Abstract
Background
Resistant hypertension is a salt‐retaining condition possibly attributable to inappropriate aldosterone secretion.
Methods and Results
This study was a secondary analysis of the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial. Patients with heart failure with preserved ejection fraction (HFpEF) with (n=1004) and without (n=2437) resistant hypertension were included. Resistant hypertension was defined as systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥80 mm Hg in a patient with hypertension, despite the concurrent use of a renin‐angiotensin system blocker (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker), a calcium channel blocker, and a diuretic; or as those patients using ≥4 classes of antihypertensive medication. The primary outcome was a composite of cardiovascular death, aborted cardiac arrest, or heart failure hospitalization. We analyzed hazard ratios (HRs) for outcomes with 95% CIs in the spironolactone group and compared them with the placebo group using Cox proportional hazard models. The risk of primary outcome events in patients with HFpEF with resistant hypertension was significantly lower in the spironolactone group than in the placebo group (HR, 0.70; 95% CI, 0.53–0.91; P=0.009), whereas the risk of primary outcome events in patients with HFpEF without resistant hypertension was not significantly different between the 2 groups (HR, 1.00; 95% CI, 0.83–1.20; P=0.97). There was a significant interaction between spironolactone use and resistant hypertension (P=0.03). Similar associations were also observed in patients with HFpEF from the Americas (United States, Canada, Brazil, and Argentina) only.
Conclusions
Spironolactone may be an effective add‐on medication for patients with HFpEF with resistant hypertension taking angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, calcium channel blockers, and diuretics.
Keywords: heart failure, heart failure with preserved ejection fraction, hypertension, spironolactone
Subject Categories: Hypertension, Heart Failure
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- PATHWAY‐2
Prevention and Treatment of Hypertension With Algorithm‐Based Therapy 2
- SPRINT
Systolic Blood Pressure Intervention Trial
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
Clinical Perspective
What Is New?
A recent study revealed that resistant hypertension is a salt‐retaining condition, most likely attributable to inappropriate aldosterone secretion.
Based on the pathophysiology of resistant hypertension, spironolactone use in patients with heart failure with preserved ejection fraction (HFpEF) with resistant hypertension, in addition to lowering blood pressure, may reduce the risk of volume overload, resulting in a reduced risk of heart failure and cardiovascular events.
The present study demonstrated that spironolactone use led to a decreased risk of composite cardiovascular events, all‐cause mortality, and heart failure hospitalization in patients with HFpEF with resistant hypertension, but this trend was not observed in those without resistant hypertension.
What Are the Clinical Implications?
Previous clinical trials involving patients with HFpEF have not produced favorable results, and guidelines for patients with HFpEF do not suggest specific medications.
Spironolactone use may be an effective add‐on medication for patients with HFpEF with resistant hypertension who are already taking angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, calcium‐channel blockers, and diuretics.
The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing; however, mortality in these patients remains unchanged. 1 Clinical trials involving patients with HFpEF have not produced favorable results, and guidelines for patients with HFpEF do not suggest specific medications. 2 , 3 , 4
Hypertension is highly prevalent in patients with HFpEF. 5 , 6 The SPRINT (Systolic Blood Pressure Intervention Trial) study revealed that intensive blood pressure treatment was associated with a decreased risk of cardiovascular events, particularly heart failure in high‐risk patients. However, patients with resistant hypertension are commonly encountered, 7 and blood pressure management in these patients is difficult. The PATHWAY‐2 (Prevention and Treatment of Hypertension With Algorithm‐Based Therapy 2) trial reported that spironolactone was the most effective add‐on medication for the treatment of resistant hypertension defined as above‐goal elevated blood pressure, despite concurrent use of an angiotensin‐converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB), a calcium‐channel blocker (CCB), and a diuretic. 8 The PATHWAY‐2 study also revealed that resistant hypertension is a salt‐retaining condition, most likely attributable to inappropriate aldosterone secretion. 9 Based on these clinical findings and the pathophysiology of resistant hypertension, spironolactone use in patients with HFpEF with resistant hypertension, in addition to lowering blood pressure, may reduce the risk of volume overload, resulting in a reduced risk of heart failure and cardiovascular events. This study aims to assess whether spironolactone use leads to improved cardiovascular outcomes in patients with HFpEF with resistant hypertension.
METHODS
The anonymized data from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) study have been made publicly available at the National Heart, Lung, and Blood Institute and can be accessed. 10
Study Design and Patients
We assessed the effects of spironolactone in patients with HFpEF with resistant hypertension using data from the TOPCAT study. 11 A detailed description of the study protocol, design, and patient characteristics has been reported previously. 6 , 11 , 12 Briefly, TOPCAT was an international, multicenter, randomized, double‐blind, placebo‐controlled trial. From August 10, 2006, to January 31, 2012, a total of 3445 patients were enrolled at 233 sites in 6 countries (the United States [n=1151], Russia [n=1066], Georgia [n=612], Canada [n=326], Brazil [n=167], and Argentina [n=123]). Participants were randomly assigned to receive spironolactone (n=1722) or placebo (n=1723). Patients aged ≥50 years were included if they had at least 1 symptom and 1 sign of heart failure from a prespecified list: a left ventricular ejection fraction ≥45% measured at the local site by echocardiography or radionuclide ventriculography, controlled systolic blood pressure (defined as <140 mm Hg, or ≤160 mm Hg if the patient were taking ≥3 antihypertensive medications), and a serum potassium level <5.0 mmol/L. Eligible patients had a history of heart failure hospitalization in the previous 12 months, or an elevated natriuretic peptide level in the 2 months before randomization (N‐terminal pro‐B‐type natriuretic peptide level ≥360 pg/mL or brain natriuretic peptide level ≥100 pg/mL). Patients were excluded if they had known infiltrative or hypertrophic obstructive cardiomyopathy or known pericardial constriction; severe renal dysfunction (defined as an estimated glomerular filtration rate <30 mL/min or serum creatinine level ≥2.5 mg/dL); known chronic hepatic disease (defined as aspartate aminotransferase and alanine aminotransferase levels >3.0 times the upper limit of the normal range); severe pulmonary disease, such as chronic pulmonary disease requiring home oxygen; severe systemic illness with life expectancy judged to be <3 years; or had undergone a heart transplant. 6 The TOPCAT study previously reported that spironolactone use did not significantly reduce the incidence of the primary composite outcome of cardiovascular death, aborted cardiac arrest, or heart failure hospitalization. 11 In our current study, we excluded patients with missing information regarding resistant hypertension (n=4), which resulted in the final sample size of 3441 patients. This study was approved by the Institutional Review Board of the National Center for Global Health and Medicine, while our use of TOPCAT data was approved by the National Heart, Lung, and Blood Institute. This study did not require informed consent of study participants.
