Abstract
Amyloids are a class of protein aggregates that have been historically characterized by their relationship with human disease. Indeed, amyloids can be the result of misfolded proteins that self-associate to form insoluble, extracellular plaques in diseased tissue. For the first 150 years of their study, the pathogen-first definition of amyloids was sufficient. However, new observations of amyloids foster an appreciation for non-pathological roles for amyloids in cellular systems. There is now evidence from all domains of life that amyloids can be non-pathogenic and functional, and that their formation can be the result of purposeful and controlled cellular processes. So-called functional amyloids fulfill an assortment of biological functions including acting as structural scaffolds, regulatory mechanisms, and storage mechanisms. The conceptual convergence of amyloids serving a functional role has been repeatedly confirmed by discoveries of additional functional amyloids. With dozens already known, and with the vigorous rate of discovery, the biology of amyloids is robustly represented by non-pathogenic amyloids.
Keywords: pathogenic amyloids, functional amyloids, curli, fap
1. Introduction
Amyloids are fibril protein aggregates originally identified in 1854 by Rudolph Virchow when he observed iodine-stained plaques in abnormal brain tissues [1,2,3]. Since the nineteenth century, amyloids, and the extracellular bodies they form in human tissue, have been inextricably connected to human disease and neurological dysfunction. More recent observations have led to an expansion of the definition of amyloids to include a class of non-pathogenic aggregates [1,4]. Functional amyloids are created by organisms intentionally, in order to exploit their many useful properties [5]. In the last 20 years, the list of functional amyloid proteins has blossomed (See Table 1). This has shifted views of amyloid biology from singularly pathogenic and cytotoxic structures to a protein fold that contributes positively to cellular biology. Our thesis put forth here is that functional amyloids are the principle class of amyloids found in nature.
Table 1.
Known functional amyloids and their wide range of putative functions.
| Amyloid | Gene or Protein | Amyloid Function | Species | Year | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| Curli | CsgA | Biofilm formation | E. coli, Salmonella spp., and other Enterobacteriacae | 2002 | [6] |
| Microcin E492 | Mcc | Toxin storage | Klebsiella pneumoniae | 2005 | [7] |
| Hairpins | HpaG | Virulence Factor | Xanthanomas spp. | 2007 | [8] |
| Phenol-soluble modulins | PSM | Biofilm formation and virulence | Staphylococcus aureus | 2007 | [9] |
| MTP | mtp | Pili formation | Mycobacterium tuberculosis | 2007 | [10] |
| FapC | FapC | Biofilm formation | Pseudomonas fluorescens and other Pseudomonads | 2010 | [11] |
| TasA | TasA | Biofilm formation | Bacillus subtilis | 2010 | [12] |
| P1 | P1 | Adhesin | Streptococcus mutans | 2012 | [13] |
| Listeriolysin | LLO | Phagolysosome | Listeria monocytogenes | 2012 | [14] |
| RepA | RepA | Plasmid replication regulator | Pseudomonas aeruginosa | 2016 | [15] |
| Chaplins | ChpA-H | Decreasing surface tension | Streptomyces coelicolor | 2017 | [16] |
| Rho | Rho | Transcriptional regulator | Clostridium botulinum | 2017 | [17] |
| Protists | |||||
| MSP2 | msp2 | Erythrocyte invasion | Plasmodium falciparum | 2009 | [18] |
| Fungi | |||||
| Het-s | het-s | Heterokaryon formation | Podospora anserina | 1997 | [19] |
| Ure2 † | Ure2 | Nitrogen catabolism regulator | Saccharomyces cerevisiae | 1997 | [20] |
| Sup35 † | Sup35 | Translation regulator | Saccharomyces cerevisiae | 1997 | [20] |
| Hydrophobins | SC3, etc | Breaking water surface tension | Schizophyllum commune, basidiomycetes, etc. | 2000 | [21] |
| Adhesions | Als Proteins | Biofilm formation | Candida albicans and other fungi | 2004 | [22] |
| Plants | |||||
| Adhesive substance | unknown | EPS ‡ component | Coccomyxa spp., Glaphyrella trebouxiodes, and other microalgae | 2008 | [23] |
| Rubber elongation factor | HevB1 | latex biosynthesis | Hevea brasiliensis | 2012 | [24] |
| Defensins | RsAFP-19 | Antifungal defense | Raphanus sativus | 2013 | [25] |
| AMP2 | Cn-AMP2 | Antimicrobial defense | Cocos nucifera | 2016 | [26] |
| Animals | |||||
| Chorions | Chorions | Egg protection | Bombyx mori, Papilio xuthus, etc. | 2000 | [27] |
| Spidroin | Spidroin | Silk production | Nephila edulis, Araneus diadematus, etc. | 2002 | [28] |
| CPEB | CPEB | Translation regulator | Aplysia californica | 2003 | [29] |
| Pmel17 | Pmel17 | Melanin synthesis | Homo sapiens | 2005 | [30] |
| Cement | cp-100k | surface adhesion | Megabalanus rosa and other barnacles | 2006 | [31] |
| Peptide hormones | GLP-2, VIP, etc. | Storage | Homo sapiens | 2009 | [32] |
| Anionic dermaseptin | aDrs | host defense | Pachymedusa dacnicolor | 2012 | [33] |
| Orb2 | Orb2 | Memory persistence | Drosophila melanogaster | 2012 | [34] |
| epididymal cystatin | cst8, etc. | Sperm maturation | Mus musculus | 2012 | [35] |
| RIP kinases | RIP1/3 | Necrosis regulator | Homo sapiens | 2012 | [36] |
| Uperin 3.5 | uperin 3.5 | Antimicrobial defense | Uperoleia mjobergii | 2016 | [37] |
| Archaea | |||||
| Biofilm amyloid protein | HVO_143 | Biofilm formation | Haloferax volcanii | 2014 | [38] |
† Only the two first yeast prions that were identified are mentioned here. This is not an inclusive list; there are many more examples of prions performing important functions in yeast. ‡ Extracellular polymeric substance.
