectively expressed in the medioadventitial border zone. Desmin was selectively expressed in arteriole‐type vasa vasorum, whereas Smoothelin was specifically expressed in venule‐type vasa vasorum.
Although h1‐Calponin was expressed in virtually all spindle‐shaped cells in the intima and media in the early stages of atherosclerosis, spatial expression of h1‐Calponin was more pronounced in LFA, shown by h1‐Calponin−/αSMA+ cells in the cap (Figure S11). Telokin and Tropomyosin were not fully contractile cell specific, as round triple Telokin+/vimentin+/CD45+ and Tropomyosin+/vimentin+/CD45+ cells were present in the adventitia (Figure S12.1/2).
Heterogeneous responses were seen for the auxiliary contractile markers in the context of the atherosclerotic disease progression: Smoothelin and Desmin expression related inversely to disease progression, whereas medial expression of h1‐Calponin, h‐Caldesmon, SM22α, Tropomyosin, and Telokin remained stable. H1‐Calponin+, Tropomyosin+, Telokin+, and SM22α+ were present in subsets of mesenchymal cells in the cap/shoulder, and in the neointima regions in LFA and FCP. A subpopulation (<10%) of spindle‐shaped Tropomyosin+ αSMA– cells was observed in the cap of the LFA lesion (Figure S13).
Focal adhesion proteins: vinculin and paxillin
The fourth cluster of markers included Vinculin and Paxillin, molecules involved in cell‐cell and cell‐matrix interactions (Figure 5D). In AIT, they were both abundantly expressed in the intimal and outer medial zone and to a lesser extent in the inner and middle media. Although Paxillin expression was also observed in double CD45+/vimentin+ cells in the adventitia (Figure S12.3), Vinculin expression was absent in the adventitia. Although Paxillin expression remained stable during atherogenic progression, Vinculin expression decreased during disease progression.
Functional markers
Apart from their phenotypical identities, mesenchymal cells can be actively involved in matrix deposition and homeostasis (synthetic phenotype), and may adapt an inflammatory phenotype. We evaluated several markers for a synthetic or an inflammatory phenotype (results are summarized in Table 4).
On the basis of an evaluation of compatibility with immunohistochemistry‐based subtyping, which involved a preferably intracellular staining pattern and availability of antibodies compatible with paraffin‐embedded material, the signal/background ratio, and specificity, it was decided for P4HB and interleukin 6 (IL‐6) or interleukin 8 (IL‐8; aka, CXCL8) as preferred markers for a secretory inflammatory phenotype. Motivations for refraining from the other candidate markers are provided in Figure S14.
IL‐6 and IL‐8 expression was used to visualize inflammatory status of the mesenchymal cell population (Figure 5E): in AIT, IL‐6 and IL‐8 expression was observed for infiltrating mesenchymal cells in the intima, and in subsets of adventitial mesenchymal cells. IL‐8 was expressed in the medioadventitial border as well. Increased medial IL‐6 and IL‐8 expression, and significant expression of IL‐6 and IL‐8 in the cap and shoulder regions, was observed during atherogenic progression.
Expression of these inflammatory markers in the mesenchymal cell population may reflect the inflammatory character of atherosclerosis, an aspect that is illustrated by macrophage and T‐cell stainings on the reference sections (Figure S15).
P4HB, an enzyme involved in (pre) collagen processing, was identified as preferred marker for a synthetic phenotype (Figure 5F). Expression of P4HB was confined to the intima and, with the exception of some vasa vasorum absent in the media and adventitia, in early‐stage atherosclerosis. In more advanced stages of atherosclerosis, P4HB expression was observed in the cap and shoulder regions, as well as in the adventitial venule‐like and arteriole‐like vasa vasorum. P4HB remained absent in the entire media.
On the basis of the tentative results of the review and the inventory, a proposed marker set was compiled for an explorative evaluation of the vascular mesenchymal landscape (Tables 5 and 6).
Table 5.
Proposed Marker Set for Standard Inventory of Mesenchymal Cell Populations in Human Vasculature
| Phenotype | Function | Pathological Conditions | ||
|---|---|---|---|---|
| Generic mesenchymal: vimentin+/CD31−/CD45− | Contractile, generic*: αSMA+ | Synthetic: P4HB+ | Proinflammatory: IL‐6+/IL‐8+ | EndoMT/stem cells: CD34+ † CD31+ |
| Noncontractile: αSMA− | LeucoMT: CD45+ | |||
Construction scheme of proposed mesenchymal marker set selection. Suggestions for well‐working antibodies are provided in Table 1. Vimentin, in a marker set with CD31−/CD45−, will identify ≈95% of mesenchymal cells. αSMA will identify ≈95% of contractile mesenchymal cells. CD indicates cluster of differentiation; EndoMT, Endothelial‐to‐Mesenchymal cell Transition; IL‐6, interleukin 6; IL‐8, interleukin 8; LeucoMT, Leuccyte‐to‐Mesenchymal cell Transition; P4HB, prolyl 4‐hydroxylase β; and αSMA, α‐smooth muscle actin.
For a list of accessory contractile and focal adhesion markers, see Table 3.
OR,/ AND.
Table 6.
Proposed Marker Set for Comprehensive Inventory of Mesenchymal Cell Populations in Human Vasculature
| Phenotype | Function | Pathological Conditions | ||
|---|---|---|---|---|
| Generic mesenchymal: FAP+/vimentin+/CD31−/CD45− | Contractile, generic*: αSMA+/tropomyosin+ |
Synthetic: P4HB+ |
Proinflammatory: IL‐6+/IL‐8+ | EndoMT/stem cells: CD34+ † CD31+ |
| Noncontractile: αSMA−/tropomyosin− | LeucoMT: CD45+ | |||
Construction scheme of proposed mesenchymal marker set selection. Suggestions for well‐working antibodies are provided in Table 1. To acquire ≈99% inclusivity for mesenchymal cells, a dual‐marker set of vimentin+/FAP+ (possibly as costaining, stained by the same chromogen) is needed. Likewise, for contractile mesenchymal cells, the dual‐marker set αSMA/Tropomyosin reaches ≈99% inclusivity. CD indicates cluster of differentiation; EndoMT, Endothelial‐to‐Mesenchymal cell Transition; FAP, fibroblast activation protein; IL‐6, interleukin 6; IL‐8, interleukin 8; LeucoMT, Leuccyte‐to‐Mesenchymal cell Transition; P4HB, prolyl 4‐hydroxylase β; and αSMA, α‐smooth muscle actin.
For a list of accessory contractile and focal adhesion markers, see Table 3.
OR,/ AND.
Discussion
The vascular mesenchymal landscape appears to be highly dynamic, diverse, and complex. It extends far beyond the classic tripartite classification scheme of fibroblasts, myofibroblasts, and SMCs. Furthermore, there is no evidence for a clear separation along the lines of myofibroblast and SMC populations. These findings for the human context confirm and extend observations for murine models of atherosclerosis that imply an extremely diverse spectrum of mesenchymal cells within the vessel wall. 129 , 130
Mesenchymal cells are the pivotal cellular component of load‐bearing structures and organs. They are the principle component of blood vessels, where they modulate vascular tone and maintain vascular integrity through deposition and maintenance of the extracellular matrix. 131 , 132 As a consequence, mesenchymal cells are in the center of vascular pathological conditions, such as atherosclerosis and aneurysmal disease. 133 , 134 In fact, mesenchymal cell activation and migration in response to intimal lipoprotein deposition is the initiating step in the human atherosclerotic process. 135 Data from murine atherosclerotic models suggest that SMCs contribute the majority of foam cells. 136 In the more advanced stages of atherosclerotic disease, mesenchymal cells critically contribute to plaque stability, as well as to aspects such as vascular calcification and intimal hyperplasia. 137 , 138 Indeed, an exploratory inventory implied clear qualitative changes in the cap during plaque progression with a nadir in cell density in thin cap lesions, but recovery of mesenchymal cell density (elongated vimentin+ cells) in the cell‐rich/matrix‐rich granulation tissue of a healed rupture.
Along similar lines, mesenchymal cells are key players in both genetic (eg, thoracic aneurysms associated with bicuspid valve disease 139 ) and degenerative aneurysms, such as the abdominal aortic aneurysm. 140 For the latter, impaired mesenchymal differentiation has been directly linked to aneurysm rupture. 141
The vascular mesenchymal landscape is particularly complex, not only as a reflection of the heterogeneous embryological origin of the vascular tree, 142 and the vascular layers, 143 but also because SMCs are nonterminally differentiated, 144 , 145 thus allowing a high degree of phenotypical plasticity. Moreover, it is now clear that processes, such as endothelial‐to‐mesenchymal transition, 146 contribute to the vascular mesenchymal population.
Evidence was also found for the presence of fibrocytes. Ample elongated double CD45+/vimentin+ cells were observed in the process of cap healing following plaque rupture, and in the neointima overlaying a fibrous lesions. Moreover, we observed subpopulations of round and spindle‐shaped double CD45+/vimentin+ cells in the cap of LFA. This observation is consistent with the (co)existence of distinct subpopulations of double CD45+/vimentin+ cells in the atherosclerotic process: spindle‐shaped fibrocytes, which could be consistent with the phenomenon of leukocyte‐mesenchymal transition, 147 and the round cells possibly representing a subclass of macrophages. 148
The immunological field has benefited enormously from the introduction of the consensus classification of leukocyte subtypes, based on well‐defined marker sets (CD markers); such classification system does not exist for mesenchymal cells. In a first attempt toward a mesenchymal cell classification for the vasculature, we inventoried candidate subtype markers through a literature review, and mapped the identified markers on a set human aorta specimens with successive stages of the atherosclerotic process.
The literature review identified 4 mesenchymal lineage markers: vimentin, FSP‐1/S100A4, Thy‐1/CD90, and FAP. Validation stainings disqualified FSP‐1/S100A4 and Thy‐1/CD90 as universal mesenchymal lineage markers. Therefore, conclusions based on studies relying on these markers may be incomplete.
On the basis of its performance in this evaluation, and on the assumption that most spindle‐shaped cells are mesenchymal cells, vimentin classified as the preferred mesenchymal lineage marker for the vasculature because this was the most inclusive marker for spindle‐shaped cells in the media. However, histological stainings identified small subsets of vimentin–/FAP+ cells in specific niches, suggesting that vimentin may not be fully inclusive and that a comprehensive appreciation of the full mesenchymal spectrum may rely on the vimentin/FAP dual‐marker set.
