Abstract
Purpose
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and estrogen-related receptor alpha (ERRα) play a vital role in various human cancers. The purpose of this study was to investigate whether the PGC-1α/ERRα axis could serve as an effective prognostic marker in ovarian cancer (OC).
Patients and Methods
We investigated the expression of both PGC-1α and ERRα in 42 ovarian cancer and 31 noncancerous ovarian samples by immunohistochemistry (IHC). The relationship between the expression of PGC-1α and ERRα in OC and the clinical characteristics of patients was evaluated. In addition, data from the Human Protein Atlas (HPA) database were collected to validate the prognostic significance of PGC-1α and ERRα mRNA expression in OC.
Results
PGC-1α and ERRα showed notably higher expression in OC tissues than in noncancerous tissues (P=0.0059, P=0.002). Moreover, in patients with OC, high ERRα and PGC-1α/ERRα expression significantly correlated with tumor differentiation (P=0.027; P=0.04), lymph node status (P=0.023; P=0.021), CA125 (P=0.036; P=0.021), and HE4 (P=0.021; P=0.05), while high PGC-1α expression was only significantly associated with tumor differentiation (P=0.029). The combined analysis of high PGC-1α and ERRα expression revealed a tendency towards poor cancer-specific survival (P=0.1276).
Conclusion
PGC-1α and ERRα are overexpressed in OC and might be significant prognostic factors for this cancer.
Keywords: prognostic significance, estrogen-related receptor alpha, immunohistochemistry, ovarian malignance, peroxisome proliferator-activated receptor gamma coactivator 1-alpha
Introduction
Ovarian cancer is one of the most fatal malignancies in women and poses a serious threat to women’s health. Despite some progress achieved with extensive clinical and basic research, the etiology and tumorigenesis of OC are not fully understood. Tumor heterogeneity leads to different stages and characteristics of disease development. Different molecular drivers may exist in patients with OC at the same stage, leading to heterogeneous treatment responses and prognosis.1 It is therefore urgent to investigate the molecular markers underlying ovarian cancer and identify novel therapeutic targets for OC treatment.
Estrogen-related receptor alpha (ESRRA, also known as ERRα), a member of the ligand-independent orphan nuclear receptor superfamily, is expressed primarily in tissues with high metabolic demand and controls the expression of genes involved in cellular energy metabolism.2–4 Notably, ERRα has been reported to be overexpressed in breast, colon, and endometrial cancers, and its overexpression is related to poor prognosis.5–8 Our prior studies have demonstrated that the mRNA levels of ERRα increase with the clinical stage of ovarian cancer, thus suggesting ERRα as a prognostic factor for ovarian cancer.7 ERRα also has potential diagnostic and therapeutic value in OC. ERRα activity highly relies on the presence of coregulatory proteins, most notably that of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A, also known as PGC-1α).9,10 The PGC-1α/ERRα axis has been implicated in controlling the expression of metabolic gene networks and mitochondrial biogenesis.11,12 Accumulating evidence suggests that the PGC-1α/ERRα axis plays a vital role in various cancer development and progression, which are accompanied by metabolic dysfunction.10,13 Our recent study indicated that PGC-1α and ERRα positively correlate with more advanced myometrial invasion in endometrial cancer, and were robust predictors for myometrial invasion.14
Currently, the suitability of PGC-1α and ERRα as biomarkers in OC tissues has not been thoroughly examined. To investigate whether the PGC-1α/ERRα axis acts as an effective prognostic marker for patients with OC, we detected the expression of these proteins in human OC tissues using immunohistochemistry and investigated their clinical significance in OC.
Patients and Methods
The HPA Database
First, we explored mRNA expression of PGC-1α and ERRα in normal tissues and ovarian cancer tissues from the HPA database (https://www.proteinatlas.org/). The HPA database provides abundant transcriptome and proteome data in specific human tissues through RNA-sequencing analysis and immunohistochemistry analysis.15,16 In addition, we analyzed the prognostic significance of PGC-1α and ERRα mRNA in patients with OC.