2. The Established Perspective of Amyloid Proteins
Since their discovery, amyloids have been most closely associated with human disease. The amyloid field was ushered into medical science in 1854 when the German physician Rudolph Virchow observed iodine-stained “copora amylacae” in nervous tissue [39], characterizing the bodies as being starch-like (Latin for starch is amylum). Seven decades later, the textile dye Congo Red was identified as a useful compound for specifically staining amyloids in histological samples taken from diseased brain tissues [40]. In the same 1927 publication, the Belgian physician Paul Divry observed that Congo Red stained Alois Alzheimer’s plaques, which he associated with presenile dementia, thereby connecting these plaques to amyloids [1,40]. Divry’s publication sparked an exploration for amyloids in histological samples that continued through the twentieth century, during which, scientists found evidence of amyloids related to both localized and systematic syndromes [1,2]. The Pras extraction method introduced in 1968 allowed for a greater biochemical and structural characterization of amyloids [1,41]. Efficient fiber extraction coupled with amino acid sequencing lead researchers to determine that amyloid fibers associated with neuropathic or systemic disorders were composed of different proteins, each with their own clinical manifestations [2].
At the turn of the twenty-first century, several groups identified roles for amyloids other than causing disease in biology. In the late 1990s, yeast geneticists finally solved the strange issue of non-Mendelian heritable traits propagating in yeast [20,42,43,44]. The infective (i.e., prion) amyloid assemblies of the protein Sup35 was shown to have epigenetic-like control over protein expression based on the presence of the amyloid or soluble form of the protein [20,42,43,44]. In 2000, it was discovered that amyloid rodlets made up of hydrophobins allow fungi to escape an aquatic environment [21]. In the same year, amyloid proteins called chorions were identified as the major protective component surrounding silkmoth eggs [27]. In 2002, curli, the main proteinaceous component of the E. coli biofilm, were revealed to share biophysical characteristics with pathogenic amyloid fibers [6]. However, unlike pathogenic amyloids, these functional amyloids are not the product of stochastic protein misfolding and are often assembled via tightly regulated and controlled pathways. Since the early 2000s, many functional amyloids proteins have been discovered and described. The functional amyloids share biophysical characteristics with their pathogenic counterparts, including tinctorial properties, ability to self-assemble, and their fibrous 3D structure (Figure 1). There are now approximately 35 new functional amyloid proteins, and new ones are continuing to be described [45].
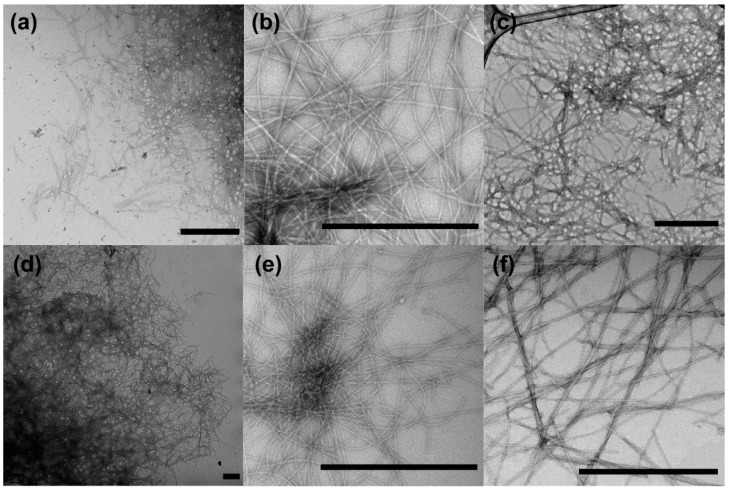
Figure 1.
Negatively stained transmission electron micrographs of pathogenic and functional amyloid fibrils. (a) α-Synuclein fibers. (b) Aβ1–40 fibrils. (c) Amylin fibrils. (d) Curli fibrils. (e) HET-s218–289 fibrils. (f) IIKIIK fibrils associated with PSMα. Scale bars represent 200 nm. Published with permission from Robert Tycko and Neha Jain.
3. The Amyloid Fold Is Intrinsic to Polypeptides
Amyloids are highly ordered protein aggregates that are in a low energy conformation and are highly stable and resistant to denaturation [46,47]. Classically, amyloids have been defined by their biophysical characteristics, including the cross-β structure, where β-strands align perpendicular to a fibril axis [3]. Recently, the amyloid structural catalog was expanded to include the cross-α structure of phenol-soluble modulins, a functional amyloid produced by S. aureus [48]. Regardless, the amyloid fold consisting of repeated, structural units that engender stability and conformity is a hallmark of protein folding. It has been theorized that the amyloid fold could be a primordial structural motif, and in a prebiotic world, it represents an early form of self-propagation and information transfer [49].
It has also been suggested that the amyloid fold can be achieved by peptides, regardless of their specific amino acid composition. Anfinsen’s dogma describes protein folding, wherein the 3D structure of a fully folded protein is determined by its primary sequence [50]. Certainly, this is true of globular proteins that adopt their native fold under optimal conditions. However, the native fold is only one of several thermodynamic minima [47,51]. Indeed, even a natively folded protein exists in a metastable state and, with enough energy proteins, can fold into the amyloid-specific, cross β-sheet conformation that is the lowest energy state [52] (Figure 2). Experiments that observed human lysozyme and transthyretin adopting the amyloid fold found that partially unstable or unfolded domains were the focal point of amyloidogenesis [53,54,55]. However, even stably folded proteins, such as the SH3 domain or acylphosphatase, can form amyloid after partial denaturation [56,57,58]. Even chains of polyalanine and polyglutamate have been shown to adopt the amyloid fold during in silico modeling experiments [3,59,60]. Therefore, given the ubiquity of the amyloid conformation in protein structure, it is not surprising that amyloids are part of normal cellular biology.