Definition of Resistant Hypertension and Outcome Measurements
The definition of resistant hypertension was recently updated in the 2018 American Heart Association Scientific Statement, 13 which highlighted the significance of the use of 3 antihypertensive medications; an ACEI/ARB, a CCB, and a diuretic. On the basis of the recent blood pressure target and definitions of resistant hypertension in the 2018 American Heart Association Scientific Statement and the PATHWAY‐2 trial, we defined resistant hypertension as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg in a hypertensive patient despite the concurrent use of 3 recommended antihypertensive medication classes, which had to be an ACEI/ARB, a CCB, and a diuretic. 8 , 13 , 14 , 15 , 16 Patients with resistant hypertension also included patients with hypertension using ≥4 classes of antihypertensive medication. 13
The primary outcome was a composite of cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure; as was the main outcome of the TOPCAT study. 11 The secondary outcome was all‐cause death, hospitalization for heart failure, major cardiovascular events, fatal or nonfatal myocardial infarction, or fatal or nonfatal stroke. Major cardiovascular events were defined as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Cardiovascular and noncardiovascular mortality was also assessed. According to prespecified criteria, all events were adjudicated by a clinical end‐point committee at Brigham and Women's Hospital. 12 Patients were evaluated every 4 months during their first year in the study and every 6 months thereafter. More detailed information about outcome evaluation has previously been reported. 11
Statistical Analysis
Demographic data are presented as proportions (percentages) or means with SDs. In the TOPCAT study data, all patients >90 years of age were rounded down to 90 years old, with age presented as the median and interquartile range. Categorical variables were compared using chi‐squared tests, and continuous variables were compared using t tests. Patients were divided into 2 groups: those with and without resistant hypertension. Using the randomized design of the TOPCAT study, we used the Cox proportional hazards model to analyze the hazard ratios (HRs) for primary and secondary outcomes with 95% CIs, in the spironolactone group compared with the placebo group, separately in patients with and without resistant hypertension. In a previous post hoc analysis, large differences in baseline characteristics and outcomes between patients from the Americas (United States, Canada, Brazil, and Argentina) and Russia/Georgia were identified.17 Many patients from Russia/Georgia did not have clear evidence of HFpEF. Furthermore, it has been shown that many patients from this area did not take their study drug. Therefore, we verified all primary and secondary outcomes in patients from the Americas only. Kaplan–Meier survival curves were constructed for primary and secondary outcomes in the spironolactone group and placebo group, respectively. Additional analyses for primary and secondary outcomes were performed using the traditional definition of resistant hypertension (defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg in a hypertensive patient despite the concurrent use of an ACEI/ARB, a CCB, and a diuretic or as hypertension with ≥4 antihypertensive medication classes) considering the management of blood pressure at that time.8, 18, 19 Furthermore, additional analyses for primary and secondary outcomes were performed in patients with uncontrolled blood pressure despite the concurrent use of 3 recommended classes of antihypertensive medication (defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg despite the concurrent use of an ACEI/ARB, a CCB, and a diuretic).
The association between spironolactone use and primary outcome in patients with or without resistant hypertension was analyzed according to the following subgroups: age (<70 or ≥70 years); sex (male or female); New York Heart Association classification (I/II or III/IV); obesity (nonobese or obese); diabetes mellitus (no diabetes mellitus or diabetes mellitus); chronic kidney disease (estimated glomerular filtration rate <60 mL/min per 1.73 m2 or estimated glomerular filtration rate ≥60 mL/min per 1.73 m2); and cardiovascular disease (no history of cardiovascular disease or prior history of cardiovascular disease). Cardiovascular disease was defined as myocardial infarction, angina pectoris, treatment of percutaneous coronary intervention, coronary artery bypass graft surgery, stroke, or peripheral artery disease. In addition, we tested for interactions between the spironolactone use and these subgroups. Based on previous studies regarding the safety of spironolactone use, 11 , 20 the association between spironolactone use and clinically important adverse events such as hyperkalemia, breast tenderness/gynecomastia, and anaphylactoid reaction/intolerance were also assessed in patients with HFpEF with or without resistant hypertension.
All statistical analyses were conducted using Stata software (version 14.1, StataCorp, College Station, TX). P<0.05 was considered statistically significant for all tests.