Patients and Tissue Samples
A total of 42 OC and 31 noncancer ovarian samples with related clinical data were obtained for immunohistochemistry from patients who received surgical therapy between September 2012 and April 2019. None of the patients received preoperative chemotherapy or radiotherapy. This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital affiliated with Fujian Medical University (approval number 2013-004), and performed in compliance with the Declaration of Helsinki. Informed consent was obtained from all patients. The patients were followed up postoperatively by their surgeons at 3-month intervals for 5 years and yearly thereafter. Overall survival (OS) was defined as the interval between surgery and mortality or the last follow-up (censored data for living patients). At the end of the follow-up period, seven patients (16.7%) died of OC.
Immunohistochemistry
To examine the expression of PGC-1α and ERRα in OC, we performed a tissue microarray constructed by Shanghai Zhuoli Biotechnology Co., Ltd (Zhuoli Biotechnology Co, Shanghai, China). Rabbit polyclonal anti-ERRα (ab93173, Abcam) and rabbit polyclonal anti-PGC-1 alpha-N-terminal (ab191838, Abcam) antibodies were used. In each case, 1–2 µm-thick sections from paraffin tissue blocks were cut, dewaxed, pretreated, and transferred to glass slides using an adhesive tape transfer system, in order to conduct ultraviolet cross linkage. All reactions were performed using an automated staining device. Two pathologists independently evaluated the quantitation of immunostaining for PGC-1α and ERRα, who were blinded to patient details. The expression of PGC-1α and ERRα in tumor parenchyma was semi-quantified by immunoreactivity score (IR score) based on intensity and heterogeneity. The IR score was determined as the sum of heterogeneity and intensity. The percentage of positive cells was scored as 0 points (0%), 1 point (1–25%), 2 points (26–50%), 3 points (51–75%), and 4 points (76–100%). Positive staining intensity was scored as 0 points (none), 1 point (low), 2 points (medium), and 3 points (high).
Statistical Analysis