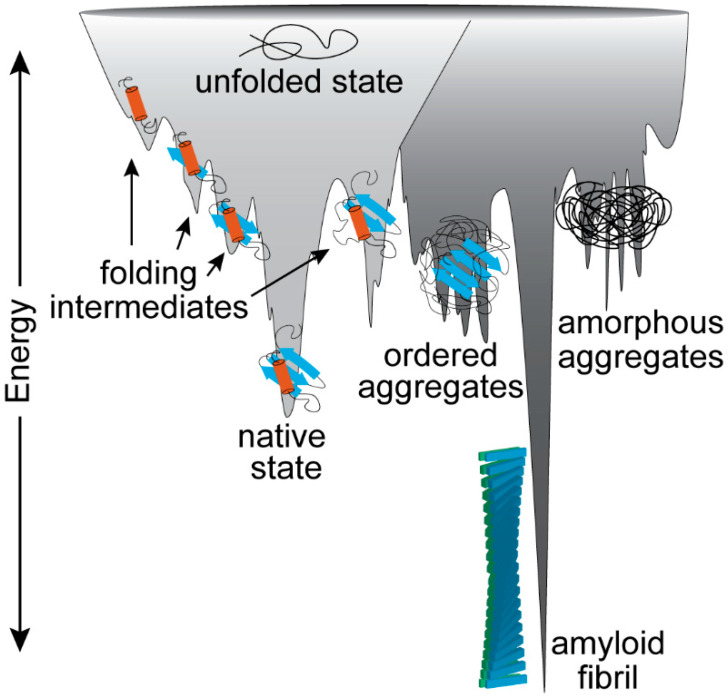
Figure 2.
Cartoon showing the complex protein folding landscape. Nascent, unfolded proteins travel down an energy gradient seeking the lowest energy conformation. Proteins can sample various energy minima on this journey including folding intermediates and the native fold state. However, every protein has the potential to reach other energy minima, including unfolded or partially ordered aggregates. In addition, all proteins can adopt the true lowest energy state, the amyloid fibril. Adapted from Jahn and Radford [50].
4. Curli and Fap Are Bacterial Amyloids Whose Assembly Is Highly Orchestrated
Arguably the most-studied bacterial functional amyloids are curli and the related Fap amyloids, made by E. coli and Pseudomonas spp., respectively. Both curli and Fap amyloids are major structural components of the biofilm matrix. Biofilms are entrenched colonies of bacteria that secrete a dense matrix of polysaccharides, amyloid fibers, and nucleic acids that collectively make up the extracellular matrix [61]. Within a biofilm, bacteria can continue to grow and survive some of the harshest environments, making biofilms an important factor in bacterial infection and pathogenesis [62]. When it comes to Proteobacteria, curli amyloids are essential biofilm components, illustrated by Reichhardt et al. who estimate that curli make up as much as 85% of the total carbon in the E. coli extracellular matrix [63]. Indeed, the use of small molecule inhibitors targeting curli production can have a destructive effect on biofilm formation [64]. Outside of proteobacteria, Firmicutes such as S. aureus and B. subtilis utilize PSM’s [9] and TasA [12] amyloids as notable biofilm components. Even in eukaryotes such as fungi [22] and microalgae [23], amyloids are the conduit that give biofilms their adhesive properties.
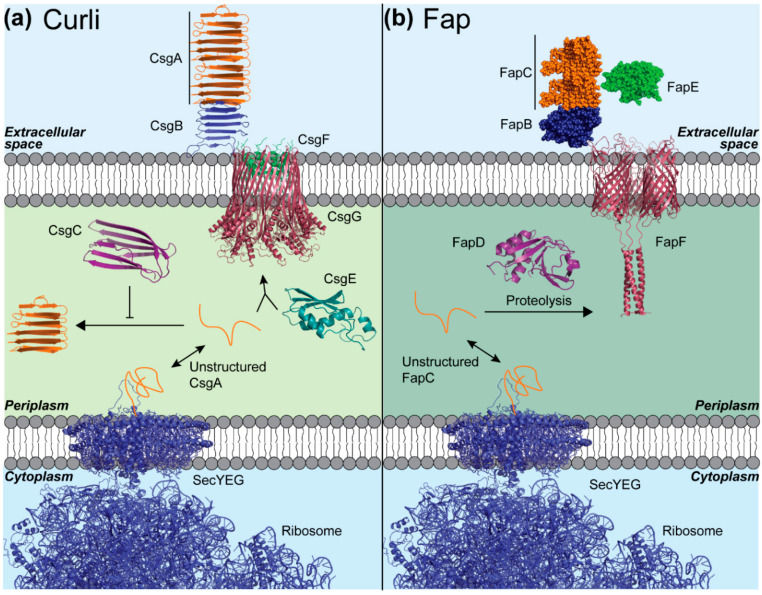
Curli amyloids are produced in an exquisitely controlled process [5] (Figure 3a). The E. coli curli-specific operon (csg) contains seven proteins including the master biofilm regulator protein CsgD which regulates curli production by responding to changes in the expression of hundreds of genes and external stimuli [65]. CsgA, the major curli subunit and functional amyloid protein, is translated and translocated directly into the periplasm through the SecYEG complex on the E. coli inner membrane [6]. Inside the periplasm, the nascent and unfolded CsgA is stabilized in an unstructured state by a chaperone-like protein called CsgC [66]. A second chaperone, CsgE, ferries CsgA to an outer membrane pore composed of the homo-nonameric CsgG [67]. CsgA then passes through the pore to be fully secreted into the extracellular space [68]. CsgB, the curli nucleator protein, is also secreted in the same fashion [69], however, it becomes anchored to the cell surface by CsgF [70]. Finally, curli fibers form on the cell surface after extracellular CsgA amyloid formation is templated by CsgB nuclei [71]. It is through the action of all these proteins that E. coli can assemble amyloid fibers at the correct time and space so that cellular fitness is not compromised.
Figure 3.
The bacterial amyloid systems called curli in E. coli and fap in P. aeruginosa are controlled through complex, multi-protein mechanisms. (a) The curli operon contains 7 proteins (6 shown here), each playing a necessary role in maintaining spatiotemporal control over polymerization of the major curli subunit CsgA [72]. CsgA is translated and translocated directly into the periplasm using the SecYEG secretion pore (PDB: 4V6M). CsgC (PDB: 2y2y) is a chaperone-like protein which inhibits CsgA aggregation within the periplasm. CsgE (PDB: 2NA4) is another periplasmic chaperone which fosters CsgA translocation through the nonameric curli assembly pore CsgG (PDB: 6L7A). Lastly, CsgF (PDB: 6L7A) and CsgB (REF [73]) both help to localize curli formation to the CsgG pore and the outer membrane, respectively. (b) In Pseudomonas, the major fap component FapC, is secreted into the periplasm using a SecYEG pore. FapD (modeled after the homologous C39 peptidase domain of ABC transporter PCAT1, PDB: 4RY2) is a peptidase which performs an essential proteolytic modification to one or more of the fap proteins. FapC is passed through the outer membrane using FapF, a trimeric polypeptide transporter (PDB: 5O67). Finally, FapB and FapE are essential minor components of fap amyloids, with FapB potentially playing a nucleator role similar to CsgB. Models shown of FapC, FapB, and FapE are structural predictions produced by the FALCON@home server, since there is no putative structural data in the literature.
