Abstract
Heart disease is the number one mortality disease in the world. In particular, cardiac fibrosis is considered as a major factor causing myocardial infarction and heart failure. In particular, oxidative stress is a major cause of heart fibrosis. In order to control such oxidative stress, the importance of nuclear factor erythropoietin 2 related factor 2 (NRF2) has recently been highlighted. In this review, we will discuss the activation of NRF2 by docosahexanoic acid (DHA), eicosapentaenoic acid (EPA), and the specialized pro-resolving lipid mediators (SPMs) derived from polyunsaturated lipids, including DHA and EPA. Additionally, we will discuss their effects on cardiac fibrosis via NRF2 activation.
Keywords: cardiac fibrosis, NRF2, lipoxins, resolvins, maresins, neuroprotectins
1. Introduction
Cardiovascular disease is the leading cause of death worldwide [1]. Cardiac fibrosis is a major factor leading to the progression of myocardial infarction and heart failure [2]. Cardiac fibrosis is characterized by the net accumulation of extracellular matrix proteins in the cardiac stroma and ultimately impairs cardiac function [3]. Therefore, interest in substances with cardioprotective activity continues. It has been emphasized that antioxidant activity by nuclear factor erythropoietin 2 related factor 2 (NRF2) is essential for cardiac protection by unsaturated essential fatty acids such as DHA and EPA. Recently, there are increasing reports that NRF2 is regulated by specialized pro-resolving lipids (SPMs) originated from docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA). However, there are not many reports on the effect of NRF2 regulation on cardiac fibrosis by SPMs. Thus, in this review, we would like to summarize the relationship between SPMs and NRF2 in cardiac fibrosis so far.
2. Cardiac Fibrosis and Its Mediators
2.1. Cardiac Fibrosis
Cardiac fibrosis is a natural compensatory process that occurs before the symptoms of heart failure. This is indicated by changes in the structure of the ventricles with increased volume and altered chamber configuration. This condition exhibits several characteristic histological features, such as cardiomyocyte hypertrophy and apoptosis, myofibroblast proliferation, and extracellular matrix (ECM) alterations.
Cardiac fibrosis occurs when fibroblasts are activated into myofibroblasts, and the amount of ECM protein increases, altering scar tissue formation and the remodeling of the ECM [4] (Figure 1). Collagen accumulated through these processes affects both systolic and diastolic function and continuously reduces heart function [3]. Cardiac fibrosis is part of the normal ageing process. However, this process is accelerated by many cardiovascular diseases, including diabetes, high blood pressure, and myocardial infarction (MI), as well as ischemic, dilated, and hypertrophic cardiomyopathy [5].
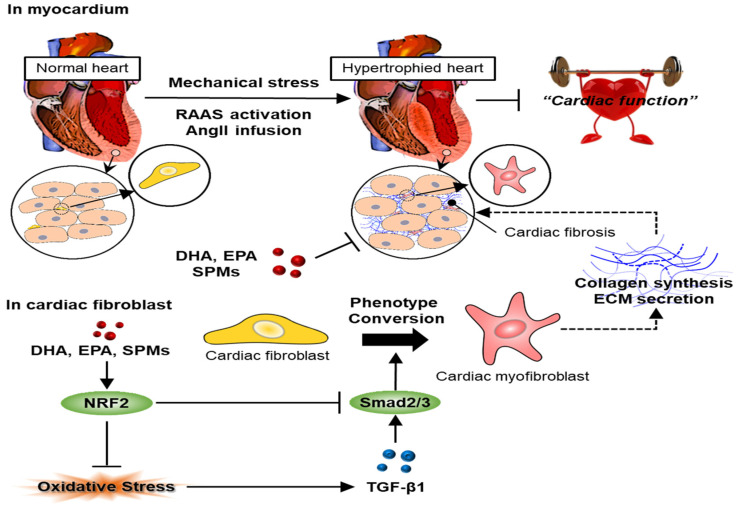
Figure 1.
Schematic diagrams depicting the mechanisms of SPMs in alleviating cardiac fibrosis in Ang-II-infused mice. Irisin activated and accelerated the translocation of Nrf2 to the nucleus, thereby reducing oxidative stress and countering the ROS/TGF-β1/Smad2/3 pro-fibrotic pathway (modified from Chen et al. [6]).
Cardiac fibrosis can be divided into three types. Regional fibrosis (also known as reparative fibrosis) occurs when the affected area is replaced by scar tissue after cardiomyocyte death. Myocardial infarction (MI) occurs due to the prolonged death of cardiomyocytes following a coronary artery occlusion. MI causes a wound healing process characterized by excessive inflammatory reactions and scarring [7]. Forming an appropriate replacement scar at the site of infarction and cardiomyocytes necrosis is an essential response to maintaining the structure of the heart and preventing myocardial rupture. Moreover, diffuse fibrosis (also known as reactive fibrosis) occurs when the affected area is replaced by scar tissue after cardiomyocytes death. This type of fibrosis can be stimulated by pro-fibrotic mediators or prolonged stress, even in the absence of specific cardiomyocyte death. Conditions that apply constant pressure to the heart, such as aortic stenosis or systemic hypertension, have been shown to increase the wall stress in the left ventricle and promote reactive fibrosis in the chamber.
Pathophysiological fluctuations that can trigger the inflammatory response of the heart, such as obesity, diabetes, metabolic syndrome, heart infections, and drugs, cause systemic or local fibrosis [8]. Additionally, invasive interstitial fibrosis leads to the gradual deposition of insoluble proteins (amyloidosis) or lipid hyperglycosylation (Anderson-Fabri’s disease) in the heart stroma [9]. In a normal heart, a well-defined fibrous network helps to convert the function of individual cardiomyocytes into an effective organ pump. However, the ECM, which is overproduced and deposited in pathological conditions, can interfere with the normal electrical conduction pathway between individual cardiomyocytes, which can adversely affect the heart’s contractile performance. Additionally, excess fibrous tissue in the heart affects the transfer of the power of individual cardiomyocytes to a powerful, well-tuned pump function [10]. The accumulation of fibrous tissue around the coronary vessels in the heart’s small myocardium (i.e., perivascular fibrosis) can lead to localized micro ischemic sites, further impairing heart function [11]. Additionally, excessive collagen production in the ECM can affect diastolic function by impairing the elastic rebound of the myocardium as the myocardial cells relax. The accumulation of global or local ECM in the heart can lead to arrhythmia through reentry and other mechanisms [12].
2.2. Mediators of Cardiac Fibrosis
Increases in various circulatory hormones, cytokines and proteins due to stress or injury lead to fibroblast activation and differentiation and contribute to cardiac fibrosis [2]. Several studies have confirmed that various substances are involved in this process, suggesting that the renin-angiotensin system (RAS), transforming growth factor (TGF)-beta, endothelin (ET), and inflammation are key. Among them, inflammation will be the focus of the discussion [13].
To briefly explain factors other than inflammation, extensive pieces of evidence, based on various studies, link neurohormonal pathways to the pathogenesis of cardiac fibrosis. The activation of RAS is often found in the fibrotic heart and is associated with the regulation of fibroblast production and activity. Macrophages and fibroblasts that penetrate the damaged heart produce renin and angiotensin-converting enzymes (ACE) and induce the production of angiotensin II (AngII) [14]. Locally released AngII stimulates cardiac fibroblast proliferation and enhances collagen synthesis activity through angiotensin II subtype 1 receptor (AT1R) receptor-dependent interactions [15]. On the other hand, AT2R acts as a negative regulator of the pro-fibrotic reaction. It inhibits AT1R-mediated pathophysiological effects and exerts anti-fibrotic, anti-proliferative, and anti-inflammatory effects [16].
Aldosterone is also known as a mediator of fibrosis [17]. It is known to mediate fibrosis through the stimulation of various cell types. The activation of cytokine and chemokine expression in vascular cells, induction of fibrotic phenotype in macrophages, activation of fibrosis signals in cardiomyocytes, induction of fibroblast proliferation, and increased collagen synthesis have been proposed as mechanisms mediating fibrosis [18].
TGF-β1 is the most characteristic fibrogenic growth factor to date, and in mammals, TGF-β1 exists in three isotypes (TGF-β1, 2, and 3) and is encoded by different genes. Of these, TGF-β1 is the predominant isoform in the cardiovascular system, and so far, most of the information related to cardiac fibrosis is limited to TGF-β1 [19]. Experimental models and previous studies of the human fibrotic heart have shown that TGF-β1 expression increases and continues to be activated during cardiac fibrosis [20]. The activation of TGF-β1 may include heart damage, the production of reactive oxygen species, AngII, hyperglycemia, pH changes and specific proteases, such as plasmin, matrix metalloproteinase-2 (MMP-2), and MMP-9 [21,22]. Activated TGF-β1 has a potent and diverse effect on many cell types associated with cardiac fibrosis, such as macrophages, lymphocytes and cardiomyocytes. TGF-β1 may increase ECM production by stimulating fibroblasts, inducing transformation into myofibroblasts [23]. TGF-β1 also reduces ECM degradation by regulating levels of protease inhibitors, such as Plasminogen Activator Inhibitor (PAI)-1 and Tissue Inhibitors of Metalloproteinases (TIMP) [24]. Besides, TGF-β1 was involved in the synthesis and secretion of other pro-fibrotic factors, such as connective tissue growth factor (CTGF; also known as CCN2) [4].
ET is a protein secreted by heart endothelial cells and is known to play an essential role in the pathogenesis of chronic heart failure [4]. ET has three isotypes (ET-1, 2, 3), of which ET-1 is generally produced in endothelial cells and can be expressed in various cells, such as cardiomyocytes and fibroblasts [25]. ET-1 is known to increase ECM production in fibroblasts, as well as promote the differentiation of fibroblasts into myofibroblasts [26]. Various previous studies have shown that ET-1 is synergistic with other pro-fibrotic agonists [4]. For example, AngII increased ET-1 expression through ERK and reactive oxygen species, and TGF-β1 induced ET-1 through the c-Jun N-terminal kinase (JNK) [27].
Inflammatory cytokines or chemokines can also directly or indirectly affect heart fibrosis. During heart injury, inflammation-related factors are released by cardiomyocytes or fibroblasts [28]. In particular, circulating levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-2, and IL-1β are known to correlate with disease severity in heart failure patients [29]. Additionally, many T helper 2 (Th2) cytokines, such as IL-4 and IL-13, were first recognized as mediators of pro-fibrotic mediators. IL-4 is known to increase collagen and matrix protein synthesis in fibroblasts, and IL-13 is also known to play a role in fibrosis by directly stimulating fibroblasts [30]. Additionally, the direct and indirect fibrosis properties of IL-17, a Th17 cytokine, have been reported [31].
2.3. Cellular Interaction in Cardiac Fibrosis
2.3.1. Cardiac Fibroblasts and Myofibroblasts
Most extracellular materials are secreted by cardiac fibroblasts and make up about 90% of the cells that make up the adult heart. Collagen, one of the crucial proteins secreted into the cardiac extracellular matrix, connects to form fibrous tissue, surrounds the cardiomyocytes, acts as structural support, and has a profound effect on the relaxation of the heart. Cardiac fibroblasts differentiate into myofibroblasts during heart disease in response to certain stress factors, and the amount and composition of extracellular secretions are greatly affected by the proliferation and differentiation of cardiac fibroblasts [32]. Vascular endothelial cells also can differentiate into “active fibroblasts” (i.e., myofibroblasts). Myofibroblasts overproduce and secrete extracellular matrix components, such as collagen, and the expression of intracellular alpha-smooth muscle actin (α-SMA) exhibits the typical features of contractile cells [33]. Additionally, myofibroblasts promote a vicious cycle of fibrosis through their sustained proliferation, self-secretion of TGF-β1 and AngII, expression of AngII type I receptors, and activation of anti-apoptotic genes [34].
2.3.2. Inflammatory Cells
The heart of healthy adult mice contains various types of immune cells such as mononuclear phagocytes, neutrophils, B cells, and T cells. They are contained at a frequency of about 12 times higher than that of skeletal muscle [35]. Cardiac macrophages, similar to macrophages in other tissues, act as guardians to monitor the cause of damage and infection [13]. Cardiac macrophages are energy-intensive and can act as members of a network of steady-state cells needed to maintain the main functions of the heart, which are mechanically dynamic. Moreover, cardiac macrophages can interact electrically with cardiomyocytes by forming gap junctions containing connexin 43 (CX43; GJA1) with cardiomyocytes in addition to typical macrophage activity [36]. In a damaged heart, various macrophage populations, including cardiac resident macrophages and bone marrow progenitor-derived macrophages, may be involved in the initiation, maintenance, and resolution of fibrotic reactions [37]. Similar to the modification of the macrophage phenotype in the normal inflammatory response, during the onset of cardiac inflammation, macrophages acquire a “classic activated M1 phenotype” and release the pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. In later stages, macrophages acquire a restorative phenotype through phenotypic transformation and produce anti-inflammatory cytokines, chemokines, AngII, and growth factors [38].