Frequency and percentage were calculated for the classified variables. We used the Chi-square test to evaluate the differences in PGC-1α and ERRα expression between the two groups of clinicopathological features. The comparison between IR score in cancer foci and in noncancer lesions was analyzed by Student’s t-test. Cancer-specific survival curves were obtained using the Kaplan–Meier method and verified by the log-rank (Mantel–Cox) test. All statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism Version 8.0 software (GraphPad Software, Inc., La Jolla, CA, USA). All P-values in the statistical analysis were two-tailed, and P<0.05 was considered statistically significant.
Result
Expression and Prognostic Significance of PGC-1α and ERRα mRNA in the HPA Database
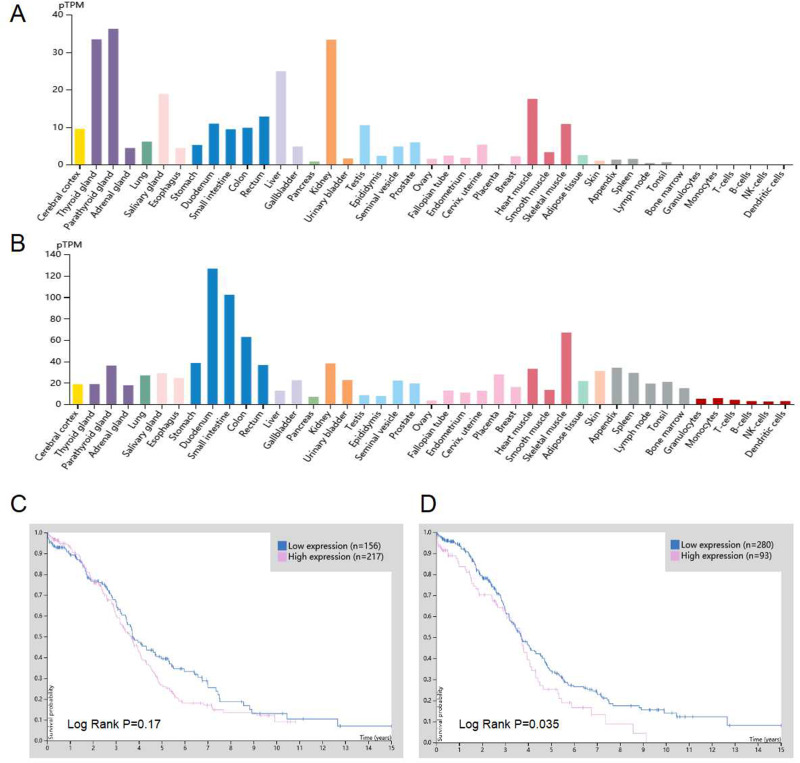
The mRNA expression profiles retrieved from HPA (https://www.proteinatlas.org/ENSG00000109819-PPARGC1A/tissue, https://www.proteinatlas.org/ENSG00000173153-ESRRA/tissue) revealed low mRNA expression of PGC-1α and ERRα in normal ovarian tissues (Figure 1A and B). We then explored the association between mRNA expression of PGC-1α and ERRα and survival outcome in OC from the HPA database (https://www.proteinatlas.org/ENSG00000109819-PPARGC1A/pathology, https://www.proteinatlas.org/ENSG00000173153-ESRRA/pathology). As shown in Figure 1C, the 5‑year OS rates in patients with high and low ERRα expression were 25% and 34%, respectively. Patients with high ERRα expression had significantly lower OS rates than those with low ERRα expression (P=0.035; Figure 1D). The 5‑year OS rates in patients with high and low PGC-1α expression were 27% and 39%, respectively. However, while patients with high PGC-1α expression tended to show poor cancer-specific survival rates, this association was not significant (P = 0.17; Figure 1C).
Figure 1.
Expression and prognostic significance of PGC-1α and ERRα mRNA in HPA database. (A and B) PGC-1α and ERRα mRNA expression in different normal human tissues. (C) Patients with high PGC-1α mRNA expression tend to have a poorer overall rate compared with those with low PGC-1α mRNA expression in OC (P = 0.17). (D) Patients with high ERRα mRNA expression exhibited a poorer overall rate compared with those with low ERRα mRNA expression in OC (P=0.035). All the pictures were downloaded from HPA database.