RESULTS
Patient Characteristics and Blood Pressure Changes
In the present study, a total of 3146 (91.4%) patients with HFpEF had hypertension. Among those with hypertension, 1004 (31.9%) patients with HFpEF had resistant hypertension. Mean (±SD) systolic and diastolic blood pressure levels in patients with HFpEF with resistant hypertension were 134.2 (13.3) and 76.4 (11.2) mm Hg, respectively, while those in patients with HFpEF without resistant hypertension were 127.2 (13.7) and 75.5 (10.4) mm Hg, respectively. Table 1 displays the baseline characteristics of patients with HFpEF with and without resistant hypertension. In patients with HFpEF with resistant hypertension, the median age (interquartile range) of patients was 69 (61–76) years, and 51.9% were female. No significant difference was observed in in the baseline characteristics, including blood pressure level, between the spironolactone and placebo groups of patients with HFpEF with resistant hypertension. Similarly, the baseline characteristics of those without resistant hypertension did not significantly differ between the 2 groups.
Table 1.
Baseline Characteristics of Patients With HFpEF With or Without Resistant Hypertension*
| Resistant Hypertension (+) | Resistant Hypertension (−) | |||||
|---|---|---|---|---|---|---|
| Placebo | Spironolactone | P Value | Placebo | Spironolactone | P Value | |
| n=499 | n=505 | n=1221 | n=1216 | |||
| Age, y | ||||||
| Median (interquartile range) | 69 (61–75) | 69 (61–76) | 0.43 | 69 (61–76) | 69 (61–76) | 0.68 |
| Female sex, % | 50.9 | 52.9 | 0.53 | 51.8 | 51.1 | 0.73 |
| Race and ethnicity, % | 0.56 | 0.54 | ||||
| White | 83.3 | 84.7 | 91.2 | 89.9 | ||
| Black | 14.1 | 12.1 | 6.5 | 7.5 | ||
| Asian | 0.4 | 1.0 | 0.6 | 0.4 | ||
| Others | 2.2 | 2.2 | 1.7 | 2.2 | ||
| Region of enrollment, % | 0.71 | 0.91 | ||||
| United States | 38.3 | 34.5 | 31.7 | 32.7 | ||
| Russia | 24.1 | 26.1 | 34.1 | 32.6 | ||
| Georgia | 19.0 | 20.4 | 17.2 | 16.8 | ||
| Canada | 11.2 | 12.9 | 8.5 | 8.3 | ||
| Brazil | 5.6 | 4.8 | 4.3 | 5.0 | ||
| Argentina | 1.8 | 1.4 | 4.2 | 4.6 | ||
| Current smoking, % | 9.6 | 8.1 | 0.40 | 11.2 | 11.1 | 0.97 |
| Alcohol drinks/wk, % | 0.32 | 0.71 | ||||
| 0 | 78.9 | 79.8 | 76.6 | 78.2 | ||
| 1–5 | 16.1 | 17.2 | 17.8 | 16.1 | ||
| 6–10 | 3.4 | 2.4 | 3.9 | 4.1 | ||
| 11– | 1.6 | 0.6 | 1.7 | 1.6 | ||
| NYHA functional classification, % | 0.97 | 0.59 | ||||
| I/II | 64.9 | 65.0 | 68.3 | 67.3 | ||
| III/IV | 35.1 | 35.0 | 31.7 | 32.7 | ||
| Body mass index † , kg/m2, % | 0.98 | 0.42 | ||||
| <18.5 | 0.2 | 0.2 | 0.7 | 0.4 | ||
| 18.5–24.9 | 10.1 | 9.3 | 12.6 | 14.4 | ||
| 25.0–29.9 | 25.9 | 26.2 | 35.1 | 33.1 | ||
| ≥30.0 | 63.8 | 64.2 | 51.6 | 52.1 | ||
| Diabetes mellitus, % | 40.7 | 42.8 | 0.50 | 28.7 | 28.7 | 0.99 |
| Hypertension, % | 100 | 100 | … | 88.5 | 87.3 | 0.36 |
| Dyslipidemia, % | 69.5 | 70.3 | 0.79 | 68.0 | 65.1 | 0.12 |
| History of cardiovascular events, % | ||||||
| Myocardial infarction | 26.3 | 24.8 | 0.58 | 26.1 | 26.2 | 0.92 |
| Angina pectoris | 50.9 | 47.5 | 0.28 | 47.2 | 44.7 | 0.20 |
| Stroke | 9.4 | 9.7 | 0.87 | 7.4 | 6.5 | 0.39 |
| Peripheral arterial disease | 11.2 | 10.7 | 0.78 | 7.7 | 9.5 | 0.12 |
| Atrial fibrillation | 28.1 | 33.5 | 0.06 | 38.0 | 36.3 | 0.39 |
| Percutaneous coronary intervention | 14.6 | 15.5 | 0.71 | 14.7 | 14.0 | 0.62 |
| CABG surgery | 13.6 | 12.9 | 0.72 | 12.8 | 12.7 | 0.92 |
| Implanted cardioverter defibrillator | 1.0 | 0.6 | 0.46 | 1.2 | 1.7 | 0.30 |
| Pacemaker | 6.8 | 5.4 | 0.33 | 8.0 | 9.1 | 0.29 |
| COPD, % | 14.8 | 13.5 | 0.53 | 9.8 | 11.5 | 0.18 |
| Asthma, % | 8.4 | 7.3 | 0.52 | 6.0 | 5.8 | 0.88 |
| Medications, % | ||||||
| ACEIs/ARBs | 98.0 | 97.8 | 0.84 | 78.5 | 78.8 | 0.88 |
| Calcium‐channel blockers | 87.0 | 84.6 | 0.27 | 19.3 | 16.3 | 0.06 |
| Diuretics | 99.0 | 98.6 | 0.57 | 75.5 | 74.2 | 0.44 |
| Beta blockers | 77.4 | 76.0 | 0.62 | 77.3 | 79.1 | 0.28 |
| Other antihypertensive medications | 32.7 | 36.4 | 0.20 | 4.8 | 5.4 | 0.50 |
| Aspirin | 72.6 | 67.7 | 0.09 | 62.7 | 64.1 | 0.47 |
| Statin | 54.1 | 57.4 | 0.37 | 51.2 | 51.0 | 0.92 |
| Estimated GFR, mL/min per 1.73 m2 | 66.0 (19.2) | 66.0 (20.1) | 0.99 | 68.2 (20.6) | 68.5 (20.1) | 0.64 |
| Blood pressure | ||||||
| Systolic blood pressure, mm Hg | 135.0 (13.9) | 133.4 (12.6) | 0.06 | 127.1 (13.7) | 127.2 (13.8) | 0.93 |
| Diastolic blood pressure, mm Hg | 76.4 (11.7) | 76.3 (10.8) | 0.87 | 75.5 (10.5) | 75.6 (10.3) | 0.75 |
| <130/80 mm Hg, % | 19.5 | 19.8 | 0.89 | 33.6 | 34.1 | 0.80 |
| Heart rate (beats per minute) | 68.3 (10.3) | 67.5 (11.0) | 0.23 | 69.5 (10.1) | 69.5 (10.7) | 0.96 |
ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; and NYHA, New York Heart Association.