On the other hand, “alternatively activated M2” macrophages regulate the decomposition of extracellular matrix components through MMP release, activate cardiac fibroblasts by secreting TGF-β1, and induce differentiation into myofibroblasts that secrete collagen [39]. Many macrophages accumulate in areas where heart damage has occurred, which can directly contribute to scar collagen production. The overexpression of the C-C Motif Chemokine Ligand 2 (CCL2), which plays an essential role in macrophage recruitment, has been demonstrated to be associated with increased macrophage invasion, extended remodeling, and fibrosis of the heart muscle [40].
Mechanisms that influence macrophage activation include efferocytosis, damage-related molecular pattern (DAMP) production, hypoxia, and ECM remodeling [41]. Cardiomyocyte death under stress conditions, such as severe infarction or prolonged ischemic injury, promotes a phenotype of macrophages dedicated to removing dead cell debris. After swallowing dead cell phagocytes, macrophages secrete TGF-β1 and IL-10 and reduce IL-12 release, resulting in pro-regenerative/fibrotic activity [42]. However, despite the removal of dead cell debris by macrophages, persistent dead cell debris increases the secretion of DAMP and causes a persistent inflammatory response. DAMP activates macrophages through the Toll-like receptor 4 (TLR4) signaling cascade, known as the major inflammatory signaling pathway in macrophages, TLR4/TLR6-IRAK4/1 signaling increases cardiac oxidative stress, and the NLRP3 inflammasome activates the production of IL-1β [43].
Mast cells (MCs) are best known as the primary cells that mediate the anaphylactic reaction in allergies and are characterized by cytoplasmic secretory granules equipped with effector molecules that can be released immediately upon activation. These include molecules, such as various types of chemokines and cytokines, proteases, heparin, histamine, growth factors, and fatty acid metabolites [43]. Pathogen-associated molecular patterns (PAMPs) and DAMPs, which can result from damaged or infarcted cardiomyocytes under stress conditions, can mediate pattern recognition receptors (PRRs) expressed in MCs to induce MCs activation. MC is present in the heart or is recruited to the heart under stress. Although the number of MCs accumulating in the heart is small, it secretes renin, chymase, and tryptase by various pro-fibrotic stimuli to stimulate cardiac fibroblasts and promote fibrosis [44].
The heart muscle is made up of a variety of cell types, and cardiomyocytes make up about 25% to 35% of the cells that make up the adult heart muscle, but 90% of the adult heart’s mass [45]. Cardiomyocytes begin to differentiate and multiply from cardiac progenitor cells during the early stages of heart development, and fetal heart growth is attributed to cardiomyocyte proliferation. However, in the adult heart, cardiomyocytes exit the cell cycle and are known to have minimal cell turnover [45]. Cardiomyocyte death under pathological conditions triggers an inflammatory reaction and promotes fibroblast activation and the production of fibrous tissue to replace dead cardiomyocytes [45]. During this process, large numbers of DAMP signaling factors such as high mobility group proteins B1 (HMGB1), ATP, S100A8-S100A9, and DNA are released from dead cardiomyocytes. Besides, peripheral cardiomyocytes can contribute to interstitial fibrosis by activating interstitial fibroblasts under stress conditions. However, the specific molecular mechanism is not yet clear. Cardiomyocytes secrete IL-6 to induce fibroblast proliferation, and CTGF and VEGF are secreted by protein kinase A stimulation, and collagen production and fibroblast proliferation are induced [46]. Cardiomyocytes also secreted micro-RNA-like molecules in co-culture with cardiac fibroblasts and stimulated collagen production through the activation of the TGF-β1 pathway [47].
3. Role of NRF2 in Cardiac Fibrosis
Oxidative stress plays an essential role in the etiology of heart disease. The NRF2/Keap1/ARE pathway, the center of our body’s antioxidant system, is of increasing importance in the treatment of heart diseases, especially heart fibrosis [48,49,50]. We will first describe what NRF2 is and discuss its role in cardiac fibrosis.
3.1. NRF2
NRF2 is a transcription factor encoded by the NFE2L2 gene in humans [51]. NRF2 is a basic leucine zipper (bZIP) protein that regulates the expression of an antioxidant protein that protects against oxidative damage from injury and inflammation [52]. NRF2 is at the heart of a complex regulatory network and plays many essential roles in the regulation of metabolism, inflammation, autophagy, protein retention, mitochondrial physiology, and immune responses [53]. Several drugs that stimulate the NFE2L2 pathway are being studied for the treatment of diseases caused by oxidative stress [54].
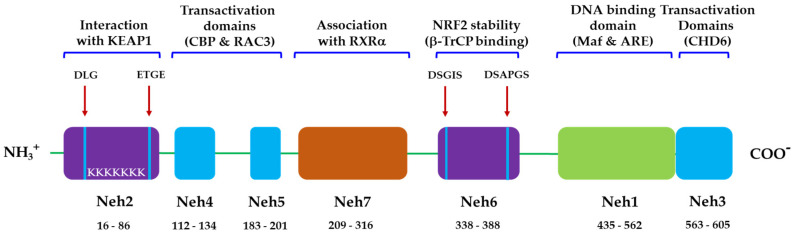
NRF2 is a basic leucine zipper (bZip) transcription factor with a Cap “n” Collar (CNC) structure [51]. NRF2 has six highly conserved domains called the NRF2-ECH homology (Neh) domains (Figure 2). The Neh1 domain is a CNC-bZIP domain that allows NRF2 to heterodimerize with small molecular weight Maf proteins (MAFF, MAFG, MAFK) [55]. The Neh2 domain allows NRF2 to bind to the cytoplasmic inhibitor Keap1 [56]. The Neh3 domain can play a role in NRF2 protein stability and can act as a trans-activating domain that interacts with components of the transcription device [57]. The Neh4 and Neh5 domains also act as transactivation domains but bind to other proteins, called cAMP-reactive element binding proteins (CREB) that have unique histone acetyltransferase activity [56]. The Neh6 domain may contain degrons, which are involved in the process of NRF2 degradation, which is not sensitive to redox. NRF2 degradation also occurs in stressed cells, which typically prolongs the half-life of the NRF2 protein compared to unstressed conditions by inhibiting other pathways of degradation [58]. When encountering stress conditions, such as oxidative stress, NRF2 is activated by our body’s defense mechanism and enters the nucleus, increasing the transcription of antioxidant-related genes, heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO-1), superoxide dismutase (SOD), and thioredoxin (TXN).
Figure 2.
Domain structure of NRF2. Functional Neh domains (amino acid positions): Neh1 (16–86) is the binding site for small Maf proteins and ARE. Neh2 (435–562), the binding site for Keap1 with low-affinity DLG and the high affinity ETGE motifs. Neh3 (653–605), Neh4 (112–134), and Neh5 (183–201) are transactivation domains for NRF2 (CHD6, CBP, and RAC3). Neh6 (338–388) is a serine-rich domain that negatively regulates NRF2 stability by β-TrCP interaction with DSGIS and DSAPGS motifs. Neh7 (209–316) interacts with RXRα, a nuclear receptor responsible for the suppression of the NRF2/ARE signaling pathway.
3.2. Role of NRF2 in Cardiac Fibrosis
Some examples of experimental evidence that demonstrates the potential involvement of NRF2 in cardiac fibrosis will be discussed below. Additionally, we will discuss the role of pharmacological activation of NRF2 in reversing many pathological processes caused by diabetic cardiomyopathy.
The treatment of PM 2.5, an air pollutant, in Nrf2-deficient mice exacerbates cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation through RIPK3-regulated mitochondrial disorders [59]. Knockout of Nrf2 (Nrf2 (-/-)) did not cause apparent structural and functional abnormalities in the unstressed heart. However, after transverse aortic constriction (TAC), Nrf2 (-/-) mice developed pathological cardiac hypertrophy, severe myocardial fibrosis and apoptosis, overt heart failure, and increased mortality, which was associated with increased myocardial levels of 4-hydroxy-2-nonenal and 8-hydroxydeoxyguanosine, coupled with the complete inhibition of several antioxidant genes in the myocardial tissue [60].
Three microRNAs (miRs), including miR-27a, miR-28-3p, and miR-34a, were highly expressed in the left ventricle of the heart where myocardial infarction occurred compared to other organs, and cultured cardiomyocytes and fibroblasts responded to TNF-α stimulation, leading to the expression of these three miRs. These miRs are preferentially integrated into exosomes, and miR-rich exosomes appear to contribute to intercellular communication and impaired NRF2 regulation [61]. miR-155 is upregulated in cardiomyocytes in cardiac fibrosis. miR-155 inhibitors significantly restored NRF2 and HO-1 expression levels in hyper glucose-induced cardiac fibrosis, reduced oxidative stress levels, mitochondrial damage, and the number of cells undergoing apoptosis. Additionally, miR-155 inhibitors improved fibrosis by significantly reversing the expression levels of collagen I and α-SMA [62]. Irisin, a hormone released by muscle cells, suppresses the ROS/TGF-β1/Smad2/3 signaling axis through NRF2 to relieve angiotensin II-induced cardiac fibrosis [6] (Figure 1).
Research results on the improvement of cardiac fibrosis by the activation of NRF2 by natural substances have also been reported one after another. For example, puerarin shows the therapeutic effects against AngII-induced cardiac fibrosis. Puerarin prevents cardiac fibrosis via the activation of NRF2 [63]. UGTA1, a metabolic enzyme of puerarin, is increased by NRF2, and puerarin-7-O-glucuronide, produced by UGT1A1, also exhibits similar therapeutic effects to puerarin. Galanthamine activates the AMPK/NRF2 pathway in mice, improving cardiac dysfunction due to myocardial ischemia-reperfusion, endoplasmic reticulum stress-related cell death, and myocardial fibrosis [64]. Isorhynchophylline, an alkaloid derived from Uncaria rhynchophylla, enhanced NRF2 in phenylephrine-induced cardiac hypertrophy [65]. Mangiferin degrades Keap1 to enhance stability, promoting NRF2 protein accumulation, leading to NRF2 activation.
By activating NRF2, mangiferin promoted the synthesis of glutathione (GSH) in cardiac fibroblasts, thereby reducing the supply of glutamate to fibroblasts to inhibit activation. Through this action, mangiferin improved cardiac fibrosis induced by transverse aortic constriction [66]. Luteolin prevented cardiac fibrosis, hypertrophy, and dysfunction in streptozotocin-induced diabetic mice through activation of NRF2 and inhibition of nuclear factor-kappa B (NF-κB) [67].
4. Specialized Pro-Resolving Lipid Mediators and Their Receptors
The possible involvement of NRF2 in the cardioprotective action of DHA and EPA has been reported. Moreover, SPMs are created from DHA and EPA to induce inflammation resolution. Various physiological and pharmacological actions by DHA and EPA are also likely to be mediated by NRF2. In this section, we will explore these possibilities. Before that, we will briefly introduce what SPMs are and what types of receptors mediate their actions. Furthermore, we would like to present the action they have on NRF2.
4.1. Types of Specialized Pro-Resolving Lipids
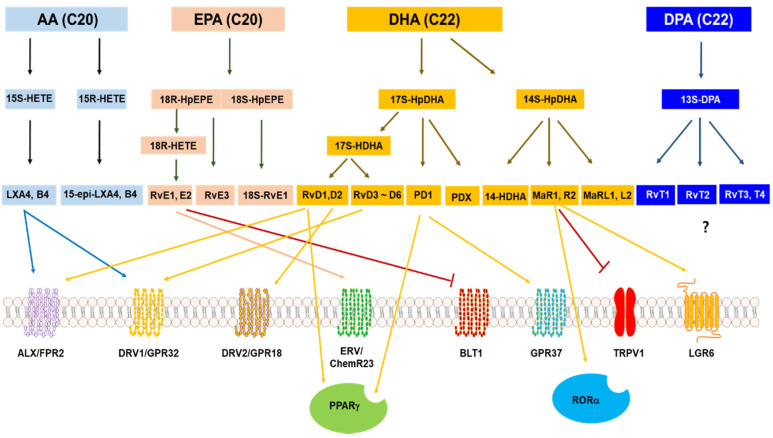
SPMs include lipoxins (LXs) made from AA, resolvins (Rvs) made from DHA and EPA, maresins (MaRs), and neuroprotectins (NPD) derived from DHA (Figure 3).
Figure 3.
SPMS and their receptors. Arrow heads of lines represent activation of receptors, and bar heads of lines indicate the inhibition of receptors? Means that no receptors are reported for each SPM (modified from Pirault et al. [68]).
4.1.1. Lipoxins (LXs)
The biosynthesis of LXs differs from other SPMs originated from DHA and EPA in that LXs are produced from arachidonic acid (AA). It is a unique feature that allows different intercellular metabolic pathways to make LXs. For example, 5-lipoxygenase in neutrophils (i.e., ALOX5) and 15-lipoxygenase-1 in immature red blood cells and reticulocytes (i.e., ALOX15) work in chains to form LxA44 and LxB44. This pathway also occurs in chain interactions between neutrophils and eosinophils, epithelial or M2 macrophages/monocytes and neutrophils, endothelial or skeletal muscle and neutrophils [69,70,71]. As LXs were discovered earlier than other SPMs, derivatives for LXs with increased stability were also synthesized, and studies are currently underway in various inflammation-related indications, including cardiac fibrosis.
