Abstract
Background
Esophageal squamous cell carcinoma (ESCC) is a common and fatal malignancy, which has posed a great challenge to public health, especially in China. Dysregulation of long non-coding RNAs is involved in the occurrence, development, invasion, and metastasis of multiple cancers including ESCC. However, little is known about the function of MIR205HG in ESCC.
Methods
We used qRT-PCR to detect the expression level of MIR205HG, miR-214, and SOX4 in human ESCC tissues and cell lines. Loss-of-functional assays were performed to test the impact of MIR205HG on cell proliferation, metastasis, and apoptosis process via CCK-8, transwell, and flow cell cytometry assays. Additionally, the downstream molecular mechanism of MIR205HG in ESCC was explored.
Results
Here, we found MIR205HG was substantially up-regulated in ESCC, and there was a positive correlation between MIR205HG expression and tumor size and lymphatic metastasis of ESCC patients. Inhibition of MIR205HG attenuated cell proliferation, migration, and invasion. Silencing MIR205HG increased G1 phase cell counts and decreased S phase cell counts, along with increased apoptotic cell populations. Notably, the rescue assays indicated that miR-214 could partly reverse the influence of MIR205HG on ESCC cell migration. We also found that SOX4 was a direct target mRNA of miR-214, and MIR205HG could act as a molecular sponge to regulate SOX4 expression in ESCC.
Conclusion
Taken together, our findings demonstrate that MIR205HG promotes ESCC progression by regulating the miR-214/SOX4 axis. MIR205HG may be a novel candidate target for ESCC diagnosis and therapy.
Keywords: MIR205HG, lncRNA, ESCC, miR-214, SOX4
Introduction
Esophageal cancer (EC) is one of the most aggressive tumors with high cancer-related mortality worldwide, among which esophageal squamous cell carcinoma (ESCC) is the most prevalent subtype of EC in China.1,2 Despite intensive clinical advances including chemotherapy, radio-chemotherapy and esophagectomy, the overall 5-year survival rate of ESCC remains poor, due to the high recurrence and metastasis rate.3 Therefore, a deeper understanding of the mechanisms underlying ESCC progression is imperative to improve clinical outcomes for ESCC patients.
Recently, global transcriptional analyses have identified that mammalian genomes contain numerous long non-coding RNA (lncRNA), which is defined as a class of non-coding RNAs whose length is longer than 200 nt.4,5 Existing researches revealed that lncRNA could serve as the biomarker for prognosis and diagnosis in multiple cancers.6,7 Notably, studies over the past years have demonstrated that lncRNAs are frequently dysregulated and play a pivotal role in tumor metastasis, including ESCC. For instance, Chu et al found that lncRNA MNX1-AS1 was up-regulated in ESCC tissues and MNX1-AS1 expression was positively associated with lymphatic metastasis. Further analysis suggested MNX1-AS1 drove ESCC growth by negatively regulating SIRT1 expression via sponging miR-34a.8 Yang et al found that FEZF1-AS1 was strongly expressed in ESCC and FEZF1-AS1 facilitated ESCC metastasis progression by regulating the Wnt/β-catenin pathway.9 These findings highlighted the far-reaching impact of lncRNAs during the ESCC carcinogenesis.
MIR205HG, also named as LINC00510, whose transcript length is 4173 bp. It has been reported that MIR205HG functioned as an oncogenic regulator to promote certain types of cancer progression.10,11 For example, MIR205HG depletion suppressed cell proliferation, migration and clonogenic capacity through sponging miR-590-3p in head and neck squamous cell carcinoma.12 Besides, MIR205HG promoted proliferation malignant progression of cervical cells by sponging miR-122 to upregulate FOXP2 expression.13 However, the function and the concrete molecular mechanism of MIR205HG in ESCC development and progression were still unknown at present.
Herein, we found that MIR205HG was remarkably increased in ESCC tissues and MIR205HG was positively associated with tumor size and lymphatic metastasis of ESCC patients. The receiver operating characteristic (ROC) analysis showed MIR205HG possessed a great potential to act as a diagnostic biomarker for ESCC. Functionally, MIR205HG promoted ESCC cell growth, migration, and invasion, and regulated cell cycle and apoptotic process. Moreover, we also unveiled that MIR205HG was involved in the ESCC progression by regulating the miR-214/SOX4 axis.
Materials and Methods
Tissue Samples
A total of 45 ESCC patients who did not receive chemotherapy or radiotherapy in The First Affiliated Hospital of Zhengzhou University from July 2019 to December 2019 were enrolled in the current study. Fresh ESCC tissues and matched adjacent non-tumor tissues were obtained after the surgery and stored at −80°C till RNA extraction, and the clinical characteristics of ESCC patients were collected. The protocol was approved by The First Affiliated Hospital of Zhengzhou University and informed consents from all patients were obtained before implementation.
Cell Lines and Cell Culture
ESCC cell lines (KYSE30 and EC109) used in this study were purchased from Shanghai institutes of life science cell bank center (Shanghai, China) and were preserved in DMEM medium (Hyclone, UT, USA) which contained 10% fetal bovine serum (FBS, Hyclone, UT, USA), and 1% penicillin-streptomycin. Cell lines were maintained at 37°C in a humidified atmosphere with 5% CO2 and confirmed without mycoplasma contamination. ESCC cells were routinely stored at the cell saving (New Cell& Molecular Biotech, Suzhou, China) and cell culture dishes were purchased from Hangzhou Xinyou Biotechnology Co., Ltd.
RNA Extraction and qRT-PCR Analysis
TriZol reagent (Takara, Dalian, China) was used to extract total RNA from frozen tissues and cells according to the manufacturer’s instruction. Then, cDNA of lncRNA and mRNA was reversed using the PrimeScript™RT reagent Kit with gDNA Eraser (Takara, Dalian, China). While cDNA of miRNA was synthesized with miRNA 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). Then, qRT-PCR was performed with HieffR qPCR SYBRR Green Master Mix (Yeasen, Shanghai, China) on QuautStudio-5 manufacture. Relative expression levels of lncRNA and mRNA or miRNA were normalized to the β-Actin or U6, respectively.
