Abstract
Background
Severe conduction delay and inter/intra‐atrial dissociation may occur in patients who undergo an extensive catheter ablation or a maze procedure for atrial tachyarrhythmia. We report a series of patients with inter/intra‐atrial dissociation that mimicked complete atrioventricular block or ventricular tachycardia.
Methods and Results
We retrospectively reviewed the medical records of 7 patients who were referred for the evaluation of atrioventricular block (patients 1–6) or ventricular tachycardia (patient 7) that occurred after biatrial maze procedure and valvular surgery. During the electrophysiologic study, slow atrial or junctional escape rhythm dissociated from isolated atrial activity mimicked complete atrioventricular blocks. Intra‐atrial dissociation of the right atrium or left atrium was observed. Atrioventricular nodal conduction from the nondissociated atrium to the ventricle was preserved in all patients, while the conduction from the dissociated atrium was blocked. In patient 7, the pacing of the ventricle by tracking of atrial tachycardia from the nondissociated left atrium/coronary sinus mimicked ventricular tachycardia during pacemaker interrogation. A total of 5 patients received new permanent pacemaker implantations during the index hospitalization for the surgery (n=2) or as a deferred procedure (n=3) according to the treatment for sick sinus syndrome.
Conclusions
Pseudo‐atrioventricular block or pseudo‐ventricular tachycardia may occur because of inter/intra‐atrial dissociation after a maze procedure. The selection of patients for permanent pacemaker implantation should be determined based on the patient’s symptoms and the status of the escape pacemaker and not on the apparent atrioventricular block. Proper diagnosis is important to avoid unnecessary implantation of a pacemaker or a defibrillator.
Keywords: AV block, intra‐atrial dissociation, maze, pacemaker
Subject Categories: Arrhythmias, Atrial Fibrillation, Electrophysiology
Nonstandard Abbreviations and Acronyms
- CS
coronary sinus
- HRA
high right atrium
- PPM
permanent pacemaker
- RA
right atrium
Clinical Perspective
What Is New?
Inter/intra‐atrial dissociation may occur in patients who undergo a maze procedure and a valvular surgery.
Inter/intra‐atrial dissociation may mimic complete/paroxysmal atrioventricular block in the ECG, or masquerade as ventricular tachycardia during pacemaker interrogation.
What Are the Clinical Implications?
The possibility of a pseudo‐atrioventricular block or pseudo‐ventricular tachycardia should be considered in patients with atrial dissociations occurring after a maze procedure.
Unnecessary implantation of a pacemaker or a defibrillator may be avoided by careful analysis of electrophysiologic studies.
Electrophysiologic studies may also aid in choosing the optimal atrial lead position to reduce unnecessary ventricular pacing.
Maze procedures are often performed concomitantly during open‐heart surgeries to control atrial fibrillation (AF). 1 , 2 However, surgical lesions create conduction delays or blocks in the atrium, and the interpretation of postmaze complex arrhythmias by surface ECG alone is often challenging because of the reduced P‐wave amplitudes and the complex interplay between dissociated atrial activity. 3 , 4 We report a series of patients who showed atrial dissociations that mimicked complete atrioventricular blocks in the surface ECGs, and atrial tachycardia that mimicked ventricular tachycardia (VT) in pacemaker interrogation.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author at gbnam@amc.seoul.kr.
Patient Characteristics
Patients consulted for the evaluation of atrioventricular block after the maze procedure were recommended to undergo an electrophysiologic study if they showed unusual ECG findings, which included highly fluctuating ventricular rate or dual P waves in the presence of an atrioventricular block, regular slow escape–echo bigeminy during AF, or new‐onset paroxysmal atrioventricular block. Inter/intra‐atrial dissociation was defined as spontaneously dissociated electrograms and conduction block between the isolated atrial portion and the remaining nonisolated atrium, which was confirmed by differential pacing maneuvers at multiple atrial sites. The institutional review board of Asan Medical Center approved this retrospective study and waived the requirement for informed consent (approval number: 2020–0634).
Electrophysiology Study
Patients were brought to the electrophysiology laboratory in the fasting and alert state. The femoral vein was percutaneously cannulated under local anesthesia with 1.0% lidocaine. Fentanyl and midazolam were used for analgesia and sedation. A 6‐Fr quadripolar electrode catheter was positioned at the His bundle region and a 7‐Fr deflectable duodecapolar catheter was advanced into the coronary sinus (CS) and placed in the right atrium (RA). In 1 patient with a suspected VT on pacemaker interrogation, programmed electrical stimulation was performed in the right ventricular apex and outflow tract using up to triple extrastimuli under isoproterenol infusion. Multiple electrocardiographic leads (lead I, aVF, and V1, filtered between 0.05 and 100 Hz) and intracardiac bipolar electrograms (filtered between 30 and 500 Hz) were simultaneously displayed and recorded on a digital electrophysiologic recording system (CardioLab, Prucka, GE Healthcare, WI). The stimuli were delivered by a programmable digital stimulator (Bloom DTU 215B, Fischer Medical Technologies, CO) at twice the diastolic threshold and a pulse width of 2 ms.
Results
Study Population
The study population consisted of 7 patients with inter/intra‐atrial dissociation and pseudo‐atrioventricular conduction block or pseudo‐VT. The clinical and demographic characteristics of the patients are summarized in Table 1. The mean age of the patients was 56±12 years, and 3 (42.9%) patients were male. All patients underwent biatrial maze procedures, and 4 patients (patients 2, 3, 4, 6) underwent mitral valve repair/replacement with or without aortic valve surgery as a result of rheumatic valvular heart disease. Two patients underwent valvular repairs because of mitral regurgitation with chordae rupture (patient 1) or aortic regurgitation with annuloaortic ectasia (patient 5). One patient (patient 7) with a previous correction of tetralogy of Fallot and an atrial septal defect underwent mitral valve replacement and maze procedure because of mitral regurgitation and AF, and received a permanent pacemaker (PPM) implantation on postoperative day 9 for a third‐degree atrioventricular block.