On the same token, vimentin expression was seen in subset(s) of vascular macrophages, as well as the endothelial lining of vasa vasorum, indicating that vimentin is not fully mesenchymal cell specific, and that full specificity relies on costaining of exclusion markers (eg, CD31 and CD68). This study also identified solitary double vimentin+/CD31+ and vimentin+/CD34+ cells in the vicinity of adventitial vasa vasorum, possibly identifying vascular stem cells or cells in Endothelial‐to‐Mesenchymal cell Transition. 149
Next to the lineage markers, the review identified several subclass markers. Markers for contractile phenotype were subdivided in 2 closely related subgroups: principal constituents of the contractile apparatus (αSMA, SM‐MHC, and Smemb) and its auxiliary molecules (ie, actin/myosin interaction regulating [h1‐Calponin, Desmin, h‐Caldesmon, Tropomyosin, Telokin, Smoothelin, and SM22α]).
αSMA classified as the most inclusive marker for presence of a professional contractile machinery. However, coverage of the full spectrum of contractile mesenchymal cells may require a dual‐marker set of αSMA/Tropomyosin, as the histological evaluation identified small specific niches in the cap of LFA that contained spindle‐shaped αSMA−/Tropomyosin+ cells.
Smemb has been linked to a synthetic phenotype. Indeed, a subset of elongated Smemb+ in the shoulder and cap of progressive atherosclerotic lesions also expressed P4HB. Yet, Smemb+/P4HB− elongated cells were abundantly present in the media of early‐stage atherosclerosis. These observations characterize Smemb as a mere differentiation marker.
A considerable degree of coexpression was observed for the auxiliary contractile markers in early atherosclerotic disease (AIT). However, increased heterogeneity was observed for the progressive stages. Clear spatial distribution of these subpopulations implies some form of synchronization in the processes of subdifferentiation. Exploration of underlying molecular synchronization pathways and functional diversity of the subdifferentiated cells is beyond the scope of this inventorying exploration.
The literature review further identified the focal adhesion proteins Vinculin and Paxillin, a binding partner of Vinculin, 150 as markers of mesenchymal differentiation. These proteins do not associate with the contractile apparatus, but are involved in environmental sensing, 151 and are abundantly expressed by mesenchymal cells in the normal vessel wall. 152 We observed downregulation of Vinculin in spindle‐shaped cells in the media during atherogenic progression, a phenomenon that has been interpreted as an indication of disturbed intermesenchymal or mesenchymal–extracellular matrix interaction. 153
The identification of functional markers set for histological phenotyping came with several technical challenges. The inflammatory spectrum is notably broad, thus interfering with the identification of a generic marker. Moreover, by virtue of the responsive and adaptive nature of the inflammatory, protein expression can be extremely low and volatile, thus creating suboptimal conditions for immunohistochemistry. The cytokines/chemokines IL‐6 and IL‐8 (both essentially controlled by nuclear factor‐κB activity) can be present as intracellular stores, and thus are well identifiable by immunohistochemistry staining. On this basis, we evaluated their potential as markers of (aspects of) an inflammatory phenotype. Indeed, IL‐6 and IL‐8 were both particularly upregulated in lesional intimas, such as the cap and shoulder regions of LFA. The dynamics of the innate and adaptive cellular immune response in human atherosclerosis have been extensively reported previously. 154 , 155
Along similar lines, challenges exist for markers of a synthetic, secretory phenotype. Histological staining of deposited matrix products results in a profound extracellular staining pattern that interferes with the interpretation of intracellular stainings. Our evaluation identified the (pre)collagen processing enzyme PH4B as the optimal marker for mapping a synthetic phenotype in immunohistochemistry. In AIT, P4HB expression was confined to the intima, with the exception of some vasora, and showed upregulation during atherogenic progression in lesional intimas, such as the cap and shoulder regions in LFA.
Because our literature review did not provide conclusive evidence with respect to a discriminatory marker set identifying the classic smooth muscle phenotype and myofibroblast phenotype, it was reasoned that the arteriolar smooth muscle cell of the vasa vasorum in the adventitia constitutes the best reference to the classic, functionally contractile SMC phenotype. On basis of this premise, we could not establish a clear separation along the lines of myofibroblastic and SMC populations based on the (auxillary) contractile markers.
As (myo)fibroblastic cells are characterized by their ability to synthesize collagen, P4HB was explored as discriminative factor. However, spindle‐shaped P4HB+ cells covering the vasa vasorum were found as well.
This may suggest that myofibroblasts are rather cell states of fibroblastic cells or SMCs than a discrete cell type.
This evaluation of the mesenchymal landscape on the basis of a parallel evaluation of >25 markers indicates a spatially diverse, highly dynamic, and heterogeneous panorama. The spatial diversity and extreme granularity, and the relative long protein half‐lives for most markers, pose particular challenges to RNA‐based analysis, and to techniques relying on tissue dissection and clustering, such as single‐cell analysis. Although immunohistochemistry has a clear advantage to these challenges, this explorative study has some limitations as well. First, the study is based on the results of a literature review. As such, the evaluation may be incomplete and findings from in vitro studies may not apply to the in vivo context (eg, we did not encounter a clear myofibroblast phenotype). Although specific cell isolation studies may add a further level of information about the in vivo context, studies on isolated cells were considered outside the scope of this inventory. Moreover, immunohistochemistry is semiqualitative at best, and heavily relies on the quality of the antibodies. Although quality control was performed, the specificity of antibodies for formalin‐fixed, paraffin‐embedded samples cannot be guaranteed. For this reason, we performed validation studies with alternative antibodies for the potentially controversial positive findings of vimentin positivity in nonmesenchymal cell lineages (fibrocytes and macrophages). Other stainings were not validated by staining with a different antibody. However, decisions to refrain from a candidate marker were only taken when multiple clones produced negative or nonspecific staining. The impact of nonspecific staining on data interpretation is clearly illustrated by the consistently observed nonspecific staining pattern when using rabbit polyclonal antibodies in concentrations beyond 1 µg/mL (1:1000 for most antibodies) on formalin‐fixed, paraffin‐embedded vessel sections.
The purpose of the study was to establish a marker set for mapping the mesenchymal landscape. The extreme granularity and spatial variation were unexpected. The full extent of the landscape can only be appreciated through systematic cataloging of the phenotypical diversity through a process that will rely on multiparameter imaging of samples covering the full disease spectra, targeted expression profiling, and functional evaluation. We consider this aspect beyond the scope of this inventorying study. However, this study provides the groundwork for a consensus cluster classification.
Sources of Funding
None.
Disclosures
None.
Supporting information
Acknowledgments
The authors wish to kindly thank J.W. Schoones for his assistance in composing the search strategy.
(J Am Heart Assoc. 2020;9:e017094 DOI: 10.1161/JAHA.120.017094.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017094
For Sources of Funding and Disclosures, see page 22.
References
- 1. Topouzis S, Majesky M. Smooth muscle lineage diversity in the chick embryo. Dev Biol. 1996;178:430–445. [PubMed] [Google Scholar]
- 2. Lacolley P, Regnault V, Nicoletti A, Li Z, Michel J. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. [DOI] [PubMed] [Google Scholar]
- 3. Riches K, Clark E, Helliwell RJ, Angelini TG, Hemmings KE, Bailey MA, Bridge KI, Scott DJA, Porter KE. Progressive development of aberrant smooth muscle cell phenotype in abdominal aortic aneurysm disease. J Vasc Res. 2018;55:35–46. [DOI] [PubMed] [Google Scholar]
- 4. Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow‐derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. [DOI] [PubMed] [Google Scholar]
- 6. Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular "myofibroblast"? J Am Soc Nephrol. 1992;2:S190–S197. [DOI] [PubMed] [Google Scholar]
- 7. Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibbons G, Dzau V. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. [DOI] [PubMed] [Google Scholar]
- 9. Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in‐stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. [DOI] [PubMed] [Google Scholar]
- 11. Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re‐epithelialization, contraction, and angiogenesis. Ann Surg. 2011;254:1066–1074. [DOI] [PubMed] [Google Scholar]
- 12. Chai X, Sun D, Han Q, Yi L, Wu Y, Liu X. Hypoxia induces pulmonary arterial fibroblast proliferation, migration, differentiation and vascular remodeling via the PI3K/Akt/p70S6K signaling pathway. Int J Mol Med. 2018;41:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilcox JN, Okamoto EI, Nakahara KI, Vinten‐Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: a role for circulating myofibroblast precursors? Ann N Y Acad Sci. 2001;947:68–90. [DOI] [PubMed] [Google Scholar]