Data are presented as number of participants, percent, or mean (standard deviation).
Body mass index was calculated as weight in kilograms divided by the square of height in meters.
Figure S1 shows the systolic and diastolic blood pressure changes in patients with HFpEF with and without resistant hypertension. In patients with HFpEF with resistant hypertension, the mean systolic and diastolic blood pressure levels at 12 months after randomization were significantly lower in the spironolactone group compared with those in the placebo group (systolic blood pressure, 129.3 [15.1] versus 133.4 [16.9] mm Hg; P<0.001 and diastolic blood pressure: 73.8 [10.7] versus 76.1 [11.1] mm Hg; P=0.001 [Figure S1A and S1C]). Similarly, in patients with HFpEF without resistant hypertension, the mean systolic and diastolic blood pressure levels at 12 months after randomization were significantly lower in the spironolactone group (systolic blood pressure, 125.6 [15.8] versus 127.8 [15.4] mm Hg; P=0.001; and diastolic blood pressure, 74.0 [10.3] versus 75.5 [10.1] mm Hg; P<0.001 [Figure S1B and S1D]). The mean difference in systolic blood pressure between baseline and 12 months after using spironolactone was significantly larger in patients with HFpEF with resistant hypertension compared with those without resistant hypertension (−4.4 versus −1.8 mm Hg; P=0.006).
Primary and Secondary Outcomes in Patients With HFpEF With or Without Resistant Hypertension
In patients with HFpEF with or without resistant hypertension, the mean (SD) follow‐up periods were 3.1 (1.7) and 3.2 (1.7) years, respectively, and 210 and 461 patients had at least 1 confirmed primary outcome event, respectively. Kaplan–Meier survival curves and cumulative event rates for primary outcome events in patients with HFpEF with or without resistant hypertension are shown in Figure 1 and Table 2, respectively. In patients with HFpEF with resistant hypertension, primary outcome event rates (number of events per 1000 person‐years) in the spironolactone and placebo groups were 81.7 and 56.0, respectively. The risk of primary outcome events in patients with HFpEF with resistant hypertension was significantly lower in the spironolactone group than in the placebo group (HR, 0.70; 95% CI, 0.53–0.91; P=0.009 [Figure 1A]), whereas the risk of primary outcome events in patients with HFpEF without resistant hypertension was not significantly different between the 2 groups (HR, 1.00; 95% CI, 0.83–1.20; P=0.97 [Figure 1B]). There was a significant interaction between spironolactone use and resistant hypertension (P=0.03). Kaplan–Meier survival curves for all‐cause death and hospitalization for heart failure are shown in Figure 2. The risk of all‐cause death and hospitalization for heart failure in patients with HFpEF with resistant hypertension were significantly lower in the spironolactone group than in the placebo group (HR for all‐cause death, 0.64; 95% CI, 0.44–0.91; P=0.01 [Figure 2A]; and HR for heart failure hospitalization, 0.69; 95% CI, 0.51–0.94; P=0.01 [Figure 2B], respectively), whereas those risks in patients with HFpEF without resistant hypertension did not significantly differ between the 2 groups (HR for all‐cause death, 1.05; 95% CI, 0.86–1.27; P=0.63 [Figure 2C]; and HR for heart failure hospitalization, 0.91; 95% CI, 0.72–1.15; P=0.41 [Figure 2D], respectively). The risk of other outcome events in patients with or without resistant hypertension was not significantly different between the 2 groups (Table 2).
Figure 1. Kaplan–Meier survival curves for primary outcome in patients with HFpEF with or without resistant hypertension.
