Abstract
Porphyromonas gingivalis Mfa1 fimbriae are thought to act as adhesion factors and to direct periodontal tissue destruction but their immunomodulatory actions are poorly understood. Here, we investigated the effect of Mfa1 stimulation on the immune and metabolic mechanisms of gingival fibroblasts from periodontal connective tissue. We also determined the role of Toll-like receptor (TLR) 2 and TLR4 in Mfa1 recognition. Mfa1 increased the expression of genes encoding chemokine (C-X-C motif) ligand (CXCL) 1, CXCL3, intercellular adhesion molecule (ICAM) 1 and Selectin endothelium (E) in gingival fibroblasts, but did not have a significant effect on genes that regulate metabolism. Mfa1-stimulated up-regulation of genes was significantly suppressed in Tlr4 siRNA-transfected cells compared with that in control siRNA-transfected cells, which indicates that recognition by TLR4 is essential for immunomodulation by Mfa1. Additionally, suppression of Tlr2 expression partially attenuated the stimulatory effect of Mfa1. Overall, these results help explain the involvement of P. gingivalis Mfa1 fimbriae in the progression of periodontal disease.
Keywords: Porphyromonas gingivalis, Mfa1, Toll-like receptors, gingival fibroblast
1. Introduction
Periodontitis is a chronic inflammatory disease caused by multiple periodontal pathogenic bacterial species [1]. Socransky et al. indicated that three bacteria, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, are the main cause of the onset and progression of periodontitis and they suggested that these three species be called “red complexes” [2].
Among the three species, P. gingivalis is the major pathogen associated with periodontitis. Riviere et al. found that P. gingivalis was more prevalent at diseased sites than at healthy sites in diseased subjects [3]. The number of P. gingivalis in plaque samples was associated with the plaque score and clinical attachment level [4]. From the perspective of oral flora, P. gingivalis is considered to act as a keystone pathogen that creates a dysbiosis between the host and the dental biofilm. This altered oral commensal microbiota is responsible for initiating pathological bone loss [5]. P. gingivalis expresses a number of potential virulence factors, including lipopolysaccharides, gingipines, and fimbriae [6].
Fimbriae are filamentous proteinaceous appendages on the surface of P. gingivalis bacteria that play a pivotal role in colonization through association with other bacteria and host tissues [7,8]. P. gingivalis ATCC strain 33,277 has fimbriae consisting of either FimA (fimA gene product) or Mfa1 (mfa1 gene product), with apparent molecular masses of approximately 38 and 75 kDa, respectively [9].
Mfa1, encoded by the mfa1 gene, is a structural subunit of a protein complex whose length varies from 60 to 500 nm [10]. In addition to the primary Mfa1 protein, mature fimbriae also have affiliated Mfa2–5 proteins. Mfa2 plays an anchor role, while Mfa3 can bind with Mfa1/2/4/5 in vitro to connect with other fimbrial subunits [11]. Recent data indicate that the C-terminal domain of Mfa1, rather than Mfa3, affects the aggregation and maturation of downstream fimbrial proteins [12].
Several studies have demonstrated different roles for FimA and Mfa1 fimbriae. FimA fimbriae act as an adhesive that mediates periodontal tissue colonization and host cell invasion [8,13,14]. They also induce inflammatory processes in periodontal tissues through several mechanisms [15,16,17]. FimA fimbriae promote early biofilm formation in a single species of P. gingivalis, whereas Mfa1 plays an inhibitory role in the formation of a homotypic biofilm in P. gingivalis [18]. However, although there are few reports on the host immune response to Mfa1 fimbriae, an Mfa1-deficient strain causes almost no alveolar bone resorption in a mouse model of oral infection [19].
The present study aimed to examine the effect of P. gingivalis Mfa1 stimulation on the immune and metabolic mechanisms of mouse gingival fibroblasts (MGFs) and to examine the effects of Toll-like receptor (TLR) 2 and TLR4 knock down on P. gingivalis Mfa1-stimulated MGFs. Our results show that Mfa1 fimbriae had a large effect on immunomodulation exerted by gingival fibroblasts, but did not have a significant effect on metabolic regulation. Our results also indicate that recognition of Mfa1 by TLR4 on MGFs is essential for the expression of genes related to cell migration and cell adhesion. Overall, these results help to explain how P. gingivalis Mfa1 fimbriae are involved in the progression of periodontal disease.
2. Materials and Methods
2.1. Cell Culture
MGFs were isolated from healthy gingival tissue from the palate of BALB/c mice which were purchased from CLEA Japan, Inc. (Tokyo, Japan). The MGFs were cultured in Minimum Essential Medium α (Thermo Fisher Scientific, Wilmington, DE, USA) containing 10% fetal bovine serum (Hyclone Laboratories Inc, Logan, UT, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 ℃ in a CO2 incubator. When the cells reached confluency, they were separated by treatment with 0.25% trypsin-EDTA (Thermo Fisher Scientific) and collected by centrifugation. This study was approved by the Institutional Animal Care and Use Committees of Aichi Gakuin University (AGUD438; approved date 31 March 2019) and all animal experiments were conducted following the national guidelines and the relevant national laws on the protection of animals. Ultrapure lipopolysaccharide from P. gingivalis was purchased for experiments (InvivoGen, San Diego, CA, USA).
2.2. Purification of Mfa1 Fimbriae
In this study, we used P. gingivalis mutant strains derived from ATCC 33,277. Mfa1 fimbriae were purified from JI-1, in which fimA was deleted, as described previously [20,21]. Mfa1 fimbriae were also purified from mfa5 mutant FMFA5, in which mfa5 was disrupted by ermF-B, and genetic complementation strain FMFA5C, as described previously [21,22]. Briefly, P. gingivalis cells were collected from 2 L of culture, suspended in 40 mL of 20 mM Tris/HCl buffer at pH 8.0, supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride and 1 mM Nα-p-tosyl-L-lysine chloromethyl ketone). The cells were disrupted by a French press. Unbroken cells were removed by centrifugation. The supernatant was subjected to precipitation at 50% ammonium sulfate saturation. The precipitate was dialyzed with 20 mM Tris/HCl buffer at pH 8.0, and then applied to DEAE Sepharose Fast Flow chromatography (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) with 50 mL of bed volume. After washing thoroughly with the buffer, sample was fractionated by a linear gradient elution with 400 mL of NaCl (0 to 0.3 M) in the buffer. Purity and identity were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA) and transmission electron microscopy. Details are described in the Supplementary Material.