- 14. Onuta G, van Ark J, Rienstra H, Boer MW, Klatter FA, Bruggeman CA, Zeebregts CJ, Rozing J, Hillebrands JL. Development of transplant vasculopathy in aortic allografts correlates with neointimal smooth muscle cell proliferative capacity and fibrocyte frequency. Atherosclerosis. 2010;209:393–402. [DOI] [PubMed] [Google Scholar]
- 15. Kuwabara JT, Tallquist MD. Tracking adventitial fibroblast contribution to disease: a review of current methods to identify resident fibroblasts. Arterioscler Thromb Vasc Biol. 2017;37:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witt W, Büttner P, Jannasch A, Matschke K, Waldow T. Reversal of myofibroblastic activation by polyunsaturated fatty acids in valvular interstitial cells from aortic valves: role of RhoA/G-actin/MRTF signalling. J Mol Cell Cardiol. 2014;74:127–138. [DOI] [PubMed] [Google Scholar]
- 17. Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015;593:3013–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene‐expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. [DOI] [PubMed] [Google Scholar]
- 19. Qian J, Tian W, Jiang X, Tamosiuniene R, Sung YK, Shuffle EM, Tu AB, Valenzuela A, Jiang S, Zamanian RT, et al. Leukotriene B4 activates pulmonary artery adventitial fibroblasts in pulmonary hypertension. Hypertension. 2015;66:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, Baker AH. Endothelial to mesenchymal transition in cardiovascular disease: JACC state‐of-the‐art review. J Am Coll Cardiol. 2019;73:190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evrard SM, Lecce L, Michelis KC, Nomura‐Kitabayashi A, Pandey G, Purushothaman KR, D'Escamard V, Li JR, Hadri L, Fujitani K, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kahounová Z, Kurfürstová D, Bouchal J, Kharaishvili G, Navrátil J, Remšík J, Šimečková Š, Študent V, Kozubík A, Souček K. The fibroblast surface markers FAP, anti‐fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial‐to‐mesenchymal transition. Cytometry A. 2018;93:941–951. [DOI] [PubMed] [Google Scholar]
- 23. Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts: busy multitaskers. Am J Pathol. 2016;186:2519–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalajzic I, Kalajzic Z, Wang L, Jiang X, Lamothe K, San Miguel SM, Aguila HL, Rowe DW. Pericyte/myofibroblast phenotype of osteoprogenitor cell. J Musculoskelet Neuronal Interact. 2007;7:320–322. [PubMed] [Google Scholar]
- 25. Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Stenmark KR. Circulating mononuclear cells with a dual, macrophage‐fibroblast phenotype contribute robustly to hypoxia‐induced pulmonary adventitial remodeling. Chest. 2005;128:583S–584S. [DOI] [PubMed] [Google Scholar]
- 26. Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. [DOI] [PubMed] [Google Scholar]
- 27. Beamish JA, He P, Kottke‐Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karamariti E, Zhai C, Yu B, Qiao L, Wang Z, Potter CMF, Wong MM, Simpson RML, Zhang Z, Wang X, et al. DKK3 (Dickkopf 3) alters atherosclerotic plaque phenotype involving vascular progenitor and fibroblast differentiation into smooth muscle cells. Arterioscler Thromb Vasc Biol. 2018;38:425–437. [DOI] [PubMed] [Google Scholar]
- 29. Starke RM, Thompson JW, Ali MS, Pascale CL, Martinez Lege A, Ding D, Chalouhi N, Hasan DM, Jabbour P, Owens GK, et al. Cigarette smoke initiates oxidative stress‐induced cellular phenotypic modulation leading to cerebral aneurysm pathogenesis. Arterioscler Thromb Vasc Biol. 2018;38:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirai H, Yang B, Garcia‐Barrio MT, Rom O, Ma PX, Zhang J, Chen YE. Direct reprogramming of fibroblasts into smooth muscle‐like cells with defined transcription factors‐brief report. Arterioscler Thromb Vasc Biol. 2018;38:2191–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2‐Wnt7b signaling reciprocally regulate the endothelial‐mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casella S, Bielli A, Mauriello A, Orlandi A. Molecular pathways regulating macrovascular pathology and vascular smooth muscle cells phenotype in type 2 diabetes. Int J Mol Sci. 2015;16:24353–24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plekhanova OS, Stepanova VV, Ratner EI, Bobik A, Tkachuk VA, Parfyonova YV. Urokinase plasminogen activator in injured adventitia increases the number of myofibroblasts and augments early proliferation. J Vasc Res. 2006;43:437–446. [DOI] [PubMed] [Google Scholar]
- 34. Wilcox JN, Cipolla GD, Martin FH, Simonet L, Dunn B, Ross CE, Scott NA. Contribution of adventitial myofibroblasts to vascular remodeling and lesion formation after experimental angioplasty in pig coronary arteries. Ann N Y Acad Sci. 1997;811:437–447. [DOI] [PubMed] [Google Scholar]
- 35. Holm A, Heumann T, Augustin HG. Microvascular mural cell organotypic heterogeneity and functional plasticity. Trends Cell Biol. 2018;28:302–316. [DOI] [PubMed] [Google Scholar]
- 36. Lepreux S, Guyot C, Billet F, Combe C, Balabaud C, Bioulac‐Sage P, Desmoulière A. Smoothelin, a new marker to determine the origin of liver fibrogenic cells. World J Gastroenterol. 2013;19:9343–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Eys GJ, Niessen PM, Rensen SS. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc Med. 2007;17:26–30. [DOI] [PubMed] [Google Scholar]
- 38. Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. [DOI] [PubMed] [Google Scholar]
- 39. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. [DOI] [PubMed] [Google Scholar]
- 40. Cecchettini A, Rocchiccioli S, Boccardi C, Citti L. Vascular smooth‐muscle‐cell activation: proteomics point of view. Int Rev Cell Mol Biol. 2011;288:43–99. [DOI] [PubMed] [Google Scholar]
- 41. Kawai‐Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59–C69. [DOI] [PubMed] [Google Scholar]
- 42. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 43. Shi N, Chen SY. Smooth muscle cell differentiation: model systems, regulatory mechanisms, and vascular diseases. J Cell Physiol. 2016;231:777–787. [DOI] [PubMed] [Google Scholar]
- 44. Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Régent A, Ly KH, Groh M, Khifer C, Lofek S, Clary G, Chafey P, Baud V, Broussard C, Federici C, et al. Molecular analysis of vascular smooth muscle cells from patients with giant cell arteritis: targeting endothelin‐1 receptor to control proliferation. Autoimmun Rev. 2017;16:398–406. [DOI] [PubMed] [Google Scholar]
- 46. Lefebvre P, Nusgens BV, Lapière CM. Cultured myofibroblasts display a specific phenotype that differentiates them from fibroblasts and smooth muscle cells. Dermatology. 1994;189:65–67. [DOI] [PubMed] [Google Scholar]
- 47. Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. [DOI] [PubMed] [Google Scholar]
- 48. Malashicheva A, Kostina D, Kostina A, Irtyuga O, Voronkina I, Smagina L, Ignatieva E, Gavriliuk N, Uspensky V, Moiseeva O. Phenotypic and functional changes of endothelial and smooth muscle cells in thoracic aortic aneurysms. Int J Vasc Med. 2016;2016:3107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding Y, Zhang M, Zhang W, Lu Q, Cai Z, Song P, Okon IS, Xiao L, Zou MH. AMP‐activated protein kinase alpha 2 deletion induces VSMC phenotypic switching and reduces features of atherosclerotic plaque stability. Circ Res. 2016;119:718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte‐derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu T, Guevara OE, Warburton RR, Hill NS, Gaestel M, Kayyali US. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am J Physiol Cell Physiol. 2010;299:C363–C373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coen M, Burkhardt K, Bijlenga P, Gabbiani G, Schaller K, Kövari E, Rüfenacht DA, Ruíz DS, Pizzolato G, Bochaton‐Piallat ML. Smooth muscle cells of human intracranial aneurysms assume phenotypic features similar to those of the atherosclerotic plaque. Cardiovasc Pathol. 2013;22:339–344. [DOI] [PubMed] [Google Scholar]
- 53. Chaabane C, Heizmann CW, Bochaton‐Piallat ML. Extracellular S100A4 induces smooth muscle cell phenotypic transition mediated by RAGE. Biochim Biophys Acta. 2015;1853:2144–2157. [DOI] [PubMed] [Google Scholar]
- 54. Brisset AC, Hao H, Camenzind E, Bacchetta M, Geinoz A, Sanchez JC, Chaponnier C, Gabbiani G, Bochaton‐Piallat ML. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circ Res. 2007;100:1055–1062. [DOI] [PubMed] [Google Scholar]
- 55. Martínez‐González J, Berrozpe M, Varela O, Badimon L. Heterogeneity of smooth muscle cells in advanced human atherosclerotic plaques: intimal smooth muscle cells expressing a fibroblast surface protein are highly activated by platelet‐released products. Eur J Clin Invest. 2001;31:939–949. [DOI] [PubMed] [Google Scholar]
- 56. Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chung KM, Hsu SC, Chu YR, Lin MY, Jiaang WT, Chen RH, Chen X. Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation. PLoS One. 2014;9:e88772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tchou J, Zhang PJ, Bi Y, Satija C, Marjumdar R, Stephen TL, Lo A, Chen H, Mies C, June CH, et al. Fibroblast activation protein expression by stromal cells and tumor‐associated macrophages in human breast cancer. Hum Pathol. 2013;44:2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prunotto M, Bruschi M, Gunning P, Gabbiani G, Weibel F, Ghiggeri G, Petretto A, Scaloni A, Bonello T, Schevzov G, et al. Stable incorporation of α‐smooth muscle actin into stress fibers is dependent on specific tropomyosin isoforms. Cytoskeleton. 2015;62:257–267. [DOI] [PubMed] [Google Scholar]
- 61. Gomez D, Owens G. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mack C, Thompson M, Lawrenz‐Smith S, Owens G. Smooth muscle α‐actin CArG elements coordinate formation of a smooth muscle cell‐selective, serum response factor‐containing activation complex. Circ Res. 2002;86:221–232. [DOI] [PubMed] [Google Scholar]
- 63. Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage‐like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. [DOI] [PubMed] [Google Scholar]