Abbreviations: ERRα, estrogen‑related receptor α; HPA, Human Protein Atlas; OC, ovarian cancer; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Expression of PGC-1α and ERRα in OC Tissues
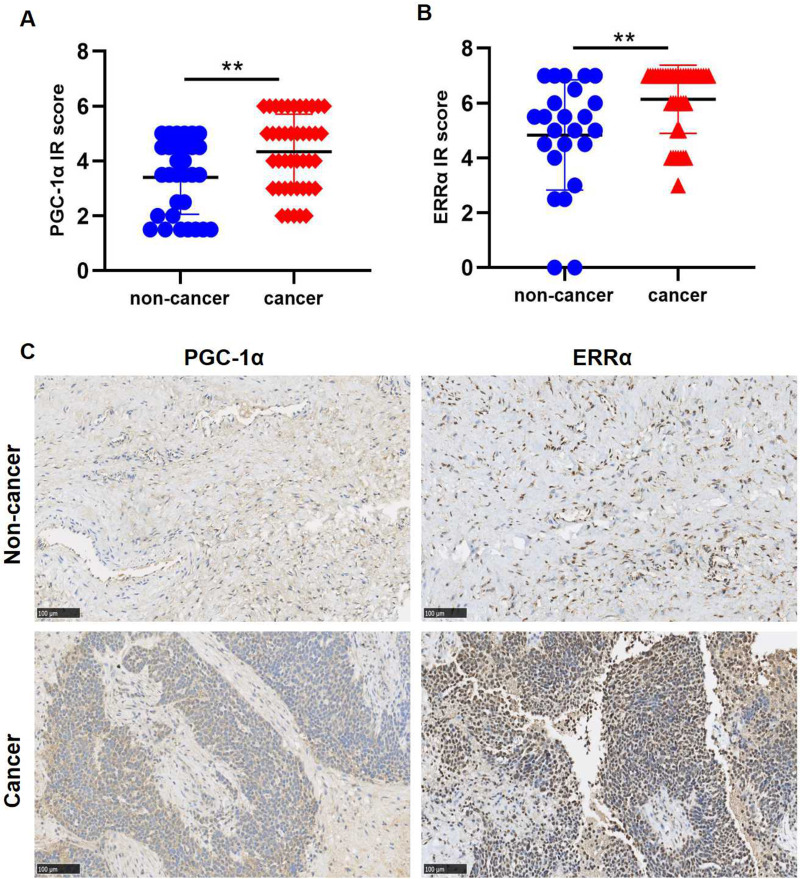
Next, we determined the protein expression of PGC-1α and ERRα in 42 OC tissues and 31 noncancerous ovarian tissues by IHC. Statistical analysis showed that OC tissues exhibited significantly higher PGC-1α and ERRα expression than noncancerous ovarian tissues (P=0.0059, P=0.002; Figure 2A and B). As shown in Figure 2C, ERRα was primarily detected in the nuclei of tumor cells, while PGC-1α was mainly detected in the cytoplasm. IHC also revealed that the expression of PGC-1α and ERRα was low in noncancerous tissues, but elevated in OC tissues.
Figure 2.
The expression of PGC-1α and ERRα in OC by IHC. (A and B) Ovarian tissues exhibited significantly higher PGC-1α and ERRα expression compared with noncancer ovarian tissues (P=0.0059, P=0.002). (C) PGC-1α and ERRα presented low expression in noncancer tissues while elevated PGC-1α and ERRα expression was recorded in the OC tissues. Original magnification, x200.
Note: **p<0.01.
Abbreviations: ERRα, estrogen‑related receptor α; IHC, Immunohistochemistry; IR score, immunoreactivity score; OC, ovarian cancer; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Association Between PGC-1α/ERRα Expression and Clinical Parameters in OC
To investigate the clinical significance of PGC-1α and ERRα in ovarian cancer, we analyzed the association between expression of these proteins and clinical characteristics of 42 patients with OC (Table 1). Almost all tumor foci showed IR scores ≥ 3 and ≥ 4 for PGC-1α and ERRα, respectively (Figure 2A and B). Thus, we defined an IR score of 4 and 7 as the cut-off for high PGC-1α and ERRα, respectively, in order to identify a potential correlation between PGC-1α/ERRα expression and patient clinical characteristics. Significant associations were identified between high ERRα and PGC-1α/ERRα expression and tumor differentiation (P=0.027; P=0.04), lymph node status (P=0.023; P=0.021), CA125 (P=0.036; P=0.021), and HE4 (P=0.021; P=0.05). However, high PGC-1α expression was only significantly associated with tumor differentiation (P=0.029).
Table 1.