2.3. RT2 Profiler PCR Array Analysis
MGF (1 × 106 cells/dish) were seeded onto 60-mm dishes. When the cells reached confluency, they were incubated for 2 hours in the presence or absence of 1 μg/mL Mfa1, FMFA5, FMFA5C, FimA, or LPS of P. gingivalis. Total RNA was then collected. Complementary deoxyribonucleic acid (cDNA) was synthesized using the RT2 First Strand Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions and then applied to the Mouse Antibacterial Response RT2Profile PCR Array (Qiagen) and the Mouse Extracellular Matrix & Adhesion Molecules RT2Profile PCR Array (Qiagen). Amplification was performed using the RT2 SYBR Green/ROX qPCR master mix (Qiagen) and the StepOnePlusTM Real-time PCR system (Thermo Fisher Scientific) with associated software (version 2.3; Thermo Fisher Scientific). CT values were transferred to an Excel file to build a table of CT values, which was then uploaded onto the data analysis web portal at http://www.qiagen.com/geneglobe.
2.4. Real-Time PCR
MGFs (1 × 106 cells/dish) were seeded onto 60-mm dishes. When the cells reached confluency, they were incubated for 2 hours in the presence or absence of 1 μg/mL JI-1, FMFA5, FMFA5C, FimA or LPS of P. gingivalis. Total RNA was then extracted with a Nucleospin RNA kit (Macherey-Nagel Inc., Bethlehem, PA, USA) according to the manufacturer’s instructions, and purity and concentration were assessed by calculating the A230/A260 and A260/A280 ratios using a NanoDrop Lite (Thermo Fisher Scientific). To quantify mRNA, quantitative PCR was performed using the TaqMan gene expression assay (Thermo Fisher Scientific) for mouse Cxcl1 (Mm04207460_m1), Cxcl3 (Mm01701838), Icam1 (Mm00516023_m1), Sele (Mm00441278_m1), Tlr2 (Mm00442346_m1) and Tlr4 (Mm00445273_m1) with the TaqMan Universal PCR Master Mix (Thermo Fisher Scientific). mRNA levels were normalized to the level of eukaryotic 18S rRNA (Hs99999901_s1). Quantitative PCR was performed using the StepOnePlus Real-Time System. PCR conditions were 10 minutes at 95 °C, followed by 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. The relative amounts of target mRNAs were determined by subtracting the cycle threshold (CT) value for 18S rRNA from that for the gene (ΔCT). Then, the ΔCT value for the control group was subtracted from that for the experimental group (ΔΔCT). The results are expressed as the fold change (2-ΔΔCT) between the mRNA levels of control and experimental groups, where ΔΔCT was calculated as follows: ((CT for the target mRNA − CT for 18S rRNA) for the experimental group)–((CT for the target mRNA − CT for 18S rRNA) for the control group).
2.5. siRNA Transfection
MGFs were transfected with siRNA targeting TLR2 and TLR4 (Silencer Select Pre-designed siRNAs, Ambion, Austin, TX, USA) or non-targeting control siRNA (Ambion) using Lipofectamine RNAiMAX (Thermo Fisher Scientific) according to the manufacturer’s protocol. Twenty-four hours after transfection, cells were stimulated with 1 μg/mL Mfa1, FMFA5, FMFA5C, FimA or LPS of P. gingivalis for 2 hours. The cells were then collected and TLR2 and TLR4 protein levels were determined by flow cytometry. Similarly, gene expression levels were determined by Real-Time PCR.
2.6. Flow Cytometry
MGFs (1 × 106 cells in 100 μL) were incubated with anti-mouse CD282 (TLR2) phycoerythrin (PE) (BioLegend, San Diego, CA, USA, Cat: 148604), anti-mouse CD284 (TLR4) phycoerythrin (PE) (BioLegend, Cat: 117605), or isotype control antibody phycoerythrin (PE) (BioLegend, Cat: 400508) and analyzed by flow cytometry using a MACSQuant analyzer and MACSQuantify software version 2.4 (Miltenyi Biotec, Tokyo, Japan).
2.7. Statistical Analysis
Data were analyzed using PASW Statistics software (version 18.0; SPSS Japan, Tokyo, Japan). Differences among groups were examined by one-factor analysis of variance (ANOVA) and Bonferroni’s multiple comparison test. Comparisons of two independent groups were performed using Student’s t-test. Data are expressed as the mean ± standard deviation (SD). Significance was accepted at p < 0.05.
3. Results
3.1. Analysis of Antibacterial Response-Associated Genes in Gingival Fibroblasts to Various Fimbriae
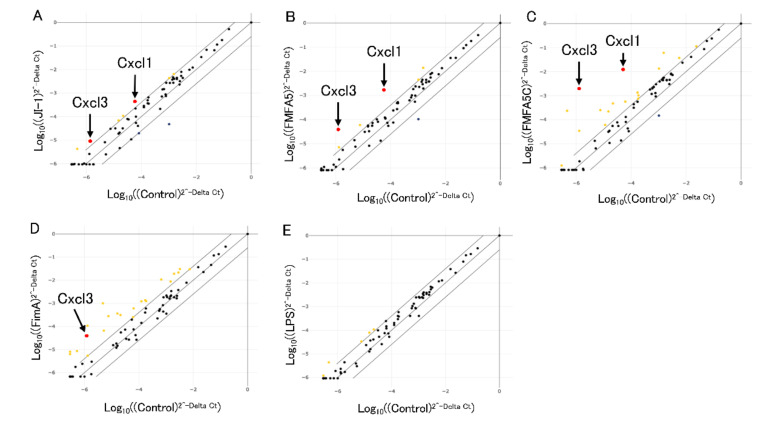
A mouse antibacterial response PCR array was used to investigate differences in the expression of 84 genes involved in bacteria-cell interactions. Figure 1 shows the fold changes in expression between control and 1 μg/mL various fimbriae or P. gingivalis LPS after stimulation for 2 hours. Among the 84 genes, Cxcl1 and Cxcl3 were upregulated in common by 4-fold in JI-1, FMFA5 or FMFA5C-stimulated cells compared with non-stimulated cells (Figure 1A–C). Other genes that were elevated include Nfkbia, Jun, Ccl5, Tlr6, Nod2 in JI-1 stimulated cells, Nfkbia, Jun, Irf5, Birc3 in FMFA5-stimulated cells, and Hsp90aa1, Nfkb1, Nfkbia, Jun, Nod1, Slpi, Tirap, Rela, Ccl5, Tlr2, Irf5, Irf7, Nod2, Birc3, Lcn2 in FMFA5C-stimulated cells. Many genes (Tnfrsf1a, Mapk1, Tollip, Irak1, Fadd, Map3k7, Map2k4, Slpi, Ripk2, Il18, Rela, Irak3, Card6, Camp, Irf5, Birc3, Slc11a1, Tlr9, Casp1, Crp), including Cxcl3 were up-regulated in FimA-stimulated cells compared with non-stimulated cells (Figure 1D). P. gingivalis LPS stimulation changed several genes expression (Ccl5, Tlr6, Irf5, Nod2, Casp1) compared with non-stimulated cells (Figure 1E).