Fap is another bacterial biofilm amyloid that displays a well-controlled mechanism of formation in P. fluorescens and other Pseudomonads [11]. In fact, the mechanism for amyloid formation in P. fluorescens is quite similar to the mechanism responsible for curli production in E. coli [74] (Figure 3b). Fap production is controlled by a larger operon composed of 6 genes, named fapABCDEF, in which the dominant amyloid forming protein is FapC [75]. FapA acts as a regulator of transcription, which alters the distribution of FapB and FapC in the amyloid product [75]. FapB is a nucleator protein that assists FapC as it assembles on the outer membrane of the bacterium [11], similar to CsgA-CsgB in E. coli [76]. FapE is also incorporated at the end of the amyloid fibrils, potentially serving as a site for protein–protein interaction [75]. FapF forms a channel that shuttles FapB, FapC, and FapE to the outside of the cell membrane [77], like CsgG in E. coli [67]. The role of FapD is still a little unclear, though it has essential proteolytic activity necessary for FapC secretion [77] and may potentially be involved in cleavage of FapF [77].
5. Other Functional Amyloids Are also Assembled in a Controlled Manner
Yeast cells have adapted multiple ways to control the formation of amyloid fibers associated with the yeast prion Sup35 and its commonly observed phenotype [PSI+] [78]. Sup35 prion formation is dependent on another yeast prion called [PIN+], the insoluble amyloid form of Rnq1, a protein of unknown function [79]. The mechanism for the regulation of [PSI+] by [PIN+] is still unclear as it relies on an inefficient and inconsistent process called “seeding”. Seeding describes the de novo construction of one prion through the interaction with a preexisting prion [79]. Though [PIN+] is required for the de novo formation of [PSI+], [PIN+] is not required for the extensive propagation of [PSI+] [80]. In fact, once the [PSI+] state has been established, [PIN+] is no longer necessary [80]. Once amyloid formation has started, the chaperone protein Hsp104 is required for the maintenance and propagation of [PSI+] [81]. There exists a critical concentration of Hsp104 that is necessary for [PSI+] formation. Too little chaperone prevents prion formation entirely, and if the concentration of Hsp104 is too high the chaperone will dissociate from the unfolded prion intermediately and prevent proper aggregation [81]. A lack of Hsp104 has been proven to cure yeast cells of [PSI+] and return them to the [psi−] state [81]. The manner in which Hsp104 facilitates aggregation is not fully clear, though it is possible that Hsp104 cleaves the Sup35 protein into smaller fragments that are necessary for their inheritance and propagation as amyloids [44]. Sup35 aggregation is also controlled by association with Sup45, a binding partner that is essential for translation termination behavior [82]. When Sup45 is overexpressed, [PSI+] formation is inhibited [83].
Sup35 plays an interesting role in yeast biology, acting as a method to quickly increase genetic variation in response to swaying environmental conditions. [PSI+] is a yeast prion that represents the inactivated, aggregated state of Sup35, a ribosomal elongation factor [84]. When Sup35 is soluble and active, the predominant phenotype is known as [psi−], and the yeast ribosome correctly recognizes stop codons and terminates translation [85]. Cells can undergo a transition to the [PSI+] state using the controlled mechanism discussed above, which decreases nonsense suppression [86]. When yeast cells are challenged to grow under stressful growth conditions, [PSI+] cells are capable of creating novel, heritable phenotypes more fit to survive in the new environment [84]. The functionality of [PSI+] formation is controversial, as it has been argued that the resulting decrease in translational fidelity is toxic rather than beneficial [87]. While the usefulness of [PSI+] remains debated, there is good evidence to support the evolutionary benefits of transient decreases in translational fidelity. Additionally, there are other examples of functional amyloids in yeast, including Rim4 [88] and Cdc19 [89].
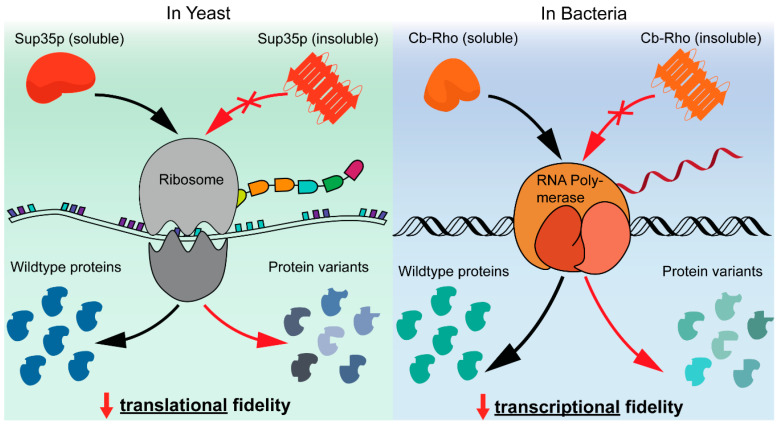
In a recent publication, Yuan et al. showed that bacteria also use functional amyloids to speed up the development of new protein variants [17]. Clostridium botulinum has a transcription factor Rho that was found to contain a well-conserved candidate prion-like domain (cPrD) [17]. Chimeric proteins containing Cb-Rho cPrD produced phenotypes identical to [psi−] and [PSI+] in recombinant E. coil [17]. Interestingly, while [PSI+] decreases translational fidelity in yeast, Rho prions decrease transcriptional fidelity in bacteria, creating genetic variation in distinct yet similar manners (Figure 4).
Figure 4.
The insoluble amyloid forms of Sup35p and Cb-Rho allow the creation of protein variants better suited to survive in sudden environmental fluctuations. In yeast, the loss of an active translation termination factor Sup35p leads to a stop codon read-through, giving rise to new phenotypes. In bacteria, the same result is accomplished through the loss of the transcriptional terminator factor Rho and, thereby, a decrease in transcriptional fidelity.