4.1.2. Resolvins (Rvs)
Rvs is a specialized pro-resolving mediator that is derived from omega-3 fatty acids, primarily EPA and DHA, docosapentaenoic acid (DPA) and clupanodonic acid. Rvs are divided into several subclasses based on the unique aspects of the structure and the straight-chain polyunsaturated fatty acids (PUFAs) from which they are formed. RvDs are metabolites of 22-carbon PUFA, DHA (i.e., 4Z, 7Z, 10Z, 13Z, 16Z, 19Z)-docosahexaenoic acid.
RvE is a 20-carbon metabolite of EPA (i.e., 5Z, 8Z, 11Z, 14Z, 17Z-5,8,11,14,17-eicosapentaenoic acid); Resolvin Dn-6DPA (RvDsn-6DPA) is a DPA isomer, a metabolite of osbond acids (i.e., 4Z, 7Z, 10Z, 13Z, 16Z-docosapentaenoic acid); Resolvin Dn-3DPA (RvDn-3DPA) is a metabolite of the DPA isomer, clupanodonic acid (i.e., 7Z, 10Z, 13Z, 16Z, 19Z)-docosapentaenoic acid; RvT is a metabolite of clupanodonic acid with 17R-hydroxyl residues, as opposed to RvDsn-3DPA (all have 17S-hydroxyl residues). Because AT-RvDs are synthesized by drug-modified cyclooxygenase 2 enzymes to form 17 (R)-hydroxyl rather than the 17 (S)-hydroxyl residue of RvE, specific isomers of RvD are converted to aspirin-triggered RvDs (AT-RvDs). All mentioned Rvs, except RvDsn-6DPA, are metabolites of omega-3 fatty acids [72,73].
4.1.3. Maresins (MaRs) and Neuroprotectins (NPDs)
MaR1 (7R, 14S-dihydroxy-4Z, 8E, 10E, 12Z, 16Z, 19Z-docosahexaenoic acid) is a member of the SPMs produced in macrophages. MaR1 and recently defined MaR2 are the 12-lipoxygenase-derived metabolites of the omega-3 fatty acid DHA metabolites and they have anti-inflammatory, protective and healing-promoting properties, similar to the other SPM members mentioned earlier in the class. MaR1 was first defined as a product derived from DHA formed by human monocyte-derived macrophage [4]. At the same time, macrophages also convert DHA to 13 (R), 14 (S)-dihydroxy-4Z, 7Z, 9E, 11E, 16Z, 19Z-docosapentaenoic acid—i.e., MaR2 [74].
Neuroprotectin D1 (NPD1) (10R, 17S-dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid), also known as Protectin D1 (PD1), is a docosanoid derived from the PUFA docosahexaenoic acid. Like other members of the specialized pro-resolving mediators, NPD1 exerts potent anti-inflammatory and anti-apoptotic/neuroprotective biological activities [75,76]. Other neuroprotective agents with similar activity exist: PDX (10R, 17S-dihydroxy-4Z, 7Z, 11E, 13Z, 15E, 19Z-docosahexaenoic acid); 20-hydroxy-PD1 (10R, 17S, 20-trihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid); 10-epi-PD1 (10R, 17S-Dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid) [77,78]. The activity of the neuroprotectin-like metabolite 17-epi-PD1 (10R, 17R-dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid) has not yet been reported.
4.2. SPM Receptors
With the discovery of SPMs that promote the termination of inflammation, new receptors that mediate the action of SPMs have also become known, providing opportunities for new drug targets to stop inflammation. The SPMs and their respective SPM receptors, which mediate their actions, are briefly described in this section (Figure 2). Enthusiastic researchers have demonstrated that the pro-resolving activity of SPM occurs through the activation of one or more G protein-coupled receptors (GPCRs) present in the plasma membrane. However, there are still unidentified receptors that mediate the action of some types of SPMs.
Four GPCRs have been reported as receptors for RvD1 and RvE1. However, it has not been confirmed whether other Rvs and PDs, such as RvE2, RvE4, RvD2, RvD3, and PDX, act on these GPCRs [79,80,81]. For the recent and specific physiological actions of these receptors and detailed study data of receptor null mice, please refer to other reviews and references therein [82,83]. There are also receptor molecules that do not exist on the cell membrane but mediate the action of SPMs, which are briefly discussed in Section 4.2.7.
4.2.1. Chemerin Receptor 1
Chemerin1 was initially classified as an orphan GPCR with homology to the formyl peptide receptor and the anaphylatoxin C3a and C5a receptors [52,84,85]. It was not until the early 2000s that it was discovered that the ligand for this receptor was chemerin [86]. In addition to chemerin, RvE1 was identified as a second endogenous agonist through a screening program on the GPCR panel [80]. ChemR23 (a chemokine-like receptor 1, also known as CMKLR1) is a receptor for RvE1, which has been shown to bind more strongly than chemerin (peptide ligand) [79,87]. Besides, RvE2 is a partial agonist compared to RvE1 in CHO-chemerin1 β-arrestin recruitment [88].
Chemerin receptor 1 (chemerin1, ChemR23, or ERV1) is expressed on a wide range of immune cells, including monocytes, macrophages, NK cells, bone marrow cells, and DC. Additionally, ERV1 has been identified in adipocytes and endothelial cells [89]. Expression of chemerin1 is more abundant in antigen-presenting cells (APCs), such as macrophages and dendritic cells than in neutrophils and T-lymphocytes. However, the expression is upregulated in neutrophils in various diseases, including type 2 diabetes [90]. The expression of chemerin is associated with inflammatory and fibrotic processes associated with kidney damage [91]. Serum levels of chemerin were significantly increased in non-alcoholic fatty liver disease patients compared to the controls [92]. However, there appears to be no report of a link to heart fibrosis.
4.2.2. N-Formyl Peptide Receptor 2/LXA4 Receptor (FPR2/ALX)
Originally, FPR2 was classified as an FPR receptor due to the activation by the low-affinity endogenous agonist N-formyl methionyl peptide (fMLP) [93]. Through the screening of various receptor ligands using radiolabeled [3H]-LXA4 and subsequent GTPase activity, it was confirmed that cells overexpressing FPR2/ALX cDNA (pINF154) exhibit high affinity and specificity for LXA4 binding [94]. Thus, the receptor was reclassified as FPR2/ALX, because LXA4 exhibited the highest affinity of all FPR2/ALX endogenous agonists [95]. The binding of LXA4 induces the stimulation of monocyte chemotaxis, macrophage differentiation and efferocytosis [96,97]. LXA4 upregulates FPR2/ALX by activating the receptor promoter, acting as positive feedback on FPR2/ALX expression [98]. The expression of FPR2/ALX is also markedly increased in the presence of inflammatory stimulants, including tumor necrosis factor-α (TNF-α), platelet-activating factor (PAF), and IL-8 [99].
FPR2/ALX is expressed on leukocytes, including neutrophils, monocytes, macrophages, dendritic cells, naive CD4 T cells, and Th1, -2, -17 cells. FPR2/ALX is also expressed in non-immune cells, including endothelial, keratinocytes and mesenchymal stem cells [100]. Endogenous and exogenous lipids, peptides and proteins can bind and activate FPR2/ALX to produce inflammatory and anti-inflammatory effects [101,102]. The LXs and Rvs families, including LXA4, AT-LXA4 (15-epi-LXA4), RvD1, AT-RvD1 (17-epi-RvD1) and Annexin A1 (ANXA1), all activate receptors with high potency. Meanwhile, serum amyloid A (SAA) and cathelicidin (LL-37) have been identified as endogenous antagonists [103,104].
The positive control of FPR2 can also improve pulmonary fibrosis, dermal fibrosis, and cystic fibrosis [105,106,107]. Impaired inflammatory control after myocardial infarction (MI) promotes left ventricular (LV) remodeling and loss of function. In left ventricle (LV) and splenic remodeling post-myocardial infarction (MI), the RvD1-group showed an early exit of neutrophils from LV and spleen at day 5 after MI with the increased expression of the LXA4 receptor (ALX; synonym formyl peptide receptor; FPR2) compared to the MI-saline group [108]. Cmpd43, a dual FPR1/FPR2 agonist, stimulated FPR1/2-mediated signaling, enhanced pro-resolving cellular function, and regulated cytokines. Cmpd43 also increased the pro-resolving macrophage marker while increasing LV function and reducing chamber remodeling. From these results, it is likely that the positive control of FPR2 by RvD and LXA4, which are ligands of FPR2, can be a significant inhibitory means of general fibrosis, including cardiac fibrosis.
4.2.3. GPR18
GPR18 was discovered as a receptor for RvD2 through GPCR-β-arrestin-based screening and the receptor was called DRV2/GPR18 [109,110,111]. In any case, several other ligands activate DRV2/GPR18. These include endogenous ligands, such as n-arachidonylglycine (NAGly), anandamide, a metabolite of the endocannabinoid anandamide, synthetic ligands such as abnormal-cannabidiol (Abn-CBD), and O-1918, a partial agonist. These substances serve as useful pharmacological tools to inhibit DRV2/GPR18 signaling [112,113]. GPR18 is abundantly expressed in polymorphonuclear neutrophils (PMNs), monocytes and macrophages [114]. RvD2 treatment increased the expression of CD163 and CD206, a classic marker of anti-inflammatory macrophages, and the upregulation of GPR18 expression [109,110]. Moreover, the activation of GPR18 by RvD2 reduced lipopolysaccharide (LPS) and ATP-stimulated inflammation in macrophages, and this effect was lost by the GPR18/GPR55 antagonist O-1918 [115]. In patients with sepsis, GPR18 is significantly reduced in PMN, indicating a role in resolving inflammation [116]. To the best of our knowledge, the potential role of GPR18 in the development of fibrosis has not been characterized in any previous study. This gene is known to be involved in the termination of inflammation and, therefore, it is likely to play an essential role in the control of fibrosis.
4.2.4. GPR32
GPR32 is primarily expressed in human PMN, monocytes, adipose tissue and vascular endothelial cells [117]. RvD1 has been identified as a potential agonist due to the activation of GPR32, where [3H]-RvD1 binds to human leukocytes and significantly lowers TNF-α-stimulated NF-κB signaling in GPR32 overexpressing cells [81]. RvD1 has a higher affinity for GPR32 than FPR2/ALX, but its interaction with GPR32 has not been extensively studied [118]. This may be because GPR32 exists as a pseudogene in rodents, making animal testing, in principle, inappropriate.
The treatment of inflammatory macrophages expressing GPR32 with RvD1 enhances the pro-resolving phenotype to increase phagocytosis and reduce the secretion of inflammatory cytokines [119]. Additionally, GPR32 mediates the inhibition of epithelial mesenchymal transition (EMT) in lung cancer cell lines by RvD1 [120]. Anti-GPR32 antibodies reversed the reduction in PMN migration. However, anti-FPR2/ALX antibodies did not show similar effects. After increasing the RvD1 concentration, the effect was reversed, confirming that RvD1 had a higher efficacy against GPR32 than FPR2/ALX [118]. Besides, RvD3, AT-RvD3 and RvD5 have all been shown to activate GPR32 in a recombinant system of β-arrestin recruitment [121,122]. This fact suggests the potential redundancy of ligands acting on GPCRs. GPR32 is involved in the inhibition of TGF-β1-induced EMT by RvD1 in lung cancer cells and primary alveolar type II (ATII) cells [120,123]. In particular, it reduced fibroproliferation and collagen production in ATII cells [123]. These results show that GPR32 may be a target for fibrosis regulation.
4.2.5. GPR37
GPR37 or Parkin-associated endothelin-like receptor (Pael-R) was originally discovered through genomic library screening to find new neuropeptide receptors [124]. The GPR37 receptor is primarily expressed in the brain and is associated with neurological disorders, such as Parkin’s disease, and autism [125]. Mutations within GPR37 affect a variety of autism spectrum disorders, dopamine reuptake regulation, and oligodendrocyte differentiation [126,127,128]. PD1 is considered a ligand for GPR37 because it induced a significant increase in intracellular calcium in HEK293 cells overexpressing GPR37 and in murine peritoneal macrophages [129]. Based on the fact that Gpr37 (-/-) mice exhibited increased apoptosis and infarct size, it has recently been suggested that GPR37 is also involved in cell damage protection and inflammation after ischemic stroke [130]. There seems to be no report of whether GPR37 regulates fibrosis.