Figure 1.
Analysis of antibacterial response-associated genes in mouse gingival fibroblasts in response to Mfa1, FimA, or Porphyromonas gingivalis LPS. Graphs show the fold changes of gene expression in cells stimulated with JI-1 (A), FMFA5 (B), FMFA5C (C), FimA (D), and LPS (E) compared with non-stimulated cells. Cxcl1 and Cxcl3 were up-regulated over 4-fold in JI-1, FMFA5 or FMFA5C-stimulated cells.
3.2. Confirmation of PCR Array Data for Selected Antibacterial Response-Associated Genes by Quantitative Real Time-PCR
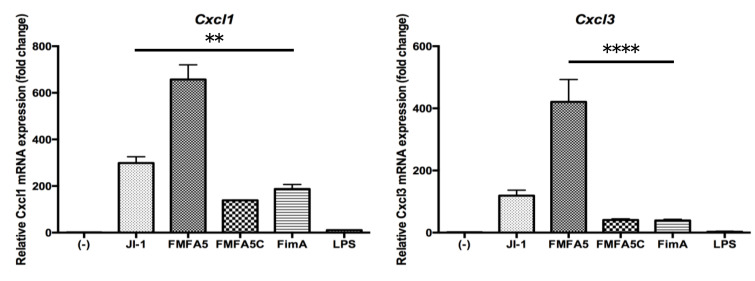
To validate the PCR array data, Real Time-PCR showed that JI-1 stimulation increased the gene expression of the cell migration factors, Cxcl1 and Cxcl3, compared with FimA stimulation (Figure 2). Furthermore, the highest increase in gene expression was observed with FMFA5 stimulation (Figure 2).
Figure 2.
Mfa1 induces Cxcl1 and Cxcl3 in mouse gingival fibroblasts. Mouse gingival fibroblasts (MGFs) were cultured for 2 hours in the presence or absence of 1 μg/mL JI-1, FMFA5, FMFA5C, FimA, or LPS of P. gingivalis and then mRNA levels were examined using real-time PCR. Values are expressed as fold changes. Differences among groups were analyzed by one-way ANOVA. Data represent the mean + SD (n = 3). ** p < 0.01, **** p < 0.0001.
3.3. Analysis of Extracellular Matrix and Adhesion Molecule-Associated Genes in Gingival Fibroblasts in Response to Various Fimbriae
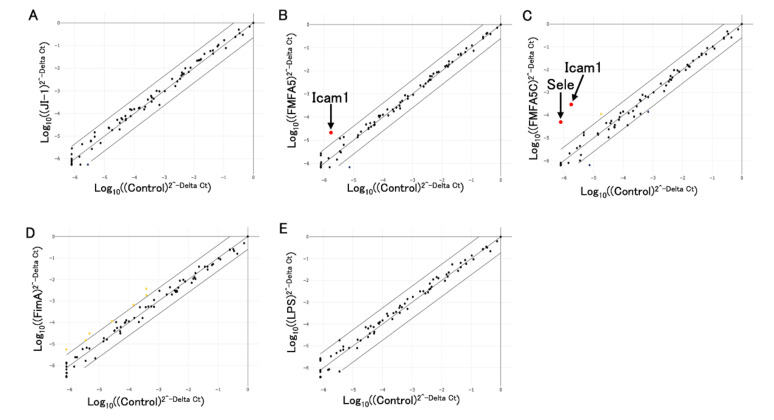
A mouse extracellular matrix and adhesion molecules PCR array was used to investigate differences in the expression of 84 genes involved in cell–cell and cell–matrix interactions. Figure 3 shows the fold changes in expression between control and 1 μg/mL various fimbriae or P. gingivalis LPS after stimulation for 2 hours. Among the 84 genes, Icam1 expression was upregulated 4-fold in FMFA5-stimulated cells compared with non-stimulated cells (Figure 3B). FMFA5C stimulation similarly induced Icam1 and also Selectin E (Sele) (Figure 3C). No obvious changes in gene expression were observed with JI-1, FimA or P. gingivalis LPS stimulation compared with unstimulated cells (Figure 3A,D,E).
Figure 3.
Analysis of extracellular matrix and adhesion molecule-associated genes in mouse gingival fibroblasts in response to Mfa1, FimA, or P. gingivalis LPS. Graphs show fold changes of gene expression in cells stimulated with JI-1 (A), FMFA5 (B), FMFA5C (C), FimA (D), and LPS (E) compared with non-stimulated cells. Icam1 and Sele were up-regulated over 4-fold in FMFA5C-stimulated cells.