Cell Transfection
Small interfering RNA targeting MIR205HG (si-MIR205HG), scramble siRNA of MIR205HG (si-NC), miR-214 inhibitor, and miR-214 negative control (control) sequences were designed by GenePharma (Shanghai, China). ESCC cells were seeded in 6-well plates and incubated for 24 h, then cells were transfected with Lipofectamine 3000 (Invitrogen) according to manufacturer’s recommendation. Following transfection 48h, we collected cells to perform further experiments. The sequences of the 3 siRNAs were listed as follows:
si-MIR205HG#1 Sense 5ʹ-UCUCCUUCAAUUCCACUUUTT-3ʹ, Anti-sense 5ʹ-AAAGUGGAAUUGAAGGAGATT-3ʹ; si-MIR205HG#2 Sense 5ʹ-GCUGAACUGGGUGCUUUAUTT-3ʹ, Anti-sense 5ʹ-AUAAAGCACCCAGUUCAGCTT-3ʹ; si-MIR20HG#3 Sense 5ʹ-GAGACAGCCAGAGAGAAAUTT-3ʹ, Anti-sense 5ʹ-AUUUCUCUCUGGCUGUCUCTT-3ʹ.
Cell Proliferation Assay
ESCC cells’ growth rate was assessed by CCK-8 Kit (Dojindo, Japan) as previously described.14 According to manufacturer’s protocol, cells transfected with si-MIR205HG and si-NC were seeded in 96-well plates (2000 cells per well). After a continuous incubation for 24h, 48h, 72 h and 96 h, 10 μL CCK-8 reagent was then added into each well at 37°C. Finally, the absorbance of the cell medium at 450 nm was detected using Molecular Devices (SpectraMax M5, USA).
Transwell Assays
We performed Transwell migration and invasion assays to explore the metastatic potential of ESCC cells as described before.15,16 A number of 2×105 cells were placed in the upper chamber covered or uncovered with a Matrigel in 200 μL serum-free DMEM accompanied with the 650 μL medium containing 15% FBS at the lower chamber. After incubation for 24h at 37°C, the cells remaining on the top side of the membrane were scratched away. Then, we used 4% paraformaldehyde to fix cells and stained with 0.1% crystal violet. Finally, cells were calculated and discerned by the microscope.
Cell Cycle and Cell Apoptosis Assays
Cell cycle and apoptosis assays were performed according to the Cell Cycle and Apoptosis Analysis Kit (Beyotime, China) instructions. Cells cultured in the six-well plate were transfected with siRNAs. After transfection for 48h, cells were collected and cleaned with cooling PBS. For cell cycle assay, cells were fixed with 70% cooling ethanol at 4°C overnight. Afterwards, cells were stained with propidium iodide (PI) solution containing RNase A after washed with cold PBS for two times. After incubation for 30min at room temperature in the dark, cell cycle was examined by flow cytometry (BD Accuri™ C6 Plus). For apoptosis assay, cells were stained with annexin V/PI double staining and the cell suspension was measured by flow cytometry according to the manufacturer’s protocol. ModFit 4.1 and FlowJo10.0 were used to analyze cell cycle and apoptosis data, respectively.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism8. For comparisons, one-way analyses of variance, Student’s t-tests, Fisher exact test, χ2 test were performed. Pearson’s correlation analysis was used to analyze correlations between two variances. Survival curves were plotted with the Kaplan–Meier method and were analyzed with the Log rank test. P <0.05 were considered as statistically significant.
Results
MIR205HG is Significantly Upregulated in ESCC Tissues
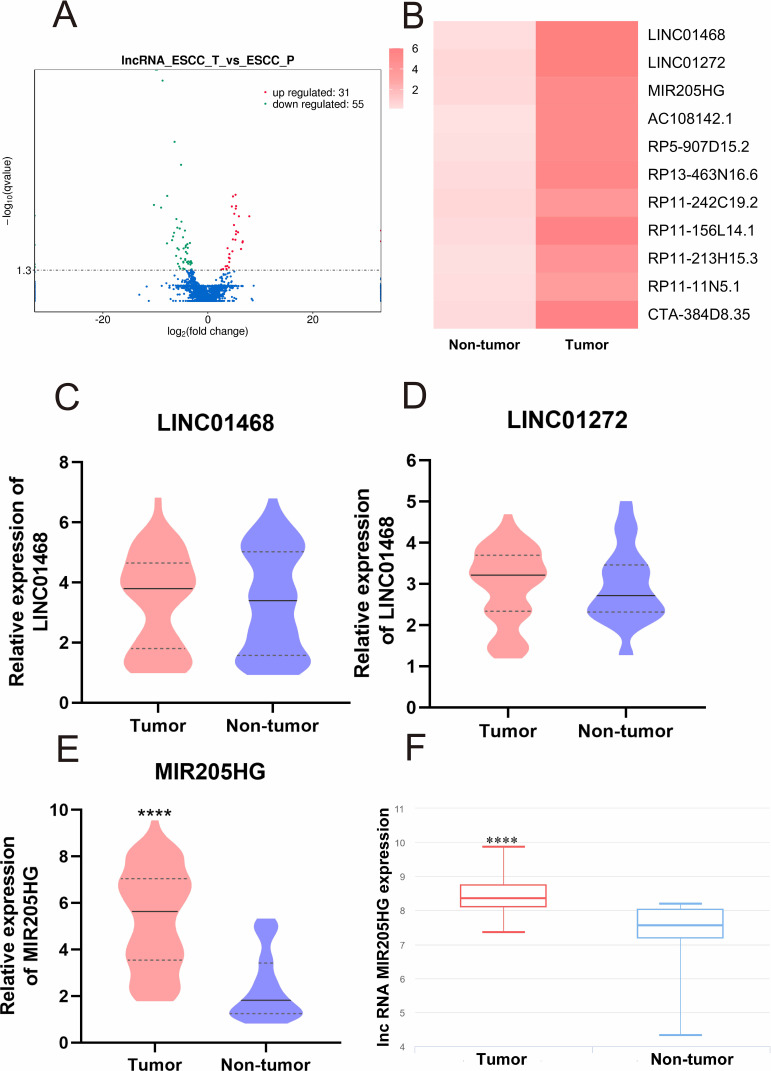
The whole transcriptome analysis of three ESCC and adjacent normal tissues was performed with RNA-sequencing. We found that a total of 31 upregulated and 55 downregulated lncRNAs in our ESCC samples (Figure 1A). The top 10 upregulated lncRNAs in ESCC tissues are shown in Figure 1B. Next, we chose three up-regulated statistically lncRNAs (LINC01468, LINC01272, MIR205HG) to verify their expression in 45 paired ESCC tissues by qRT-PCR. The expression of LINC01468 and LINC01272 in ESCC tissues and adjacent non-tumor tissues had no statistical significance (Figure 1C and D). However, MIR205HG was robustly upregulated in ESCC tissues compared to adjacent non-tumor tissues (Figure 1E). Bioinformatic data from lnCAR17 further corroborated the high expression of MIR205HG in ESCC (Figure 1F). Therefore, we focused on MIR205HG for the following studies.