Association Between PGC-1α/ERRα Expression and Clinicopathological Parameters in Patients with Ovarian Cancer
| Clinical Features | Total (n) | PGC-1α Expression | χ2 | P | ERRα Expression | χ2 | P | PGC-1α/ERRα Expression | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | High/High | Neither High | ||||||||
| Age, years | 0.032 | 0.859 | 0.095 | 0.758 | 0.033 | 0.856 | |||||||
| <55 | 42 | 17 | 8 | 15 | 10 | 11 | 14 | ||||||
| ≥55 | 12 | 5 | 11 | 6 | 7 | 10 | |||||||
| Tumor size, cm | 3.459 | 0.063 | 0.119 | 0.73 | 1.073 | 0.3 | |||||||
| <10 | 41 | 21 | 5 | 17 | 9 | 13 | 13 | ||||||
| ≥10 | 8 | 7 | 9 | 6 | 5 | 10 | |||||||
| Tumor differentiation | 4.786 | 0.029 | 6.275a | 0.027 | 5.451a | 0.04 | |||||||
| Well, moderate | 37 | 6 | 8 | 6 | 8 | 3 | 11 | ||||||
| Poor | 18 | 5 | 19 | 4 | 14 | 9 | |||||||
| FIGO stage | 0.149 | 0.699 | 1.036 | 0.309 | 0.717 | 0.397 | |||||||
| Ⅰ–Ⅱ | 41 | 14 | 5 | 10 | 9 | 7 | 12 | ||||||
| III–Ⅳ | 15 | 7 | 15 | 7 | 11 | 11 | |||||||
| Lymph node status | 1.332a | 0.425 | 5.772a | 0.023 | 7.161a | 0.021 | |||||||
| Absent | 32 | 13 | 8 | 10 | 11 | 5 | 16 | ||||||
| Present | 9 | 2 | 10 | 1 | 8 | 3 | |||||||
| CA125, U/mL | 0.497 | 0.481 | 4.388 | 0.036 | 5.802a | 0.021 | |||||||
| <78 | 42 | 8 | 5 | 5 | 8 | 2 | 11 | ||||||
| ≥78 | 21 | 8 | 21 | 8 | 16 | 13 | |||||||
| CA153, U/mL | 2.844 | 0.092 | 2.085 | 0.149 | 3.635a | 0.078 | |||||||
| <14.8 | 38 | 5 | 6 | 5 | 6 | 2 | 9 | ||||||
| ≥14.8 | 20 | 7 | 19 | 8 | 14 | 13 | |||||||
| HE4, U/mL | 0.296 | 0.67 | 5.625a | 0.025 | 4.568a | 0.05 | |||||||
| <75.5 | 34 | 6 | 3 | 2 | 7 | 1 | 8 | ||||||
| ≥75.5 | 19 | 6 | 17 | 8 | 13 | 12 | |||||||
Notes: aFisher test was performed. The rest of the scores were from Chi-square test.
Abbreviations: ERRα, estrogen-related receptor α; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Prognostic Significance of PGC-1α and ERRα in OC
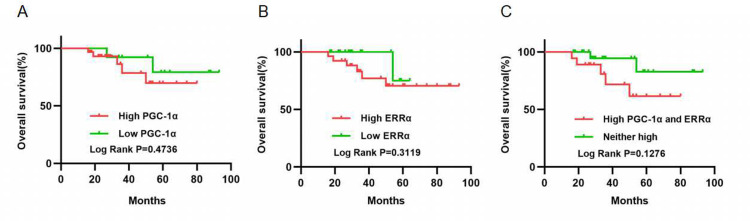
The Kaplan-Meier estimator model was employed to evaluate the prognostic significance of PGC-1α and ERRα expression (Figure 3). Seven (16.7%) patients died of ovarian cancer during the follow-up period. No significant relationship was observed between expression of PGC-1α and ERRα and cancer-specific survival rate (P=0.4736, P=0.3119; Figure 3A and B). However, combined analysis of high PGC-1α and ERRα expression revealed a tendency towards poor cancer-specific survival (P=0.1276; Figure 3C).
Figure 3.
The prognostic significance of PGC-1α and ERRα in OC. (A and B) No significant relation was observed between PGC-1α and ERRα expression and the cancer-specific survival rate (P=0.4736, P=0.3119). (C) Combined analysis of high PGC-1α and ERRα expression tended to show poor cancer-specific survival (P = 0.1276).