3.4. Confirmation of PCR Array Data for Selected Extracellular Matrix and Adhesion Molecule-Associated Genes by Quantitative RT-PCR
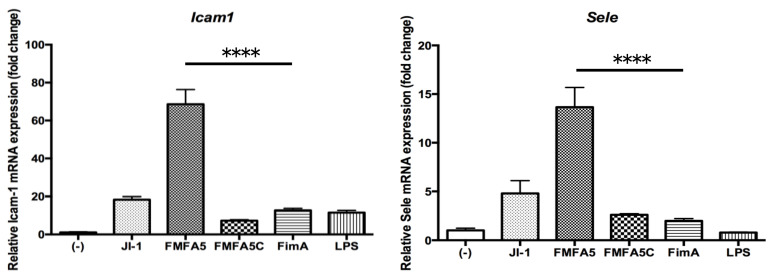
Expression of cell adhesion factors, Icam1 and Sele, was increased to a greater extent by JI-1 stimulation compared with FimA stimulation (Figure 4). The highest increase in gene expression was observed with FMFA5 stimulation (Figure 4).
Figure 4.
Mfa1 induces Icam1 and Sele in mouse gingival fibroblasts. MGFs were cultured for 2 hours in the presence or absence of 1 μg/mL JI-1, FMFA5, FMFA5C, FimA, or LPS of P. gingivalis and then mRNA levels were examined using real-time PCR. Values are expressed as fold changes. Differences among groups were analyzed by one-way ANOVA. Data represent the mean + SD (n = 3). **** p < 0.0001.
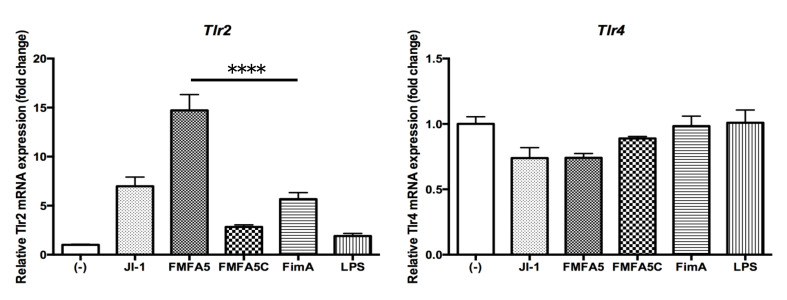
3.5. Induction of Tlr2 and Tlr4 Gene Expression by Various Fimbriae and LPS Stimulation in Gingival Fibroblasts
Tlr2 showed the same change in expression as the other factors, such as Cxcl1, but Tlr4, the receptor for LPS, did not show any significant variation in gene expression in response to various stimulants (Figure 5).
Figure 5.
Mfa1 induces TLR2 but not TLR4 in mouse gingival fibroblasts. MGFs were cultured for 2 hours in the presence or absence of 1 μg/mL JI-1, FMFA5, FMFA5C, FimA or LPS of P. gingivalis and then mRNA levels were examined using real-time PCR. Values are expressed as fold changes. Differences among groups were analyzed by one-way ANOVA. Data represent the mean + SD (n = 3). **** p < 0.0001.
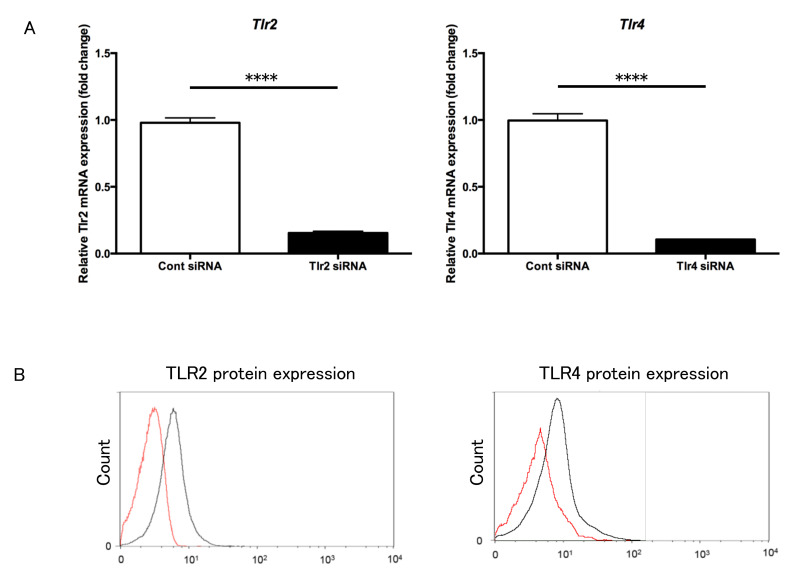
3.6. Transfection of Tlr2 and Tlr4 siRNA into Gingival Fibroblasts
Tlr2 and Tlr4siRNA-transfected gingival fibroblast cells showed obvious knockdown of Tlr2 and Tlr4mRNA, respectively, compared with control siRNA-transfected gingival fibroblast cells (Figure 6A). FACS analysis confirmed a decrease in the surface expression of Tlr2 and Tlr4 in the respective siRNA-transfected gingival fibroblast cells compared with control cells (Figure 6B).
Figure 6.
Suppression of Tlr2 and Tlr4 in mouse gingival fibroblasts using siRNA. (A) Tlr2 (left) and Tlr4 (right) mRNA levels were examined using real-time PCR. Tlr2 and Tlr4 siRNA-transfected cells showed clear knockdown of Tlr2 and Tlr4 mRNAs, respectively, compared with control siRNA-transfected cells. (B) TLR2 (left) and TLR4 (right) protein levels were examined using flow cytometry. Tlr2 or Tlr4 siRNA transfected cells (red line) showed decreased levels of the respective receptors compared with control siRNA-transfected cells (black line). **** p < 0.0001.