Human cells have established a well-controlled mechanism to post-translationally regulate PMel17 amyloid formation in melanosomes. After synthesis, PMel17 associates with intraluminal vesicles (ILVs) of multivesicular bodies, where further processing and amylogenesis take place [90]. The tetraspanin protein CD63 ensures proper association of pre-processed PMel17 with ILVs by protecting it from degradation pathways [91]. Apolipoprotein E (ApoE) is also important for amyloid formation after CD63 has carried out its function [92]. Though its role is not fully understood, ApoE acts downstream of CD63 and likely functions to assist in the sorting of PMel17 as it associates with ILVs [92]. Once associated with ILVs, PMel17 is cleaved into two subunits, Mα and Mβ, in a specified Golgi compartment [93] by a furin-like proprotein convertase [94]. The two subunits remain connected by a disulfide bond [93] until the endosomal sheddase BACE2 catalyzes the release of the Mβ subunit from the membrane-bound Mα complex [95]. The Mβ subunit is subsequently degraded by γ-secretase activity [96]. The larger fragment, Mα, remains membrane-bound to the membranes of ILVs and acts as a nucleation site upon which amyloid formation takes place [93]. Mα is further cleaved into 3 subdomains by lysosomal proteases, fragments which form the core of PMel17 amyloids [97].
While the formation of functional amyloids is tightly regulated and predictable, pathogenic amyloid formation is stochastic and unpredictable. The inappropriate accumulation of amyloid deposits and their associated pathologies are often age-dependent processes [98,99]. Amyloid formation and the resulting protein folding diseases can be coupled to the natural decline in chaperone activity and proteosome capacity in the cell [99]. Amyloidoses most often begin with a spontaneous event during which normal proteins go above a critical concentration and transition into a pathogenic state [52]. In other cases, some amyloidoses are the result of infection. Prusiner’s protein-only theory postulated that the infective agent transferred between individuals in prion diseases were misfolded proteins [100]. Regarding sporadic Parkinson Disease, Braak’s hypothesis suggests that alpha-synuclein aggregation could be triggered by outside pathogens that introduce amyloids to distal nervous tissue [101]. Interestingly, several recent publications suggest that Braak’s pathogens could be bacterial amyloids from the microbiome [102,103,104,105]. In the case of dialysis-related amyloidosis, interventional medicine is to blame for the buildup of β2-microglobulin amyloids at needle injection sites [106]. These examples illustrate the sometimes random nature of pathogenic amyloidogenesis, which is in contrast to the controlled and predictable ways that functional amyloids form.
6. Conclusions
Historically, amyloids have been conceptually tied to the devastating human diseases that they can cause. However, in the last twenty years there have also been dozens of functional amyloids described that have helped usher in a new appreciation of amyloid biology. Since the amyloid conformation is a structure that is intrinsically available to all polypeptides, it is not surprising that nature has found many uses for the amyloid state. Indeed, examples of beneficial amyloids can be found all over biology, performing a wide range of tasks. Evidence of the longevity and usefulness of functional amyloids can be seen in their widespread stewardship. Where functional amyloids used to represent the exceptions in amyloid biology, they are now robustly represented and provide a template for understanding how amyloid formation can occur without causing cellular toxicity and death.
Acknowledgments
We thank all the members of the Chapman lab for their helpful discussions. We thank Robert Tycko (Panels b, c, and e) and Neha Jain (Panels a, d, and f) for generously sharing the TEM micrographs used in Figure 1.
Author Contributions
A.B. and M.C. contributed the conception of the review. A.B. and E.G. wrote the manuscript; A.B., E.G., and M.C. contributed to the editing and revising of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health R01GM118651 and by the BSF AWD007203 grants.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tanskanen M. Amyloidosis. InTech; Rijeka, Croatia: 2013. “Amyloid”—Historical Aspects. [Google Scholar]
- 2.Sipe J.D., Cohen A.S. Review: History of the Amyloid Fibril. J. Struct. Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Pham C.L.L., Kwan A.H., Sunde M. Functional amyloid: Widespread in Nature, diverse in purpose. Essays Biochem. 2014;56:207–219. doi: 10.1042/BSE0560207. [DOI] [PubMed] [Google Scholar]
- 5.Otzen D. Functional amyloid: Turning swords into plowshares. Prion. 2010;4:256–264. doi: 10.4161/pri.4.4.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman M.R., Robinson L.S., Pinkner J.S., Roth R., Heuser J., Hammar M., Normark S., Hultgren S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieler S., Estrada L., Lagos R., Baeza M., Castilla J., Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- 8.Oh J., Kim J.G., Jeon E., Yoo C.H., Jae S.M., Rhee S., Hwang I. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J. Biol. Chem. 2007;282:13601–13609. doi: 10.1074/jbc.M602576200. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz K., Syed A.K., Stephenson R.E., Rickard A.H., Boles B.R. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus aureus Biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alteri C.J., Xicohténcatl-Cortes J., Hess S., Caballero-Olin G., Girón J.A., Friedman R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. USA. 2007;104:5145–5150. doi: 10.1073/pnas.0602304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dueholm M.S., Petersen S.V., Sonderkaer M., Larsen P., Christiansen G., Hein K.L., Enghild J.J., Nielsen J.L., Nielsen K.L., Nielsen P.H., et al. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010;77:1009–1020. doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- 12.Romero D., Aguilar C., Losick R., Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oli M.W., Otoo H.N., Crowley P.J., Heim K.P., Nascimento M.M., Ramsook C.B., Lipke P.N., Brady L.J. Functional amyloid formation by Streptococcus mutans. Microbiology. 2012;158:2903–2916. doi: 10.1099/mic.0.060855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bavdek A., Kostanjšek R., Antonini V., Lakey J.H., Dalla Serra M., Gilbert R.J.C., Anderluh G. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 2012;279:126–141. doi: 10.1111/j.1742-4658.2011.08405.x. [DOI] [PubMed] [Google Scholar]