4.2.6. Leukotriene B4 Receptor 1 (BLT1)
BLT1 is a receptor for RvE1 and leukotriene B4 (LTB4). However, high-affinity LTB4 is a potent lipid inflammatory chemoattractant, but induces T helper cell chemotaxis and early effector T cell recruitment through BLT1 [80,131]. BLT1 shares 21% sequence identity with chemerin1. Although this value is relatively low, the selective BLT1 antagonist U-75302 has been demonstrated to replace the binding of [3H]-RvE1 to human PMN membranes [80]. The activation of BLT1 by RvE1 also acts as a feedback mechanism for other SPMs, including increased production of LXA4 in the FPR2/ALX-mediated resolution of allergic pulmonary inflammation [132]. RvE2 was identified as an additional BLT1 agonist, and β-arrestin analysis showed that RvE2 blocks LTB4-mediated β-arrestin signaling with similar efficacy to RvE1, indicating direct competition with LTB4 [133]. Various pro-solving roles of RvE2 have been proposed, including the regulation of PMN infiltration and IL-10 production [133]. However, while RvE1 promotes NADPH oxidase-mediated ROS production through the BLT1 receptor, RvE2 and RvE3 do not exhibit this effect [134]. The activation of BLT1 via LTB4 is involved in promoting fibrosis [135,136,137]. Research on whether fibrosis is inhibited by RvE1, a ligand related to the termination of inflammation of BLT1, has not yet been conducted. Exploring this topic would provide insights into the role of BLT1, RvE1, and LTB4 in fibrosis.
4.2.7. Miscellaneous SPMs Receptors
Several studies have reported other GPCR-related possibilities that SPMs activate. Among them, GPR101 mediates the resolving effects of RvD5n-3 DPA in arthritis and infection [138]. Furthermore, SPMs has been reported to activate non-GPCR receptors such as nuclear receptors. Dose-dependently, PD1 enhances peroxisome proliferator-activated receptor γ (PPAR-γ) transcription-activated reporter activity in human neuron-glia (HNG) cells co-transfected with hPPAR-γ-GAL4 and MH100-tk-luc [139]. The transcriptional activity of PPAR-γ increased significantly after treatment with 100 nM PD1. This suggests that PD1 may enhance the PPAR-γ. RvD1 was also hypothesized as a ligand for PPAR-γ. It inhibited IκBα degradation and NF-κB p65 nuclear translocation in an LPS-induced lung injury model, which was partially reversed by the PPAR-γ inhibitor GW9662 [140]. Interestingly, PPAR-γ has the action of improving fibrosis, including cardiac fibrosis [141,142]. GPCRs that act directly on MaR1 have not yet been identified. However, MaR1 blocks TRPV1-mediated currents in neurons, acts as a ligand for the retinoid-associated orphan receptor α (RORα), and inhibits TLR4 signaling [143], Chiang et al. found that MaR1 can activate leucine rich repeat containing G protein-coupled receptor 6 (LGR6), a member of the glycoprotein hormone receptor subfamily of this rhodopsin-like GPCR. LGR6 initiates cAMP, impedance changes and stimulates innate immune responses to PMN, monocytes and macrophages [144].
5. Specialized Pro-Resolving Lipid Mediators and Cardiac Fibrosis
There were not many reports on the improvement of cardiac fibrosis by SPMs resulting from DHA and EPA. As mentioned above, in many papers, the activation of NRF2 has been reported as a major mechanism of action for improving cardiac fibrosis. There have been many reports on the relationship between NRF2 activation and SPMs. Thus, in this section, we will first describe the relationships between NRF2 and DHA, EPA, and SPMs.
5.1. Connection between NRF2 and SPMs
We mentioned earlier that activation of NRF2 in cardiac fibrosis can improve cardiac fibrosis (see Section 3.2). This section summarizes the relationship between the DHA, EPA, and SPMs reported so far and NRF2.
5.1.1. Connection between NRF2 and DHA/EPA
What is the relationship between DHA and EPA and SPMs derived from them with NRF2? Antarctic krill oil (AKO) contains abundant EPA and DHA, accounting for more than 27% of its total fatty acid profile [145]. The serum levels of EPA and DHA, and relative protein levels of KEAP1, and NRF2 in the peripheral blood leukocytes from intervention group (IG) patients taking AKO were higher than those in the control group (CG) of patients not taking the AKO (p < 0.05) [145]. Serum levels of reactive oxygen species (ROS), 8-hydroxy-2-deoxyguanosine (8-OHdG), nitric oxide (NO) and malondialdehyde (MDA) of the IG group were lower than those of the CG group. The levels of superoxide dismutase (SOD), glutathione (GSH) and glutathione peroxidase (GPx) in the IG group were higher than in the CG group (p < 0.05) [145].
Omega-3 polyunsaturated fatty acids exert antioxidant effects through the NRF2 pathway in immortalized mouse Schwann cells [146]. The mechanism of action appears to be that the n-3 fatty acid oxidation product destabilizes the bond between Keap1 and Cullin3, thereby activating NRF2 [147]. Omega-3 polyunsaturated fatty acids also showed antioxidant effects in 3T3-L1 adipocytes and murine astrocytes through the NRF2/HO-1 pathway [148,149]. Omega-3 fatty acids activate NRF2 and upregulate HO-1 to protect the brain from ischemic damage [150]. N-3 PUFA induces inflammatory resistance through the formation of KEAP1-containing cytosolic speckles of the autophagic receptor SQSTM1/p62 (sequestosome 1) SQSTM1/p62-body and activation of NFE2L2 [151]. n-3 PUFA induces acute myeloid leukaemia cell death associated with mitochondrial glycolytic switch from oxidative respiration and the activation of the NRF2 pathway [152]. EPA treatment also increased NRF2 nuclear translocation and antioxidant activity, leading to the upregulation of HO-1 expression, and treatment with EPA reduced apoptosis due to hydrogen peroxide [153]. EPA regulates NRF2 to prevent oxidative stress and inflammatory responses caused by tetrachlorodibenzo-p-dioxin (TCDD) [154]. EPA prevents salt sensitivity and reduces oxidative stress in diabetic mice [155].
17-Oxo-docosahexaenoic acid induces the NRF2-mediated expression of HO-1 in mouse skin and cultured rat epidermal cells in vivo [156]. DHA increases the expression of oxidative stress-induced growth inhibitor 1 through the PI3K/AKT/NRF2 signaling pathway in breast cancer cells [157]. Marine n-3 PUFA DHA induces autophagy and NFE2L2 in human retinal pigment epithelial cells, leading to cellular protection against oxidative stress and protein misfolding [158]. The inhibition of inflammation by DHA is, in part, through communication between the NRF2/HO-1 and the IKK/NF-κB pathway [159]. DHA protects against traumatic brain injury by regulating NADPH oxidase 2 production through the NRF2-ARE signaling pathway [160,161]. DHA and MeHg treatment markedly induced the expression of endoplasmic reticulum (ER) stress markers’ (CHOP and DNAJB9) and NRF2 target genes’ (p62 and HMOX-1) mRNA levels and, unexpectedly, adding EPA to DHA and MeHg treatment attenuated DHA and MeHg-induced apoptosis and inhibited ER stress and the expression of NRF2 target genes [162]. DHA can also block nerve damage and prevent stroke, and its inhibitory effect on nerve cell damage is achieved through DHA’s antioxidant (through induction of the NRF2/HO-1 system) and anti-inflammatory effects (through JNK/AP-1 signaling) [163].
5.1.2. Connection between NRF2 and LXs
LXA4-induced HO-1 protects cardiomyocytes from hypoxia/reoxygenation damage through p38 MAPK activation and NRF2/ARE complexes [164]. NRF2 is translocated to the nucleus and binds to the HO-1 ARE and E1 enhancer, leading to upregulation of HO-1. LXA4 inhibited oxidative stress-induced vascular endothelial cell (EC) damage and the expression of thrombosis-related factors by the same mechanism [165]. LXA4 inhibits EC hyperpermeability induced by LPS in human umbilical vein endothelial cells (HUVEC), and NRF2 is also involved [166]. LXA4 also exhibits various pharmacological actions through NRF2 in nerve-related cells. In other words, it improves cellular damage through NRF2 in cultured cortical astrocytes exposed to glucose deficiency/reperfusion stimulation [167]. Besides, LXA4 upregulates NRF2 to protect against brain ischemia-reperfusion injury and spinal cord injury [168,169,170]. LXA4 induced NRF2 expression and nuclear translocation, HO-1 expression, and GSH synthesis. LXA4 reduced LPS-induced mouse acute lung injury through NRF2-mediated E-cadherin expression [171]. LXA4 further promoted the dissociation of NRF2 and Keap1 in LPS-treated 16HBE cells, and LXA4 activated NRF2 by inducing the phosphorylation of Ser40 of NRF2 to induce nuclear translocation (Figure 4). LXA4 also improved acute lung injury related to acute pancreatitis through the antioxidant and anti-inflammatory effects of the NRF2 pathway [172]. In Nrf2-/- mice, the effect of LXA4 on the reduction in inflammatory factors was partially attenuated [172]. This result suggested that NRF2 is involved in the LXA4-induced reduction in the level of inflammatory factors.
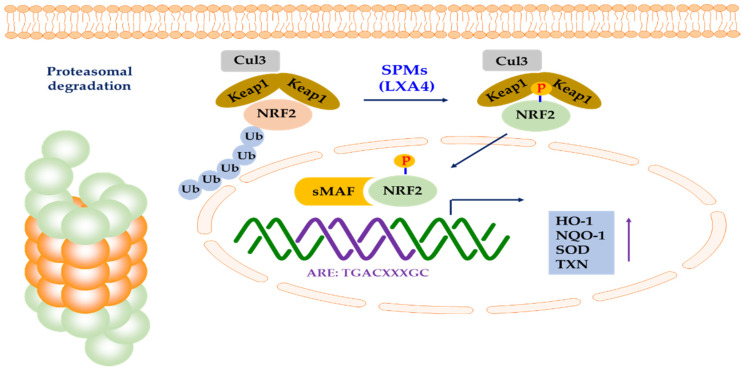
Figure 4.
Activation of NRF2 by SPMs. LXA4 can activate NRF2 by inducing the phosphorylation of Ser40 of NRF2 to induce nuclear translocation. Phosphorylated NRF2 can form a heterodimer with sMAF and bind to ARE, leading to the transcription of antioxidant genes, such as HO-1, NQO-1, SOD, and TXN (modified from Lin et al. [173] and Hiebert et al. [52]).
LXA4 preconditioning attenuates intestinal ischemia-reperfusion injury via the KEAP1/NRF2 pathway in a LXA4 receptor-independent manner [174]. The LXA4 receptor antagonist Boc-2 reversed the protective effect of LXA4 on intestinal damage but did not affect oxidative stress and the associated NRF2 pathway [174]. Additionally, the NRF2 antagonist brusatol reversed the antioxidant effects imparted by LXA4, exacerbating oxidative stress and apoptosis in intestinal epithelial cells, eventually reducing the survival rate of mice [174]. LXA4 treatment upregulated p38 MAPK activation, Nrf2 nuclear translocation, and the binding activity of NRF2 to HO-1 ARE and E1 enhancers in human kidney cells exposed to H/R injury [175]. The treatment of bone marrow-derived macrophages with 15-epi-LXA4 (e-LXA4) inhibits the LPS-dependent activation of NF-κB and IκB kinase β activities, protects against LPS activation-dependent apoptosis, and enhances the accumulation of NRF2 transcription factors. Moreover, the treatment of LPS-stimulated bone marrow-derived macrophages with e-LXA4 dramatically reduces the Kv current, which is compatible with the attenuation of the inflammatory response [176].
LXs were discovered before other SPMs, resulting in synthetic derivatives with increased chemical stability, and the possibility of NRF2 involvement in pharmacological action by these compounds is suggested. In other words, LXA4 methyl ester activates the ERK/NRF2 signaling pathway in rats, improving cognitive impairment due to chronic decreased cerebral perfusion [167]. The LXA4 Receptor/FPR2 Agonist BML-111 protected mouse skin from ultraviolet B radiation, and an increase in NRF2 signaling was observed [177]. BML-111 treatment prevents cardiac cell death and oxidative stress in an autoimmune myocarditis mouse model [178]. These beneficial effects were mediated by the activation of the NRF2 pathway via the CaMKK2-AMPKα kinase pathway. BML-111 also attenuates high glucose-induced inflammation, oxidative stress, and reduces extracellular matrix accumulation by targeting NRF2 in murine glomerular media cells [179]. Finally, BML-111 exerts anti-inflammatory effects in a chronic obstructive pulmonary disease (COPD) mouse model by preventing NLRP3 inflammasome activation and inhibiting ROS production through NRF2 upregulation [180].
5.1.3. Connection between NRF2 and Rvs
RvD1 prevents memory impairment and hippocampal damage in mice fed a corn oil-based high fat diet through NRF2 upregulation and p66 Shc downregulation and inactivation [181]. RvD1 attenuates ventilator-induced lung damage by reducing HMGB1 release in the HO-1 dependent pathway [182]. At this condition, RvD1 increases the expression of NRF2 and inhibits the activation of NF-κB. AT-RvD1 inhibited TGF-β1-induced EndMT (endothelial to mesenchymal transition) by increasing the expression of Smad7 [183]. AT-RvD1 restored the decrease in NRF2 by TGF-β1 and decreased the increase in vimentin expression. AT-RvD1 recovers mouse lungs after tobacco smoke-induced emphysema through downregulation of oxidative stress by the NRF2/KEAP1 pathway [184]. AT-RvD1 also alleviated paraquat-induced acute lung injury in mice [185]. At this time, AT-RvD1 activated NRF2 and upregulated the expression of its downstream genes (NQO-1 and HO-1). This action was also observed when Rvs suppressed skin inflammation and UV-induced oxidative stress [186]. Besides, RvD1 promotes corneal epithelial wound healing and mechanical sensation recovery in diabetic mice [187].