Abbreviations: ERRα, estrogen‑related receptor α; OC, ovarian cancer; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Discussion
Increasing evidence has suggested that ERRα promotes tumor cell proliferation, angiogenesis, metastasis, and drug resistance in various cancers.10,17 Willy et al18 and Chisamore et al19 revealed that suppression of ERRα blocks the cell cycle in the G1/S transition, hindering the growth of breast tumor cell lines and their tumorigenicity in vivo. Epithelial-mesenchymal transition (EMT) plays an essential role in OC cell invasion and metastasis.20,21 Wang et al22 reported that suppression of ERRα expression inhibits tumor metastasis and migration in OC cell lines by restraining mitochondrial activity and preventing EMT in vitro. Moreover, in an orthotopic model of OC, Lam et al23 found that inhibition of ERRα dramatically reduces tumor burden, ascites formation, and metastatic peritoneal nodules in vivo. Our recent research also demonstrated that inhibition of ERRα reduces EMT phenotypes, thereby significantly inhibiting invasion and migration in endometrial cancer cells.14 ERRα expression has also been associated with negative outcome in various human cancers. For example, in breast cancer, the mRNA and protein expression of ERRα positively correlates with node status, increased risk of recurrence, and metastatic status.24,25 Our previous studies have demonstrated that a high mRNA level of ERRα is associated with advanced FIGO stage and histological grade, and is thus associated with poor prognosis and shorter median overall survival time in OC.7
However, ERRα activity is primarily controlled by its coactivators, especially PGC-1α. The PGC-1α/ERRα axis also has been implicated in regulating several genes involved in energy metabolism, and increased mRNA and protein levels of ERRα in tissues are accompanied by high expression of PGC-1α.26,27 Expression of PGC-1α and ERRα is sensitive to physiological and pathological changes, which strengthens their crucial role in energy homeostasis in health and disease.9 Compounds affecting either the coactivator or the receptor could regulate signaling activity.9,10 Moreover, several studies indicate that PGC-1α/ERRα efficiently induce vascular endothelial growth factor (VEGF), and promote angiogenesis, mitochondrial biogenesis, and OXPHOS, leading to tumor angiogenesis, invasion, and metastasis.28–30 Numerous studies have focused on the potential mechanism of action of PGC-1α/ERRα,31–33 and multiple potential novel agonists have been identified, such as genistein, apigenin, resveratrol, daidzein, flavone, and cholesterol.18,34–36 Our latest research detected ERRα levels in uterine tumors by IHC and found that high expression of ERRα is associated with myometrial invasion. In addition, we identified a novel role for PGC-1α and ERRα as positive regulators of EMT, and showed that disruption of PGC-1α/ERRα signaling could reverse EMT and inhibit endometrial cancer invasion and migration.14
Currently, there are no reports in the literature on the analysis of PGC-1α and ERRα expression in OC tissues by IHC. To verify the clinical significance of PGC-1α/ERRα expression in OC, we first performed analyzed the mRNA levels of PGC-1α and ERRα in OC tissues using data from the public database HPA. We found that patients with high ERRα mRNA expression had significantly lower OS rates compared with patients with low ERRα expression, which supported our prior observations.7 Similar trends were observed between PGC-1α mRNA levels and patients with OC in HPA. According to the central dogma of molecular biology,37 the sequential information transfer residue-by-residue, thus mRNA expression correlates to protein expression. Consistent with these results, immunohistochemical staining indicated that PGC-1α and ERRα were remarkably overexpressed in OC tissues rather than in non-cancer ovarian tissues. We further investigated the correlation between PGC-1α and ERRα expression and the clinical features of patients with OC. High ERRα expression was significantly associated with tumor differentiation, lymph node status, CA125, and HE4, whereas high PGC-1α expression only significantly correlated with tumor differentiation. Moreover, Kaplan-Meier survival analysis revealed that the prognostic value of PGC-1α and ERRα in OC was not significant. Interestingly, combined analyses of the expression of the two proteins enhanced their clinical significance in OC as compared with the analysis of their expression alone. Poor differentiation and positive lymph node predict poor outcomes in OC. CA125 and HE4 are crucial biomarkers in the diagnostic and therapeutic monitoring phase.38 These data enhance the role of PGC-1α and ERRα in the development and progression of ovarian cancer, as with what we observed in the experiments of cell lines and animals.22,23
This study has several limitations. Firstly, this is a retrospective study and might have selection bias. Secondly, our current study was based on small sample size and lack of adequate samples for further validation of protein biomarkers. Thirdly, future studies are necessary to address the mechanism that regulates the development and progression of OC.