Figure 1.
MIR205HG was significantly elevated in ESCC tissues. (A) The volcano plot showed the differential expression lncRNAs in ESCC tissues. (B) Heatmap analysis showed the top 10 up-regulated lncRNAs in ESCC tissues. (C and D) LINC01468 and LINC01272 were not statistically up-regulated in 45 ESCC tissues. (E) MIR205HG expression abundance was significantly increased in ESCC tissues. (F) The expression of MIR205HG in ESCC from the lnCAR database. ****P<0.0001.
The Association Between MIR205HG Expression and Clinical-Pathological Factors in ESCC
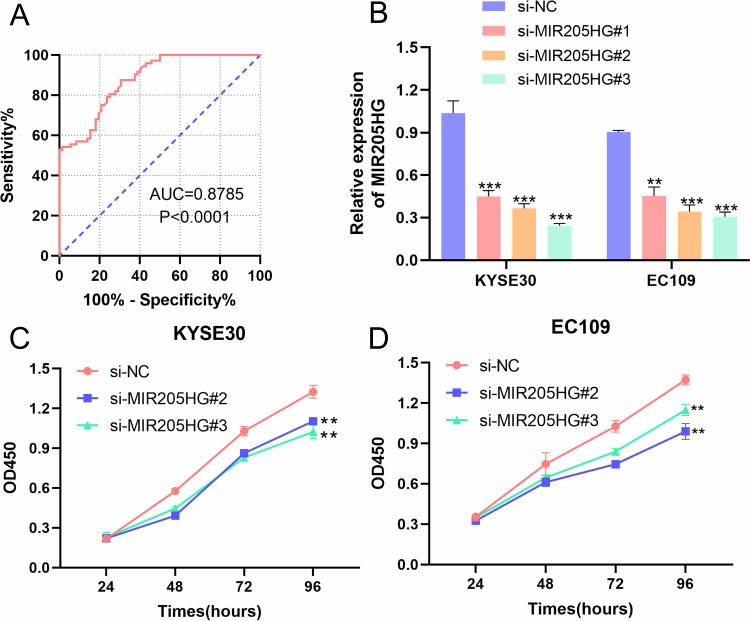
We collected clinical-pathological data of ESCC patients and dissected the association between MIR205HG expression and clinical features of ESCC patients. We observed that MIR205HG expression was significantly related to lymph node metastasis (P=0.0251, Table 1) and tumor size (P=0.0106, Table 1), but there was no relationship between MIR205HG expression level and differentiation grade, TNM stage, age or gender (Table 1). Moreover, we performed the ROC analysis to evaluate the diagnostic accuracy of MIR205HG in ESCC. Our investigations demonstrated that MIR205HG could be a diagnosis biomarker for ESCC (AUC = 0.8785; Youden index J=0.5694, P<0.0001, 95% confidence interval, CI=0.8260–0.9310, Figure 2A). The sensitivity and specificity were 87.5% and 69.44%, respectively. Overall, these findings implied the oncogenic property of MIR205HG in ESCC development.
Table 1.
The Association of MIR205HG Expression and Clinical Features of ESCC
| Variables | N | MIR205HG Expression | P-value | ||
|---|---|---|---|---|---|
| High (23) | Low (22) | ||||
| Gender | Male | 36 | 16 | 20 | 0.1346 |
| Female | 9 | 7 | 2 | ||
| Age | ≤65 years | 25 | 9 | 16 | 0.0955 |
| >65 years | 20 | 14 | 6 | ||
| Tumor size | ≤3cm | 14 | 3 | 11 | 0.0106* |
| >3cm | 31 | 20 | 11 | ||
| Differentiation grade | Poor | 9 | 5 | 4 | 1.0000 |
| Moderate | 36 | 18 | 18 | ||
| TNM stage | I+II | 18 | 11 | 7 | 0.2732 |
| III+IV | 27 | 12 | 15 | ||
| Lymphatic metastasis | Negative | 23 | 8 | 15 | 0.0251* |
| Positive | 22 | 15 | 7 | ||
Note: *P<0.05.
Figure 2.
The association between MIR205HG and clinical features of ESCC patients. (A) The results of the ROC analysis of MIR205HG in ESCC. (B) The si-MIR205HG#2 and si-MIR205HG #3 suppressed MIR205HG expression in KYSE30 and EC109 cells. (C) MIR205HG knockdown inhibited KYSE30 cell proliferation. (D) The cell growth rate of the EC109 cell was reduced when silencing of MIR205HG. **P<0.01, ***P<0.001.