- 64. Sandison ME, Dempster J, McCarron JG. The transition of smooth muscle cells from a contractile to a migratory, phagocytic phenotype: direct demonstration of phenotypic modulation. J Physiol. 2016;594:6189–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res. 2000;48:89–100. [DOI] [PubMed] [Google Scholar]
- 66. Aikawa M, Sivam PN, Kuro‐o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000–1012. [DOI] [PubMed] [Google Scholar]
- 67. Garanich JS, Mathura RA, Shi ZD, Tarbell JM. Effects of fluid shear stress on adventitial fibroblast migration: implications for flow‐mediated mechanisms of arterialization and intimal hyperplasia. Am J Physiol Heart Circ Physiol. 2007;292:H3128–H3135. [DOI] [PubMed] [Google Scholar]
- 68. Madsen CS, Regan CP, Hungerford JE, White SL, Manabe I, Owens GK. Smooth muscle‐specific expression of the smooth muscle myosin heavy chain gene in transgenic mice requires 5'‐flanking and first intronic DNA sequence. Circ Res. 1998;82:908–917. [DOI] [PubMed] [Google Scholar]
- 69. Liu R, Jin JP. Calponin isoforms CNN1, CNN2 and CNN3: regulators for actin cytoskeleton functions in smooth muscle and non‐muscle cells. Gene. 2016;585:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miano JM, Olson EN. Expression of the smooth muscle cell calponin gene marks the early cardiac and smooth muscle cell lineages during mouse embryogenesis. J Biol Chem. 1996;271:7095–7103. [DOI] [PubMed] [Google Scholar]
- 71. Frid MG, Shekhonin BV, Koteliansky VE, Glukhova MA. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992;153:185–193. [DOI] [PubMed] [Google Scholar]
- 72. Perez‐Montiel MD, Plaza JA, Dominguez‐Malagon H, Suster S. Differential expression of smooth muscle myosin, smooth muscle actin, H‐caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105–111. [DOI] [PubMed] [Google Scholar]
- 73. Bär H, Strelkov SV, Sjöberg G, Aebi U, Herrmann H. The biology of desmin filaments: how do mutations affect their structure, assembly, and organisation? J Struct Biol. 2004;148:137–152. [DOI] [PubMed] [Google Scholar]
- 74. Singh SR, Robbins J. Desmin and cardiac disease: an unfolding story. Circ Res. 2018;122:1324–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. [DOI] [PubMed] [Google Scholar]
- 76. Kaplan‐Albuquerque N, Garat C, Van Putten V, Nemenoff RA. Regulation of SM22 alpha expression by arginine vasopressin and PDGF‐BB in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1444–H1452. [DOI] [PubMed] [Google Scholar]
- 77. Zhong L, He X, Si X, Wang H, Li B, Hu Y, Li M, Chen X, Liao W, Liao Y, et al. SM22α (smooth muscle 22α) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-κB (nuclear factor‐κB). Arterioscler Thromb Vasc Biol. 2019;39:e10–e25. [DOI] [PubMed] [Google Scholar]
- 78. Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci. 2009;84:394–401. [DOI] [PubMed] [Google Scholar]
- 79. Coco DP, Hirsch MS, Hornick JL. Smoothelin is a specific marker for smooth muscle neoplasms of the gastrointestinal tract. Am J Surg Pathol. 2009;33:1795–1801. [DOI] [PubMed] [Google Scholar]
- 80. Van Eys G, Niessen P, Rensen S. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc Med. 2007;17:26–30. [DOI] [PubMed] [Google Scholar]
- 81. Hitchcock‐DeGregori SE, Barua B. Tropomyosin structure, function, and interactions: a dynamic regulator. Subcell Biochem. 2017;82:253–284. [DOI] [PubMed] [Google Scholar]
- 82. Marston S, El‐Mezgueldi M. Role of tropomyosin in the regulation of contraction in smooth muscle. Adv Exp Med Biol. 2008;644:110–123. [DOI] [PubMed] [Google Scholar]
- 83. Herring BP, Smith AF. Telokin expression is mediated by a smooth muscle cell‐specific promoter. Am J Physiol. 1996;270:C1656–C1665. [DOI] [PubMed] [Google Scholar]
- 84. Madden JA, Dantuma MW, Sorokina EA, Weihrauch D, Kleinman JG. Telokin expression and the effect of hypoxia on its phosphorylation status in smooth muscle cells from small and large pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1166–L1173. [DOI] [PubMed] [Google Scholar]
- 85. Herring BP, El‐Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol. 2006;291:C817–C827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schaller MD. Paxillin: a focal adhesion‐associated adaptor protein. Oncogene. 2001;20:6459–6472. [DOI] [PubMed] [Google Scholar]
- 87. Yuminamochi T, Yatomi Y, Osada M, Ohmori T, Ishii Y, Nakazawa K, Hosogaya S, Ozaki Y. Expression of the LIM proteins paxillin and Hic‐5 in human tissues. J Histochem Cytochem. 2003;51:513–521. [DOI] [PubMed] [Google Scholar]
- 88. Peng X, Nelson ES, Maiers JL, DeMali KA. New insights into vinculin function and regulation. Int Rev Cell Mol Biol. 2011;287:191–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Belkin AM, Ornatsky OI, Kabakov AE, Glukhova MA, Koteliansky VE. Diversity of vinculin/meta-vinculin in human tissues and cultivated cells: expression of muscle specific variants of vinculin in human aorta smooth muscle cells. J Biol Chem. 1988;263:6631–6635. [PubMed] [Google Scholar]
- 90. Chen D, Ma L, Tham EL, Maresh S, Lechler RI, McVey JH, Dorling A. Fibrocytes mediate intimal hyperplasia post‐vascular injury and are regulated by two tissue factor‐dependent mechanisms. J Thromb Haemost. 2013;11:963–974. [DOI] [PubMed] [Google Scholar]
- 91. Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest. 2002;122:326S–334S. [DOI] [PubMed] [Google Scholar]
- 92. Imoto K, Okada M, Yamawaki H. Characterization of fibroblasts from hypertrophied right ventricle of pulmonary hypertensive rats. Pflugers Arch. 2018;470:1405–1417. [DOI] [PubMed] [Google Scholar]
- 93. Rodriguez A, Karen J, Gardner H, Gerdin B, Rubin K, Sundberg C. Integrin alpha1beta1 is involved in the differentiation into myofibroblasts in adult reactive tissues in vivo. J Cell Mol Med. 2009;13:3449–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Messier RH Jr, Bass BL, Aly HM, Jones JL, Domkowski PW, Wallace RB, Hopkins RA. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J Surg Res. 1994;57:1–21. [DOI] [PubMed] [Google Scholar]
- 95. Jin X, Fu GX, Li XD, Zhu DL, Gao PJ. Expression and function of osteopontin in vascular adventitial fibroblasts and pathological vascular remodeling. PLoS One. 2011;6:e23558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hu WY, Fukuda N, Ikeda Y, Suzuki R, Tahira Y, Takagi H, Matsumoto K, Kanmatsuse K, Mugishima H. Human‐derived vascular smooth muscle cells produce angiotensin II by changing to the synthetic phenotype. J Cell Physiol. 2003;196:284–292. [DOI] [PubMed] [Google Scholar]
- 97. Khaw BA, Carrio I, Pieri PL, Narula J. Radionuclide imaging of the synthetic smooth muscle cell phenotype in experimental atherosclerotic lesions. Trends Cardiovasc Med. 1996;6:226–232. [DOI] [PubMed] [Google Scholar]
- 98. Kudryavtseva O, Aalkjaer C, Matchkov VV. Vascular smooth muscle cell phenotype is defined by Ca2+‐dependent transcription factors. FEBS J. 2013;280:5488–5499. [DOI] [PubMed] [Google Scholar]
- 99. Karoor V, Fini MA, Loomis Z, Sullivan T, Hersh LB, Gerasimovskaya E, Irwin D, Dempsey EC. Sustained activation of Rho GTPases promotes a synthetic pulmonary artery smooth muscle cell phenotype in neprilysin null mice. Arterioscler Thromb Vasc Biol. 2018;38:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Saleh Al‐Shehabi T, Iratni R, Eid AH. Anti‐atherosclerotic plants which modulate the phenotype of vascular smooth muscle cells. Phytomedicine. 2016;23:1068–1081. [DOI] [PubMed] [Google Scholar]
- 101. Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, Reutelingsperger C, Jacobs M, Mees B, Schurgers L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019;39:1351–1368. [DOI] [PubMed] [Google Scholar]
- 102. Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015;214:33–50. [DOI] [PubMed] [Google Scholar]
- 103. Furgeson SB, Simpson PA, Park I, Vanputten V, Horita H, Kontos CD, Nemenoff RA, Weiser‐Evans MC. Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro‐inflammatory phenotype and enhances neointima formation. Cardiovasc Res. 2010;86:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen PY, Simons M. Fibroblast growth factor‐transforming growth factor beta dialogues, endothelial cell to mesenchymal transition, and atherosclerosis. Curr Opin Lipidol. 2018;29:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA. 2008;105:9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang Y, Bao S, Kuang Z, Ma Y, Hu Y, Mao Y. Urotensin II promotes monocyte chemoattractant protein‐1 expression in aortic adventitial fibroblasts of rat. Chin Med J (Engl). 2014;127:1907–1912. [PubMed] [Google Scholar]
- 107. Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton‐Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chung SW, Park JW, Lee SA, Eo SK, Kim K. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun. 2010;396:748–754. [DOI] [PubMed] [Google Scholar]
- 110. Kwansa AL, De Vita R, Freeman JW. Tensile mechanical properties of collagen type I and its enzymatic crosslinks. Biophys Chem. 2016;214–215:1–10. [DOI] [PubMed] [Google Scholar]
- 111. Nadkarni SK, Bouma BE, de Boer J, Tearney GJ. Evaluation of collagen in atherosclerotic plaques: the use of two coherent laser‐based imaging methods. Lasers Med Sci. 2009;24:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41:376–384. [DOI] [PubMed] [Google Scholar]
- 113. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liu X, Huang X, Chen L, Zhang Y, Li M, Wang L, Ge C, Wang H, Zhang M. Mechanical stretch promotes matrix metalloproteinase‐2 and prolyl‐4-hydroxylase α1 production in human aortic smooth muscle cells via Akt‐p38 MAPK‐JNK signaling. Int J Biochem Cell Biol. 2015;62:15–23. [DOI] [PubMed] [Google Scholar]
- 115. Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Icer MA, Gezmen‐Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. [DOI] [PubMed] [Google Scholar]
- 117. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. [DOI] [PubMed] [Google Scholar]
- 118. Hamill KJ, Kligys K, Hopkinson SB, Jones JC. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci. 2009;122:4409–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. [DOI] [PubMed] [Google Scholar]