- 15.Molina-García L., Gasset-Rosa F., Moreno-Del Álamo M., Fernández-Tresguerres M.E., De La Espina S.M., Lurz R., Giraldo R. Functional amyloids as inhibitors of plasmid DNA replication. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragoš A., Kovács Á.T., Claessen D. The Role of Functional Amyloids in Multicellular Growth and Development of Gram-Positive Bacteria. Biomolecules. 2017;7:60. doi: 10.3390/biom7030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan A.H., Hochschild A. A bacterial global regulator forms a prion. Science. 2017;355:198–201. doi: 10.1126/science.aai7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adda C.G., Murphy V.J., Sunde M., Waddington L.J., Schloegel J., Talbo G.H., Vingas K., Kienzle V., Masciantonio R., Howlett G.J., et al. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol. Biochem. Parasitol. 2009;166:159–171. doi: 10.1016/j.molbiopara.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coustou V., Deleu C., Saupe S., Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickner R.B. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 21.Wösten H.A.B., De Vocht M.L. Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta Rev. Biomembr. 2000;1469:79–86. doi: 10.1016/S0304-4157(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Rauceo J.M., Gaur N.K., Lee K.G., Edwards J.E., Klotz S.A., Lipke P.N. Global cell surface conformational shift mediated by a Candida albicans adhesin. Infect. Immun. 2004;72:4948–4955. doi: 10.1128/IAI.72.9.4948-4955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostaert A.S., Higgins M.J., Fukuma T., Rindi F., Jarvis S.P. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 2006;32:393–401. doi: 10.1007/s10867-006-9023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berthelot K., Lecomte S., Estevez Y., Coulary-Salin B., Bentaleb A., Cullin C., Deffieux A., Peruch F. Rubber Elongation Factor (REF), a Major Allergen Component in Hevea brasiliensis Latex Has Amyloid Properties. PLoS ONE. 2012;7:e48065. doi: 10.1371/journal.pone.0048065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvey M., Meehan S., Gras S.L., Schirra H.J., Craik D.J., Van Der Weerden N.L., Anderson M.A., Gerrard J.A., Carver J.A. A radish seed antifungal peptide with a high amyloid fibril-forming propensity. Biochim. Biophys. Acta Proteins Proteom. 2013;1834:1615–1623. doi: 10.1016/j.bbapap.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Gour S., Kaushik V., Kumar V., Bhat P., Yadav S.C., Yadav J.K. Antimicrobial peptide (Cn-AMP2) from liquid endosperm of Cocos nucifera forms amyloid-like fibrillar structure. J. Pept. Sci. 2016;22:201–207. doi: 10.1002/psc.2860. [DOI] [PubMed] [Google Scholar]
- 27.Iconomidou V.A., Vriend G., Hamodrakas S.J. Amyloids protect the silkmoth oocyte and embryo. FEBS Lett. 2000;479:141–145. doi: 10.1016/S0014-5793(00)01888-3. [DOI] [PubMed] [Google Scholar]
- 28.Kenney J.M., Knight D., Wise M.J., Vollrath F. Amyloidogenic nature of spider silk. Eur. J. Biochem. 2002;269:4159–4163. doi: 10.1046/j.1432-1033.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- 29.Si K., Lindquist S., Kandel E.R. A Neuronal Isoform of the Aplysia CPEB Has Prion-Like Properties. Cell. 2003;115:879–891. doi: 10.1016/S0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- 30.Fowler D.M., Koulov A.V., Alory-Jost C., Marks M.S., Balch W.E., Kelly J.W. Functional Amyloid Formation within Mammalian Tissue. PLoS Biol. 2005;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamino K. Biological Adhesives. Springer; Berlin/Heidelberg, Germany: 2006. Barnacle Underwater Attachment; pp. 145–166. [Google Scholar]
- 32.Maji S.K., Perrin M.H., Sawaya M.R., Jessberger S., Vadodaria K., Rissman R.A., Singru P.S., Nilsson K.P.R., Simon R., Schubert D., et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gößler-Schöfberger R., Hesser G., Reif M.M., Friedmann J., Duscher B., Toca-Herrera J.L., Oostenbrink C., Jilek A. A stereochemical switch in the aDrs model system, a candidate for a functional amyloid. Arch. Biochem. Biophys. 2012;522:100–106. doi: 10.1016/j.abb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumdar A., Cesario W.C., White-Grindley E., Jiang H., Ren F., Khan M., Li L., Choi E.M.-L., Kannan K., Guo F., et al. Critical Role of Amyloid-like Oligomers of Drosophila Orb2 in the Persistence of Memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Whelly S., Johnson S., Powell J., Borchardt C., Hastert M.C., Cornwall G.A. Nonpathological Extracellular Amyloid Is Present during Normal Epididymal Sperm Maturation. PLoS ONE. 2012;7:e36394. doi: 10.1371/journal.pone.0036394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., McQuade T., Siemer A.B., Napetschnig J., Moriwaki K., Hsiao Y.S., Damko E., Moquin D., Walz T., McDermott A., et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese A.N., Liu Y., Wang T., Musgrave I.F., Pukala T.L., Tabor R.F., Martin L.L., Carver J.A., Bowie J.H. The Amyloid Fibril-Forming Properties of the Amphibian Antimicrobial Peptide Uperin 3.5. ChemBioChem. 2016;17:239–246. doi: 10.1002/cbic.201500518. [DOI] [PubMed] [Google Scholar]
- 38.Chimileski S., Franklin M.J., Papke R.T. Biofilms formed by the archaeon Haloferax volcaniiexhibit cellular differentiation and social motility, and facilitate horizontal gene transfer. BMC Biol. 2014;12:65. doi: 10.1186/s12915-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virchow R. Ueber eine im Gehirn und Rückenmark des Menschen aufgefundene Substanz mit der chemischen Reaction der Cellulose. Arch. Pathol. Anat. Physiol. Klin. Med. 1854;6:135–138. doi: 10.1007/BF01930815. [DOI] [Google Scholar]
- 40.Divry P. Étude histo-chimique des plaques seniles. J. Neurol. Psychiatr. 1927;27:643–657. [Google Scholar]