Table 1.
Baseline Demographic and Echocardiographic Characteristics of the Patients
| Patients | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Sex | Male | Female | Female | Female | Male | Female | Male |
| Age, y | 53 | 60 | 55 | 73 | 64 | 46 | 38 |
| AF | LsPeAF | PAF | LsPeAF | PAF | PeAF | LsPeAF | PAF |
| VHD | MR with chordae rupture | Rheumatic | Rheumatic | Rheumatic | AR with AAE | Rheumatic | AR, MR, PR, TR |
| Surgery | MVP, TAP | MVR, TVP | MVR, TAP | AVR, MVR, TVP | AVP, MVP | AVR, MVR, TAP* | AVR, MVR, PVR, TAP † |
| Maze | Biatrial | Biatrial | Biatrial | Biatrial | Biatrial | Biatrial | Biatrial |
| Prior ablation | No | No | No | No | No | No | CTI |
| Prior CIED | No | No | No | No | No | No | PPM |
| LV EF, % | 56 | 73 | 60 | 74 | 57 | 54 | 48 |
| LA size, mm | 53 | 56 | 43 | 54 | 42 | 59 | 58 |
AAE indicates annuloaortic ectasia; AF, atrial fibrillation; AR, aortic regurgitation; ASD, atrial septal defect; AVP, aortic valvuloplasty; AVR, aortic valve replacement; CIED, cardiovascular implantable electronic device; CTI, cavotricuspid isthmus; LA, left atrium; LsPeAF, long‐standing persistent atrial fibrillation; LV EF, left ventricular ejection fraction; MR, mitral regurgitation; MVP, mitral valvuloplasty; MVR, mitral valve replacement; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; PPM, permanent pacemaker; PR, pulmonic regurgitation; PVR, pulmonic valve replacement; TAP, tricuspid annuloplasty; TOF, tetralogy of Fallot; TR, tricuspid regurgitation; TVP, tricuspid valvuloplasty; and VHD, valvular heart disease.
Patient 6 had undergone percutaneous mitral balloon valvotomy twice.
Patient 7 had undergone TOF correction and ASD closure.
Electrocardiographic Findings
Table 2 summarizes the electrocardiographic and electrophysiological findings of the study subjects. Among the 7 patients, 6 (all but patient 6) showed apparent complete atrioventricular blocks with small but distinct P waves dissociated from the QRS complexes (Figures 1A, 2A, and Figures S1A‐D). In patient 1, junctional rhythm during complete atrioventricular block intermittently accelerated at heart rates faster than 70 to 80 beats per minute. In patient 2, AF with slow bigeminal junctional rhythm (Figure 2B) alternating with a rapid ventricular response (Figure 2C) was documented. In patients 3 and 6, paroxysmal atrioventricular blocks with nonconducted P waves were documented during Holter monitoring, and these findings were correlated with their symptoms such as dizziness or presyncope (Figure 3A and Figure S1F). Patient 6 showed 2 distinct, dissociated P waves: 1 had a lower amplitude and was associated with the QRS complex, and the other had a higher amplitude and was dissociated from the QRS complex (Figure S1E). Patient 7 was admitted for the evaluation of an apparent VT (rapid sensed ventricular activity dissociated from slow atrial activity), which was documented during pacemaker interrogation.
Table 2.
Electrocardiography and Electrophysiologic Study
| Patients | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Heart rate, bpm | 50 | 50 | 50 | 60 | 60 | 60 | 40 |
| QRS duration, ms | 100 | 130 | 100 | 90 | 100 | 130 | 140 |
| QRS morphology | NL | RBBB | NL | NL | NL | RBBB | RBBB |
| EPS indication | CAVB | CAVB | CAVB | CAVB | CAVB | PAVB | VT |
| Time interval* | 2.5 m | 12 d | 10 d | 9 d | 18 d | 6.5 m | 7 m |
| RR interval, ms | 1546 | 1570 | 1284 | 1096 | 990 | 948 | 1490 |
| HV interval, ms | 44 | 44 | 38 | 44 | 40 | 46 | 40 |
| Atrioventricular block | – | – | – | – | – | – | – |
| AVN BCL, ms | 400 | 300 | 340 | N/A | 380 | 440 | N/A |
| AVN ERP, ms | 260 | 200 | N/A | N/A | 300 | 290 | 220 |
AVN BCL indicates atrioventricular nodal block cycle length; AVN ERP, atrioventricular nodal effective refractory period; CAVB, complete atrioventricular block; d, days; EPS, electrophysiologic study; HV, His to ventricular; m, months; N/A, not available; NL, normal; PAVB, paroxysmal atrioventricular block; RBBB, right bundle branch block; and VT, ventricular tachycardia.
Time interval between maze procedure and electrophysiologic study.
Figure 1. Recordings from patient 1.