- 120. Uchio K, Tuchweber B, Manabe N, Gabbiani G, Rosenbaum J, Desmoulière A. Cellular retinol‐binding protein‐1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619–628. [DOI] [PubMed] [Google Scholar]
- 121. Liu T, Ma W, Xu H, Huang M, Zhang D, He Z, Zhwang L, Brem S, O’Rourke D, Gong Y, et al. PDGF‐mediated mesenchymal transformation renders endothelial resistance to anti‐VEGF treatment in glioblastoma. Nat Commun. 2018;9:3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kimani PW, Holmes AJ, Grossmann RE, McGowan S. PDGF‐Rα gene expression predicts proliferation, but PDGF‐A suppresses transdifferentiation of neonatal mouse lung myofibroblasts. Respir Res. 2009;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liu T, Zhang L, Joo D. NF‐κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Reiss A, Siegart N, De Leon J. Interleukin‐6 in atherosclerosis: atherogenic or atheroprotective? Clin Lipidol. 2017;12:14–23. [Google Scholar]
- 125. Hunter C, Jones S. IL‐6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 126. Van der Velde AR, Meijers WC, de Boer RA. Cardiovascular biomarkers: translational aspects of hypertension, atherosclerosis, and heart failure in drug development In: Wehling M, ed. Principles of Translational Science in Medicine, 2nd ed Amsterdam: Elsevier; 2015:167–183. [Google Scholar]
- 127. Bester J, Pretorius E. Effects of IL‐1β, IL‐6 and IL‐8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lindeman JH, Abdul‐Hussien H, Schaapherder AF, Van Bockel JH, Von der Thüsen JH, Roelen DL, Kleemann R. Enhanced expression and activation of pro‐inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL‐6- and IL‐8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond). 2008;114:687–697. [DOI] [PubMed] [Google Scholar]
- 129. Wirka RC, Wagh D, Paik DT, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single‐cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze‐Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage‐like cells during atherogenesis. Circ Res. 2014;115:662–667. [DOI] [PubMed] [Google Scholar]
- 131. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bochaton‐Piallat ML, Bäck M. Novel concepts for the role of smooth muscle cells in vascular disease: towards a new smooth muscle cell classification. Cardiovasc Res. 2018;114:477–480. [DOI] [PubMed] [Google Scholar]
- 133. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lindeman JH. The pathophysiologic basis of abdominal aortic aneurysm progression: a critical appraisal. Expert Rev Cardiovasc Ther. 2015;13:839–851. [DOI] [PubMed] [Google Scholar]
- 135. Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ, Francis GA. Smooth muscle cells contribute the majority of foam cells in ApoE (apolipoprotein E)‐deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Evrard SM, Lecce L, Michelis KC, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grewal N, Gittenberger‐de Groot AC, Poelmann RE, Klautz RJ, Lindeman JH, Goumans MJ, Palmen M, Mohamed SA, Sievers HH, Bogers AJ, et al. Ascending aorta dilation in association with bicuspid aortic valve: a maturation defect of the aortic wall. J Thorac Cardiovasc Surg. 2014;148:1583–1590. [DOI] [PubMed] [Google Scholar]
- 140. Kim HW, Weintraub NL. Aortic aneurysm: in defense of the vascular smooth muscle cell. Arterioscler Thromb Vasc Biol. 2016;36:2138–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Doderer SA, Gäbel G, Kokje VBC, Northoff BH, Holdt LM, Hamming JF, Lindeman JHN. Adventitial adipogenic degeneration is an unidentified contributor to aortic wall weakening in the abdominal aortic aneurysm. J Vasc Surg. 2018;67:1891–1900. [DOI] [PubMed] [Google Scholar]
- 142. Roostalu U, Wong JK. Arterial smooth muscle dynamics in development and repair. Dev Biol. 2018;435:109–121. [DOI] [PubMed] [Google Scholar]
- 143. Gittenberger‐de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. [DOI] [PubMed] [Google Scholar]
- 144. Madsen CS, Hershey JC, Hautmann MB, White SL, Owens GK. Expression of the smooth muscle myosin heavy chain gene is regulated by a negative‐acting GC‐rich element located between two positive‐acting serum response factor‐binding elements. J Biol Chem. 1997;272:6332–6340. [DOI] [PubMed] [Google Scholar]
- 145. Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, Giachelli CM, Parmacek MS, Raines EW, Rusch NJ, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gäbel G, Northoff BH, Weinzierl I, Ludwig S, Hinterseher I, Wilfert W, Teupser D, Doderer SA, Bergert H, Schönleben F, et al. Molecular fingerprint for terminal abdominal aortic aneurysm disease. J Am Heart Assoc. 2017;6:e006798 DOI: 10.1161/JAHA.117.006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Håversen L, Sundelin JP, Mardinoglu A, Rutberg M, Ståhlman M, Wilhelmsson U, Hultén LM, Pekny M, Fogelstrand P, Bentzon JF, et al. Vimentin deficiency in macrophages induces increased oxidative stress and vascular inflammation but attenuates atherosclerosis in mice. Sci Rep. 2018;8:16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Piera‐Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99:1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. [DOI] [PubMed] [Google Scholar]
- 151. Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE‐cadherin junctions to control force‐dependent remodeling. J Cell Biol. 2012;196:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fang S, Sharma RV, Bhalla RC. Enhanced recovery of injury‐caused downregulation of paxillin protein by eNOS gene expression in rat carotid artery: mechanism of NO inhibition of intimal hyperplasia? Arterioscler Thromb Vasc Biol. 1999;19:147–152. [DOI] [PubMed] [Google Scholar]
- 153. von Essen M, Rahikainen R, Oksala N, et al. Talin and vinculin are downregulated in atherosclerotic plaque: Tampere Vascular Study. Atherosclerosis. 2016;255:43–53. [DOI] [PubMed] [Google Scholar]
- 154. van Dijk RA, Rijs K, Wezel A, Hamming JF, Kolodgie FD, Virmani R, Schaapherder AF, Lindeman JHN. Systematic evaluation of the cellular innate immune response during the process of human atherosclerosis. J Am Heart Assoc. 2016;5:e002860 DOI: 10.1161/JAHA.115.002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. van Dijk RA, Duinisveld AJ, Schaapherder AF, Mulder‐Stapel A, Hamming JF, Kuiper J, de Boer OJ, van der Wal AC, Kolodgie FD, Virmani R, et al. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc. 2015;4:e001403 DOI: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao CY, Li JC. Inhibition of reactive oxygen species generation attenuates TLR4‐mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab Invest. 2013;93:880–887. [DOI] [PubMed] [Google Scholar]
- 159. Sava P, Ramanathan A, Dobronyi A, Peng X, Sun H, Ledesma‐Mendoza A, Herzog EL, Gonzalez AL. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight. 2017;2:e96352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Xu JY, Chang NB, Li T, Jiang R, Sun XL, He YZ, Jiang J. Endothelial cells inhibit the angiotensin II induced phenotypic modulation of rat vascular adventitial fibroblasts. J Cell Biochem. 2017;118:1921–1927. [DOI] [PubMed] [Google Scholar]
- 161. Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, Dong XR, Poczobutt J, Nemenoff RA, Weiser‐Evans MC. Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ Res. 2017;120:296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hegner B, Schaub T, Catar R, Kusch A, Wagner P, Essin K, Lange C, Riemekasten G, Dragun D. Intrinsic deregulation of vascular smooth muscle and myofibroblast differentiation in mesenchymal stromal cells from patients with systemic sclerosis. PLoS One. 2016;11:e0153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bahnson ES, Vavra AK, Flynn ME, Vercammen JM, Jiang Q, Schwartz AR, Kibbe MR. Long‐term effect of PROLI/NO on cellular proliferation and phenotype after arterial injury. Free Radic Biol Med. 2016;90:272–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. An SJ, Liu P, Shao TM, Wang ZJ, Lu HG, Jiao Z, Li X, Fu JQ. Characterization and functions of vascular adventitial fibroblast subpopulations. Cell Physiol Biochem. 2015;35:1137–1150. [DOI] [PubMed] [Google Scholar]
- 165. Wang Z, Ren Z, Hu Z, Hu X, Zhang H, Wu H, Zhang M. Angiotensin‐II induces phosphorylation of ERK1/2 and promotes aortic adventitial fibroblasts differentiating into myofibroblasts during aortic dissection formation. J Mol Histol. 2014;45:401–412. [DOI] [PubMed] [Google Scholar]
- 166. Chen WD, Chu YF, Liu JJ, Hong MN, Gao PJ. RhoA‐Rho kinase signaling pathway mediates adventitial fibroblasts differentiation to myofibroblasts induced by TGF‐β1. Sheng Li Xue Bao. 2013;65:113–121. [PubMed] [Google Scholar]
- 167. Li Y, Tao J, Zhang J, Tian X, Liu S, Sun M, Zhang X, Yan C, Han Y. Cellular repressor E1A‐stimulated genes controls phenotypic switching of adventitial fibroblasts by blocking p38MAPK activation. Atherosclerosis. 2012;225:304–314. [DOI] [PubMed] [Google Scholar]
- 168. Forte A, Della Corte A, Grossi M, Bancone C, Provenzano R, Finicelli M, De Feo M, De Santo LS, Nappi G, Cotrufo M, et al. Early cell changes and TGFβ pathway alterations in the aortopathy associated with bicuspid aortic valve stenosis. Clin Sci (Lond). 2013;124:97–108. [DOI] [PubMed] [Google Scholar]
- 169. Zhang YG, Hu YC, Mao YY, Wei RH, Bao SL, Wu LB, Kuang ZJ. Transforming growth factor‐β1 involved in urotensin II‐induced phenotypic differentiation of adventitial fibroblasts from rat aorta. Chin Med J (Engl). 2010;123:3634–3639. [PubMed] [Google Scholar]
- 170. Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, et al. Platelet‐derived growth factor receptor signaling activates pericyte‐myofibroblast transition in obstructive and post‐ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. [DOI] [PubMed] [Google Scholar]
- 171. Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, Zhu DL, Gao PJ. MicroRNA‐155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010;400:483–488. [DOI] [PubMed] [Google Scholar]