5.1.4. Connection between NRF2 and MaRs
MaR1, another member of SPMs, promotes the nuclear translocation of NRF2 in Sprague–Dawley mice, improves hepatic ischemia-reperfusion injury and stimulates hepatocyte proliferation [188]. MaR1 also modulates the NRF2 and TLR4/NF-κB signaling pathways to relieve dextran sulfate sodium-induced ulcerative colitis [189]. MaR1 activated NRF2 signaling and reduced TLR4/NF-κB activation, and ML385, an inhibitor of NRF2, significantly suppressed these effects of MaR1. MaR1 alleviates renal ischemia/reperfusion injury in mice through inhibition of the TLR4/MAPK/NF-κB pathway and activation of the NRF2 pathway [190]. MaR1 decreased the nuclear translocation of NF-κB and increased the nuclear translocation of NRF2 in this model. MaR1 also improves pulmonary ischemia/reperfusion injury by inhibiting oxidative stress through the activation of the NRF2 mediated HO-1 signaling pathway [191]. Specifically, MaR1 significantly reduces the production of ROS, methane dicarboxylic acid aldehyde and 15-F2t-isoprostane, and restores antioxidant enzymes (superoxide dismutase, glutathione peroxidase and catalase) activity. This appears to be through the expression of nuclear NRF2 and cytoplasmic HO-1.
5.2. Therapeutic Effects of DHA and EPA, and SPMs on Cardiac Fibrosis
5.2.1. Therapeutic Effects of DHPA and EPA on Cardiac Fibrosis
Before examining the effects of SPMs on cardiac fibrosis, we would like first to mention the effects of DHA and EPA on cardiac fibrosis. DHA and EPA do not only impact cardiac fibrosis but show improvement in various fibrosis. In other words, for example, krill oil (KO), which is high in DHA and EPA, relieves oxidative stress, iron accumulation and fibrosis in the liver and spleen of iron-overloaded mice [192]. In clinical trials, high-dose EPA (4 g/day) reduced the incidences of clinical events by 25% in statin-treated patients with established CVD or diabetes and other cardiovascular risk factors [193]. The cardioprotective effect of omega-3 fatty acids and ascorbic acid improves the regenerative capacity of embryonic stem cell-derived cardiac lineage cells [194]. Pre-incubation of cells with EPA + DHA + AA before H2O2 treatment weakened the production of reactive oxygen species (ROS), improved cell viability and increased HO-1 and CX43 (connexin43) expression, leading to a marked rise in cardiac troponin (TNNT2) positive cells and a decrease in vimentin-positive cells [194]. In particular, the injection of EPA + DHA + AA pretreated ESC (embryonic stem cell)-derived cardiac lineage cells into the ischemic myocardium of the MI rat model significantly reduced fibrosis compared to the vehicle group [194].
FO, which is rich in omega-3 polyunsaturated fatty acids, reduced the arachidonic acid (AA) to DHA plasma ratios [195]. In the rat diabetes model (DM), FO reduced cardiac nitrite and MPO and plasma ET-1 levels and increased cardiac glutathione, catalase, and SOD activities. FO prevented DM-related cardiac fibrosis and reduced TGF-β1 and p38 MAP kinases in the heart [195]. ω3-PUFA exhibits cardioprotective action by signaling through the free fatty acid receptor 4 (Ffar4), a G-protein coupled receptor (GPR) for long-chain fatty acids (FA) [196]. EPA, not DHA, prevents fibrosis in heart failure due to pressure overload: a potential role of Ffar4 is suggested [197]. The superphysiological levels of ω3-PUFA achieved by 12 weeks of dietary supplementation prevented fibrosis and diastolic function following pressure overload (transverse artery contraction (TAC)), which was meant to mimic heart failure with preserved ejection fraction [198].
DHA reverses AngII-induced RECK inhibition and migration of cardiac fibroblast [198]. EPA and DHA reversed AngII-mediated RECK inhibition, and DHA appeared to be more effective. EPA and DHA reversed AngII-induced miR-21 expression, RECK inhibition, MMP2 induction, and cardiac fibroblast migration. Therefore, it may exert its potential beneficial effects in cardiac fibrosis. Treatment with omega-3 fatty acids inhibited the loss of CX43 protein by IL-1β, and more importantly, suppressed the loss of CX43 function by inhibiting the translocation of NF-κB. Diets rich in omega-3 fatty acids in the intact heart limited the loss of CX43 in the disc inserted into the heart after MI [199].
DHA supplementation reduced susceptibility to atrial fibrillation, and in dogs, DHA also reduced atrial fibrosis compared to No-PUFA [200]. Long-term administration of EPA improves cardiac remodeling after myocardial infarction in mice by modulating macrophage polarization [201]. Highly purified EPA improves heart damage and adipose tissue inflammation in a rat model of metabolic syndrome [202]. EPA suppresses the side effects of C-reactive protein overexpression on cardiac remodeling due to pressure overload [203]. EPA prevents atrial fibrillation associated with heart failure in rabbit models [204].
5.2.2. Therapeutic Effects of SPMs on Cardiac Fibrosis
Compared to the effects of DHA and EPA on improving cardiac fibrosis, there are few reports on the therapeutic effect on cardiac fibrosis by pro-resolving lipids mediators derived from them. Chagas disease, caused by the protozoan Trypanosoma cruzi, progresses to chronic progressive inflammatory cardiomyopathy, which leads to heart failure and death during the acute asymptomatic stage if an appropriate treatment is not administered [205]. Statins can regulate chagasic myocarditis by inducing the production of 15-epi-lipoxin A4 (15-epi-LXA4), a pro-resolving lipid mediator in inflammatory cardiomyopathy. Ticagrelor, which acts as a platelet aggregation inhibitor by antagonizing the P2Y12 receptor [206], protects the heart from reperfusion injury and improves remodeling after myocardial infarction [207]. Ticagrelor alleviated fibrosis and decreased collagen-III mRNA levels in ischemia/reperfusion models [207]. Besides, ticagrelor attenuated the increase in pro-inflammatory tumor necrosis factor-α, interleukin-1β, and interleukin-18, and increased anti-inflammatory 15-epi-LXA4 levels, which suggested that 15-epi-LXA4 was involved in ticagrelor-induced improving cardiac fibrosis [207].
BML-111 treatment prevents cardiac cell death and oxidative stress in an autoimmune myocarditis mouse model [178]. In vivo and in vitro studies have demonstrated that these beneficial effects are mediated by the activation of the NRF2 pathway via the CaMKK2-AMPKα kinase pathway. Cardiac dysfunction, hypertrophy, and neofibrosis found in experimental autoimmune myocarditis mice were prevented by BML-111 treatment [178].
RvD1 improves ventricular function by activating the pro-resolving response in the ventricular region after myocardial infarction [108]. In this method, 8–12-week-old male C57BL/6J mice were coronary ligated and injected with Lipo-RvD1 or RvD1 (3 μg/kg/day) for 3 h after MI (d) for 1 day or until fifth day. The RvD1-group showed increased expression of the LXA4 receptor (ALX; synonym formyl peptide receptor; FPR2) compared to the MI-saline group. RvD1 stabilized the extracellular matrix by reducing the pro-fibrotic genes (colla1, coll2a1 and tnc (all; p < 0.05)) and reducing collagen deposition, thereby reducing fibrosis after MI.
6. Conclusions
Cardiac fibrosis is a serious disease that is difficult to cure. DHA and EPA have therapeutic effects on cardiac fibrosis through the activation of NRF2. Recently, the collection of results on SPMs produced from DHA and EPA have proved the potential against various chronic inflammatory diseases. Cardiac fibrosis, in which inflammation plays an important role, can also be such a disease. Among the SPMs, BML-111, an LX derivative, and RvD1 have been effective against cardiac fibrosis, but many other SPMs, such as RvEs, MaRs, and PDs, have not been reported with regard to their efficacy in treating cardiac fibrosis. This is likely not because SPMs may not have a therapeutic effect on cardiac fibrosis, and because it is not easy to get enough SPMs to evaluate the therapeutic effects for cardiac fibrosis.
There are many reports that DHA and EPA, the precursors of SPMs, effectively treat cardiac fibrosis through the activation of NRF2 and that SPMs can activate the NRF2. Therefore, it is expected for other SPMs to show a therapeutic effect. Accordingly, there are many reports that SPMs are already effective in fibrosis in other organs, such as lung. Therefore, the therapeutic effect of SPMs via NRF2 in cardiac fibrosis is predicted to be sufficiently effective, and studies on this prediction are expected to proceed actively. In particular, the activation of NRF2 through novel SPMs and their derivatives seems to be a promising new strategy for the treatment of cardiac fibrosis.
Acknowledgments
We would like to thank Grace Lee (Rice University at Houston, TX, USA) for critically reading the manuscript.