- 41.Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E.C. The characterization of soluble amyloid prepared in water. J. Clin. Invest. 1968;47:924–933. doi: 10.1172/jci105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuite M.F., Staniforth G.L., Cox B.S. [PSI+] turns 50. Prion. 2015;9:318–332. doi: 10.1080/19336896.2015.1111508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patino M.M., Liu J.J., Glover J.R., Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- 44.Paushkin S.V., Kushnirov V.V., Smirnov V.N., Ter-Avanesyan M.D. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 1996;15:3127–3134. doi: 10.1002/j.1460-2075.1996.tb00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizhnikov A.A., Antonets K.S., Inge-Vechtomov S.G. Amyloids: From pathogenesis to function. Biochemistry. 2015;80:1127–1144. doi: 10.1134/S0006297915090047. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg D.S., Sawaya M.R. Structural Studies of Amyloid Proteins at the Molecular Level. Annu. Rev. Biochem. 2017;86:69–95. doi: 10.1146/annurev-biochem-061516-045104. [DOI] [PubMed] [Google Scholar]
- 47.Jahn T.R., Radford S.E. Folding versus aggregation: Polypeptide conformations on competing pathways. Arch. Biochem. Biophys. 2008;469:100–117. doi: 10.1016/j.abb.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tayeb-Fligelman E., Salinas N., Tabachnikov O., Landau M. Staphylococcus aureus PSMα3 Cross-α Fibril Polymorphism and Determinants of Cytotoxicity. Structure. 2020;28:301–313.e6. doi: 10.1016/j.str.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Maury C.P.J. Self-Propagating β-sheet polypeptide structures as prebiotic informational molecular entities: The amyloid world. Orig. Life Evol. Biosph. 2009;39:141–150. doi: 10.1007/s11084-009-9165-6. [DOI] [PubMed] [Google Scholar]
- 50.Anfinsen C.B. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 51.Tsai C.J., Kumar S., Ma B., Nussinov R. Folding funnels, binding funnels, and protein function. Protein Sci. 1999;8:1181–1190. doi: 10.1110/ps.8.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazit E. The “Correctly Folded” State of Proteins: Is It a Metastable State? Angew. Chem. Int. Ed. 2002;41:257. doi: 10.1002/1521-3773(20020118)41:2<257::AID-ANIE257>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Kelly J.W. Amyloid fibril formation and protein misassembly: A structural quest for insights into amyloid and priori diseases. Structure. 1997;5:595–600. doi: 10.1016/S0969-2126(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 54.Booth D.R., Sundetll M., Bellotti V., Robinson C.V., Hutchinson W.L., Fraser P.E., Hawkins P.N., Dobson C.M., Radford S.E., Blaket C.C.F., et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature. 1997;385:787–793. doi: 10.1038/385787a0. [DOI] [PubMed] [Google Scholar]
- 55.Buxbaum J.N., Tagoe C., Gallo G., Walker J.R., Kurian S., Salomon D.R. Why are some amyloidoses systemic? Does hepatic “chaperoning at a distance” prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J. 2012;26:2283–2293. doi: 10.1096/fj.11-189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 57.Guijarro J.I., Sunde M., Jones J.A., Campbell I.D., Dobson C.M. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. USA. 1998;95:4224–4228. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiti F., Webster P., Taddei N., Clark A., Stefani M., Ramponi G., Dobson C.M. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. USA. 1999;96:3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen H.D., Hall C.K. Kinetics of fibril formation by polyalanine peptides. J. Biol. Chem. 2005;280:9074–9082. doi: 10.1074/jbc.M407338200. [DOI] [PubMed] [Google Scholar]
- 60.Marchut A.J., Hall C.K. Side-Chain Interactions Determine Amyloid Formation by Model Polyglutamine Peptides in Molecular Dynamics Simulations. Biophys. J. 2006;90:4574–4584. doi: 10.1529/biophysj.105.079269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 62.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 63.Reichhardt C., Cegelski L. Solid-state NMR for bacterial biofilms. Mol. Phys. 2014;112:887–894. doi: 10.1080/00268976.2013.837983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cegelski L., Pinkner J.S., Hammer N.D., Cusumano C.K., Hung C.S., Chorell E., Åberg V., Walker J.N., Seed P.C., Almqvist F., et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith D., Price J., Burby P., Blanco L., Chamberlain J., Chapman M. The Production of Curli Amyloid Fibers Is Deeply Integrated into the Biology of Escherichia coli. Biomolecules. 2017;7:75. doi: 10.3390/biom7040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans M.L., Chorell E., Taylor J.D., Åden J., Götheson A., Li F., Koch M., Sefer L., Matthews S.J., Wittung-Stafshede P., et al. The Bacterial Curli System Possesses a Potent and Selective Inhibitor of Amyloid Formation. Mol. Cell. 2015;57:445–455. doi: 10.1016/j.molcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nenninger A.A., Robinson L.S., Hammer N.D., Epstein E.A., Badtke M.P., Hultgren S.J., Chapman M.R. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol. Microbiol. 2011;81:486–499. doi: 10.1111/j.1365-2958.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goyal P., Krasteva P.V., Van Gerven N., Gubellini F., Van den Broeck I., Troupiotis-Tsailaki A., Jonckheere W., Pehau-Arnaudet G., Pinkner J.S., Chapman M.R., et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature. 2014;516:250–253. doi: 10.1038/nature13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammer N.D., Schmidt J.C., Chapman M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nenninger A.A., Robinson L.S., Hultgren S.J. Localized and efficient curli nucleation requires the chaperone-like amyloid assembly protein CsgF. Proc. Natl. Acad. Sci. USA. 2009;106:900–905. doi: 10.1073/pnas.0812143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X., Hammer N.D., Chapman M.R. The molecular basis of functional bacterial amyloid polymerization and nucleation. J. Biol. Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian P., Boomsma W., Wang Y., Otzen D.E., Jensen M.H., Lindorff-Larsen K. Structure of a Functional Amyloid Protein Subunit Computed Using Sequence Variation. J. Am. Chem. Soc. 2015;137:22–25. doi: 10.1021/ja5093634. [DOI] [PubMed] [Google Scholar]
- 73.DeBenedictis E.P., Ma D., Keten S. Structural predictions for curli amyloid fibril subunits CsgA and CsgB. RSC Adv. 2017;7:48102–48112. doi: 10.1039/C7RA08030A. [DOI] [Google Scholar]