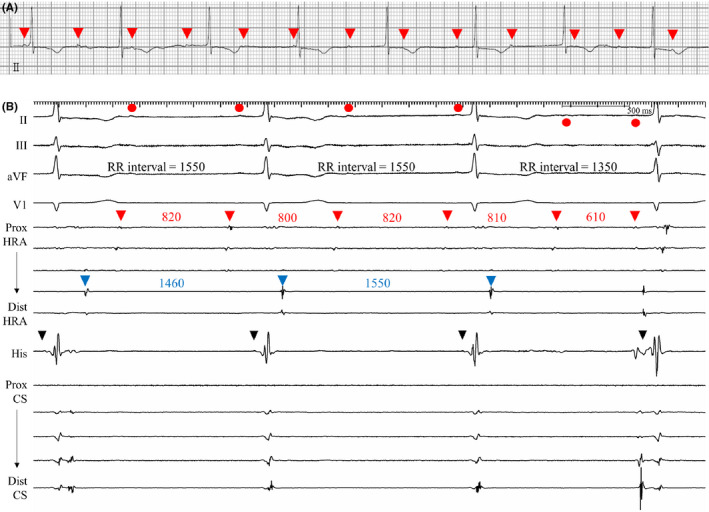
A, Electrocardiographic rhythm strip showing small P waves (red arrowheads) dissociated from the QRS complex, suggesting complete atrioventricular block. B, In the electrophysiologic study, atrial electrograms of the proximal HRA electrodes (posterolateral wall of RA, red arrowheads) were dissociated from the electrograms of the distal HRA (RA free wall, blue arrowheads), His (RA septum), and CS electrodes, indicating dissociation of posterolateral wall of the RA. His (black arrowhead)‐to‐V interval was within the normal range (HV interval=38 ms). P waves on the surface leads (red circles) were consistent with the dissociated atrial electrograms of the posterolateral RA wall. Junctional escape rhythm conducted both to the septal RA and to the ventricle. CS indicates coronary sinus; HRA, high right atrium; HV, His to ventricular; and RA, right atrium.
Figure 2. Recordings from patient 2.
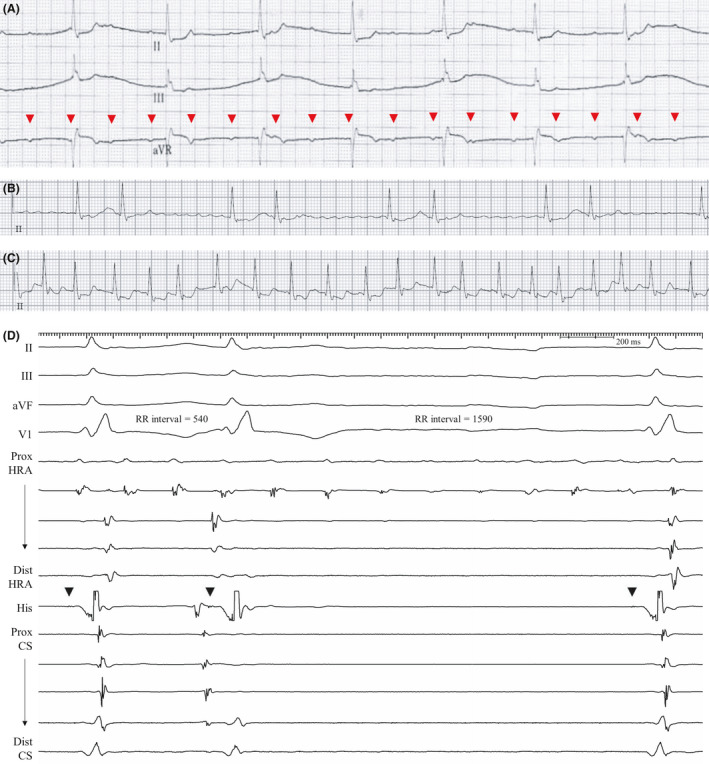
Complete atrioventricular block (A), AF with slow bigeminal ventricular response (B), and rapid ventricular response (C) were observed. Red arrowheads in Figure 2A indicate dissociated P waves. In the electrophysiologic study, AF rhythm was observed at the proximal HRA electrodes located at the posterolateral RA wall. D, Junctional escape rhythm with possible escape‐echo bigeminy was recorded as in Figure 2B. The HV interval was 44 ms (His signal is marked with black arrowheads). AF indicates atrial fibrillation; CS, coronary sinus; HRA, high right atrium; HV, His to ventricular; and RA, right atrium.
Figure 3. Recordings from patient 3.
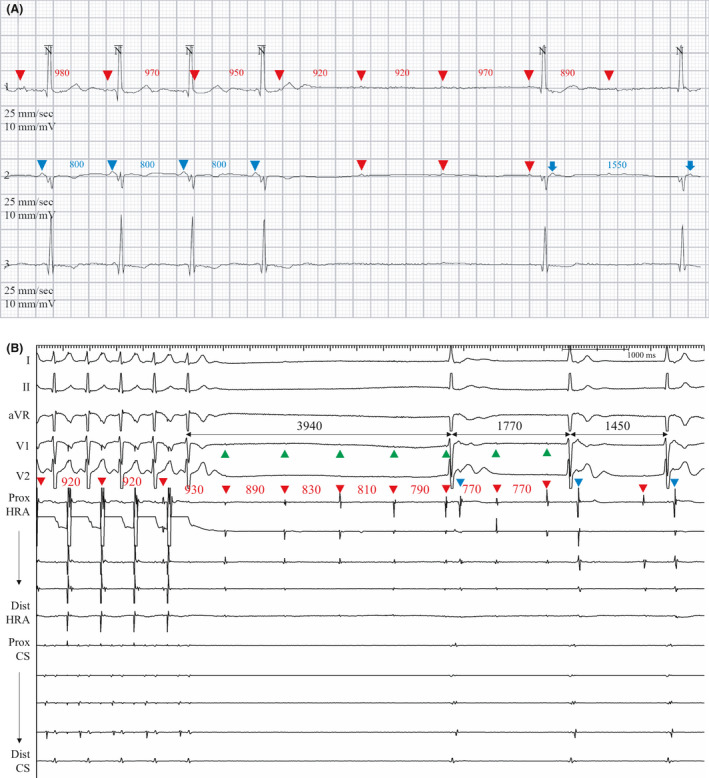
A, Holter monitoring that mimicked sinus pause or paroxysmal atrioventricular block with nonconducted P waves (red arrowheads) and RR intervals of 3.18 s. The nonconducted P waves (red arrowheads) during the apparent atrioventricular block period represent depolarization from the dissociated atrium. The initial 4 conducted P waves (blue arrowheads) with a shorter cycle length (800 ms) represent atrial depolarization from the nondissociated atrium with 1:1 atrioventricular conduction. The dissociated P waves continued with a cycle length of ≈900 ms, regardless of the presence of atrial activity from the nondissociated atrium, although it is quite difficult to distinguish the dissociated P waves because of the very low amplitude. After the apparent atrioventricular block, junctional escape beats with retrogradely conducted P waves (blue arrows) were observed. Therefore, the pause (loss of firing) of the nondissociated atrium and the continuation of the independent but dissociated atrium seem to have mimicked a paroxysmal atrioventricular block. B, After termination of pacing at the RA posterior wall (PCL 500 ms), suppression of atrial activity at the CS electrodes was followed by junctional escape rhythm. During the pause, dissociated atrial electrograms of the posterolateral RA wall (red arrowheads) masqueraded as complete atrioventricular block in the surface ECG. Green arrowheads indicate dissociated P waves at the I, V1, and V2 leads. During the junctional escape, retrogradely conducted atrial activities (blue arrowheads) were observed at the proximal HRA electrodes, suggesting that the proximal HRA electrodes were located at the line of conduction block where double potentials were recorded. The capture of the nondissociated RA (RA posterior wall) resulted in transient overdrive suppression and ventricular pause while the dissociated activity from the posterolateral RA wall continued throughout the tracing. CS indicates coronary sinus; HRA, high right atrium; PCL, pacing cycle length; and RA, right atrium.