Author Contributions
G.J.K. and E.J.K. contributed to writing the original draft. C.H.L. contributed to conceptualization, supervision, and writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Basic Science Research Program, through the NRF (NRF-2018R1E1A2A02057995, NRF-2018R1A5A2023127, NRF-2020R1A2C 3004973, and NRF-2020M3E5E2038356) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (HP20C0131).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Tao H., Shi K.H., Yang J.J., Huang C., Zhan H.Y., Li J. Histone deacetylases in cardiac fibrosis: Current perspectives for therapy. Cell Signal. 2014;26:521–527. doi: 10.1016/j.cellsig.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 5.Piek A., de Boer R.A., Sillje H.H. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016;21:199–211. doi: 10.1007/s10741-016-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R.R., Fan X.H., Chen G., Zeng G.W., Xue Y.G., Liu X.T., Wang C.Y. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFbeta1/Smad2/3 signaling axis. Chem. Biol. Interact. 2019;302:11–21. doi: 10.1016/j.cbi.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen S.H., Mouton A.J., DeLeon-Pennell K.Y., Genovese F., Karsdal M., Lindsey M.L. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. 2019;75–76:43–57. doi: 10.1016/j.matbio.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mewton N., Liu C.Y., Croisille P., Bluemke D., Lima J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brower G.L., Gardner J.D., Forman M.F., Murray D.B., Voloshenyuk T., Levick S.P., Janicki J.S. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur. J. Cardiothorac. Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Dai Z., Aoki T., Fukumoto Y., Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J. Cardiol. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Nattel S. How does fibrosis promote atrial fibrillation persistence: In silico findings, clinical observations, and experimental data. Cardiovasc. Res. 2016;110:295–297. doi: 10.1093/cvr/cvw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baci D., Bosi A., Parisi L., Buono G., Mortara L., Ambrosio G., Bruno A. Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis. Int. J. Mol. Sci. 2020;21:7165. doi: 10.3390/ijms21197165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 15.Crabos M., Roth M., Hahn A.W., Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J. Clin. Investig. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurisu S., Ozono R., Oshima T., Kambe M., Ishida T., Sugino H., Matsuura H., Chayama K., Teranishi Y., Iba O., et al. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.HYP.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 17.Lijnen P., Petrov V. Induction of cardiac fibrosis by aldosterone. J. Mol. Cell. Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 18.Messaoudi S., Gravez B., Tarjus A., Pelloux V., Ouvrard-Pascaud A., Delcayre C., Samuel J., Launay J.M., Sierra-Ramos C., Alvarez de la Rosa D., et al. Aldosterone-specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension. 2013;61:361–367. doi: 10.1161/HYPERTENSIONAHA.112.198986. [DOI] [PubMed] [Google Scholar]
- 19.Biernacka A., Dobaczewski M., Frangogiannis N.G. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarreal F.J., Dillmann W.H. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am. J. Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 21.Lyons R.M., Keski-Oja J., Moses H.L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Desmouliere A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller M., Javelaud D., Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Shi-Wen X., Rodriguez-Pascual F., Lamas S., Holmes A., Howat S., Pearson J.D., Dashwood M.R., du Bois R.M., Denton C.P., Black C.M., et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: Evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol. Cell. Biol. 2006;26:5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leask A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008;20:1409–1414. doi: 10.1016/j.cellsig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Shi-wen X., Kennedy L., Renzoni E.A., Bou-Gharios G., du Bois R.M., Black C.M., Denton C.P., Abraham D.J., Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 28.Gevaert A.B., Shakeri H., Leloup A.J., Van Hove C.E., De Meyer G.R.Y., Vrints C.J., Lemmens K., Van Craenenbroeck E.M. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ. Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003806. [DOI] [PubMed] [Google Scholar]
- 29.Testa M., Yeh M., Lee P., Fanelli R., Loperfido F., Berman J.W., LeJemtel T.H. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996;28:964–971. doi: 10.1016/S0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann U., Knorr S., Vogel B., Weirather J., Frey A., Ertl G., Frantz S. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ. Heart Fail. 2014;7:822–830. doi: 10.1161/CIRCHEARTFAILURE.113.001020. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Wu Y., Zhang C., Li P., Cui W., Hao J., Ma X., Yin Z., Du J. gammadeltaT Cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64:305–314. doi: 10.1161/HYPERTENSIONAHA.113.02604. [DOI] [PubMed] [Google Scholar]
- 32.Kanisicak O., Khalil H., Ivey M.J., Karch J., Maliken B.D., Correll R.N., Brody M.J., Suh-Chin J.L., Aronow B.J., Tallquist M.D., et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos G.C., van den Berg A., Nunes-Silva V., Weirather J., Peters L., Burkard M., Friedrich M., Pinnecker J., Abesser M., Heinze K.G., et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl. Acad. Sci. USA. 2017;114:E2420–E2429. doi: 10.1073/pnas.1621047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulsmans M., Clauss S., Xiao L., Aguirre A.D., King K.R., Hanley A., Hucker W.J., Wulfers E.M., Seemann G., Courties G., et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169:510–522.e20. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dick S.A., Macklin J.A., Nejat S., Momen A., Clemente-Casares X., Althagafi M.G., Chen J., Kantores C., Hosseinzadeh S., Aronoff L., et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019;20:29–39. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 39.Song E., Ouyang N., Horbelt M., Antus B., Wang M., Exton M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 40.Simoes F.C., Cahill T.J., Kenyon A., Gavriouchkina D., Vieira J.M., Sun X., Pezzolla D., Ravaud C., Masmanian E., Weinberger M., et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020;11:600. doi: 10.1038/s41467-019-14263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K., Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S., Weinberg S., DeBerge M., Gainullina A., Schipma M., Kinchen J.M., Ben-Sahra I., Gius D.R., Yvan-Charvet L., Chandel N.S., et al. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019;29:443–456.e5. doi: 10.1016/j.cmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swirski F.K., Nahrendorf M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 44.Gentek R., Hoeffel G. The Innate Immune Response in Myocardial Infarction, Repair, and Regeneration. Adv. Exp. Med. Biol. 2017;1003:251–272. doi: 10.1007/978-3-319-57613-8_12. [DOI] [PubMed] [Google Scholar]
- 45.Frangogiannis N.G. The immune system and cardiac repair. Pharmacol. Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuamnaichati N., Sato V.H., Moongkarndi P., Parichatikanond W., Mangmool S. Sustained beta-AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation. Life Sci. 2018;193:257–269. doi: 10.1016/j.lfs.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 47.Zhao D., Li C., Yan H., Li T., Qian M., Zheng N., Jiang H., Liu L., Xu B., Wu Q., et al. Cardiomyocyte Derived miR-328 Promotes Cardiac Fibrosis by Paracrinely Regulating Adjacent Fibroblasts. Cell. Physiol. Biochem. 2018;46:1555–1565. doi: 10.1159/000489201. [DOI] [PubMed] [Google Scholar]
- 48.Ge Z.D., Lian Q., Mao X., Xia Z. Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy. Int. Heart J. 2019;60:512–520. doi: 10.1536/ihj.18-476. [DOI] [PubMed] [Google Scholar]
- 49.Wu J., Xia S., Kalionis B., Wan W., Sun T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res. Int. 2014;2014:615312. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parim B., Sathibabu Uddandrao V.V., Saravanan G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019;24:279–299. doi: 10.1007/s10741-018-9749-1. [DOI] [PubMed] [Google Scholar]
- 51.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 53.He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dodson M., de la Vega M.R., Cholanians A.B., Schmidlin C.J., Chapman E., Zhang D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motohashi H., Katsuoka F., Engel J.D., Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. USA. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Nioi P., Nguyen T., Sherratt P.J., Pickett C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahn J.H., Hwang S.H., Cho H.S., Lee M. Differential Gene Expression Common to Acquired and Intrinsic Resistance to BRAF Inhibitor Revealed by RNA-Seq Analysis. Biomol. Ther. 2019;27:302–310. doi: 10.4062/biomolther.2018.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ge C., Hu L., Lou D., Li Q., Feng J., Wu Y., Tan J., Xu M. Nrf2 deficiency aggravates PM2.5-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging. 2020;12:4836–4865. doi: 10.18632/aging.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Ichikawa T., Villacorta L., Janicki J.S., Brower G.L., Yamamoto M., Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arter. Thromb. Vasc. Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 61.Tian C., Gao L., Zimmerman M.C., Zucker I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H928–H939. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Duan J.Z., He Q., Wang C.Q. miR155 modulates high glucoseinduced cardiac fibrosis via the Nrf2/HO1 signaling pathway. Mol. Med. Rep. 2020 doi: 10.3892/mmr.2020.11495. [DOI] [PubMed] [Google Scholar]
- 63.Cai S.A., Hou N., Zhao G.J., Liu X.W., He Y.Y., Liu H.L., Hua Y.Q., Li L.R., Huang Y., Ou C.W., et al. Nrf2 Is a Key Regulator on Puerarin Preventing Cardiac Fibrosis and Upregulating Metabolic Enzymes UGT1A1 in Rats. Front. Pharmacol. 2018;9:540. doi: 10.3389/fphar.2018.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou X., Fu M., Cheng B., Kang Y., Xie D. Galanthamine improves myocardial ischemia-reperfusion-induced cardiac dysfunction, endoplasmic reticulum stress-related apoptosis, and myocardial fibrosis by suppressing AMPK/Nrf2 pathway in rats. Ann. Transl. Med. 2019;7:634. doi: 10.21037/atm.2019.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Cui Y., Dai S., Deng W., Wang H., Qin W., Yang H., Liu H., Yue J., Wu D., et al. Isorhynchophylline enhances Nrf2 and inhibits MAPK pathway in cardiac hypertrophy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:203–212. doi: 10.1007/s00210-019-01716-0. [DOI] [PubMed] [Google Scholar]
- 66.Song J., Meng Y., Wang M., Li L., Liu Z., Zheng K., Wu L., Liu B., Hou F., Li A. Mangiferin activates Nrf2 to attenuate cardiac fibrosis via redistributing glutaminolysis-derived glutamate. Pharmacol. Res. 2020;157:104845. doi: 10.1016/j.phrs.2020.104845. [DOI] [PubMed] [Google Scholar]
- 67.Li L., Luo W., Qian Y., Zhu W., Qian J., Li J., Jin Y., Xu X., Liang G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 2019;59:152774. doi: 10.1016/j.phymed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 68.Pirault J., Back M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018;9:1273. doi: 10.3389/fphar.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romano M., Cianci E., Simiele F., Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 70.Markworth J.F., Maddipati K.R., Cameron-Smith D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016;22:110–134. [PubMed] [Google Scholar]
- 71.Chandrasekharan J.A., Sharma-Walia N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015;8:181–192. doi: 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serhan C.N., Chiang N., Dalli J., Levy B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7:a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duvall M.G., Levy B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng B., Wang C.W., Arnardottir H.H., Li Y., Cheng C.Y., Dalli J., Serhan C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE. 2014;9:e102362. doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong S., Gronert K., Devchand P.R., Moussignac R.L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 76.Bazan N.G. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 77.Shinohara M., Mirakaj V., Serhan C.N. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front. Immunol. 2012;3:81. doi: 10.3389/fimmu.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balas L., Durand T. Dihydroxylated E,E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 2016;61:1–18. doi: 10.1016/j.plipres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arita M., Ohira T., Sun Y.P., Elangovan S., Chiang N., Serhan C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 81.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.H., Yang R., Petasis N.A., Serhan C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park J., Langmead C.J., Riddy D.M. New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci. 2020;3:88–106. doi: 10.1021/acsptsci.9b00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Im D.S. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog. Lipid Res. 2012;51:232–237. doi: 10.1016/j.plipres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Gantz I., Konda Y., Yang Y.K., Miller D.E., Dierick H.A., Yamada T. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet. Cell Genet. 1996;74:286–290. doi: 10.1159/000134436. [DOI] [PubMed] [Google Scholar]
- 85.Samson M., Edinger A.L., Stordeur P., Rucker J., Verhasselt V., Sharron M., Govaerts C., Mollereau C., Vassart G., Doms R.W., et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 86.Meder W., Wendland M., Busmann A., Kutzleb C., Spodsberg N., John H., Richter R., Schleuder D., Meyer M., Forssmann W.G. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003;555:495–499. doi: 10.1016/S0014-5793(03)01312-7. [DOI] [PubMed] [Google Scholar]