The Influence of MIR205HG on ESCC Cell Proliferation
To depict the biological function of MIR205HG in ESCC cells, we designed three siRNAs targeting MIR205HG to inhibit MIR205HG expression in KYSE30 and EC109 cells. We named them as si-MIR205HG#1-3. Our results showed that si-MIR205HG#2 and si-MIR205HG#3 could remarkably inhibit MIR205HG expression in ESCC cells, as evidenced by qRT-PCR results (Figure 2B). Therefore, we chose these two siRNAs for the following assays. We conducted CCK-8 assays to detect ESCC cells (KYSE30 and EC109) growth rate at designed time intervals (24 h, 48 h, 72 h, 96 h). The results suggested that the cell proliferation ability was significantly reduced after the silencing of MIR205HG in ESCC cells (Figure 2C and D).
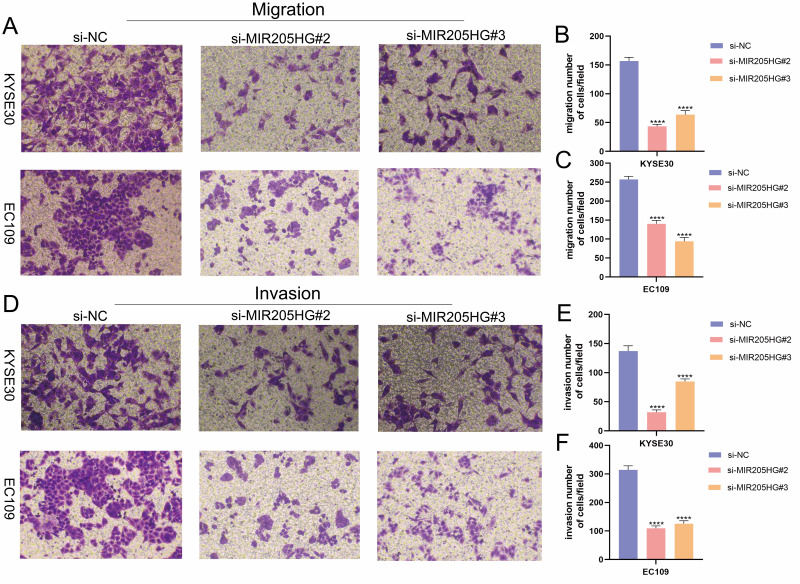
MIR205HG Increases ESCC Cell Migration and Invasion
To further investigate the role of MIR205HG in ESCC metastasis, we performed transwell migration and invasion experiments. Notably, our research identified that the downregulation of MIR205HG evidently inhibited ESCC cell migration (Figure 3A–C). Meanwhile, MIR205HG depletion prominently attenuated ESCC cell invasion, as evidenced by Matrigel-coated transwell experiments (Figure 3D–F). Taken together, these findings suggested that MIR205HG played a key role in regulating the mobility of ESCC cells.
Figure 3.
MIR205HG increased ESCC cell metastasis. (A–C) The results of the transwell invasion assay in KYSE30 and EC109 cell. (D–F) The results of the transwell migration assay in KYSE30 and EC109 cell. ****P<0.0001.
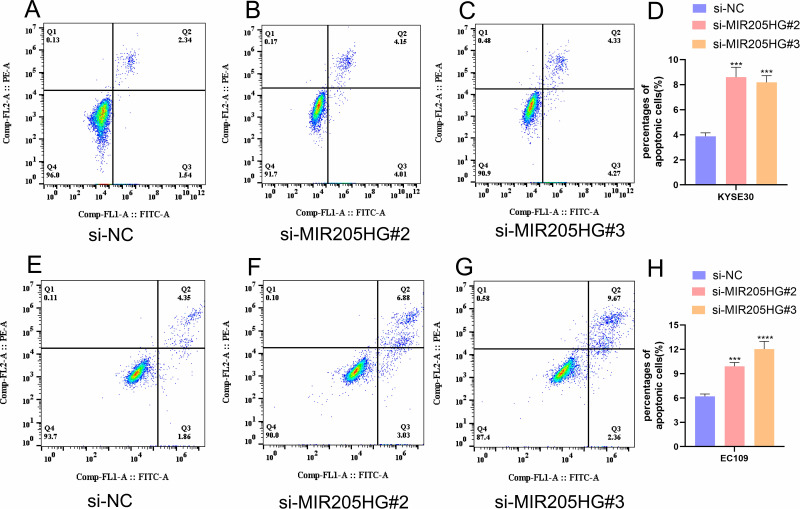
MIR205HG Regulates ESCC Cells Apoptosis Progression
To further study the association between MIR205HG expression and ESCC cells apoptosis, we detected apoptosis percentages in ESCC cell transfected with si-MIR205HG#2 or si-MIR205HG#3 through flow cytometry. We found that the number of apoptotic cells in the MIR205HG silenced group was increased compared with the negative group in KYSE30 cells (Figure 4A–D). The cell apoptosis assays of EC109 cell lines yielded the similar results (Figure 4E–H). These findings confirmed that MIR205HG could negatively regulate the ESCC cell apoptosis process.
Figure 4.
MIR205HG inhibited ESCC cell apoptosis. (A–D) The number of apoptosis cells was remarkably elevated in the si-MIR205HG group for KYSE30 cells. (E–H) The proportion of apoptosis cells increased in the si-MIR205HG group for EC109 cells. ***P<0.001, ****P<0.0001.