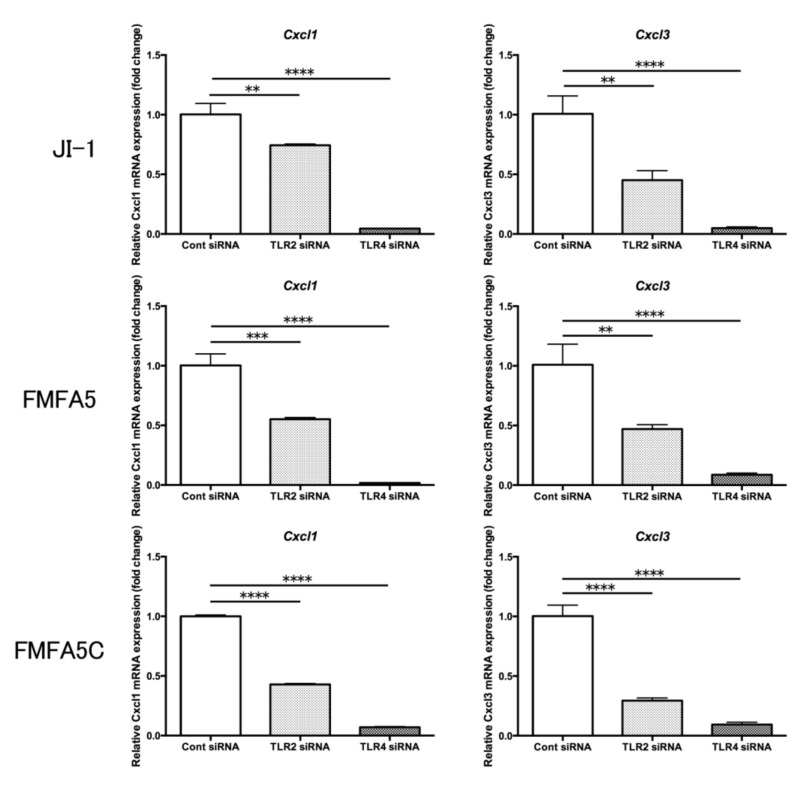
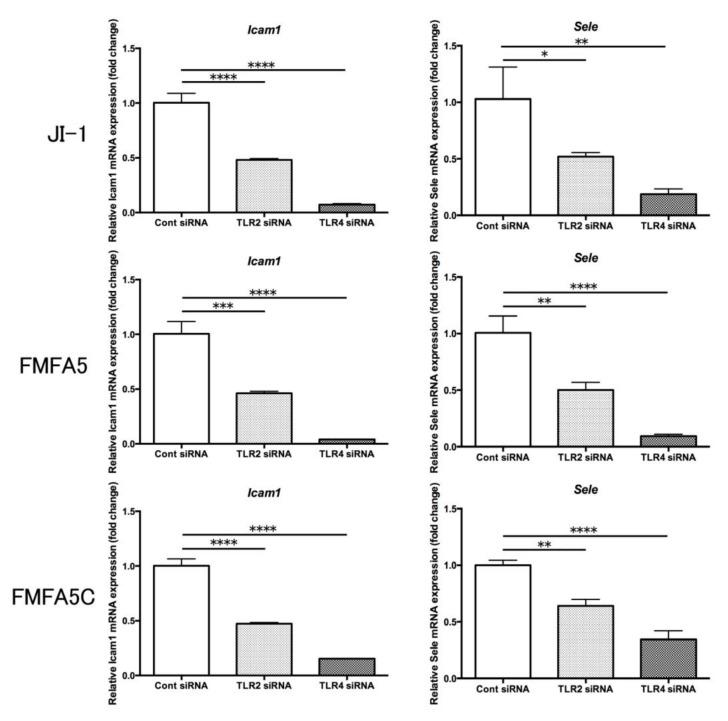
3.7. Expression of Selected Genes in Tlr2 and Tlr4 siRNA-Transfected Cells
Expression of the cell migration-related factor and cell adhesion factor genes in JI-1, FMFA5, and FMFA5C-stimulated Tlr4 siRNA-transfected cells were significantly suppressed compared with control siRNA-transfected cells (Figure 7). Also, the suppression of Tlr2 expression partially attenuated the stimulation effect (Figure 7).
Figure 7.
Involvement of TLR2 and TLR4 in the expression of Mfa1-induced cell migration and cell adhesion-related factors. Tlr2 siRNA, Tlr4 siRNA, or control siRNA-transfected mouse gingival fibroblasts were cultured for 2 hours in the presence or absence of 1 μg/mL JI-1, FMFA5, or FMFA5C and then mRNA levels were examined using real-time PCR. Values are expressed as fold changes. Differences among groups were analyzed by one-way ANOVA. Data represent the mean + SD (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
4. Discussion
In this study, we demonstrated that Mfa1 fimbriae stimulation markedly increased the expression of cell migration/cell adhesion-related genes in mouse gingival fibroblasts, and that the increase was more pronounced than with FimA stimulation. In addition, the ability of Mfa1 to regulate cell migration and cell adhesion was significantly attenuated in mouse gingival fibroblasts in which Tlr4 expression was suppressed.
When the effect of JI-1 on gingival fibroblasts, which constitute the gingival connective tissue, was examined, a remarkable increase in the expression of Cxcl1 and Cxcl3, which are involved in cell migration, was observed. Higher levels of CXCL1 were found in human and rat gingiva from periodontitis sites compared with periodontally healthy sites [23]. There was also a significant difference in the level of CXCL1 in gingival crevicular fluid between healthy and periodontitis subjects [24]. CXCL1 stimulates gingiva fibroblast migration [25] and it may be related to periodontal tissue healing. There is no literature reporting the relationship between CXCL3 and periodontitis, but its activity is similar to that of CXCL2, which may play an important role in the initiation of inflammation and subsequent periodontal tissue destruction [26], which suggests that it is involved in the progression of periodontitis. JI-1 stimulation produced a higher increase in Cxcl1 and Cxcl3 expression compared with FimA stimulation. FimA induces Cxcl1 expression in mouse peritoneal macrophages [27]. It is possible that the immunomodulatory capacity of Mfa1 outperforms that of FimA for some factors in certain cell types.
Next, we investigated which part of the fimbriae structure is important for the immunomodulatory ability of Mfa1. FMFA5, with the Mfa3–5 tip structure of JI-1 removed, and FMFA5C, with the tip structure was complemented in FMFA5, were compared for their ability to induce immunomodulation. Compared with JI-1, a markedly higher increase in gene expression was observed with FMFA5 stimulation, while that of FMFA5C was significantly reduced. From this result, it is indicated that the incomplete fimbriae deficient in tip structure increase the stimulation ability.
When we confirmed the effects of Mfa1 on extracellular matrix and adhesion molecules, we found a marked increase in the expression of the cell adhesion factor genes, Icam1 and Sele. ICAM1 deficiencies increase susceptibility to and severity of alveolar bone loss after P. gingivalis infection [28]. Selectin Platelet/Selectin E-deficient mice exhibit spontaneous early onset alveolar bone loss [29]. FimA and Mfa1 induce ICAM1 and Selectin E in human aortic endothelial cells [30]. These fimbriae appear to have similar effects on gingival fibroblasts. Similar to the results for cell migration factors, a markedly higher increase in cell adhesion factor gene expression was observed with FMFA5 stimulation and the stimulation ability of FMFA5C was significantly reduced compared with JI-1 stimulation. These results indicate that the regulation of cell adhesion factors by Mfa1 is also greatly influenced by the Mfa1 molecule in the shaft portion.