- 172. Ji J, Xu F, Li L, Chen R, Wang J, Hu WC. Activation of adventitial fibroblasts in the early stage of the aortic transplant vasculopathy in rat. Transplantation. 2010;89:945–953. [DOI] [PubMed] [Google Scholar]
- 173. Gan Q, Yoshida T, Li J, Owens GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha‐actin expression. Circ Res. 2007;101:883–892. [DOI] [PubMed] [Google Scholar]
- 174. Guo SJ, Wu LY, Shen WL, Chen WD, Wei J, Gao PJ, Zhu DL. Gene profile for differentiation of vascular adventitial myofibroblasts. Sheng Li Xue Bao. 2006;58:337–344. [PubMed] [Google Scholar]
- 175. Butcher JT, Barrett BC, Nerem RM. Equibiaxial strain stimulates fibroblastic phenotype shift in smooth muscle cells in an engineered tissue model of the aortic wall. Biomaterials. 2006;27:5252–5258. [DOI] [PubMed] [Google Scholar]
- 176. Jiang YL, Dai AG, Li QF, Hu RC. Transforming growth factor‐beta1 induces transdifferentiation of fibroblasts into myofibroblasts in hypoxic pulmonary vascular remodeling. Acta Biochim Biophys Sin (Shanghai). 2006;38:29–36. [DOI] [PubMed] [Google Scholar]
- 177. Miyazaki H, Hayashi K, Hasegawa Y. Tensile properties of fibroblasts and vascular smooth muscle cells. Biorheology. 2003;40:207–212. [PubMed] [Google Scholar]
- 178. Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976–L986. [DOI] [PubMed] [Google Scholar]
- 179. Zhong A, Mirzaei Z, Simmons CA. The roles of matrix stiffness and ß‐catenin signaling in endothelial‐to‐mesenchymal transition of aortic valve endothelial cells. Cardiovasc Eng Technol. 2018;9:158–167. [DOI] [PubMed] [Google Scholar]
- 180. Zhang L, Chen Y, Li G, Chen M, Huang W, Liu Y, Li Y. TGF‐β1/FGF-2 signaling mediates the 15‐HETE-induced differentiation of adventitial fibroblasts into myofibroblasts. Lipids Health Dis. 2016;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Castellano G, Franzin R, Stasi A, Divella C, Sallustio F, Pontrelli P, Lucarelli G, Battaglia M, Staffieri F, Crovace A, et al. Complement activation during ischemia/reperfusion injury induces pericyte‐to‐myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front Immunol. 2018;9:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen C, Han X, Fan F, Liu Y, Wang T, Wang J, Hu P, Ma A, Tian H. Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF‐β1/Smad3‐mediated fibrotic responses through 5‐HT(2A) receptors. Mol Cell Biochem. 2014;397:267–276. [DOI] [PubMed] [Google Scholar]
- 183. Lin S, Ma S, Lu P, Cai W, Chen Y, Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF‐β1 from rat aorta in vitro. Int J Clin Exp Pathol. 2014;7:2199–2208. [PMC free article] [PubMed] [Google Scholar]
- 184. Okayama K, Azuma J, Dosaka N, Iekushi K, Sanada F, Kusunoki H, Iwabayashi M, Rakugi H, Taniyama Y, Morishita R. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial‐mesenchymal transition. Hypertension. 2012;59:958–965. [DOI] [PubMed] [Google Scholar]
- 185. Yang W, Zhang J, Wang H, Gao P, Singh M, Shen K, Fang N. Angiotensin II downregulates catalase expression and activity in vascular adventitial fibroblasts through an AT1R/ERK1/2‐dependent pathway. Mol Cell Biochem. 2011;358:21–29. [DOI] [PubMed] [Google Scholar]
- 186. Jones JA, Beck C, Barbour JR, Zavadzkas JA, Mukherjee R, Spinale FG, Ikonomidis JS. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast‐mediated vascular remodeling. Am J Pathol. 2009;175:1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Che ZQ, Gao PJ, Shen WL, Fan CL, Liu JJ, Zhu DL. Angiotensin II‐stimulated collagen synthesis in aortic adventitial fibroblasts is mediated by connective tissue growth factor. Hypertens Res. 2008;31:1233–1240. [DOI] [PubMed] [Google Scholar]
- 188. Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad‐mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008;22:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Merryman WD, Lukoff HD, Long RA, Engelmayr GC Jr, Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor‐beta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. An SJ, Boyd R, Zhu M, Chapman A, Pimentel DR, Wang HD. NADPH oxidase mediates angiotensin II‐induced endothelin‐1 expression in vascular adventitial fibroblasts. Cardiovasc Res. 2007;75:702–709. [DOI] [PubMed] [Google Scholar]
- 191. Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, Schultz GS, Ozaki CK, Berceli SA. TGF‐beta- and CTGF‐mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H482–H488. [DOI] [PubMed] [Google Scholar]
- 192. Karasek MA. Does transformation of microvascular endothelial cells into myofibroblasts play a key role in the etiology and pathology of fibrotic disease? Med Hypotheses. 2007;68:650–655. [DOI] [PubMed] [Google Scholar]
- 193. Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, , Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin‐1. Am J Pathol. 2006;168:1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tomas JJ, Stark VE, Kim JL, Wolff RA, Hullett DA, Warner TF, Hoch JR. Beta‐galactosidase‐tagged adventitial myofibroblasts tracked to the neointima in healing rat vein grafts. J Vasc Res. 2003;40:266–275. [DOI] [PubMed] [Google Scholar]
- 195. Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59:212–221. [DOI] [PubMed] [Google Scholar]
- 196. Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial‐mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. [DOI] [PubMed] [Google Scholar]
- 197. Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. [DOI] [PubMed] [Google Scholar]
- 198. Hirose M, Kosugi H, Nakazato K, Hayashi T. Restoration to a quiescent and contractile phenotype from a proliferative phenotype of myofibroblast‐like human aortic smooth muscle cells by culture on type IV collagen gels. J Biochem. 1999;125:991–1000. [DOI] [PubMed] [Google Scholar]
- 199. Shi Y, O'Brien JE Jr, Fard A, Zalewski A. Transforming growth factor‐beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. [DOI] [PubMed] [Google Scholar]
- 200. Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. [DOI] [PubMed] [Google Scholar]
- 201. Zhang YG, Li J, Li YG, Wei RH. Urotensin II induces phenotypic differentiation, migration, and collagen synthesis of adventitial fibroblasts from rat aorta. J Hypertens. 2008;26:1119–1126. [DOI] [PubMed] [Google Scholar]
- 202. Patel S, Shi Y, Niculescu R, Chung EH, Martin JL, Zalewski A. Characteristics of coronary smooth muscle cells and adventitial fibroblasts. Circulation. 2000;101:524–532. [DOI] [PubMed] [Google Scholar]
- 203. Frösen J, Joutel A. Smooth muscle cells of intracranial vessels: from development to disease. Cardiovasc Res. 2018;114:501–512. [DOI] [PubMed] [Google Scholar]
- 204. Majesky MW. Vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2016;36:e82–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Yuan SM. α‐Smooth muscle actin and ACTA2 gene expressions in vasculopathies. Braz J Cardiovasc Surg. 2015;30:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lao KH, Zeng L, Xu Q. Endothelial and smooth muscle cell transformation in atherosclerosis. Curr Opin Lipidol. 2015;26:449–456. [DOI] [PubMed] [Google Scholar]
- 207. Chaabane C, Coen M, Bochaton‐Piallat ML. Smooth muscle cell phenotypic switch: implications for foam cell formation. Curr Opin Lipidol. 2014;25:374–379. [DOI] [PubMed] [Google Scholar]
- 208. Davis‐Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chan‐Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, Kang GC, Greisler HP. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue‐engineered small‐diameter blood vessels. J Biomed Mater Res A. 2009;88:1104–1121. [DOI] [PubMed] [Google Scholar]
- 210. Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–1382. [DOI] [PubMed] [Google Scholar]
- 211. Halka AT, Turner NJ, Carter A, Ghosh J, Murphy MO, Kirton JP, Kielty CM, Walker MG. The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovasc Pathol. 2008;17:98–102. [DOI] [PubMed] [Google Scholar]
- 212. Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45(suppl A):A25–A32. [DOI] [PubMed] [Google Scholar]
- 213. McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–1441. [DOI] [PubMed] [Google Scholar]
- 214. Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260:192–210. [DOI] [PubMed] [Google Scholar]
- 215. Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321–2327. [DOI] [PubMed] [Google Scholar]
- 216. Hao H, Gabbiani G, Bochaton‐Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. [DOI] [PubMed] [Google Scholar]
- 217. Stenmark KR, Frid MG. Smooth muscle cell heterogeneity: role of specific smooth muscle cell subpopulations in pulmonary vascular disease. Chest. 1998;114:82S–90S. [DOI] [PubMed] [Google Scholar]
- 218. Shanahan CM, Weissberg PL. Smooth muscle cell heterogeneity: patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1998;18:333–338. [DOI] [PubMed] [Google Scholar]
- 219. Frid MG, Dempsey EC, Durmowicz AG, Stenmark KR. Smooth muscle cell heterogeneity in pulmonary and systemic vessels: importance in vascular disease. Arterioscler Thromb Vasc Biol. 1997;17:1203–1209. [DOI] [PubMed] [Google Scholar]
- 220. Archer SL. Diversity of phenotype and function of vascular smooth muscle cells. J Lab Clin Med. 1996;127:524–529. [DOI] [PubMed] [Google Scholar]
- 221. Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. [DOI] [PubMed] [Google Scholar]
- 222. Sartore S, Scatena M, Chiavegato A, Faggin E, Giuriato L, Pauletto P. Myosin isoform expression in smooth muscle cells during physiological and pathological vascular remodeling. J Vasc Res. 1994;31:61–81. [DOI] [PubMed] [Google Scholar]
- 223. Harrison OJ, Visan AC, Moorjani N, Modi A, Salhiyyah K, Torrens C, Ohri S, Cagampang FR. Defective NOTCH signaling drives increased vascular smooth muscle cell apoptosis and contractile differentiation in bicuspid aortic valve aortopathy: a review of the evidence and future directions. Trends Cardiovasc Med. 2019;29:61–68. [DOI] [PubMed] [Google Scholar]
- 224. Walsh K, Takahashi A. Transcriptional regulation of vascular smooth muscle cell phenotype. Z Kardiol. 2001;90(suppl 3):12–16. [DOI] [PubMed] [Google Scholar]