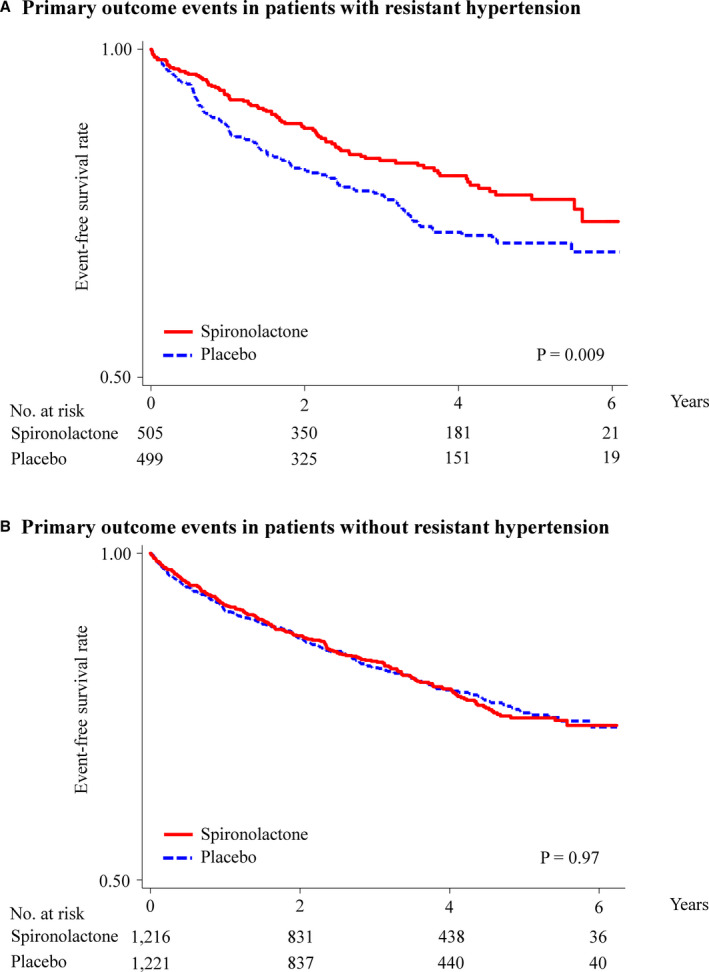
Rates of freedom from primary outcome events in patients with HFpEF with (A) and without (B) resistant hypertension. The primary outcome was a composite of cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure. HFpEF indicates heart failure with preserved ejection fraction.
Table 2.
Primary and Secondary Outcomes in Patients With HFpEF With or Without Resistant Hypertension*
| Resistant Hypertension (+) | Resistant Hypertension (−) | |||||
|---|---|---|---|---|---|---|
| Placebo | Spironolactone | P Value | Placebo | Spironolactone | P Value | |
| n=499 | n=505 | n=1221 | n=1216 | |||
| Event | ||||||
| Primary outcome events † | ||||||
| No. of patients | 120 | 90 | 231 | 230 | ||
| Event rate (per 1000 person‐years) | 81.7 | 56.0 | 59.9 | 59.8 | ||
| HR (95% CI) | 1.00 (ref) | 0.70 (0.53–0.91) ‡ | 0.009 ‡ | 1.00 (ref) | 1.00 (0.83–1.20) | 0.97 |
| All‐cause death | ||||||
| No. of patients | 74 | 49 | 199 | 207 | ||
| Event rate (per 1000 person‐years) | 44.4 | 28.2 | 48.4 | 50.7 | ||
| HR (95% CI) | 1.00 (ref) | 0.64 (0.44–0.91) ‡ | 0.01 ‡ | 1.00 (ref) | 1.05 (0.86–1.27) | 0.63 |
| Cardiovascular death | ||||||
| No. of patients | 44 | 31 | 132 | 129 | ||
| Event rate (per 1000 person‐year) | 26.4 | 17.9 | 32.1 | 31.6 | ||
| HR (95% CI) | 1.00 (ref) | 0.68 (0.43–1.07) | 0.09 | 1.00 (ref) | 0.99 (0.77–1.26) | 0.90 |
| Noncardiovascular death | ||||||
| No. of patients | 30 | 18 | 67 | 78 | ||
| Event rate (per 1000 person‐years) | 18.0 | 10.4 | 16.3 | 19.1 | ||
| HR (95% CI) | 1.00 (ref) | 0.58 (0.32–1.03) | 0.06 | 1.00 (ref) | 1.17 (0.84–1.62) | 0.34 |
| Hospitalization for heart failure | ||||||
| No. of patients | 97 | 72 | 148 | 134 | ||
| Event rate (per 1000 person‐years) | 65.8 | 44.8 | 38.3 | 34.9 | ||
| HR (95% CI) | 1.00 (ref) | 0.69 (0.51–0.94) ‡ | 0.01 ‡ | 1.00 (ref) | 0.91 (0.72–1.15) | 0.41 |
| Major cardiovascular events § | ||||||
| No. of patients | 70 | 58 | 194 | 183 | ||
| Event rate (per 1000 person‐years) | 43.6 | 34.4 | 48.8 | 45.9 | ||
| HR (95% CI) | 1.00 (ref) | 0.79 (0.56–1.12) | 0.19 | 1.00 (ref) | 0.94 (0.77–1.15) | 0.56 |
| Myocardial infarction | ||||||
| No. of patients | 19 | 20 | 45 | 45 | ||
| Event rate (per 1000 person‐years) | 11.7 | 11.8 | 11.2 | 11.3 | ||
| HR (95% CI) | 1.00 (ref) | 1.01 (0.54–1.09) | 0.96 | 1.00 (ref) | 1.00 (0.66–1.52) | 0.98 |
| Stroke | ||||||
| No. of patients | 14 | 17 | 46 | 40 | ||
| Event rate (per 1000 person‐years) | 8.6 | 10.0 | 11.4 | 9.9 | ||
| HR (95% CI) | 1.00 (ref) | 1.18 (0.58–2.39) | 0.64 | 1.00 (ref) | 0.87 (0.57–1.33) | 0.52 |
HFpEF indicates heart failure with preserved ejection fraction; and HR, hazard ratio.
Data are presented as number or HR (95% CI).
The primary outcome was a composite of cardiovascular death, aborted cardiac arrest, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for the management of heart failure.
Denotes significance.
Major cardiovascular events included cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Figure 2. Kaplan–Meier survival curves for all‐cause death and heart failure hospitalization in patients with HFpEF with or without resistant hypertension.