Cells use pattern recognition receptors, such as TLRs, to recognize pathogen-associated molecular patterns (PAMPs). Ten TLRs have been identified in humans and 12 in mice and the host innate immune response to pathogens is largely mediated via TLR signaling [31]. TLR2 and TLR4 are the most widely studied extracellular innate receptors that recognize various PAMPs and are likely to play a role in the pathogenesis of periodontitis [32]. TLR2 has been shown to be important for P. gingivalis to produce inflammatory cytokines [33,34]. TLR4 recognizes LPS (from Escherichia coli), which is a bacterial cell wall component [31]. Uniquely, P. gingivalis LPS is recognized by both TLR2 and TLR4 [35]. The LPS1435/1449 and LPS1690 isoforms can produce opposite effects on TLR2 and TLR4 activation [36,37]. P. gingivalis LPS and E. coli LPS regulate cytokine production differently in human gingival fibroblasts [38]. The heterogeneity of P. gingivalis LPS might contribute to one of the strategies used by P. gingivalis to evade the innate host defense in gingival tissues [39]. FimA activates human peripheral blood monocytes via TLR2 and CD14 [40]. TLR2-dependent signaling leads to CD11b-CD18 activation in human monocytes upon recognition of FimA through CD14 [41]. However, the receptor for Mfa1 is still unclear. The gingival fibroblasts constitutively express TLR2 and TLR4 [42]. When we examined the expression of Tlr2 and Tlr4 genes in MGFs stimulated by various fimbriae and LPS, Tlr2 showed the same changes in expression as the other factors, but Tlr4 did not show any significant changes in expression in response to various fimbriae or LPS. Therefore, to clarify the receptor of Mfa1, MGFs in which Tlr2 and Tlr4 were knocked down were stimulated with Mfa1 and their reactivity was assessed. Control siRNA, Tlr2 siRNA and Tlr4 siRNA were introduced into MGFs, and cells were stimulated with JI-1, FMFA5, and FMFA5C. Gene expression of cell migration-related genes, Cxcl1 and Cxcl3, and cell adhesion genes, Icam1 and Sele, were analyzed. The suppression of TLR4 was significantly reduced by Mfa1 fimbriae stimulation. Also, suppression of Tlr2 partially attenuated this stimulation. Recognition of Mfa1 by TLR4 is suggested to be essential for the expression of genes related to cell migration and cell adhesion. This result is different from previous reports in which anti-TLR2 antibody pretreatment significantly inhibited pro-inflammatory cytokines in mouse macrophages stimulated with Mfa1 fimbriae [43]. Conversely, Hajishengallis et al. reported that native Mfa1 induced proinflammatory cytokines in a CD14- and TLR2-dependent mode, which was likely due to a fimbriae-associated 12-kDa lipoprotein [44]. There is a similar report on FimA-like lipoproteins or lipopeptides associated with FimA that could, at least in part, account for signaling via TLR2 and subsequent TNF-α production in macrophages [45]. Furthermore, the following reports demonstrate the stimulation ability of LPS. A lipoprotein from P. gingivalis LPS was shown to be a principal component for TLR2-mediated cell activation [46]. A lipopolysaccharide preparation extracted from a P. gingivalis lipoprotein-deficient mutant showed a marked decrease in TLR2-mediated signaling [47]. Recombinant FimA stimulated cytokine release in THP-1 mononuclear cells via CD14 and TLR4 but not TLR2 [44], while recombinant FimA induced an inflammatory response via the TLR4/NF-kB signaling pathway in human peripheral blood mononuclear cells [48]. P. gingivalis lipid A and its synthetic counterpart activate cells through a TLR4-dependent pathway [46,49]. From our results, we speculate that wild-type purified Mfa1 is mainly recognized by TLR4, but TLR2 might recognize the lipoprotein of fimbriae and contributes to the overall action. In the future, it is necessary to confirm the reactivity of purified Mfa1 from which lipoprotein has been removed.
There are several reports on the importance of TLR4 in periodontitis. TLR4- but not TLR2-mediated stimulation, was positively associated with plaque score and bleeding on probing score of teeth from which the plaque samples were taken [4]. The ratio of TLR4/TLR2-mediated stimulation activity was also positively associated with probing depth and clinical attachment level [4]. TLR4- but not TLR2-stimulation of subgingival plaque is associated with plaque index [50]. Therefore, TLR4 may play an important role in the progression of periodontitis, in which stimulation by Mfa1 may play a role.
Recent reports suggest that intracellular DC-SIGN, an intracellularly expressed pattern recognition receptor, could be critical for recognition of Mfa1 by dendritic cells [51]. We assayed expression of DC-SIGN in MGFs after stimulation with various fimbriae, but we could not detect its expression. This may be because of differences between immunocompetent cells and periodontal tissue constituent cells.
In conclusion, Mfa1 fimbriae have a significant effect on immunomodulation in gingival fibroblasts of periodontal tissue. We also suggest that recognition of Mfa1 by TLR4 on MGFs is essential for the expression of genes related to cell migration and cell adhesion. More detailed analysis, such as using an animal infection model, is needed to assess the immunomodulatory capacity of Mfa1 fimbriae in the progression of periodontitis.
Acknowledgments
We thank Jeremy Allen, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/12/4004/s1, Figure S1: Purity of Mfa1 fimbriae.