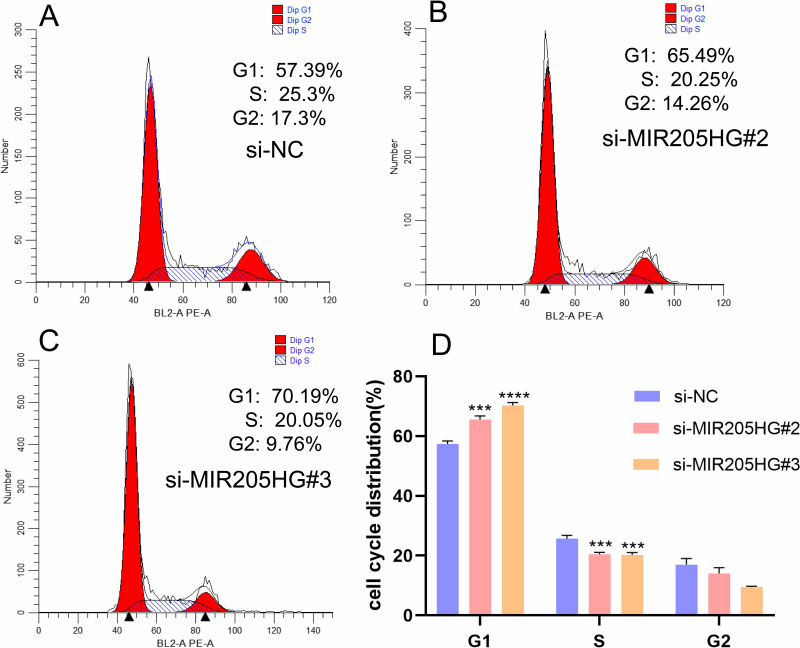
MIR205HG Expedites ESCC Cell Cycle Distribution
To further elucidate the role of MIR205HG-mediated ESCC cells growth, we detected cell cycle distribution after inhibiting MIR205HG by flow cytometry assay. The result showed that G1 phase cell percentages were significantly increased in the MIR205HG silenced group compared to the negative control group, while the proportion of the S phase was reduced after knocking down of MIR205HG (Figure 5A–D). Taken together, these data indicated that MIR205HG could efficiently accelerate the cell cycle progression in ESCC to control cell proliferation.
Figure 5.
MIR205HG accelerated ESCC cell cycle progression. (A–D) The counts of G1 phase cell was significantly increased in the MIR205HG silence group along with reduced counts of S phase cell according to flow cytometry results. ***P<0.001, ****P<0.0001.
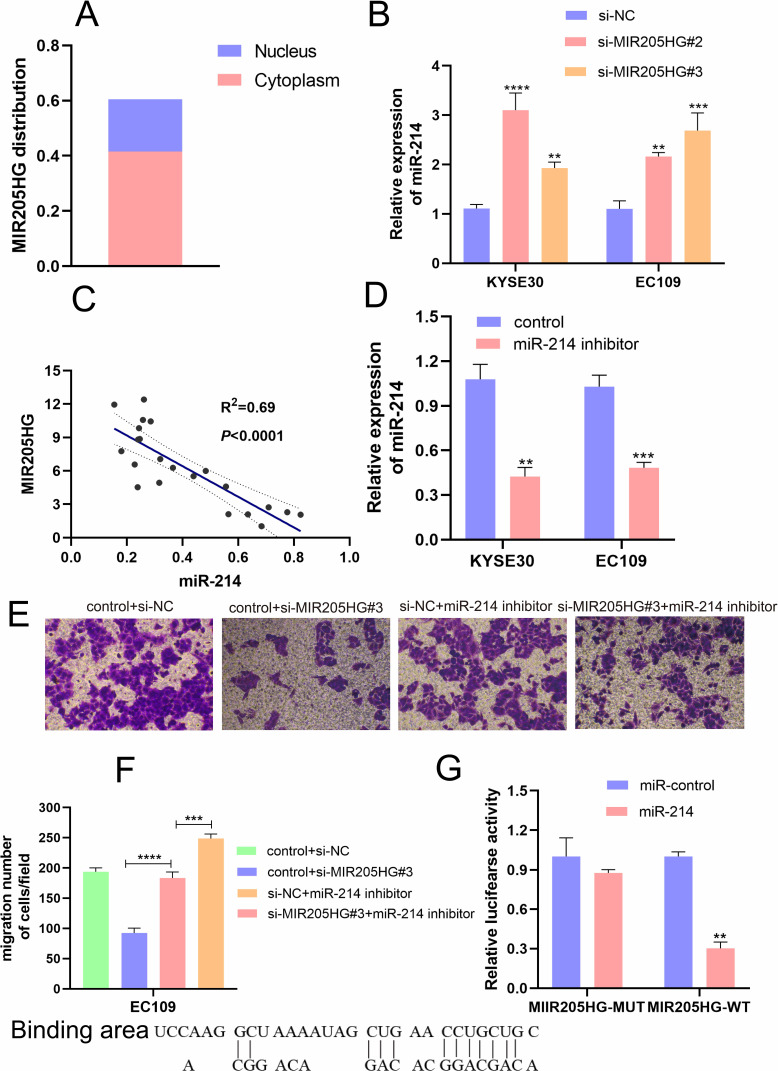
MIR205HG Promotes ESCC Cell Migration via Targeting miR-214
Recently, accumulating evidence has testified that lncRNAs could function as molecular sponge of miRNAs to regulate the expression and biological function of mRNAs. Firstly, we found that MIR205HG was located in the cytoplasm of cells using lncLocator (http://www.csbio.sjtu.edu.cn) to confirm the possibility of MIR205HG as a molecular sponge in ESCC cells (Figure 6A). Moreover, we found that miR-214 was a direct target miRNA of MIR205HG by the Annolnc database (http://annolnc.gao-lab.org/). We performed qRT-PCR to test the regulations between MIR205HG and miR-214. Our results demonstrated that miR-214 expression was remarkably increased after inhibiting MIR205HG (Figure 6B). Meanwhile, we found that miR-214 expression was significantly negatively correlated with MIR205HG expression in ESCC tissues (R2=0.69, P<0.05, Figure 6C). Previous research identified miR-214 served as an important tumor suppressor, which predominantly inhibited cell migration in multiple cancers including ESCC.18 To further test the negative regulation of MIR205HG on miR-214, we conducted rescue assays. Our results exhibited that miR-214 expression was remarkably reduced after the cell was transfected with miR-214 inhibitor (Figure 6D). Furthermore, knockdown MIR205HG inhibited cell migration while inhibiting miR-214 could partly abolish this suppression in EC109 cells (Figure 6E and F). In addition, MIR205HG could bind with miR-214 (Figure 6G). Collectively, MIR205HG enhanced ESCC cell migration via targeting miR-214.