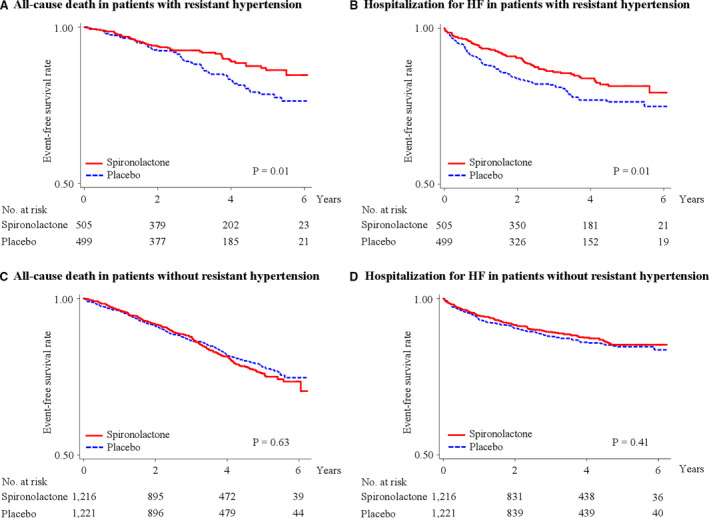
Rates of freedom from all‐cause death (A and C) and hospitalization for heart failure (B and D). HF indicates heart failure; and HFpEF, heart failure with preserved ejection fraction.
Kaplan–Meier survival curves for primary outcome events, all‐cause death, and hospitalization for heart failure in patients with HFpEF with or without traditional resistant hypertension, are shown in Figures S2 and S3. The risk of primary outcome events, all‐cause death, and hospitalization for heart failure in patients with HFpEF with traditional resistant hypertension were significantly lower in the spironolactone group than in the placebo group (HR for primary outcome events, 0.66; 95% CI, 0.50–0.88; P=0.004 [Figure S2A]; HR for all‐cause death, 0.56; 95% CI, 0.38–0.82; P=0.002 [Figure S3A]; and HR for heart failure hospitalization, 0.71; 95% CI, 0.38–0.82; P=0.002 [Figure S3B], respectively), whereas those risks were not significantly different between the 2 groups (HR for primary outcome events, 1.01; 95% CI, 0.84–1.21; P=0.90 [Figure S2B], HR for all‐cause death, 1.07; 95% CI, 0.88–1.30; P=0.47 [Figure S3C]; and HR for heart failure hospitalization, 0.89; 95% CI, 0.71–1.13; P=0.34 [Figure S3D], respectively). In addition, the risk of cardiovascular death and major cardiovascular events in patients with HFpEF with traditional resistant hypertension were significantly lower in the spironolactone group compared with the placebo group (HR for cardiovascular death, 0.53; 95% CI, 0.32–0.87; P=0.01; and HR for major cardiovascular events, 0.68; 95% CI, 0.47–0.99; P=0.04), whereas those risks in patients with HFpEF without traditional resistant hypertension were not significantly different between the 2 groups (Table S1).
Table S2 shows the HRs for primary and secondary outcomes in patients with HFpEF with uncontrolled blood pressure, despite the concurrent use of an ACEI/ARB, a CCB, and a diuretic. Similar to the results of patients with HFpEF with resistant hypertension, the risk of primary outcome events and hospitalization for heart failure was significantly lower in the spironolactone group compared with the placebo group (HR for primary outcome events, 0.68; 95% CI, 0.47–0.98; P=0.04; and HR for heart failure hospitalization, 0.62; 95% CI, 0.41–0.94; P=0.02, respectively).
Primary and Secondary Outcomes in Patients With HFpEF From the Americas
In patients with HFpEF from the Americas, Kaplan–Meier survival curves and cumulative event rates for primary and secondary outcomes are shown in Figures S4 and S5 and Table S3. In patients with resistant hypertension, the primary outcome event rates (number of events per 1000 person‐years) in the spironolactone and placebo groups were 102.4 and 156.0, respectively. The risk of primary outcome events in patients with HFpEF with resistant hypertension was significantly lower in the spironolactone group than in the placebo group (HR, 0.66; 95% CI, 0.48–0.89; P=0.006 [Figure S4A]), whereas the risk of primary outcome events in patients with HFpEF without resistant hypertension was not significantly different between the 2 groups (HR, 0.92; 95% CI, 0.74–1.13; P=0.50 [Figure S4B]). Kaplan–Meier survival curves for all‐cause death and hospitalization for heart failure in patients with HFpEF from the Americas are shown in Figure S5. The risks of all‐cause death and hospitalization for heart failure in patients with HFpEF with resistant hypertension were significantly lower in the spironolactone group than in the placebo group (HR for all‐cause death, 0.53; 95% CI, 0.35–0.80; P=0.002 [Figure S5A]; and HR for heart failure hospitalization, 0.72; 95% CI, 0.51–0.98; P=0.04 [Figure S5B], respectively), whereas the risks in patients with HFpEF without resistant hypertension did not significantly differ between the 2 groups (HR for all‐cause death, 1.00; 95% CI, 0.79–1.26; P=0.96 [Figure S5C]; and HR for heart failure hospitalization, 0.88; 95% CI, 0.68–1.13; P=0.30 [Figure S5D], respectively). Furthermore, the risks of cardiovascular death and major cardiovascular events in patients from the Americas with resistant hypertension were significantly lower in the spironolactone group than in the placebo group (Table S3). The risk of other outcome events in patients with or without resistant hypertension was not significantly different between the 2 groups.
Association Between Spironolactone Use and Primary Outcome Events in Various Subgroups With or Without Resistant Hypertension
Further analyses were performed to assess the HRs for primary outcome events in the spironolactone group compared with the placebo group in various subgroups, with or without resistant hypertension (Figure 3). The analyses showed that spironolactone use also tended to be better in each subgroup with resistant hypertension. There were no significant interactions between the use of spironolactone and age, sex, New York Heart Association classification, obesity, diabetes mellitus, chronic kidney disease, or history of cardiovascular disease. In addition, there were no significant interactions between the use of spironolactone and these subgroups in patients with HFpEF without resistant hypertension.