Electrophysiology Study
Before proceeding to pacemaker implantation, 6 patients (patients 1–6) underwent electrophysiology studies despite apparent atrioventricular dissociation considering the unusual ECG findings such as accelerated or highly fluctuating junctional rhythm, intermittent AF with a rapid ventricular response, or bigeminal junctional rhythm. In patient 7, the electrophysiology study was performed as a work‐up for VT observed during the pacemaker interrogation.
In 4 patients (patients 1–4), the RA posterior wall or the sinus venosa was dissociated from the RA free wall, RA septum, and CS. Atrial pacing from the CS or RA septum showed preserved atrioventricular conduction with a mean cycle length of 347±50 ms. Specifically, the atrial electrograms recorded from the posterolateral RA or sinus venosa (where proximal high right atrium [HRA] electrodes were located) were dissociated from the electrograms of the RA septum, RA free wall (where distal HRA electrodes were located), and CS electrodes, indicating intra‐RA dissociation between the posterolateral wall and the rest of the RA (septal and free‐wall RA) (patient 1; Figure 1B and Figures S2A‐C).
In patient 2, the baseline intracardiac electrograms showed localized AF at the posterolateral RA and simultaneous junctional escape or atrial rhythm in the His (Figure 2D) or CS electrodes. After cardioversion, the posterolateral RA was electrically dissociated from the septal and free wall of RA and the CS as in patient 1 (Figures S3A‐C). Based on the above findings, we inferred that the surface ECG findings had resulted from the junctional escape rhythm dissociated from the RA activity (Figure 2A). AF localized at the dissociated RA was simultaneously recorded with junctional escape beats or escape echo bigeminy (Figure 2B), and AF localized at nonisolated RA (septum) and left atrium (LA)/CS resulted in a rapid ventricular response via atrioventricular nodal conduction (Figure 2C and Figure S3D).
In patient 3 in whom paroxysmal atrioventricular block was observed during Holter monitoring (Figure 3A), intra‐RA dissociation of a similar pattern was observed during the electrophysiology study (Figures S4A‐C). When a rapid atrial pacing was performed at the RA posterior wall, a pause (3.9 s) with dissociated RA potentials was observed (Figure 3B), which was caused by the absence of ectopic escape beat from the nondissociated atrium and atrioventricular junction (overdrive suppression, compatible with tachycardia–bradycardia syndrome), thereby suggesting the possibility of poor ectopic pacemaker function in the nondissociated atrium. This may explain the sudden loss of escape ventricular beat in the presence of continuing right atrial activity during paroxysmal atrioventricular block (Figure 3A).
Likewise, in patient 4, the posterolateral RA was dissociated from the RA septum and CS. Atrioventricular conduction from the LA/CS to the ventricle was preserved, while the same was blocked from the posterolateral RA (Figures S5A–C).
A similar pattern of dissociation of atrial activity in the HRA electrodes was also observed in patient 5. However, unlike the other cases, the greater parts of the RA and LA were dissociated from the septal wall, and only the RA septum and proximal CS showed preserved atrioventricular conduction (Figure 4). The RA free wall and the LA were electrically coupled via the Bachmann’s bundle (Figures S6A through 6E).
Figure 4. Recordings from patient 5.
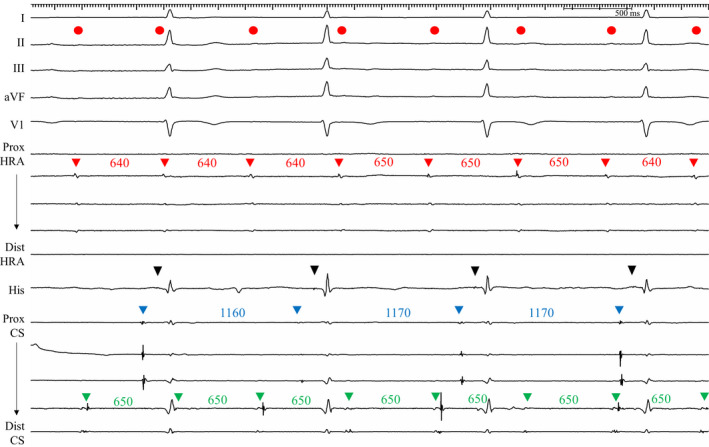
Baseline intracardiac electrograms showed that atrial electrograms of the HRA electrodes (red arrowheads) were dissociated from the electrograms of the His and proximal CS electrodes (blue arrowheads). P waves on the surface ECG leads (red circles) coincided with the dissociated RA (red arrowheads) and LA (distal CS; green arrowheads) electrograms. The RA and LA activities were coupled at an interval of 100 ms, most likely via the Bachmann’s bundle, and the atrial activity from the proximal CS (blue arrowheads) conducted to the ventricle. His (black arrowheads) to the ventricular interval was within the normal range (HV interval=40 ms). CS indicates coronary sinus; HRA, high right atrium; HV, His to ventricular; LA; left atrium; and RA, right atrium.