Figure 6.
MIR205HG regulated miR-214 expression. (A) The localization of MIR205HG. (B) MIR205HG depletion increased miR-214 expression. (C) MIR205HG expression was negatively related to miR-214 expression in ESCC tissues. (D) The miR −214 inhibitor reduced miR-214 expression in KYSE30 and EC109 cells (control represents the negative control of inhibitor). (E and F) miR-214 partly compromised the migration of MIR205HG in ESCC cells. (G) The binding sites and the result of the dual-luciferase assay of MIR205HG and miR-214. **P<0.01, ***P<0.001, ****P<0.0001.
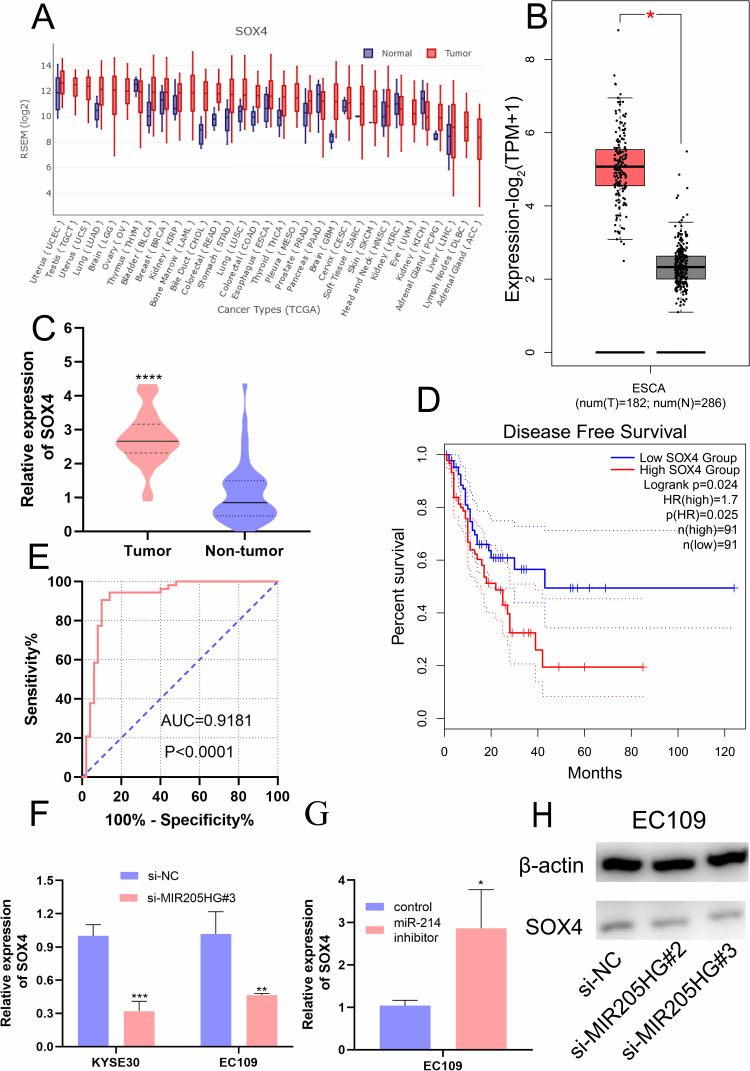
MIR205HG Upregulates SOX4 Expression by miR-214
Previous study identified that SOX4 was a direct downstream gene of miR-214 in ESCC cells.19 We found that SOX4 was up-regulated in multiple cancers including ESCC in the GEDS database (Figure 7A).20 Similarly, there was a remarkable increase of SOX4 mRNA expression in ESCC tissues based on the analysis of GEPIA database and Oncomine (https://www.oncomine.org/) (Figure 7B and C). In addition, we identified high expression of SOX4 was closely linked to disease-free survival for ESCC patients (Figure 7D) according to GEPIA. ROC analysis indicated the high accuracy of SOX4 in ESCC diagnosis (AUC=0.9181, 95% CI=0.8574 to 0.9789, Figure 7E). Moreover, we found that SOX4 was significantly downregulated at mRNA level after silencing of MIR205HG (Figure 7F). On the contrary, inhibiting miR-214 could elevate SOX4 level in ESCC cells (Figure 7G). There was a decrease of SOX4 protein in MIR205HG depletion in EC109 cells (Figure 7H). Taken together, these data demonstrated that MIR205HG drives ESCC progression via regulating the miR-214/SOX4 axis (Figure 8).
Figure 7.
The high expression of SOX4 in ESCC and the association between SOX4 and MIR205HG. (A) The expression profile of SOX4 in the pan-cancer analysis. (B and C) GEPIA and Oncomine analysis showed SOX4 was significantly elevated in ESCC. (D) The disease-free survival analysis of SOX4 in ESCC. (E) The ROC analysis of SOX4 in ESCC. (F) SOX4 expression was remarkably reduced after knocking down MIR205HG. (G) The miR −214 inhibitor increased SOX4 expression in EC109 cell. (H) The SOX4 protein was reduced after MIR205HG silence in EC109 cell. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
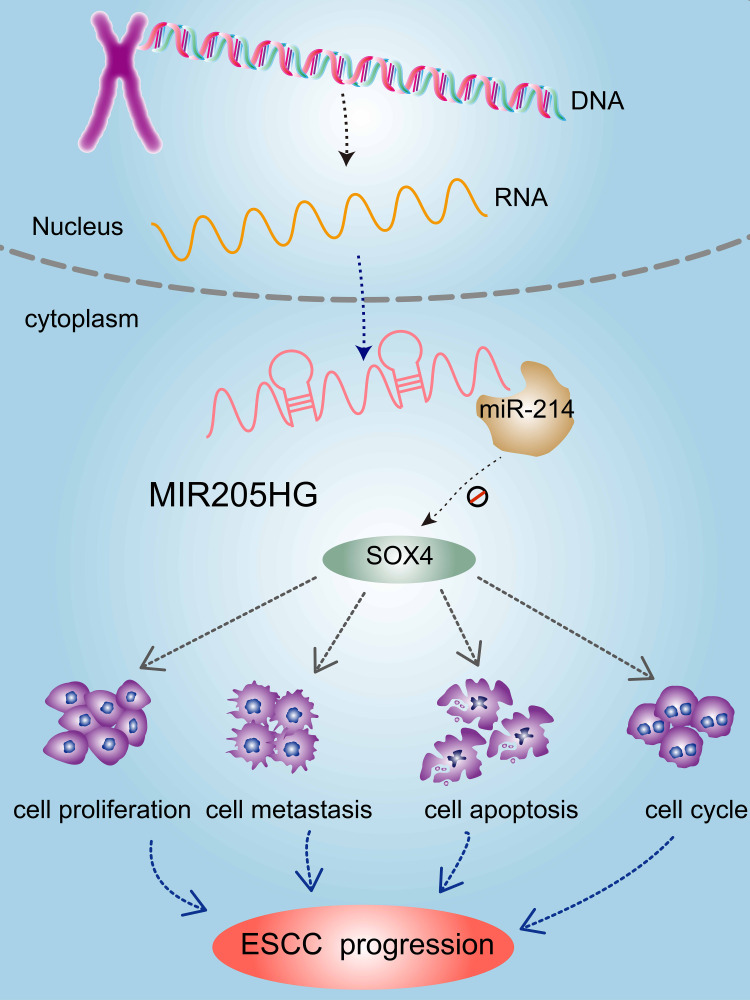
Figure 8.