- 87.Wittamer V., Gregoire F., Robberecht P., Vassart G., Communi D., Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 88.Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., Taguchi R., Masuda K., Sasaki K., Urabe D., et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luangsay S., Wittamer V., Bondue B., De Henau O., Rouger L., Brait M., Franssen J.D., de Nadai P., Huaux F., Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 90.Freire M.O., Dalli J., Serhan C.N., Van Dyke T.E. Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes. J. Immunol. 2017;198:718–728. doi: 10.4049/jimmunol.1601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mocker A., Hilgers K.F., Cordasic N., Wachtveitl R., Menendez-Castro C., Woelfle J., Hartner A., Fahlbusch F.B. Renal Chemerin Expression is Induced in Models of Hypertensive Nephropathy and Glomerulonephritis and Correlates with Markers of Inflammation and Fibrosis. Int. J. Mol. Sci. 2019;20:6240. doi: 10.3390/ijms20246240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yilmaz Y., Kurt R., Gurdal A., Alahdab Y.O., Yonal O., Senates E., Polat N., Eren F., Imeryuz N., Oflaz H. Circulating vaspin levels and epicardial adipose tissue thickness are associated with impaired coronary flow reserve in patients with nonalcoholic fatty liver disease. Atherosclerosis. 2011;217:125–129. doi: 10.1016/j.atherosclerosis.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 93.Ye R.D., Cavanagh S.L., Quehenberger O., Prossnitz E.R., Cochrane C.G. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 1992;184:582–589. doi: 10.1016/0006-291X(92)90629-Y. [DOI] [PubMed] [Google Scholar]
- 94.Fiore S., Maddox J.F., Perez H.D., Serhan C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brink C., Dahlen S.E., Drazen J., Evans J.F., Hay D.W., Nicosia S., Serhan C.N., Shimizu T., Yokomizo T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 96.Maderna P., Cottell D.C., Toivonen T., Dufton N., Dalli J., Perretti M., Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maddox J.F., Serhan C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. J. Exp. Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simiele F., Recchiuti A., Mattoscio D., De Luca A., Cianci E., Franchi S., Gatta V., Parolari A., Werba J.P., Camera M., et al. Transcriptional regulation of the human FPR2/ALX gene: Evidence of a heritable genetic variant that impairs promoter activity. FASEB J. 2012;26:1323–1333. doi: 10.1096/fj.11-198069. [DOI] [PubMed] [Google Scholar]
- 99.Waechter V., Schmid M., Herova M., Weber A., Gunther V., Marti-Jaun J., Wust S., Rosinger M., Gemperle C., Hersberger M. Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J. Immunol. 2012;188:1856–1867. doi: 10.4049/jimmunol.1101788. [DOI] [PubMed] [Google Scholar]
- 100.Chen K., Bao Z., Gong W., Tang P., Yoshimura T., Wang J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017;85:64–77. doi: 10.1016/j.jaut.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takano T., Fiore S., Maddox J.F., Brady H.R., Petasis N.A., Serhan C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooray S.N., Gobbetti T., Montero-Melendez T., McArthur S., Thompson D., Clark A.J., Flower R.J., Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bozinovski S., Uddin M., Vlahos R., Thompson M., McQualter J.L., Merritt A.S., Wark P.A., Hutchinson A., Irving L.B., Levy B.D., et al. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wan M., Godson C., Guiry P.J., Agerberth B., Haeggstrom J.Z. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 105.Kim H., Park S.H., Han S.Y., Lee Y.S., Cho J., Kim J.M. LXA4-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-beta/Smad signaling. Cell Death Dis. 2020;11:653. doi: 10.1038/s41419-020-02846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park G.T., Kwon Y.W., Lee T.W., Kwon S.G., Ko H.C., Kim M.B., Kim J.H. Formyl Peptide Receptor 2 Activation Ameliorates Dermal Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Front. Immunol. 2019;10:2095. doi: 10.3389/fimmu.2019.02095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Higgins G., Fustero Torre C., Tyrrell J., McNally P., Harvey B.J., Urbach V. Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L1053–L1061. doi: 10.1152/ajplung.00368.2015. [DOI] [PubMed] [Google Scholar]
- 108.Kain V., Ingle K.A., Colas R.A., Dalli J., Prabhu S.D., Serhan C.N., Joshi M., Halade G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chiang N., Dalli J., Colas R.A., Serhan C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiang N., de la Rosa X., Libreros S., Serhan C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017;198:842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiang N., Barnaeva E., Hu X., Marugan J., Southall N., Ferrer M., Serhan C.N. Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions. Cell Chem. Biol. 2019;26:244–254.e4. doi: 10.1016/j.chembiol.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Offertaler L., Mo F.M., Batkai S., Liu J., Begg M., Razdan R.K., Martin B.R., Bukoski R.D., Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 113.Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 114.Wang X., Sumida H., Cyster J.G. GPR18 is required for a normal CD8alphaalpha intestinal intraepithelial lymphocyte compartment. J. Exp. Med. 2014;211:2351–2359. doi: 10.1084/jem.20140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kurihara T., Jones C.N., Yu Y.M., Fischman A.J., Watada S., Tompkins R.G., Fagan S.P., Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang L., Qiu C., Yang L., Zhang Z., Zhang Q., Wang B., Wang X. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019;217:49–56. doi: 10.1016/j.lfs.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 117.Sansbury B.E., Spite M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016;119:113–130. doi: 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Norling L.V., Dalli J., Flower R.J., Serhan C.N., Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arter. Thromb. Vasc. Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmid M., Gemperle C., Rimann N., Hersberger M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J. Immunol. 2016;196:3429–3437. doi: 10.4049/jimmunol.1501701. [DOI] [PubMed] [Google Scholar]
- 120.Lee H.J., Park M.K., Lee E.J., Lee C.H. Resolvin D1 inhibits TGF-beta1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int. J. Biochem. Cell Biol. 2013;45:2801–2807. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Dalli J., Winkler J.W., Colas R.A., Arnardottir H., Cheng C.Y., Chiang N., Petasis N.A., Serhan C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiang N., Fredman G., Backhed F., Oh S.F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng S., Wang Q., D’Souza V., Bartis D., Dancer R., Parekh D., Gao F., Lian Q., Jin S., Thickett D.R. ResolvinD1 stimulates epithelial wound repair and inhibits TGF-beta-induced EMT whilst reducing fibroproliferation and collagen production. Lab. Investig. 2018;98:130–140. doi: 10.1038/labinvest.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marazziti D., Golini E., Gallo A., Lombardi M.S., Matteoni R., Tocchini-Valentini G.P. Cloning of GPR37, a gene located on chromosome 7 encoding a putative G-protein-coupled peptide receptor, from a human frontal brain EST library. Genomics. 1997;45:68–77. doi: 10.1006/geno.1997.4900. [DOI] [PubMed] [Google Scholar]
- 125.Lopes J.P., Morato X., Souza C., Pinhal C., Machado N.J., Canas P.M., Silva H.B., Stagljar I., Gandia J., Fernandez-Duenas V., et al. The role of parkinson’s disease-associated receptor GPR37 in the hippocampus: Functional interplay with the adenosinergic system. J. Neurochem. 2015;134:135–146. doi: 10.1111/jnc.13109. [DOI] [PubMed] [Google Scholar]
- 126.Fujita-Jimbo E., Yu Z.L., Li H., Yamagata T., Mori M., Momoi T., Momoi M.Y. Mutation in Parkinson disease-associated, G-protein-coupled receptor 37 (GPR37/PaelR) is related to autism spectrum disorder. PLoS ONE. 2012;7:e51155. doi: 10.1371/journal.pone.0051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marazziti D., Mandillo S., Di Pietro C., Golini E., Matteoni R., Tocchini-Valentini G.P. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc. Natl. Acad. Sci. USA. 2007;104:9846–9851. doi: 10.1073/pnas.0703368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang H.J., Vainshtein A., Maik-Rachline G., Peles E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016;7:10884. doi: 10.1038/ncomms10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bang S., Xie Y.K., Zhang Z.J., Wang Z., Xu Z.Z., Ji R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018;128:3568–3582. doi: 10.1172/JCI99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCrary M.R., Jiang M.Q., Giddens M.M., Zhang J.Y., Owino S., Wei Z.Z., Zhong W., Gu X., Xin H., Hall R.A., et al. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. 2019;33:10680–10691. doi: 10.1096/fj.201900070R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 132.Haworth O., Cernadas M., Yang R., Serhan C.N., Levy B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh S.F., Dona M., Fredman G., Krishnamoorthy S., Irimia D., Serhan C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Unno Y., Sato Y., Fukuda H., Ishimura K., Ikeda H., Watanabe M., Tansho-Nagakawa S., Ubagai T., Shuto S., Ono Y. Resolvin E1, but not resolvins E2 and E3, promotes fMLF-induced ROS generation in human neutrophils. FEBS Lett. 2018;592:2706–2715. doi: 10.1002/1873-3468.13215. [DOI] [PubMed] [Google Scholar]
- 135.Liang M., Lv J., Jiang Z., He H., Chen C., Xiong Y., Zhu X., Xue Y., Yu Y., Yang S., et al. Promotion of Myofibroblast Differentiation and Tissue Fibrosis by the Leukotriene B4 -Leukotriene B4 Receptor Axis in Systemic Sclerosis. Arthritis Rheumatol. 2020;72:1013–1025. doi: 10.1002/art.41192. [DOI] [PubMed] [Google Scholar]
- 136.Kamata M., Amano H., Ito Y., Fujita T., Otaka F., Hosono K., Kamata K., Takeuchi Y., Yokomizo T., Shimizu T., et al. Role of the high-affinity leukotriene B4 receptor signaling in fibrosis after unilateral ureteral obstruction in mice. PLoS ONE. 2019;14:e0202842. doi: 10.1371/journal.pone.0202842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lv J., Xiong Y., Li W., Yang W., Zhao L., He R. BLT1 Mediates Bleomycin-Induced Lung Fibrosis Independently of Neutrophils and CD4+ T Cells. J. Immunol. 2017;198:1673–1684. doi: 10.4049/jimmunol.1600465. [DOI] [PubMed] [Google Scholar]
- 138.Flak M.B., Koenis D.S., Sobrino A., Smith J., Pistorius K., Palmas F., Dalli J. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J. Clin. Investig. 2020;130:359–373. doi: 10.1172/JCI131609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Y., Calon F., Julien C., Winkler J.W., Petasis N.A., Lukiw W.J., Bazan N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS ONE. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liao Z., Dong J., Wu W., Yang T., Wang T., Guo L., Chen L., Xu D., Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir. Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kokeny G., Calvier L., Legchenko E., Chouvarine P., Mozes M.M., Hansmann G. PPARgamma is a gatekeeper for extracellular matrix and vascular cell homeostasis: Beneficial role in pulmonary hypertension and renal/cardiac/pulmonary fibrosis. Curr. Opin. Nephrol. Hypertens. 2020;29:171–179. doi: 10.1097/MNH.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 142.Zhuang Y., Li T., Zhuang Y., Li Z., Yang W., Huang Q., Li D., Wu H., Zhang G., Yang T., et al. Involvement of lncR-30245 in Myocardial Infarction-Induced Cardiac Fibrosis Through Peroxisome Proliferator-Activated Receptor-gamma-Mediated Connective Tissue Growth Factor Signalling Pathway. Can. J. Cardiol. 2019;35:480–489. doi: 10.1016/j.cjca.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Park C.K. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediat. Inflamm. 2015;2015:275126. doi: 10.1155/2015/275126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiang N., Libreros S., Norris P.C., de la Rosa X., Serhan C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019;129:5294–5311. doi: 10.1172/JCI129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wen C., Jiang M., Huang W., Liu S. Antarctic Krill Oil Attenuates Oxidative Stress via the KEAP1-NRF2 Signaling in Patients with Coronary Heart Disease. Evid. Based Complement Altern. Med. 2020;2020:9534137. doi: 10.1155/2020/9534137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tatsumi Y., Kato A., Sango K., Himeno T., Kondo M., Kato Y., Kamiya H., Nakamura J., Kato K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019;10:602–612. doi: 10.1111/jdi.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao L., Wang J., Sekhar K.R., Yin H., Yared N.F., Schneider S.N., Sasi S., Dalton T.P., Anderson M.E., Chan J.Y., et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007;282:2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 148.Kusunoki C., Yang L., Yoshizaki T., Nakagawa F., Ishikado A., Kondo M., Morino K., Sekine O., Ugi S., Nishio Y., et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013;430:225–230. doi: 10.1016/j.bbrc.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 149.Zgorzynska E., Dziedzic B., Gorzkiewicz A., Stulczewski D., Bielawska K., Su K.P., Walczewska A. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol. Rep. 2017;69:935–942. doi: 10.1016/j.pharep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 150.Zhang M., Wang S., Mao L., Leak R.K., Shi Y., Zhang W., Hu X., Sun B., Cao G., Gao Y., et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014;34:1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mildenberger J., Johansson I., Sergin I., Kjobli E., Damas J.K., Razani B., Flo T.H., Bjorkoy G. N-3 PUFAs induce inflammatory tolerance by formation of KEAP1-containing SQSTM1/p62-bodies and activation of NFE2L2. Autophagy. 2017;13:1664–1678. doi: 10.1080/15548627.2017.1345411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Picou F., Debeissat C., Bourgeais J., Gallay N., Ferrie E., Foucault A., Ravalet N., Maciejewski A., Vallet N., Ducrocq E., et al. n-3 Polyunsaturated fatty acids induce acute myeloid leukemia cell death associated with mitochondrial glycolytic switch and Nrf2 pathway activation. Pharmacol. Res. 2018;136:45–55. doi: 10.1016/j.phrs.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 153.Lee S.E., Kim G.D., Yang H., Son G.W., Park H.R., Cho J.J., Ahn H.J., Park C.S., Park Y.S. Effects of Eicosapentaenoic Acid on the Cytoprotection Through Nrf2-Mediated Heme Oxygenase-1 in Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2015;66:108–117. doi: 10.1097/FJC.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 154.Palanisamy K., Krishnaswamy R., Paramasivan P., Chih-Yang H., Vishwanadha V.P. Eicosapentaenoic acid prevents TCDD-induced oxidative stress and inflammatory response by modulating MAP kinases and redox-sensitive transcription factors. Br. J. Pharmacol. 2015;172:4726–4740. doi: 10.1111/bph.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vara-Messler M., Mukdsi J.H., Osieki N.I., Benizio E., Repossi G.M., Ajayi E.I.O., Garcia N.H. Eicosapentaenoic acid prevents salt sensitivity in diabetic rats and decreases oxidative stress. Nutrition. 2020;72:110644. doi: 10.1016/j.nut.2019.110644. [DOI] [PubMed] [Google Scholar]