Conclusion
In conclusion, the present study showed the expression and clinical significance of PGC-1α and ERRα in human OC. The combined analysis of PGC-1α and ERRα expression could be a useful prognostic indicator of OC.
Acknowledgments
This study was supported in part by grant YCXZ18-01 from Fujian Provincial Maternity and Children’s Health Hospital, China.
Abbreviations
ERRα, estrogen-related receptor alpha; HPA, Human Protein Atlas; IHC, Immunohistochemistry; OC, ovarian cancer; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kopper O, de Witte CJ, Lõhmussaar K, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–849. doi: 10.1038/s41591-019-0422-6 [DOI] [PubMed] [Google Scholar]
- 2.Crevet L, Vanacker J-M. Regulation of the expression of the estrogen related receptors (ERRs). Cell Mol Life Sci. 2020;77:4573–4579. doi: 10.1007/s00018-020-03549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Q, Lin T, Kamarajugadda S, Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013;32(16):2079–2086. doi: 10.1038/onc.2012.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13(2):139–148. doi: 10.1016/j.cmet.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernier M, McGuirk S, Dufour CR, et al. Inhibition of DNMT1 and ERRα crosstalk suppresses breast cancer via derepression of IRF4. Oncogene. 2020;39(41):6406–6420. doi: 10.1038/s41388-020-01438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, Xia H, Xu H, et al. ERRα suppression enhances the cytotoxicity of the MEK inhibitor trametinib against colon cancer cells. J Exp Clin Cancer Res. 2018;37(1):218. doi: 10.1186/s13046-018-0862-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun P, Sehouli J, Denkert C, et al. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med (Berl). 2005;83(6):457–467. doi: 10.1007/s00109-005-0639-3 [DOI] [PubMed] [Google Scholar]
- 8.Gao M, Sun P, Wang J, Zhao D, Wei L. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16(2):827–833. doi: 10.1111/j.1525-1438.2006.00527.x [DOI] [PubMed] [Google Scholar]
- 9.Lynch C, Zhao J, Huang R, et al. Identification of estrogen-related receptor α agonists in the Tox21 compound library. Endocrinology. 2018;159(2):744–753. doi: 10.1210/en.2017-00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deblois G, St-Pierre J, Giguère V. The PGC-1/ERR signaling axis in cancer. Oncogene. 2013;32(30):3483–3490. doi: 10.1038/onc.2012.529 [DOI] [PubMed] [Google Scholar]
- 11.Chang C-Y, McDonnell DP. Molecular pathways: the metabolic regulator estrogen-related receptor α as a therapeutic target in cancer. Clin Cancer Res. 2012;18(22):6089–6095. doi: 10.1158/1078-0432.CCR-11-3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19(8):269–276. doi: 10.1016/j.tem.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audet-Walsh É, Papadopoli DJ, Gravel S-P, et al. The PGC-1α/ERRα axis represses one-carbon metabolism and promotes sensitivity to anti-folate therapy in breast cancer. Cell Rep. 2016;14(4):920–931. doi: 10.1016/j.celrep.2015.12.086 [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Mao X, Huang M, Lei H, Xue L, Sun P. PGC-1α and ERRα in patients with endometrial cancer: a translational study for predicting myometrial invasion. Aging (Albany NY). 2020;12(17):16963–16980. doi: 10.18632/aging.103611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 16.Pontén F, Jirström K, Uhlen M. The human protein atlas–a tool for pathology. J Pathol. 2008;216(4):387–393. doi: 10.1002/path.2440 [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Sun P, Dong B, Sehouli J. Key regulator of cellular metabolism, estrogen-related receptor α, a new therapeutic target in endocrine-related gynecological tumor. Cancer Manag Res. 2018;10:6887–6895. doi: 10.2147/CMAR.S182466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willy PJ, Murray IR, Qian J, et al. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc Natl Acad Sci U S A. 2004;101(24):8912–8917. doi: 10.1073/pnas.0401420101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chisamore MJ, Cunningham ME, Flores O, Wilkinson HA, Chen JD, Bauer JA. Characterization of a novel small molecule subtype specific estrogen-related receptor alpha antagonist in MCF-7 breast cancer cells. PLoS One. 2009;4(5):e5624. doi: 10.1371/journal.pone.0005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergara D, Merlot B, Lucot J-P, et al. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291(1):59–66. doi: 10.1016/j.canlet.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 21.Loret N, Denys H, Tummers P, Berx G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers (Basel). 2019;11(6):838. doi: 10.3390/cancers11060838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C-W, Hsu W-H, Tai C-J. Antimetastatic effects of cordycepin mediated by the inhibition of mitochondrial activity and estrogen-related receptor α in human ovarian carcinoma cells. Oncotarget. 2017;8(2):3049–3058. doi: 10.18632/oncotarget.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY, Wong AS. Targeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol Ther. 2014;22(4):743–751. doi: 10.1038/mt.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Li Y, Lou G, et al. MiR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PLoS One. 2012;7(6):e39102. doi: 10.1371/journal.pone.0039102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein RA, Gaillard S, McDonnell DP. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114(1–2):106–112. doi: 10.1016/j.jsbmb.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deblois G, Giguère V. Functional and physiological genomics of estrogen-related receptors (ERRs) in health and disease. Biochim Biophys Acta. 2011;1812(8):1032–1040. doi: 10.1016/j.bbadis.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 27.Eichner LJ, Giguère V. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion. 2011;11(4):544–552. doi: 10.1016/j.mito.2011.03.121 [DOI] [PubMed] [Google Scholar]
- 28.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10). doi: 10.1038/ncb3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arany Z, Foo S-Y, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613 [DOI] [PubMed] [Google Scholar]
- 30.Dwyer MA, Joseph JD, Wade HE, et al. WNT11 expression is induced by estrogen-related receptor alpha and beta-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010;70(22):9298–9308. doi: 10.1158/0008-5472.CAN-10-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan M, Audet-Walsh É, Manteghi S, et al. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016;30(9):1034–1046. doi: 10.1101/gad.281410.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YK, Park JH, Baek -Y-Y, et al. Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca channel-mediated PGC-1α/ERRα activation. Biochem Biophys Res Commun. 2016;479(2):297–304. doi: 10.1016/j.bbrc.2016.09.063 [DOI] [PubMed] [Google Scholar]
- 33.Craige SM, Kröller-Schön S, Li C, et al. PGC-1α dictates endothelial function through regulation of eNOS expression. Sci Rep. 2016;6:38210. doi: 10.1038/srep38210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng CT, Hsieh J-H, Zhao J, et al. Development of novel cell lines for high-throughput screening to detect estrogen-related receptor alpha modulators. SLAS Discov. 2017;22(6):720–731. doi: 10.1177/2472555216689772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei W, Schwaid AG, Wang X, et al. Ligand activation of ERRα by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23(3):479–491. doi: 10.1016/j.cmet.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng CT, Beames B, Alex Merrick B, Martin N, Romeo C, Jetten AM. Development of a stable cell line with an intact PGC-1α/ERRα axis for screening environmental chemicals. Biochem Biophys Res Commun. 2014;444(2):177–181. doi: 10.1016/j.bbrc.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strasser BJ. A world in one dimension: Linus Pauling, Francis Crick and the central dogma of molecular biology. Hist Philos Life Sci. 2006;28(4):491–512. [PubMed] [Google Scholar]
- 38.Potenza E, Parpinel G, Laudani ME, Macchi C, Fuso L, Zola P. Prognostic and predictive value of combined HE-4 and CA-125 biomarkers during chemotherapy in patients with epithelial ovarian cancer. Int J Biol Markers. 2020;1724600820955195. doi: 10.1177/1724600820955195 [DOI] [PubMed] [Google Scholar]