- 74.Mann E.E., Wozniak D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dueholm M.S., Søndergaard M.T., Nilsson M., Christiansen G., Stensballe A., Overgaard M.T., Givskov M., Tolker-Nielsen T., Otzen D.E., Nielsen P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen. 2013;2:365–382. doi: 10.1002/mbo3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hammar M., Bian Z., Normark S. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2007;93:6562–6566. doi: 10.1073/pnas.93.13.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rouse S.L., Hawthorne W.J., Berry J.-L., Chorev D.S., Ionescu S.A., Lambert S., Stylianou F., Ewert W., Mackie U., Morgan R.M.L., et al. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat. Commun. 2017;8:263. doi: 10.1038/s41467-017-00361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox B.S. Ψ, A cytoplasmic suppressor of super-suppressor in yeast. Heredity. 1965;20:505–521. doi: 10.1038/hdy.1965.65. [DOI] [Google Scholar]
- 79.Derkatch I.L., Bradley M.E., Hong J.Y., Liebman S.W. Prions Affect the Appearance of Other Prions: The Story of [PIN+] Cell. 2001;106:171–182. doi: 10.1016/S0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 80.Derkatch I.L., Bradley M.E., Masse S.V., Zadorsky S.P., Polozkov G.V., Inge-Vechtomov S.G., Liebman S.W. Dependence and independence of [PSI(+)] and [PIN(+)]: A two-prion system in yeast? EMBO J. 2000;19:1942–1952. doi: 10.1093/emboj/19.9.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chernoff Y.O., Lindquist S.L., Ono B., Inge-Vechtomov S.G., Liebman S.W. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 82.Frolova L., Le Goff X., Rasmussen H.H., Cheperegin S., Drugeon G., Kress M., Arman I., Haenni A.-L., Celis J.E., Phllippe M., et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994;372:701–703. doi: 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- 83.Derkatch I.L., Bradley M.E., Liebman S.W. Overexpression of the SUP45 gene encoding a Sup35p-binding protein inhibits the induction of the de novo appearance of the [PSI] prion. Proc. Natl. Acad. Sci. USA. 1998;95:2400–2405. doi: 10.1073/pnas.95.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.True H.L., Lindquist S.L. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- 85.Tuite M.F., Cox B.S., McLaughlin C.S. A ribosome-associated inhibitor of in vitro nonsense suppression in [psi-] strains of yeast. FEBS Lett. 1987;225:205–208. doi: 10.1016/0014-5793(87)81158-4. [DOI] [PubMed] [Google Scholar]
- 86.Tuite M.F., Cox B.S., McLaughlin C.S. In vitro nonsense suppression in [psi+] and [psi-] cell-free lysates of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1983;80:2824–2828. doi: 10.1073/pnas.80.10.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGlinchey R.P., Kryndushkin D., Wickner R.B. Suicidal [PSI+] is a lethal yeast prion. Proc. Natl. Acad. Sci. USA. 2011;108:5337–5341. doi: 10.1073/pnas.1102762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berchowitz L.E., Kabachinski G., Walker M.R., Carlile T.M., Gilbert W.V., Schwartz T.U., Amon A. Regulated Formation of an Amyloid-like Translational Repressor Governs Gametogenesis. Cell. 2015;163:406–418. doi: 10.1016/j.cell.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saad S., Cereghetti G., Feng Y., Picotti P., Peter M., Dechant R. Reversible protein aggregation is a protective mechanism to ensure cell cycle restart after stress. Nat. Cell Biol. 2017;19:1202–1213. doi: 10.1038/ncb3600. [DOI] [PubMed] [Google Scholar]
- 90.Hurbain I., Geerts W.J.C., Boudier T., Marco S., Verkleij A.J., Marks M.S., Raposo G. Electron tomography of early melanosomes: Implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc. Natl. Acad. Sci. USA. 2008;105:19726–19731. doi: 10.1073/pnas.0803488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., Marks M.S., Rubinstein E., Raposo G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Niel G., Bergam P., Di Cicco A., Hurbain I., Lo Cicero A., Dingli F., Palmulli R., Fort C., Potier M.C., Schurgers L.J., et al. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015;13:43–51. doi: 10.1016/j.celrep.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 93.Berson J.F., Harper D.C., Tenza D., Raposo G., Marks M.S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berson J.F., Theos A.C., Harper D.C., Tenza D., Raposo G., Marks M.S. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rochin L., Hurbain I., Serneels L., Fort C., Watt B., Leblanc P., Marks M.S., De Strooper B., Raposo G., van Niel G. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc. Natl. Acad. Sci. USA. 2013;110:10658–10663. doi: 10.1073/pnas.1220748110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kummer M.P., Maruyama H., Huelsmann C., Baches S., Weggen S., Koo E.H. Formation of Pmel17 amyloid is regulated by juxtamembrane metalloproteinase cleavage, and the resulting C-terminal fragment is a substrate for γ-secretase. J. Biol. Chem. 2009;284:2296–2306. doi: 10.1074/jbc.M808904200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watt B., Van Niel G., Fowler D.M., Hurbain I., Luk K.C., Stayrook S.E., Lemmon M.A., Raposo G., Shorter J., Kelly J.W., et al. N-terminal Domains Elicit Formation of Functional Pmel17 Amyloid Fibrils. J. Biol. Chem. 2009;284:35543–35555. doi: 10.1074/jbc.M109.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartl F.U. Protein Misfolding Diseases. Annu. Rev. Biochem. 2017;86:21–26. doi: 10.1146/annurev-biochem-061516-044518. [DOI] [PubMed] [Google Scholar]
- 99.Labbadia J., Morimoto R.I. The Biology of Proteostasis in Aging and Disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 101.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sampson T.R., Challis C., Jain N., Moiseyenko A., Ladinsky M.S., Shastri G.G., Thron T., Needham B.D., Horvath I., Debelius J.W., et al. A gut bacterial amyloid promotes a-synuclein aggregation and motor impairment in mice. Elife. 2020;9 doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Challis C., Hori A., Sampson T.R., Yoo B.B., Challis R.C., Hamilton A.M., Mazmanian S.K., Volpicelli-Daley L.A., Gradinaru V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020;23:327–336. doi: 10.1038/s41593-020-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen C., Ahn E.H., Kang S.S., Liu X., Alam A., Ye K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020;6:eaba0466. doi: 10.1126/sciadv.aba0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T., et al. A new form of amyloid protein associated with chronic hemodialysis was identified as β2-microglobulin. Biochem. Biophys. Res. Commun. 1985;129:701–706. doi: 10.1016/0006-291X(85)91948-5. [DOI] [PubMed] [Google Scholar]