Author Contributions
Conceptualization, Y.T., T.K., Y.H., and A.M.; methodology, Y.T., T.K., Y.H., and A.M.; validation, Y.T., T.K., and A.M.; formal analysis, Y.T., H.G., I.O., Y.S. (0000-0002-7536-5931), N.S., and T.O. (Tasuku Ohno); investigation, Y.T., Y.N., H.G., K.O., I.O., Y.K., Y.S. (0000-0002-7536-5931), N.S., T.O. (Teppei Okabe), and Y.S.(0000-0003-3660-6867); data curation, Y.K., Y.S. (0000-0003-3660-6867), and S.K.; writing—original draft preparation, Y.T., T.K., and A.M.; writing—review and editing, Y.T., T.K., Y.H., J.-I.H., and A.M.; visualization, Y.T. and T.K.; supervision, T.K., Y.H., J.H., and A.M.; project administration, A.M.; funding acquisition, T.K., Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (No.20K09985, No. 20K09931 and No.17K11999) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Riviere G.R., Smith K.S., Tzagaroulaki E., Kay S.L., Zhu X., DeRouen T.A., Adams D.F. Periodontal status and detection frequency of bacteria at sites of periodontal health and gingivitis. J. Periodontol. 1996;67:109–115. doi: 10.1902/jop.1996.67.2.109. [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka H., Yoshimura A., Kaneko T., Golenbock D.T., Hara Y. Analysis of the activity to induce toll-like receptor (TLR)2- and TLR4-mediated stimulation of supragingival plaque. J. Periodontol. 2008;79:920–928. doi: 10.1902/jop.2008.070516. [DOI] [PubMed] [Google Scholar]
- 5.Darveau R.P., Hajishengallis G., Curtis M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012;91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamont R.J., Jenkinson H.F. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/MMBR.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hospenthal M.K., Costa T.R.D., Waksman G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017;15:365–379. doi: 10.1038/nrmicro.2017.40. [DOI] [PubMed] [Google Scholar]
- 8.Lamont R.J., Jenkinson H.F. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000;15:341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura F., Murakami Y., Nishikawa K., Hasegawa Y., Kawaminami S. Surface components of Porphyromonas gingivalis. J. Periodontal. Res. 2009;44:1–12. doi: 10.1111/j.1600-0765.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 10.Enersen M., Nakano K., Amano A. Porphyromonas gingivalis fimbriae. J. Oral Microbiol. 2013;5:20265. doi: 10.3402/jom.v5i0.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikai R., Hasegawa Y., Izumigawa M., Nagano K., Yoshida Y., Kitai N., Lamont R.J., Yoshimura F., Murakami Y. Mfa4, an Accessory Protein of Mfa1 Fimbriae, Modulates Fimbrial Biogenesis, Cell Auto-Aggregation, and Biofilm Formation in Porphyromonas gingivalis. PLoS ONE. 2015;10:e0139454. doi: 10.1371/journal.pone.0139454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall M., Hasegawa Y., Yoshimura F., Persson K. Structural and functional characterization of shaft, anchor, and tip proteins of the Mfa1 fimbria from the periodontal pathogen Porphyromonas gingivalis. Sci. Rep. 2018;8:1793. doi: 10.1038/s41598-018-20067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yilmaz O., Watanabe K., Lamont R.J. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4:305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Amano A., Nakagawa I., Okahashi N., Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontol. Res. 2004;39:136–142. doi: 10.1111/j.1600-0765.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 15.Hajishengallis G., Wang M., Liang S., Shakhatreh M.A., James D., Nishiyama S., Yoshimura F., Demuth D.R. Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Adv. Exp. Med. Biol. 2008;632:203–219. doi: 10.1007/978-0-387-78952-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amano A. Host-parasite interactions in periodontitis: Subgingival infection and host sensing. Periodontology 2000. 2010;52:7–11. doi: 10.1111/j.1600-0757.2009.00328.x. [DOI] [PubMed] [Google Scholar]
- 17.Amano A. Host-parasite interactions in periodontitis: Microbial pathogenicity and innate immunity. Periodontology 2000. 2010;54:9–14. doi: 10.1111/j.1600-0757.2010.00376.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuboniwa M., Amano A., Hashino E., Yamamoto Y., Inaba H., Hamada N., Nakayama K., Tribble G.D., Lamont R.J., Shizukuishi S. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009;9:105. doi: 10.1186/1471-2180-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umemoto T., Hamada N. Characterization of biologically active cell surface components of a periodontal pathogen. The roles of major and minor fimbriae of Porphyromonas gingivalis. J. Periodontol. 2003;74:119–122. doi: 10.1902/jop.2003.74.1.119. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa Y., Nagano K., Ikai R., Izumigawa M., Yoshida Y., Kitai N., Lamont R.J., Murakami Y., Yoshimura F. Localization and function of the accessory protein Mfa3 in Porphyromonas gingivalis Mfa1 fimbriae. Mol. Oral Microbiol. 2013;28:467–480. doi: 10.1111/omi.12040. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa Y., Nagano K., Murakami Y., Lamont R.J. Purification of Native Mfa1 Fimbriae from Porphyromonas gingivalis. Methods Mol. Biol. 2021;2210:75–86. doi: 10.1007/978-1-0716-0939-2_8. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa Y., Iijima Y., Persson K., Nagano K., Yoshida Y., Lamont R.J., Kikuchi T., Mitani A., Yoshimura F. Role of Mfa5 in Expression of Mfa1 Fimbriae in Porphyromonas gingivalis. J. Dent. Res. 2016;95:1291–1297. doi: 10.1177/0022034516655083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath-Deschner B., Memmert S., Damanaki A., Nokhbehsaim M., Eick S., Cirelli J.A., Gotz W., Deschner J., Jager A., Nogueira A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues-in vitro and in vivo studies. Clin. Oral Investig. 2020;24:3661–3670. doi: 10.1007/s00784-020-03244-1. [DOI] [PubMed] [Google Scholar]
- 24.Sakai A., Ohshima M., Sugano N., Otsuka K., Ito K. Profiling the cytokines in gingival crevicular fluid using a cytokine antibody array. J. Periodontol. 2006;77:856–864. doi: 10.1902/jop.2006.050340. [DOI] [PubMed] [Google Scholar]