Figure 3. Primary outcome according to several subgroups with or without resistant hypertension.
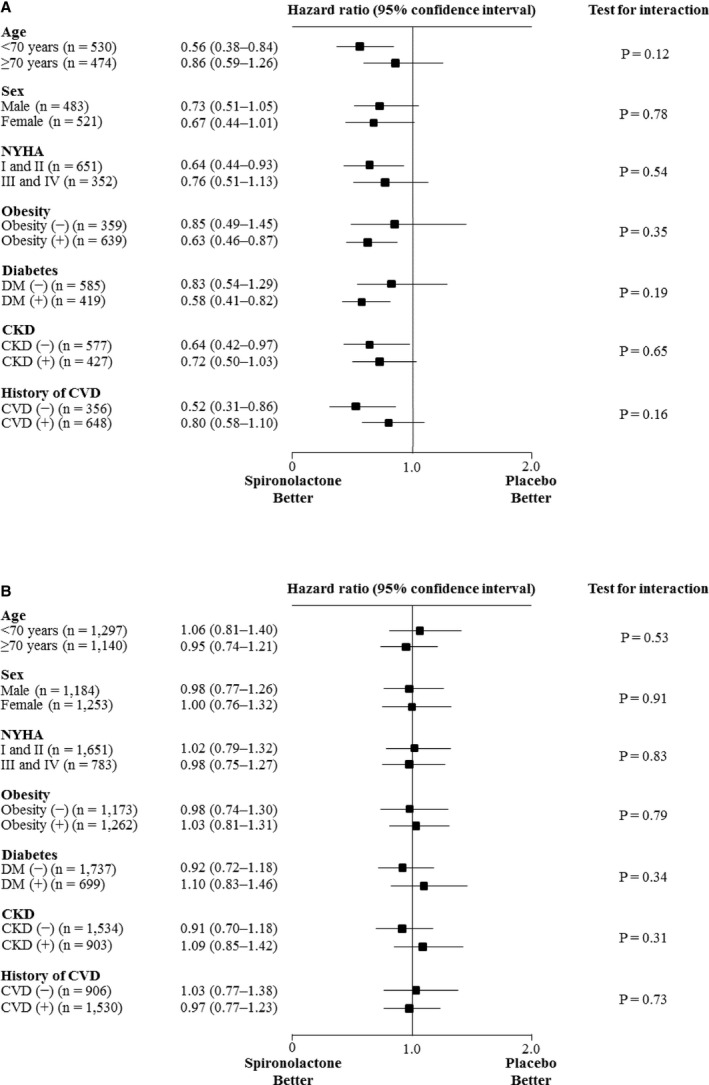
Hazard ratios for primary outcome in patients with HFpEF, with (A) and without (B) resistant hypertension. CVD was defined as myocardial infarction, angina pectoris, percutaneous coronary intervention, coronary artery bypass graft surgery, stroke, or peripheral artery disease. CKD indicates chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HFpEF, heart failure with preserved ejection fraction; and NYHA, New York Heart Association.
Spironolactone Use and Study Discontinuation in Patients With HFpEF With or Without Resistant Hypertension
In patients with HFpEF with resistant hypertension, the risk of serum potassium ≥5.5 mmol/L on the lowest spironolactone dose and serious hyperkalemia were significantly higher in the spironolactone group than in the placebo group (HR for serum potassium ≥5.5 mmol/L, 9.97; 95% CI, 3.57–27.88; P<0.001; and HR for serious hyperkalemia, 8.66; 95% CI, 2.00–37.50; P=0.003, respectively) (Table S4). The risk of breast tenderness or enlargement was also significantly higher in the spironolactone group than in the placebo group (HR, 9.15; 95% CI, 1.16–72.23; P<0.03). The risk of anaphylactoid reaction or intolerance did not significantly differ between the 2 groups. Similar associations were observed in patients with HFpEF without resistant hypertension.
DISCUSSION
The present study revealed that spironolactone use in patients with HFpEF with resistant hypertension was associated with a decreased risk of composite cardiovascular events, whereas spironolactone use in those without resistant hypertension did not decrease the composite cardiovascular events. There was a significant interaction between spironolactone use and resistant hypertension in patients with HFpEF. In addition, spironolactone use in patients with HFpEF with resistant hypertension led to a decreased risk of all‐cause death and hospitalization for heart failure. These findings were confirmed in patients with HFpEF from the Americas. This was an essential fact to support the results of the present study. Beneficial effects of spironolactone use were also observed in patients with HFpEF with traditional resistant hypertension or in those with uncontrolled blood pressure, despite the concurrent use of an ACEI/ARB, a CCB, and a diuretic. Similar associations between spironolactone use and a decreased risk of composite cardiovascular events were observed in the various subgroups with resistant hypertension. Although spironolactone use reduced blood pressure in patients both with and without resistant hypertension, a larger reduction of blood pressure was observed in patients with HFpEF with resistant hypertension.