- 225. Shanahan CM, Weissberg PL. Smooth muscle cell phenotypes in atherosclerotic lesions. Curr Opin Lipidol. 1999;10:507–513. [DOI] [PubMed] [Google Scholar]
- 226. Vendrov AE, Sumida A, Canugovi C, Lozhkin A, Hayami T, Madamanchi NR, Runge MS. NOXA1‐dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019;21:101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Sun QR, Zhang X, Fang K. Phenotype of vascular smooth muscle cells (VSMCs) is regulated by miR‐29b by targeting sirtuin 1. Med Sci Monit. 2018;24:6599–6607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 228. Zeng M, Luo Y, Xu C, Li R, Chen N, Deng X, Fang D, Wang L, Wu J, Luo M. Platelet‐endothelial cell interactions modulate smooth muscle cell phenotype in an in vitro model of type 2 diabetes mellitus. Am J Physiol Cell Physiol. 2019;316:C186–C197. [DOI] [PubMed] [Google Scholar]
- 229. Yi B, Shen Y, Tang H, Wang X, Li B, Zhang Y. Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl Mater Interfaces. 2019;11:6867–6880. [DOI] [PubMed] [Google Scholar]
- 230. Wu W, Zhang W, Choi M, Zhao J, Gao P, Xue M, Singer HA, Jourd'heuil D, Long X. Vascular smooth muscle‐MAPK14 is required for neointimal hyperplasia by suppressing VSMC differentiation and inducing proliferation and inflammation. Redox Biol. 2019;22:101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single‐cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Malhotra R, Mauer AC, Lino Cardenas CL, Guo X, Yao J, Zhang X, Wunderer F, Smith AV, Wong Q, Pechlivanis S, et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat Genet. 2019;51:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sun L, Zhao M, Liu A, Lv M, Zhang J, Li Y, Yang X, Wu Z. Shear stress induces phenotypic modulation of vascular smooth muscle cells via AMPK/mTOR/ULK1‐mediated autophagy. Cell Mol Neurobiol. 2018;38:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wu B, Zhang L, Zhu YH, Zhang YE, Zheng F, Yang JY, Guo LY, Li XY, Wang L, Tang JM, et al. Mesoderm/mesenchyme homeobox gene l promotes vascular smooth muscle cell phenotypic modulation and vascular remodeling. Int J Cardiol. 2018;251:82–89. [DOI] [PubMed] [Google Scholar]
- 235. Fu Y, Chang Y, Chen S, Li Y, Chen Y, Sun G, Yu S, Ye N, Li C, Sun Y. BAG3 promotes the phenotypic transformation of primary rat vascular smooth muscle cells via TRAIL. Int J Mol Med. 2018;41:2917–2926. [DOI] [PubMed] [Google Scholar]
- 236. Charles R, Bourmoum M, Claing A. ARF GTPases control phenotypic switching to vascular smooth muscle cells through the regulation of actin function and actin dependent gene expression. Cell Signal. 2018;46:64–75. [DOI] [PubMed] [Google Scholar]
- 237. Zhang L, Xu Z, Wu Y, Liao J, Zeng F, Shi L. Akt/eNOS and MAPK signaling pathways mediated the phenotypic switching of thoracic aorta vascular smooth muscle cells in aging/hypertensive rats. Physiol Res. 2018;67:543–553. [DOI] [PubMed] [Google Scholar]
- 238. Rizzo S, Coen M, Sakic A, De Gaspari M, Thiene G, Gabbiani G, Basso C, Bochaton‐Piallat ML. Sudden coronary death in the young: evidence of contractile phenotype of smooth muscle cells in the culprit atherosclerotic plaque. Int J Cardiol. 2018;264:1–6. [DOI] [PubMed] [Google Scholar]
- 239. Davis‐Knowlton J, Turner JE, Turner A, Damian‐Loring S, Hagler N, Henderson T, Emery IF, Bond K, Duarte CW, Vary CPH, et al. Characterization of smooth muscle cells from human atherosclerotic lesions and their responses to Notch signaling. Lab Invest. 2019;99:290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Misra A, Feng Z, Chandran RR, Kabir I, Rotllan N, Aryal B, Sheikh AQ, Ding L, Qin L, Fernández‐Hernando C, et al. Integrin beta3 regulates clonality and fate of smooth muscle‐derived atherosclerotic plaque cells. Nat Commun. 2018;9:2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Smyth LCD, Rustenhoven J, Scotter EL, Schweder P, Faull RLM, Park TIH, Dragunow M. Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat. 2018;92:48–60. [DOI] [PubMed] [Google Scholar]
- 242. Gao P, Wu W, Ye J, Lu YW, Adam AP, Singer HA, Long X. Transforming growth factor β1 suppresses proinflammatory gene program independent of its regulation on vascular smooth muscle differentiation and autophagy. Cell Signal. 2018;50:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ma S, Motevalli SM, Chen J, Xu MQ, Wang Y, Feng J, Qiu Y, Han D, Fan M, Ding M, et al. Precise theranostic nanomedicines for inhibiting vulnerable atherosclerotic plaque progression through regulation of vascular smooth muscle cell phenotype switching. Theranostics. 2018;8:3693–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Liu Z, Zhang M, Zhou T, Shen Q, Qin X. Exendin‐4 promotes the vascular smooth muscle cell re‐differentiation through AMPK/SIRT1/FOXO3a signaling pathways. Atherosclerosis. 2018;276:58–66. [DOI] [PubMed] [Google Scholar]
- 245. Xie SA, Zhang T, Wang J, Zhao F, Zhang YP, Yao WJ, Hur SS, Yeh YT, Pang W, Zheng LS, et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: role of DNA methyltransferase 1. Biomaterials. 2018;155:203–216. [DOI] [PubMed] [Google Scholar]
- 246. Dale M, Fitzgerald MP, Liu Z, Meisinger T, Karpisek A, Purcell LN, Carson JS, Harding P, Lang H, Koutakis P, et al. Premature aortic smooth muscle cell differentiation contributes to matrix dysregulation in Marfan syndrome. PLoS One. 2017;12:e0186603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lakhkar A, Dhagia V, Joshi SR, Gotlinger K, Patel D, Sun D, Wolin MS, Schwartzman ML, Gupte SA. 20-HETE‐induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose‐6-phosphate dehydrogenase inhibition. Am J Physiol Heart Circ Physiol. 2016;310:H1107–H1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll‐like receptor 4 signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1069–H1076. [DOI] [PubMed] [Google Scholar]
- 249. Krug AW, Allenhöfer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal‐regulated kinase 1/2 mitogen‐activated protein kinase and epidermal growth factor receptor‐dependent pathways. Hypertension. 2010;55:1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Liu R, Lo L, Lay AJ, Zhao Y, Ting KK, Robertson EN, Sherrah AG, Jarrah S, Li H, Zhou Z, et al. ARHGAP18 protects against thoracic aortic aneurysm formation by mitigating the synthetic and proinflammatory smooth muscle cell phenotype. Circ Res. 2017;121:512–524. [DOI] [PubMed] [Google Scholar]
- 251. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age‐associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non‐human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous interleukin‐1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927–H2934. [DOI] [PubMed] [Google Scholar]
- 253. Yang X, Murthy V, Schultz K, Tatro JB, Fitzgerald KA, Beasley D. Toll‐like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H2334–H2343. [DOI] [PubMed] [Google Scholar]
- 254. Zucker MM, Wujak L, Gungl A, Didiasova M, Kosanovic D, Petrovic A, Klepetko W, Schermuly RT, Kwapiszewska G, Schaefer L, et al. LRP1 promotes synthetic phenotype of pulmonary artery smooth muscle cells in pulmonary hypertension. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1604–1616. [DOI] [PubMed] [Google Scholar]
- 255. Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C, et al. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 2017;121:1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Patel R, Cardneau JD, Colles SM, Graham LM. Synthetic smooth muscle cell phenotype is associated with increased nicotinamide adenine dinucleotide phosphate oxidase activity: effect on collagen secretion. J Vasc Surg. 2006;43:364–371. [DOI] [PubMed] [Google Scholar]
- 257. Rama A, Matsushita T, Charolidi N, Rothery S, Dupont E, Severs NJ. Up‐regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF‐beta-treated human aortic smooth muscle cells. Eur J Cell Biol. 2006;85:375–386. [DOI] [PubMed] [Google Scholar]
- 258. Acampora KB, Nagatomi J, Langan EM III, LaBerge M. Increased synthetic phenotype behavior of smooth muscle cells in response to in vitro balloon angioplasty injury model. Ann Vasc Surg. 2010;24:116–126. [DOI] [PubMed] [Google Scholar]
- 259. Augstein A, Mierke J, Poitz DM, Strasser RH. Sox9 is increased in arterial plaque and stenosis, associated with synthetic phenotype of vascular smooth muscle cells and causes alterations in extracellular matrix and calcification. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2526–2537. [DOI] [PubMed] [Google Scholar]
- 260. Alshanwani AR, Riches‐Suman K, O'Regan DJ, Wood IC, Turner NA, Porter KE. MicroRNA‐21 drives the switch to a synthetic phenotype in human saphenous vein smooth muscle cells. IUBMB Life. 2018;70:649–657. [DOI] [PubMed] [Google Scholar]
- 261. Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45:837–846. [DOI] [PubMed] [Google Scholar]
- 262. Thyberg J, Hultgårdh‐Nilsson A. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 1994;276:263–271. [DOI] [PubMed] [Google Scholar]
- 263. Anwar A, Li M, Frid MG, Kumar B, Gerasimovskaya EV, Riddle SR, McKeon BA, Thukaram R, Meyrick BO, Fini MA, et al. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1–L11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Giachelli C, Bae N, Lombardi D, Majesky M, Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991;177:867–873. [DOI] [PubMed] [Google Scholar]
- 265. Okada Y, Katsuda S, Matsui Y, Watanabe H, Nakanishi I. Collagen synthesis by cultured arterial smooth muscle cells during spontaneous phenotypic modulation. Acta Pathol Jpn. 1990;40:157–164. [DOI] [PubMed] [Google Scholar]
- 266. Ang AH, Tachas G, Campbell JH, Bateman JF, Campbell GR. Collagen synthesis by cultured rabbit aortic smooth‐muscle cells: alteration with phenotype. Biochem J. 1990;265:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Chiarini A, Onorati F, Marconi M, Pasquali A, Patuzzo C, Malashicheva A, Irtyega O, Faggian G, Pignatti PF, Trabetti E, et al. Studies on sporadic non‐syndromic thoracic aortic aneurysms, 1: deregulation of Jagged/Notch 1 homeostasis and selection of synthetic/secretor phenotype smooth muscle cells. Eur J Prev Cardiol. 2018;25:42–50. [DOI] [PubMed] [Google Scholar]