In patient 6, the atrial activity from the posterolateral RA (lower P‐wave amplitude) normally propagated to the RA septum, proximal CS, and the ventricle. However, dissociated electrograms were observed in the RA free wall, posterior part of RA septum (split electrograms of the proximal HRA and His electrodes), and the distal CS with a shorter cycle length that coincided with the P waves of higher amplitudes on the surface ECG (Figure 5A). The atrial activity of the posterolateral RA was easily suppressed by rapid atrial pacing at the proximal HRA, and the postpacing pause of escape beat was longer than 12 s. During the suppression of atrial escape, the dissociated atrial rhythm was not affected, and pseudoparoxysmal atrioventricular block was observed (Figure 5B).
Figure 5. Recordings from patient 6.
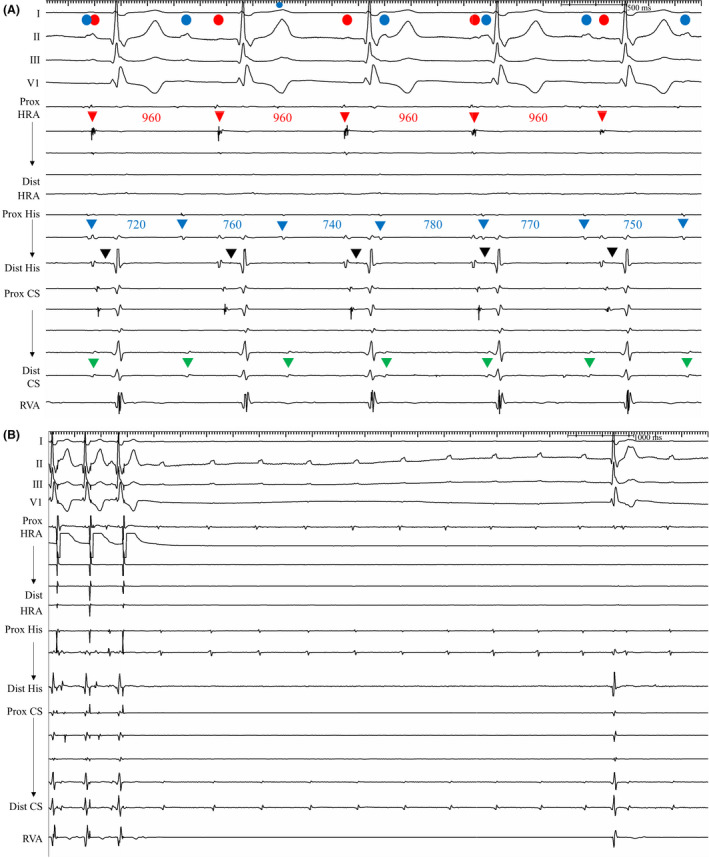
A, Baseline rhythm strip showed dual P waves. Atrial activity from the PL RA (red circle and arrowheads, cycle length=960 ms) coincided with the smaller P wave and was conducted to the ventricle. His (black arrowheads)‐to‐V interval was within the normal range (HV interval=44 ms). The larger P waves in the ECG (blue circles) coincided with the dissociated atrial electrograms, which were observed at the RA FW, posterior part of RA septum (split potentials of the proximal HRA and proximal His electrodes), and distal CS (green arrowheads) with a shorter cycle length (720‐780 ms). B, Atrial pacing at proximal HRA at PCL 600 ms was carried out for 1 minute, and postpacing pause of escape atrial beat was longer than 12 s. During the pause, dissociated atrial rhythm was observed at the distal CS, proximal His, and proximal HRA electrodes. This was consistent with the pseudo‐paroxysmal atrioventricular block documented in the Holter monitoring (Figure S1F). CS indicates coronary sinus; FW, free wall; HRA, high right atrium; HV, His to ventricular; PCL, pacing cycle length; PL, posterolateral; RA, right atrium; and RVA, right ventricular apex.
Patient 7 underwent an electrophysiology study for the evaluation of VT detected during the pacemaker interrogation (Figure 6A and 6B). During the electrophysiology study, the entire RA except for the mid‐/posteroseptum was dissociated from the LA/CS and the ventricle (Figures S7A‐J). VT was not inducible during the stimulation test with or without isoproterenol infusion. Atrial dissociation was observed between the RA and the LA/CS. Atrial activity from the RA did not conduct to the ventricle, while electrical conduction from the LA/CS to the ventricle was preserved. Spontaneous atrial tachycardia from the LA/CS resulted in rapid ventricular response with the right bundle branch block morphology during isoproterenol infusion (Figure 6C). The isolated RA activity dissociated from the LA/CS and the ventricle mimicked complete atrioventricular block, while atrial tachycardia from the LA conducted rapidly to the ventricle and mimicked VT.
Figure 6. Recordings from patient 7.