Mechanistic model of oncogenic function of MIR205HG in ESCC. LncRNA MIR205HG promoted ESCC progression by regulating the miR-214/SOX4 axis.
Discussion
It was reported that MIR205HG could serve as an oncogenic modulator to contribute to cell growth, epithelial-to-mesenchymal transition (EMT), and inhibit cell apoptosis in lung cancer.10 In melanoma, the abnormal expression of MIR205HG caused the dysregulation of immune response, ultimately accelerating cancer development.21 Herein, we carried out the first study to investigate the expression profile and biological functions of MIR205HG in ESCC. We observed that MIR205HG was robustly increased in ESCC tissues through RNA-seq and qRT-PCR assays. Besides, we discovered that MIR205HG expression was tightly related to the tumor size and lymphatic metastasis of ESCC samples. ROC analysis showed the availability of MIR205HG as a potential biomarker in ESCC diagnosis. Therefore, we postulated that MIR205HG could serve as a critical target in ESCC progression. More importantly, we confirmed that deficiency of MIR205HG reduced cell proliferation, migration, and invasion capacities in ESCC. Notably, knocking down MIR205HG significantly decreased the cell numbers in the S phase and increased G1 phase cell numbers, along with elevated the numbers of apoptosis cells. All these findings confirmed our hypothesis that MIR205HG is a pivotal driving factor during the ESCC development.
A great part of literature has pinpointed that lncRNA exerts various biological functions through binding with miRNA.22,23 LncRNA PVT1 promoted cell growth and migration through competitively binding to miR-143 to modulates HK2 expression in gallbladder cancer.24 In the current study, we identified that miR-214 is a target of MIR205HG from the Annolnc database. The previous study demonstrated that miR-214 suppressed cell proliferation, migration, and invasion in ESCC.18,25 From our results, we noticed that miR-214 expression was raising sharply after the suppression of MIR205HG in ESCC cells. MIR205HG expression was negatively related to miR-214 expression in ESCC tissues. Dual-luciferase assay indicated that MIR205HG could bind with miR-214. Functionally, inhibiting miR-214 partially rescued the suppression of cell migration induced by MIR205HG depletion. Taken together, our data suggested that miR-214 is a pivotal downstream effector of MIR205HG in regulating ESCC progression.
To gain a deeper understanding of the molecular mechanism of MIR205HG in ESCC, we analyzed the target mRNA of miR-214. Interestingly, SOX4 was reported to be a direct target gene of miR-214 and SOX4 served as an important oncogene to promote ESCC development.19,26 Here, we found SOX4 was elevated in ESCC tissues and patients with high SOX4 expression had shorter overall survival rates. These findings were consistent with previous reports. More importantly, we noted that MIR205HG silencing decreased SOX4 expression at mRNA and protein levels while inhibiting miR-214 induced SOX4 expression. Altogether, these results indicated that MIR205HG promotes ESCC progression by regulating the miR-214/SOX4 axis. However, more experiments will be needed to elucidate this regulation.
Conclusion
In summary, our study demonstrated that the oncogenic phenotypes of MIR205HG in ESCC for the first time. Moreover, we identified that MIR205HG promoted ESCC progression by regulating the miR-214/SOX4 axis. These investigations implied that MIR205HG could serve as a potential efficient diagnosis biomarker and therapeutic target for ESCC.