- 268. Denger S, Jahn L, Wende P, Watson L, Gerber SH, Kübler W, Kreuzer J. Expression of monocyte chemoattractant protein‐1 cDNA in vascular smooth muscle cells: induction of the synthetic phenotype: a possible clue to SMC differentiation in the process of atherogenesis. Atherosclerosis. 1999;144:15–23. [DOI] [PubMed] [Google Scholar]
- 269. Wang TM, Chen KC, Hsu PY, Lin HF, Wang YS, Chen CY, Liao YC, Juo SH. microRNA let‐7g suppresses PDGF‐induced conversion of vascular smooth muscle cell into the synthetic phenotype. J Cell Mol Med. 2017;21:3592–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Pahk K, Joung C, Jung SM, Young Song H, Yong Park J, Woo Byun J, Lee YS, Chul Paeng J, Kim C, Kim S, et al. Visualization of synthetic vascular smooth muscle cells in atherosclerotic carotid rat arteries by F‐18 FDG PET. Sci Rep. 2017;7:6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ghosh S, Kollar B, Nahar T, Suresh Babu S, Wojtowicz A, Sticht C, Gretz N, Wagner AH, Korff T, Hecker M. Loss of the mechanotransducer zyxin promotes a synthetic phenotype of vascular smooth muscle cells. J Am Heart Assoc. 2015;4:e001712 DOI: 10.1161/JAHA.114.001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Lee HS, Yun SJ, Ha JM, Jin SY, Ha HK, Song SH, Kim CD, Bae SS. Prostaglandin D2 stimulates phenotypic changes in vascular smooth muscle cells. Exp Mol Med. 2019;51:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Jaminon A, Reesink K, Kroon A, Schurgers L. The role of vascular smooth muscle cells in arterial remodeling: focus on calcification‐related processes. Int J Mol Sci. 2019;20:5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, et al. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler Thromb Vasc Biol. 2019;39:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Owens GK, Pasterkamp G. PlaqOmics Leducq Fondation Trans‐Atlantic Network: defining the roles of smooth muscle cells and other extracellular matrix‐producing cells in late‐stage atherosclerotic plaque pathogenesis. Circ Res. 2019;124:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Liu M, Gomez D. Smooth muscle cell phenotypic diversity. Arterioscler Thromb Vasc Biol. 2019;39:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Yuan SM, Wu N. Aortic alpha‐smooth muscle actin expressions in aortic disorders and coronary artery disease: an immunohistochemical study. Anatol J Cardiol. 2018;19:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lin CS, Hsieh PS, Hwang LL, Lee YH, Tsai SH, Tu YC, Hung YW, Liu CC, Chuang YP, Liao MT, et al. The CCL5/CCR5 axis promotes vascular smooth muscle cell proliferation and atherogenic phenotype switching. Cell Physiol Biochem. 2018;47:707–720. [DOI] [PubMed] [Google Scholar]
- 279. Zhong L, He X, Si X, Wang H, Li B, Hu Y, Li M, Chen X, Liao W, Liao Y, et al. SM22 (smooth muscle 22) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-kappaB (nuclear factor‐kappaB). Arterioscler Thromb Vasc Biol. 2019;39:e10–e25. [DOI] [PubMed] [Google Scholar]
- 280. Choi S, Park M, Kim J, Park W, Kim S, Lee DK, Hwang JY, Choe J, Won MH, Ryoo S, et al. TNF‐alpha elicits phenotypic and functional alterations of vascular smooth muscle cells by miR‐155-5p‐dependent down‐regulation of cGMP‐dependent kinase 1. J Biol Chem. 2018;293:14812–14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Schwartz SM, Virmani R, Majesky MW. An update on clonality: what smooth muscle cell type makes up the atherosclerotic plaque? F1000Res. 2018;7:F1000 Faculty Rev‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Goikuria H, del Mar FM, Manrique RV, Sastre M, Elizagaray E, Lorenzo A, Vandenbroeck K, Alloza I. Characterization of carotid smooth muscle cells during phenotypic transition. Cells. 2018;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Rensen SSM, Doevendans PAFM, Van Eys GJJM. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Mahoney WM Jr, Schwartz SM. Defining smooth muscle cells and smooth muscle injury. J Clin Invest. 2005;115:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Li J, Zhang YG, Luo LM, Dong X, Ding WH, Dang SY. Urotensin II promotes aldosterone expression in rat aortic adventitial fibroblasts. Mol Med Rep. 2018;17:2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Yin L, Cai W, Sheng J, Sun Y. Hypoxia induced changes of SePP1 expression in rat preadipocytes and its impact on vascular fibroblasts. Int J Clin Exp Med. 2014;7:41–50. [PMC free article] [PubMed] [Google Scholar]
- 287. Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984–1995. [DOI] [PubMed] [Google Scholar]
- 288. Banks MF, Gerasimovskaya EV, Tucker DA, Frid MG, Carpenter TC, Stenmark KR. Egr‐1 antisense oligonucleotides inhibit hypoxia‐induced proliferation of pulmonary artery adventitial fibroblasts. J Appl Physiol (1985). 2005;98:732–738. [DOI] [PubMed] [Google Scholar]
- 289. Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3‐kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia‐induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol (1985). 2005;98:722–731. [DOI] [PubMed] [Google Scholar]
- 290. Das M, Stenmark KR, Dempsey EC. Enhanced growth of fetal and neonatal pulmonary artery adventitial fibroblasts is dependent on protein kinase C. Am J Physiol Lung Cell Mol Physiol. 1995;269:L660–L667. [DOI] [PubMed] [Google Scholar]
- 291. Moulton KS, Li M, Strand K, Burgett S, McClatchey P, Tucker R, Furgeson SB, Lu S, Kirkpatrick B, Cleveland JC, et al. PTEN deficiency promotes pathological vascular remodeling of human coronary arteries. JCI Insight. 2018;3:e97228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wesseling M, Sakkers TR, de Jager SCA, Pasterkamp G, Goumans MJ. The morphological and molecular mechanisms of epithelial/endothelial‐to‐mesenchymal transition and its involvement in atherosclerosis. Vasc Pharmacol. 2018;106:1–8. [DOI] [PubMed] [Google Scholar]
- 293. Kim M, Choi SH, Jin YB, Lee HJ, Ji YH, Kim J, Lee YS, Lee YJ. The effect of oxidized low‐density lipoprotein (ox‐LDL) on radiation‐induced endothelial‐to‐mesenchymal transition. Int J Radiat Biol. 2013;89:356–363. [DOI] [PubMed] [Google Scholar]
- 294. Tang R, Li Q, Lv L, Dai H, Zheng M, Ma K, Liu B. Angiotensin II mediates the high‐glucose‐induced endothelial‐to‐mesenchymal transition in human aortic endothelial cells. Cardiovasc Diabetol. 2010;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Muchaneta‐Kubara EC, El Nahas AM. Myofibroblast phenotypes expression in experimental renal scarring. Nephrol Dial Transplant. 1997;12:904–915. [DOI] [PubMed] [Google Scholar]
- 296. Ikawati M, Kawaichi M, Oka C. Loss of HtrA1 serine protease induces synthetic modulation of aortic vascular smooth muscle cells. PLoS One. 2018;13:e0196628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Foster LC, Arkonac BM, Sibinga NES, Shi C, Perrella MA, Haber E. Regulation of CD44 gene expression by the proinflammatory cytokine interleukin‐1beta in vascular smooth muscle cells. J Biol Chem. 1998;273:20341–20346. [DOI] [PubMed] [Google Scholar]
- 298. Clement N, Gueguen M, Glorian M, Blaise R, Andreani M, Brou C, Bausero P, Limon I. Notch3 and IL‐1beta exert opposing effects on a vascular smooth muscle cell inflammatory pathway in which NF‐kappaB drives crosstalk. J Cell Sci. 2007;120:3352–3361. [DOI] [PubMed] [Google Scholar]
- 299. Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc. Res. 2007;75:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Orekhov AN, Bobryshev YV, Chistiakov DA. The complexity of cell composition of the intima of large arteries: focus on pericyte‐like cells. Cardiovasc Res. 2014;103:438–451. [DOI] [PubMed] [Google Scholar]
- 301. Murashov IS, Volkov AM, Kazanskaya GM, Kliver EE, Savchenko SV, Klochkova SV, Lushnikova EL. Immunohistochemical phenotypes of stable and unstable occlusive atherosclerotic plaques in coronary arteries. Bull Exp Biol Med. 2018;165:798–802. [DOI] [PubMed] [Google Scholar]
- 302. Okamoto E, Suzuki T, Aikawa M, Imataka K, Fujii J, Kuro‐o M, Nakahara K, Hasegawa A, Yazaki Y, Nagai R. Diversity of the synthetic‐state smooth‐muscle cells proliferating in mechanically and hemodynamically injured rabbit arteries. Lab Invest. 1996;74:120–128. [PubMed] [Google Scholar]
- 303. Yutani C, Fujita H, Takaichi S, Yamamoto A. The role of vascular smooth muscle cell phenotypic modulation at the aortic branch in atherogenesis. Front Med Biol Eng. 1993;5:143–146. [PubMed] [Google Scholar]
- 304. Owens GK, Wise G. Regulation of differentiation/maturation in vascular smooth muscle cells by hormones and growth factors. Agents Actions Suppl. 1997;48:3–24. [DOI] [PubMed] [Google Scholar]
- 305. Forte A, Della Corte A, Grossi M, Bancone C, Maiello C, Galderisi U, Cipollaro M. Differential expression of proteins related to smooth muscle cells and myofibroblasts in human thoracic aortic aneurysm. Histol Histopathol. 2013;28:795–803. [DOI] [PubMed] [Google Scholar]
- 306. Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol Histopathol. 1998;13:871–891. [DOI] [PubMed] [Google Scholar]
- 307. Weissberg PL, Cary NR, Shanahan CM. Gene expression and vascular smooth muscle cell phenotype. Blood Press Suppl. 1995;2:68–73. [PubMed] [Google Scholar]
- 308. Ci W, Wang T, Li T, Wan J. T‐614 inhibits human aortic adventitial fibroblast proliferation and promotes interleukin‐8 production in vitro. Vascular. 2020;28:314–320. [DOI] [PubMed] [Google Scholar]
- 309. Kuro‐o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem. 1991;266:3768–3773. [PubMed] [Google Scholar]
- 310. Aslam MI, Hettmer S, Abraham J, Latocha D, Soundararajan A, Huang ET, Goros MW, Michalek JE, Wang S, Mansoor A. Dynamic and nuclear expression of PDGFRα and IGF‐1R in alveolar rhabdomyoscarcoma. Mol Cancer Res. 2013;11:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch MT, Xu Y, Per AK. Temporal, spatial and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol. 2017;425:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