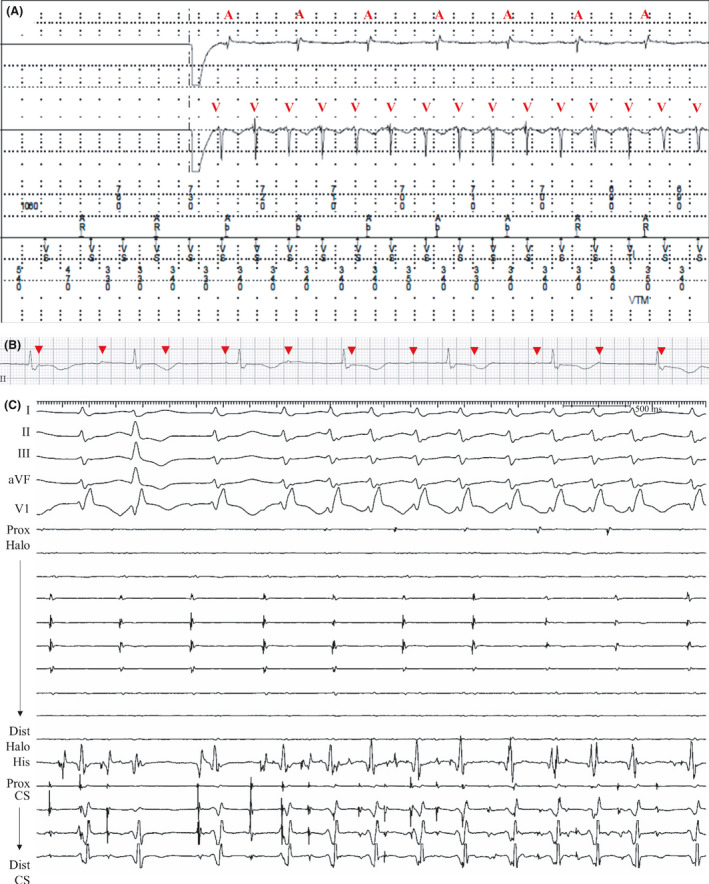
A, During the interrogation of a permanent pacemaker, a tachycardia with an atrioventricular dissociation was recorded. Red symbols of A and V indicate electrogram of atrium and ventricle, respectively. B, Baseline rhythm strips after pacing‐off showed dissociated P waves (red arrowheads) and atrioventricular dissociation, suggesting complete atrioventricular block. C, Intracardiac electrograms showed atrial dissociation between the Halo (placed at the RA) and the CS electrodes. Under isoproterenol infusion (10 µg/min), an irregular atrial tachycardia localized at the CS electrodes spontaneously occurred and rapidly conducted to the ventricle. This atrial tachycardia with rapid ventricular response showed a wide QRS complex with RBBB aberrancy, and reproduced the VA dissociation recorded during pacemaker interrogation. Electrical activity of the Halo electrodes represented the atrial electrograms recorded at the atrial leads of the pacemaker. aVF indicates augmented vector foot; CS coronary sinus; RA, right atrium; RBBB, right bundle branch block; and VA, ventriculoatrial.
Figure 7 shows the schematic view of the inter/intra‐atrial dissociation and atrioventricular dissociation by pacing at different RA regions during the electrophysiology study. To summarize the electrophysiology findings, 6 patients (patients 1–5 and 7) showed that the posterolateral RA wall was dissociated from the RA septum and CS, and there was a small difference in the range of dissociated atrial regions among the patients. Patient 7 showed a relatively broader area of dissociated RA, and the intra‐RA dissociation of the lateral wall with preserved automatic sinoatrial activity was commonly observed in all 7 patients. Differential pacing maneuvers were performed at multiple sites of the RA and CS in all patients as described in the Supplemental Figures.
Figure 7. Schematic diagrams showing the bi‐atrium and patterns of intra‐ and interatrial dissociation.
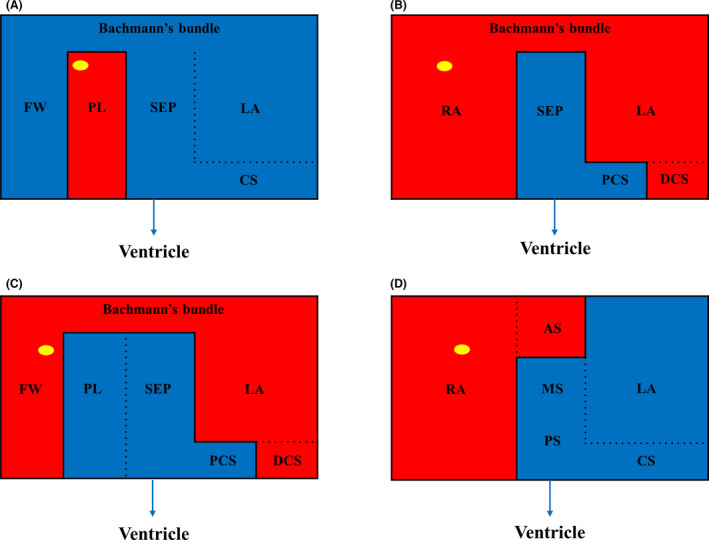
A, Pattern in patients 1‐4. The PL wall of the RA was dissociated from the RA FW, SEP, and CS/LA. At the PL wall of RA, dissociated atrial activity (marked with yellow circle) was observed. Atrioventricular nodal conduction from the nondissociated atrium to ventricle was preserved. Conduction between RA FW and septum, LA/CS was preserved via the BB. B, Pattern in patient 5. Septal RA and the PCS were dissociated from the rest of the atria, and atrioventricular conduction from the SEP and PCS to ventricle was preserved. Conduction between the PCS and DCS was blocked. Interatrial conduction between RA and LA/DCS via BB was preserved. Dissociated atrial activity (yellow circle) was observed at the RA. C, Pattern in patient 6. The PL RA, SEP, and PCS were dissociated from the rest of atria, and atrioventricular conduction from RA PL, SEP, and PCS to ventricle was preserved. Interatrial conduction between RA FW and LA/DCS via BB was preserved and dissociated atrial activity (yellow circle) was observed at the RA FW. Conduction between the PCS and DCS was blocked. D, Pattern in patient 7. The whole RA except the mid‐to‐posteroseptum was dissociated from the LA/CS. Dissociated atrial activity (yellow circle) was observed at the RA. Atrioventricular conduction from the SEP and CS to the ventricle was preserved. AS indicates anteroseptum; BB, Bachmann’s bundle; CS, coronary sinus; DCS, distal coronary sinus; FW, free wall; LA, left atrium; MS, midseptum; PCS, proximal coronary sinus; PL, posterolateral; PS, posteroseptum; RA, right atrium; and SEP, septum.