Acknowledgments
The authors thank for the sample support from Nan Huang, Junqi Liu, Biwei Yang at the Department of Pathology from the First Affiliated Hospital of Zhengzhou University. The authors also acknowledged the Academy of Medical Sciences of the Zhengzhou University Translational Medicine platform. The study was supported by grants from the Science and Technology Project of Henan province of China (Grant No. 192102310133 and Grant No. 202102310112). Medical Science and Technology Joint Construction Project of Henan province of China (Grant No. 201901102 and Grant No. 20190325).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Teng H, Xue M, Liang J, et al. Inter- and intratumor DNA methylation heterogeneity associated with lymph node metastasis and prognosis of esophageal squamous cell carcinoma. Theranostics. 2020;10(7):3035–3048. doi: 10.7150/thno.42559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Chen G, Song Y, et al. POLE2 knockdown reduce tumorigenesis in esophageal squamous cells. Cancer Cell Int. 2020;20:388. doi: 10.1186/s12935-020-01477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly RJ. Emerging multimodality approaches to treat localized esophageal cancer. J Natl Compr Canc Netw. 2019;17(8):1009–1014. doi: 10.6004/jnccn.2019.7337 [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Chen Q, Zhao Y, et al. lncRNA CRNDE is upregulated in glioblastoma multiforme and facilitates cancer progression through targeting miR-337-3p and ELMOD2 axis. OncoTargets Ther. 2020;13:9225–9234. doi: 10.2147/OTT.S249887 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Wang F, Yang Q. Long non-coding RNA LINC01089 enhances the development of gastric cancer by sponging miR-145-5p to mediate SOX9 expression. OncoTargets Ther. 2020;13:9213–9224. doi: 10.2147/OTT.S249392 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Shen FF, Pan Y, Yang HJ, et al. Decreased expression of SPINT1-AS1 and SPINT1 mRNA might be independent unfavorable prognostic indicators in esophageal squamous cell carcinoma. OncoTargets Ther. 2019;12:4755–4763. doi: 10.2147/OTT.S206448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Yin ZH. Diagnostic value of long non-coding RNA H19, UCA1, and HOTAIR as promising biomarkers in human bladder cancer. Int J Clin Exp Pathol. 2017;10(12):11659–11665. [PMC free article] [PubMed] [Google Scholar]
- 8.Chu J, Li H, Xing Y, et al. LncRNA MNX1-AS1 promotes progression of esophageal squamous cell carcinoma by regulating miR-34a/SIRT1 axis. Biomed Pharmacother. 2019;116:109029. doi: 10.1016/j.biopha.2019.109029 [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Ye Y, Chu J, et al. Long noncoding RNA FEZF1-AS1 promotes the motility of esophageal squamous cell carcinoma through Wnt/beta-catenin pathway. Cancer Manag Res. 2019;11:4425–4435. doi: 10.2147/CMAR.S196004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Li Y, Zhang R, et al. MIR205HG acts as a ceRNA to expedite cell proliferation and progression in lung squamous cell carcinoma via targeting miR-299-3p/MAP3K2 axis. BMC Pulm Med. 2020;20(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y, Xue X, Li C, et al. MIR205HG facilitates carcinogenesis of lung squamous cell carcinoma in vitro revealed by long noncoding RNA profiling. Acta Biochim Biophys Sin (Shanghai). 2020;52(4):371–381. doi: 10.1093/abbs/gmaa006 [DOI] [PubMed] [Google Scholar]
- 12.Di Agostino S, Valenti F, Sacconi A, et al. Long non-coding MIR205HG depletes Hsa-miR-590-3p leading to unrestrained proliferation in head and neck squamous cell carcinoma. Theranostics. 2018;8(7):1850–1868. doi: 10.7150/thno.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Wang H, Huang H. Long non-coding RNA MIR205HG function as a ceRNA to accelerate tumor growth and progression via sponging miR-122-5p in cervical cancer. Biochem Biophys Res Commun. 2019;514(1):78–85. doi: 10.1016/j.bbrc.2019.04.102 [DOI] [PubMed] [Google Scholar]
- 14.Duan Y, Wang Z, Xu L, et al. lncRNA SNHG3 acts as a novel tumor suppressor and regulates tumor proliferation and metastasis via AKT/mTOR/ERK pathway in papillary thyroid carcinoma. J Cancer. 2020;11(12):3492–3501. doi: 10.7150/jca.42070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan J, Liu H, Yang L, et al. JMJD6 promotes hepatocellular carcinoma carcinogenesis by targeting CDK4. Int J Cancer. 2019;144(10):2489–2500. doi: 10.1002/ijc.31816 [DOI] [PubMed] [Google Scholar]
- 16.Wan J, Liu H, Feng Q, et al. HOXB9 promotes endometrial cancer progression by targeting E2F3. Cell Death Dis. 2018;9(5):509. doi: 10.1038/s41419-018-0556-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Xu Q, Liu M, et al. lnCAR: a comprehensive resource for lncRNAs from cancer arrays. Cancer Res. 2019;79(8):2076–2083. doi: 10.1158/0008-5472.CAN-18-2169 [DOI] [PubMed] [Google Scholar]
- 18.Huang S-D, Yuan Y, Zhuang C-W, et al. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer. 2012;11:51. doi: 10.1186/1476-4598-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Zhao W, Gao X, et al. HNF1A-AS1 promotes growth and metastasis of esophageal squamous cell carcinoma by sponging miR-214 to upregulate the expression of SOX-4. Int J Oncol. 2017;51(2):657–667. doi: 10.3892/ijo.2017.4034 [DOI] [PubMed] [Google Scholar]
- 20.Xia M, Liu C-J, Zhang Q, et al. GEDS: a gene expression display server for mRNAs, miRNAs and proteins. Cells. 2019;8(7):675. doi: 10.3390/cells8070675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Liu Z, Liu X, et al. Comprehensive analysis of a competing endogenous RNA network identifies seven-lncRNA signature as a prognostic biomarker for melanoma. Front Oncol. 2019;9:935. doi: 10.3389/fonc.2019.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu S-W, Zhang Y, Li S, et al. LncRNA TTN-AS1 promotes the progression of oral squamous cell carcinoma via miR-411-3p/NFAT5 axis. Cancer Cell Int. 2020;20:415. doi: 10.1186/s12935-020-01378-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao C, Sun G, Liu C. Long non-coding RNA SNHG6 regulates the sensitivity of prostate cancer cells to paclitaxel by sponging miR-186. Cancer Cell Int. 2020;20:381. doi: 10.1186/s12935-020-01462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JA, Yu Y, Li H, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18:16. doi: 10.1186/s12943-019-0947-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Wang L, Zhang M, et al. MiR-214 inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by targeting CDC25B. Biomed Pharmacother. 2017;95:1678–1683. doi: 10.1016/j.biopha.2017.09.048 [DOI] [PubMed] [Google Scholar]
- 26.Koumangoye RB, Andl T, Taubenslag KJ, et al. SOX4 interacts with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal cancer cells. Mol Cancer. 2015;14:24. doi: 10.1186/s12943-014-0284-y [DOI] [PMC free article] [PubMed] [Google Scholar]