Worldwide, the prevalence of hypertension is increasing, and patients with HFpEF are frequently complicated by hypertension. However, a large proportion of patients with hypertension fail to achieve their target blood pressure levels. 7 Resistant hypertension is a common clinical problem faced by many clinicians. In our current study, most of the patients with HFpEF had hypertension, with a high proportion of resistant hypertension. A secondary analysis of the SPRINT study suggested that intensive blood pressure treatment resulted in a decreased incidence of cardiovascular events and death even in patients with resistant hypertension, 21 although a low number of patients with resistant hypertension achieved their target blood pressure levels. A previous study, using part of the TOPCAT study data, reported similar benefits of spironolactone use in patients with HFpEF both with and without resistant hypertension (defined as systolic blood pressure between 140 and 160 mm Hg on ≥3 antihypertensive medications) 22 However, the present study showed that the beneficial effects of spironolactone were mainly observed in patients with HEpEF with resistant hypertension. The differences observed between our current study and the previous study may be attributable to using different definitions of resistant hypertension. In this study, we used the recent definition of resistant hypertension in the PATHWAY‐2 trial as a reference and focused on inappropriate aldosterone secretion. 8 , 13 , 14 , 15 , 16 Taking the salt‐retaining condition associated with inappropriate aldosterone secretion into consideration, 8 , 9 spironolactone use may be effective in reducing the risk of volume overload as well as lowering the blood pressure of patients with HFpEF with resistant hypertension who are taking an ACEI/ARB, a CCB, and a diuretic. Fluid management is very important in both HFpEF and resistant hypertension. In fact, this study demonstrated that spironolactone use in patients with HFpEF with resistant hypertension, but not in those without resistant hypertension, led to a significant decrease in all‐cause mortality and heart failure hospitalization. Thus, spironolactone use may be beneficial for patients with HFpEF with resistant hypertension, particularly in those with inappropriate aldosterone secretion. Further studies are necessary to investigate the association between spironolactone use and cardiovascular outcomes in patients with HFpEF. In our current study, we found that spironolactone use was associated with an increased risk of hyperkalemia and breast tenderness/enlargement. Spironolactone use requires closer laboratory monitoring and careful follow‐up of patients. 23 In addition, resistant hypertension can be caused by various factors including obstructive sleep apnea, several classes of pharmacologic agents such as nonsteroidal anti‐inflammatory agents, endocrine disorders such as primary aldosteronism, or a genetic predisposition. 13 Therefore, the cause of resistant hypertension should be carefully assessed before spironolactone is used.
This study, however, has several limitations. First, it was a secondary analysis of the TOPCAT study. Therefore, a randomized controlled trial would be required to confirm the results of this study, to evaluate if the use of spironolactone is beneficial and safe in patients with HFpEF with resistant hypertension. Second, the TOPCAT study included patients with HFpEF with controlled blood pressure. Thus, it remains unclear whether similar results would be observed in patients with HFpEF with uncontrolled high blood pressure. Third, we could not clarify the doses of antihypertensive medications such as ACEIs, ARBs, CCBs, and diuretics. In addition, this study did not include information regarding other antihypertensive medication classes other than ACEIs, ARBs, CCBs, diuretics, and beta blockers. Further investigation is needed to verify our findings using data with more detailed information, including the doses and classes of antihypertensive medications taken.
In conclusion, the results of our study demonstrated that spironolactone use led to a decreased risk of composite cardiovascular events, all‐cause mortality, and heart failure hospitalization in patients with HFpEF with resistant hypertension, but this trend was not observed in those without resistant hypertension. Spironolactone use may be an effective add‐on medication for patients with HFpEF with resistant hypertension who are already taking ACEIs/ARBs, CCBs, and diuretics.
Sources of Funding
The present study was supported by a Grants‐in‐Aid for Research from the National Center for Global Health and Medicine (Grant Number: 30‐1001) and a Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant Number: 18K16219). The study funders had no role in the study design; data collection, analysis, or interpretation; writing of the manuscript; or decision to submit the manuscript.
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S5
Acknowledgments
This manuscript was prepared using TOPCAT Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT or the National Heart, Lung, and Blood Institute.
Author contributions: Study concept and design: Dr Tsujimoto; data acquisition: Dr Tsujimoto; data analysis and interpretation: Drs Tsujimoto and Kajio; drafting the manuscript: Dr Tsujimoto; and statistical analysis: Dr Tsujimoto. Dr Tsujimoto had full access to all data in the study and is responsible for the integrity and accuracy of the data analysis.
(J Am Heart Assoc. 2020;9:e018827 DOI: 10.1161/JAHA.120.018827.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018827
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;251–259. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Writing Committee Members , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;e240–e327. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;776–803. [DOI] [PubMed] [Google Scholar]
- 5. Teo LY, Chan LL, Lam CS. Heart failure with preserved ejection fraction in hypertension. Curr Opin Cardiol. 2016;410–416. [DOI] [PubMed] [Google Scholar]
- 6. Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012;1594–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015;2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY‐2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biologic Specimen and Data Repositories Information Coordinating Center. National Heart, Lung, and Blood Institute; https://biolincc.nhlbi.nih.gov/studies/topcat/. Accessed October 14, 2020. [Google Scholar]
- 11. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;1383–1392. [DOI] [PubMed] [Google Scholar]
- 12. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;966–972.e910. [DOI] [PubMed] [Google Scholar]
- 13. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison‐Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR III, Polonsky T, Thompson‐Paul AM, Vupputuri S. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;e116–e135. [DOI] [PubMed] [Google Scholar]
- 15. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;1269–1324. [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;3021–3104. [DOI] [PubMed] [Google Scholar]
- 17. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;34–42. [DOI] [PubMed] [Google Scholar]
- 18. Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, et al. Treatment‐resistant hypertension and the incidence of cardiovascular disease and end‐stage renal disease: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;1012–1021. [DOI] [PubMed] [Google Scholar]
- 19. Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;653–658. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;709–717. [DOI] [PubMed] [Google Scholar]
- 21. Tsujimoto T, Kajio H. Intensive blood pressure treatment for resistant hypertension. Hypertension. 2019;415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossignol P, Claggett BL, Liu J, Vardeny O, Pitt B, Zannad F, Solomon S. Spironolactone and resistant hypertension in heart failure with preserved ejection fraction. Am J Hypertens. 2018;407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;543–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S5