Management
Patient 1 underwent PPM implants for symptomatic bradycardia and improvement of quality of life. By referring to the electrophysiology study findings, we fixed the tip of the atrial lead to the septal RA posterior to the CS ostium, where the 1:1 atrioventricular conduction was observed at a pacing cycle length of 400 ms (Figure S2D). Patient 2 underwent a PPM implant for medical treatment of symptomatic AF with rapid ventricular response combined with tachycardia–bradycardia syndrome. Electrophysiology studies revealed that the apparent paroxysmal atrioventricular blocks in patient 3 and patient 6 were the result of intra‐atrial dissociation. In patient 6, a PPM was implanted immediately after the electrophysiology study according to the treatment of sick sinus syndrome. In patient 3, PPM implantation was initially deferred and was carried out at 40 months after the electrophysiology study because of recurrent fainting or syncope combined with more prolonged pauses (during apparent paroxysmal atrioventricular block). In patient 5, the PPM implant was initially deferred because of the lack of symptoms after the electrophysiology study; however, apparent paroxysmal atrioventricular block (15 s of longest RR intervals) correlated with fainting symptoms (Figure S8) was later documented, and a PPM was implanted. In patient 4, we have been deferring the PPM implantation for 4 years after the electrophysiology study, considering the patient’s asymptomatic state on stable junctional rhythm.
Discussion
We retrospectively reviewed the medical records of 7 patients who were referred for the evaluation of atrioventricular block (patients 1–6) or VT (patient 7) that occurred after biatrial maze procedure and valvular surgery. The major findings of our study are that (1) apparent atrioventricular block after a maze procedure may occur as a result of inter/intra‐atrial dissociation, and (2) supraventricular tachycardia originating from the nondissociated atrium could masquerade as VT during pacemaker interrogation.
Postoperative complete atrioventricular block is an important indication for pacemaker implantation. Our results suggest that surface ECG diagnosis of complete atrioventricular blocks in patients with maze procedure may not always indicate conduction system abnormalities. Electrocardiographic findings that were not compatible with ordinary complete atrioventricular blocks included accelerated and highly variable junctional rhythm, AF with rapid ventricular response alternating with bigeminal junctional rhythm, and a new‐onset paroxysmal atrioventricular block with otherwise normal conduction system function. During the electrophysiology study, intra‐atrial dissociation of RA or LA was observed in all patients. Six patients (patients 1‐5 and 7) showed dissociation at the posterolateral RA, while 1 patient (patient 6) showed dissociation of the RA free wall and LA/distal CS. Atrial activity originating from the dissociated atrial tissue was isolated, while atrial depolarization from the nondissociated atrium was conducted to the ventricle.
Atrial dissociation has been most commonly described in patients with cardiac transplantation, 5 , 6 and only a few reports have described atrial dissociation occurring after maze procedure or catheter ablation for atrial tachyarrhythmia. 7 , 8 , 9 Eleid et al reported a case with delayed atrial sensing after an RA maze procedure, which showed intermittent intra‐atrial conduction block to the pacing tip because of surgical ablation lines. 10 To the best of our knowledge, our current study is the first to describe postmaze atrial dissociations that mimicked complete atrioventricular block or VT.
In our study, some patients showed atrial tachyarrhythmia that was either spontaneous or induced by pacing stimulation. AF localized to the nondissociated atrium was observed in patients 2, 3, and 5 (Figure 2C, Figures S3D and 6E); in these cases, the absence of an ectopic escape beat (the nondissociated atrium) immediately after the termination of atrial tachyarrhythmia resulted in ventricular asystole, while P waves from the dissociated atrium (but not conducting to the ventricle) continued during the pause, thereby mimicking paroxysmal atrioventricular block in the surface ECG (Figure 3A).
Based on the electrophysiology studies, we deferred immediate PPM implantation, considering the patients’ symptoms and the severity of bradycardia. In patient 7, atrioventricular dissociation with junctional rhythm occurred after valve surgery, and a PPM was implanted under what we had misdiagnosed as a complete atrioventricular block. Thus, implantation of a PPM might have been unnecessary in this patient if an electrophysiology study had been performed before the implantation. In addition, unnecessary ventricular pacing could have been avoided by placing the atrial lead in the RA septum where the normal atrioventricular conduction was preserved, rather than in the dissociated RA appendage area. Thus, electrophysiology studies seem to have an important role not only in identifying atrioventricular blocks but also in determining the ideal implant locations of atrial leads.
When a supraventricular tachycardia occurs in the nondissociated atrium with intact atrioventricular conduction, it could be mistaken as VT because of the apparent atrioventricular dissociation. In patient 7, interrogation of the pacemaker revealed tachycardia with VA dissociation. Because the atrial lead only recorded the activity of the electrically dissociated RA appendage, the rapid atrial tachycardia from the nondissociated atrium was obscured.
A detailed description of the modified method of the maze procedure was previously reported. 11 We reviewed the surgery records of the patients and found that an extended transseptal atriotomy of LA was used in all patients. We assume that the intra‐RA dissociation might be caused by the extensive surgical lesion sets, although it is generally regarded that the extended transseptal approach does not increase postoperative arrhythmia. 12 , 13 Presumably, the fibrotic change of the atrium because of the progression of AF and valvular heart disease may also have contributed to this phenomenon.