- 25.Buskermolen J.K., Roffel S., Gibbs S. Stimulation of oral fibroblast chemokine receptors identifies CCR3 and CCR4 as potential wound healing targets. J. Cell. Physiol. 2017;232:2996–3005. doi: 10.1002/jcp.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyauchi M., Kitagawa S., Hiraoka M., Saito A., Sato S., Kudo Y., Ogawa I., Takata T. Immunolocalization of CXC chemokine and recruitment of polymorphonuclear leukocytes in the rat molar periodontal tissue after topical application of lipopolysaccharide. Histochem. Cell. Biol. 2004;121:291–297. doi: 10.1007/s00418-004-0636-6. [DOI] [PubMed] [Google Scholar]
- 27.Hanazawa S., Murakami Y., Takeshita A., Kitami H., Ohta K., Amano S., Kitano S. Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: Role of protein kinase C. Infect. Immun. 1992;60:1544–1549. doi: 10.1128/IAI.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker P.J., DuFour L., Dixon M., Roopenian D.C. Adhesion molecule deficiencies increase Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000;68:3103–3107. doi: 10.1128/IAI.68.6.3103-3107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederman R., Westernoff T., Lee C., Mark L.L., Kawashima N., Ullman-Culler M., Dewhirst F.E., Paster B.J., Wagner D.D., Mayadas T., et al. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J. Clin. Periodontol. 2001;28:569–575. doi: 10.1034/j.1600-051x.2001.028006569.x. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y., Davey M., Yumoto H., Gibson F.C., 3rd, Genco C.A. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell Microbiol. 2006;8:738–757. doi: 10.1111/j.1462-5822.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 31.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 32.Hajishengallis G., Lambris J.D. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns E., Bachrach G., Shapira L., Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe N., Yokoe S., Ogata Y., Sato S., Imai K. Exposure to Porphyromonas gingivalis Induces Production of Proinflammatory Cytokine via TLR2 from Human Respiratory Epithelial Cells. J. Clin. Med. 2020;9:3433. doi: 10.3390/jcm9113433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darveau R.P., Pham T.T., Lemley K., Reife R.A., Bainbridge B.W., Coats S.R., Howald W.N., Way S.S., Hajjar A.M. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen I., Singhrao S.K. Importance of heterogeneity in Porhyromonas gingivalis lipopolysaccharide lipid A in tissue specific inflammatory signalling. J. Oral Microbiol. 2018;10:1440128. doi: 10.1080/20002297.2018.1440128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herath T.D.K., Darveau R.P., Seneviratne C.J., Wang C.Y., Wang Y., Jin L. Heterogeneous Porphyromonas gingivalis LPS modulates immuno-inflammatory response, antioxidant defense and cytoskeletal dynamics in human gingival fibroblasts. Sci Rep. 2016;6:29829. doi: 10.1038/srep29829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrukhov O., Ertlschweiger S., Moritz A., Bantleon H.P., Rausch-Fan X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontol. Scand. 2014;72:337–345. doi: 10.3109/00016357.2013.834535. [DOI] [PubMed] [Google Scholar]
- 39.Herath T.D., Darveau R.P., Seneviratne C.J., Wang C.Y., Wang Y., Jin L. Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PLoS ONE. 2013;8:e58496. doi: 10.1371/journal.pone.0058496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa T., Asai Y., Hashimoto M., Uchida H. Bacterial fimbriae activate human peripheral blood monocytes utilizing TLR2, CD14 and CD11a/CD18 as cellular receptors. Eur. J. Immunol. 2002;32:2543–2550. doi: 10.1002/1521-4141(200209)32:9<2543::AID-IMMU2543>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Harokopakis E., Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur. J. Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 42.Tabeta K., Yamazaki K., Akashi S., Miyake K., Kumada H., Umemoto T., Yoshie H. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect. Immun. 2000;68:3731–3735. doi: 10.1128/IAI.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiramine H., Watanabe K., Hamada N., Umemoto T. Porphyromonas gingivalis 67-kDa fimbriae induced cytokine production and osteoclast differentiation utilizing TLR2. FEMS Microbiol. Lett. 2003;229:49–55. doi: 10.1016/S0378-1097(03)00788-2. [DOI] [PubMed] [Google Scholar]
- 44.Hajishengallis G., Martin M., Sojar H.T., Sharma A., Schifferle R.E., DeNardin E., Russell M.W., Genco R.J. Dependence of bacterial protein adhesins on toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 2002;9:403–411. doi: 10.1128/CDLI.9.2.403-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki Y., Tabeta K., Murakami Y., Yoshimura F., Yamazaki K. Analysis of immunostimulatory activity of Porphyromonas gingivalis fimbriae conferred by Toll-like receptor 2. Biochem. Biophys. Res. Commun. 2010;398:86–91. doi: 10.1016/j.bbrc.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto M., Asai Y., Ogawa T. Separation and structural analysis of lipoprotein in a lipopolysaccharide preparation from Porphyromonas gingivalis. Int. Immunol. 2004;16:1431–1437. doi: 10.1093/intimm/dxh146. [DOI] [PubMed] [Google Scholar]
- 47.Asai Y., Hashimoto M., Fletcher H.M., Miyake K., Akira S., Ogawa T. Lipopolysaccharide preparation extracted from Porphyromonas gingivalis lipoprotein-deficient mutant shows a marked decrease in toll-like receptor 2-mediated signaling. Infect. Immun. 2005;73:2157–2163. doi: 10.1128/IAI.73.4.2157-2163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai J., Chen J., Guo H., Pan Y., Zhang Y., Zhao W., Li X., Li Y. Recombinant fimbriae protein of Porphyromonas gingivalis induces an inflammatory response via the TLR4/NFkappaB signaling pathway in human peripheral blood mononuclear cells. Int. J. Mol. Med. 2019;43:1430–1440. doi: 10.3892/ijmm.2019.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reife R.A., Coats S.R., Al-Qutub M., Dixon D.M., Braham P.A., Billharz R.J., Howald W.N., Darveau R.P. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: Differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell Microbiol. 2006;8:857–868. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 50.Ziauddin S.M., Montenegro Raudales J.L., Sato K., Yoshioka H., Ozaki Y., Kaneko T., Yoshimura A., Hara Y. Analysis of Subgingival Plaque Ability to Stimulate Toll-Like Receptor 2 and 4. J. Periodontol. 2016;87:1083–1091. doi: 10.1902/jop.2016.150573. [DOI] [PubMed] [Google Scholar]
- 51.El-Awady A.R., Miles B., Scisci E., Kurago Z.B., Palani C.D., Arce R.M., Waller J.L., Genco C.A., Slocum C., Manning M., et al. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 2015;10:e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.