- 156.Jamil M.U., Kim J., Yum H.W., Kim S.H., Kim S.J., Kim D.H., Cho N.C., Na H.K., Surh Y.J. 17-Oxo-docosahexaenoic acid induces Nrf2-mediated expression of heme oxygenase-1 in mouse skin in vivo and in cultured murine epidermal cells. Arch. Biochem. Biophys. 2020;679:108156. doi: 10.1016/j.abb.2019.108156. [DOI] [PubMed] [Google Scholar]
- 157.Tsai C.H., Shen Y.C., Chen H.W., Liu K.L., Chang J.W., Chen P.Y., Lin C.Y., Yao H.T., Li C.C. Docosahexaenoic acid increases the expression of oxidative stress-induced growth inhibitor 1 through the PI3K/Akt/Nrf2 signaling pathway in breast cancer cells. Food Chem. Toxicol. 2017;108:276–288. doi: 10.1016/j.fct.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 158.Johansson I., Monsen V.T., Pettersen K., Mildenberger J., Misund K., Kaarniranta K., Schonberg S., Bjorkoy G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy. 2015;11:1636–1651. doi: 10.1080/15548627.2015.1061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Y.C., Lii C.K., Wei Y.L., Li C.C., Lu C.Y., Liu K.L., Chen H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J. Nutr. Biochem. 2013;24:204–212. doi: 10.1016/j.jnutbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 160.Zhu W., Cui G., Li T., Chen H., Zhu J., Ding Y., Zhao L. Docosahexaenoic Acid Protects Traumatic Brain Injury by Regulating NOX2 Generation via Nrf2 Signaling Pathway. Neurochem. Res. 2020;45:1839–1850. doi: 10.1007/s11064-020-03078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhu W., Ding Y., Kong W., Li T., Chen H. Docosahexaenoic Acid (DHA) Provides Neuroprotection in Traumatic Brain Injury Models via Activating Nrf2-ARE Signaling. Inflammation. 2018;41:1182–1193. doi: 10.1007/s10753-018-0765-z. [DOI] [PubMed] [Google Scholar]
- 162.Takanezawa Y., Nakamura R., Hamaguchi M., Yamamoto K., Sone Y., Uraguchi S., Kiyono M. Docosahexaenoic acid enhances methylmercury-induced endoplasmic reticulum stress and cell death and eicosapentaenoic acid potentially attenuates these effects in mouse embryonic fibroblasts. Toxicol. Lett. 2019;306:35–42. doi: 10.1016/j.toxlet.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Yamagata K. Dietary docosahexaenoic acid inhibits neurodegeneration and prevents stroke. J. Neurosci. Res. 2020 doi: 10.1002/jnr.24728. [DOI] [PubMed] [Google Scholar]
- 164.Chen X.Q., Wu S.H., Zhou Y., Tang Y.R. Lipoxin A4-induced heme oxygenase-1 protects cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE complex. PLoS ONE. 2013;8:e67120. doi: 10.1371/journal.pone.0067120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang S., Zheng Y., Hou X. Lipoxin A4 restores oxidative stress-induced vascular endothelial cell injury and thrombosis-related factor expression by its receptor-mediated activation of Nrf2-HO-1 axis. Cell. Signal. 2019;60:146–153. doi: 10.1016/j.cellsig.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Pang H., Yi P., Wu P., Liu Z., Liu Z., Gong J., Hao H., Cai L., Ye D., Huang Y. Effect of lipoxin A4 on lipopolysaccharide-induced endothelial hyperpermeability. Sci. World J. 2011;11:148208. doi: 10.1100/tsw.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu L., Li H.H., Wu Q., Miao S., Liu Z.J., Wu P., Ye D.Y. Lipoxin A4 Activates Nrf2 Pathway and Ameliorates Cell Damage in Cultured Cortical Astrocytes Exposed to Oxygen-Glucose Deprivation/Reperfusion Insults. J. Mol. Neurosci. 2015;56:848–857. doi: 10.1007/s12031-015-0525-6. [DOI] [PubMed] [Google Scholar]
- 168.Guo Y., Dong C., Tang J., Deng X., Wang G., Wang W., Xu H. Lipoxin A4 alleviates cerebral ischemia-reperfusion injury through up-regulating Nrf2. J. Neurosurg. Sci. 2018;62:225–226. doi: 10.23736/S0390-5616.17.04164-9. [DOI] [PubMed] [Google Scholar]
- 169.Lu T., Wu X., Wei N., Liu X., Zhou Y., Shang C., Duan Y., Dong Y. Lipoxin A4 protects against spinal cord injury via regulating Akt/nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 signaling. Biomed. Pharmacother. 2018;97:905–910. doi: 10.1016/j.biopha.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 170.Wu L., Liu Z.J., Miao S., Zou L.B., Cai L., Wu P., Ye D.Y., Wu Q., Li H.H. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol. Res. 2013;35:968–975. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- 171.Cheng X., He S., Yuan J., Miao S., Gao H., Zhang J., Li Y., Peng W., Wu P. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic. Biol. Med. 2016;93:52–66. doi: 10.1016/j.freeradbiomed.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 172.Ye W., Zheng C., Yu D., Zhang F., Pan R., Ni X., Shi Z., Zhang Z., Xiang Y., Sun H., et al. Lipoxin A4 Ameliorates Acute Pancreatitis-Associated Acute Lung Injury through the Antioxidative and Anti-Inflammatory Effects of the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2019;2019:2197017. doi: 10.1155/2019/2197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin H., Zhang J., Ni T., Lin N., Meng L., Gao F., Luo H., Liu X., Chi J., Guo H. Yellow Wine Polyphenolic Compounds prevents Doxorubicin-induced cardiotoxicity through activation of the Nrf2 signalling pathway. J. Cell. Mol. Med. 2019;23:6034–6047. doi: 10.1111/jcmm.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han X., Yao W., Liu Z., Li H., Zhang Z.J., Hei Z., Xia Z. Lipoxin A4 Preconditioning Attenuates Intestinal Ischemia Reperfusion Injury through Keap1/Nrf2 Pathway in a Lipoxin A4 Receptor Independent Manner. Oxidative Med. Cell. Longev. 2016;2016:9303606. doi: 10.1155/2016/9303606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu S.H., Wang M.J., Lu J., Chen X.Q. Signal transduction involved in lipoxin A4induced protection of tubular epithelial cells against hypoxia/reoxygenation injury. Mol. Med. Rep. 2017;15:1682–1692. doi: 10.3892/mmr.2017.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moreno C., Prieto P., Macias A., Pimentel-Santillana M., de la Cruz A., Traves P.G., Bosca L., Valenzuela C. Modulation of voltage-dependent and inward rectifier potassium channels by 15-epi-lipoxin-A4 in activated murine macrophages: Implications in innate immunity. J. Immunol. 2013;191:6136–6146. doi: 10.4049/jimmunol.1300235. [DOI] [PubMed] [Google Scholar]
- 177.Martinez R.M., Fattori V., Saito P., Pinto I.C., Rodrigues C.C.A., Melo C.P.B., Bussmann A.J.C., Staurengo-Ferrari L., Bezerra J.R., Vignoli J.A., et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules. 2020;25:2953. doi: 10.3390/molecules25122953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaen R.I., Fernandez-Velasco M., Terron V., Sanchez-Garcia S., Zaragoza C., Canales-Bueno N., Val-Blasco A., Vallejo-Cremades M.T., Bosca L., Prieto P. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 2020 doi: 10.1096/fj.202000611R. [DOI] [PubMed] [Google Scholar]
- 179.Wu X., Pan C., Chen R., Zhang S., Zhai Y., Guo H. BML-111 attenuates high glucose-induced inflammation, oxidative stress and reduces extracellular matrix accumulation via targeting Nrf2 in rat glomerular mesangial cells. Int. Immunopharmacol. 2020;79:106108. doi: 10.1016/j.intimp.2019.106108. [DOI] [PubMed] [Google Scholar]
- 180.Cao Y., Zhou X., Yin Z., Yu X., Yang Q., Guo Q., Tian D., Xiong X., Xu G., Kuang X. The anti-inflammatory effect of BML-111 on COPD may be mediated by regulating NLRP3 inflammasome activation and ROS production. Prostaglandins Other Lipid Mediat. 2018;138:23–30. doi: 10.1016/j.prostaglandins.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 181.Mostafa D.G., Satti H.H. Resolvin D1 Prevents the Impairment in the Retention Memory and Hippocampal Damage in Rats Fed a Corn Oil-Based High Fat Diet by Upregulation of Nrf2 and Downregulation and Inactivation of p(66)Shc. Neurochem. Res. 2020;45:1576–1591. doi: 10.1007/s11064-020-03022-1. [DOI] [PubMed] [Google Scholar]
- 182.Sun Z., Wang F., Yang Y., Wang J., Sun S., Xia H., Yao S. Resolvin D1 attenuates ventilator-induced lung injury by reducing HMGB1 release in a HO-1-dependent pathway. Int. Immunopharmacol. 2019;75:105825. doi: 10.1016/j.intimp.2019.105825. [DOI] [PubMed] [Google Scholar]
- 183.Shu Y., Liu Y., Li X., Cao L., Yuan X., Li W., Cao Q. Aspirin-Triggered Resolvin D1 Inhibits TGF-beta1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress. Biomol. Ther. 2016;24:132–139. doi: 10.4062/biomolther.2015.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Posso S.V., Quesnot N., Moraes J.A., Brito-Gitirana L., Kennedy-Feitosa E., Barroso M.V., Porto L.C., Lanzetti M., Valenca S.S. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int. Immunopharmacol. 2018;56:330–338. doi: 10.1016/j.intimp.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 185.Hu X., Shen H., Wang Y., Zhang L., Zhao M. Aspirin-triggered resolvin D1 alleviates paraquat-induced acute lung injury in mice. Life Sci. 2019;218:38–46. doi: 10.1016/j.lfs.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 186.Saito P., Melo C.P.B., Martinez R.M., Fattori V., Cezar T.L.C., Pinto I.C., Bussmann A.J.C., Vignoli J.A., Georgetti S.R., Baracat M.M., et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018;9:1242. doi: 10.3389/fphar.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Z., Hu X., Qi X., Di G., Zhang Y., Wang Q., Zhou Q. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol. Vis. 2018;24:274–285. [PMC free article] [PubMed] [Google Scholar]
- 188.Soto G., Rodriguez M.J., Fuentealba R., Treuer A.V., Castillo I., Gonzalez D.R., Zuniga-Hernandez J. Maresin 1, a Proresolving Lipid Mediator, Ameliorates Liver Ischemia-Reperfusion Injury and Stimulates Hepatocyte Proliferation in Sprague-Dawley Rats. Int. J. Mol. Sci. 2020;21:540. doi: 10.3390/ijms21020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qiu S., Li P., Zhao H., Li X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020;78:106018. doi: 10.1016/j.intimp.2019.106018. [DOI] [PubMed] [Google Scholar]
- 190.Qiu Y., Wu Y., Zhao H., Sun H., Gao S. Maresin 1 mitigates renal ischemia/reperfusion injury in mice via inhibition of the TLR4/MAPK/NF-kappaB pathways and activation of the Nrf2 pathway. Drug Des. Dev. Ther. 2019;13:739–745. doi: 10.2147/DDDT.S188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun Q., Wu Y., Zhao F., Wang J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017;2017:9634803. doi: 10.1155/2017/9634803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Helal M.G., El-Kashef D.H. Krill oil alleviates oxidative stress, iron accumulation and fibrosis in the liver and spleen of iron-overload rats. Environ. Sci. Pollut. Res. Int. 2020;27:3950–3961. doi: 10.1007/s11356-019-06983-1. [DOI] [PubMed] [Google Scholar]
- 193.O’Connell T.D., Mason R.P., Budoff M.J., Navar A.M., Shearer G.C. Mechanistic insights into cardiovascular protection for omega-3 fatty acids and their bioactive lipid metabolites. Eur. Heart J. Suppl. 2020;22:J3–J20. doi: 10.1093/eurheartj/suaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shabani P., Ghazizadeh Z., Gorgani-Firuzjaee S., Molazem M., Rajabi S., Vahdat S., Azizi Y., Doosti M., Aghdami N., Baharvand H. Cardioprotective effects of omega-3 fatty acids and ascorbic acid improve regenerative capacity of embryonic stem cell-derived cardiac lineage cells. Biofactors. 2019;45:427–438. doi: 10.1002/biof.1501. [DOI] [PubMed] [Google Scholar]
- 195.Mayyas F., Jaradat R., Alzoubi K.H. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr. Metab. Cardiovasc. Dis. 2018;28:592–599. doi: 10.1016/j.numecd.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 196.O’Connell T.D., Block R.C., Huang S.P., Shearer G.C. omega3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017;103:74–92. doi: 10.1016/j.yjmcc.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Eclov J.A., Qian Q., Redetzke R., Chen Q., Wu S.C., Healy C.L., Ortmeier S.B., Harmon E., Shearer G.C., O’Connell T.D. EPA, not DHA, prevents fibrosis in pressure overload-induced heart failure: Potential role of free fatty acid receptor 4. J. Lipid Res. 2015;56:2297–2308. doi: 10.1194/jlr.M062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siddesha J.M., Valente A.J., Yoshida T., Sakamuri S.S., Delafontaine P., Iba H., Noda M., Chandrasekar B. Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration. Cell Signal. 2014;26:933–941. doi: 10.1016/j.cellsig.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baum J.R., Dolmatova E., Tan A., Duffy H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol. 2012;3:272. doi: 10.3389/fphys.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ramadeen A., Connelly K.A., Leong-Poi H., Hu X., Fujii H., Laurent G., Domenichiello A.F., Bazinet R.P., Dorian P. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012;5:978–983. doi: 10.1161/CIRCEP.112.971515. [DOI] [PubMed] [Google Scholar]
- 201.Takamura M., Kurokawa K., Ootsuji H., Inoue O., Okada H., Nomura A., Kaneko S., Usui S. Long-Term Administration of Eicosapentaenoic Acid Improves Post-Myocardial Infarction Cardiac Remodeling in Mice by Regulating Macrophage Polarization. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ito S., Sano Y., Nagasawa K., Matsuura N., Yamada Y., Uchinaka A., Murohara T., Nagata K. Highly purified eicosapentaenoic acid ameliorates cardiac injury and adipose tissue inflammation in a rat model of metabolic syndrome. Obes. Sci. Pract. 2016;2:318–329. doi: 10.1002/osp4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nagai T., Anzai T., Mano Y., Kaneko H., Anzai A., Sugano Y., Maekawa Y., Takahashi T., Yoshikawa T., Fukuda K. Eicosapentaenoic acid suppresses adverse effects of C-reactive protein overexpression on pressure overload-induced cardiac remodeling. Heart Vessel. 2013;28:404–411. doi: 10.1007/s00380-012-0270-5. [DOI] [PubMed] [Google Scholar]
- 204.Kitamura K., Shibata R., Tsuji Y., Shimano M., Inden Y., Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1814–H1821. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- 205.Guzman-Rivera D., Liempi A., Gonzalez-Herrera F., Fuentes-Retamal S., Carrillo I., Abarca P., Castillo C., Kemmerling U., Pesce B., Maya J.D. Simvastatin Improves Cardiac Function through Notch 1 Activation in BALB/c Mice with Chronic Chagas Cardiomyopathy. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02141-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jacobson K.A., Boeynaems J.M. P2Y nucleotide receptors: Promise of therapeutic applications. Drug Discov. Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ye Y., Birnbaum G.D., Perez-Polo J.R., Nanhwan M.K., Nylander S., Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arter. Thromb. Vasc. Biol. 2015;35:1805–1814. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]