This study has several limitations. It is a retrospective review of data collected from a single tertiary hospital and did not systematically include all consecutive patients presenting with complete atrioventricular blocks after maze procedures. Therefore, the electrophysiology study was performed as needed in selected patients with unusual ECG manifestations. We identified the dissociated atrium through conventional fluoroscopy mapping without a 3‐dimensional electroanatomical system. Although we tried to map the atrium in detail, it was not feasible to perform the mapping and differential pacing maneuvers in all parts of the atrium, and there was a limitation in terms of the mapping density. Also, we indirectly evaluated the electrical activity of the LA by electrogram analysis recorded in the CS according to routine practice, and not by the transseptal approach.
In summary, inter/intra‐atrial dissociations may mimic complete atrioventricular blocks in patients who undergo maze procedures and valvular surgeries. Pseudo‐atrioventricular blocks should be suspected, especially when they present with unusual electrocardiographic findings that are not compatible with ordinary complete atrioventricular blocks. Implantation of a PPM could be deferred based on the patient’s symptoms according to the treatment principle for sick sinus syndrome. Electrophysiology study has a crucial role in making the correct diagnosis and selecting the optimal location of the atrial lead in such patients. Atrioventricular block is an important indication of PPM implantation after the maze procedure. 14 , 15 It may be necessary to consider the possibility of a pseudo‐atrioventricular block (atrial dissociation) in addition to true conduction system disease in apparent atrioventricular blocks occurring after a maze procedure, especially lone maze procedure or maze with coronary artery bypass surgery.
Conclusions
Inter/intra‐atrial dissociations may mimic complete/paroxysmal atrioventricular blocks in patients who undergo maze procedures and valvular surgeries. These pseudo‐atrioventricular blocks may also masquerade as VT in pacemaker interrogation. The selection of patients for PPM implantation should be determined based on the patient’s symptoms and the status of the escape pacemaker and not on the apparent atrioventricular block.
Sources of Funding
This study was supported by a grant from the Asan Heart Institute (2018–0231).
Disclosures
None.
Supporting information
Figures S1‐S8
(J Am Heart Assoc. 2020;9:e018241 DOI: 10.1161/JAHA.120.018241.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018241
For Sources of Funding and Disclosures, see page 12.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badhwar V, Rankin JS, Damiano RJ Jr, Gillinov AM, Bakaeen FG, Edgerton JR, Philpott JM, McCarthy PM, Bolling SF, Roberts HG, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2017;103:329–341. [DOI] [PubMed] [Google Scholar]
- 3. Pasic M, Musci M, Edelmann B, Siniawski H, Bergs P, Hetzer R. Identification of P waves after the Cox‐maze procedure: significance of right precordial leads V3R through V6R. Ann Thorac Surg. 1999;67:1292–1294. [DOI] [PubMed] [Google Scholar]
- 4. Park HE, Kim KH, Kim KB, Ahn H, Choi YS, Oh S. Characteristics of P wave in patients with sinus rhythm after maze operation. J Korean Med Sci. 2010;25:712–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowery CM, Lewkowiez L, Sauer WH. P‐wave rejection in a transplanted heart. Ann Noninvasive Electrocardiol. 2011;16:308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorla SR, Raja KR, Sokoloski MC, Swaminathan S. Sinus node dysfunction masquerading as complete atrioventricular block in the setting of atrial parasystole after heart transplantation. J Electrocardiol. 2018;51:555–558. [DOI] [PubMed] [Google Scholar]
- 7. Chugh A, Yokokawa M, Baman T, Bogun F, Wu A. Intra‐atrial conduction block mimicking atrioventricular nodal block after multiple catheter ablation procedures for atrial tachycardia in a patient with cardiomyopathy. J Cardiovasc Electrophysiol. 2012;23:1258–1261. [DOI] [PubMed] [Google Scholar]
- 8. Markowitz SM, Choi DY, Daian F, Liu CF, Cheung JW, Thomas G, Ip JE, Lerman BB. Regional isolation in the right atrium with disruption of intra‐atrial conduction after catheter ablation of atrial tachycardia. J Cardiovasc Electrophysiol. 2019;30:1773–1785. [DOI] [PubMed] [Google Scholar]
- 9. Deger FT, Greenberg RM. Right atrial electrical isolation: left atrial connection to the atrioventricular node. Heart Rhythm. 2005;2:643–645. [DOI] [PubMed] [Google Scholar]
- 10. Eleid MF, Dearani JA, Shen WK. Isolated atrial lead conduction delay following right atrial radiofrequency maze procedure. ISRN Cardiol. 2011;2011:475796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JB, Bang JH, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial ablation versus biatrial ablation in the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2011;92:1397–1404. [DOI] [PubMed] [Google Scholar]
- 12. Mujtaba SS, Clark SC. Extended trans‐septal versus left atrial approach in mitral valve surgery: 1017 patients' experience. Heart Asia. 2018;10:e011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura T, Ota T, Takayama H, Naka Y. Extended trans‐septal and left atrial dome incision combined with Manouguian's aortotomy for complex double valve surgery. Eur J Cardiothorac Surg. 2014;46:127–128. [DOI] [PubMed] [Google Scholar]
- 14. Robertson JO, Cuculich PS, Saint LL, Schuessler RB, Moon MR, Lawton J, Damiano RJ, Maniar HS. Predictors and risk of pacemaker implantation after the Cox‐maze IV procedure. Ann Thorac Surg. 2013;95:2015–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ad N, Holmes SD, Ali R, Pritchard G, Lamont D. A single center's experience with pacemaker implantation after the Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2017;154(139–146):e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S8


